Abstract
We analyzed the immune responses evoked by a series of overlapping peptides to better understand the molecular basis for respiratory syncytial virus (RSV) G protein–induced eosinophilia in BALB/c mice. In vitro stimulation of spleen cells from natural G protein–primed mice showed dominant proliferative and cytokine (interferon [IFN]-γ and interleukin [IL]-5) responses to a peptide encompassing amino acids 184–198. Mice vaccinated with peptide 184– 198 conjugated to keyhole limpet hemocyanin showed significant pulmonary eosinophilia (39.5%) after challenge with live RSV. In contrast, mice immunized with a peptide (208–222) conjugate associated with induction of IFN-γ secreting spleen cells did not exhibit pulmonary eosinophilia after challenge. The in vivo depletion of CD4+ cells abrogated pulmonary eosinophilia in mice vaccinated with the peptide 184–198 conjugate, whereas the depletion of CD8+ cells had a negligible effect. Therefore, we have identified an association between peptide 184– 198 of natural G protein and the CD4+ T cell–mediated induction of pulmonary eosinophilia after live RSV challenge. Out of 43 human donors, 6 provided peripheral blood mononuclear cells that showed reactivity to G protein from RSV A2, 3 of which responded to peptide 184– 198. The results have important implications for the development of a vaccine against RSV.
Keywords: respiratory syncytial virus, G protein, eosinophilia, T helper cell, peptide
The continued interest in the attachment (G) glycoprotein as a component of any subunit vaccine strategy is predicated by its ability to induce and augment protective immune responses to respiratory syncytial virus (RSV) infection (1–3). However, a correlation between immunization with purified natural G protein or recombinant vaccinia constructs and priming for atypical pulmonary eosinophilia was observed in the BALB/c mouse model of RSV disease (1, 4). This phenomenon was reminiscent of the enhanced disease observed in human recipients of formalin-inactivated RSV (FI-RSV) upon subsequent infection with live RSV (5). Both FI-RSV and natural G protein have been shown to induce Th2 immune responses, whereas live attenuated viral vaccines are associated with Th1 responses (1, 6–10). Accordingly, the depletion of IL-5, a cytokine associated with Th2 immune responses, significantly reduced eosinophilia in bronchoalveolar lavages (BAL) of mice that had been vaccinated with G protein before challenge with live RSV (1). The response was shown to be T cell mediated by transfer of G protein–specific CD4+ T cell lines into naive recipient mice, resulting in atypical pulmonary inflammatory responses after challenge (4, 10). The gene encoding RSV G protein contains alternative initiation codons resulting in both full-length and truncated forms of the protein, the latter of which is secreted (11). Recent evidence suggests that the secreted form of G protein is involved in immunopathology and pulmonary eosinophilia (12). Therefore, the goal of this study was to dissect the molecular mechanisms underlying the immunopathology of natural G protein.
Materials and Methods
Mice.
Female BALB/c mice (aged 7–9 wk) were purchased from Taconic Farms, Inc. (Germantown, NY). All mice were housed in a facility approved by the American Association for Accreditation of Laboratory Animal Care.
Preparation and Use of Protein Antigens.
RSV G protein was immunoaffinity purified (1) from the A2 strain of RSV grown in Vero cells (ATCC CCL 81 [American Type Culture Collection, Rockville, MD]). Intramuscular immunizations were performed at 0 and 4 wk with 0.1 ml of PBS containing 1 μg of purified G protein and 20 μg QS-21 (Aquila Biopharmaceuticals, Inc., Worcester, MA) as adjuvant. For RSV vaccinations and challenges, 1–2 × 106 PFU of infectious RSV A2 was clarified from HEp-2 cells (ATCC CCL 23) by low speed centrifugation and administered intranasally in a 50 μl vol.
Preparation and Use of Peptide Antigens.
A series of overlapping peptides was synthesized by Genosys Biotechnologies, Inc. (The Woodlands, TX). The peptides were synthesized as 15-mers to overlap by seven amino acids. The resultant series encompassed the secreted form (amino acids 48–294) of G protein (9, 11). In addition, peptide 17 was synthesized as a 17-mer to span amino acids 171–187. The purity of the peptides was determined by mass spectometry to be >90%.
Peptides 19 and 22 were conjugated to maleimide-activated (13) keyhole limpet hemocyanin (KLH) using an Imject® activated conjugation kit (Pierce Chemical Co., Rockford, IL). Since the mechanism of conjugation was dependent upon a chemical reaction between maleimide groups in KLH and sulfhydryl groups in the peptide, a cysteine was added to the 3′ end of peptide 22 (208–222). The degree to which the peptides were conjugated was determined by loss of thiol groups in the peptide using Ellman's reagent (Pierce Chemical Co.). The extent of conjugation (typically 50–80 μg peptide per mg of KLH) compared favorably with that previously seen for the attachment of peptides to KLH (14). For study of the induction of eosinophilia, 250 μg of each peptide–KLH conjugate was adjuvanted with 20 μg QS-21 in 0.1 ml of PBS and injected intramuscularly at 0 and 4 wk. The mice were challenged 2 wk after secondary vaccination. BAL cells were isolated 7 d later and stained as described previously (1). The proportion of eosinophils was enumerated by analyzing a minimum of 400 leukocytes per slide. The results are expressed as mean percentage of eosinophils (± 1 SD).
In Vivo Depletion of T Cell Subsets.
mAbs to murine CD4, GK1.5 (ATCC TIB 207), and murine CD8, 53-6.72 (ATCC TIB 105), were purified from hybridoma culture supernatants over a recombinant protein G column (Amersham Pharmacia Biotech, Piscataway, NJ). As control, purified rat IgG was purchased from Calbiochem (San Diego, CA). mAbs were administered at 14 and 20 d after final immunization in doses of 750 and 250 μg per mouse, respectively. The effectiveness of the depletion regime was monitored on a FACScan® (Becton Dickinson, Mountain View, CA) using PE-conjugated anti–mouse CD4 (L3T4) and FITC-conjugated anti–mouse CD8 (Ly-2) (PharMingen, San Diego, CA).
In Vitro Expansion of Immunocytes and Cytokine Analyses.
Spleens were isolated from groups of five mice 2 wk after secondary vaccination with G/QS-21 and were converted to single cell suspensions as previously described (1). As controls, purified G protein, diphtheria toxin cross-reactive protein (CRM197), and Con A were added at final concentrations of 2.5 and 0.5 μg/ml, 10 μg/ml, and 1 μg/ml, respectively. After 3 d in culture, supernatants were pooled from triplicate wells and assayed for IFN-γ and IL-5 by antigen-capture ELISA (1, 15). Data are presented in picograms per milliliter for IL-5 and units per milliliter for IFN-γ, where 1 U of IFN-γ bioactivity is defined as the reciprocal of the endpoint dilution that protects 50% of mouse L cells from viral destruction (16). The remaining cultures were pulsed with 1 μCi of [3H]thymidine and subsequently harvested to quantify [3H]thymidine incorporation into DNA.
Heparinized human peripheral blood was collected from normal adult donors and separated using Ficoll-Hypaque (Amersham Pharmacia Biotech) centrifugation. Cells were cultured with peptides in RPMI 1640 medium containing 10% AB− serum (Biocell, Rancho Dominguez, CA) at a concentration of 50 μg/ml. As controls, cells were cultured with CRM 197 (30 μg/ml), PHA (5 μg/ml), or medium alone.
Statistical Analyses.
Significant differences between groups were determined by the Tukey-Kramer honestly significant difference multiple comparisons test using JMP® statistical discovery software (SAS Institute Inc., Cary, NC).
Results
Lymphoproliferative and Cytokine Responses of Natural G Protein Primed Spleen Cells Stimulated with Synthetic Peptides.
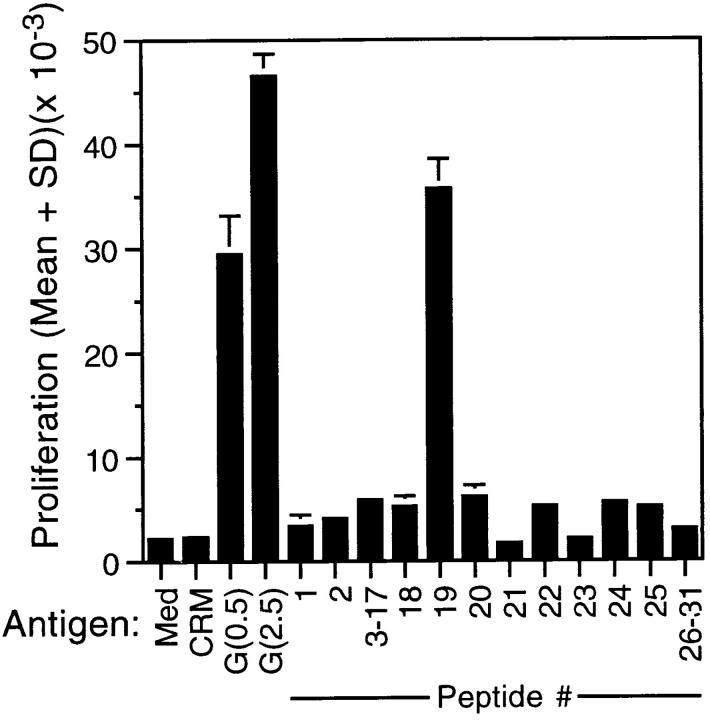
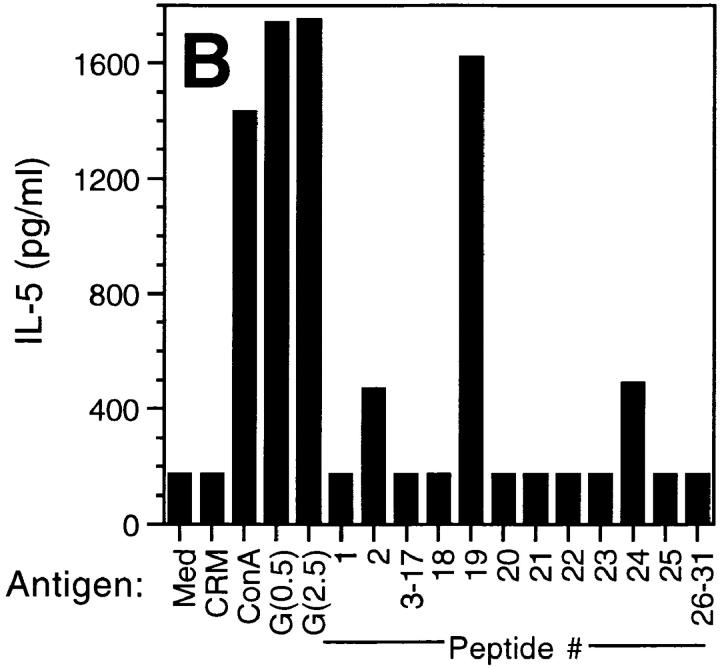
To localize T cell epitopes, spleen cells from G protein–vaccinated mice were stimulated with a series of 15-mers encompassing the secreted form of RSV G protein (11). The data (Fig. 1) demonstrated that maximal proliferation was obtained after stimulation with peptide 19 (amino acids 184–198). Proliferation was 16-fold above background levels and far exceeded that attained by other G protein–derived peptides. In addition, the magnitude of the response to peptide 19 (3.6 × 104 cpm) was comparable to that attained by purified natural G protein, which yielded mean proliferation levels of 3 and 4.7 × 104 cpm for concentrations of 0.5 and 2.5 μg/ml, respectively. The proliferative data for peptides 3–17 and 26–31 were at or near background levels. The highest levels of IFN-γ and IL-5 were also observed after stimulation with peptide 19 (Fig. 2). Moreover, the levels were equivalent to or greater than those obtained after restimulation with natural G protein. Several other peptides (20, 22, 24, and 25) appeared to induce lower but noteworthy levels of IFN-γ secretion in the absence of demonstrable IL-5 production (Fig. 2). IL-5 was also observed at lower levels after stimulation with peptides 2 and 24. Both IFN-γ and IL-5 were observed in cultures that were stimulated with purified natural G protein and Con A. Collectively, the data suggest that a region spanning amino acids 184–198 contains the dominant epitope(s) in natural G protein recognized by T cells in BALB/c mice.
Figure 1.
Proliferative responses of BALB/c splenocytes to peptides of RSV G protein. BALB/c mice were vaccinated at 0 and 4 wk with 1 μg G protein adjuvanted with QS-21 (20 μg per dose). 2 wk after secondary vaccination, splenocytes were isolated, pooled, and cultured for 4 d with synthetic peptides (50 μg/ml), natural G protein (0.5 [G(0.5)] or 2.5 [G(2.5)] μg/ml), Con A, CRM197 (CRM), or medium alone (Med). Con A stimulation of splenocytes resulted in mean cpm of 94,746 ± 8,005. Data are presented as the mean cpm (± 1 SD) of triplicate wells. The experiment is representative of five independent experiments with qualitatively similar results.
Figure 2.
The secretion of IFN-γ and IL-5 after stimulation with peptides of RSV G protein. Pooled supernatants from triplicate wells (Fig. 1) were collected after 3 d of stimulation. For IFN-γ (A), the lower limit of detection was 0.84 U/ml. For IL-5 (B), the lower limit of detection was 173 pg/ml.
Peptide 19 Conjugated to KLH Primes for Pulmonary Eosinophilia.
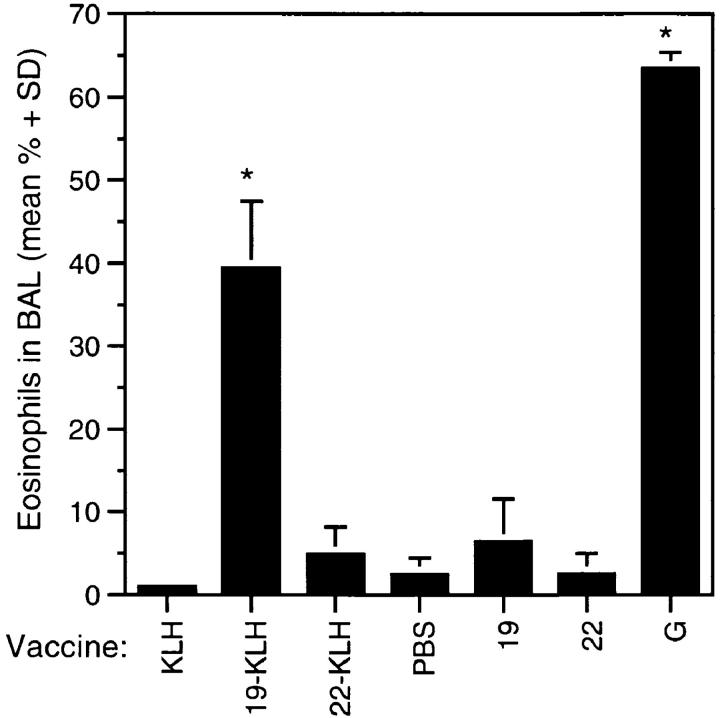
To affirm a direct role in priming for pulmonary eosinophilia, peptide 19 was compared with peptide 22, which stimulated IFN-γ production in the absence of IL-5 (Fig. 2). To ensure sufficient immunogenicity, the peptides were conjugated to maleimide-activated KLH. Statistically significant pulmonary eosinophilia was observed in mice primed with either peptide 19–KLH (39.5 ± 8.0%) or G protein (63 ± 1.9%) when compared with mice vaccinated with adjuvant alone (2.5 ± 2.0%) (Fig. 3). In contrast, the level of eosinophilia associated with peptide 22–KLH (4.9 ± 3.3%) remained at background levels. It was noteworthy that peptide 22 was immunogenic. 2 wk after final vaccination, the geometric mean endpoint anti-RSV G protein IgG antibody titers of mice vaccinated with peptide 19– KLH or peptide 22–KLH were 1517 and 5611, respectively. Thus, although humoral immune responses were generated to each of the peptide conjugates, the induction of aberrant eosinophilia was limited to those mice that received natural G protein or peptide 19–KLH.
Figure 3.
Peptide 19 conjugated to KLH primes for pulmonary eosinophilia. BALB/c mice (five mice per group) were vaccinated intramuscularly at 0 and 4 wk. The vaccines consisted of 250 μg of KLH (KLH); 250 μg KLH containing either 13 μg peptide 19 (19-KLH) or 11 μg peptide 22 (22-KLH); 250 μg of unconjugated peptides 19 or 22 (19 and 22, respectively), 1 μg natural G protein, or PBS alone. Each vaccine was adjuvanted with QS-21 (20 μg/dose). 2 wk after secondary vaccination, mice were challenged with RSV. Data are presented as the mean percent (± 1 SD) eosinophils in BAL fluids 7 d after challenge. Asterisk denotes significant differences for G protein or 19–KLH–vaccinated mice compared to control mice injected with either PBS or KLH. The data are representative of three experiments.
In Vivo Depletion of T Cell Subsets and the Induction of Eosinophilia.
To determine whether peptide 19–KLH–associated eosinophilia was mediated by CD4+ cells, a series of depletion experiments were performed using mAbs to CD4 or CD8 surface molecules. The depletion of CD4+ cells resulted in a significant reduction in pulmonary eosinophilia in mice vaccinated with either G/QS-21 or peptide 19–KLH/QS-21 (Table 1). Specifically, treatment with anti-CD4 mAb significantly reduced pulmonary eosinophilia from 67.2 ± 8.5% and 29.6 ± 13.3% to 8.1 ± 4.7% and 0.75 ± 0.6%, respectively. The corresponding effect of anti-CD8 mAb treatment had minimal impact. After challenge, eosinophilia persisted at 63.8 ± 6.4 and 32.8 ± 10.3% for G- and peptide 19–KLH–vaccinated mice, respectively. The data demonstrate that CD4+ cells are required for the pulmonary eosinophilic response in natural G protein– or peptide 19–KLH–immunized mice.
Table 1.
CD4 T Cells Mediate the Eosinophilic Response Induced by RSV G Protein and Peptide 19–KLH
| Vaccine Antigen | Antibody treatment | Percentage of CD4+ cells | Percentage of CD8+ cells | Percentage of BAL eosinophils | ||||
|---|---|---|---|---|---|---|---|---|
| G protein | rat Ig | 21.2 | 8.5 | 67.2 ± 8.5 | ||||
| G protein | anti-CD4 | 1.5 | 15.0 | 8.1 ± 4.7** | ||||
| G protein | anti-CD8 | 24.4 | 2.7 | 63.8 ± 6.4 | ||||
| Peptide 19–KLH | rat Ig | 19.0 | 7.7 | 29.6 ± 13.3 | ||||
| Peptide 19–KLH | anti-CD4 | 0.3 | 20.0 | 0.75 ± 0.6** | ||||
| Peptide 19–KLH | anti-CD8 | 27.4 | 2.8 | 32.8 ± 10.3 | ||||
| RSV | none | 25.8 | 11.2 | 0.7 ± 1.0 |
BALB/c mice (five mice per group) were vaccinated intramuscularly at 0 and 4 wk with natural G protein (1 μg) or 250 μg KLH containing 18 μg peptide 19, or intranasally with RSV (106 PFU). The indicated antibodies were administered intraperitoneally at 14 and 20 d after immunization. The mice were challenged with RSV the next day and pulmonary eosinophilia was assessed 7 d later. Data are presented as mean percentage eosinophils in BAL (± 1 SD), and percentage of CD4+ and CD8+ cells relative to total splenic lymphocytes. Significant differences (**) are indicated compared to control mice given rat IgG. A second experiment yielded similar results.
Human Peripheral Blood Cell Responses after Stimulation with Synthetic Peptides.
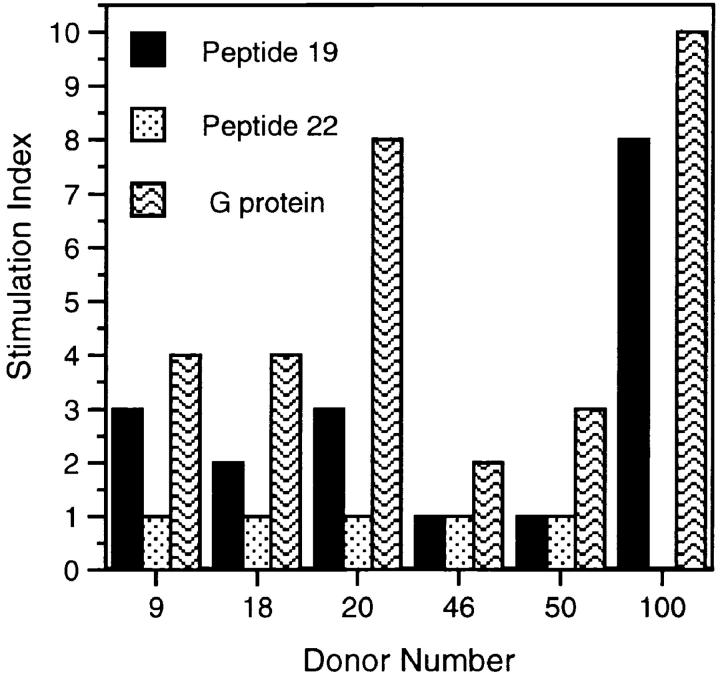
The PBMCs from a panel of donors were tested for reactivity to purified natural G protein from the A2 strain of RSV. Subsequently, the cells from six reactive donors were cultured in the presence of the synthetic peptides (Fig. 4). A strong proliferative response to peptide 19 was observed for donor 100 (mean stimulation index, 8.5). A variety of specific proliferative responses were apparent to peptide 19 in donors 9 and 20 with mean stimulation indices of threefold in each case. In addition to peptide 19, a variety of specific proliferative responses were also seen to other G protein–derived peptides. For example, PBMCs isolated from donor 100 also responded to peptides 2 and 15 with mean stimulation indices of 13.5 and 10.0, respectively. Responses to peptides 4, 9, and 29 were also noteworthy for donor 100, achieving a mean stimulation index of at least fivefold in each case (data not shown).
Figure 4.
Proliferative responses of human PBMCs to peptides of RSV G protein. PBMCs from 6 out of 43 donors with reactivity to G protein from the A2 strain of RSV were cultured in the presence of peptide 19 (50 μg/ml), natural G protein (3 μg/ml), PHA (5 μg/ml), or medium alone. PHA stimulation of PBMCs ranged from 22,945 to 55,619 cpm. Data are presented as the mean SI obtained from triplicate cultures.
Discussion
This study provides the first evidence that a specific amino acid sequence (184–198) is sufficient to mimic purified natural G protein and predispose BALB/c mice for atypical pulmonary eosinophilia after challenge. Collectively, the data show that this peptide (AICKRIPNKKPGKKT) primes for pulmonary eosinophilia by stimulating the expansion of CD4+ T cells destined to secrete IL-5, a cytokine associated with the induction and recruitment of eosinophils (17). We propose a model of immune priming in which one or more Th cell epitopes within peptide 19 control the qualitative nature of subsequent immune responses, resulting in a profound skewing toward the Th2 phenotype. That the peptide component of the TCR– MHC interaction can modulate the quality of the immune response between Th1 and Th2 phenotypes has previously been shown by altering peptide sequences (18, 19). However, it remains possible that the 15 amino acids that comprise peptide 19 contain more than one T cell epitope, each with a discrete ability to stimulate a Th1 versus Th2 response. In favor of this hypothesis, an analysis of the sequence of peptide 19 indicates that it contains three potential T cell epitopes restricted to MHC class II I-Ed that align closely at the critical 1, 4, 6, and 9 anchor residues (I, K or R, I, and K, respectively) (20). Each of these putative sequences are consistent with class II binding based upon the publication of known ligands generated by the biochemical isolation of MHC-associated peptides or by peptide binding assays (20). The mutation of peptide 19 (AICKRIPNKKPGKKT) to one that disrupts the critical MHC-binding anchor regions of two of the potential T cell epitopes (AICGRGPNGKPGKKT) completely abrogated the ability of this peptide to predispose mice for pulmonary eosinophilia (data not shown).
We originally identified peptide 19 as the specific amino acid sequence that stimulated a dominant proliferative response to natural G protein (21). The data presented here provide a positive correlation between the peptide encompassing amino acids 184–198 of G protein and the predisposition for pulmonary eosinophilia. As an extension to our studies, recent experiments showed that the predisposition for pulmonary eosinophilia in BALB/c mice was lost in a vaccinia virus construct containing a frameshift mutation in the G protein gene at amino acids 193–273 (22). The results further demonstrated that the mutated G protein gene was able to protect mice against subsequent virus challenge (22), possibly due to the presence of previously identified protective B cell epitopes that persist in the truncated construct (23). In this regard, it is interesting to speculate that once the dominant T cell epitope(s) contained within peptide 19 is deleted, subdominant T cell epitopes will provide the requisite T cell help necessary for protective immunity in the absence of priming for pulmonary pathology. The murine data presented here identify a number of peptide candidates that stimulate IFN-γ secretion (e.g., peptides 20, 22, 24, and 25) and may function in this capacity. Similarly, our human data suggested a number of peptides (e.g., for donor 100; peptides 2, 4, 9, 15, and 29) that stimulated proliferation of PBMCs. Thus, for seronegative populations, the results argue that a vaccine for RSV should be genetically modified at amino acids 184–198 of G protein. This vaccine would not bias recipients for atypical pulmonary disease, but would retain an ability to protect against subsequent RSV challenge.
Although it is possible for the TCR ligand to effect a Th1 versus Th2 transition, other mechanisms of immune control may be at play. The appearance of both IFN-γ and IL-5 in the supernatants of G protein–primed splenocytes restimulated with peptide 19 may result from the generation of both Th1 and Th2 clones, or Th0 cells (24, 25). The phenomenon may also be explained by the relative absence of CD8+ T cells. CD8+ T cells are an important source of IFN-γ and may modulate CD4+ T cell immune responses (26, 27). Indeed, we have previously shown only a low level of splenic cytotoxic T cell activity in response to G/QS-21 vaccination (1). Thus, the most favorable RSV vaccine strategy for seronegative populations would consist of components that, while not priming for immunopathological sequelae, achieve a balanced immune response mediated by protective CD4+ and CD8+ cells.
In this study, we have shown that vaccination with a specific peptide of RSV G protein is sufficient to bias atypical pulmonary eosinophilia after challenge, although contributions from other amino acid sequences within the secretory form of RSV G protein cannot be ruled out. For example, peptides 2 and 24 stimulated the production of low levels of IL-5 from G protein–primed splenocytes. However, recent studies suggest that peptide 2 is not sufficient to predispose mice for pulmonary eosinophilia (22). The data do imply that peptide 19 contains the antigenic determinants responsible for atypical pulmonary eosinophilia in mice. The proliferative response of human PBMCs suggests that peptide 19 is important in human immune responses to RSV (28).
Footnotes
The authors wish to acknowledge the excellent efforts of Jason Smith in the purification of natural G protein. We also thank Kristen Heers, Natisha LaPierre, Christine Reilly, and Catherine Unczur for technical assistance, and Drs. J.H. Eldridge and P.R. Paradiso for constructive review of the manuscript.
References
- 1.Hancock GE, Speelman DJ, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J Virol. 1996;70:7783–7791. doi: 10.1128/jvi.70.11.7783-7791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 3.Murphy BR, Alling DW, Snyder MH, Walsh EE, Prince GA, Chanock RM, Hemming VG, Rodriguez WJ, Kim HW, Graham BS, Wright PF. Effect of age and preexisiting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol. 1986;24:894–898. doi: 10.1128/jcm.24.5.894-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alwan W, Openshaw P. Distinct patterns of T- and B-cell immunity to respiratory syncytial virus induced by individual viral proteins. Vaccine. 1993;11:431–437. doi: 10.1016/0264-410x(93)90284-5. [DOI] [PubMed] [Google Scholar]
- 5.Kim HW, Canchola JG, Brandt CD, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 6.Connors M, Giese NA, Kulkarni AB, Firestone C-Y, Morse HC, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of IL-4 and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham BS. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 1996;4:290–294. doi: 10.1016/0966-842x(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 8.Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wertz GW, Collins PL, Huang Y, Gruber C, Levine S, Ball LA. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci USA. 1985;82:4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alwan WH, Kozlowska WJ, Openshaw PJM. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts SR, Lichtenstein D, Ball LA, Wertz GW. The membrane-associated and secreted forms of the respiratory syncytial virus attachment glycoprotein G are synthesized from alternative initiation codons. J Virol. 1994;68:4538–4546. doi: 10.1128/jvi.68.7.4538-4546.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BS. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments Interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partis MD, Griffifths DG, Roberts GC, Beechey RB. Cross-linking of protein by ω-maleimido alkanoyl N-hydroxysuccinimido esters. J Prot Chem. 1983;2:263–277. [Google Scholar]
- 14.Tsao J, Lin X, Lackland H, Tous G, Wu Y, Stein S. Internally standardized amino acid analysis for determining peptide/carrier protein coupling ratio. Anal Biochem. 1991;197:137–142. doi: 10.1016/0003-2697(91)90369-5. [DOI] [PubMed] [Google Scholar]
- 15.Curry RC, Kiener PA, Spitalny GL. A sensitive immunochemical assay for biologically active murine IFN-γ. J Immunol Methods. 1987;104:137–142. doi: 10.1016/0022-1759(87)90497-2. [DOI] [PubMed] [Google Scholar]
- 16.Familletti PC, Rubinstein S, Pestka S. A convenient and rapid cytopathic effect inhibition assay for interferon. Methods Enzymol. 1981;78:387–394. doi: 10.1016/0076-6879(81)78146-1. [DOI] [PubMed] [Google Scholar]
- 17.Coffman RL, Seymour WP, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 18.Pfieffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995;181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray JS, Ferrandis-Edwards D, Wolfe CJ, Schountz T. Major histocompatibility complex regulation of T helper functions mapped to a peptide C terminus that controls ligand density. Eur J Immunol. 1994;24:2337–2344. doi: 10.1002/eji.1830241012. [DOI] [PubMed] [Google Scholar]
- 20.Rammensee H-G, Friede T, Stevanovic S. MHC ligand and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 21.Tebbey, P.W., and G.E. Hancock. 1997. Characterization of immune responses to the attachment (G) protein of respiratory syncytial virus in inbred mice. 10th International Conference on Negative Strand Viruses. A152 (Abstr.)
- 22.Sparer TE, Matthews S, Hussell T, Rae AJ, Garcia-Barreno B, Melero JA, Openshaw PJM. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J Exp Med. 1998;187:1921–1926. doi: 10.1084/jem.187.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trudel M, Nadon F, Seguin C, Binz H. Protection of BALB/c mice from respiratory syncytial virus infection by immunization with a synthetic peptide derived from the G glycoprotein. Virology. 1991;185:749–757. doi: 10.1016/0042-6822(91)90546-n. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 25.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 26.Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussell T, Baldwin CJ, O'Garra A, Openshaw PJM. CD8+T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- 28.Welliver JR, Welliver RC. Bronchiolitis. Pediatr Rev. 1993;14:134–139. doi: 10.1542/pir.14-4-134. [DOI] [PubMed] [Google Scholar]