Abstract
The β2 family of integrins, CD11a, CD11b, CD11c, and αd, are expressed on most leukocytes. We show that the newest member of this family, αd, is expressed on human eosinophils in peripheral blood, and surface expression can be upregulated within minutes by phorbol ester or calcium ionophore A23187. Culture of eosinophils with interleukin 5 (IL-5) leads to a two- to fourfold increase in αd levels by 3–7 d without a change in α4 integrin expression. Eosinophils isolated from late phase bronchoalveolar lavage fluids express αd at levels similar to that seen after 3 d of IL-5 culture. Regarding αdβ2 ligands, in both freshly isolated and IL-5–cultured eosinophils, as well as αdβ2-transfected Chinese hamster ovary cells, αdβ2 can function as a ligand for vascular cell adhesion molecule 1 (VCAM-1). This conclusion is based on the ability of monoclonal antibodies to αd, β2, or VCAM-1 to block cell attachment in static adhesion assays. In experiments with eosinophils, the relative contribution of αdβ2 integrin– mediated adhesion is enhanced after IL-5 culture. These experiments demonstrate that αdβ2 is an alternative ligand for VCAM-1, and this integrin may play a role in eosinophil adhesion to VCAM-1 in states of chronic inflammation.
Keywords: β2 integrin, eosinophil, adhesion, vascular cell adhesion molecule 1
Eosinophils have been shown to play an important role in a variety of inflammatory diseases (1). Besides their postulated importance in parasitic infections, these cells are felt to participate in the pathogenesis of allergic disease. In asthma, for example, eosinophils are selectively recruited into the lung, where release of their products, such as granule proteins and leukotrienes, contributes to the airway damage seen in asthma (2, 3). Indeed, one of the possible mechanisms by which corticosteroids work in asthma is that they substantially decrease eosinophil numbers both in the lung and peripheral circulation (4, 5).
Integrins are a class of heterodimeric surface molecules involved in cellular adhesion (6). They are expressed on leukocytes and other cells, and are composed of both an α and a β chain. Based on shared β subunits these molecules can be classified into families. Eosinophils express members of the β1, β2, and β7 integrin families, and in many respects their integrin expression resembles that of other leukocytes (7). However, because human eosinophils express α4β1 and α4β7 integrins, but normal human neutrophils do not, their interaction with one of their ligands, vascular cell adhesion molecule 1 (VCAM-1), is felt to be a mechanism by which selective recruitment of eosinophils into sites of allergic inflammation occurs (8–10).
Recently a fourth β2 integrin, αdβ2, was identified and found to be most homologous to CD11b/CD18 and CD11c/CD18 (11). αdβ2 is expressed on most human leukocytes, including neutrophils, monocytes, and, to a lesser extent, lymphocytes (11). Van der Vieren et al., using αdβ2-expressing chinese hamster ovary (CHO) transfectants, demonstrated binding of αdβ2 to a human intercellular adhesion molecule 3 (ICAM-3) chimeric protein (11). Whether αdβ2 is expressed on eosinophils, and how it functions on these cells, were not examined.
The goal of these studies was to examine the expression and function of αdβ2 integrins on human eosinophils. We report that eosinophils express αdβ2, that its surface expression can be acutely and chronically regulated by various stimuli, and that, like α4 integrins, they can function as a ligand for VCAM-1.
Materials and Methods
Reagents.
The following murine IgG1 mAbs were used: irrelevant control IgG1 mAb (Coulter Corp., Hialeah, FL), CD11a mAb (MHM24; courtesy of Dr. James Hildreth, Johns Hopkins University School of Medicine, Baltimore, MD [12]), CD11b mAb (H4C2, Dr. Hildreth [13]; and clone 44, R&D Systems, Minneapolis, MN), CD11c mAb (BU-15; Immunotech, Inc., Westbrook, ME), αd mAb (169A, nonblocking, used for flow cytometry [11]; and 240I, used in adhesion assays because of its blocking ability [our unpublished observations]), CD18 mAb (H52, Dr. Hildreth [14]; and 7E4, Immunotech, Inc.), α4 (CD49d) mAb (HP2/1; Immunotech, Inc.), CD16 mAb (3G8; Medarex, Inc., Annandale, NJ), and blocking F(ab′)2 anti– VCAM-1 mAb (IG11b1; Caltag Laboratories, Inc., Burlingame, CA). Also used was an IgG2a FITC–anti-CD9 mAb (3B5; Coulter Corp.), polyclonal human IgG (Sigma Chemical Co., St. Louis, MO), R-PE–conjugated F(ab′)2 goat anti–mouse IgG (BioSource International, Camarillo, CA), murine polyclonal IgG (Sigma Chemical Co.), and FITC-conjugated polyclonal goat anti–human IgE (Kirkegaard & Perry, Gaithersburg, MD). Soluble recombinant human VCAM-1 and E-selectin (R&D Systems, Inc.) and BSA (Sigma Chemical Co.) were also purchased.
The following stimuli were used: PMA and calcium ionophore A23187 (Sigma Chemical Co.). Several C-C chemokines were also used, including macrophage-derived chemokine (MDC; Gryphon Sciences, South San Francisco, CA), RANTES (regulated upon activation, normal T cell expressed and secreted), and Eotaxin (R&D Systems, Inc.).
Cell Isolation.
Normodense (specific gravity ≥ 1.090) eosinophils were isolated from peripheral blood of allergic volunteers by density gradient centrifugation, hypotonic erythrocyte lysis, and immunomagnetic negative selection as described previously, while neutrophils were purified from peripheral blood of normal volunteers using density gradient centrifugation and hypotonic erythrocyte lysis alone (15, 16). Respective purities always exceeded 95%. Enrichment of peripheral blood for basophils was performed using a double-percoll density gradient separation, increasing the number of basophils to 3–10% of the total leukocyte count (17).
In some experiments, purified eosinophils were cultured for up to 7 d in RPMI 1640 (Biofluids, Inc., Rockville, MD) with 1% l-glutamine, 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, 500 ng/ml amphotericin (GIBCO BRL, Gaithersburg, MD), supplemented with 10 ng/ml recombinant human IL-5 (R&D Systems, Inc.) as described (18). Viability after 1 d or less of culture was ≥95%, whereas viability by 7 d was 80 ± 1% (mean ± SEM). Culture preparations in which ≤50% of cells were viable were excluded from analysis (5 of 42 experiments). In other experiments, eosinophils were incubated with optimal concentrations of various stimuli (50 ng/ml PMA or calcium ionophore A23187, 100 nM MDC, 100 ng/ml RANTES, or 100 nM Eotaxin in PBS/0.1% BSA) for up to 15 min at 37°C.
Bronchoalveolar lavage (BAL) cells were obtained from allergic patients who had undergone an endobronchial segmental allergen challenge with either ragweed or Dermatophagoides pteronyssinus extract 18 h previously as described elsewhere (19). Eosinophil purity in the late phase BAL fluid was 19 ± 4% (mean ± SEM, n = 5).
CHO Transfectants.
CHO cells were transfected with both the human αd and β2 integrin chains as described previously (11). αdβ2-transfected CHO cells were cultured in DMEM/F12 media with 1 mM pyruvate and 2 mM l-glutamine (Biofluids, Inc.) supplemented with 10% dialyzed FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 600 μg/ml G418 (all from GIBCO BRL). Transfected CHO cells that failed to maintain their αdβ2 integrin expression with serial passage were used as controls.
Flow Cytometry.
Expression of integrins on the CHO cell transfectants or on freshly isolated cells from blood after stimulation or culture was evaluated using single color indirect immunofluorescence and flow cytometry as described previously (17, 18). Dual color detection of basophils (using anti-IgE) and lower purity eosinophils in BAL fluids (using anti-CD9) was also performed. All samples were fixed in 1% paraformaldehyde (Sigma Chemical Co.) and analyzed using a flow cytometer (EPICS Profile II; Coulter Corp.). Approximately 10,000 events were collected and displayed on a 4-log scale, yielding values for mean fluorescence intensity (MFI).
Adhesion Assays.
For eosinophils, both freshly purified and cultured, 51Cr-labeled cell adhesion to VCAM-1 (250 ng/ml) or BSA (1%)–coated wells was performed for 30 min at 37°C as described previously (20). In some experiments, cells were preincubated for 30 min at 4°C with saturating concentrations of one or more of the following blocking mAbs before examining their adhesion: CD18 (7E4), CD11a (MHM24), CD11b (clone 44), CD11c (BU-15), αd (240I), and α4 integrin (HP2/1).
For transfected and control CHO cells, adhesion was performed using coated plates identical to those used for eosinophil adhesion. However, because the interaction between CHO transfectants and VCAM-1 was not as strong as that between eosinophils and VCAM-1 (data not shown), a modification of a previously described (21) gentle washing technique was used. This technique allowed nonadherent cells to be dislodged from the inverted plate at 1 g for 30 min at 20°C. Remaining adherent cells were then removed using 0.1 M EDTA (Sigma Chemical Co.) and counted by flow cytometry. Percent adhesion was determined from the number of adherent cells compared with the total number of cells added. In addition to VCAM-1, E-selectin (100 ng/ml) was also used to coat wells in some adhesion experiments. Besides the blocking mAbs used in the eosinophil studies, in certain experiments plates were pretreated with an appropriate dilution of F(ab′)2 anti–VCAM-1 mAb before the addition of CHO cells.
Statistical Analyses.
Statistical analyses were performed using an analysis of variance (ANOVA) with a Fisher post hoc t test. Significance was set at P < 0.05 for all tests.
Results
Expression of αd Integrins on Human Granulocytes and Regulation of Its Surface Expression on Human Eosinophils.
Using indirect immunofluorescence and flow cytometry, eosinophils were found to consistently express all four of the β2 integrins, including αdβ2, with the rank order of MFI values as follows: IgG control (3) < CD11c (15) < αd (22) < α4 (42) < CD11a (88) < CD11b (111) (values in parentheses representative of n = 7). Eosinophils and neutrophils had roughly similar levels of αd expression, whereas basophils had approximately twice the levels of surface expression (n = 4, data not shown).
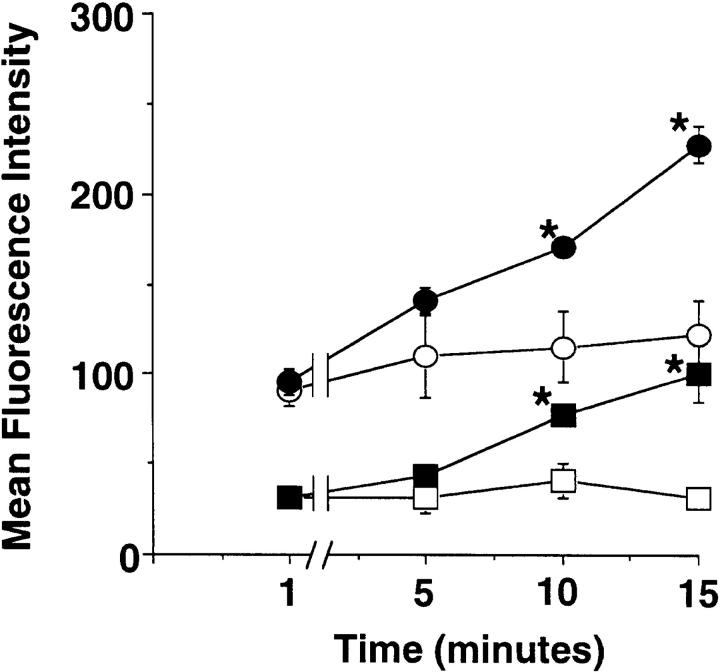
Subsequent studies were performed to determine whether eosinophils could rapidly mobilize intracellular stores of αdβ2 as has been reported for neutrophils (11). Purified peripheral blood eosinophils were incubated for 15 min with either PMA or the calcium ionophore A23187, and the surface expression of several α chains of the β2 integrins was then measured by indirect immunofluorescence. Fig. 1 shows the kinetics of this upregulation with phorbol ester. Both PMA (50 ng/ml) and calcium ionophore (1 μM, data not shown) significantly increased the expression of αd integrin and CD11b. Within minutes of adding PMA, expression increases, reaching significantly increased levels by 10 min. Therefore, eosinophils appear to have preformed stores of αdβ2 which, similar to CD11b stores, can be rapidly mobilized to the cell surface. Other eosinophil-active stimuli were tested for their acute effects on αdβ2 expression. Incubation of eosinophils for 15 min with MDC (100 nM), IL-5 (10 ng/ml), RANTES (100 ng/ml), and Eotaxin (100 nM) failed to alter αd integrin expression (data not shown).
Figure 1.
Expression of both αd integrin (squares) and CD11b (circles) is upregulated rapidly in peripheral blood eosinophils incubated with PMA (50 ng/ml; filled symbols) but not with buffer alone (open symbols). Values are expressed as average MFI ± SEM, n = 3. Irrelevant isotype control antibody fluorescence (1.7 ± 0.2) was unchanged throughout these experiments. *P < 0.05 for treated vs. untreated samples.
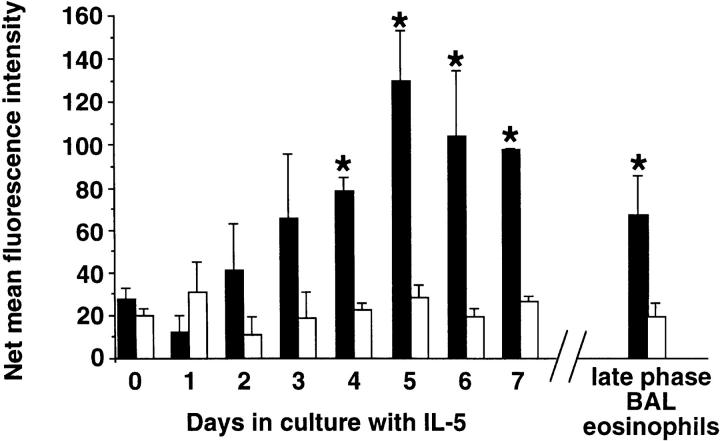
Many eosinophil responses can be enhanced by prolonged exposure to certain cytokines, such as IL-5, a phenomenon referred to as “priming” (22). Therefore, we determined whether eosinophil culture with IL-5 would lead to changes in surface expression of αd integrin. The kinetics of this effect on αd integrin expression is shown in Fig. 2. As can be seen, the level of αd integrin increases gradually, with statistically significant increases in levels at days 4–7 of culture. In contrast, levels of α4 integrin did not change significantly. Because late phase BAL eosinophils express many characteristics of cytokine-primed eosinophils (19, 23), their levels were also compared. Indeed, late phase BAL eosinophils also showed a statistically significant increase in the level of αd integrin expression, with levels similar to those seen after 3 d of culture in IL-5 (Fig. 2, right).
Figure 2.
Kinetics of changes in surface expression of αd (black bars) versus α4 (white bars) integrins on eosinophils cultured with 10 ng/ml of IL-5. Levels on eosinophils obtained from late phase BAL fluid after allergen challenge are also displayed. Values are expressed as net MFI values after subtraction of the irrelevant IgG1 control MFI values (3.1 ± 0.2, range 0.6–5.7). *P < 0.05 vs. day 0 value, n ≥ 3.
Eosinophil αdβ2 Integrin Binds to VCAM-1.
Although αd integrin has been shown to bind ICAM-3 and mediate leukocyte–leukocyte adhesion (11), the next series of experiments were designed to examine other possible αd ligands for eosinophils. In part because of previous studies suggesting β2 integrin–dependent, CD11b-independent eosinophil adhesion to VCAM-1 (20; and our unpublished observations), initial studies were performed using immobilized recombinant VCAM-1.
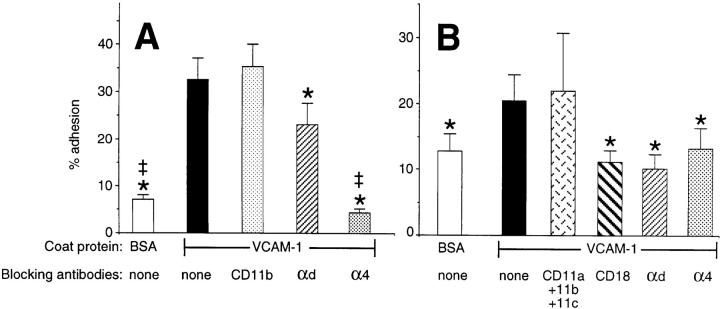
As shown in Fig. 3 A, freshly isolated eosinophils adhered to VCAM-1, and mAb blockade of α4 integrin effectively inhibited adhesion, whereas CD11b blockade had no effect. However, adhesion could also be significantly and consistently inhibited by the αd mAb 240I, albeit to a lesser degree (∼30% inhibition). Even more striking were results of VCAM-1 adhesion experiments in which IL-5– cultured eosinophils were used. Data in Fig. 3 B show that under these conditions, mAbs to CD18, αd, or α4 integrins were equally effective in reducing adhesion to background levels, whereas a combination of blocking mAbs to CD11a, CD11b, and CD11c had no effect. Note also that IL-5–cultured eosinophils displayed enhanced background adhesion and reduced VCAM-1 adhesion compared with those seen with freshly isolated eosinophils.
Figure 3.
Adhesion of freshly isolated (A, n ≥ 5) or IL-5–cultured (10 ng/ml for 4–7 d; B, n ≥ 3) eosinophils to immobilized recombinant VCAM-1. Blocking mAbs used included MHM24 (CD11a), clone 44 (CD11b), BU-15 (CD11c), 7E4 (CD18), 240I (αd integrin), and HP2/1 (α4 integrin). Results represent mean ± SEM for percent adhesion; *P < 0.05 vs. VCAM-1 adhesion without mAb, ‡ P < 0.05 vs. VCAM-1 adhesion in the presence of mAb 240I.
To further verify that αdβ2 functions as a ligand for VCAM-1, we generated CHO transfectants expressing the human αd and β2 integrin chains and used them in adhesion assays. These transfected cells, unlike the control CHO cells, expressed both the αd and β2 integrin chains at levels similar to that on eosinophils (depending on passage number: an MFI for each subunit of 10–50, compared with an MFI using an irrelevant IgG control mAb of 0.4–1.0; n = 5). Transfected CHO cells expressing αdβ2 adhered to VCAM-1–coated wells (16.0 ± 3.6% adhesion, mean ± SEM; n = 11), and adhesion was effectively blocked by an F(ab′)2 mAb against the first domain of VCAM-1 (80.1 ± 5.2% inhibition; P < 0.0005 vs. αdβ2-transfected CHO cell adhesion to VCAM-1, n = 3) as well as by mAbs (n = 3) against either CD18 (79.8 ± 18.1% inhibition; P < 0.0005) or αd integrin (59.6 ± 21% inhibition; P < 0.001). In contrast, control nontransfected CHO cells failed to adhere to VCAM-1 (1.9 ± 0.9% adhesion; n = 3), and neither the transfected nor the control CHO cells displayed significant adherence to wells coated with another adhesion protein, E-selectin (6.1 ± 3 and 1.5 ± 0.5% adhesion respectively, n = 2–5).
Discussion
These studies have shown that αdβ2, like other β2 integrins, is expressed on human eosinophils, basophils, and neutrophils. On peripheral blood eosinophils, the level of αd integrin expression is similar to that of α4 integrins, greater than that of CD11c, and less than that of CD11a and CD11b. Stimuli such as PMA and the calcium ionophore A23187 rapidly upregulated eosinophil surface expression of αd integrins, whereas a more gradual increase in surface expression was seen after 4–7 d of culture in media containing IL-5. In adhesion assays, αdβ2 integrin was shown to function as a ligand for VCAM-1 in both freshly isolated and IL-5–cultured eosinophils; these results were corroborated in adhesion assays using αdβ2-transfected CHO cells. Based on mAb blocking studies with freshly isolated eosinophils, adhesion to VCAM-1 was mainly mediated through α4 integrins, the other known ligand for VCAM-1. However, in IL-5–cultured eosinophils, adhesion to VCAM-1 was equally mediated by α4 and αd integrins. Together, these data are the first to demonstrate activation-dependent regulation of αdβ2 integrin expression and function on human eosinophils and document a novel function for eosinophil αdβ2 as an alternative ligand for VCAM-1.
There appear to be preformed stores of αd integrin in eosinophils, as evidenced by the rapid upregulation of surface expression with exposure to PMA or calcium ionophore. These results are similar to those observed for αd integrin and neutrophils (11). The kinetics of enhanced expression with PMA exposure was similar to that of CD11b, suggesting that these two leukointegrins might exist in similar or identical intracellular compartments. The location of this compartment for either integrin in eosinophils is not known; however, in neutrophils, preformed stores of CD11b have been localized to specific granules (24, 25). The immunolocalization of these preformed β2 integrin pools, as well as effects of more physiologic activators of eosinophils on integrin expression, are currently under investigation.
In contrast to the rapid mobilization by PMA, a gradual increase in surface αd integrin expression was seen during IL-5 culture. Whether this represents events occurring at the level of transcription or translation, rather than slow mobilization from preformed pools, is not yet known, due in part to difficulties encountered in isolating eosinophil mRNA as well as adverse effects of inhibitors of transcription and translation on eosinophil survival. In examining levels of αd integrin on late phase BAL eosinophils, which have already undergone cell adhesion and migration to get to the airway lumen, levels of expression intermediate to those seen on freshly isolated and IL-5–cultured eosinophils were observed. These data suggest that at least a portion of the elevated levels of αd found after IL-5 culture are likely due to increased transcription and translation of αd integrin.
A particularly novel aspect of this study was the determination that αdβ2 integrin, expressed on eosinophils and CHO transfectants, can function as a ligand for VCAM-1. Although the exact binding site on VCAM-1 is unknown, it is interesting that an mAb to the α4 integrin binding site in the first domain of VCAM-1 completely blocked αdβ2 integrin–dependent VCAM-1 adhesion. While this suggests that the αdβ2 binding site is near or identical to that for α4 integrins, additional studies are required to examine this issue more directly. Whether αdβ2 integrins can bind to other α4 integrin ligands, such as fibronectin or mucosal addressin cell adhesion molecule 1, is unknown. The finding that αdβ2 integrin can function as a ligand for VCAM-1 appears to conflict with data presented in the first report on human αdβ2 integrin by Van der Vieren et al. (11). In that paper it was shown that these same αdβ2-transfected CHO cells bound to a soluble ICAM-3 construct but not to a VCAM-1–Ig chimeric protein. Possible explanations for this discrepancy include a lower affinity for soluble ligand binding as well as other differences in assays, such as temperature.
Depending on the experimental conditions, both α4 and αd integrins can mediate eosinophil adhesion to VCAM-1. As we have shown in IL-5–cultured eosinophils, the relative contribution of αd integrin–dependent versus α4 integrin–dependent adhesion increases in parallel with an increase in αd integrin surface expression. However, because regulation of integrin affinity also influences adhesive function, additional studies are needed to define the mechanisms responsible for these IL-5–induced changes. Another potential paradox from our findings is that although neutrophils express αdβ2, they do not adhere to seven-domain VCAM-1. The most plausible explanation for this again appears to be related to integrin activation state. Freshly isolated eosinophils and neutrophils express similar levels of αd integrins and, at least for eosinophils, much lower αd integrin–dependent adhesion responses than those seen with activated cells. Whether this can be overcome with neutrophil activation is not yet known. Finally, the function of αd integrin in vivo is currently under investigation. Based on our results, it is plausible that because αd integrins on eosinophils bind to VCAM-1 and are upregulated with IL-5 in vitro and in BAL fluids in vivo, this leukointegrin may play a role in cytokine-primed eosinophil recruitment to inflammatory sites. However, evaluation of this hypothesis will require further investigation.
Acknowledgments
This work was supported in part by grants HL49545 and AI41472 from the National Institutes of Health and by a Developing Investigator Award to B.S. Bochner from the Burroughs Wellcome Fund.
References
- 1.Wardlaw AJ, Moqbel R, Kay AB. Eosinophils: biology and role in disease. Adv Immunol. 1995;60:151–266. doi: 10.1016/s0065-2776(08)60586-6. [DOI] [PubMed] [Google Scholar]
- 2.Seminario MC, Gleich GJ. The role of eosinophils in the pathogenesis of asthma. Curr Opin Immunol. 1994;6:860–864. doi: 10.1016/0952-7915(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 3.Broide DH, Gleich GJ, Cuomo AJ, Coburn DA, Federman EC, Schwartz LB, Wasserman SI. Evidence of ongoing mast cell and eosinophil degranulation in symptomatic asthma airway. J Allergy Clin Immunol. 1991;88:637–648. doi: 10.1016/0091-6749(91)90158-k. [DOI] [PubMed] [Google Scholar]
- 4.Evans PM, O'Connor BJ, Fuller RW, Barnes PJ, Chung KF. Effect of inhaled corticosteroids on peripheral blood eosinophil counts and density profiles in asthma. J Allergy Clin Immunol. 1993;91:643–650. doi: 10.1016/0091-6749(93)90270-p. [DOI] [PubMed] [Google Scholar]
- 5.Gleich, G.J., L.W. Hunt, B.S. Bochner, and R.P. Schleimer. 1996. Glucocorticoid effects on human eosinophils. In Inhaled Glucocorticoids in Asthma: Mechanisms and Clinical Actions. R.P. Schleimer, W.W. Busse, and P. O'Byrne, editors. Marcel Dekker, Inc., New York. 279–308.
- 6.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 7.Bochner BS. Cellular adhesion and its antagonism. J Allergy Clin Immunol. 1997;100:581–585. doi: 10.1016/s0091-6749(97)70158-1. [DOI] [PubMed] [Google Scholar]
- 8.Weller PF, Rand TH, Goelz SE, Chi-Rosso G, Lobb RR. Human eosinophil adherence to vascular endothelium mediated by binding to vascular cell adhesion molecule 1 and endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci USA. 1991;88:7430–7433. doi: 10.1073/pnas.88.16.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh GM, Symon FA, Lazarovits AI, Wardlaw AJ. Integrin α4β7 mediates human eosinophil interaction with MAdCAM-1, VCAM-1 and fibronectin. Immunology. 1996;89:112–119. doi: 10.1046/j.1365-2567.1996.d01-713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster CA. VCAM-1/alpha 4-integrin adhesion pathway: therapeutic target for allergic inflammatory disorders. J Allergy Clin Immunol. 1996;98:S270–S277. doi: 10.1016/s0091-6749(96)70075-1. [DOI] [PubMed] [Google Scholar]
- 11.Van der Vieren M, Letrong H, Wood CL, Moore PF, St. John T, Staunton DE, Gallatin WM. A novel leukointegrin, αdβ2, binds preferentially to ICAM-3. Immunity. 1995;3:683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 12.Hildreth JEK, Gotch FM, Hildreth PDK, McMichael AJ. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983;13:202–208. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- 13.Hildreth JEK, August JT. The human lymphocyte function-associated (HLFA) antigen and a related macrophage differentiation antigen (HMac-1): functional effects of subunit-specific monoclonal antibodies. J Immunol. 1985;134:3272–3280. [PubMed] [Google Scholar]
- 14.Law SKA, Gagnon J, Hildreth JEK, Wells CE, Willis AC, Wong AJ. The primary structure of the beta subunit of the cell surface adhesion glycoproteins LFA-1, CR3 and p150,95 and its relationship to the fibronectin receptor. EMBO (Eur Mol Biol Organ) J. 1987;6:915–919. doi: 10.1002/j.1460-2075.1987.tb04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansel TT, Vries IJMD, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 16.Bochner BS, McKelvey AA, Sterbinsky SA, Hildreth JEK, Derse CP, Klunk DA, Lichtenstein LM, Schleimer RP. Interleukin-3 augments adhesiveness for endothelium and CD11b expression in human basophils but not neutrophils. J Immunol. 1990;145:1832–1837. [PubMed] [Google Scholar]
- 17.Bochner BS, McKelvey AA, Schleimer RP, Hildreth JEK, MacGlashan DW., Jr Flow cytometric methods for analysis of human basophil surface antigens and viability. J Immunol Methods. 1989;125:265–271. doi: 10.1016/0022-1759(89)90102-6. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto K, Appiah-Pippim J, Schleimer RP, Bickel CA, Beck LA, Bochner BS. CD44 and CD69 represent different types of cell surface activation markers for human eosinophils. Am J Respir Cell Mol Biol. 1998;18:860–866. doi: 10.1165/ajrcmb.18.6.3159. [DOI] [PubMed] [Google Scholar]
- 19.Kroegel C, Liu MC, Hubbard WM, Lichtenstein LM, Bochner BS. Blood and bronchoalveolar eosinophils in allergic subjects following segmental antigen challenge: surface phenotype, density heterogeneity, and prostanoid production. J Allergy Clin Immunol. 1994;93:725–734. doi: 10.1016/0091-6749(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto K, Sterbinsky SA, Bickel CA, Zhou DW, Kovach NL, Bochner BS. Regulation of α4 integrin-mediated adhesion of human eosinophils to fibronectin and vascular cell adhesion molecule-1 (VCAM-1) J Allergy Clin Immunol. 1997;99:648–656. doi: 10.1016/s0091-6749(97)70027-7. [DOI] [PubMed] [Google Scholar]
- 21.Yang LJ, Zeller CB, Schnaar RL. Detection and isolation of lectin-transfected COS cells based on cell adhesion to immobilized glycosphingolipids. Anal Biochem. 1996;236:161–167. doi: 10.1006/abio.1996.0145. [DOI] [PubMed] [Google Scholar]
- 22.Walsh GM, Hartnell A, Wardlaw AJ, Kurihara K, Sanderson CJ, Kay AB. IL-5 enhances the in vitro adhesion of human eosinophils, but not neutrophils, in a leucocyte integrin (CD11/18)-dependent manner. Immunology. 1990;71:258–265. [PMC free article] [PubMed] [Google Scholar]
- 23.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–3718. [PubMed] [Google Scholar]
- 24.Todd RF, III, Arnaout MA, Rosin RE, Crowley CA, Peters WA, Babior BM. Subcellular localization of the large subunit of Mo1 (Mo1α: formerly gp 110), a surface glycoprotein associated with neutrophil adhesion. J Clin Invest. 1984;74:1280–1290. doi: 10.1172/JCI111538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bainton DF, Miller LJ, Kishimoto TK, Springer TA. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987;166:1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]