Abstract
The development of T cell–mediated autoimmune diseases hinges on the balance between effector and regulatory mechanisms. Using two transgenic mouse lines expressing identical myelin basic protein (MBP)–specific T cell receptor (TCR) genes, we have previously shown that mice bearing exclusively MBP-specific T cells (designated T/R−) spontaneously develop experimental autoimmune encephalomyelitis (EAE), whereas mice bearing MBP-specific T cells as well as other lymphocytes (designated T/R+) did not. Here we demonstrate that T/R− mice can be protected from EAE by the early transfer of total splenocytes or purified CD4+ T cells from normal donors. Moreover, whereas T/R+ mice crossed with B cell–deficient, γ/δ T cell–deficient, or major histocompatibility complex class I–deficient mice did not develop EAE spontaneously, T/R+ mice crossed with TCR-α and -β knockout mice developed EAE with the same incidence and severity as T/R− mice. In addition, MBP-specific transgenic mice that lack only endogenous TCR-α chains developed EAE with high incidence but reduced severity. Surprisingly, two-thirds of MBP-specific transgenic mice lacking only endogenous TCR-β chains also developed EAE, suggesting that in T/R+ mice, cells with high protective activity escape TCR-β chain allelic exclusion. Our study identifies CD4+ T cells bearing endogenous α and β TCR chains as the lymphocytes that prevent spontaneous EAE in T/R+ mice.
Keywords: T helper cells, T cell receptor rearrangements, allelic exclusion, allelic inclusion, autorreactivity
Autoreactive CD4+ T cells are believed to play a major role in chronic inflammatory diseases such as multiple sclerosis, diabetes, and rheumatoid arthritis. However, the mere presence of CD4+ autoreactive T cells is not sufficient for development of autoimmune diseases (1), as a number of investigators have detected self-antigen–specific T cells in normal individuals (2–5). Moreover, the frequency of such self-reactive T cells is often similar between normal individuals and those afflicted with autoimmune diseases. For instance, myelin basic protein (MBP)-reactive1 T cells constituted 7.18 ± 2.38% of T cell lines derived from patients with multiple sclerosis, and 4.70 ± 1.58% of the T cell lines derived from normal individuals, a difference considered not significant (6). More surprisingly, even in systems with an artificially high frequency of self-reactive CD4+ T cells, such as TCR transgenic mice, only a small proportion of mice develop spontaneous autoimmune diseases under normal, pathogen-free conditions (7, 8). In the latter cases, self-reactive T cells were shown to be generated in large numbers, migrated normally to the peripheral lymphoid organs, and responded well to the self-antigen in vitro and in vivo. Similar observations were reported by Scott et al. (9) using another self-reactive TCR transgenic mouse model in a BALB/c genetic background.
The presence of nontolerant self-reactive T cells in normal individuals and transgenic animals suggests a role for regulatory cells. Thus, the development of autoimmune disease depends not only on the generation of self-reactive T cells, but also on the presence or absence of the appropriate number of functional regulatory cells.
In several experimental and natural systems, T cell– dependent autoimmune diseases appear to arise, paradoxically, as a consequence of immunodeficiency. For instance, neonatal thymectomy induces multiple organ-specific autoimmune diseases (10, 11). One such disease, autoimmune oophoritis, is caused by CD4+ effector cells and can be prevented by transfer of normal splenocytes (12). The cells that suppress disease have been characterized as CD4+/CD25+ T cells (13, 14). Omenn's syndrome, a Th2-induced autoimmune condition (15), was recently shown to be caused by impaired activity of both recombination-activating gene (RAG)-1 and RAG-2 proteins (16). In these two conditions, alterations induced by neonatal thymectomy or impaired V(D)J recombination, respectively, may lead to a reduced generation of regulatory cells.
Modigliani et al. (17) showed, in a model of transplantation, that mice receiving allogeneic thymic epithelium accepted skin grafts of the same origin as the thymic epithelium, but rejected third party skin grafts. Tolerance could be transferred to nude recipient mice with total peripheral lymphocytes or purified CD4+, but not CD8+, T cells. Interestingly, if only a small number of cells were transferred from the tolerant host, regulatory cells could be lost, whereas effector functions always remained (17). These examples indicate that regulatory cells are relatively rare as compared with effector cells. Therefore, after a general reduction in lymphocyte numbers, the balance between the two cell types is tilted in favor of effector cells and against regulatory cells.
We previously generated mice transgenic for the α and β chains of an MBP-specific TCR (8). We showed that these transgenic mice, designated T/R+, had a T cell repertoire largely dominated by MBP-specific T cells in the peripheral lymphoid organs, and that these cells were not anergic. Despite the lack of tolerance toward MBP, the vast majority of T/R+ mice never developed experimental autoimmune encephalomyelitis (EAE) spontaneously. However, when T/R+ mice were crossed with mice mutant for the RAG-1 gene, all the mice (designated T/R−) developed EAE spontaneously (8). Although the number of MBP-specific T cells is roughly equal in T/R+ and T/R− mice, T/R+ mice bear, in addition, a low proportion of T cells expressing TCRs encoded by the endogenous (nontransgenic) α and β TCR genes (hereafter referred to as endogenous α or β chains), γ/δ T cells, and normal numbers of B cells. Due to the RAG-1 mutation (18), T/R− mice contain only MBP-specific T cells and no other lymphocytes.
Because the immune system of T/R− mice is strictly monoclonal, it was possible that spontaneous EAE in T/R− mice was triggered by cross-reactivity with components of the normal microbial flora, which would become more aggressive in the absence of an adaptive immune system. However, the differences in EAE susceptibility between T/R− and T/R+ mice were reproduced in mice kept under germ-free conditions (Lafaille, J.J., F. Van de Keere, and S. Tonegawa, unpublished data). We therefore concluded that lymphocytes present in T/R+ mice but absent in T/R− mice were responsible for the prevention of EAE in T/R+ mice.
Using genetic ablation of lymphocyte populations as well as adoptive transfer of total or purified subpopulations of splenocytes, we here identify CD4+ T cells expressing endogenous TCR chains as the cells that prevent EAE in T/R+ mice.
Materials and Methods
Mice.
MBP-specific TCR transgenic mice have been described previously (5). In brief, genomic clones of the TCR-α (Vα4) and -β (Vβ8.2) chains obtained from an encephalomyelitogenic CD4+ T cell clone (19) were microinjected into C57/ BL6 fertilized eggs. MHC H-2u was introduced through breeding with C57/BL10.PL mice (The Jackson Laboratory, Bar Harbor, ME). To generate T/R− mice in C57/BL genetic background, RAG-1 KO mice (18) were backcrossed for nine generations with C57BL/6 mice at Susumu Tonegawa's laboratory (Massachusetts Institute of Technology [MIT], Cambridge, MA). RAG-1 KO-C57/BL6 mice were then crossed with T/R+ mice in a C57/BL genetic background. Similar procedure was followed with TCR-δ KO mice (20), which were backcrossed eight times with C57BL/6 mice at MIT. TCR-α KO (21), TCR-β KO (21), β2-microglobulin (β2m) KO (22), μMT KO (23), and IL-4 KO (24), all backcrossed with C57BL/6 were purchased from The Jackson Laboratory. CD4 KO mice (25) backcrossed with C57/BL6 were obtained from Dr. Dan Littman (New York University Medical Center). B6.Thy1.1 mice were purchased from The Jackson Laboratory and crossed with C57/ BL10.PL mice to yield H-2u/u Thy1.1 animals in a C57/BL genetic background. All mice were kept in the specific pathogen– free facility at the Skirball Institute, New York University Medical Center.
Disease Evaluation.
EAE was scored as described by Baron et al. (19): level 1, limp tail; level 2, weak or partial hind leg paralysis; level 3, total hind leg paralysis; level 4, hind leg paralysis and weak or partial front leg paralysis; level 5, moribund. All mice were observed weekly for EAE level and general status of the mice (e.g., weight). Moribund animals were killed. All procedures involving mice were approved by New York University's Institutional and Animal Care Use Committee (IACUC).
Cell Sorting and Cell Transfer Experiments.
Donor splenocytes from mice up to 6 wk of age were obtained by rupturing the lymphoid organ following conventional procedures. Cells were washed with PBS, counted, filtered through a nylon membrane, and then injected intravenously. CD4+ cells were purified by magnetically depleting CD8+ and B220+ cells using MACS microbeads and the magnetic cell separator VarioMACS (Miltenyi Biotec Inc., Sunnyvale, CA). Purity of the separation was assessed by FACS® (Becton Dickinson, San Jose, CA) analysis (see below). CD4+ cell purity was >93%. Cells were adoptively transferred as mentioned above.
Generation and Specificity of mAb 3H12.
Anti-MBP transgenic TCR mAb 3H12 was generated by priming subcutaneously normal B10.PL (H-2u/u) mice with 2 × 107 in vitro–cultured resting transgenic MBP-specific T cells in complete Freund's adjuvant. Animals were subsequently boosted intravenously with 2 × 107 in vitro–cultured resting transgenic MBP-specific T cells or total splenocytes from T/R− mice. 3 d after the last immunization, splenocytes from immune animals were fused with the myeloma partner P3L1 and incubated in selection medium following standard procedures. Supernatants were then tested for their ability to specifically stain MBP-specific transgenic T cells by FACS®.
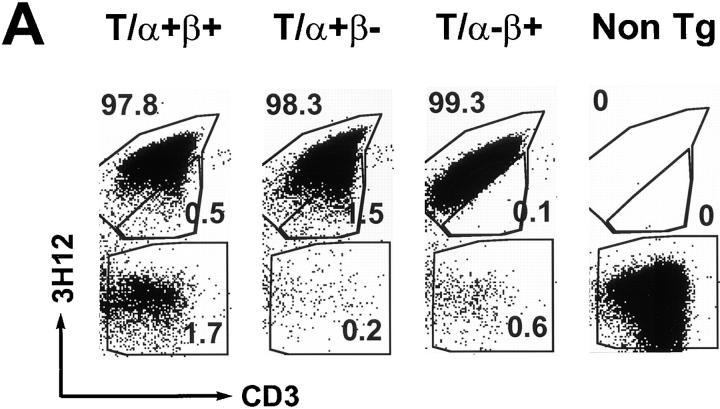
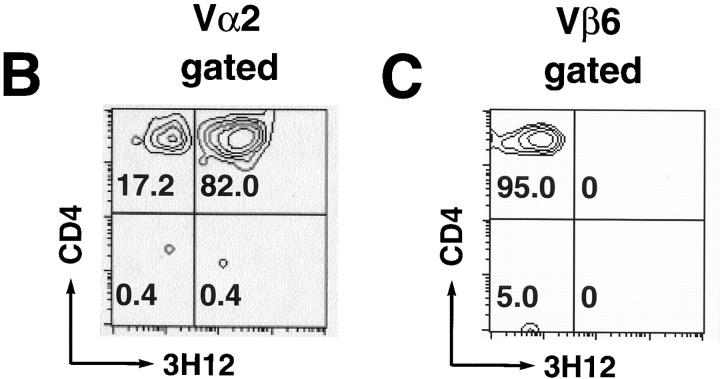
The staining specificity of 3H12 is defined in the following way: 3H12 stains MBP-specific T cells from the TCR transgenic mice, but does not stain cells from either nontransgenic syngeneic mice (see Fig. 5 A), or D011.10 TCR transgenic mice (data not shown), which, like the MBP-specific TCR transgenic mice, express Vβ8.2. Moreover, 3H12 recognizes neither the transgenic-encoded α chain paired with any endogenous β chain, nor the transgenic-encoded β chain paired with any endogenous α chain. The former was concluded after we observed no 3H12 staining on T cells derived from a cross between TCR-α KO mice and a mouse strain that is transgenic only for the MBP-specific TCR-α chain (data not shown). The latter was concluded because some endogenous Vα-expressing T cells in T/α+β− mice (which can only express the transgenic-derived β chain) are negative for 3H12 staining (see Fig. 5 B). We have not ruled out the possibility that the 3H12 antibody stains, in addition to the MBP-specific TCR, a subpopulation of cells expressing the transgenic-encoded β chain paired with selected endogenous α chains that are structurally related to the transgenic-encoded TCR-α chain.
Figure 5.
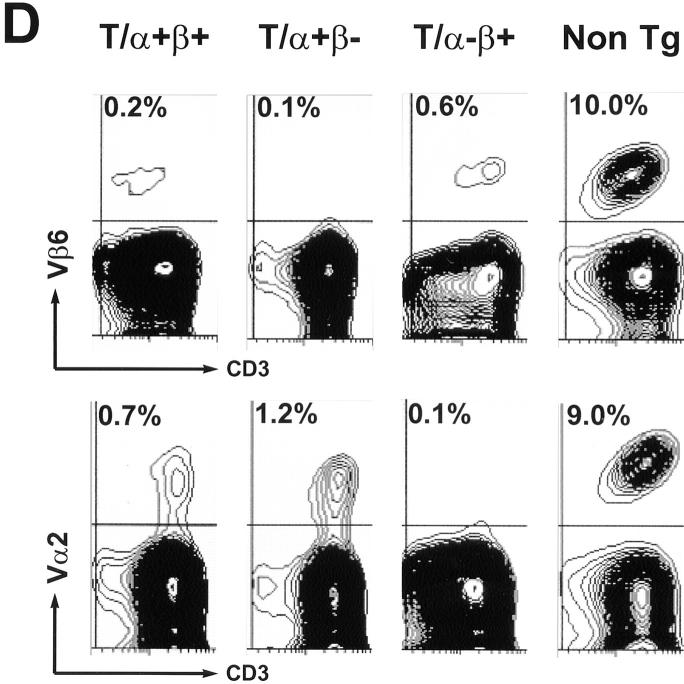
Repertoire analysis of T/α+β+, T/α+β−, T/α−β+, and nontransgenic mice. (A) Peripheral blood from a group of 6-wk-old littermates was stained with anti-CD3 and the anti-TCR clonotypic mAb 3H12. The double-expressing cells are contained in the middle gate. (B) Blood from one T/α+β− mouse (different than the ones in A) was stained for Vα2, CD3, 3H12, and CD4. CD3 and Vα2-positive lymphocytes are shown. (C) Blood from one T/α−β+ mouse (different than the ones in A) was stained for Vβ6, CD3, 3H12, and CD4. CD3 and Vβ6 positive lymphocytes are shown. (D) Anti-CD3 and anti-Vβ6 (top) or Vα2 (bottom) blood staining from the same mice as shown in A. Note that most Vα2-positive cells in the T/α+β+ and T/α+β− samples are off the diagonal, indicating that these cells express two TCR-α chains (36, 52). At least 50,000 lymphocytes were acquired.
Other Antibodies, Cell Reconstitution, and FACS® Analysis.
3H12 was generated as described in the preceding section. Anti-TCR Vβ4, Vβ7, and Vβ10b antibodies were purchased from Caltag Labs. (Burlingame, CA). All other antibodies were purchased from PharMingen (San Diego, CA). Recipient mice were bled (50 μl each time) through the retro-orbital plexus using a small capillary tube. Blood was stained with anti-CD4, anti-CD8, anti-B220, anti-Thy1.1, and anti-Thy1.1 antibodies. TCR Vβ and α usage was determined by staining peripheral blood with 3H12, anti-Thy1.1, anti-CD3, and the anti-TCR antibodies against Vβ2-, -Vβ3-, -Vβ4-, -Vβ6-, -Vβ7-, -Vβ8.1.2-, -Vβ8.3-, Vβ9-, -Vβ10b-, -Vβ11-, -Vβ13-, -Vβ14-, -Vα2-, -Vα3.2-, -Vα8-, and Vα11. To determine the proportion of CD8+ T cells and γ/δ T cells in donor-derived and recipient splenocytes, cells were stained with anti-CD8, anti-CD4, anti-TCR-γ/δ, 3H12, and anti-B220 antibodies. Peripheral blood was stained for 45 min at room temperature with the antibody cocktail, lysed, and fixed with FACS® lysing solution (Becton Dickinson), and then washed twice with PBS. When biotinylated antibodies were used, an additional 20 min of incubation in the presence of Streptavidin (PharMingen) was included. Samples were resuspended in PBS and analyzed in a FACScalibur® instrument (Becton Dickinson) equipped with two lasers. Unless otherwise indicated, up to 5,000 lymphocytes were acquired. For endogenous T cell repertoire analysis of T/α−β+, T/α+β−, T/α−β− and T/α+β+ mice, at least 50,000 lymphocytes were acquired. Splenocytes were stained similarly with incubations at 4°C, and no incubation in lysis solution. Samples were washed in staining solution (PBS, 2% fetal calf serum, 0.1% NaN3), and resuspended in the same solution. Propidium iodide was included in the solution to gate out dead cells. Up to 20,000 lymphocytes were acquired.
Results
Transfer of Splenocytes Protects T/R− Mice from EAE.
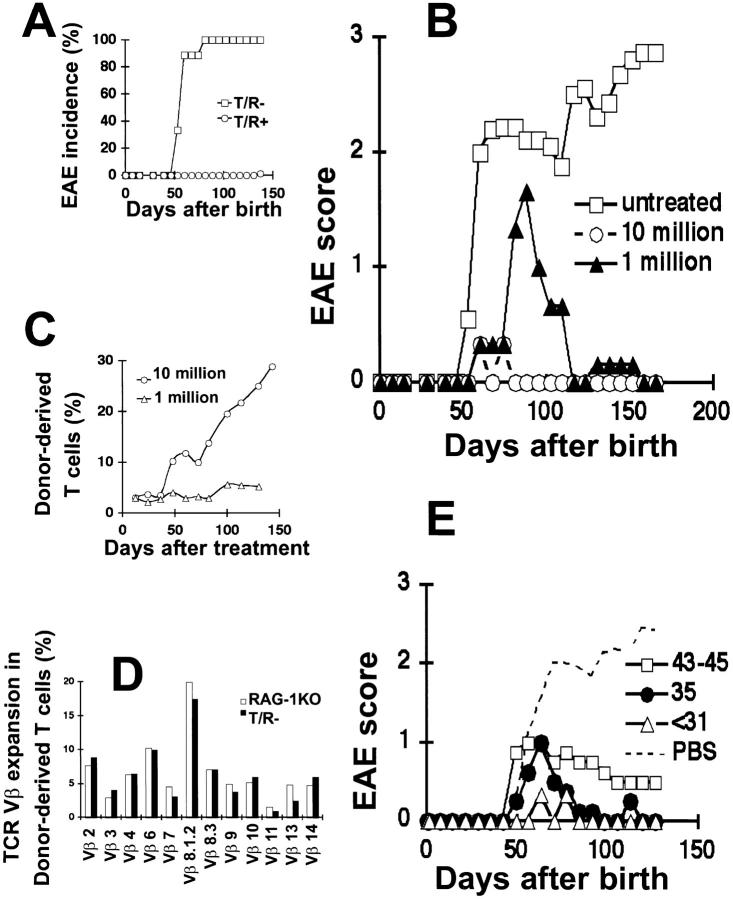
We have shown previously that MBP-specific TCR transgenic mice crossed with RAG-1 KO mice spontaneously develop EAE (8). At that time, the genetic makeup of these mice was comprised of three strains. Therefore, individual mice differed in minor histocompatibility antigens and cell transfer experiments could not be performed without a potential graft versus host reaction. To eliminate this problem, we crossed the T/R+ mice (originally made in C57BL oocytes) with RAG-1 KO mice that had been backcrossed for nine generations into the C57BL background. All T/R− mice in the C57BL background also developed EAE spontaneously (Fig. 1 A). However, the disease onset was accelerated, with ∼80% of the C57BL mice first exhibiting signs of EAE by the age of 60 d, and all mice developing EAE by 90 d of age. In contrast, we only observed signs of EAE in one of the 70 T/R+ littermates included in this study, and this mouse did not display the first signs of EAE until the age of 130 d. This low EAE incidence was observed only in T/R+ animals heterozygous for the TCR transgenes. T/R+ mice homozygous for the TCR transgenes develop EAE spontaneously (our unpublished data), and the 14% incidence of spontaneous EAE in T/R+ mice that we initially reported reflects the inclusion of some animals homozygous for the TCR transgenes. All work described henceforth pertains to mice on the C57BL background, with MHC H-2u/u and heterozygous for the TCR transgenes.
Figure 1.
Total splenocytes from normal donors protect T/R− mice from spontaneous EAE. (A) EAE incidence in untreated B10.PL H-2u/u T/R− (□; n = 9) and T/R+ (○; n = 70). Data indicate the percentage of mice that developed EAE within each group. (B) 3-wk-old T/R− mice were transferred with 107 (○ and ---; n = 3) or 106 (▴; n = 3) total splenocytes from normal donors (Thy1.1), or left untreated (□; n = 9). Mice were observed for a period of 150 d and EAE was scored as described in Materials and Methods. Data represent the average of each group. (C) Thy1.1+ T cell reconstitution was followed biweekly in T/R− mice treated as in B (107: ○, n = 3; 106: ▵, n = 3) by staining of peripheral blood from recipient mice with anti-Thy1.1-PE and anti-Thy1.2-FITC antibodies. Data represent the average of each group. (D) TCR Vβ expression in the donor-derived population 150 d after transfer of 107 total splenocytes into T/R− (black bars) or RAG-1 KO (white bars) mice. Data from one mouse per group is shown. (E) T/R− mice were treated with 107 total normal splenocytes before 31 (▵; n = 3), at 35 (•; n = 4), and between 43 and 45 (□; n = 4) d after birth, or treated with PBS between 31 and 45 days (---). Data represent the average of the groups.
To study the regulation of spontaneous EAE, we intravenously transferred 106 and 107 total splenocytes from normal Thy1.1 mice into 3-wk-old T/R− (Thy1.2) recipients. Administration of 106 splenocytes had a protective effect on T/R− mice, as EAE onset was delayed, its severity diminished, and the animals recovered from the disease (Fig. 1 B). An even more dramatic protective effect was observed upon injection of 107 splenocytes (Fig. 1 B). Although mice receiving either 106 or 107 normal splenocytes were both free of EAE at the end of the observation period, the beneficial effect of 106 normal splenocytes was clearly delayed in comparison to that of 107 normal splenocytes.
To monitor the repopulation of recipient mice by the donor-derived T cells, we used the allotypic marker Thy1.1. Repopulation of the B cell compartment could be monitored by direct staining with anti-B220 antibodies, since T/R− mice lack mature B cells. Blood samples were obtained approximately every 12 d for a period of 150 d, and stained for Thy1.1, Thy1.2, CD8, CD4, and B220. Donor-derived T cells plateaued at ∼6% of the total circulating T cells in mice injected with 106 splenocytes. In contrast, after injection of 107 splenocytes, donor-derived T cells expanded continuously, reaching 30% of the circulating T cells 150 d after injection (Fig. 1 C). Repopulation by B cells was modest in all recipient mice when compared with that of T cells. The proportion of lymphocytes expressing B220 at 150 d was only 4.5 and 1%, respectively, in T/R− mice receiving 107 and 106 splenocytes (data not shown). However, those recipients in which donor-derived T cells expanded the best also supported greater expansion of B cells.
Analysis of lymphocyte compartments in individual mice revealed no strict correlation between the expansion of donor-derived cells and EAE protection. In some cases, fully protected animals displayed slightly lower proportions of donor-derived lymphocytes than animals afflicted with a mild form of the disease. Therefore, the protection of T/R− mice from EAE is not determined only by the quantity of donor-derived lymphocytes.
Because T cells expanded considerably more than B cells in the recipient mice, we investigated whether particular donor-derived T cell subpopulations were enriched in the protected recipients as compared with recipients in which T cell expansion is independent of EAE protection. Toward that end, we determined whether the Vβ usage of T cells recovered from T/R− mice that had been protected from EAE differed from that of T cells recovered from nontransgenic RAG-1 KO recipients. No major skewing on the TCR Vβ usage was observed (Fig. 1 D), indicating that protection from EAE does not entail a large expansion of T cells bearing particular Vβ chains.
Based on these results, we conclude that normal splenocytes are able to protect T/R− mice from EAE. The protection is more effective as more cells are injected, but does not always correlate with the degree of expansion of the donor-derived cells within the recipient mice.
Older T/R− Mice Are Less Susceptible to Protection by Splenocytes than Younger Mice.
The experiments described in the preceding section were performed using 21-d-old mice. To determine the effectiveness of splenocyte cell transfer in older T/R− mice, 107 normal splenocytes were injected into 31-, 35-, and 43–45-d-old T/R− recipients that had not yet developed EAE. The protective effect of the transferred cells decreased as the age of the recipients increased (Fig. 1 E). Younger recipients (31 d old) either showed no disease, or only mild disease with delayed onset followed by complete recovery (no sequelae). In contrast, older recipients (43–45 d old) had no delay in the onset of EAE and frequently did not recover completely. However, some beneficial effects of splenocyte injection were apparent even when animals were injected at 43–45 d of age.
Due to the effect of age on the effectiveness of protection by cell transfers, all subsequent experiments were carried out using recipients <31 d old.
CD4+ T Cells Protect T/R− Mice from EAE.
The major populations of lymphocytes in the spleen are B cells, CD4+ T cells, CD8+ T cells, γ/δ T cells, and NK T cells. To establish which cell type(s) was more important in protecting T/R− mice from EAE, we transferred total splenocytes from H-2u/u CD4 KO mice and μMT KO mice backcrossed into the C57BL background. Due to the mutation in the CD4 coreceptor, CD4 KO mice display reduced T helper activity (25, 26), whereas μMT KO mice lack mature B cells (23). Cell injections were normalized according to the number of CD8+ T cells. As shown in Table 1, transfer of splenocytes from μMT KO animals protected against EAE very effectively. In a total of eight recipients, four never developed EAE, two that did develop disease recovered completely, and the remaining two had only a mild form of EAE at the end of the experiment. In contrast, all 12 mice that received splenocytes from CD4 KO mice developed EAE, with only 1 mouse recovering completely. However, compared with PBS-injected T/R− mice, there was a small protective effect of splenocytes from CD4 KO mice at the end of the experiment.
Table 1.
Splenocytes from μMT KO Mice but not from CD4 KO Mice Confer Protection from EAE
| Treatment* | n | Mice without EAE‡ | Maximum EAE | Total recovery§ | Final EAE‖ | CD8+ cells reconstitution¶ | γ/δ+ cells reconstitution¶ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 × 106 CD4 KO | 7 | 0/7 | 2.4 ± 1.36 | 1/7 | 1.5 ± 1.7 | ND | ND | |||||||
| 2 × 106 CD4 KO | 5 | 0/5 | 2.4 ± 0.5 | 0/5 | 1.9 ± 0.5 | 0.2 | 0.03 | |||||||
| 3 × 106 μMT KO | 3 | 2/3 | 0.7 ± 1.2 | 0/1 | 0.2 ± 0.3 | ND | ND | |||||||
| 2 × 106 μMT KO | 5 | 2/5 | 0.8 ± 0.8 | 2/3 | 0.2 ± 0.3 | 0.4 | 0.02 | |||||||
| PBS | 4 | 0/4 | 2.8 ± 1.5 | 0/4 | 2.5 ± 1.7 | ND | ND |
T/R− mice (<31 d old) transferred with different numbers of CD4 KO or μMT KO splenocytes, or injected with PBS. Mice were observed for a period of 140 d and EAE was scored as described in Materials and Methods.
Injected cells correspond to the number of total CD8+ cells.
Observed mice with no signs of EAE.
Observed mice that recovered completely from EAE.
Average EAE score at the end of the experiment.
Number of CD8+ or γ/δ+ cells per one anti-MBP-specific TCR transgenic cell after 140 d of treatment.
Since T/R− animals that received splenocytes from μMT KO mice did not develop EAE, B cells can be excluded from acting as the protective lymphocytes. In addition, because the numbers of CD8+ cells and γ/δ T cells were equal in the protective (μMT KO) or nonprotective (CD4 KO) inoculum, and similar in the recipient mice at the end of the experiment (Table 1), we can also exclude CD8+ T cells and γ/δ cells. These data suggest that CD4+ T cells are the protective lymphocytes.
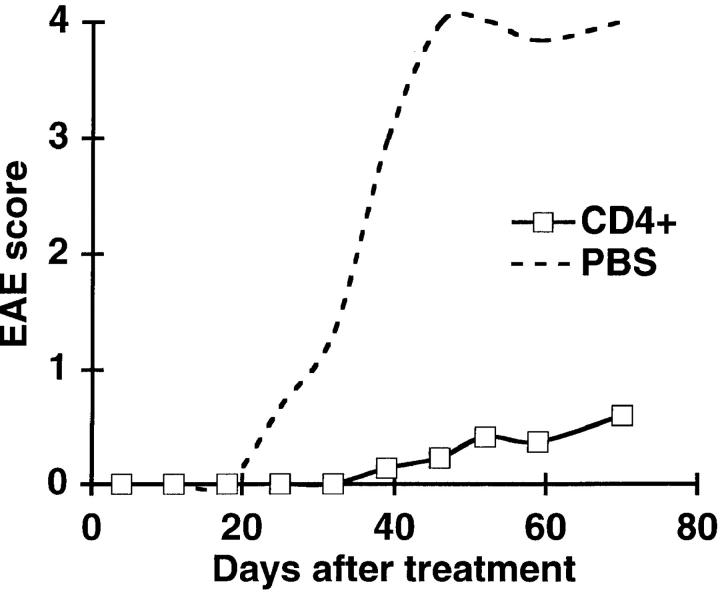
To demonstrate that CD4+ T cells themselves are protective, and do not act indirectly through, for example, CD8+ T cells, we transferred 5 × 106 magnetically sorted CD4+ T cells from normal mouse spleens into T/R− mice. CD4+ T cells had a clear protective capacity (Fig. 2). Of the 11 T/R− mice injected with purified CD4+ T cells, 7 (64%) remained disease free, whereas all of the PBS-injected mice developed EAE. Similar results were obtained with mature single positive CD4+ thymocytes (data not shown). Therefore, we conclude that CD4+ T cells present in normal individuals have the capacity to prevent the development of EAE in T/R− mice, independently of CD8+ T cells or B cells.
Figure 2.
Purified CD4+ T cells protect T/R− mice from spontaneous EAE. Total splenocytes from normal mice were depleted of CD8+ T cells and B cells with anti-CD8 and anti-B220 magnetic beads (see Materials and Methods). 5 × 106 purified CD4+ T cells were transferred into T/R− mice (□; n = 11). As a control, age- and sex-matched mice were treated with PBS (---; n = 3). Data represent the average of each group.
IL-4 Is Not Required for Protection of T/R− Mice.
CD4 KO mice have ∼15% of the number of helper T cells found in normal mice, as determined by expression of a human CD2 cDNA regulated by CD4 cis-acting sequences (27).
Although generally deficient in helper T cell activity, some helper T cell responses are unaffected in CD4 KO mice, particularly Th1 responses (28). On the other hand, Th2 responses are severely impaired in these mice (29).
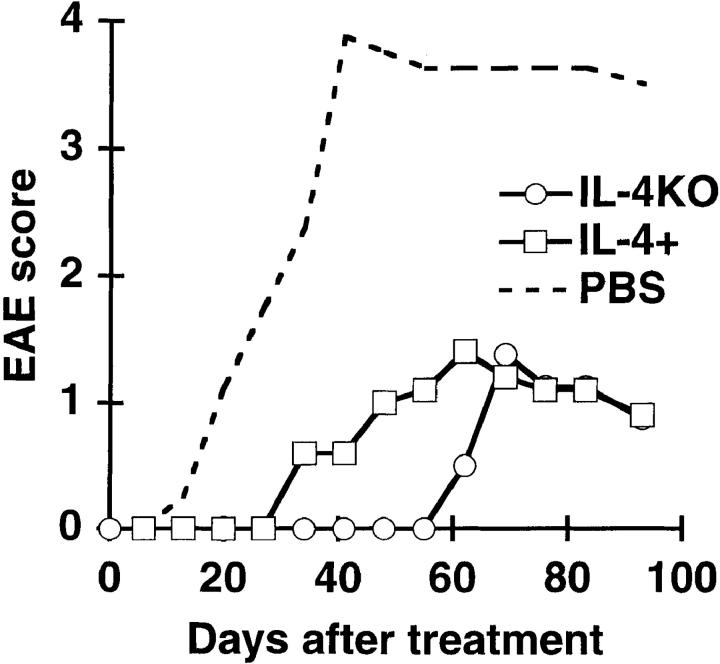
To determine whether the lack of protection by CD4 KO splenocytes was caused by their inability to mount Th2 responses, we transferred total splenocytes from IL-4 KO mice into T/R− mice. Although IL-4 KO mice are known to be defective in Th2 responses, splenocytes from these mice were found to have a protective capacity similar to that of IL-4–normal littermates (Fig. 3), indicating that conventional Th2 responses are not necessary for protection against EAE in T/R− mice.
Figure 3.
Splenocytes from IL-4 KO mice protect T/R− mice from spontaneous EAE as effectively as do splenocytes from IL-4+ mice. T/R− mice were transferred with 107 total splenocytes from IL-4 KO (○; n = 4) or IL-4+ (□; n = 5) mice, or were treated with PBS (---; n = 4). Data represent the average of each group.
Mice Lacking Endogenous TCR-α or -β Chains Develop EAE Spontaneously.
The spontaneous EAE phenotype was originally described comparing unmanipulated T/R+ and T/R− mice. To confirm that CD4+ T cells with endogenous TCR rearrangements are the lymphocytes that prevent EAE in T/R+ mice, we crossed T/R+ mice with μMT KO (lacking B cells), β2m KO (lacking MHC class I–restricted T cells, such as CD8+ and NK T cells), TCR-δ KO (lacking γ/δ T cells), and TCR-α/β KO (lacking endogenous TCR-α and -β chains) mice. All the KO mice had been backcrossed repeatedly onto the C57BL background.
As predicted by the cell transfer experiments, MBP- specific TCR transgenic mice that congenitally lack B cells, CD8+ T cells, CD1-restricted NK T cells, and γ/δ T cells do not develop EAE (Table 2). To assess the role of endogenous TCR-α and -β chains in EAE protection, we generated MBP-specific TCR transgenic mice lacking endogenous TCR-α chains (referred to as T/α−β+), lacking endogenous TCR-β chains (T/α+β−), and lacking both TCR-α and -β chains (T/α−β−), and compared them to mice which have at least one wild-type allele at both the TCR-α and β loci (T/α+β+). These four kinds of mice were generated from the same type of breeding pairs, in which MBP-specific transgenic males heterozygous for both α and β TCR mutations were crossed with nontransgenic females homozygous for the mutations at both the TCR-α and -β loci. Mice were scored for EAE once a week for a period of at least 4 mo.
Table 2.
EAE Incidence in the Offspring of Anti-MBP TCR-specific Transgenic Mice Crossed into Different KO Backgrounds
| Anti-MBP TCR transgenic RAG-1+ mice crossed into | Mice with EAE/ mice observed | |
|---|---|---|
| TCR-α−β− | 16/16 | |
| TCR-α−β+ | 19/20 | |
| TCR-α+β− | 15/21 | |
| TCR-α+β+ | 0/32 | |
| TCR-δ KO | 0/26 | |
| β2m KO | 0/18 | |
| μMT KO | 1/19 |
None of the 32 T/α+β+ mice developed EAE spontaneously. In contrast, all 16 T/α−β− mice and 19 out of 20 T/α−β+ mice developed EAE spontaneously. Interestingly, 15 out of 21 T/α+β− mice also developed EAE, whereas the remaining 6 mice were completely free of clinical signs of EAE for at least 5 mo (Table 2). Because we expected that some T/α+β− mice would be heterozygous for the TCR-α KO mutation whereas others would be homozygous for the wild-type TCR-α locus, we speculated that the six healthy animals might be homozygous for the wild-type TCR-α allele. This homozygosity could enable the mice to have a greater diversification of the endogenous TCR-α repertoire. However, this prediction was not confirmed: although two of the healthy mice had the wild-type allele in both chromosomes, the remaining four mice were heterozygous for the TCR-α mutation (data not shown). The EAE susceptibility of T/α+β− mice was unexpected, because allelic exclusion is highly effective at the TCR-β locus (30).
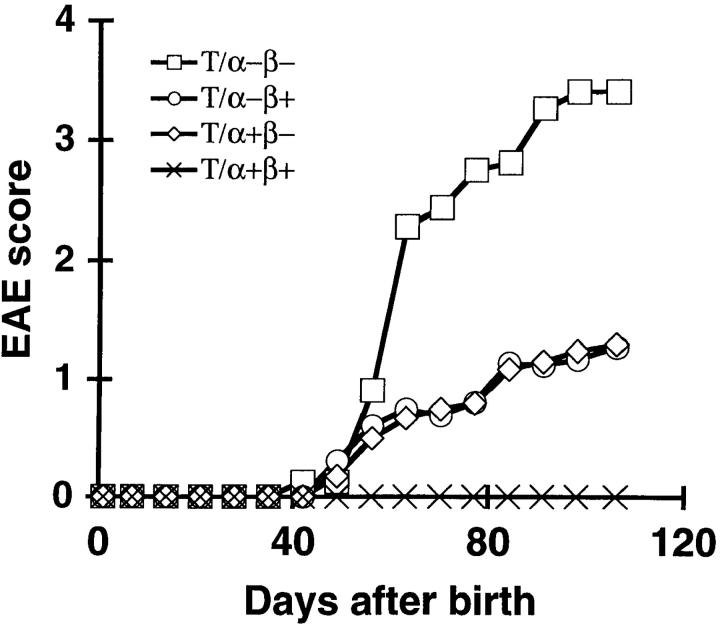
Further analysis of disease in a subgroup of the above T/α−β−, T/α−β+, T/α+β−, and T/α+β+ mice revealed that the mean EAE score in mice lacking either one of the endogenous TCR chains (T/α−β+ or T/α+β−) was reduced in comparison to mice lacking both endogenous TCR chains (T/α−β−). These latter mice behaved similarly to T/R− mice (Fig. 4).
Figure 4.
T/α−β+ and T/α+β− mice develop less severe EAE than T/α−β− mice. T/α−β− (□; n = 16), T/α−β+ (○; n = 18), T/α+β− (⋄; n = 19), and T/α+β+ mice (×; n = 22) were monitored for spontaneous EAE as described in Materials and Methods.
The reduction of the mean EAE score in T/α−β+ or T/α+β− animals could reflect a delayed onset of EAE in some mice, and/or a less severe disease. In the case of T/α−β− mice, 13% of mice developed EAE by day 50 after birth, 87% of the mice had EAE by day 60 after birth (Table 3), and none of the mice with EAE showed improvement of more than one clinical level during the observation period. Analysis of 17 T/α−β+ mice showed that only 8 animals (47%) first displayed clinical signs of EAE before 60 d of age (Table 3). Analysis of 18 T/α+β− mice indicated that only 7 (39%) of these animals had EAE before 60 d of age, whereas 6 mice did not develop EAE even up to 5 mo of age (Table 3). In summary, many animals unable to generate either of the endogenous TCR-α or -β chains showed a delayed onset of EAE, which was more pronounced in T/α+β− than in T/α−β+ mice. Interestingly, 3 out of 17 T/α−β+ mice, and 3 out of 18 T/α+β− mice, developed EAE before 50 d of age, a proportion similar to that found in T/α−β− or T/R− animals. Thus, T/α−β+ and T/α+β− mice displayed a greater variance in the age of EAE onset. Mortality was also reduced in T/α−β+ and T/α+β− animals: 5 out of 15 T/α−β− mice died as a consequence of EAE, whereas none of T/α−β+ or T/α+β− animals did (Table 3). Finally, T/α−β+ and T/α+β− mice experienced a delay in disease onset as well as reduced disease severity and mortality in comparison with T/α−β− mice.
Table 3.
Longitudinal Analysis of Spontaneous EAE in TCR-α/β Mutant Mice
| Strain | n | Observed EAE* | EAE onset before day 50‡ | EAE onset before day 60‡ | EAE onset before day 90‡ | Mortality due to EAE§ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T/α−β− | 15 | 15 | 2 (13.3) | 13 (86.6) | 15 (100) | 5 (33.3) | ||||||
| T/α−β+ | 17 | 16‖ | 3 (17.6) | 8 (47) | 13 (76.5) | 0 | ||||||
| T/α+β− | 18 | 12 | 3 (16.6) | 7 (38.8) | 9 (50) | 0 |
Number of mice that developed EAE during the 160 d of observation.
Mice that developed EAE during the indicated time frame. Percentage is indicated in parentheses.
Mice that died of EAE during the course of the experiment. Percentage is indicated in parentheses.
The only mouse that did not develop EAE died at 4 mo of age.
T Cell Repertoire Analysis of T/α−β+, T/α−β+, and T/α+β+ Mice.
Since T/α−β+, T/α+β−, T/α−β−, and T/α+β+ mice differ only in their capacity to express TCR chains encoded by the endogenous TCR-α or -β genes, the differences in EAE susceptibility that we observed between the various kinds of mutant mice must be attributed to differences in the α/β T cell repertoire. Depending on the kind of TCR transgenic mice, the α/β T cell repertoire may consist of up to four types of cells: (a) cells expressing exclusively the transgene-encoded MBP-specific TCR (αt-gβtg); (b) cells expressing a transgenic chain paired with an endogenous chain (αtgβend or αendβtg); (c) cells expressing both α and β chains not encoded by the transgenes (αendβend); and (d) cells expressing the transgene-encoded α/β chains in addition to a TCR chain encoded by the endogenous loci (double-expressing cells).
To correlate the EAE susceptibility with the T cell repertoire, we stained peripheral blood samples of T/α−β+, T/ α+β−, T/α−β−, and T/α+β+ mice with an anti-TCR clonotypic antibody (3H12; for a description of its specificity see Materials and Methods), together with anti-CD3 and a panel of anti-TCR Vα and Vβ antibodies. The data is shown in Fig. 5 and the results are summarized in Table 4. We observed that the number of TCR clonotype-negative cells was ∼2% in 6–8-wk-old T/α+β+ mice, 0.6% in T/α−β+ mice, 0.2% in T/α+β− mice, and 0.1% in T/α−β− mice (Fig. 5 A and data not shown). The proportion of clonotype-negative cells was relatively constant among mice of the same genotype and age, and increased as the animals aged.
Table 4.
Repertoire Diversity in the Different TCR-α/β KO Mice*
| T/α−β− | T/α−β+ | T/α+β− | T/α+β+ | |||||
|---|---|---|---|---|---|---|---|---|
| TCR-clonotype negative cells | 0.1% | 0.6% | 0.2% | 2.0% | ||||
| Double-TCR expressing cells | 0% | <0.1% | ∼10% | ∼10% |
6-wk-old mice were analyzed.
Using four different anti-TCR Vα antibodies that stain 21% of the T cells in wild-type syngeneic mice, we observed staining of ∼2% of the T cells in both T/α+β+ and T/α+β− young mice. Therefore, ∼10% of the T cells in both types of mice express endogenous TCR-α chains. The vast majority of T cells expressing endogenous Vα chains coexpress the MBP-specific α/β TCR (Fig. 5 B), whereas most T cells expressing endogenous Vβ chains are negative for the MBP-specific TCR (Fig. 5 C). An example of Vα2 and Vβ6 staining of the same group of mice as in Fig. 5 A is shown in Fig. 5 D.
In summary, although T/α−β+ mice have slightly more MBP-TCR− T cells than T/α+β− mice, they lack T cells coexpressing endogenous β chains with the transgene- encoded TCR. On the other hand, the repertoire diversity in T/α+β− mice is provided largely by cells that coexpress the MBP-specific TCR and endogenous TCR-α chains. Finally, the repertoire diversity in disease-free T/α+β+ mice is provided by both MBP-TCR−negative cells and double- expressing T cells. We conclude that the susceptibility of T/α−β+, T/α+β−, T/α−β−, and T/α+β+ mice to EAE correlates inversely with the diversity of their T cell repertoire.
Discussion
We have used genetic depletion and adoptive transfer methods to identify a lymphocyte population, present in T/R+ mice and absent in T/R− mice, capable of preventing EAE. Both approaches indicate that CD4+ T cells have a protective effect on spontaneous EAE. A similar conclusion has been reached in the paper by Van de Keere et al. appearing in this issue (29a).
α/β T Cells Bearing Endogenous Receptors Are Required for Protection against Spontaneous EAE.
MBP-specific TCR transgenic mice that congenitally lack B cells, CD8+ T cells, CD1-restricted NK T cells, or γ/δ T cells do not develop spontaneous EAE (Table 2), indicating that none of these absent cell types is the primary protective cell population in our experimental model. However, mice lacking T cells that express endogenous α and β TCR chains do spontaneously develop EAE. In conjunction with adoptive transfer experiments, these results demonstrate that CD4+ T lymphocytes bearing endogenous α or β T cell receptors protect T/R+ mice from EAE.
In TCR transgenic mice, expression of transgenic TCR-β chains leads to allelic exclusion of endogenous TCR-β chains (30), whereas expression of transgenic TCR-α chains does not. However, further rearrangement of endogenous TCR-α chains is halted after positive selection (31, 32). Therefore, in a positively selecting MHC environment, most TCR transgenic mice bear a T cell repertoire dominated by the transgene-encoded specificity, with most of the endogenously derived repertoire comprised of cells expressing the transgenic β chain paired with endogenous α chains (for review see reference 33).
Crosses of T/R+ mice with TCR-α KO and TCR-β KO mice generated three main observations. First, spontaneous EAE develops in 100% of mice lacking both endogenous TCR chains (T/α−β−) and is almost as high in mice lacking only endogenous α chains (T/α−β+). In contrast, one-third of mice lacking endogenous β chains (T/α+β−) never show signs of EAE, and mice capable of generating both endogenous TCR chains never develop EAE. Second, the age of EAE onset in T/α−β− mice is similar to that observed in T/R− mice, with 80% of the mice showing first signs of EAE within a 2-wk period. On the other hand, the age of EAE onset in T/α−β+ and T/α+β− animals follows a much broader distribution (Table 3). Finally, EAE severity and mortality are greater in afflicted T/α−β− mice than in T/α−β+ and T/α+β− animals (Table 3 and Fig. 4).
To understand these observations, we have begun examining the endogenous TCR repertoire in T/α−β+, T/α+β−, and T/α+β+ mice. MBP clonotype–negative T cells comprise ∼2% of total T cells in young (6-wk-old) T/α+β+ animals, ∼0.6% in T/α−β+ mice, ∼0.2% in T/α+β− mice, and ∼0.1% in T/α−β− mice. Further TCR diversity is provided by cells coexpressing MBP-specific TCR with endogenous TCR-α chains. These coexpressing cells comprise ∼10% of the total T cell population in both T/α+β− and T/α+β+ mice. On the other hand, we observed only a small proportion of cells coexpressing endogenous TCR-β chains and the MBP-specific TCR, and therefore the repertoire diversity of T/α−β+ mice is largely provided by cells that do not express the MBP-specific clonotype. The potential for TCR diversity is therefore greatest in T/α+β+ mice, followed by T/α+β− mice, then T/α−β+ mice, and finally T/α−β− mice (Table 4).
Despite the relatively equal proportions of T cells expressing endogenous TCR-α chains in T/α+β+ and T/α+β− mice, the incidence of EAE in these two groups is very different. Thus, it is likely that T cells bearing TCRs consisting of two endogenous TCR chains (αendβend) are the most effective at achieving EAE protection. For instance, although <2% of the T cells in young T/α+β+ mice express both endogenous TCR-α and β chains, these cells are sufficient to induce long-lasting protection in all mice. Some degree of protection is also conferred by T cells expressing endogenous TCR-α chains with transgenic-derived TCR-β chains (αendβtg), since one-third of the T/α+β−, but none of the T/α−β− mice, remained free of EAE.
On the other hand, T cells expressing the MBP-specific transgenic-encoded α chain with endogenous β chains (αtgβend) appear to have a very low protective capacity. However, as αtgβend cells constitute a very small proportion of total T cells in T/α−β+ mice, the possibility exists that αtgβend T cells could confer protection were they present in higher numbers. Adoptive transfer experiments with sorted subpopulations of CD4+ T cells from T/α−β+, T/α+β−, and T/α+β+ should clarify this point.
Stochastic Generation of Regulatory Cells May Determine the EAE Outcome of T/α+β− Mice.
It is remarkable that, of the T/α+β− mice, approximately one-third developed an early, severe EAE, one-third developed a late form, and one-third did not develop EAE at all. The increased heterogeneity in the age of EAE onset was also observed, albeit to a lesser extent, in T/α−β+ mice (Table 3). This variability contrasts sharply with the relatively homogeneous behavior of T/α−β− and T/α+β+ mice, and can not be explained by differences in the genetic makeup of the T/α+β− mice, since all KO mice were backcrossed several times into a C57BL background.
The presence of a single TCR-β chain in T/α+β− mice restricts the potential diversity of the T cells, since endogenous TCR-α chains have to pair appropriately with the transgenic β chain. In addition, positive selection of MBP-specific T cells, which prevents further TCR-α rearrangements (31), is very effective in H-2u/u mice. These two factors generate a highly restrictive environment for the rearrangement and selection of endogenous TCR-α- encoded chains, a situation that enables important repertoire variations between genetically identical individuals. The resulting biased T cell repertoire may bear T cells expressing TCRs with poor, intermediate, or good avidity to putative ligands required for the activation of regulatory cells. Based on our data, approximately one-third of the T/α+β− mice appear to successfully generate those rare CD4+ αendβtg cells and one-third may be unable to generate those cells, at least at a young enough age to prevent disease. The remaining third of T/α+β− animals may generate regulatory cells with low avidity for the required ligand, resulting in ineffective regulation and a later onset of EAE. A similar explanation can be applied to the behavior of T/α−β+ mice. However, the need for endogenous TCR-α chains seems to be more important than the need for endogenous TCR-β chains for ligand recognition, because all but one T/α−β+ mice developed EAE (Table 2).
The endogenous TCR repertoire of individual T/α+β− and T/α−β+ mice in connection to EAE susceptibility is currently under investigation.
Is Allelic Inclusion of TCR-α Chains Protective?
Recently, Sarukhan et al. (34) described a pathogenic role for T cells expressing two TCR-α chains in a TCR transgenic mouse diabetes model. In adoptive transfer experiments, T cells expressing endogenous TCR-α chains and low levels of transgenic-encoded TCR-α chains were shown to be more diabetogenic than those expressing high levels of the transgenic-encoded (autoreactive) TCR, which did not express endogenous TCR-α chains. There are several differences between this diabetes model and our model of EAE. In EAE, elimination of endogenous TCR and Ig gene-rearrangements dramatically increases the frequency of disease, whereas the same changes in the diabetes model produce the opposite effect (35). The reduction in the incidence of diabetes may reflect the fact that other cell types (such as CD8+ T cells) are required to trigger disease in this experimental model. It has also been shown that T cells expressing endogenous TCR-α chains are not required for the initiation of disease in another TCR transgenic diabetes model (36). Furthermore, the relatively low incidence of diabetes observed in TCR transgenic mice heterozygous for the selecting MHC H-2g7 increased upon crossing those mice with TCR-α KO mice (36).
Due to the presence of T cells coexpressing endogenous TCR-α chains with the MBP-specific TCR in H-2u/u T/α+β+ mice, which do not develop EAE, and their absence in T/α−β+ and T/α−β− mice, which do, it is possible that allelic inclusion of a second TCR on MBP-specific cells would, actually, make the mice less susceptible to EAE. This is unlikely for the following reasons. First, although it is possible that MBP-specific cells coexpressing endogenous TCR-α chains have a higher threshold of activation than cells expressing only the MBP-specific TCR, the majority of MBP-specific T cells would have to be double expressors in order for an increased activation threshold to affect the incidence of EAE, which is clearly not the case. In addition, transfer of normal CD4+ cells does protect T/R− mice from EAE in the absence of endogenous TCR chains on the MBP-specific cells. Finally, two out of three T/α+β− mice develop EAE despite bearing similar numbers of T cells that coexpress endogenous TCR-α chains and the MBP-specific receptor as the disease-free T/α+β+ mice.
Lymphocytes Other Than the Primary Regulatory CD4+ T Cells May Modulate EAE at Later Stages.
EAE onset and severity differ between T/α−β− mice and T/α−β+ or T/α+β− mice. As previously stated, we believe that the development of EAE is due to a lack of primary regulation by CD4+ T cells. On the other hand, the lower morbidity and mortality observed in T/α−β+ and T/α+β− mice as compared with T/α−β− mice could be due to lymphocytes other than the CD4+ primary regulatory cells. In various EAE models it has been shown that B cells (37), CD8+ T cells (38–40), and CD4+ T cells expressing Vβ14 and, to a lesser extent, Vβ3 (41, 42), are involved in the recovery from acute EAE or in the control of relapses. Importantly, if B cells ameliorate the course of spontaneous EAE, they do so in an α/β T cell–dependent manner, because none of these beneficial effects are apparent in T/α−β− mice, which behave very similarly to T/R− mice. We are currently crossing the T/α−β+ and T/α+β− with μMT KO mice and β2m KO mice to investigate the possible “secondary” protective role of cells absent in those mutant mice.
Regulatory Function of CD4+ T Cells.
Splenocytes from CD4 KO mice conferred virtually no protection to T/R− mice. Helper activity is not abolished in CD4 KO mice, but Th2 responses are (29). However, the lack of protection by splenocytes from CD4 KO mice is not due to deficient Th2 responses, because splenocytes from IL-4 KO mice effectively prevented EAE. Based upon these data, it is tempting to speculate that the regulatory cells need high avidity interactions with MHC-class II ligands, and this avidity level cannot be reached in the absence of CD4. Alternatively, the generation of regulatory cells in CD4 KO mice may be impaired due to holes in the T cell repertoire.
Several reports have linked CD4+ T cell populations with immune regulation. In rat EAE models, CD4+ T cells with suppressive potential were isolated at the time of recovery from this monophasic disease (43, 44). These cells were shown to secrete TGF-β (45). A regulatory type of CD4+ T cell (named Th3) was obtained after oral immunization with low doses of MBP (46). MBP-induced Th3 cells secreted large amounts of TGF-β, and, upon cell transfer, protected against EAE induced both by MBP and proteolipid protein. The protective effect of Th3 cells could be abolished with anti–TGF-β treatment. Female nonobese diabetic mice and BB rats develop diabetes spontaneously, which can be prevented by early transfer of CD4+ T cells (47, 48). In a mouse colitis model, CD4+ CD45RB-low T cells have been shown to have regulatory activity, whereas CD45RB-high T cells were pathogenic (49). This effect was also mediated by TGF-β (50). Recently, CD4+ T regulatory cells (named Tr1) secreting large amounts of IL-10 were described (51). Tr1 cells were capable of preventing colitis in an adoptive transfer system, an effect mediated synergistically by IL-10 and TGF-β. Therefore, it is possible that the CD4+ regulatory cells that we describe here also protect T/R+ mice from EAE through the secretion of immunomodulatory cytokines such as TGF-β and/or IL-10.
Because of the 100% incidence of spontaneous EAE and the relatively synchronized manner in which it arises in the C57BL genetic background, T/α−β− mice or T/R− mice display features not found in any other spontaneous autoimmunity model. In addition, this experimental system enables the clear identification of MBP-specific effector T cells in mixtures with other lymphocytes, and makes disease prevention possible upon adoptive transfer of purified subpopulations of lymphocytes, two important requirements for the further characterization of regulatory T cells.
In this report we show that protection from EAE can be accomplished without a large expansion of donor-derived cells, and that expression of both endogenous TCR-α and -β chains in CD4+ T cells is required for full protection from the disease. However, the question of whether CD4+ regulatory T cells act through specific ligand recognition, or competition for growth factors and space, remains a major unresolved issue and is the subject of ongoing studies.
Acknowledgments
We wish to thank Dan R. Littman, Maria A.C. Lafaille, Antonio Bandeira, Pablo Pereira, Mary K. Pao, and Claudia Muller for numerous suggestions during the preparation of the manuscript.
Abbreviations used in this paper
- β2m
β2-microglobulin
- EAE
experimental autoimmune encephalomyelitis
- KO
knockout
- MBP
myelin basic protein
- RAG-1
recombination activating gene-1
- T/R−
MBP-specific T cell receptor transgenic mice with mutated RAG-1 genes
- T/R+
MBP-specific T cell receptor transgenic mice with at least a normal RAG-1 gene
Footnotes
This work was partially supported by the National Institutes of Health (R21 and R01 AI41647), the Hirschl-Caulier Trust, and the Bernard B. Levine Investigatorship in Allergy and Immunology (J.J. Lafaille).
References
- 1.Mason D, Fowell D. T-cell subsets in autoimmunity. Curr Opin Immunol. 1992;4:728–732. doi: 10.1016/0952-7915(92)90053-h. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Nun A, Eisenstein S, Cohen IR. Experimental autoimmune encephalomyelitis (EAE) in genetically resistant rats: PVG rats resist active induction of EAE but are susceptible to and can generate EAE effector T cell lines. J Immunol. 1982;129:918–919. [PubMed] [Google Scholar]
- 3.Cohen IR. Regulation of autoimmune disease physiological and therapeutic. Immunol Rev. 1986;94:5–21. doi: 10.1111/j.1600-065x.1986.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 4.Fowell D, Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+T cell subset that inhibits this autoimmune potential. J Exp Med. 1993;177:627–636. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hafler DA, Weiner HL. Immunologic mechanisms and therapy in multiple sclerosis. Immunol Rev. 1995;144:75–107. doi: 10.1111/j.1600-065x.1995.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 6.Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 7.Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 8.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 9.Scott B, Liblau R, Degermann S, Marconi LA, Ogata L, Caton AJ, McDevitt HO, Lo D. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1:73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 10.Nishizuka Y, Tanaka Y, Sakakura T, Kojima A. Murine thyroiditis induced by neonatal thymectomy. Experientia (Basel) 1973;29:1396–1398. doi: 10.1007/BF01922839. [DOI] [PubMed] [Google Scholar]
- 11.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 12.Smith H, Sakamoto Y, Kasai K, Tung KS. Effector and regulatory cells in autoimmune oophoritis elicited by neonatal thymectomy. J Immunol. 1991;147:2928–2933. [PubMed] [Google Scholar]
- 13.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 15.Chilosi M, Facchetti F, Notarangelo LD, Romagnani S, Del Prete G, Almerigogna F, De Carli M, Pizzolo G. CD30 cell expression and abnormal soluble CD30 serum accumulation in Omenn's syndrome: evidence for a T helper 2-mediated condition. Eur J Immunol. 1996;26:329–334. doi: 10.1002/eji.1830260209. [DOI] [PubMed] [Google Scholar]
- 16.Villa A, Santagata S, Bozzi F, Giliani S, Frattini A, Imberti L, Gatta LB, Ochs HD, Schwarz K, Notarangelo LD, et al. Partial V(D)J recombination activity leads to Omenn syndrome. Cell. 1998;93:885–896. doi: 10.1016/s0092-8674(00)81448-8. [DOI] [PubMed] [Google Scholar]
- 17.Modigliani Y, Thomas-Vaslin V, Bandeira A, Coltey M, Le Douarin NM, Coutinho A, Salaun J. Lymphocytes selected in allogeneic thymic epithelium mediate dominant tolerance toward tissue grafts of the thymic epithelium haplotype. Proc Natl Acad Sci USA. 1995;92:7555–7559. doi: 10.1073/pnas.92.16.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 19.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of α4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 21.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 22.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 25.Killeen N, Sawada S, Littman DR. Regulated expression of human CD4 rescues helper T cell development in mice lacking expression of endogenous CD4. EMBO (Eur Mol Biol Organ) J. 1993;12:1547–1553. doi: 10.1002/j.1460-2075.1993.tb05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 27.Killeen N, Davis CB, Chu K, Crooks ME, Sawada S, Scarborough JD, Boyd KA, Stuart SG, Xu H, Littman DR. CD4 function in thymocyte differentiation and T cell activation. Philos Trans R Soc Lond B Biol Sci. 1993;342:25–34. doi: 10.1098/rstb.1993.0131. [DOI] [PubMed] [Google Scholar]
- 28.Locksley RM, Reiner SL, Hatam F, Littman DR, Killeen N. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science. 1993;261:1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- 29.Brown DR, Moskowitz NH, Killeen N, Reiner SL. A role for CD4 in peripheral T cell differentiation. J Exp Med. 1997;186:101–107. doi: 10.1084/jem.186.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Van de Keere F, Tonegawa S. CD4+T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti–myelin basic protein T cell receptor transgenic mice. J Exp Med. 1998;188:1875–1882. doi: 10.1084/jem.188.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uematsu Y, Ryser S, Dembic Z, Borgulya P, Krimpenfort P, Berns A, von Boehmer H, Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988;52:831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- 31.Borgulya P, Kishi H, Uematsu Y, von Boehmer H. Exclusion and inclusion of alpha and beta T cell receptor alleles. Cell. 1992;69:529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- 32.Couez D, Malissen M, Buferne M, Schmitt-Verhulst AM, Malissen B. Each of the two productive T cell receptor alpha2 gene rearrangements found in both the A10 and BM 3.3 T cell clones give rise to an alpha chain which can contribute to the constitution of a surface- expressed alpha beta dimer. Int Immunol. 1991;3:719–729. doi: 10.1093/intimm/3.7.719. [DOI] [PubMed] [Google Scholar]
- 33.von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- 34.Sarukhan A, Garcia C, Lanoue A, von Boehmer H. Allelic inclusion of T cell receptor alpha genes poses an autoimmune hazard due to low-level expression of autospecific receptors. Immunity. 1998;8:563–570. doi: 10.1016/s1074-7613(00)80561-0. [DOI] [PubMed] [Google Scholar]
- 35.Sarukhan A, Lanoue A, Franzke A, Brousse N, Buer J, von Boehmer H. Changes in function of antigen-specific lymphocytes correlating with progression towards diabetes in a transgenic model. EMBO (Eur Mol Biol Organ) J. 1998;17:71–80. doi: 10.1093/emboj/17.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luhder F, Katz J, Benoist C, Mathis D. Major histocompatibility complex class II molecules can protect from diabetes by positively selecting T cells with additional specificities. J Exp Med. 1998;187:379–387. doi: 10.1084/jem.187.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell–deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koh DR, Fung-Leung WP, Ho A, Gray D, Acha-Orbea H, Mak TW. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 1992;256:1210–1213. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 39.Jiang H, Zhang SI, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213–1215. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 40.Jiang H, Ware R, Stall A, Flaherty L, Chess L, Pernis B. Murine CD8+ T cells that specifically delete autologous CD4+ T cells expressing V beta 8 TCR: a role of the Qa-1 molecule. Immunity. 1995;2:185–194. doi: 10.1016/s1074-7613(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 41.Kumar V, Sercarz EE. The involvement of T cell receptor peptide-specific regulatory CD4+ T cells in recovery from antigen-induced autoimmune disease. J Exp Med. 1993;178:909–916. doi: 10.1084/jem.178.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar V, Stellrecht K, Sercarz E. Inactivation of T cell receptor peptide-specific CD4 regulatory T cells induces chronic experimental autoimmune encephalomyelitis (EAE) J Exp Med. 1996;184:1609–1617. doi: 10.1084/jem.184.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellerman KE, Powers JM, Brostoff SW. A suppressor T-lymphocyte cell line for autoimmune encephalomyelitis. Nature. 1988;331:265–267. doi: 10.1038/331265a0. [DOI] [PubMed] [Google Scholar]
- 44.Karpus WJ, Swanborg RH. CD4+ suppressor cells differentially affect the production of IFN-gamma by effector cells of experimental autoimmune encephalomyelitis. J Immunol. 1989;143:3492–3497. [PubMed] [Google Scholar]
- 45.Karpus WJ, Swanborg RH. CD4+ suppressor cells inhibit the function of effector cells of experimental autoimmune encephalomyelitis through a mechanism involving transforming growth factor-beta. J Immunol. 1991;146:1163–1168. [PubMed] [Google Scholar]
- 46.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 47.Mordes JP, Gallina DL, Handler ES, Greiner DL, Nakamura N, Pelletier A, Rossini AA. Transfusions enriched for W3/25+ helper/inducer T lymphocytes prevent spontaneous diabetes in the BB/W rat. Diabetologia. 1987;30:22–26. doi: 10.1007/BF01788902. [DOI] [PubMed] [Google Scholar]
- 48.Boitard C, Yasunami R, Dardenne M, Bach JF. T cell–mediated inhibition of the transfer of autoimmune diabetes in NOD mice. J Exp Med. 1989;169:1669–1680. doi: 10.1084/jem.169.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 50.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1–mediated colitis by CD45RBlow CD4+T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 52.Hardardottir F, Baron JL, Janeway CA., Jr T cells with two functional antigen-specific receptors. Proc Natl Acad Sci USA. 1995;92:354–358. doi: 10.1073/pnas.92.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]