Abstract
Enteropathogenic Escherichia coli (EPEC) belongs to a family of related bacterial pathogens, including enterohemorrhagic Escherichia coli (EHEC) O157:H7 and other human and animal diarrheagenic pathogens that form attaching and effacing (A/E) lesions on host epithelial surfaces. Bacterial secreted Esp proteins and a type III secretion system are conserved among these pathogens and trigger host cell signal transduction pathways and cytoskeletal rearrangements, and mediate intimate bacterial adherence to epithelial cell surfaces in vitro. However, their role in pathogenesis is still unclear. To investigate the role of Esp proteins in disease, mutations in espA and espB were constructed in rabbit EPEC serotype O103 and infection characteristics were compared to that of the wild-type strain using histology, scanning and transmission electron microscopy, and confocal laser scanning microscopy in a weaned rabbit infection model. The virulence of EspA and EspB mutant strains was severely attenuated. Additionally, neither mutant strain formed A/E lesions, nor did either one cause cytoskeletal actin rearrangements beneath the attached bacteria in the rabbit intestine. Collectively, this study shows for the first time that the type III secreted proteins EspA and EspB are needed to form A/E lesions in vivo and are indeed virulence factors. It also confirms the role of A/E lesions in disease processes.
Keywords: enteropathogenic Escherichia coli, attaching and effacing lesion, Esp proteins, type III secretion system, cytoskeletal rearrangement
Afamily of enteropathogenic Escherichia coli (EPEC)1 causes diarrhea in humans and animals (1), yet the mechanism by which they cause disease in vivo has not been defined. These pathogens cause a characteristic histopathological lesion termed an attaching/effacing (A/E) lesion (2), although the contribution of this lesion to disease has not been defined. Enterohemorrhagic Escherichia coli (EHEC) O157:H7 also causes this lesion (3–5). Several genes are involved in formation of the A/E lesion in tissue culture cells, including eae (which encodes intimin, an adhesin) and espB (which encodes a secreted protein), and these genes are conserved in EPEC, EHEC, and rabbit EPEC strains (6, 7).
The A/E lesion is defined by the intimate attachment between bacteria and the epithelial surface, and the effacement (disappearance) of host cell microvilli. Alteration of cytoskeletal components beneath adherent bacteria results in formation of a pedestal-like structure that can extend to a pseudopod. In vitro (tissue culture), the A/E lesion is mediated by bacteria–host cell interactions, including triggering of host signal transduction pathways (8–10). Initial localized adherence to epithelial cells is mediated by a plasmid-encoding, bundle-forming pilus (BFP) (11–15), followed by the insertion of translocated intimin receptor (Tir), a bacterial protein, into the host plasma membrane (10). Tir (10), formerly Hp90 (16), is delivered to tissue culture cells, and requires the secreted proteins EspA and EspB for its translocation. Tir has at least three possible functions that have been identified (10). Tir is translocated into the epithelial cell membrane and serves as a cell surface receptor for intimin. The second function of Tir is to nucleate actin after intimin binding. The third function is to transmit additional signals to host cells once Tir–intimin interaction occurs. These events trigger tyrosine phosphorylation of phospholipase Cγ (17) and other host proteins, and the resulting signals follow Tir phosphorylation and other early signaling events.
EPEC A/E lesions require products of a 35-kb locus termed the LEE (locus of enterocyte effacement; 18) that includes the esc cluster, tir, eae, espA, espD, and espB (formerly eaeB). The esc cluster encodes a type III secretion system responsible for secretion of Esp proteins (19). tir and eae encode Tir (10) and intimin (20), which are required for intimate attachment in vitro. At least three secreted proteins, EspA (21), EspB (22) and EspD (23), encoded by espA, espB, and espD, are secreted via the type III secretion pathway. These secreted proteins are essential for triggering of host signal transduction pathways in tissue culture cells. However, it is not known if these processes, and thus these secreted proteins, are needed for virulence in vivo.
To investigate the role of EspA and EspB in EPEC pathogenesis, we constructed mutant strains in E. coli serotype O103. In Europe, serotype O103 is the most common rabbit EPEC (REPEC) strain, causing heavy diarrhea in weaned rabbits, and its rhamnose fermentation negative phenotype is linked to high virulence (24–26). E. coli O103 also causes histopathological features similar to that caused by EPEC in the intestine, including A/E lesions on the intestinal mucosa (27, 28). Recently, the E. coli O103 LEE region has been studied (29). However, the role of the type III secreted proteins EspA and EspB in virulence is still unclear, as is the role of the A/E lesion in disease for this family of pathogens. Here we report characterization of disease in rabbits infected with strains that are defective for EspA and EspB using a rabbit infection model, and show that these two secreted proteins and the resulting A/E lesions formed are essential for pathogenesis.
Materials and Methods
Bacterial Strains, Plasmids, Cell Culture and Media.
E. coli serotype O103 85/150 strain causes heavy diarrhea in weaned rabbits and was provided by Dr. Johan E. Peeters (National Institute of Veterinary Research, Brussels, Belgium). The phenotypes and genotypes of this strain and other bacteria used in this study are listed in Table 1. Plasmids pAA23Δ and pAABxbΔ (7) are derivatives of a positive suicide vector pCVD442 that contains sacB, bla, and a pir-dependent R6K replicon (30). HeLa cells were maintained and assayed in MEM containing 10% (vol/vol) fetal calf sera at 37°C in a 5% CO2 incubator. For infection, REPEC and mutant strains were grown in Luria-Bertani (LB) broth overnight at 37°C without shaking.
Table 1.
Strains Used in this Study
| Strain | Relevant characteristics | Comments | Reference | |||
|---|---|---|---|---|---|---|
| 85/150 | Rabbit EPEC | Prototypic O103:K−:H2 strain, rhamnose negative | 24 | |||
| RDEC-1 | Rabbit EPEC | Prototypic O15:K−:H− strain | 43 | |||
| E2348/69 | Human EPEC | Prototypic O127:H6 EPEC strain | 44 | |||
| REPEC O103 | Rabbit EPEC | Nalidixic acid resistant strain derived from 85/150 | This study | |||
| AAF004 | REPEC O103 (EspA−) | Nonpolar mutation in REPEC O103 espA | This study | |||
| AAF005 | REPEC O103 (EspB−) | Nonpolar mutation in REPEC O103 espB | This study | |||
| SM10λpir | Thi thr leu tonA lacY supE | Donor strain for constructing REPEC | 31 | |||
| resA::RP4-2-Tc::Mu Km | Mutant strains |
Construction of Nalidixic Acid Resistant Derivative of REPEC O103.
Strain 85/150 was inoculated in 25 ml LB media and incubated overnight. After centrifugation, bacteria were plated on LB agar containing 30 μg/ml nalidixic acid. A resulting nalidixic acid resistant strain was used for further experiments and designated as REPEC O103.
Construction of Nonpolar Stop Codon Mutations in REPEC O103 espA and espB.
Plasmid pAA23Δ and pAABxbΔ are positive suicide vectors, and were designed for construction of nonpolar mutations in espA and espB, respectively (7). Plasmid pAA23Δ contains RDEC-1 espA including a stop codon and a BglII site 235 bp downstream from the espA start codon. This plasmid was introduced into E. coli SM10λpir (31) and was transconjugated into REPEC O103 as described elsewhere (30). Plasmid pAABxbΔ (7) contained a stop codon in addition to a 250-bp deletion, starting 154 bp downstream of the espB start codon. This plasmid was transformed into E. coli SM10λpir and transconjugated into REPEC O103. Both mutant strains were selected by resistance to sucrose and nalidixic acid and sensitivity to ampicillin.
Preparation of Secreted Proteins.
Bacterial overnight cultures were diluted 1:100 into DMEM and incubated for 6.5 h in a 5% CO2 incubator. Bacteria were removed by centrifugation (18,000 g for 10 min) and the supernatant was precipitated by addition of 10% ice-cold TCA, and incubated on ice for 1 h. After centrifugation, the pellets were resuspended in Laemmli sample buffer and analyzed by 12% SDS-PAGE (32).
Immunofluorescence Microscopy.
HeLa cells (105) were inoculated and grown overnight on coverslips, then infected with REPEC O103 or its mutant strains for 3 h. Cells were washed three times with PBS and fixed with 3.0% PFA in PBS (pH 7.2) for 30 min at room temperature, then washed twice with PBS. The fixed cells were permeabilized with 20 μl 0.1% Triton X-100 in PBS in the presence of phalloidin–Texas Red (TxR) (Molecular Probes, Eugene, OR) to stain filamentous actin, and antiphosphotyrosine antibodies, clone 4G10 (Upstate Biotechnology, Lake Placid, NY). FITC-conjugated anti–mouse IgG and IgM (ICN Pharmaceuticals Inc., Costa Mesa, CA) was used as the secondary antibody for antiphosphotyrosine. Stained samples were visualized and photographed as described elsewhere (33).
Rabbit Infection.
Overnight standing bacterial cultures were collected by centrifugation and resuspended in 2 ml PBS. Bacteria (7 × 108 in 2 ml PBS) were given to New Zealand white rabbits (female, 461–636 g, 28–36-d-old) using orogastric tubes, and bacteria inside the orogastric tubes were flushed with an additional 2 ml PBS wash.
Tissue Preparation and Histological Procedure.
After 1–4 d infection, tissues from ileum, Peyer's patches, cecum, and proximal colon were excised immediately after sacrifice by intravenous injection of ketamine and overdosing of sodium pentobarbital in accordance to the guidelines of the Canadian Council of Animal Care and the University of British Columbia. For light microscopy, intestinal tissues were fixed in 10% neutral buffered formalin and processed for paraffin embedding. Serial 4-μm-thick sections were cut onto glass slides and stained with hematoxylin and eosin, and examined and photographed using a light microscope (AH-2; Olympus America Inc., Melville, NY). For scanning electron microscopy (SEM) and transmission electron microscopy (TEM), tissues were washed in PBS and prefixed in 2.5% glutaraldehyde, then rinsed in 0.1 M cacodylate buffer. After postfixation in 1% osmium tetroxide, tissues were stained in 2% uranyl acetate, then dehydrated in graded ethanols. SEM sections were critical point-dried using liquid carbon dioxide and coated with gold. The resulting sections were examined with an SEM (Stereoscan 250 MK III; Leica Inc., Deerfield, IL). TEM sections were further dehydrated in graded ethanols and propylene oxide, then embedded in Spurr's resin. After polymerization, ultrathin sections (90 nm) were stained with uranyl acetate and lead citrate and examined with a TEM (EM 10A/B; Carl Zeiss Inc., Thornwood, NY).
Preparation of Thick Cryosection of Intestinal Tissues.
Intestinal tissues were fixed with 4% PFA in PBS (pH 7.2) for 2 h at room temperature, washed twice with PBS, then incubated in 20% sucrose in PBS overnight at 4°C for cryoprotection. Tissue samples were embedded in OCT Tissuetek (Miles Laboratories Inc., Elkhart, IN), then frozen in cold 3-methyl-butane (−50 to −60°C). The frozen tissues were cut at 20 μm sections on a cryostat, then kept in PBS at 4°C.
Immunochemistry of Thick Cryosections.
Sections were incubated in 10% normal goat serum in PBS for 10 min, then permeabilized with 100 μl permeabilization buffer (0.2% saponin, 10% normal goat serum in PBS) in the presence of mouse antisera against REPEC O103 (1:200) overnight at 4°C. Sections were washed three times in PBS, then incubated in 0.2% saponin and 10% normal goat serum in PBS in the presence of phalloidin-TxR (1:400; Molecular Probes) and FITC-conjugated anti– mouse IgG and IgM (ICN Pharmaceuticals Inc.) as the secondary antibody for anti-REPEC O103 antibody. Tissue samples were washed twice in PBS, then mounted on coverslips and visualized as described elsewhere (34). In brief, stained sections were visualized by a confocal laser scanning microscope (MRC-600; Bio-Rad Labs., Hercules, CA) under control of COMOS software (Bio-Rad Labs.) The resulting scanned images were analyzed by a public domain image processing and analysis program, NIH Image (National Institutes of Health, Bethesda, MD), and processed images were exported to Adobe Photoshop (Adobe Systems Inc., San Jose, CA) to assign different fluorophore images into individual RGB channels.
Nucleotide Sequence Accession Numbers.
Nucleotide sequence data used in this study are available from the EMBL/GenBank/DDBJ under the following accession numbers: RDEC-1 eae, U60002; REPEC O103 84/110/1 eae, U59502; and REPEC O103 85/150 espB, AF059713.
Results
Construction of Nonpolar Mutations in espA and espB.
To construct mutations in espA and espB, a nalidixic acid resistant strain of E. coli serotype O103 85/150 (REPEC O103) was established, then nonpolar mutations were constructed by inserting a stop codon into these genes as described in Materials and Methods. To confirm mutations in espA and espB, chromosomal DNA was prepared from each mutant strain and PCR analysis was performed with two sets of primers encompassing espA or espB. The resulting PCR products were digested with BglII to confirm the presence of this engineered restriction site (data not shown). The mutant strains containing the stop codon in espA and espB were designated as AAF004 (EspA−) and AAF005 (EspB−), respectively.
Protein Secretion Profile of REPEC O103 and Its Mutant Strains in Tissue Culture Media.
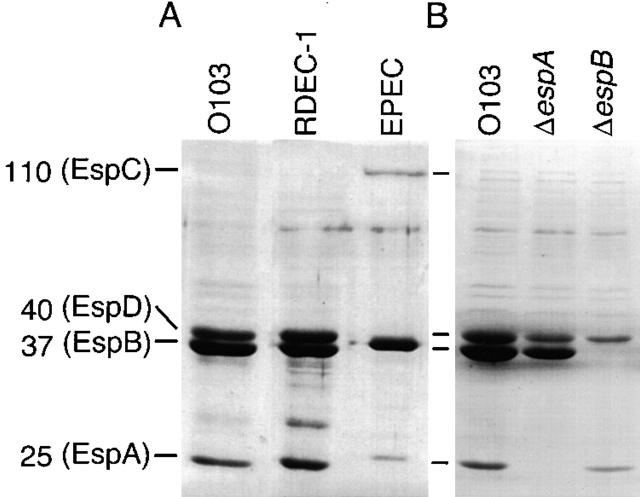
We have already shown that another rabbit EPEC strain, RDEC-1, secretes EspA and EspB into culture media (7). The secreted protein profile of REPEC O103 was quite similar to that of RDEC-1, and EspA and EspB in both rabbit EPEC strains migrated faster compared to those from the human EPEC strain (Fig. 1 A). Interestingly, EspC (110 kD) in both rabbit EPEC strains was not detectable using Coomassie blue staining. However, this protein is not involved in EPEC triggering of host signal transduction pathways and intimate adherence in vitro (35). In contrast, AAF004 (EspA−) and AAF005 (EspB−) lacked bands corresponding to EspA and EspB. Although we constructed both mutant strains by inserting a stop codon that should not affect downstream gene expression, the amounts of other secreted proteins were less compared to the wild-type O103 strain (Fig. 1 B). It is possible that lack of an Esp protein might affect the secretion of other Esp proteins by altering transcriptional, posttranslational events, and/or Esp–Esp protein interaction. However, these effects might be common features of type III secretion in EPEC, because the reduced amount of secreted proteins were also found in the EspB (36) and EspD (23) mutant strains in EPEC O127. The reduced amount of other secreted proteins in EspA and EspB mutant strains might contribute to changes in the virulence phenotypes.
Figure 1.
Secreted protein profiles of REPEC O103, RDEC-1, EPEC (A), and REPEC O103 mutant strains (B). Bacteria were grown in DMEM and the secreted proteins were precipitated by addition of 10% TCA, and then analyzed by 12% SDS-PAGE stained with Coomassie blue. Strains: REPEC O103, O103; AAF004 (EspA−), ΔespA; AAF005 (EspB−), ΔespB.
EspA and EspB Proteins Are Involved in Cytoskeletal Rearrangements in HeLa cells.
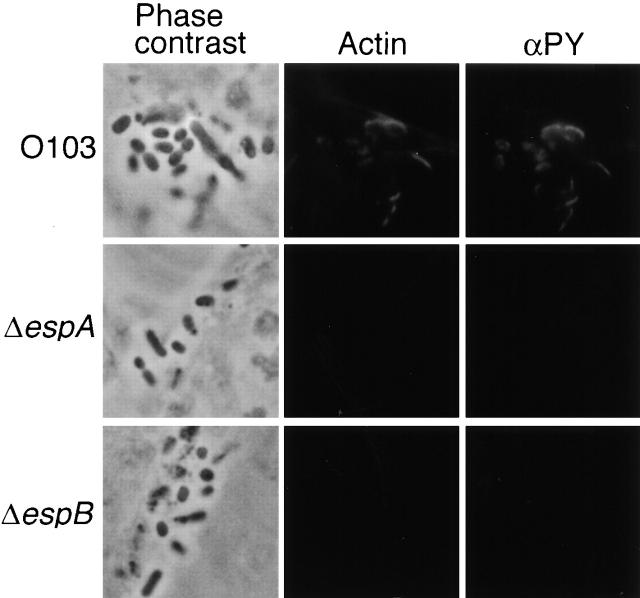
EPEC EspA and EspB are involved in accumulation of cytoskeletal actin and tyrosine-phosphorylated proteins beneath attached bacteria in tissue culture cells (21, 37). To confirm the role of REPEC O103 EspA and EspB in triggering these events in HeLa cells, cytoskeletal actin and tyrosine phosphorylated proteins were stained with phalloidin-TxR and FITC-labeled antiphosphotyrosine (Fig. 2). Unlike EPEC, REPEC O103 did not form microcolonies, but adhered diffusely on cell surfaces. This strain also accumulated cytoskeletal actin and tyrosine phosphorylated proteins beneath the attached bacteria. These accumulation patterns, including horseshoe-shaped structures, are similar to those caused by EPEC. In contrast, AAF004 (EspA−) and AAF005 (EspB−) strains did not accumulate cytoskeletal actin or tyrosine-phosphorylated proteins beneath the attached bacteria. These results suggest that EspA and EspB proteins in both EPEC and REPEC O103 are necessary for triggering of cytoskeletal rearrangements in HeLa cells.
Figure 2.
Accumulation of cytoskeletal actin and tyrosine-phosphorylated proteins beneath bacteria in cultured epithelial cells. HeLa cells (105) were infected for 3 h with REPEC O103 or isogenic mutant strains. Infected HeLa cells were fixed by the addition of paraformaldehyde, then phalloidin-TxR and antiphosphotyrosine antibodies (αPY) were used for detection of colocalization of actin and tyrosine-phosphorylated proteins by immunofluorescence microscopy. Strains: REPEC O103, O103; AAF004 (EspA−), ΔespA; AAF005 (EspB−), ΔespB.
Virulence of EspA− and EspB− Strains in Rabbits.
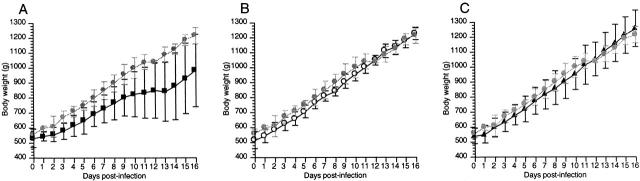
To determine if EspA and EspB are involved in disease, rabbits were infected with REPEC O103 and the mutant strains via an orogastric route, and the virulence of these mutant strains was compared to that of the wild-type strain. Rabbits (n = 5) infected with REPEC O103 (7 × 108) suffered weight loss and one rabbit died with watery diarrhea 7 d after infection. Rabbits that survived (n = 4) showed significant weight loss compared to rabbits given PBS (n = 3; Fig. 3 A). In contrast, rabbits given AAF004 (EspA−, n = 5) and AAF005 (EspB−, n = 4) did not show any diarrheagenic symptoms or weight loss (Fig. 3, B and C). These results clearly show that EspA and EspB are involved in these disease processes.
Figure 3.
Growth curves of rabbits that survived infection with REPEC O103 (A) or those infected with AAF004 (B), and AAF005 (C). Bacteria (7 × 108) were inoculated via the orogastric route and weights were measured daily. Values represent the average rabbit weight ± SD. Strains and control are indicated as follows: REPEC O103, black square; AAF004 (EspA−), white circle; AAF005 (EspB−), black triangle; PBS inoculation, gray circle.
Histological Analysis of Intestinal Tissues from Rabbits Infected with REPEC O103 and Mutant Strains.
After 72 h of infection, ileal sections were obtained from rabbits infected with REPEC O103, AAF004 (EspA−), and AAF005 (EspB−), and stained with hematoxylin and eosin. Only animals infected with REPEC O103 showed histological evidence of blunting of intestinal villi, necrosis of mucosal epithelial cells and sloughing, and increased inflammation consisting of a mixture of polymorphonuclear leukocytes, lymphocytes, and plasma cells (Fig. 4 A). In contrast, animals infected with AAF004 (EspA−) and AAF005 (EspB−) did not have altered villous architecture, nor increase inflammatory cell infiltrates in the lamina propria or muscularis layers (Fig. 4, B and C), much like the uninfected controls (Fig. 4 D). Furthermore, increased injury and inflammation were also observed in Peyer's patches when rabbits were infected with REPEC O103, but not with EspA and EspB mutant strains (data not shown). These results indicate that EspA and EspB are involved in inflammation and disruption of the mucosal epithelial surface.
Figure 4.
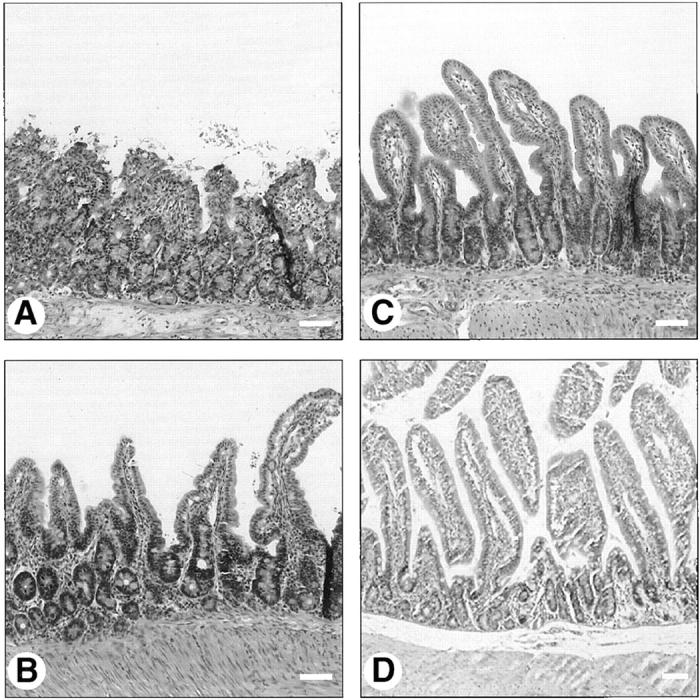
Hematoxylin and eosin–stained ileal sections of rabbits infected with REPEC O103 (A), AAF004 (B), AAF005 (C), and PBS inoculation (D). Note the extensive villous blunting, epithelial cell necrosis and sloughing, and inflammation within the lamina propria of the wild-type infected tissue (A). In contrast, villous architecture and extent of inflammation within the lamina propria infected with AAF004 (EspA−) or AAF005 (EspB−) are similar to those of control animals that were inoculated PBS. Bar, 100 μm.
Adherence of REPEC O103 and Mutant Strains to Intestinal Villi.
To analyze how EspA and EspB contribute to adherence to the mucosal surface, rabbits were killed 1–4 d after infection, and intestinal sections, including ileum, Peyer's patches, cecum, and proximal colon, were visualized by SEM. REPEC O103 was detected in all intestinal tissues (summarized in Table 2). In the ileum, bacteria were detected 24 h after infection, and showed diffuse adherence 48–72 h after infection (Fig. 5 A). The bacteria were sometimes aggregated and clumped on top of each other, and embedded into the ileal surface (Fig. 5 B). At higher magnifications, cup-like structures were also observed on the epithelial surface, presumably caused by detached bacteria (Fig. 5 C). In Peyer's patches, REPEC O103 appeared to adhere preferentially to the domed villi (follicle associated epithelium) 48 h after infection, but bacteria were also observed in normal villi of Peyer's patches 72 h after infection (data not shown). In the proximal colon, REPEC O103 showed a similar adherence pattern, but bacteria appear to bind to mucus rather than the epithelial surface. Although we could detect bacteria in the cecum, bacterial adherence was less than in other intestinal sections.
Table 2.
Phenotypes of REPEC O103 and Mutant Strains
| Strain | Adherence* | A/E lesions and cup-like structures | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL | PP | CE | PC | IL | PP | CE | PC | |||||||||
| REPEC O103 | +++ | +++ | + | +++ | + | + | – | + | ||||||||
| AAF004 (EspA−) | ++ | ++ | + | ++ | – | – | – | – | ||||||||
| AAF005 (EspB−) | ++ | ++ | + | ++ | – | – | – | – | ||||||||
Degree of adherent efficiency was assigned as follows: +, few adherent bacteria; ++, diffuse adherence; +++, intimate adherence. Tissue samples: IL, ileum; PP, Peyer's patches; CE, cecum; PC, proximal colon.
Figure 5.
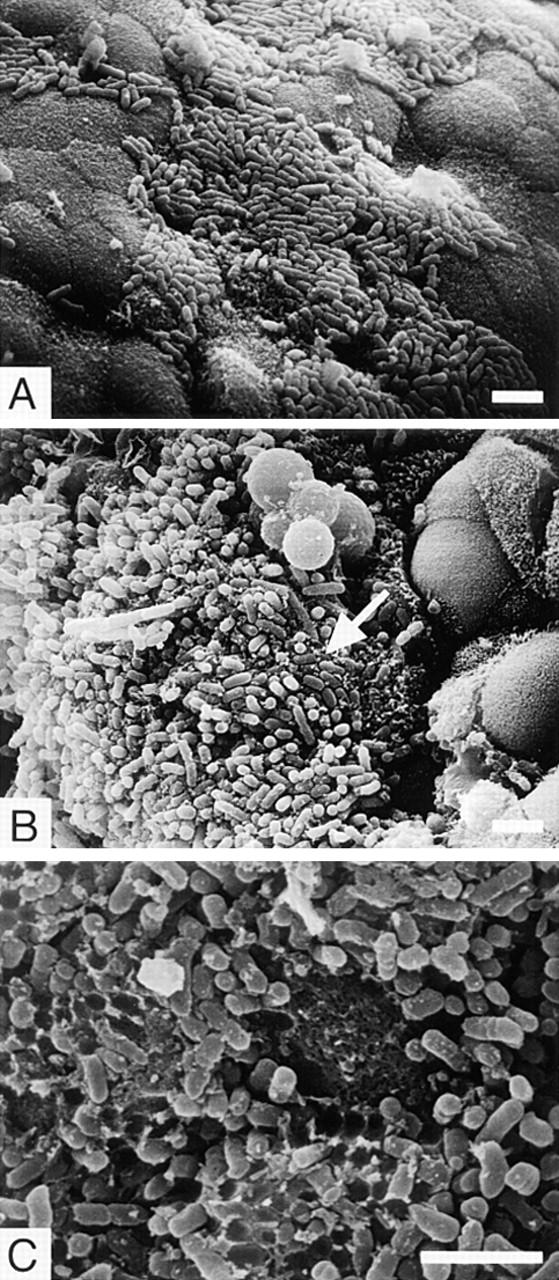
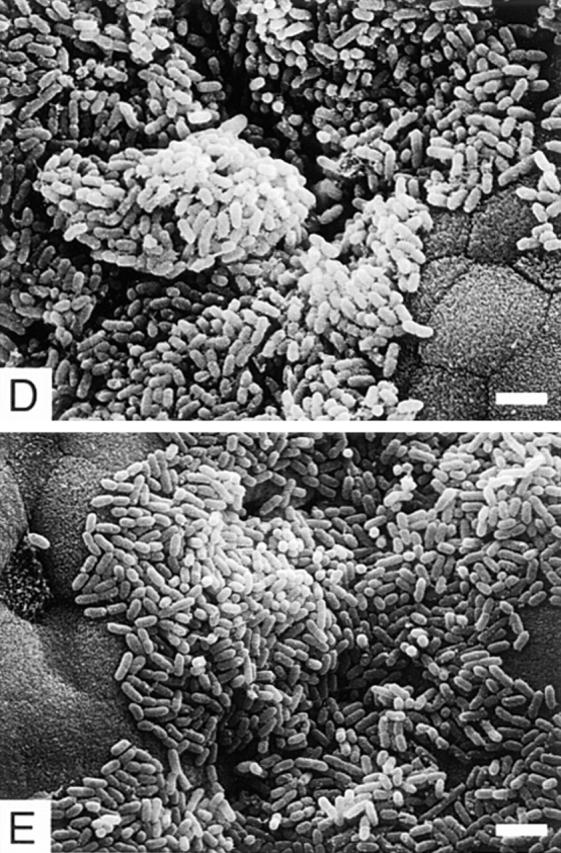
Scanning electron micrographs of the ileal villi infected with REPEC O103 (A–C), AAF004 (D), and AAF005 (E). REPEC O103, AAF004 (EspA−), and AAF005 (EspB−) adhered to the villi, and the adherence pattern is similar between wild-type and mutant strains. However, embedded bacteria as indicated by an arrow (B) and cup-like structures (C) were observed only in rabbits infected with REPEC O103. Ileal sections were taken 72 h (for REPEC O103 and AAF005) and 96 h (for AAF004) after infection. Bar, 4 μm.
Although the adherence of AAF004 (EspA−) and AAF005 (EspB−) was somewhat delayed compared to that of wild-type, both strains adhered to the ileum (Fig. 5, D and E), Peyer's patches, and proximal colon 72–96 h after infection (summarized in Table 2). However, we could not detect embedded bacteria as shown in Fig. 5 B, nor formation of cup-like structures on epithelial surfaces as in Fig. 5 C. These results suggest that formation of embedded and cup-like structures require EspA and EspB, although the initial (nonintimate) adherence of both AAF004 (EspA−) and AAF005 (EspB−) is similar to that of the parental strain.
EspA and EspB Are Required for A/E Lesions In Vivo.
To examine bacterial adherence in more detail, rabbit intestinal samples were analyzed using TEM. The results showed that REPEC O103 caused typical A/E lesions in vivo and intimately associated with the ileal surface (Fig. 6 A). Elongated and swollen microvilli and microbodies were observed closely associated with adherent bacteria. The bacteria rested on cups or pedestals, sometimes oriented perpendicularly or invaginated into host epithelial membranes. The glycocalyx and microvilli were completely disrupted beneath attached bacteria. Indicative of progressive intracellular damage, the enterocytes showed a pale vacuolated watery cytoplasm and disappearance or dysmorphy of mitochondria, ribosomes, and endoplasmatic reticulum, as well as pale nuclei, with the enterocytes often being extruded into the lumen. These typical A/E lesions were found in the ileum, Peyer's patches, and proximal colon, but not in the cecum (summarized in Table 2). In contrast, when rabbits were infected with AAF004 (EspA−) and AAF005 (EspB−), no A/E lesions were detected in ileal tissue (Fig. 6, B and C) or other intestinal sections examined. The microvilli beneath these bacteria still exhibited its normal brush border, and no sign of intracellular damage could be found. These results suggest that EspA and EspB are needed to trigger A/E lesions and cell damage in vivo.
Figure 6.
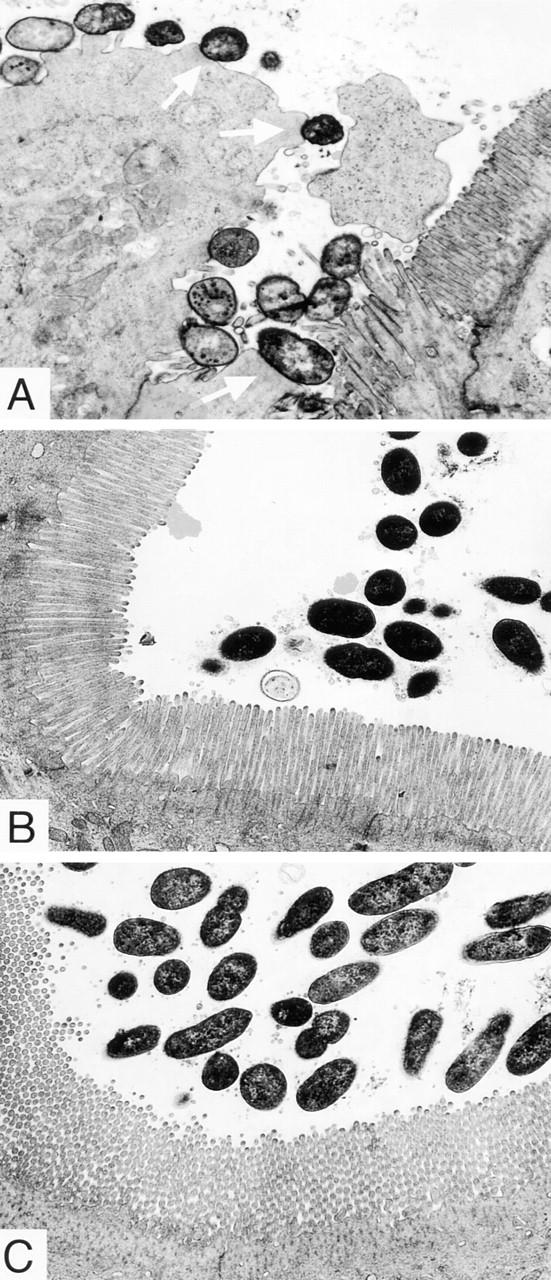
Transmission micrographs of the ileal villi infected with REPEC O103 (A), AAF004 (B), and AAF005 (C). The REPEC O103 are intimately associated with the ileal villi and form pedestal-like structures, which are indicated by arrows. Microbodies and elongated and swollen microvilli were also observed. In contrast, AAF004 (EspA−) and AAF005 (EspB−) do not cause A/E lesions, and intracellular damage was not seen. All ileal sections were taken 96 h after infection. Original magnification, ×6,300.
We have already shown that EspA and EspB were needed for efficient delivery of Tir, which was required for intimate adherence to HeLa cells (10). In this study, both mutant strains did not adhere intimately and did not make A/E lesions, presumably due to the inability to deliver Tir to the host membrane. However, these strains still adhered at a distance above the ileal surface. An adhesive factor, AF/R2, was previously identified in REPEC O103 and is needed for nonintimate adherence (38). Therefore, the initial (nonintimate) adherence at a distance from ileal surfaces (Fig. 6, B and C) is probably mediated by AF/R2 or another adhesin. Indeed, adherent patterns in EspA and EspB mutant strains were quite similar to that of the EPEC EspB mutant strain in Hep-2 cells (22), although EPEC initial adherence to epithelial cells is mediated by bundle-forming pilus.
EspA and EspB Are Required for Cytoskeletal Rearrangements and Diarrhea.
The role of cytoskeletal actin rearrangements mediated by EPEC infection in vivo is still unclear. To determine if cytoskeletal rearrangements also occur in vivo and are similar to those seen with tissue culture cells, cytoskeletal actin of epithelial surfaces were analyzed by confocal laser scanning microscopy (CLSM) using anti-O103 antibody and phalloidin-TxR, which specifically stains filamentous actin. We selected sections from ileum and Peyer's patches at 72 h after infection for examination. REPEC O103 adhered well to Peyer's patches (Fig. 7) confirming the SEM and TEM results. When tissue was stained with phalloidin-TxR, we found that the cytoskeletal actin was highly reorganized and formed cup-like structures beneath attached bacteria (Fig. 7). Bacterial colonization occurred in the ileum and Peyer's patches when rabbits were infected with AAF004 (EspA−) and AAF005 (EspB−). However, both mutant strains never triggered cytoskeletal rearrangements beneath the attached bacteria (Fig. 7). These results indicate that EspA and EspB are involved in triggering of cytoskeletal actin rearrangement and cup-like structures in vivo, processes that are needed for disease.
Figure 7.
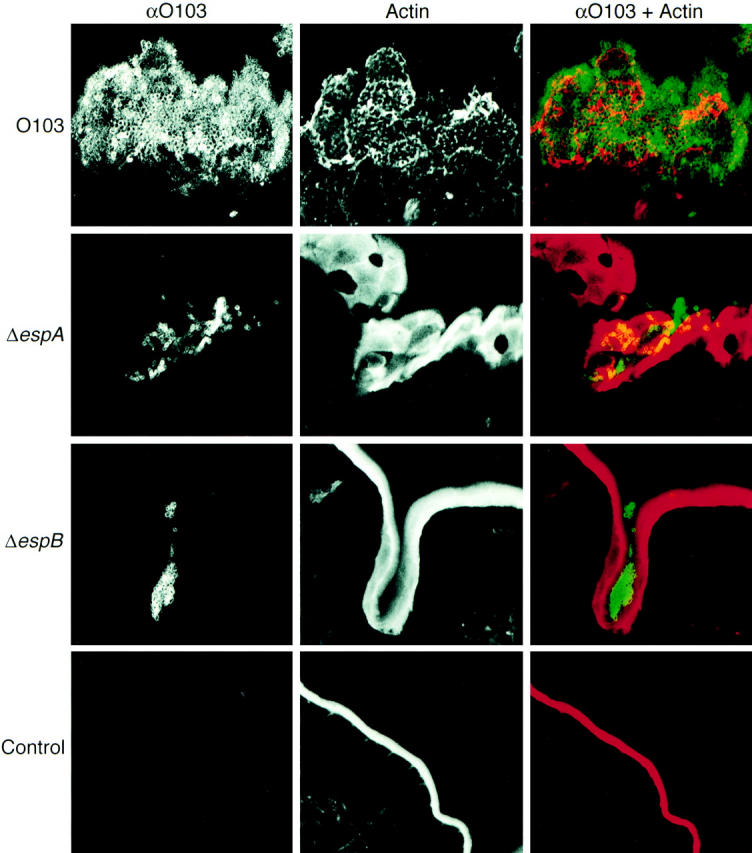
Confocal laser scanning micrographs of Peyer's patches infected with REPEC O103, AAF004, and AAF005. Peyer's patches were taken 72 h after infection and cryosections (20-μm-thick) were stained with phalloidin-TxR (red for overlay) and anti-O103 antibody (green for overlay). REPEC O103 adhered and colonized to the Peyer's patches, cytoskeletal actin beneath the attached bacteria was rearranged, and cup-like structures were also observed. In contrast, a small number of EspA and EspB mutant strains were observed in villi crypts, but no actin rearrangements occurred. Strains: REPEC O103, O103; AAF004 (EspA−), ΔespA; AAF005 (EspB−), ΔespB; PBS inoculation, Control.
Discussion
We show here that formation of A/E lesions mediated by the type III secreted proteins, EspA and EspB, is involved in virulence using histology, SEM, and TEM. Furthermore, thick tissue sections prepared from infected intestinal organs were analyzed by CLSM, revealing for the first time that the A/E lesion is formed by extensive cytoskeletal actin rearrangement in vivo.
Due to host tropism of human EPEC and their limitation to human in vivo studies, we used a natural rabbit infection model, EPEC serotype O103, to study disease events. Human EPEC serotype O127 normally infects only young children and does not infect animals. We have previously shown that maximal expression of EPEC Esp proteins occurs at 37°C, and their expression is reduced at 39°C, which corresponds to rabbit body temperature (7). The adaptation to human body temperature is one possible explanation of the narrow host range of EPEC. Although an EPEC infection study was carried out using human adult volunteers (39), this does not reflect a natural infection as adults were used and a high infection inoculum was required. In contrast, the advantages of using REPEC O103 are that this pathogen naturally infects weaned rabbits, forms the A/E lesion, and has a conserved LEE locus similar to EPEC O127. Thus the natural rabbit infection model using REPEC O103 is a useful model of human EPEC infections.
REPEC O103 secreted at least three proteins, EspA, EspB, and EspD, and its secretion profile was similar to that of another rabbit EPEC strain, RDEC-1 serotype O15. Indeed, DNA sequencing alignment analysis showed that the open reading frame encoding intimin (eae, 2820 bp) in REPEC O103 84/110/1 strain (24) is very similar to that of RDEC-1 (99.8% identity) with two nucleotide changes at positions 2608 bp (C to T) and 2709 bp (C to T). Furthermore, we have partially sequenced REPEC O103 espB and found it was identical to RDEC-1 espB from positions 22–924 bp, corresponding to 95.5% of the espB gene. These similarities indicate that other genes located in the REPEC LEE locus are highly conserved between serotype O103 and O15. We have previously established RDEC-1 EspA and EspB mutant strains using suicide vectors pAA23Δ and pAABxbΔ, which were originally constructed using RDEC-1 espA and espB (7). As expected, both vectors also functioned in REPEC O103 due to their sequence similarity. However, we were unable to produce any disease in rabbits infected with high doses of RDEC-1, and thus we switched to infection studies using REPEC O103 and its espA and espB nonpolar mutant strains.
REPEC O103 causes actin nucleation beneath the attached bacteria and forms horseshoe-like structures on HeLa cells, and this accumulation requires EspA and EspB. In contrast to our results, De Rycke et al. (29) have reported that REPEC O103 B10 strain does not accumulate actin beneath adherent bacteria 4 h after infection. Instead of actin accumulation, B10 induces a cytopathic effect (CPE) by causing vinculin accumulation, and the accumulation of vinculin forms a rod-shaped pattern 48 h after infection. Although human EPEC E2348/69 serotype O127 does not show a CPE, this CPE in B10 strains is dependent on the type III secretion system (29). De Rycke et al. suggested that the lack of EPEC-like actin accumulation is due to the diffuse adherence mediated by an AF/R2 (38, 40). However, REPEC O103 used in this study showed the same diffuse adherence pattern, and the secretion profiles of REPEC O103 and B10 appear similar. These results indicate that O103 strains might differ in causing cytoskeletal rearrangements and that the REPEC O103 strain used in this study may be more similar to the human EPEC strain.
Studies with adult human volunteers indicate that EPEC intimin mutants are decreased for virulence (39). Diarrhea still developed in 4 out of 11 volunteers who ingested the intimin mutant strain, indicating that other virulence factors are also involved in EPEC pathogenesis. In this study, the virulence of EspA and EspB mutants in REPEC O103 was greatly decreased, and growth curves from rabbits infected with both mutant strains were similar to a control group. Furthermore, the infection of REPEC O103, but not EspA and EspB mutant strains, showed histopathological features including blunting of villi, necrosis, and inflammation (Fig. 4). These results clearly indicate that both EspA and EspB proteins are virulence factors that are needed for these histopathological effects.
Using SEM, the initial adherence of both mutant strains was quite similar to that of the wild-type strains (Fig. 5), indicating that other adhesins such as AF/R2 fimbriae may contribute to initial levels of adherence. In contrast, only the wild-type strain showed formation of embedded (Fig. 5 B) and cup-like (Fig. 5 C) structures on the epithelial surface. These adherent characteristics were further analyzed by TEM. We could detect formation of the A/E lesion and this formation only occurred in rabbits infected with the wild-type, but not EspA and EspB mutant strains. Furthermore, pedestal-like structures (using TEM) were only seen in the wild-type strain. These results suggest that formation of the A/E lesion followed by pedestal-like structures required EspA and EspB. This is the first study suggesting that EspA and EspB are needed for formation of A/E lesions and pedestal-like structures in an in vivo natural infection model and that these processes are needed for disease.
CLSM studies showed that extensive cytoskeletal rearrangements beneath the attached bacteria only occurred when rabbits were infected with wild-type, but not EspA and EspB mutant, strains, indicating that EspA and EspB trigger cytoskeletal rearrangements in vivo (Fig. 7) similar to that seen in vitro. Immunostained thick sections with phalloidin-TxR revealed that heavy cytoskeletal rearrangements were composed of filamentous actin and were observed as cup-like structures beneath attached bacteria. Collectively, these results suggest that EspA and EspB are required for cytoskeletal actin rearrangements, and A/E lesions followed by pedestal-like structures are formed by actin reorganization. Therefore, continued cytoskeletal rearrangements might contribute to disease in infected animals, probably by disrupting the brush border.
We confirmed that REPEC O103 does not produce Shiga toxin (STX) I and II using an enzyme-linked assay (data not shown), since some REPEC O103 strains produce STX I or II (27, 41, 42) and this might facilitate their virulence. Thus although RDEC O103 does not encode STX and appears to have no invasive ability, this pathogen still disrupted the epithelial surface, and this was correlated to A/E lesion production.
In conclusion, we showed that EspA and EspB proteins are required for virulence and to trigger formation of the A/E lesion in vivo, which contains cytoskeletal actin rearrangements. The role of the type III secretion system and its secreted components have been extensively studied in vitro using cultured epithelial cells. We have now extended those observations to a natural infection model and confirmed that these events do indeed occur in vivo, and, more importantly, are critical for disease.
Acknowledgments
We are grateful to Dr. Johan E. Peeters for the gift of REPEC O103 85/150 strain. We would like to thank Dr. Agneta Richter-Dahlfors and Dr. Alison M.J. Buchan for helping with the CLSM analysis. We would like to thank Dr. Edgar C. Boedeker for showing us how to do animal experiments.
Abbreviations used in this paper
- A/E
attaching and effacing
- CLSM
confocal laser scanning microscopy
- CPE
cytopathic effect
- EHEC
enterohemorrhagic Escherichia coli
- EPEC
enteropathogenic Escherichia coli
- LB
Luria-Bertani
- LEE
locus of enterocyte effacement
- REPEC
rabbit EPEC
- SEM
scanning electron microscopy
- STX
Shiga toxin
- TEM
transmission electron microscopy
- Tir
translocated intimin receptor
- TxR
Texas red
Footnotes
A. Abe was supported by a fellowship from The Naito Foundation. U. Heczko was supported by an Erwin-Schrödinger-Fellowship (No. Jo1417-MOB) of the Fonds zur Förderung der wissenschaftlichen Forschung. This work was supported by operating grants to B.B. Finlay from the Medical Research Council of Canada and ID Vaccine Corporation, and a Howard Hughes International Research Scholar Award, and to R.G. Hegele from the Medical Research Council of Canada/British Columbia Lung Association Scholarship Award.
A. Abe and U. Heczko contributed equally to this paper.
References
- 1.Donnenberg MS, Kaper JB. Enteropathogenic Escherichia coli. . Infect Immun. 1992;60:3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coliin pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis DH, Collins JE, Duimstra JR. Infection of gnotobiotic pigs with an Escherichia coliO157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun. 1986;51:953–956. doi: 10.1128/iai.51.3.953-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzipori S, Wachsmuth KI, Smithers J, Jackson C. Studies in gnotobiotic piglets on non-0157:H7 Escherichia coliserotypes isolated from patients with hemorrhagic colitis. Gastroenterology. 1988;94:590–597. doi: 10.1016/0016-5085(88)90228-4. [DOI] [PubMed] [Google Scholar]
- 5.Tzipori S, Karch H, Wachsmuth KI, Robins-Browne RM, O'Brien AD, Lior H, Cohen ML, Smithers J, Levine MM. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coliO157:H7 in gnotobiotic piglets. Infect Immun. 1987;55:3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agin TS, Cantey JR, Boedeker EC, Wolf MK. Characterization of the eaeA gene from rabbit enteropathogenic Escherichia coli strain RDEC-1 and comparison to other eaeAgenes from bacteria that cause attaching-effacing lesions. FEMS Microbiol Lett. 1996;144:249–258. doi: 10.1111/j.1574-6968.1996.tb08538.x. [DOI] [PubMed] [Google Scholar]
- 7.Abe A, Kenny B, Stein M, Finlay BB. Characterization of two virulence proteins secreted by rabbit enteropathogenic Escherichia coli, EspA and EspB, whose maximal expression is sensitive to host body temperature. Infect Immun. 1997;65:3547–3555. doi: 10.1128/iai.65.9.3547-3555.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlay BB, Rosenshine I, Donnenberg MS, Kaper JB. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coliadherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenshine I, Donnenberg MS, Kaper JB, Finlay BB. Signal transduction between enteropathogenic Escherichia coli(EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO (Eur Mol Biol Organ) J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenny B, Devinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. Enteropathogenic E. coli(EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 11.Vuopio-Varkila J, Schoolnik GK. Localized adherence by enteropathogenic Escherichia coliis an inducible phenotype associated with the expression of new outer membrane proteins. J Exp Med. 1991;174:1167–1177. doi: 10.1084/jem.174.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnenberg MS, Giron JA, Nataro JP, Kaper JB. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coliassociated with localized adherence. Mol Microbiol. 1992;6:3427–3437. doi: 10.1111/j.1365-2958.1992.tb02210.x. [DOI] [PubMed] [Google Scholar]
- 13.Giron JA, Ho AS, Schoolnik GK. Characterization of fimbriae produced by enteropathogenic Escherichia coli. . J Bacteriol. 1993;175:7391–7403. doi: 10.1128/jb.175.22.7391-7403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohel I, Puente JL, Murray WJ, Vuopio-Varkila J, Schoolnik GK. Cloning and characterization of the bundle-forming pilin gene of enteropathogenic Escherichia coli and its distribution in Salmonellaserotypes. Mol Microbiol. 1993;7:563–575. doi: 10.1111/j.1365-2958.1993.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 15.Stone KD, Zhang HZ, Carlson LK, Donnenberg MS. A cluster of fourteen genes from enteropathogenic Escherichia coliis sufficient for the biogenesis of a type IV pilus. Mol Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 16.Rosenshine I, Ruschkowski S, Stein M, Reinscheid DJ, Mills SD, Finlay BB. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO (Eur Mol Biol Organ) J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny B, Finlay BB. Intimin-dependent binding of enteropathogenic Escherichia colito host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase c-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis KG, Giron JA, Jerse AE, McDaniel TK, Donnenberg MS, Kaper JB. Enteropathogenic Escherichia colicontains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerse AE, Yu J, Tall BD, Kaper JB. A genetic locus of enteropathogenic Escherichia colinecessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenny B, Lai LC, Finlay BB, Donnenberg MS. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 22.Donnenberg MS, Yu J, Kaper JB. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia colito epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai LC, Wainwright LA, Stone KD, Donnenberg MS. A third secreted protein that is encoded by the enteropathogenic Escherichia colipathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun. 1997;65:2211–2217. doi: 10.1128/iai.65.6.2211-2217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peeters JE, Geeroms R, Orskov F. Biotype, serotype, and pathogenicity of attaching and effacing enteropathogenic Escherichia colistrains isolated from diarrheic commercial rabbits. Infect Immun. 1988;56:1442–1448. doi: 10.1128/iai.56.6.1442-1448.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camguilhem R, Milon A. Biotypes and O serogroups of Escherichia coliinvolved in intestinal infections of weaned rabbits: clues to diagnosis of pathogenic strains. J Clin Microbiol. 1989;27:743–747. doi: 10.1128/jcm.27.4.743-747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milon A, Esslinger J, Camguilhem R. Adhesion of Escherichia colistrains isolated from diarrheic weaned rabbits to intestinal villi and HeLa cells. Infect Immun. 1990;58:2690–2695. doi: 10.1128/iai.58.8.2690-2695.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall GA, Dorn CR, Chanter N, Scotland SM, Smith HR, Rowe B. Attaching and effacing lesions in vivo and adhesion to tissue culture cells of Vero-cytotoxin-producing Escherichia colibelonging to serogroups O5 and O103. J Gen Microbiol. 1990;136:779–786. doi: 10.1099/00221287-136-4-779. [DOI] [PubMed] [Google Scholar]
- 28.Licois D, Reynaud A, Federighi M, Gaillard-Martinie B, Guillot JF, Joly B. Scanning and transmission electron microscopic study of adherence of Escherichia coliO103 enteropathogenic and/or enterohemorrhagic strain GV in enteric infection in rabbits. Infect Immun. 1991;59:3796–3800. doi: 10.1128/iai.59.10.3796-3800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Rycke J, Comtet E, Chalareng C, Boury M, Tasca C, Milon A. Enteropathogenic Escherichia coliO103 from rabbit elicits actin stress fibers and focal adhesions in HeLa epithelial cells, cytopathic effects that are linked to an analog of the locus of enterocyte effacement. Infect Immun. 1997;65:2555–2563. doi: 10.1128/iai.65.7.2555-2563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donnenberg MS, Kaper JB. Construction of an eae deletion mutant of enteropathogenic Escherichia coliby using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/ Technology. 1983;1:784–791. [Google Scholar]
- 32.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–585. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Finlay BB, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying Salmonellaentry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 34.Richter-Dahlfors A, Buchan A, Finlay BB. Murine salmonellosis studied by confocal microscopy: Salmonella typhimuriumresides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein M, Kenny B, Stein MA, Finlay BB. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coliwhich is homologous to members of the immunoglobulin a protease-like family of secreted proteins. J Bacteriol. 1996;178:6546–6554. doi: 10.1128/jb.178.22.6546-6554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenny B, Finlay BB. Protein secretion by enteropathogenic Escherichia coliis essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foubister V, Rosenshine I, Donnenberg MS, Finlay BB. The eaeB gene of enteropathogenic Escherichia coliis necessary for signal transduction in epithelial cells. Infect Immun. 1994;62:3038–3040. doi: 10.1128/iai.62.7.3038-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiederling F, Boury M, Petit C, Milon A. Adhesive factor/rabbit 2, a new fimbrial adhesin and a virulence factor from Escherichia coliO103, a serogroup enteropathogenic for rabbits. Infect Immun. 1997;65:847–851. doi: 10.1128/iai.65.2.847-851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donnenberg MS, Tacket CO, James SP, Losonsky G, Nataro JP, Wasserman SS, Kaper JB, Levine MM. Role of the eaeA gene in experimental enteropathogenic Escherichia coliinfection. J Clin Invest. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pillien F, Chalareng C, Boury M, Tasca C, de Rycke J, Milon A. Role of adhesive factor/rabbit 2 in experimental enteropathogenic Escherichia coliO103 diarrhea of weaned rabbit. Vet Microbiol. 1996;50:105–115. doi: 10.1016/0378-1135(96)00012-0. [DOI] [PubMed] [Google Scholar]
- 41.Ritchie M, Partington S, Jessop J, Kelly MT. Comparison of a direct fecal Shiga-like toxin assay and sorbitol-MacConkey agar culture for laboratory diagnosis of enterohemorrhagic Escherichia coliinfection. J Clin Microbiol. 1992;30:461–464. doi: 10.1128/jcm.30.2.461-464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarr PI, Fouser LS, Stapleton AE, Wilson RA, Kim HH, Vary J, Jr, Clausen CR. Hemolytic-uremic syndrome in a six-year-old girl after a urinary tract infection with Shiga-toxin-producing Escherichia coliO103:H2. N Engl J Med. 1996;335:635–638. doi: 10.1056/NEJM199608293350905. [DOI] [PubMed] [Google Scholar]
- 43.Cantey JR, Blake RK. Diarrhea due to Escherichia coliin the rabbit: a novel mechanism. J Infect Dis. 1977;135:454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- 44.Levine MM, Bergquist EJ, Nalin DR, Waterman DH, Hornick RB, Young CR, Sotman S. Escherichia colistrains that cause diarrhea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;1:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]