Abstract
During T cell development, thymocytes which are tolerant to self-peptides but reactive to foreign peptides are selected. The current model for thymocyte selection proposes that self-peptide–major histocompatibility complex (MHC) complexes that bind the T cell receptor with low affinity will promote positive selection while those with high affinity will result in negative selection. Upon thymocyte maturation, such low affinity self-peptide–MHC ligands no longer provoke a response, but foreign peptides can incidentally be high affinity ligands and can therefore stimulate T cells. For this model to work, thymocytes must be more sensitive to ligand than mature T cells. Contrary to this expectation, several groups have shown that thymocytes are less responsive than mature T cells to anti-T cell receptor for antigen (TCR)/CD3 mAb stimulation. Additionally, the lower TCR levels on thymocytes, compared with T cells, would potentially correlate with decreased thymocyte sensitivity. Here we compared preselection thymocytes and mature T cells for early activation events in response to peptide–MHC ligands. Remarkably, the preselection thymocytes were more responsive than mature T cells when stimulated with low affinity peptide variants, while both populations responded equally well to the antigenic peptide. This directly demonstrates the increased sensitivity of thymocytes compared with T cells for TCR engagement by peptide–MHC complexes.
Keywords: T cell receptor, thymus, lymphocyte development, calcium, signaling
Thymic selection is essential for generating a successful T cell repertoire which is able to respond to foreign peptides while remaining tolerant to self-peptides. Positive selection ensures differentiation and survival of thymocytes with a functional TCR while negative selection removes those thymocytes with dangerously high reactivity to self (1). Thymocyte positive and negative selection and mature T cell activation all depend on the same TCR interacting with a peptide–MHC ligand. Therefore, understanding the TCR signal in different cellular contexts is essential for understanding T cell immunity. Experiments investigating the peptide requirement for selection of MHC class I restricted TCR have found that very low concentrations of the antigenic peptide (2, 3) or variants of the antigenic peptide (4) can mediate positive selection in fetal thymus organ culture. Subsequently, the affinity of these variant peptide– MHC complexes for the OT-I TCR was determined and found to be lower than for the antigenic peptide–MHC complex (5). These data are consistent with the proposed avidity model of selection where low avidity complexes promote survival and differentiation whereas high avidity ligands drive death (6, 7).
A requirement of this model, where self-peptides positively select thymocytes which develop into T cells that are nonreactive to self, is that the thymocytes must be more sensitive to ligand than mature T cells. Many of the variant peptides (4, 8, 9) and naturally occurring self-peptides (10, 11) which are able to positively select TCR transgenic thymocytes are non or weakly stimulatory for mature T cells. In addition, thymocyte deletion has been shown to be more sensitive than peripheral T cell proliferation in response to titration of antigenic peptide in vitro (12), superantigen in vitro (13), or viral variants in vivo (14). The drawback to these comparisons is that two different systems are being analyzed, an inevitable consequence of studying late activation events given that double positive (DP)1 thymocytes die in response to agonist stimulation while T cells proliferate. By measuring early events associated with TCR stimulation that are common to both cell populations, it is possible to directly compare thymocytes and T cells in the same assay. Surprisingly, experiments comparing calcium flux in response to TCR cross-linking by mAb found that DP thymocytes are less responsive than mature thymocytes or T cells (15–17). This lower sensitivity of DP thymocytes is inconsistent with the proposed model and the biological behavior of DP thymocytes (as described above).
In this paper, we use stimulation with peptide–MHC ligands to further analyze the differences between thymocyte and T cell early responses. Three common parameters were examined: CD69 upregulation, TCR downregulation, and calcium mobilization. The central role of intracellular calcium in T cell activation has been investigated extensively (18). The CD69 molecule is a type II C-type lectin receptor expressed on a subset of DP and single positive (SP) thymocytes and a small proportion of mature T cells (19). CD69 expression is associated with activation and the molecule can be detected on T cells within 2 h after stimulation through the TCR (20). Similarly, TCR downregulation has been shown to correlate with TCR occupancy and to be proportional to the T cell's biological response (21). However, unlike CD69 modulation and calcium flux, TCR downregulation appears to be independent of TCR signal transduction (22, 23).
In this study, we found that T cells were more sensitive than thymocytes to anti-CD3 mAb stimulation, as previously described. However, in response to low affinity peptide–MHC complexes, thymocytes were more sensitive than mature T cells despite lower TCR levels.
Materials and Methods
Mice and Cells.
OT-I is a C57BL/6 transgenic strain expressing a TCR specific for the OVA peptide (OVAp, SIINFEKL) in the context of MHC class I molecule Kb (4). TAPo is a 129 × C57BL/6 strain with a targeted mutation of the TAP-1 gene on both chromosomes (gift of Anton Berns, Netherlands Cancer Institute, Amsterdam, The Netherlands). RAGo is a C57BL/6 strain with a targeted mutation of the RAG-1 gene on both chromosomes, obtained from The Jackson Laboratory (Bar Harbor, ME). TAPo and RAGo mice were crossed with OT-I to give OT-I TAPo and OT-I RAGo mice, respectively. In some experiments OT-I lymph node cells were enriched for CD8+ cells using a negative selection mouse CD8 CellectTM column (Cytovax Biotechnologies Inc., Alberta, Canada).
Peptides and Antibodies.
OVAp variant peptides were used at 1 and 10 μM. V-OVA is RGYNYEKL; E1 is EIINFEKL; G4 is SIIGFEKL; N6 is SIINFNKL. The properties of these peptides in the OT-I system have been detailed elsewhere (4, 24, 25).
The anti-CD3 mAb (clone 500A2) was used for TCR stimulation, plate bound for the in vitro assays, and cross-linked with goat anti–mouse Ig (Sigma Chemical Co., St. Louis, MO) for calcium flux experiments. For flow cytometry the following antibodies from PharMingen (San Diego, CA) were used: CD4 (RM4-5), CD8 (53-6.7), CD69 (H1.2F3), Vα2 TCR (B20.1), and VβTCR (H57-597).
In Vitro Assay for CD69 Upregulation and TCR Downregulation.
5 × 105 thymocytes, spleen, or lymph node cells from OT-I TAPo or OT-I mice and 105 APCs were prepared in RPMI/10% FCS, and peptide was added where required. The cells were pelleted together in a round bottom 96-well plate and incubated for 3 h at 37°C, 5% CO2. The APCs were either peritoneal exudate cells (PEC) from TAPo mice or the 5AKb cell line, a mouse fibroblast cell transfected with H-2Kb (26, 27). The TAPo PEC (predominantly macrophages) were generated by injecting TAPo mice intraperitoneally with 1 ml of thioglycollate 5 d before harvesting cells by peritoneal wash.
For TCR cross-linking by antibody, diluted anti-CD3 mAb in 0.1 M NaHCO3, pH 8.1, was incubated in flat bottom 96-well plates, 4°C overnight. The wells were subsequently washed twice with PBS/0.05% Tween 20 and twice with PBS before adding the cells, centrifuging plates, and incubating for 3 h at 37°C, 5% CO2. In experiments where TAPo PEC were used to present the anti-CD3 mAb, cells and mAb were incubated together for 1 h on ice, washed, and aliquoted into 96-well round bottom plates.
After the 3-h incubation the cells were simultaneously labeled for flow cytometry with CD4, CD8, CD69, and the transgenic (Tg) Vα2 TCR antibodies. Forward and side scatter gating was used to eliminate dead cells. Populations of interest were identified on the basis of CD4 and CD8 staining. CD69 and TCR staining of these gated populations were subsequently analyzed. The data were normalized to account for the lower level of TCRs on unstimulated DP thymocytes and, conversely, the greater CD69 expression attained by T cells after maximal stimulation relative to thymocytes. To normalize the TCR data for each cell subset, TCR levels in the presence of APC but no peptide were set at 100%. Therefore the percentage of TCR downregulation was calculated by dividing the mean channel fluorescence with peptide by the mean channel fluorescence without peptide and multiplying the result by 100. To normalize the CD69 data, the levels of CD69 in the presence of saturating OVAp (generally 5 nM) were set at 100%. Therefore the percentage of CD69 upregulation was calculated by dividing the mean channel fluorescence for that peptide dilution by the mean channel fluorescence for saturating OVAp and multiplying the result by 100.
Calcium Flux.
2 × 106 freshly isolated lymphocytes in 1 ml of media (RPMI/3% FCS) were incubated at 37°C for 1 h with 5 μg of Indo-1-AM (1 mg/ml in DMSO; Molecular Probes, Inc., Eugene, OR). Immediately before stimulation the cells were washed twice with warm media and resuspended at 106 cells/ml. Exposure to light was minimized during this procedure to avoid bleaching effects. The APCs were incubated with 10 μM peptide at 37°C in RPMI/10% FCS for 1 h, washed, and resuspended at 106 cells/ml.
Changes in intracellular calcium were measured using a FACSVantageTM (Becton Dickinson, San Jose, CA) equipped with a multiple wavelength 100-mW water-cooled argon laser and 395λ and 530λ filters to maximize the Rmax/Rmin ratio of Indo-1-AM (28). Calcium-bound Indo was measured by the FL4 channel and unbound Indo was measured in the FL5 channel. An attached water bath maintained the sample at 37°C throughout the assay period.
For peptide–MHC ligand stimulation, 5 × 106 lymphocytes were added to an equal number of APCs, gently agitated, and the sample applied to the FACSVantageTM for 1 min to determine the basal response. The sample was then centrifuged for 6 s at 16,000 g to maximize cell–cell contacts, and reapplied to the machine. This procedure took <1 min and appears as a break in the analysis trace (see Fig. 3 A). The sample was then read for a further 6–8 min. For mAb stimulation, the primary antibody (20 μg/ml) was applied after 1 min, the sample measured for 1 min, and then the secondary antibody was applied; there was no centrifugation of the sample. Data were analyzed using CellQuestTM software (Becton Dickinson).
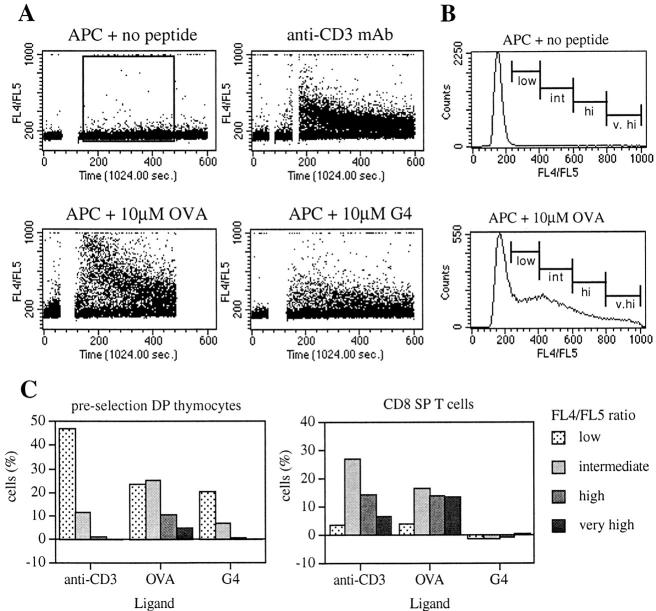
Figure 3.
Changes in intracellular calcium in DP thymocytes and T cells stimulated with various ligands. (A) Dot plots of FL4/FL5 versus time for preselection (OT-I TAPo) DP thymocytes stimulated with APC alone, anti-CD3 mAb + goat anti–mouse Ig, OVAp + APC, and G4 + APC. The break in the trace corresponds to the time where the T cells and APCs were centrifuged together before being resuspended gently to pass through the flow cytometer. The two breaks for mAb cross-linking correspond to the sequential addition of anti-CD3 mAb and the goat anti–mouse Ig. (B) Representative histograms for FL4/FL5 of preselection DP thymocytes with APC alone or 10 μM OVAp, showing nonoverlapping markers set for low, intermediate, high, and very high expressing cells corresponding to increased intracellular calcium levels. Histogram data are based on the gate shown in A. (C) Graphs of the percentage of cells in each FL4/FL5 gate for preselection DP thymocytes and OT-I CD8 SP splenocytes stimulated with anti-CD3 mAb, OVAp, and G4 peptide. Markers were set as in B. Background levels determined by the APCs alone control have been subtracted from the values graphed. The APCs used in this experiment were of the 5AKb cell line. Similar results were seen using TAPo PEC as the APCs. The data shown are representative of four independent experiments.
TCR Quantitation.
TCR quantitation was undertaken by comparing anti-Vα2 TCR-FITC labeling with calibrated microbeads of known fluorescence intensity (QuantumTM 24; Flow Cytometry Standards Corp. [FCSC], San Juan, Puerto Rico). Mean fluorescence intensity data from the flow cytometry were used to generate a value of molecules of equivalent soluble fluorochrome (MESF) using the QuickCal® software (FCSC). The number of receptors was then calculated by dividing the MESF value by the relative fluorescence/protein ratio for the mAb. The relative fluorescence/protein ratio was determined by comparing mAb fluorescence to a standard curve of freshly prepared FITC solution (5 × 10−6 to 5 × 10−9 M) in a fluorometer. The relative fluorescence/protein ratio was similar to the fluorescein/protein ratio, determined by the manufacturer. Calculation of these numbers assumes that mAb binding is monovalent at saturating levels of mAb.
Results and Discussion
Preselection Tg Thymocytes Have Fewer TCR Than Mature T Cells.
CD4+CD8+ (DP) thymocytes are a heterogeneous population of pre- and postpositive selection cells with no definitive marker to distinguish all postpositive selection cells. Therefore, to study a homogenous population of preselection DP thymocytes we took advantage of transgene technology. The OT-I TCR Tg mice express a Vα2,Vβ5 TCR specific for the OVA peptide, SIINFEKL, in the context of the MHC class I molecule, Kb (4). In TAPo mice, which have minimal MHC class I on the cell surface, the selection of CD4−CD8+ (SP) T cells is severely impaired (29). Therefore, in a TAPo mouse with the OT-I TCR, almost all the DP thymocytes are preselection and few mature CD8 SP thymocytes develop (10). Hence, OT-I TAPo thymii provide a source of preselection DP thymocytes which can be compared with OT-I TAPwt CD8 SP thymocytes and peripheral T cells.
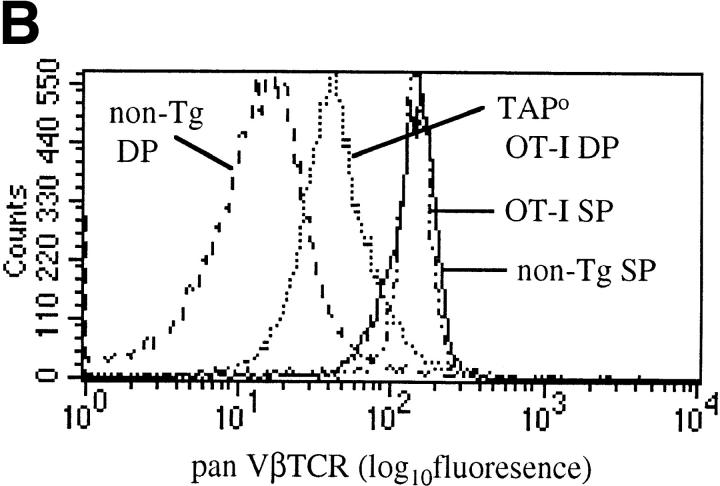
In normal (non-TCR Tg) mice, mature T cells express ∼10-fold more TCRs on the cell surface than immature thymocytes (30). This is also true in TCR Tg mice although the difference is not as large (Fig. 1 A). In part this may be due to the greater proportion of cells undergoing positive selection in the Tg mice, and hence upregulating their TCRs. In contrast, for normal mice the majority of DP thymocytes are preselection. Therefore we compared preselection TCR-Tg DP thymocytes (OT-I TAPo) with DP thymocytes from a selecting environment (OT-I). The level of TCRs on preselection OT-I DP cells was significantly lower than for selected OT-I DP cells (Fig. 1 A). However, the level on preselection OT-I DP thymocytes was still higher than for non-TCR Tg DP thymocytes (Fig. 1 B). These data confirm that DP thymocytes maintain low TCR surface levels until after positive selection, despite the fact that in αβTCR Tg mice the TCR is expressed at high levels on double negative precursor cells (31, 32). Most probably, the low TCR level on OT-I DP cells is due to degradation of the TCRα chains in the endoplasmic reticulum, as seen in wild-type mice (33).
Figure 1.
Surface TCR levels are lower on DP thymocytes than T cells from OT-I mice. (A) Flow cytometry histograms of Vα2 TCR expression on splenic T cells (solid line) or DP thymocytes (dashed line) from OT-I mice and preselection DP thymocytes (dotted line) from OT-I TAPo mice. (B) Flow cytometry histograms of pan Vβ TCR expression on splenic T cells (solid line) or DP thymocytes (dashed line) from non-TCR Tg mice, splenic T cells (dash-dot line) from OT-I mice, and preselection DP thymocytes (dotted line) from OT-I TAPo mice.
DP Thymocytes Are More Sensitive to Low Affinity Ligands.
In view of the fact that thymocytes have fewer TCRs, a comparison of their sensitivity to TCR stimulation was undertaken. To directly compare the relative sensitivity of DP thymocytes to SP thymocytes and T cells it is necessary to have an assay relevant to both immature and mature cell types. Therefore CD69 upregulation, an early activation event common to both thymocytes and T cells, was measured after in vitro stimulation with peptide–MHC complexes on APCs. TCR-dependent CD69 upregulation in mature T cells requires sustained activation of Ras (34), calcium flux, and PKC activation (20, 35).
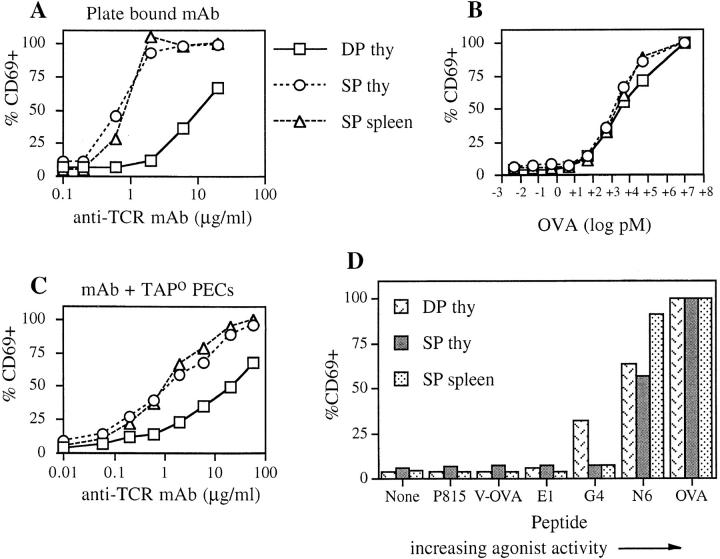
In previous experiments, when DP thymocytes or T cells were activated through TCR cross-linking, the T cells showed a greater magnitude of calcium flux than DP thymocytes (15–17). Thus, we tested whether CD69 upregulation would follow a similar pattern. In this experiment plate-bound anti-CD3 mAb was used to stimulate different T cell subsets. Fig. 2 A shows that CD69 upregulation was more readily activated in T cells or mature thymocytes compared with preselection DP thymocytes. CD8 SP thymocytes and T cells attained maximal CD69 expression when exposed to 2 μg/ml anti-CD3 antibody, whereas the preselection DP thymocytes were submaximal even at 60 μg/ml (data not shown). Given the differences in the affinity and kinetics of binding for antibody versus peptide– MHC ligands (36), it was of interest to compare anti-CD3 mAb stimulation to the more physiologically relevant peptide–MHC ligands.
Figure 2.
CD69 upregulation on preselection DP thymocytes and T cells stimulated with various ligands. Immature and mature T cells were stimulated, for 3 h at 37°C, with dilutions of plate-bound anti-CD3 mAb (A), OVAp + APC (B), anti-CD3 + APC (C), or OVA variant peptides + APC (D). For preselection (OT-I TAPo) DP thymocytes (squares), OT-I CD8 SP thymocytes (circles), and OT-I CD8 SP splenocytes (triangles), the percentage of CD69-expressing cells on CD4/CD8-defined subpopulations was determined by flow cytometry. The data have been normalized such that the percentage of CD69+ cells in the presence of 5 μM OVAp = 100% for each cell subset. The graphs shown are representative of data from six to eight independent experiments.
Three populations, preselection DP thymocytes, CD8 SP thymocytes, and CD8 SP T cells, were incubated for 3 h with titrated OVAp in the presence of TAPo PEC as the APC. Unlike the anti-CD3 mAb stimulation, each population responded similarly to the antigenic peptide–MHC complex (Fig. 2 B). Upregulation of CD69 was first detectable at 5–50 pM and was maximal at 50 nM. As the intensity of CD69 expression and number of cells which respond is different between thymocytes and T cells, the data are normalized such that 100% corresponds to CD69 levels on each population stimulated with the highest concentration of OVAp. The same result was obtained whether cells were analyzed for the percentage of CD69+ cells or the change in mean fluorescence intensity (data not shown).
Possible explanations for the lack of thymocyte response to anti-CD3 mAb may be the lower levels of TCRs on the cell surface. In contrast to the relatively low affinity binding of peptide–MHC, antibody binding has a high affinity and a slow off-rate (36). Therefore, the difference in TCR number may be more significant if the TCR cannot be serially triggered (21). Alternatively, the APCs may be providing adhesion molecules or costimulators which preferentially lower the signaling threshold for thymocytes but not T cells. To address this, FcR+ TAPo PEC were used to present and cross-link the anti-CD3 mAb, rather than using plate-bound mAb. Even under these conditions, CD8 SP T cells were still more responsive than the preselection DP thymocytes (Fig. 2 C). Therefore, the inability of thymocytes to respond optimally to anti-CD3 mAb may reflect the lower TCR numbers on the cell surface and a quantitatively different signal and/or differences in ligand binding resulting in a qualitatively different signal relative to peptide–MHC ligand stimulation.
The most interesting result was obtained when OVA variant peptides were used for stimulating the TCRs (Fig. 2 D). The selected variants were chosen to encompass a spectrum of agonist strength for the OT-I TCR, as characterized previously (4, 24, 25). A correlation between agonist strength and TCR affinity for peptide–MHC ligands has been observed (5). As seen with OVAp, the strong agonist peptide N6 induced considerable upregulation of CD69 in all the cell subsets (the greater magnitude of the CD8 SP T cell response was not consistently observed). However, for peptide G4, CD69 upregulation was observed for the preselection DP thymocytes but not the CD8 SP thymocytes or mature T cells. In some experiments a response to peptide E1 was detected for the DP thymocytes only (data not shown). V-OVA, although able to positively select OT-I T cells in fetal thymic organ culture (4), did not stimulate detectable CD69 upregulation at this time point in the assay. Identical results were obtained using a mouse fibroblast cell line transfected with Kb (26, 27) as the APC (data not shown). This enhanced sensitivity of thymocytes to variant peptide–MHC ligands was also observed at an 18-h time point (data not shown).
The greater response of DP thymocytes to OVAp– MHC ligand compared with TCR cross-linking by mAb may be explained, at least in part, by the participation of CD4 and CD8 coreceptors. Previous work has found that costimulation with TCR/CD4 over TCR cross-linking alone improved the calcium flux in thymocytes (37) and the activation of Zap-70 in T cells (38). Similarly, we observed enhanced CD69 upregulation in response to anti-CD3 and anti-CD8 mAb cross-linking on DP thymocytes relative to anti-CD3 alone (data not shown). However, when comparing the response with OVAp or variants, the involvement of coreceptors is equivalent for the thymocytes and T cells. The fact that the thymocytes and T cells were indistinguishable in their response to strong stimuli such as OVAp, but different for weak stimuli such as G4 is consistent with previous data in the OT-I system and others, which suggests that low concentrations of antigenic peptide cannot substitute for low affinity ligands in the positive selection of functionally competent mature cells (39, 40). Whether this is because low affinity ligands give a qualitatively (41, 42) or quantitatively different signal in thymocytes remains to be determined.
Preselection DP Thymocytes Flux Calcium in Response to Low Affinity Ligands.
In addition to CD69 upregulation, an increase in intracellular calcium levels is an early activation event occurring in both thymocytes and T cells. Therefore we were interested in whether the same sensitivity of preselection DP thymocytes to stimulation by peptide–MHC ligands could be seen by measuring changes in intracellular calcium. Thymocytes and T cells were loaded with the calcium binding dye, Indo-1-AM, and stimulated with anti-CD3 mAb or APCs loaded with no peptide–G4 or OVAp. Upon binding calcium, the fluorescence of Indo-1 changes and this can be measured by flow cytometry, where FL4 detects calcium bound Indo, FL5 detects unbound Indo-1, and the ratio of FL4/FL5 is determined. The magnitude of the change in FL4/FL5 correlates with the increase in intracellular calcium (28). Fig. 3 A shows the change in the FL4/FL5 ratio over time for preselection DP thymocytes exposed to different stimuli. To quantitate this data, FL4/FL5 histograms were divided into adjacent nonoverlapping regions corresponding to low, intermediate, high, and very high FL4/FL5 ratios. Fig. 3 B shows representative histograms of this analysis for DP thymocytes mixed with no peptide and OVAp-loaded APCs. A summary of this analysis for preselection DP thymocytes and CD8 SP T cells is graphed in Fig. 3 C. To avoid the potential complication of stimulating the cells through antibody labeling, preselection DP thymocytes were isolated from TAPo OT-I thymii (>85% CD4+CD8+), and used without further purification. For the CD8 SP T cells, unpurified lymph node and spleen cells from OT-I RAGo animals (80% CD4−CD8+) or OT-I negative selection column chromatography purified OT-I lymph node cells (>90% CD4−CD8+) were used. Similar results were obtained with each cell population.
Both the trace (Fig. 3 A) and graph (Fig. 3 C) show that a significant proportion of both thymocytes and mature T cells increase intracellular calcium levels in response to anti-CD3 mAb stimulation. For the preselection DP thymocytes, most of the responding cells have a low FL4/FL5 ratio in comparison with the CD8 SP T cells, where the responding cells have an intermediate to very high response. Hence, these data using TCR Tg cells are consistent with previous data in non-TCR Tg systems showing that mature T cells are more responsive to anti-TCR mAb cross-linking than DP thymocytes (15–17). For stimulation with OVAp, an increase in intracellular calcium was seen in both the preselection DP thymocytes and the mature T cells, with a broad spectrum of individual cell responses from low to very high FL4/FL5 ratios. For the preselection DP thymocytes stimulated with the OVA variant, G4, a distinct calcium flux was detected, albeit fewer cells responded and with a smaller magnitude of response relative to activation by OVAp. In contrast, no response to G4 was detected in the CD8 SP T cells. Therefore, consistent with the CD69 analysis, preselection DP thymocytes are exquisitely sensitive to stimulation by low affinity ligands but are less sensitive to mAb cross-linking of the TCR complex relative to mature SP T cells. Further examination of the calcium response at the individual cell level to assess latency and oscillations in response to peptide–MHC ligands may reveal further differences with implications for cell function (17).
TCR Engagement Is Equivalent Between Thymocytes and T Cells.
The enhanced responsiveness of thymocytes to low affinity peptide–MHC ligands could be explained by more efficient TCR signal transduction in thymocytes and/or a preferential increase in nonspecific adhesion between thymocytes and APCs relative to T cells. To distinguish between these two possibilities we measured the number of TCRs downregulated in response to stimulation. TCR downregulation has been shown to correlate with TCR occupancy and be proportional to the T cell's biological response (21, 43). Additionally, in contrast to CD69 upregulation, TCR downregulation is independent of TCR signal transduction, as it is not blocked by inhibitors of protein tyrosine kinases, protein C kinases, lipid kinases, protein synthesis, or actin polymerization (22, 23) (Schober, S.L., and S.C. Jameson, unpublished data). Therefore, if nonspecific adhesion interactions are increasing the area of contact between thymocytes and APCs then more TCRs will be downregulated in thymocytes compared with T cells upon exposure to the same ligand concentration.
The number of TCRs downregulated in response to OVAp presentation was quantitated for preselection DP thymocytes and CD8 SP splenocytes which, as shown above, respond equally in terms of CD69 upregulation (Fig. 2 B). The quantitative analysis was performed using flow cytometry and comparison to beads with known concentrations of fluorochrome. At high concentrations of peptide, the CD8 SP T cells will downregulate a greater number of TCRs as they have more TCRs than DP thymocytes. However, at lower OVAp concentrations, the initial numbers of TCRs downregulated can be determined. Table 1 shows that preselection DP thymocytes and T cells initiate TCR downregulation at a similar OVAp concentration, 1–10 pM. However, the thymocytes downregulate fewer TCRs than mature T cells at any given OVAp concentration. Hence, it is unlikely that preselection DP thymocytes are more sensitive to low affinity variant peptide ligands due to increased nonspecific adhesion to the APC. Indeed, the same biological response observed for fewer TCRs downregulated is more consistent with enhanced TCR signal transduction in the preselection DP thymocytes.
Table 1.
The Number of TCR Downregulated on Thymocytes and T Cells in Response to OVAp + APC
| OVAp | TCR number internalized‡ | |||
|---|---|---|---|---|
| Preselection DP thymocytes | CD8 SP splenocytes | |||
| pM* | ||||
| 0 | 0 | 0 | ||
| 0.5 | −10 ± 16 | 22 ± 65 | ||
| 1.4 | −61 ± 52 | 182 ± 83 | ||
| 4.1 | 76 ± 42 | 252 ± 74 | ||
| 12.3 | 158 ± 31 | 601 ± 77 | ||
| 37 | 351 ± 10 | 1,070 ± 118 | ||
| 111 | 581 ± 58 | 1,666 ± 146 | ||
| 333 | 860 ± 59 | 2,279 ± 67 | ||
| 1,000 | 1,131 ± 63 | 3,340 ± 301 | ||
| 3,000 | 1,311 ± 42 | 4,464 ± 408 | ||
Preselection (OT-I TAPo) DP thymocytes or OT-I CD8 SP splenocytes were incubated for 3 h with dilutions of OVAp and APC. The APC in these experiments was the 5AKb cell line.
TCR numbers were quantitated by flow cytometry with reference to beads of known fluorochrome levels. The number of TCR downregulated was calculated by (number of TCR for APC + no peptide) − (number of TCR at peptide dilution). Values shown are the mean ± SD for triplicate samples.
Conclusion.
For thymocytes to be positively selected by low avidity ligands, the DP thymocytes must be more sensitive than mature T cells. To the contrary, previous data directly comparing thymocytes and T cells found thymocytes were less sensitive when stimulated with anti-TCR mAb. In the experiments described here, early activation events in immature and mature T cells stimulated with peptide–MHC complexes were investigated. We have demonstrated the unique sensitivity of a defined preselection thymocyte population to low affinity ligands.
The increase in CD69 and intracellular calcium levels seen in the preselection thymocytes in response to low affinity peptide–MHC ligands is consistent with observations of in vivo positive selection. In the HY TCR Tg mice a subset of CD69+ DP cells was seen in thymocytes from female mice on a selecting MHC background (44). Likewise, the development of late stage DP thymocytes in female HY-TCR Tg mice was blocked by inhibitors of calcineurin, a component of the calcium signaling pathway (45, 46).
As seen in normal mice, TCR Tg thymocytes maintain low levels of TCRs at the DP stage. A cell with fewer receptors would be expected to be less sensitive than a cell with more receptors. On the other hand, if the thymocyte is more sensitive due to more efficient signal amplification or altered signaling pathways then low TCR levels may be a protective mechanism to prevent inappropriate negative selection.
A caveat concerning the conclusions of these experiments is that the two APCs used may express a costimulator that preferentially lowers the signaling threshold in thymocytes but not in T cells. This would make the thymocytes selectively more sensitive rather than inherently so. However, if this were the case, a difference in the response to OVAp might also be expected, whereas we found the response between thymocytes and T cells to be the same.
Therefore, the challenge is to identify the differences in the signaling pathways downstream of the TCRs which mediate this enhanced sensitivity of preselection DP thymocytes to low affinity ligands. In DP thymocytes a significant proportion of the ζ chains are tyrosine phosphorylated although the effect of this on signal transduction remains to be clarified (47, 48). In addition, a number of molecules with different expression levels between DP thymocytes and T cells such as the protein tyrosine kinases Fyn (49) and Syk (50) and the protein phosphatase, Shp-1 (Davey, G.M., and K.A. Hogquist, unpublished data) are interesting candidates for further investigation.
Acknowledgments
The authors wish to thank Caridad Rosette for advice on calcium analysis, Janet Peller for FACSVantage™ operation, Lisa Rogers for technical assistance, and lab colleagues for helpful discussion and critical review of this manuscript.
This work was supported by National Institutes of Health grants A135296, A139560, and A138903 and the American Cancer Society.
Abbreviations used in this paper
- DP
double positive
- MESF
molecules of equivalent soluble fluorescence
- PEC
peritoneal exudate cells
- SP
single positive
- Tg
transgenic
References
- 1.Jameson SC, Bevan MJ. T cell selection. Curr Opin Immunol. 1998;10:214–219. doi: 10.1016/s0952-7915(98)80251-3. [DOI] [PubMed] [Google Scholar]
- 2.Sebzda E, Wallace VA, Mayer J, Yeung RSM, Mak TW, Ohashi PS. Positive and negative thymocyte selection induced by different concentration of a single peptide. Science. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 3.Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher H-P, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 4.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 5.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NRJ. T cell receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 6.Ashton-Rickardt PG, Tonegawa S. A differential-avidity model for T-cell selection. Immunol Today. 1994;15:362–366. doi: 10.1016/0167-5699(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 7.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 8.Sebzda E, Kundig TM, Thomson CT, Aoki K, Mak SY, Mayer JP, Nathenson SG, Ohashi PS. Mature T cell reactivity altered by peptide agonist that induces positive selection. J Exp Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu CP, Crawford F, Marrack P, Kappler J. T cell positive selection by a high density, low affinity ligand. Proc Natl Acad Sci USA. 1998;95:4522–4526. doi: 10.1073/pnas.95.8.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogquist KA, Tomlinson AJ, Kieper WC, McGargill MA, Hart MC, Naylor S, Jameson SC. Identification of a naturally occurring ligand for thymic positive selection. Immunity. 1997;6:389–399. doi: 10.1016/s1074-7613(00)80282-4. [DOI] [PubMed] [Google Scholar]
- 11.Hu Q, Walker CRB, Girao C, Opferman JT, Sun J, Shabanowitz J, Hunt DF, Ashton-Rickardt PG. Specific recognition of thymic self-peptides induces the positive selection of cytotoxic T lymphocytes. Immunity. 1997;7:221–231. doi: 10.1016/s1074-7613(00)80525-7. [DOI] [PubMed] [Google Scholar]
- 12.Vasquez NJ, Kaye J, Hedrick SM. In vivo and in vitro clonal deletion of double-positive thymocytes. J Exp Med. 1992;175:1307–1316. doi: 10.1084/jem.175.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yagi J, Janeway CA., Jr Ligand thresholds at different stages of T cell development. Int Immunol. 1990;2:83–88. doi: 10.1093/intimm/2.1.83. [DOI] [PubMed] [Google Scholar]
- 14.Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 15.Havran WL, Poenie M, Kimura J, Tsien R, Weiss A, Allison JP. Expression and function of the CD3-antigen receptor on murine CD4+CD8+thymocytes. Nature. 1987;330:170–173. doi: 10.1038/330170a0. [DOI] [PubMed] [Google Scholar]
- 16.Finkel TH, McDuffie M, Kappler JW, Marrack P, Cambier JC. Both immature and mature T cells mobilize Ca2+in response to antigen receptor crosslinking. Nature. 1987;330:179–181. doi: 10.1038/330179a0. [DOI] [PubMed] [Google Scholar]
- 17.Hedin KE, Appleby MW, Clapham DE. Developmental regulation of TCR-CD3-dependent [Ca2+]i responses of individual normal and pp59fyn-deficient T lymphocytes. Immunology. 1995;84:183–192. [PMC free article] [PubMed] [Google Scholar]
- 18.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 19.Testi R, D'Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today. 1994;15:479–483. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 20.Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 21.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T cell receptors by a few peptide–MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 22.Salio M, Valitutti S, Lanzavecchia A. Agonist-induced T cell receptor downregulation: molecular requirements and dissociation from T cell activation. Eur J Immunol. 1998;27:1769–1773. doi: 10.1002/eji.1830270726. [DOI] [PubMed] [Google Scholar]
- 23.Cai Z, Kishimoto H, Brunmark A, Jackson MR, Peterson PA, Sprent J. Requirements for peptide- induced T cell receptor downregulation on naive CD8+T cells. J Exp Med. 1997;185:641–651. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jameson SC, Carbone FR, Bevan MJ. Clone-specific T cell receptor antagonists of major histocompatibility complex class I–restricted cytotoxic T cells. J Exp Med. 1993;177:1541–1550. doi: 10.1084/jem.177.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbone FR, Sterry SJ, Butler J, Rodda S, Moore MW. T cell receptor alpha-chain pairing determines the specificity of residue 262 within the Kb-restricted, ovalbumin257-264 determinant. Int Immunol. 1992;4:861–867. doi: 10.1093/intimm/4.8.861. [DOI] [PubMed] [Google Scholar]
- 26.Pullen JK, Hunt HD, Horton RM, Pease LR. The functional significance of two amino acid polymorphisms in the antigen-presenting domain of class I MHC molecules. J Exp Med. 1993;177:869–873. [PubMed] [Google Scholar]
- 27.Chen W, McCluskey J, Rodda S, Carbone FR. Changes at peptide residues buried in the major histocompatibility complex (MHC) class I binding cleft influence T cell recognition: a possible role for indirect conformational alterations in the MHC class I or bound peptide in determining T cell recognition. J Exp Med. 1993;177:869–873. doi: 10.1084/jem.177.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.June CH, Rabinovitch PS. Intracellular ionized calcium. Methods Cell Biol. 1994;41:149–174. [PubMed] [Google Scholar]
- 29.Van Kaer L, Ashton PG, Rickardt, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4−8+T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 30.Roehm N, Herron L, Cambier J, DiGuisto D, Haskins K, Kappler J, Marrack P. The major histocompatibility complex–restricted antigen receptor on T cells: distribution on thymus and peripheral T cells. Cell. 1984;38:577–584. doi: 10.1016/0092-8674(84)90512-9. [DOI] [PubMed] [Google Scholar]
- 31.Teh HS, Kishi H, Scott B, Borgulya P, von Boehmer H. Early deletion and late positive selection of T cells expressing a male-specific receptor in T cell receptor transgenic mice. Dev Immunol. 1990;1:1–10. doi: 10.1155/1990/18208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogquist KA, Bonnevier JL. Development of peptide selected CD8 T cells in fetal thymic organ culture occurs via the conventional pathway. J Immunol. 1998;161:3896–3901. [PubMed] [Google Scholar]
- 33.Bonifacino JS, McCarthy SA, Maguire JE, Nakayama T, Singer DS, Klausner RD, Singer A. Novel post-translational regulation of TCR expression in CD4+ CD8+thymocytes influenced by CD4. Nature. 1990;344:247–251. doi: 10.1038/344247a0. [DOI] [PubMed] [Google Scholar]
- 34.D'Ambrosio D, Cantrell DA, Frati L, Santoni A, Testi R. Involvement of p21ras activation in T cell CD69 expression. Eur J Immunol. 1988;24:616–620. doi: 10.1002/eji.1830240319. [DOI] [PubMed] [Google Scholar]
- 35.Bjorndahl JM, Nakamura S, Hara T, Jung LK, Fu SM. The 28-kDa/32-kDa activation antigen EA 1. Further characterization and signal requirements for its expression. J Immunol. 1988;141:4094–4100. [PubMed] [Google Scholar]
- 36.Valitutti S, Lanzavecchia A. Serial triggering of TCRs: a basis for the sensitivity and specificity of antigen recognition. Immunol Today. 1997;18:299–304. [PubMed] [Google Scholar]
- 37.Nakayama T, June CH, Munitz TI, Sheard M, McCarthy SA, Sharrow SO, Samelson LE, Singer A. Inhibition of T cell receptor expression and function in immature CD4+CD8+cells by CD4. Science. 1998;249:1558–1561. doi: 10.1126/science.2120773. [DOI] [PubMed] [Google Scholar]
- 38.Chau LA, Bluestone JA, Madrenas J. Dissociation of intracellular signaling pathways in response to partial agonist ligands of the T cell receptor. J Exp Med. 1998;187:1699–1709. doi: 10.1084/jem.187.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogquist KA, Jameson SC, Bevan MJ. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+T cells. Immunity. 1995;3:79–86. doi: 10.1016/1074-7613(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 40.Girao C, Hu Q, Sun J, Ashton-Rickardt PG. Limits to the differential affinity model of T cell selection in the thymus. J Immunol. 1997;159:4205–4211. [PubMed] [Google Scholar]
- 41.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 42.Reis e Sousa C, Levine EH, Germain RN. Partial signaling by CD8+T cells in response to antagonist ligands. J Exp Med. 1996;184:149–157. doi: 10.1084/jem.184.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachmann MF, Mariathasan S, Bouchard D, Speiser DE, Ohashi PS. Four types of Ca2+ signal in naive CD8+cytotoxic T cells after stimulation with T cell agonists, partial agonists and antagonists. Eur J Immunol. 1997;27:3414–3419. doi: 10.1002/eji.1830271241. [DOI] [PubMed] [Google Scholar]
- 44.Swat W, Dessing M, von Boehmer H, Kisielow P. CD69 expression during selection and maturation of CD4+8+thymocytes. Eur J Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- 45.Urdahl KB, Pardoll DM, Jenkins MK. Cyclosporin A inhibits positive selection and delays negative selection in alpha beta TCR transgenic mice. J Immunol. 1994;152:2853–2859. [PubMed] [Google Scholar]
- 46.Wang CR, Hashimoto K, Kubo S, Yokochi T, Kubo M, Suzuki M, Suzuki K, Tada T, Nakayama T. T cell receptor-mediated signaling events in CD4+ CD8+thymocytes undergoing thymic selection: requirement of calcineurin activation for thymic positive selection but not negative selection. J Exp Med. 1995;181:927–941. doi: 10.1084/jem.181.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Oers NS, Killeen N, Weiss A. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR zeta in murine thymocytes and lymph node T cells. Immunity. 1994;1:675–685. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 48.Wiest DL, Ashe JM, Abe R, Bolen JB, Singer A. TCR activation of ZAP70 is impaired in CD4+CD8+thymocytes as a consequence of intrathymic interactions that diminish available p56lck. Immunity. 1996;4:495–504. doi: 10.1016/s1074-7613(00)80415-x. [DOI] [PubMed] [Google Scholar]
- 49.Cooke MP, Abraham KM, Forbush KA, Perlmutter RM. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn) Cell. 1991;65:281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- 50.Chan AC, van Oers NS, Tran A, Turka L, Law CL, Ryan JC, Clark EA, Weiss A. Differential expression of ZAP-70 and Syk protein tyrosine kinases, and the role of this family of protein tyrosine kinases in TCR signaling. J Immunol. 1994;152:4758–4766. [PubMed] [Google Scholar]