Abstract
Pathogenic Yersinia cause a systemic infection in mice that is dependent on the presence of a large plasmid encoding a number of secreted virulence proteins called Yops. We previously demonstrated that a plasmid-encoded Yop, YopJ, was essential for inducing apoptosis in cultured macrophages. Here we report that YopJ is a virulence factor in mice and is important for the establishment of a systemic infection. The oral LD50 for a yopJ mutant Yersinia pseudotuberculosis increases 64-fold compared with wild-type. Although the yopJ mutant strain is able to reach the spleen of infected mice, the mutant strain seldom reaches the same high bacterial load that is seen with wild-type Yersinia strain and begins to be cleared from infected spleens on day 4 after infection. Furthermore, when in competition with wild-type Yersinia in a mixed infection, the yopJ mutant strain is deficient for spread from the Peyer's patches to other lymphoid tissue. We also show that wild-type Yersinia induces apoptosis in vivo of Mac-1+ cells from infected mesenteric lymph nodes or spleens, as measured by quantitative flow cytometry of TUNEL (Tdt-mediated dUTP–biotin nick-end labeling)-positive cells. The levels of Mac-1+, TUNEL+ cells from tissue infected with the yopJ mutant strain were equivalent to the levels detected in cells from uninfected tissue. YopJ is necessary for the suppression of TNF-α production seen in macrophages infected with wild-type Yersinia, based on previous in vitro studies (Palmer, L.E., S. Hobbie, J.E. Galan, and J.B. Bliska. 1998. Mol. Microbiol. 27:953–965). We conclude here that YopJ plays a role in the establishment of a systemic infection by inducing apoptosis and that this is consistent with the ability to suppress the production of the proinflammatory cytokine tumor necrosis factor α.
Keywords: YopJ, virulence, macrophages, TUNEL reactions, Mac-1 antibody
The genus Yersinia includes three species that are pathogenic for humans and rodents and carry the virulence plasmid, pYV (1, 2). Y. pestis is the causative agent of plague. The enteropathogenic Yersinia species, Y. enterocolitica and Y. pseudotuberculosis, cause gastrointestinal syndromes, lymphadenitis, and septicemia (2, 3). Furthermore, systemic infections can occur with formation of abscesses in the liver and spleen as well as immunopathologic sequelae such as reactive arthritis (4, 5).
In the experimental mouse model of infection, the bacteria bind to and invade the M cells within the follicle-associated epithelium overlying the lymphoid follicles of the Peyer's patches (PP),1 which are components of the gut- associated lymphoid tissue (6). The chromosomally-encoded bacterial protein, invasin, and the plasmid-encoded protein, YadA, have been shown to be involved in this binding and subsequent entry of Yersinia to the M cells within the follicle-associated epithelium (7). Once the bacteria have entered the PP, they encounter the host immune cells and serum factors. Two bacterial adhesins, Ail and YadA, also play a role in promoting Yersinia-mediated serum resistance (8–10). Several virulence factors have been implicated in the ability of Yersinia to multiply in the PP and then spread to deeper tissues (11). Many of the genes contained on the pYV plasmid encode proteins that comprise a host cell contact-dependent or type III secretory pathway. The coordinate activities of the secretion machinery and the adherence factors allows the bacteria to translocate other pYV-encoded proteins called Yops (12). Several Yops are translocated into host cells where they interfere with normal cellular processes and thus enable Y. pseudotuberculosis and Y. enterocolitica to cause systemic infections (13). YopH mediates dephosphorylation of macrophage phosphotyrosine proteins and prevents phagocytosis (14, 15). YopH has been shown to bind two focal adhesion proteins, p130Cas and FAK, and destabilize focal adhesion points within mammalian cells (16). YopE depolymerizes actin microfilaments within host cells by an unknown mechanism and also plays a role in preventing phagocytosis of Yersinia (17, 18). YopM, also a virulence determinant, has homology to a platelet glycoprotein that is a transmembrane receptor for thrombin. Culture supernatants containing YopM have been shown to competitively bind thrombin and inhibit platelet aggregation in vitro (19, 20). However, YopM has also been shown to be translocated into mammalian cells, where its function is unknown (21). YpkA (also called YopO in Y. enterocolitica) is a serine/threonine protein kinase that has been shown to cause morphological changes in cultured mammalian cells, presumably interfering with host cell signal transduction cascades (22, 23). A nonpolar ypkA mutant is attenuated in the mouse model of infection (22).
Recently, studies have shown that programmed cell death, apoptosis, is triggered in host cells in response to in vitro infection by a variety of extra- and intracellular bacterial pathogens (24–27). Apoptosis is an innate cell suicide mechanism that plays a role in homeostasis in multicellular organisms and may play a role in some infectious diseases (28). Pathogens can cause apoptosis by a variety of mechanisms including inhibition of host cell protein synthesis by bacterial A-B toxins, disruption of membrane integrity by pore-forming hemolysins, and activation of the caspase IL-1β converting enzyme (ICE) by IpaB from Shigella (27, 29). Yersinia also triggers programmed cell death in cultured macrophages (30–32). YopJ (YopP in Y. enterocolitica), another Yersinia- secreted protein, is necessary for inducing apoptosis of macrophages in vitro (31, 32). The exact mechanism by which YopJ induces apoptosis is not known, but YopJ has been shown to effect TNF-α and IL-8 production by host cells in vitro (33–35).
The cytokines, TNF-α, IFN-γ, and IL-8 play essential roles in the proinflammatory response to bacterial infection. Studies in the murine infection model of Yersiniosis provide evidence that Yops mediate suppression of the production of TNF-α and IFN-γ in vivo, and the ability of Yersinia to inhibit these proinflammatory cytokines correlates with enhanced replication within its host (36, 37). Furthermore, the addition of TNF-α and IFN-γ limits the severity of Yersinia infection (37). In cultured macrophages, Y. pseudotuberculosis and Y. enterocolitica promote deactivation of mitogen-activated protein kinases (MAPKs) p38 and JNK, and inhibit nuclear translocation of nuclear factor (NF)-κB (33–35, 38). The production of YopJ correlates with TNF-α suppression, inhibition of NF-κB, and apoptosis in cultured macrophages (35, 38).
Despite the recent advancements in molecular and cellular studies on YopJ and its effects on host cells in vitro, its role in vivo has not yet been characterized. In this study, we assess the role of YopJ in virulence by calculating the oral LD50 and measuring the colonization of tissues in mice when infected either with an equal mixture of yopJ mutant bacteria and wild-type bacteria or with the individual Yersinia strains. We also measure the amount of apoptosis in infected tissue and show that YopJ production significantly increases the levels of apoptosis in infected tissues. We conclude that the ability of wild-type Yersinia to induce apoptosis correlates with increased bacterial replication and aids in the establishment of a systemic infection.
Materials and Methods
Mice.
8–10-wk-old, female BALB/c mice were purchased from Charles River Labs. (Wilmington, MA) and kept under specific pathogen free conditions in filter-top cages. Mice were provided with sterile water and food ad libitum.
Bacterial Strains.
Several wild-type Y. pseudotuberculosis YPIIIpYV containing random Tn5 insertions in the chromosome were isolated and tested for virulence. The isolate, YPIIIpYVKm1, was shown in a competition infection in mice to colonize PP, mesenteric lymph nodes (MLNs), and spleen as well as YPIIIpYV (data not shown).
The yopJ mutant strain, YPIIIpyopJ, was constructed as follows: pypkAyopJ (32) was digested with SnaBI and HpaI and ligated, which resulted in a 1044 bp deletion spanning the start codon of yopJ. The resulting clone was digested with SstI and XbaI and ligated into the SstI and XbaI sites of a suicide vector, pCVD442 (39), containing sacB. The resulting clone, pΔyopJ, was electroporated into Escherichia coli SM10λpir and conjugated into YPIIIpYV. Exconjugants were selected on L-agar plates containing irgasan (2 μg/ml) and ampicillin (100 μg/ml). To identify double recombinants, the exconjugants were plated on L-agar with 5% sucrose and single colonies were screened by PCR and Southern blotting for the presence of the 1044 bp deletion (data not shown). The phenotype of the resulting mutants were analyzed in a macrophage cytotoxicity assay measuring the release of lactate dehydrogenase using the cytotox 96 kit (Promega Corp., Madison, WI), an In Situ Cell Death Detection Kit (Boehringer Mannheim, Indianapolis, IN) for labeling the nicked end of DNA in apoptotic cells, and by Western blotting (32). As expected, the mutant strain, YPIIIpyopJ, did not kill the infected macrophages, and the level of apoptosis as indicated by the TUNEL (Tdt-mediated dUTP–biotin nick-end labeling) reaction was the same as background levels for uninfected macrophages (data not shown). The addition of yopJ on a plasmid in trans restores the ability of the mutant strain to kill macrophages in vitro (data not shown). The growth rates of YPIIIpyopJ and YPIIIpYV in 2xYT broth were identical (data not shown).
Infection of Animals.
Bacteria were grown with aeration in 2xYT broth for 15–18 h at 26°C and resuspended in PBS before inoculating mice. For LD50 calculations, 120 mice were inoculated orogastrically, through a gastric tube, with serial 10-fold (n = 10 mice/inoculum) dilutions ranging from 3 × 106 to 3 × 1010. The health of the animals was followed for 30 d after inoculation, and deaths were recorded. For competition infections, mice were inoculated orogastrically with an equal mixture of 8 × 108 YPIIIpYVKmr and 8 × 108 YPIIIpyopJ. PP, MLNs, and spleens were homogenized in sterile stomacher bags and weighed. Dilutions of homogenized tissues were plated on L-agar plates either with or without kanamycin (40 μg/ml) to obtain bacterial counts per gram of tissue (CFU on plain L-agar − CFU on L-agar with kanamycin = CFU YPIIIpyopJ).
Flow Cytometry.
Single-cell suspensions of MLNs and spleens were prepared by homogenizing tissue in 1.5 ml PBS containing 5% FCS with the rubber end of a syringe plunger. An aliquot from each sample was plated on L-agar to assess bacterial load in the tissue. Debris was removed by passing cells through a cell strainer cap with a 35-μm mesh screen (Falcon Labs., Cockeysville, MD). Approximately 2 × 106 cells per organ from each mouse of each group were stained with a 1:100 dilution of anti– mouse CD11b Mac-1–biotin antibody (Caltag Labs., San Francisco, CA) (40) for 30 min at 4°C. The cells were fixed with 4% paraformaldehyde for 30 min at room temperature, washed with 1 ml of PBS, and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice and washed with 1 ml of PBS. Cells were then labeled with the TUNEL reaction mixture for 1 h at 37°C, labeled with a 1:100 dilution of streptavidin-PE (Caltag Labs.) for 30 min at room temperature and resuspended in 500 μl PBS for analysis by flow cytometry. From each sample, 50,000– 100,000 cells were analyzed by flow cytometry with a FACScan® and FACScan® software (Becton Dickinson, San Jose, CA).
Histology and TUNEL Reactions on Fixed Tissue.
For histological examinations and TUNEL reactions on tissue sections, PP, MLNs, and spleens were fixed in 10% buffered neutral formalin, embedded in paraffin, and cut. Some sections were then stained with hematoxylin and eosin. Sections for TUNEL reactions were processed as described for the In Situ Cell Death Detection Kit, Fluorescein (Boehringer Mannheim Biochemicals). In brief, tissue sections were heated at 60°C for 30 min followed by washing in xylene and rehydration through graded series of ethanol and redistilled water. Sections were treated with proteinase K (20 μg/ ml in 10 mM Tris-HCL, pH 7.4) for 30 min at room temperature, washed, and overlaid with premixed TUNEL reaction mixture. The sections were labeled for 1 h at 37°C, washed with PBS, incubated in anti-Yersinia polyclonal rabbit antiserum, and finally incubated with anti–rabbit-TRITC antibody (Sigma Chemical Co., St. Louis, MO). Coverslips were mounted over antiquench and sealed, and tissue sections were analyzed on a fluorescence microscope.
Statistical Analysis.
The paired differences of CFU g−1 between the yopJ mutant and wild-type strains for each animal were compared by the paired t test and Mann Whitney U test. The mean percentage of Mac-1+ cells that were TUNEL+ for cells isolated from MLNs and spleens were compared by the t test.
Results
YPIIIpyopJ Is Attenuated in the Oral Mouse Model.
To assess the role of YopJ in Yersinia virulence, we determined the oral LD50 in a susceptible strain of mice. 10 BALB/c mice per bacterial strain were inoculated with serial 10-fold dilutions of wild-type Y. pseudotuberculosis, YPIIIpYV, or of the isogenic yopJ mutant Y. pseudotuberculosis, YPIIIpyopJ. The infected animals were followed for 30 d and the estimated value for the 50% death point was calculated by the Reed-Muench method (41). The calculated LD50 for the mutant strain, YPIIIpyopJ, was 1.59 × 109, which is 64-fold higher compared with the calculated LD50 for wild-type Y. pseudotuberculosis, YPIIIpYV, 2.49 × 107. This indicated that YopJ is a virulence factor in the mouse model of infection.
Wild-type Y. pseudotuberculosis Out-competes the yopJ Mutant in a Mixed Infection in Mice.
To assess where in the course of the infection the activity of YopJ becomes important for the establishment of Yersiniosis, we first looked at the ability of the yopJ mutant to survive in the presence of competing wild-type bacteria. 30 mice were infected orogastrically with an equal mixture of wild-type Y. pseudotuberculosis, Kmr and yopJ mutant Y. pseudotuberculosis. Recoverable bacteria were obtained on days 1, 2, 3, 4, and 5 after inoculation from cecum, PP, MLNs, and spleens and plated on L-agar with or without kanamycin. Both bacterial strains were recovered in equal numbers from the cecum and PP on days 1–5 (Table 1). At 2 d after inoculation, the wild-type Y. pseudotuberculosis strain was recovered in higher numbers from the MLNs, and the mean paired difference was 2.47 ± 0.74 logs (Table 1). The wild-type strain continued to out-compete the yopJ mutant strain on days 3, 4, and 5 with the mean paired differences being 1.66 ± 0.75, 1.67 ± 0.76, and 0.97 ± 0.18 logs, respectively. Yersinia was first observed in the spleens of infected mice on day 3. At 3 d after inoculation, the wild-type strain colonized the spleen in greater numbers, with a mean paired difference of 2.68 ± 0.83 logs. This 100-fold difference between the wild-type strain and the yopJ mutant strain remained similar on days 4 and 5 after inoculation, with mean paired differences of 2.39 ± 0.76 and 1.95 ± 0.66 logs, respectively (Table 1). The P values for the mean paired differences in the MLNs and spleen were <0.05 and thus were significant (Table 1). These data suggest that the yopJ mutant bacteria were defective in their ability to spread from the PP to the MLNs and then to the spleen.
Table 1.
Wild-type Yersinia out-competes yopJ Mutant in Colonization of MLNs and Spleens
| Tissue | Day | Log mean difference ± SD* | P value‡ | |||
|---|---|---|---|---|---|---|
| Cecum | 1 | −0.37 ± 0.45 | ||||
| 2 | 3.56 ± 1.29 | 0.0399 | ||||
| 3 | 1.70 ± 1.52 | |||||
| 4 | 2.71 ± 1.52 | |||||
| 5 | 0.09 ± 0.16 | |||||
| PP | 1 | −0.03 ± 0.25 | ||||
| 2 | 2.73 ± 1.38 | |||||
| 3 | 1.43 ± 1.17 | |||||
| 4 | 1.73 ± 1.46 | |||||
| 5 | 0.33 ± 0.19 | |||||
| MLN | 2 | 2.47 ± 0.74 | 0.0204 | |||
| 3 | 1.66 ± 0.75 | 0.0404 | ||||
| 4 | 1.66 ± 0.76 | 0.0500 | ||||
| 5 | 0.97 ± 0.18 | 0.0029 | ||||
| Spleen | 3 | 2.68 ± 0.83 | 0.0481 | |||
| 4 | 2.39 ± 0.76 | 0.0254 | ||||
| 5 | 1.95 ± 0.66 | 0.0319 |
Difference = Log YPIIIpYV CFU g−1−Log YPIIIpyopJ CFU g−1.
P value is from paired two group t test and is only given when ≤0.0500.
A yopJ Mutant Does Not Colonize Spleens as Well as a Wild-type.
To determine where in the course of infection the yopJ mutant was attenuated in the absence of competing wild-type bacteria, we infected mice with two different doses of YPIIIpYV or YPIIIpyopJ. In an initial experiment, the mice were inoculated with 2 × 108 wild-type strain or yopJ mutant strain, and recoverable bacteria were quantitated. All of the mice inoculated with wild-type bacteria were colonized in all tissues tested with the mean log CFU g−1 increasing 2.7-fold in the MLNs from day 4 to day 5 after infection (Table 2). The mean CFU g−1 spleen increased 16-fold from day 4 to day 5. YPIIIpyopJ bacteria were recovered from the cecum of all mice inoculated with the mutant strain; however, the mean CFU g−1 cecum was 40-fold less than the mean CFU g−1 cecum infected with wild-type bacteria (Table 2). Although we recovered bacteria from the PP of three out of the five mice inoculated with the yopJ mutant bacteria 4 d after infection, the CFU g−1 PP were lower than the mean CFU g−1 PP from mice infected with wild-type bacteria. We recovered an average of 3.2 × 104 CFU g−1 MLNs from the three mice that contained yopJ mutant bacteria in the PP, and we did not recover any bacteria from the MLNs of those mice that did not have any bacteria in the PP. Only one of the five mice infected with the yopJ mutant strain had any viable bacteria in the spleen, with 4.56 × 102 CFU g−1. By day 5, only two of the five mice infected with the yopJ mutant bacteria contained viable bacteria in the PP and MLNs, and only one of these mice contained bacteria in the spleen. Thus, the inoculation of mice with wild-type Y. pseudotuberculosis at a dose that is 10-fold higher than the LD50 resulted in uniformly high levels of infection in the tissues tested. In striking contrast, the inoculation of mice with the same dose of the yopJ mutant strain resulted in very few of the animals showing signs of infection 4 and 5 d after inoculation (Table 2).
Table 2.
Colonization of Mice Inoculated Orally with 2 × 108 Wild-type or yopJ Mutant Y. pseudotuberculosis
| Strain | Animal | Cecum | PP | MLN | Spleen | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 4 | ||||||||||||
| YPIIIpYV | 1 | 8.20 × 107 | 5.21 × 106 | 6.69 × 102 | 1.52 × 103 | |||||||
| 2 | 6.32 × 106 | 3.56 × 105 | 4.21 × 105 | 1.10 × 102 | ||||||||
| 3 | 6.93 × 106 | 3.62 × 107 | 3.16 × 103 | 7.35 × 103 | ||||||||
| 4 | 6.62 × 107 | 1.56 × 107 | 7.79 × 103 | 1.89 × 103 | ||||||||
| 5 | 5.21 × 107 | 3.26 × 106 | 5.10 × 104 | 5.14 × 104 | ||||||||
| mean | 4.27 × 107 | 1.21 × 107 | 9.67 × 104 | 1.25 × 104 | ||||||||
| YPIIIpyopJ | 1 | 2.23 × 105 | 3.32 × 103 | 2.25 × 104 | 0.00 | |||||||
| 2 | 6.53 × 102 | <10 | 0.00 | 0.00 | ||||||||
| 3 | 2.25 × 106 | 4.38 × 102 | 0.00 | 0.00 | ||||||||
| 4 | 5.64 × 102 | <10 | 0.00 | 0.00 | ||||||||
| 5 | 5.23 × 105 | 2.25 × 104 | 7.56 × 104 | 4.56 × 102 | ||||||||
| mean | 5.99 × 105 | |||||||||||
| Day 5 | ||||||||||||
| YPIIIpYV | 1 | 7.70 × 106 | 6.00 × 104 | 3.26 × 104 | 1.55 × 104 | |||||||
| 2 | 5.63 × 106 | 3.73 × 105 | 4.89 × 104 | 8.42 × 104 | ||||||||
| 3 | 1.21 × 107 | 1.08 × 105 | 1.61 × 105 | 4.50 × 105 | ||||||||
| 4 | 3.93 × 106 | 3.27 × 105 | 9.08 × 105 | 1.65 × 105 | ||||||||
| 5 | 5.20 × 107 | 1.04 × 107 | 1.36 × 105 | 2.62 × 105 | ||||||||
| mean | 1.63 × 107 | 2.25 × 106 | 2.57 × 105 | 1.95 × 105 | ||||||||
| YPIIIpyopJ | 1 | 8.34 × 106 | <10 | 0.00 | 0.00 | |||||||
| 2 | 7.70 × 102 | <10 | 0.00 | 0.00 | ||||||||
| 3 | 6.68 × 102 | <10 | 0.00 | 0.00 | ||||||||
| 4 | 1.62 × 105 | 3.69 × 103 | 3.47 × 102 | 0.00 | ||||||||
| 5 | 1.41 × 106 | 5.18 × 104 | 1.02 × 105 | 3.58 × 103 | ||||||||
| mean | 1.98 × 106 |
Values are CFU g−1 recovered from various tissues.
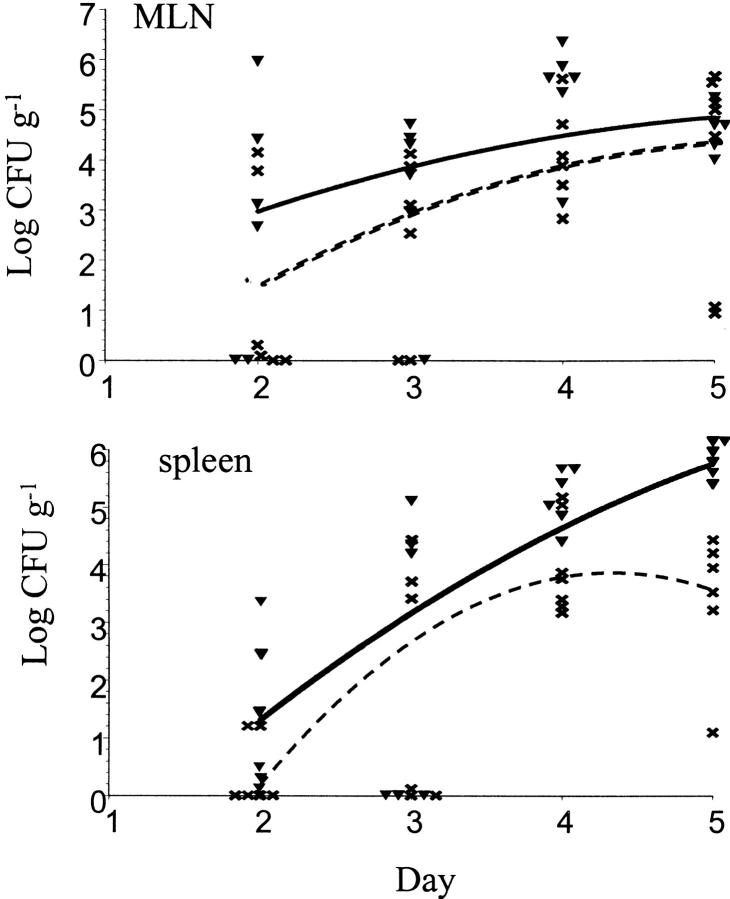
To extend these results, mice were infected with higher bacterial doses. 25 mice were inoculated with 109 wild-type YPIIIpYV and 25 mice were inoculated with 109 YPIIIpyopJ. Bacteria were recovered from PP, MLNs, and spleens on days 2, 3, 4, and 5 after inoculation. There was no difference in the ability of wild-type bacteria and yopJ mutant bacteria to colonize the PP (data not shown). In contrast to the mixed infection experiments, the yopJ mutant bacteria colonized the MLNs as well as wild-type bacteria at these high challenge doses in the absence of competing wild-type bacteria (Fig. 1). However, in the spleen the yopJ mutant never reached the same levels as wild-type bacteria. At 4 d after inoculation the mean log CFU g−1 for the yopJ mutant was 4.11 ± 0.43, whereas the mean log CFU g−1 spleen infected with wild-type was 5.07 ± 0.22 (P = 0.0500; Fig. 1). 5 d after inoculation the number of yopJ mutant bacteria recovered from the spleens decreased to 3.44 ± 0.66 log CFU g−1, whereas the number of wild-type bacteria increased to 5.75 ± 0.09 (P = 0.0100; Fig. 1). Thus, the yopJ mutant is unable to sustain growth in spleens of infected mice.
Figure 1.
The yopJ mutant does not colonize spleens as well as wild-type Y. pseudotuberculosis. Colonization kinetics from mice infected orally with 109 YPIIIpYV or 109 YPIIIpyopJ. Log CFU g−1 MLNs or spleen for YPIIIpYV, and YPIIIpyopJ, on days 2, 3, 4, and 5 after inoculation are shown (n = 6 mice/strain for each time point). Curve for YPIIIpYV colonization is a solid line. Curve for YPIIIpyopJ colonization is a dashed line.
Flow Cytometry Indicates that Yersinia Induces YopJ-dependent Apoptosis In Vivo.
YopJ is necessary for the ability of Yersinia to induce apoptosis in macrophages in vitro (31). To date there has not been any in vivo evidence demonstrating that Yersinia causes apoptosis during an infection or that the ability to induce apoptosis is important in Yersinia pathogenesis. To correlate Y. pseudotuberculosis virulence in mice with the ability of Yersinia to induce programmed cell death in cultured macrophages, we measured the levels of apoptosis in tissues infected with 8 × 108 wild-type or 5 × 109 yopJ mutant bacteria. Inoculating mice with higher numbers of yopJ mutant bacteria resulted in more viable bacteria recovered, and thus allows us to rule out a nonspecific effect of bacterial load in our analysis of Yersinia-induced apoptosis in vivo. At days 4 and 5 after inoculation, PP, MLNs, and spleen were harvested and single cell suspensions were prepared for stainings and flow cytometry. Apoptosis was measured by the TUNEL reaction where nuclei containing DNA fragmentation were labeled with dUTP-FITC by the enzyme TdT for detection by flow cytometry. Cells were also stained with a biotinylated Mac-1 antibody, an antibody that recognizes the CR3 receptor on macrophages, polymorphonuclear cells, dendritic cells, and a subset of natural killer cells. Mac-1 cells were then labeled with streptavidin-PE for detection by flow cytometry.
The overall percentage of cells isolated from uninfected PP that were TUNEL positive was relatively high and extremely variable (5.25–45.10%; data not shown). However, there was no significant difference in the percentage of TUNEL-positive cells or Mac-1+ cells that were TUNEL positive between infected PP and uninfected PP (data not shown).
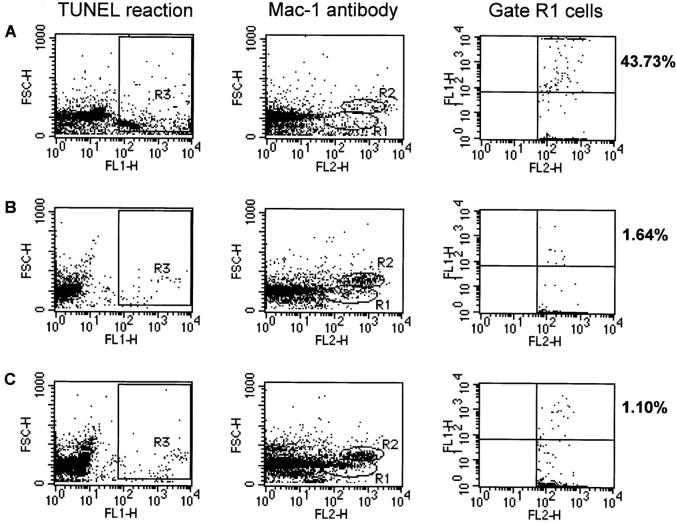
Two distinct Mac-1+ cell populations were observed based on their forward scatter. The population in gate R1 has a forward scatter consistent with that of macrophages and excludes cell debris (Fig. 2; references 42, 43). The mean percentage of gate R1 cells from YPIIIpYV-infected MLNs and spleens that were TUNEL positive on day 4 after inoculation was 9.85 ± 4.20 and 12.08 ± 4.12, respectively (Table 3). These levels increased in YPIIIpYV- infected MLNs and spleens to 21.41 ± 4.96 and 27.82 ± 5.40, respectively, on day 5 after inoculation (Table 3). Although the CFU recovered from MLNs and spleens infected with YPIIIpYV or YPIIIpyopJ were similar (Table 3), the percentage of the Mac-1+ cells within gate R1 from tissues infected with wild-type bacteria that were TUNEL positive was 5–10-fold higher than the percentages obtained from tissues infected with the yopJ mutant. The percentage of the total cell population or of gated Mac-1+ cells that were TUNEL positive isolated from MLNs and spleens infected with the yopJ mutant was never significantly greater than the background level obtained for cells isolated from uninfected control MLNs and spleens (Table 3). Thus, YopJ production correlates with the induction of apoptosis in a population of Mac-1+ cells that includes macrophages in infected MLNs and spleens.
Figure 2.
An increase in the number of TUNEL-positive cells in infected tissues correlates with YopJ production. Flow cytometry analysis of single cell suspensions prepared from spleens infected for 4 d with (A) YPIIIpYV (n for R1 = 1006) or (B) YPIIIpyopJ (n for R1 = 1546), or (C) from uninfected mice (n for R1 = 2255). TUNEL-positive cells were detected in FL1 and cells immunostained with Mac-1 antibody were detected in FL2. Gates were drawn around three populations of cells. R1 represents a population of cells that were stained with Mac-1 antibody and had a mean forward scatter of 170. R2 represents a population of cells that also stained with Mac-1 antibody and had a mean forward scatter of 350. R3 represents a population of cells that were specifically labeled in the TUNEL reaction and had a positive signal in FL1 relative to negative controls. Percentages indicate the percentage of cells within the gate R1 cell population that were TUNEL positive.
Table 3.
Increase in TUNEL+ Mac-1+ Cells from Tissue of Mice Infected with Wild-type Y. pseudotuberculosis Bacteria
| Tissue | Bacterial strain | Percentage of gate R1, TUNEL+ cells ± SE* | P value‡ | Percentage of Mac-1+ ± SE§ | P value¶ | Mean CFU recovered | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 4 | ||||||||||||||
| MLN | YPIIIpVY | 9.85 ± 4.20 | 3.38 ± 0.36 | 2.42 × 103 | ||||||||||
| MLN | YPIIIpyopJ | 2.14 ± 0.78 | 2.62 ± 0.41 | 2.48 × 103 | ||||||||||
| Spleen | YPIIIpYV | 12.08 ± 4.12 | 4.97 ± 1.38 | 5.13 × 102 | ||||||||||
| Spleen | YPIIIpyopJ | 2.17 ± 0.47 | 7.10 ± 0.92 | 3.13 × 102 | ||||||||||
| Day 5 | ||||||||||||||
| MLN | YPIIIpYV | 21.41 ± 4.96 | 0.0013 | 3.95 ± 0.25 | 0.0161 | 4.45 × 103 | ||||||||
| MLN | YPIIIpyopJ | 2.01 ± 0.37 | 3.74 ± 0.36 | 0.0434 | 1.33 × 103 | |||||||||
| Spleen | YPIIIpYV | 27.82 ± 5.40 | 0.0002 | 5.86 ± 1.05 | 3.94 × 103 | |||||||||
| Spleen | YPIIIpyopJ | 2.14 ± 0.33 | 12.32 ± 1.42 | 0.0167 | 2.25 × 103 | |||||||||
| MLN | Uninfected | 3.45 ± 1.41 | 1.91 ± 0.89 | |||||||||||
| Spleen | Uninfected | 1.74 ± 0.55 | 5.96 ± 0.92 |
Mac-1 (M1/70) antibody conjugated to biotin was used to stain single cell suspensions. Streptavidin-PE was detected in FL-2. Cells within gate R1 were analyzed to obtain the percentage of Mac-1+ cells with a forward scatter in the range of monocytes or macrophages that were TUNEL positive. n = 9 mice per inoculum/day, n = 5 uninfected mice.
P values are from a t test comparing percentage of gate R1, Mac-1+ cells that were also TUNEL positive obtained for YPIIIpYV-infected tissue compared to values obtained for YPIIIpyopJ-infected tissue. Values only given when P ≤ 0.0500.
The relative percentage of Mac-1+ cells was obtained by gating on cells positive in FL-2. Cell debris was gated out.
P values are from a t test comparing percentage Mac-1+ cells obtained for either YPIIIpYV-infected tissue or YPIIIpyopJ-infected tissue to values obtained for uninfected tissue. Values only given when P ≤ 0.0500.
The Mac-1+ population of cells within gate R2 had higher forward scatter values and are most likely polymorphonuclear and dendritic cells (Fig. 2; references 42, 43). When we analyzed this second population of cells, we did not observe a significant difference in the percentage of TUNEL-positive cells from tissue infected with either bacterial strain compared with uninfected tissue. We conclude that there appears to be a distinct Mac-1 population of cells that is targeted by Yersinia in vivo.
Does the eventual reduction in the recovery of viable yopJ mutant bacteria in the spleen correlate with an increase of phagocytic cells that could kill the bacteria? To address this we analyzed the entire population of cells that stained with the Mac-1 antibody (Fig. 2). The recruitment of Mac-1+ cells to MLNs infected with YPIIIpYV or YPIIIpyopJ was significant on day 5 after inoculation, with an increase from 2 to 4% of the total population (Table 3). The increase of Mac-1+ cells in spleens infected with YPIIIpyopJ was most noticeable at 5 d after inoculation with 12% of the total cell population staining with the Mac-1 antibody compared with 6% for uninfected spleens. In contrast, the percentage of Mac-1+ cells from spleens infected with YPIIIpYV remained the same as that of uninfected spleens (Table 3). Thus, the increase in the relative percentage of cell types capable of producing proinflammatory cytokines as well as phagocytosing and subsequently killing the yopJ mutant may be responsible for the eventual elimination of the bacteria (Fig. 1).
Histologic Studies Show Yersinia-induced Apoptosis In Vivo.
To further characterize abscesses observed in infected tissues, fixed sections of tissue from three mice inoculated orogastrically with 5 × 109 YPIIIpYV or three mice inoculated with 5 × 109 YPIIIpyopJ were stained with hematoxylin and eosin. Infection with both wild-type and yopJ mutant bacteria resulted in the recruitment of phagocytes to MLNs and spleens (days 1–2) and the subsequent formation of abscesses. These abscesses had a central region composed of cells with the morphological features of polymorphonuclear cells and a distinct border made up of predominantly large mononuclear cells (days 3–5). Often more cell debris and pyknotic/apoptotic cells were associated with abscesses from MLNs or spleen infected with wild-type Y. pseudotuberculosis compared with tissue infected with the yopJ mutant strain (Fig. 3).
Figure 3.

Histology shows cells with condensed nuclei in MLNs infected with wild-type Yersinia. Hematoxylin and eosin staining of fixed MLNs infected with 5 × 109 YPIIIpYV or YPIIIpyopJ for 3 days. (a) This photomicrograph shows an abscess typical of the inflammation seen in mice infected with wild-type Yersinia. (b) At higher magnification note the predominance of condensed nuclei and pyknotic cell debris, (arrowheads) typical of apoptotic cells. (c) Typical lymph node abscess seen in mice infected with the yopJ mutant strain which at higher magnification (d) shows numerous viable inflammatory cells (arrows) that maintain their nuclear morphology. Original magnifications: a and c, ×100; b and d, ×250 oil.
To determine which cells were apoptotic, TUNEL reactions were performed on sections from the same MLNs that were stained for histology (Fig 3). Bacteria were stained with an anti-Yersinia antibody followed by a secondary antibody-rhodamine conjugate. There was a dramatic increase in the number of TUNEL-positive cells in the MLNs infected for 3 d with wild-type bacteria compared with MLNs infected for 3 d with the yopJ mutant (Fig. 4). The microscopic data on fixed tissue sections combined with the flow cytometry data support the conclusion that the presence of YopJ correlates with an increase in apoptotic cells and that bacteria lacking YopJ are more likely to be cleared from infected tissues.
Figure 4.
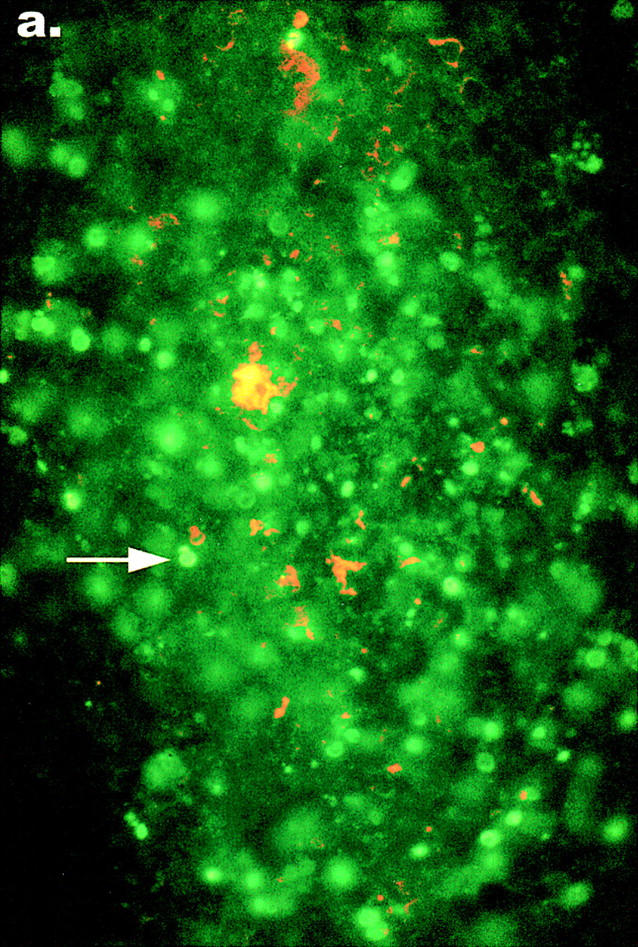

TUNEL reactions show an increase in apoptosis in MLNs infected with wild-type Y. pseudotuberculosis. Sections from the same fixed tissues used for histology were processed as described in Materials and Methods, permeabilized, and subjected to the TUNEL reaction. Nuclei of apoptotic cells in tissue infected with either (a) wild type Yersinia or (b) yopJ mutant Yersinia, were detected with an FITC filter on an epifluorescent microscope (arrows). The presence of TUNEL-positive nuclei in MLNs from the mouse infected with the yopJ mutant was similar to the background level of apoptosis seen in MLNs from uninfected mice. Bacteria were detected with an anti–Y. pseudotuberculosis antibody and a secondary TRITC-conjugated antibody with a rhodamine filter. Original magnification: ×640.
Discussion
Previously we and others have shown that YopJ is required for Yersinia-induced apoptosis in macrophages in vitro (30–32). In this study, we report that a Y. pseudotuberculosis strain that no longer makes YopJ is significantly attenuated in the mouse model of a systemic Yersinia infection with an almost 100-fold increase in the oral LD50 over wild-type Y. pseudotuberculosis in BALB/c mice. Thus, YopJ is a virulence factor. Two previous publications on mouse infections with yopJ mutants indicated that YopJ is not a virulence factor (44, 45). Galyov et al. challenged three mice orally with high undefined doses of either wild-type or yopJ mutant Y. pseudotuberculosis strains and reported that all mice died within 6 d of infection (45). Our data are not inconsistent with these results given that a high dose of the yopJ mutant did result in the death of some of our infected mice. Straley and Bowmer measured the LD50 of a Y. pestis yopJ mutant injected intravenously into Swiss mice and found that the LD50 values for the yopJ mutant and wild-type were similar (44). It is possible that a yopJ mutant is not as attenuated in Y. pestis or in Swiss mice, or that the mode of inoculation plays a role in bacterial virulence.
To investigate at what stage of a systemic Yersinia infection YopJ might be playing a role in virulence, we first tested the yopJ mutant in a competition infection in mice. To this end, mice were inoculated with an equal mixture of the wild-type bacterial strain and the isogenic yopJ mutant bacterial strain. Although the wild-type and yopJ mutant strains were recovered in equal numbers from the cecum and PP, 500-fold more wild-type bacteria than yopJ mutant bacteria were recovered from infected MLNs 2 d after inoculation. The selective advantage that YopJ confers on Yersinia is also reflected in the subsequent spread to the spleen, where wild-type had 500-fold more colonies than the yopJ mutant. A similar difference was seen in the spleens of mice infected with the individual bacterial strains. We propose that YopJ does not play a role in the ability of Yersinia to enter the PP or to replicate within the PP. Rather, we believe that early in the infectious process, the bacteria making YopJ have a selective advantage in seeding and colonizing the MLNs due to their ability to kill activated macrophages by apoptosis.
To determine if the ability of wild-type Yersinia to cause a systemic infection correlates with the ability to induce apoptosis in macrophages in vivo, we quantitated the level of apoptosis by flow cytometry from tissues infected with either the wild-type bacteria or the yopJ mutant bacteria. There was a significant increase in the number of TUNEL-positive cells in the R1 subset of Mac-1+ cells from MLNs and spleens infected with wild-type bacteria. In contrast, the levels of TUNEL-positive cells in the Mac-1+ cells within gate R1 or even in the total cell population from MLNs and spleens infected with the yopJ mutant bacteria was never significantly greater than the levels found in cells from uninfected tissues. These results indicate that the production of YopJ correlates with an increase in apoptosis in vivo and that Yersinia-induced apoptosis probably plays a role in establishing a systemic infection. We also analyzed a second Mac-1+ cell population (Fig. 2, gate R2) with a higher forward scatter, and found that the percentage of these cells that were TUNEL positive did not increase when infected with wild-type bacteria. This population of Mac-1+ cells is likely to be polymorphonuclear lymphocytes, dendritic cells, and perhaps some macrophages (42).
Previous studies focusing on host defense mechanisms against Yersinia infection have implicated both T cells and macrophages, and their secretion of the cytokines TNF-α, IFN-γ, and IL-12 as essential components in protective host responses against Yersinia (37, 46–50). Although we did not stain with antibodies that recognize macrophages exclusively, the forward scatter profile indicates that the population of cells that show an increase in the percentage of TUNEL-positive cells is likely to be macrophages. We suggest that the macrophage is being targeted and killed in the host during Yersinia infection by YopJ. Autenrieth et al. have shown that the administration of Mac-1 antibodies before Yersinia infection caused increased bacterial numbers in the PP and MLNs (51). Therefore, the ability of wild type Yersinia to neutralize a primary host immune cell, the macrophage, gives the bacterial pathogen a distinct advantage in its ability to cause a systemic infection.
We noticed a population of cells that did not stain with the Mac-1 antibody yet were TUNEL positive in tissues from animals infected with wild-type Yersinia. It is possible that the Mac-1 antibody did not stain the dying cells in this population because they no longer express the CR3 receptor. The forward scatter of these cells was slightly lower than the forward scatter of the gate R1 population of cells that showed the increase in TUNEL-positive cells when infected with wild-type bacteria. The decrease in the forward scatter of these cells could be due to cell shrinkage associated with cells undergoing apoptosis. Alternatively, the cells within this population could include T cells, which have been shown to be important for limiting Yersinia infections in mice (49). The possibility that a cell population other than macrophages is being killed by Yersinia in vivo is intriguing in light of results reported by Ruckdeschel et al. (38). They provide data that Yersinia infection in vitro confers susceptibility to programmed cell death to cell types other than macrophages (epithelial cells), provided that the appropriate death signal is delivered. Perhaps the ability of wild-type Yersinia to promote apoptosis in vivo of epithelial cells or B or T cells is important in pathogenesis.
Recent reports have correlated the production of YopJ (YopP in Y. enterocolitica) with both the suppression of TNF-α and the ability to induce apoptosis in macrophages by Yersinia (33, 34, 38). We propose that our in vivo results are consistent with the in vitro YopJ phenotype of the suppression of TNF-α production by macrophages. The result that wild-type bacteria out-compete the yopJ mutant in spreading from the PP to the MLNs suggests that the ability of Yersinia to kill macrophages early in the infection gives the bacteria an advantage in colonizing the deeper tissues, such as MLNs and spleens. This is further supported by our results that, in spleens infected with the yopJ mutant bacteria, we see a recruitment of Mac-1+ cells that are presumably clearing the bacterial infection. Recruitment of Mac-1+ cells suggests the production of a proinflammatory cytokine such as TNF-α in vivo. Preliminary results obtained from TNF-α ELISA assays performed on serum from infected mice did not show any differences in the serum levels of TNF-α between mice infected with wild-type or yopJ mutant bacteria compared with levels detected in uninfected mice. Could the levels of proinflammatory cytokines within the bacterial microenvironment ultimately decide the fate of the bacterial infection? Studies addressing this question are underway.
Clearly our data indicate that YopJ is playing a role in Yersinia pathogenesis. We believe that the ability of wild-type Yersinia to kill one of the host's first lines of defense, macrophages, gives the bacteria an advantage early in the infection upon first contact with host immune cells. Additionally, the ability to suppress the production of the proinflammatory cytokines, TNF-α and IL-8 perhaps as well as other as yet unidentified cytokines may allow the Yersinia to spread to deeper tissue such as MLNs and spleens and replicate within the deeper tissues of the host.
Although cell necrosis is a powerful stimulus for inflammation, apoptosis is a mechanism of cell death in which the cell initiates its own signal transduction pathways to cause its own demise, and in most cases the release of proinflammatory cellular contents and cytokines is suppressed (52). However, evidence from recent studies with Shigella flexneri, an enteric pathogen, have indicated that during infection with Shigella bacterial-induced apoptosis functions as a trigger for inflammation through the release of proinflammatory cytokines (53). The virulence plasmid-encoded protein, IpaB, induces apoptosis by interacting with IL-1β converting enzyme (ICE), which plays two roles. One is to initiate the apoptotic pathway and the other is to convert IL-1β to its mature, biologically active form, which is then released and initiates an acute inflammatory response (54). The role of the ability of Shigella to induce this acute inflammatory response is postulated to be aiding in the spread of Shigella through the basolateral surfaces of the intestinal epithelium (28, 55). Thus, in contrast to the in vivo role of Shigella-induced apoptosis, the role of Yersinia-induced apoptosis is to reduce the inflammatory response in infected tissues and thereby allow the bacteria to replicate extracellularly.
Acknowledgments
We would like to thank Lisa Raskin in the Department of Comparative Medicine at Stanford University for help with tissue preparation, sectioning and stainings. We thank Inna Bilis for help in processing animal tissues, Daniela Cirillo for assaying serum for cytokine levels as well as for helpful discussions, and Rachel Ettinger for helpful tips regarding FACS® experiments. We also thank Lalita Ramakrishnan, Corrella Detweiler, and David Discher for critically reading our manuscript.
This work was funded in part by Public Health Service grant RO1 AI26195 and in part by the Defense Advanced Research Projects Agency. The content of the information does not necessarily reflect the position or the policy of the Government and no official endorsements should be inferred. J. Mecsas was supported by American Cancer Society Grant No. PF-4477.
Abbreviations used in this paper
- MLNs
mesenteric lymph nodes
- PP
Peyer's patches
- TUNEL
Tdt-mediated dUTP–biotin nick-end labeling
References
- 1.Carter PB. Animal model of human disease. Yersinia enteritis. Animal model: oral Yersinia enterocoliticainfection of mice. Am J Pathol. 1975;81:703–706. [PMC free article] [PubMed] [Google Scholar]
- 2.Cornelis G, Laroche Y, Balligand G, Sory MP, Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987;9:64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- 3.Bottone EJ. Yersinia enterocolitica: a panoramic view of a charismatic microorganism. CRC Crit Rev Microbiol. 1977;5:211–241. doi: 10.3109/10408417709102312. [DOI] [PubMed] [Google Scholar]
- 4.Ahvonen P, Sievers K, Aho K. Arthritis associated with Yersinia enterocoliticainfection. Acta Rheumatol Scand. 1969;15:232–253. doi: 10.3109/rhe1.1969.15.issue-1-4.32. [DOI] [PubMed] [Google Scholar]
- 5.Bouza E, Dominguez A, Meseguer M, Buzon L, Boixeda D, Revillo MJ, de Rafael L, Martinez-Beltran J. Yersinia enterocoliticaSepticemia. Am J Clin Pathol. 1980;74:404–409. doi: 10.1093/ajcp/74.4.404. [DOI] [PubMed] [Google Scholar]
- 6.Grützkau A, Hanski C, Hahn H, Riecken EO. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocoliticaand other enteroinvasive bacteria. Gut. 1990;31:1011–1015. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pepe JC, Wachtel MR, Wagar E, Miller VL. Pathogenesis of defined invasion mutants of Yersinia enterocoliticain a BALB/c mouse model of infection. Infect Immun. 1995;63:4837–4848. doi: 10.1128/iai.63.12.4837-4848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bliska JB, Falkow S. Bacterial resistance to complement killing mediated by the Ail protein of Yersinia enterocolitica. . Proc Natl Acad Sci USA. 1992;89:3561–3565. doi: 10.1073/pnas.89.8.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.China B, Sory MP, N'Guyen BT, De Bruyere M, Cornelis GR. Role of the YadA protein in prevention of opsonization of Yersinia enterocoliticaby C3b molecules. Infect Immun. 1993;61:3129–3136. doi: 10.1128/iai.61.8.3129-3136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilz D, Vocke T, Heesemann J, Brade V. Mechanism of YadA-mediated serum resistance of Yersinia enterocoliticaserotype O3. Infect Immun. 1992;60:189–195. doi: 10.1128/iai.60.1.189-195.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanski C, Naumann M, Grützkau A, Pluschke G, Friedrich B, Hahn H, Riecken EO. Humoral and cellular defense against intestinal murine infection with Yersinia enterocolitica. . Infect Immun. 1991;59:1106–1111. doi: 10.1128/iai.59.3.1106-1111.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelis GR, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 13.Brubaker RR. Factors promoting acute and chronic diseases caused by yersiniae. Clin Microbiol Rev. 1991;4:309–324. doi: 10.1128/cmr.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bliska JB, Guan KL, Dixon JE, Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci USA. 1991;88:1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosqvist R, Bolin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black DS, Bliska JB. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO (Eur Mol Biol Organ) J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosqvist R, Forsberg A, Rimpilainen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 18.Fallman M, Andersson K, Hakansson S, Magnusson KE, Stendahl O, Wolf-Watz H. Yersinia pseudotuberculosisinhibits Fc receptor-mediated phagocytosis in J774 cells. Infect Immun. 1995;63:3117–3124. doi: 10.1128/iai.63.8.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung KY, Straley SC. The yopM gene of Yersinia pestisencodes a released protein having homology with the human platelet surface protein GPIb alpha. J Bacteriol. 1989;171:4623–4632. doi: 10.1128/jb.171.9.4623-4632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung KY, Reisner BS, Straley SC. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestisin mice. Infect Immun. 1990;58:3262–3271. doi: 10.1128/iai.58.10.3262-3271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boland A, Sory MP, Iriarte M, Kerbourch C, Wattiau P, Cornelis GR. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocoliticais internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO (Eur Mol Biol Organ) J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 22.Galyov EE, Hakansson S, Forsberg A, Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosisis an indispensable virulence determinant. Nature. 1993;361:730–732. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
- 23.Hakansson S, Galyov EE, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 24.Guzman CA, Domann E, Rohde M, Bruder D, Darji A, Weiss S, Wehland J, Chakraborty T, Timmis KN. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. . Mol Microbiol. 1996;20:119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen LM, Kaniga K, Galan JE. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 26.Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimuriuminvasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Zychlinsky A. Apoptosis induced by bacterial pathogens. Microb Pathog. 1994;17:203–212. doi: 10.1006/mpat.1994.1066. [DOI] [PubMed] [Google Scholar]
- 28.Zychlinsky A, Sansonetti P. Perspectives series: host/pathogen interactions. Apoptosis in bacterial pathogenesis. J Clin Invest. 1997;100:493–495. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zychlinsky A, Kenny B, Menard R, Prevost MC, Holland IB, Sansonetti PJ. IpaB mediates macrophage apoptosis induced by Shigella flexneri. . Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 30.Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monack DM, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills SD, Boland A, Sory MP, van der Smissen P, Kerbourch C, Finlay BB, Cornelis GR. Yersinia enterocoliticainduces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer LE, Hobbie S, Galan JE, Bliska JB. YopJ of Yersinia pseudotuberculosisis required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 34.Boland A, Cornelis GR. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect Immun. 1998;66:1878–1884. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schesser K, Splik A, Dukuzumuremyi J, Neurath M, Petterson S, Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima R, Brubaker RR. Association between virulence of Yersinia pestisand suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Autenrieth IB, Heesemann J. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocoliticainfection in mice. Med Microbiol Immunol. 1992;181:333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- 38.Ruckdeschel K, Harb S, Roggenkamp A, Hornef M, Zumbihl R, Köhler S, Heesemann J, Rouot B. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor α production. J Exp Med. 1998;187:1069–1079. doi: 10.1084/jem.187.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donnenberg MS, Kaper JB. Construction of an eae deletion mutant of enteropathogenic Escherichia coliby using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Springer T, Galfre G, Secher DS, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979;9:301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- 41.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 42.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro, H.M. Practical Flow Cytometry. Third ed. 1995. John Wiley & Sons, Inc., New York. 542 pp.
- 44.Straley SC, Bowmer WS. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestisinclude structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galyov EE, Hakansson S, Wolf-Watz H. Characterization of the operon encoding the YpkA Ser/Thr protein kinase and the YopJ protein of Yersinia pseudotuberculosis. . J Bacteriol. 1994;176:4543–4548. doi: 10.1128/jb.176.15.4543-4548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heesemann J, Gaede K, Autenrieth IB. Experimental Yersinia enterocoliticainfection in rodents: a model for human yersiniosis. APMIS. 1993;101:417–429. [PubMed] [Google Scholar]
- 47.Bohn E, Autenrieth IB. IL-12 is essential for resistance against Yersinia enterocoliticaby triggering IFN-gamma production in NK cells and CD4+ T cells. J Immunol. 1996;156:1458–1468. [PubMed] [Google Scholar]
- 48.Bohn E, Schmitt E, Bielfeldt C, Noll A, Schulte R, Autenrieth IB. Ambiguous role of interleukin-12 in Yersinia enterocoliticainfection in susceptible and resistant mouse strains. Infect Immun. 1998;66:2213–2220. doi: 10.1128/iai.66.5.2213-2220.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Autenrieth IB, Tingle A, Reske-Kunz A, Heesemann J. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. . Infect Immun. 1992;60:1140–1149. doi: 10.1128/iai.60.3.1140-1149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Autenrieth IB, Beer M, Bohn E, Kaufmann SH, Heesemann J. Immune responses to Yersinia enterocoliticain susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994;62:2590–2599. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Autenrieth IB, Kempf V, Sprinz T, Preger S, Schnell A. Defense mechanisms in Peyer's patches and mesenteric lymph nodes against Yersinia enterocoliticainvolve integrins and cytokines. Infect Immun. 1996;64:1357–1368. doi: 10.1128/iai.64.4.1357-1368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 53.Zychlinsky A, Sansonetti PJ. Apoptosis as a proinflammatory event: what can we learn from bacteria- induced cell death? . Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]
- 54.Zychlinsky A, Fitting C, Cavaillon JM, Sansonetti PJ. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. . J Clin Invest. 1994;94:1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perdomo OJ, Cavaillon JM, Huerre M, Ohayon H, Gounon P, Sansonetti PJ. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994;180:1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]