Abstract
The c-maf protooncogene is a T helper cell type 2 (Th2)-specific transcription factor that activates the interleukin (IL)-4 promoter in vitro. Although it has been postulated that c-maf directs the Th2-specific expression of the IL-4 gene in vivo, direct evidence that c-maf functions during the differentiation of normal, primary T cells is lacking. We now demonstrate that overexpression of c-maf in vivo skews the Th immune response along a Th2 pathway, as evidenced by increased production of Th2 cytokines and the IL-4–dependent immunoglobulins, IgG1 and IgE. The overproduction of IgGl and IgE in the CD4 promoter/c-maf transgenic mice was IL-4 dependent since this was not observed in c-maf transgenic mice bred onto an IL-4–deficient background. Ectopic expression of c-maf in mature Th1 cells did not confer on them the ability to produce IL-4, but did decrease the production of IFN-γ. The attenuation of Th1 differentiation by c-maf overexpression occurred by a mechanism that was independent of IL-4 and other Th2 cytokines, and could be overcome by IL-12. These studies demonstrate that c-maf promotes Th2 differentiation by IL-4–dependent mechanisms and attenuates Th1 differentiation by Th2 cytokine-independent mechanisms.
Keywords: interleukin 4, T helper cells, c-maf, transcription factor
The cytokine IL-4 is known to regulate a broad spectrum of biologic activities including activation, growth, and differentiation of T and B lymphocytes, macrophages, and cells of the inflammatory and hematopoietic systems (1–4). The receptor for IL-4 is expressed on most cells of hematopoietic lineage and, when occupied with its ligand, induces a program of gene activation mediated primarily by the Stat6 and insulin receptor substrate (IRS)-2 transcription factors (5–7).
One of the most important consequences of IL-4 production is the generation of a selected subset of CD4 T helper cells termed Th2 (8, 9). These cells secrete a panoply of cytokines, including IL-4 itself, IL-5, IL-10, and IL-13, that are critical in the allergic response and in mounting an effective immune response to parasites and nematodes. Furthermore, the relative ratio of IL-4–producing Th2 cells to the opposing CD4 IFN-γ–producing Th1 subset has dramatic consequences for the immune response to diverse antigens, including pathogens, autoantigens, tumor antigens, and allergens. For example, in the nonobese diabetic mouse, a mouse model of the human disease insulin-dependent diabetes mellitus, Th1 cells are pathogenic (10, 11). Enhancing the formation of Th2 cells at the expense of Th1 cells, in such mice, either through the direct administration of recombinant IL-4 or through altering T cell/costimulatory molecule interactions, results in disease amelioration (12, 13). Furthermore, IL-4 is necessary to initiate and sustain in vivo IgE responses as demonstrated by the failure of IL-4–deficient animals to mount IgE responses to infection (14–16).
IL-4 is a highly tissue-specific gene whose expression is limited to Th2 cells, a small population of CD4+NK1.1+ T cells, basophils, and mast cells (17–19). Resting T cells do not transcribe IL-4 until activated through the TcR or with pharmacologic agents such as PMA and Ca2+ ionophores. The minimal IL-4 promoter sufficient to confer Th2 specificity and inducibility has been identified and characterized (20). It contains functionally critical binding sites for a family of transcription factors called nuclear factor of activated T cells (NFAT),1 and for activator protein (AP)–1 family members. However, none of these factors can explain the Th2 cell specificity of the IL-4 gene (21). Recently, we have shown that the protooncogene c-maf is expressed in Th2 but not in Th1 clones and is induced during the differentiation of normal Thp along a Th2 but not a Th1 lineage. c-maf is a basic region/leucine zipper transcription factor that belongs to the subfamily of AP-1/ CREB/ATF proteins and, like them, forms homo- and heterodimers (22). Homodimers of c-maf can bind to a sequence adjacent to a Th2-specific footprint and immediately downstream of an NFAT site in the proximal IL-4 promoter. Ectopic expression of c-maf in Th1 cells, B cells, and HepG2 cells can transactivate an exogenous IL-4 promoter. Furthermore, c-maf, in synergy with NFATp, can initiate endogenous IL-4 production in B cells. These observations led us to conclude that c-maf directs tissue-specific IL-4 expression in Th2 cells (21).
Although we have shown that c-maf is a potent transactivator of the IL-4 gene in vitro, the function of c-maf during Th cell differentiation in vivo was unknown. Furthermore, it was possible that c-maf may regulate genes in addition to IL-4. To address these questions, we have generated transgenic mice overexpressing c-maf in both immature and mature T cells. Here we report that c-maf transgenic mice have an increased Th2 immune response in vivo and in vitro that can be ablated by backcrossing onto an IL-4–deficient background. In addition, c-maf, in the absence of IL-12, attenuates the differentiation of Th1 cells by a mechanism that is independent of Th2 cytokines. Taken together, these observations provide strong evidence for a critical role of c-maf in mediating Th2 cell differentiation through both IL-4–dependent and –independent mechanisms.
Materials and Methods
Generation of c-maf Transgenic Mice.
A 4-kb full-length cDNA encoding the murine c-maf gene was cloned into the SalI site of the transgenic expression vector, p37.1 (gift of Dr. D. Littman, New York University Medical School, New York, NY), which contains the CD4 promoter/enhancer and the first intron without the silencer element (23). c-maf transgenic mice were identified by digesting genomic DNA with SalI, followed by Southern analysis using a c-maf–specific probe from the 3′UTR. Subsequent screening of c-maf transgenic mice was carried out by genomic PCR using the following primers: c-maf primer: 5′-TGTTGTGGTGCAGAACTGGAT-3′. p37-1 primer; 5′-GTTTCAGGTTCAGGGGGAGGT-3′.
Northern Analysis and Reverse Transcription PCR.
Total RNA was prepared from thymi or spleens of wild-type or c-maf transgenic mice using the Trizol reagent according to the manufacturer's instructions (GIBCO BRL, Gaithersburg, MD). 10 μg of each RNA sample was fractionated on 1.2% agarose gel, transferred to a Hybond membrane, and subsequently hybridized with specific probes in QuickHyb buffer. The probe for the c-maf transgene contains part of the first exon of the CD4 gene and the first 347 bp of the c-maf cDNA. Reverse transcription (RT)– PCR was performed by using the Access RT-PCR kit (Promega, Madison, WI). The sequence of the primers used to amplify the spliced c-maf transgenic mRNA were the CD4 upstream primer, 5′-ACACACACCTGTGCAAGAAGC-3′, and 5′-CTCTCCTCTTCTGCCTGGCTCTTATGGTTA-3′. Primers used to amplify CD4 were the CD4 upstream primer and the CD4 downstream primer 5′-TCCTCTGGTCAGAGAACTTCC-3′. The c-maf–specific internal primer used for southern analysis was 5′-AGCCGAGAGCAAAAGGGTTGG-3′.
Purification of Naive CD4+ T Cells.
Lymph node cells were harvested, stained with FITC-conjugated anti–CD4 antibody and PE-conjugated Mel-14 antibody, resuspended in unsupplemented RPMI at 106 cells per ml, and subjected to sorting by a MO FLO (Cytomation, Fort Collins, CO) cell sorter. The CD4+ and Mel-14 high cells were collected as naive cells and used in in vitro differentiation assays.
In Vitro Differentiation of Th Cells.
Spleen cells were harvested and stimulated in vitro with plate-bound anti–CD3 mAb (2C11) at 1 μg/ml alone and/or anti–CD28 (1 μg/ml) for naive T cells (nonskewing conditions), or with anti–IL-12 mAb (5C3) at 20 μg/ml (Th2 skewing conditions), anti–IL-4 mAb (11B11) at 5 μg/ml (Th1 skewing conditions), anti–IL-10 mAb (R & D Systems, Inc., Minneapolis, MN) at 0.2 μg/ml, or anti–IL-13 mAb (R & D Systems, Inc.) at 0.2 μg/ml. 24 h poststimulation, IL-2 at 50 U/ml was added to all cultures. In addition, IL-4 at 500 U/ml or IL-12 at 50 U/ml was added into Th2 or Th1 cultures, respectively. 7 d after stimulation, cells were harvested, washed thoroughly, and restimulated with plate-bound anti–CD3. 24 h after restimulation, supernatants were harvested and subjected to ELISA. Under these conditions, we find that ∼80% of the population at the end of the secondary stimulation are CD4+ T cells.
Intracellular Cytokine Staining.
Cells were first incubated with 2 μM Monesin in complete medium for 5–6 h, and subsequently fixed with 4% paraformaldehyde and permeabilized with Saponin. The fixed and permeabilized cells were incubated with PE- or FITC-conjugated anticytokine antibodies (PharMingen, San Diego, CA) or control antibodies for 30 min on ice, washed with 0.1% BSA in PBS, and subjected to flow cytometric analysis on a FACS® (Becton Dickinson & Co., Mountain View, CA), and analyzed with Cellquest software.
Results
Generation of c-maf Transgenic Mice.
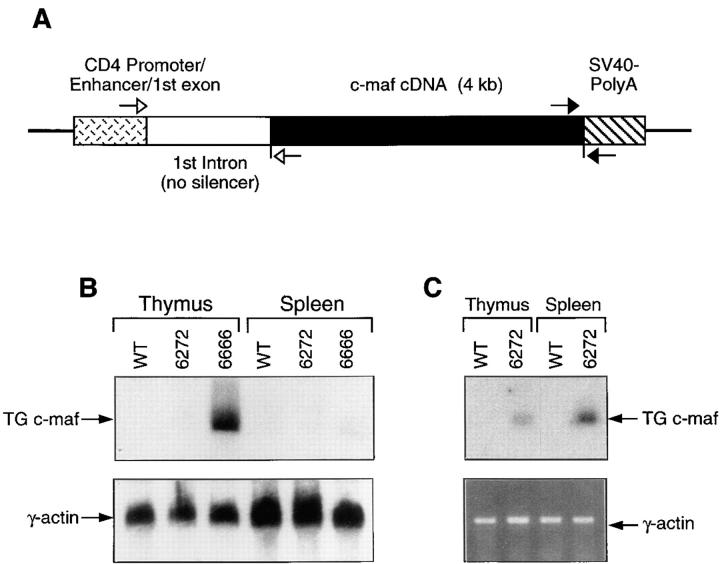
To study the function of c-maf in vivo, we have generated mouse strains overexpressing c-maf in immature and mature T cells. A full-length cDNA encoding murine c-maf was inserted into a transgenic cassette that contained the CD4 promoter/enhancer and first intron without the silencer element, shown to be expressed in all T cells (23) (Fig. 1 A). The resulting c-maf transgenic construct was injected into C57BL/6 blastocysts. From twenty independent offspring analyzed, six founders that had incorporated the c-maf transgene as determined by genomic Southern analysis were identified. Three of the 6 founder lines, all low copy number transgenics, transmitted the transgene to offspring, and all three lines displayed the phenotype which will be described below. Line 6666 expressed moderately high levels of transgenic c-maf mRNA of the expected size in thymus but only low levels in spleen (Fig. 1 B). Line 6272 expressed very low levels of transgenic c-maf which could only be detected by RT-PCR in both thymus and spleen (Fig. 1 C). The third line, 6669, was lost before a careful documentation of its level of expression of the c-maf transgene, but also exhibited the phenotype described below. The transgenic mice were born healthy and survived normally up to 12 months. It is curious that none of several high copy c-maf transgenic founder lines successfully transmitted the transgene to offspring (Ho, I-C., unpublished observations). Although the reason for this is unknown, it is reminiscent of unsuccessful attempts to produce IL-4 transgenic mice using a very potent Ig promoter that failed secondary to neonatal lethality until an attenuated promoter was used (24).
Figure 1.
Generation of c-maf transgenic mice. (A) Schematic diagram of the c-maf transgenic construct. Filled arrows represent primers used to screen c-maf transgenic mice. Open arrows represent primers used for RT-PCR. (B) Northern analysis of c-maf transgenic mice. Total RNA prepared from thymi or spleens of c-maf transgenic or wild-type mice was probed with a c-maf–specific probe. The same blot was also hybridized with a γ-actin–specific probe to quantify loading of RNA. (C) RT-PCR analysis of c-maf transgenic mice. Total RNA prepared from thymi or spleen of line 6272 or from wild-type mice was used as template in RT-PCR analysis using the primers described above. The 409-bp RT-PCR product, containing the spliced c-maf transgenic transcript, was further confirmed by Southern blot analysis using an internal oligonucleotide as probe. A 266-bp fragment of the spliced CD4 transcript was also amplified by RT-PCR as a control. Sequences of all the primers used are described in Materials and Methods.
c-maf Transgenic Mice Display a Th2 Phenotype.
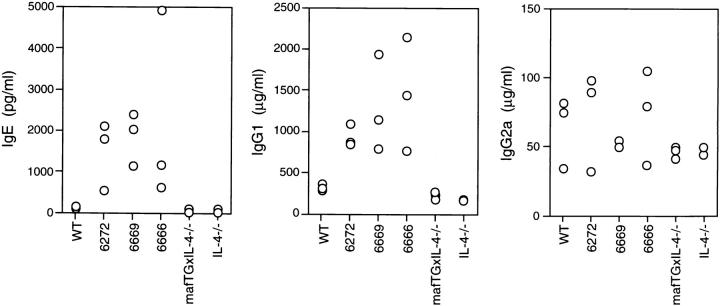
Previously, we have shown that c-maf is a Th2 cell-specific transcription factor that can by itself transactivate both an exogenous IL-4 promoter (21) and the endogenous IL-4 gene in T cells (our unpublished data) and that, together with NFAT and NIP45 proteins, can activate the endogenous IL-4 promoter in non-T cells. Given these data, it was our expectation that provision of c-maf to normal Thp cells by transgenesis would allow them to transcribe the IL-4 gene. Such mice might overproduce IL-4 and other Th2 cytokines and have increased levels of the IL-4–dependent immunoglobulins IgG1 and IgE. We examined sera from unimmunized C57Bl/6 wild-type mice and from the three lines of c-maf transgenic mice by ELISA to quantitate the basal levels of IL-4 and immunoglobulin isotypes. Although levels of serum IL-4 were below detection in both wild-type and c-maf transgenic mice, all three c-maf transgenic lines had significantly higher levels of IgE and IgG1 (5–10-fold) than did wild-type littermates (Fig. 2). In contrast, there was no difference in levels of IFN-γ–dependent IgG2a between wild-type and transgenic mice (Fig. 2).
Figure 2.
ELISA analysis of serum immunoglobulin. Sera obtained from unimmunized wild-type (WT), three c-maf transgenic (6666, 6669, 6272), c-maf transgenic/IL-4–deficient (mafTGxIL-4-/-) and IL-4–deficient (IL-4-/-) mice were analyzed by ELISA for IgE, IgG1, and IgG2a.
In addition to controlling the isotype switch to IgG1 and IgE, IL-4 is essential for promoting the differentiation of Th2 cells. To test whether Th cells derived from c-maf transgenic mice preferentially develop along a Th2 lineage upon TcR-mediated stimulation, an in vitro differentiation assay was performed. Splenocytes obtained from both wild-type and c-maf transgenic mice were stimulated in vitro with plate-bound anti–CD3 for 7 d, washed thoroughly, and restimulated with anti–CD3. 24 h after the secondary stimulation, supernatant was harvested and cytokine levels were quantitated by ELISA. Under these “nonskewing” conditions, splenocytes derived from wild-type C57BL/6 became “classical” Th1 cells secreting high levels of IFN-γ and very low, if not undetectable, levels of the Th2 cytokines, IL-4, IL-5, and IL-10 (Fig. 3 and Table 1). In contrast, under the same conditions, splenocytes derived from the c-maf transgenic line 6666 differentiated into classical Th2 cells producing high levels of Th2 cytokines and significantly lower levels of IFN-γ than wild type. A clear gene dosage effect of the c-maf transgene was apparent since splenocytes derived from the lower expresser c-maf transgenic line 6272 matured into Th1-like cells secreting high levels of IFN-γ and very low levels of IL-4 and IL-5. Interestingly, 6272 splenocytes did produce high levels of IL-10 comparable with that of wild-type Th2 cells generated under Th2-skewing conditions.
Figure 3.
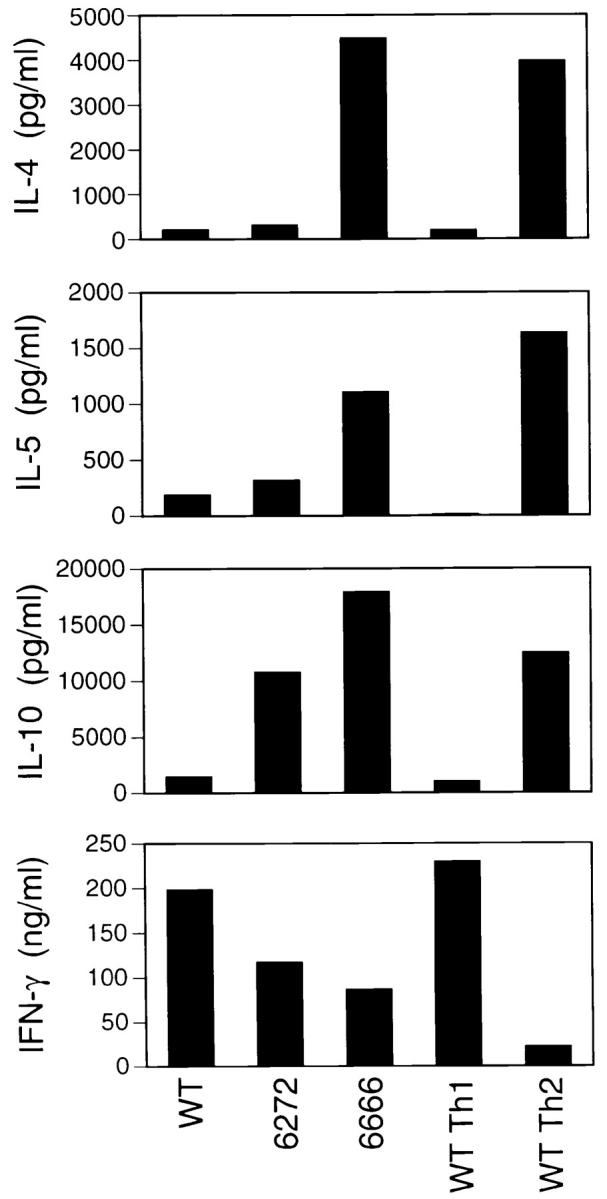
Th cells of c-maf transgenic mice are skewed toward a Th2 pathway upon stimulation. Splenocytes derived from wild-type (WT) and two c-maf transgenic (6666, 6272) mice were differentiated in vitro under nonskewing conditions according to the protocol described in Materials and Methods. Supernatants were harvested 24 h after the secondary stimulation and cytokine profile quantitated by ELISA. As controls, wild-type splenocytes were also differentiated in vitro under Th1-skewing (WT Th1) or Th2-skewing (WT Th2) conditions.
Table 1.
Summary of In Vitro Differentiation of Th Cells
| IL-4 | IL-15 | IL-10 | IFN-γ | |||||
|---|---|---|---|---|---|---|---|---|
| Exp 1 | ||||||||
| WT Th1 (%) | 5 | 1 | 8 | 100 | ||||
| WT Th2 (%) | 100 | 100 | 100 | 10 | ||||
| WT (%) | 5 | 11 | 12 | 86 | ||||
| 6272 (%) | 8 | 19 | 86 | 51 | ||||
| 6666 (%) | 113 | 68 | 144 | 38 | ||||
| Exp 2 | ||||||||
| WT Th1 (%) | 0 | 0 | 0 | 100 | ||||
| WT Th2 (%) | 100 | 100 | 100 | 80 | ||||
| WT (%) | 0 | 0 | 0 | 103 | ||||
| 6272 (%) | 0 | 0 | 12 | 121 | ||||
| 6666 (%) | 14 | 88 | 99 | 82 | ||||
| Exp 3 | ||||||||
| WT Th1 (%) | 23 | 36 | 11 | 100 | ||||
| WT Th2 (%) | 100 | 100 | 100 | 28 | ||||
| WT (%) | 15 | 39 | 9 | 119 | ||||
| 6272 (%) | 28 | 40 | 33 | 63 | ||||
| 6666 (%) | 214 | 114 | 93 | 51 | ||||
| Three independent experiments using in vitro differentiation of Th cells were performed as described in Fig. 3. The levels of IFN-γ obtained from wild-type Th1 (WT Th1) cells and the levels of Th2 cytokines (IL-4, IL-5, and IL-10) obtained from wild-type Th2 (WT Th2) cells were arbitrarily normalized to 100%. The actual concentration of cytokines from experiment 1 is shown in Fig. 3. | ||||||||
The Th2 Phenotype of c-maf Transgenic Mice Is IL-4 Dependent.
Since the basal serum level of IL-4 in both wild-type and c-maf transgenic mice was below the detectable range, it was important to establish that the Th2 phenotype of these mice was due to overproduction of IL-4. IL-4– deficient mice do not mount Th2-type responses and have impaired production of IgG1 and IgE (14–16). We therefore introduced the c-maf transgene (line 6666) onto an IL-4−/− background, and these doubly transgenic mice are henceforward called mafTG×IL-4−/− mice. As shown in Fig. 2, in contrast to c-maf transgenic mice, mafTG×IL-4−/− mice had normal levels of serum IgE and IgG1. Furthermore, similar to splenocytes derived from wild-type mice, splenocytes derived from mafTG×IL-4−/− mice differentiated into Th1 cells under nonskewing conditions (Fig. 4). Similar results were obtained when splenocytes derived from line 6666 were stimulated in vitro in the presence of anti–IL-4 antibody (Fig. 5 A). These results confirm that the Th2 phenotype of the c-maf transgenic mice is dependent on IL-4 and also demonstrate that Th1 cells can be generated normally in vitro from c-maf transgenic mice in the absence of IL-4.
Figure 4.
The Th2-skewing effect is dependent on IL-4. Splenocytes derived from wild-type (WT) and c-maf transgenic/ IL-4–deficient (mafTGxIL-4-/-) mice were differentiated under nonskewing conditions in vitro and the cytokine profiles quantitated by ELISA as described in Fig. 3. The transgenic line used is line 6666.
Figure 5.
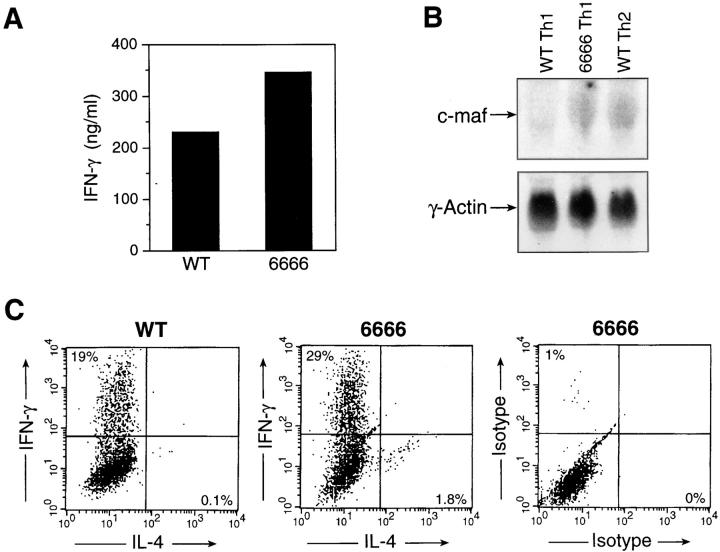
c-maf alone is insufficient to transactivate the endogenous IL-4 promoter in mature Th1 cells. (A) Levels of IFN-γ produced by mature Th1 cells, derived from splenocytes of wild-type (WT) and c-maf transgenic (6666) mice, generated by in vitro differentiation under Th1-skewing conditions. (B) Northern analysis of c-maf expression in wild-type Th1 (WT Th1), c-maf transgenic Th1 (6666 Th1), and wild-type Th2 (WT Th2) cells generated by in vitro differentiation. (C) Intracellular cytokine staining of wild-type (WT) or c-maf transgenic (6666) Th1 cells using FITC-conjugated anti–IFN-γ and PE-conjugated anti–IL-4 antibodies. FITC- and PE-conjugated isotype control antibodies were also used to determine background staining.
Overexpression of c-maf Alone Is Not Sufficient to Induce the Production of IL-4 by Normal Th1 Cells.
Previously, we have demonstrated that ectopic expression of c-maf in mature, effector Th1 clones allows them to transactivate an exogenous IL-4 promoter in in vitro assays. Given the low transfection efficiency of these clones, we were not able to test whether ectopic expression of c-maf permits endogenous production of IL-4 by established Th1 cells. The overproduction of IL-4 in the c-maf transgenic line could be ascribed to enhanced skewing of Thp cells towards a Th2 pathway, to increased production of IL-4 by mature Th2 cells and/or to production of IL-4 by mature effector Th1 cells. To distinguish among these possibilities, Th1 cells were generated from c-maf transgenic mice, hence called transgenic Th1 cells, by using an in vitro differentiation protocol under Th1-skewing conditions. We could not detect any IL-4 production by ELISA by transgenic Th1 cells under these conditions (data not shown). However, it was possible that, on a single cell basis, a small percentage of the transgenic Th1 cells might be producing both IFN-γ and IL-4. To test this hypothesis, intracellular cytokine staining was used to evaluate the production of IFN-γ and IL-4 by transgenic Th1 cells. Although a small number of mature transgenic Th cells stained positive for IL-4, no IL-4/IFN-γ double positive cells were detected (Fig. 5 C). One possible explanation is that the level of c-maf in transgenic Th1 cells was below the physiological level required for transactivation of the endogenous IL-4 gene. To rule out this possibility, levels of c-maf mRNA were assessed by Northern analysis. As shown in Fig. 5 B, the level of c-maf transcripts in transgenic Th1 cells was comparable to that of wild-type Th2 cells. We conclude that overexpression of c-maf alone, to a level comparable to that of wild-type Th2 cells, is not sufficient to transactivate the endogenous IL-4 promoter in Th1 cells.
c-maf, Independent of IL-4, Attenuates the Th1 Pathway in the Absence of IL-12.
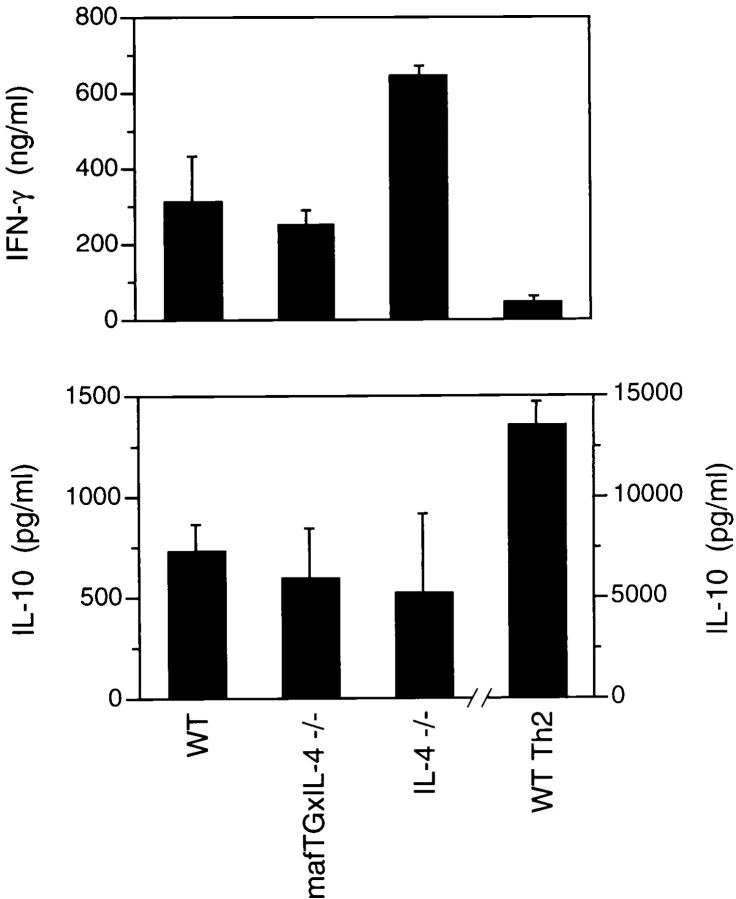
We have demonstrated that the Th2 phenotype of c-maf transgenic mice is IL-4 dependent, and that Th1 cells can be generated normally in vitro from these mice in the presence of exogenous IL-12 (Fig. 3 and Table 1). However, we noted that the amount of IFN-γ produced by c-maf transgenic Th cells under nonskewed conditions was less than that of wild-type Th cells. The impaired production of IFN-γ was not secondary to overproduction of IL-4 since mature nonskewed mafTG×IL-4−/− Th cells also produced ∼50% less IFN-γ than was produced by mature IL-4−/− Th cells (Fig. 4). This difference between double transgenic and IL-4−/− Th cells was also reflected in the smaller number of IFN-γ–positive cells detected by intracellular staining of the nonskewed populations (data not shown). Thus, ectopic expression of c-maf in the absence of IL-4 resulted in impaired IFN-γ production. One possible explanation for this result was that unfractionated mafTG×IL-4−/− splenocytes might contain increased numbers of Th2 memory cells that had been generated in vivo. Another potential explanation was that mafTG×IL-4−/− antigen presenting cells, such as macrophages and dendritic cells, might produce less IL-12, which is required for Th1 differentiation. Alternatively, overexpression of c-maf in Th cells might transactivate a yet-to-be-defined target gene, other than IL-4, able to attenuate the development of Th1 cells.
To distinguish among these hypotheses, naive Thp cells derived from IL-4−/− and maf TG×IL-4−/− mice were purified by cell sorting and stimulated with plate-bound anti–CD3 and anti–CD28 in vitro in the presence or absence of recombinant IL-12. As shown in Fig. 6 A, naive IL-4−/− Thp cells differentiated into Th1 cells producing high levels of IFN-γ in the absence of recombinant IL-12. Addition of recombinant IL-12 did not further enhance the production of IFN-γ (data not shown). In contrast, naive c-mafTG×IL-4−/− Thp cells that had been differentiated in the absence of IL-12 produced significantly lower levels of IFN-γ (∼30% of control) than that produced by IL-4−/− mice. Of interest, no such difference was noted when IL-12 was added into the culture (Fig. 6 B). Consistent with the lower levels of IFN-γ, differentiated c-mafTG×IL-4−/− Th cells produced significantly higher levels of IL-10 than did differentiated IL-4−/− Th cells (Fig. 6 A). This result strongly argues against a role for Th2 memory cells or IL-12 production as responsible for the impaired production of IFN-γ. Rather, it argues for the presence of c-maf target genes, other than IL-4, in Th cells that can attenuate the Th1 pathway via an IL-4–independent mechanism.
Figure 6.
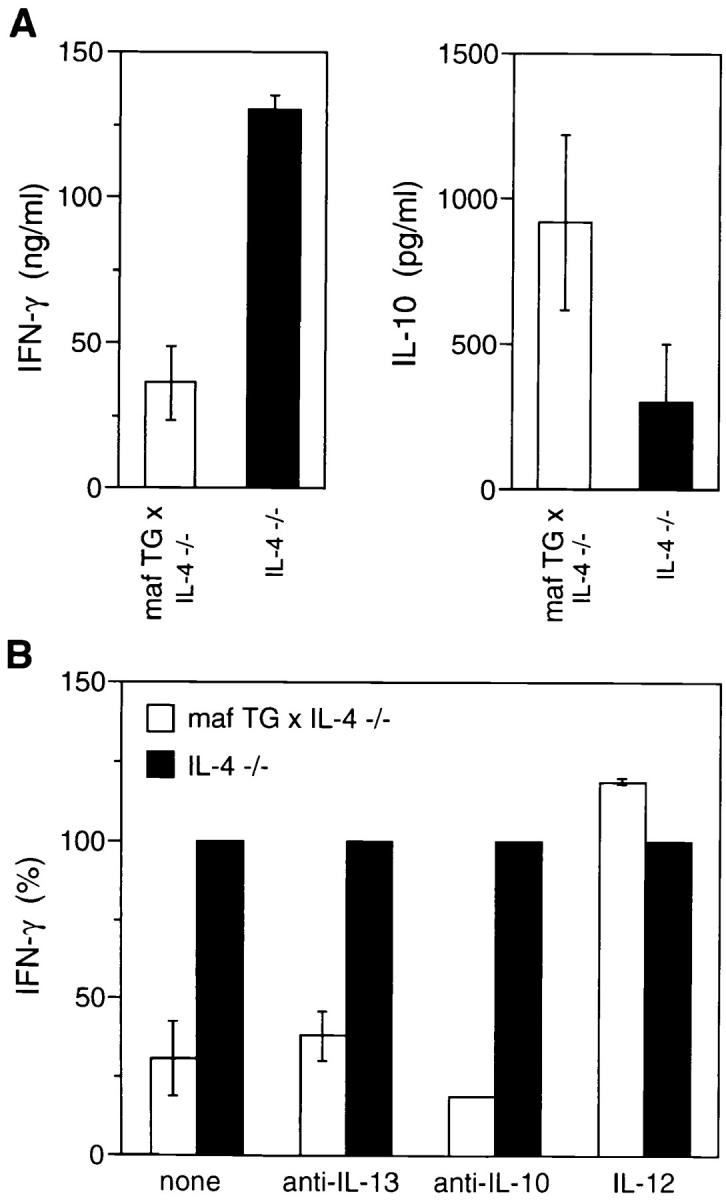
c-maf is able to attenuate the Th1 pathway by IL-4–independent mechanism(s). Levels of IFN-γ and IL-10 produced by naive Th cells of c-maf transgenic/IL-4–deficient (mafTGx IL-4-/-) and IL-4–deficient (IL-4-/-) mice, subjected to in vitro differentiation under nonskewing conditions in the absence (A) or presence (B) of anti–IL-13, anti–IL-10, or IL–12. The level of IFN-γ produced by mature IL-4−/− Th cells in B was arbitrarily normalized to 100%.
However, it has been demonstrated that other Th2 cytokines, such as IL-13 and IL-10, can also promote the differentiation of Th2 cells (25–27). Thus, yet another possible explanation was that the c-maf transgene induced the production of Th2 cytokine genes other than IL-4 that attenuated the Th1 pathway. To test this hypothesis, in vitro differentiation experiments similar to those described above were performed in the absence or presence of anti–IL-13 or anti–IL-10 antibodies. As shown in Fig. 6 B, addition of anti–IL-13 or anti–IL-10 could not restore the ability of mafTG×IL-4−/− Th cell to produce normal levels of IFN-γ. Taken together, these experiments demonstrate that, in the absence of IL-4, c-maf is able to attenuate the Th1 pathway via a Th2-cytokine–independent mechanism.
Discussion
Previously, we have demonstrated that the c-maf proto-oncogene, a Th2-specific transcription factor, can transactivate both exogenous and endogenous IL-4 promoters in Th clones and in lymphoid tumor cells. In this report, we now provide evidence that provision of c-maf to normal T cells drives them to increased production of IL-4. Transgenic mice that overexpress c-maf in normal T cells displayed an IL-4–dependent enhancement of Th2 cell differentiation as manifested by increased levels of IgG1 and IgE and preferential development of Th2 cells in in vitro differentiation assays. Interestingly, ectopic expression of c-maf in mature Th1 cells did not confer on them the ability to produce IL-4, but did attenuate the production of IFN-γ. These results establish that c-maf is a potent transactivator of the IL-4 gene both in vivo and in vitro. In addition, these data suggest the presence of additional c-maf target gene(s) in T cells that function to attenuate the Th1 pathway by a mechanism that is Th2-cytokine independent.
Although c-maf transgenic mice have significantly higher basal levels of serum IgE and IgG1 than nontransgenic littermates, these levels are considerably lower than those found in mice transgenic for the IL-4 gene itself (24). This difference is likely explained by an IL-4 gene dosage effect. In c-maf transgenic mice, levels of IgE and IgG1 are determined by the amount of endogenous IL-4. Since the c-maf founder lines were low copy number and low expresser transgenics, levels of endogenous IL-4 were unlikely to be greatly elevated; indeed, we did not detect serum IL-4 by ELISA. In contrast, much higher levels of IgE and IgG1 can be expected in IL-4 transgenic mice that carry multiple copies of an exogenous IL-4 gene driven by a fairly strong Ig promoter. The differences in IL-4 levels may also explain the lack of allergic blepharitis in c-maf compared with IL-4–transgenic mice. In addition to increased IgE and IgG1 levels, c-maf transgenic mice, in particular line 6666, also displayed preferential Th2 responses upon stimulation in vitro. This effect was very noticeable since T cells from the C57BL/6 background into which the transgene was introduced are strongly directed along a Th1 pathway. Line 6272, expressing very low levels of the c-maf transgene, only detectable by RT-PCR, did not have elevated IL-4 production in vitro. Nevertheless, serum levels of IgE and IgG1 were elevated, suggesting that the isotype switch is a very sensitive biological marker of functional IL-4. The phenotype of this line further suggests that a very modest increase in levels of c-maf can effect a Th2 shift in vivo. It will be of interest to determine whether c-maf transgenic mice are more resistant to the development of autoimmune diseases using models such as the NOD and MRL/lpr strains.
What is the source of the increased IL-4 produced by c-maf transgenic T cells? The CD4 promoter/enhancer drives expression of c-maf in naive Thp cells as well as in mature Th1 and Th2 cells. The elevated IL-4 production observed could be secondary to maf-induced preferential skewing of naive Thp cells along a Th2 pathway, and/or to overproduction of IL-4 by mature Th2 and Th1 cells. Our data demonstrate that preferential skewing of Thp cells and increased production of IL-4 by mature Th2 cells is the likely explanation, as discussed below.
c-maf is a potent transactivator of the IL-4 promoter-reporter construct in mature effector Th1 clones, and its provision is sufficient to allow endogenous IL-4 production in the Jurkat human Th1 cell line (our unpublished observations). Nevertheless, ectopic expression of c-maf in our transgenic lines, in normal, mature Th1 cells, to a level comparable to that present in mature wild-type Th2 cells, did not permit them to express IL-4. This result implies either that positive transacting factors in addition to c-maf are required to drive IL-4 expression in normal Th1 cells, or that an active repressor mechanism exists in Th1 cells in vivo. The first possibility is consistent with the identification of two other Th2-specific transcriptional factors, GATA-3 and NF-IL-6 (C/EBPβ) (28–30). Both factors have been demonstrated to transactivate the IL-4 promoter in vivo or in vitro. It is intriguing in this regard that provision of c-maf alone to Jurkat cells allows them to produce endogenous IL-4 since Jurkat cells do in fact express GATA-3 (31). A requirement for the presence of more than one Th2-specific factor to allow IL-4 production by Th1 cells can be best tested by generating c-maf/GATA-3 or c-maf/NF-IL6 double transgenic mice. Alternatively, there may be active inhibitory mechanisms that repress the IL-4 promoter in Th1 cells. This hypothesis is consistent with the recent identification of a dominant silencer element that actively suppresses the activity of the IL-4 promoter in Th1 cells, but it does not fit well with our own observation that transient Th1–Th2 heterokaryons produce both IL-2 and IL-4 (21, 32).
Overexpression of c-maf resulted in impaired IFN-γ production, an effect that was Th2-cytokine independent, and could be overcome by the addition of exogenous IL-12. It is possible that c-maf directly represses the transcription of the IFN-γ gene, although this possibility cannot be tested since the Th1-specific regulatory regions of the IFN-γ gene have yet to be identified. A direct repressor mechanism would be consistent with recent reports demonstrating that ectopic expression of maf family members can repress gene expression. Thus, c-maf/c-Myb dimers suppress the expression of the myeloid-specific gene, CD13/APN, and a mafB/Ets complex inhibits erythroid differentiation (33–35). The physiologic import of these studies, however, is unclear since they involve inappropriate expression of maf proteins. We would hypothesize that if c-maf is a biologically relevant repressor of IFN-γ, it must be acting very early in Th cell differentiation, specifically at the Thp stage since c-maf is expressed at very low levels in Thp cells (our unpublished observations). This is consistent with our data that the repressive effect of c-maf on IFN-γ production was overridden by the addition of rIL-12. Thus, mature effector transgenic Th1 cells produced normal levels of IFN-γ. Therefore, if c-maf indeed directly represses IFN-γ gene transcription, it only does so in Thp and not in mature Th1 cells. Another explanation for the Th1-attenuating, IL-4– independent effect of c-maf is that it controls the expression of other genes in Thp cells that repress the Th1 program. These genes are not the Th2 cytokines IL-5, IL-10, and IL-13 since we demonstrated no effect of blocking these cytokines on IFN-γ production. Although IFN-γ itself may be one such c-maf target gene, there may well be others. The availability of lymphoid tissue from c-maf overexpresser transgenic and c-maf–deficient strains should allow the isolation of such genes.
In conclusion, we have shown that overexpression of c-maf in vivo is able to skew the Th immune response to a Th2 type via both IL-4–dependent and –independent mechanisms. The overproduction of IL-4 is consistent with our previous report that c-maf is a potent transactivator of the IL-4 gene in vitro. The mechanism by which c-maf attenuates the Th1 differentiation program is still unclear, but clearly is Th2-cytokine independent. Taken together, this study demonstrates that c-maf is a critical and pluripotent transcription factor that promotes the differentiation of Th2 cells.
Acknowledgments
We thank S. Smale and T. Baumruker for the IL-10 and IL-5 reporter plasmids; T. Laufer, M.Oukka, A. Wurster, and M. Grusby for careful review of the manuscript; and C. Freedman for help with manuscript preparation.
This work was supported by grants from The Arthritis Foundation (I-C. Ho), the National Multiple Sclerosis Society (L.H. Glimcher), the G. Harold and Leila Y. Mathers Charitable Foundation (L.H. Glimcher), the Juvenile Diabetes Foundation International (D. Lo), and by National Institutes of Health grant AI29689 (D. Lo).
Abbreviations used in this paper
- NFAT
nuclear factor of activated T cells
- RT
reverse transcription
References
- 1.Crawford RM, Finbloom DS, Ohara J, Paul WE, Meltzer MS. B cell stimulating factor-1 (interleukin 4) activates macrophages for increased tumoricidal activity and expression of Ia antigens. J Immunol. 1987;139:135–141. [PubMed] [Google Scholar]
- 2.Crawford RM, Finbloom DS, Ohara J, Paul WE, Meltzer MS. Regulation of macrophage effector function by B cell stimulatory factor-1 (BSF-1) Adv Exp Med Biol. 1988;239:223–229. doi: 10.1007/978-1-4757-5421-6_22. [DOI] [PubMed] [Google Scholar]
- 3.Paul WE. Interleukin 4: signalling mechanisms and control of T cell differentiation. Ciba Found Symp. 1997;204:208–216. doi: 10.1002/9780470515280.ch14. [DOI] [PubMed] [Google Scholar]
- 4.Takatsu K. Cytokines involved in B-cell differentiation and their sites of action. Proc Soc Exp Biol Med. 1997;215:121–133. doi: 10.3181/00379727-215-44119. [DOI] [PubMed] [Google Scholar]
- 5.Ohara J, Paul WE. Receptors for B-cell stimulatory factor-1 expressed on cells of haematopoietic lineage. Nature. 1987;325:537–540. doi: 10.1038/325537a0. [DOI] [PubMed] [Google Scholar]
- 6.Hou J, Schindler U, Henzel WJ, Ho TC, Brasseur M, McKnight SL. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994;265:1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 7.Welham MJ, Bone H, Levings M, Learmonth L, Wang LM, Leslie KB, Pierce JH, Schrader JW. Insulin receptor substrate-2 is the major 170-kDa protein phosphorylated on tyrosine in response to cytokines in murine lymphohemopoietic cells. J Biol Chem. 1997;272:1377–1381. doi: 10.1074/jbc.272.2.1377. [DOI] [PubMed] [Google Scholar]
- 8.Seder, R.A., and W.E. Paul. 1994. Acquisition of lymphokine-producing phenotype by CD4+ T cells. In Annual Review of Immunology, Vol. 12. W.E. Paul, C.G. Fathman, and H. Metzger, editors. Annual Reviews, Inc., Palo Alto, CA. 635–673. [DOI] [PubMed]
- 9.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 10.Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity. 1997;7:727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 11.Fox CJ, Danska JS. IL-4 expression at the onset of islet inflammation predicts nondestructive insulitis in nonobese diabetic mice. J Immunol. 1997;158:2414–2424. [PubMed] [Google Scholar]
- 12.Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 1996;184:1093–1099. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arreaza GA, Cameron MJ, Jaramillo A, Gill BM, Hardy D, Laupland KB, Rapoport MJ, Zucker P, Chakrabarti S, Chensue SW, et al. Neonatal activation of CD28 signaling overcomes T cell anergy and prevents autoimmune diabetes by an IL-4-dependent mechanism. J Clin Invest. 1997;100:2243–2253. doi: 10.1172/JCI119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelman FD, Katona IM, Urban JF, Jr, Holmes J, Ohara J, Tung AS, Sample JV, Paul WE. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- 15.Katona IM, Urban JF, Jr, Kang SS, Paul WE, Finkelman FD. IL-4 requirements for the generation of secondary in vivo IgE responses. J Immunol. 1991;146:4215–4221. [PubMed] [Google Scholar]
- 16.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimoto T, Paul WE. CD4pos, NK1.1posT cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown MA, Pierce JH, Watson CJ, Falco J, Ihle JN, Paul WE. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987;50:809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- 19.Brunner T, Heusser CH, Dahinden CA. Human peripheral blood basophils primed by interleukin 3 (IL-3) produce IL-4 in response to immunoglobulin E receptor stimulation. J Exp Med. 1993;177:605–611. doi: 10.1084/jem.177.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabo SJ, Glimcher LH, Ho I-C. Genes that regulate interleukin-4 expression in T cells. Curr Opin Immunol. 1997;9:776–781. doi: 10.1016/s0952-7915(97)80177-x. [DOI] [PubMed] [Google Scholar]
- 21.Ho I-C, Hodge MR, Rooney JW, Glimcher LH. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–983. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- 22.Blank V, Andrews NC. The Maf transcription factors: regulators of differentiation. Trends Biochem Sci. 1997;22:437–441. doi: 10.1016/s0968-0004(97)01105-5. [DOI] [PubMed] [Google Scholar]
- 23.Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 24.Tepper RI, Levinson DA, Stanger BZ, Campos-Torres J, Abbas AK, Leder P. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990;62:457–467. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- 25.Bancroft AJ, McKenzie AN, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 26.Liu L, Rich BE, Inobe J, Chen W, Weiner HL. A potential pathway of Th2 development during primary immune response. Adv Exp Med Biol. 1997;417:375–381. [PubMed] [Google Scholar]
- 27.Barner M, Mohrs M, Brombacher F, Kopf M. Differences between IL-4Rα-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr Biol. 1998;8:669–672. doi: 10.1016/s0960-9822(98)70256-8. [DOI] [PubMed] [Google Scholar]
- 28.Zheng W-P, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 29.Davydov IV, Krammer PH, Li-Weber M. Nuclear factor-IL6 activates the human IL-4 promoter in T cells. J Immunol. 1995;155:5273–5279. [PubMed] [Google Scholar]
- 30.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 31.Ho IC, Vorhees P, Marin N, Oakley BK, Tsai SF, Orkin SH, Leiden JM. Human GATA-3: a lineage-restricted transcription factor that regulates the expression of the T cell receptor alpha gene. EMBO (Eur Mol Biol Organ) J. 1991;10:1187–1192. doi: 10.1002/j.1460-2075.1991.tb08059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo M, Ransom J, Webb D, Hashimoto Y, Tada T, Nakayama T. T-cell subset-specific expression of the IL-4 gene is regulated by a silencer element and STAT6. EMBO (Eur Mol Biol Organ) J. 1997;16:4007–4020. doi: 10.1093/emboj/16.13.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedge SP, Kumar A, Kurschner C, Shapiro LH. c-Maf interacts with c-Myb to regulate transcription of an early myeloid gene during differentiation. Mol Cell Biol. 1998;18:2729–2737. doi: 10.1128/mcb.18.5.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieweke MH, Tekotte H, Frampton J, Graf T. MafB represses erythroid genes and differentiation through direct interaction with c-Ets-1. Leukemia. 1997;11:486–488. [PubMed] [Google Scholar]
- 35.Sieweke MH, Tekotte H, Frampton J, Graf T. MafB is an interaction partner and repressor of Ets-1 that inhibits erythroid differentation. Cell. 1996;85:49–60. doi: 10.1016/s0092-8674(00)81081-8. [DOI] [PubMed] [Google Scholar]