Abstract
The extracellular signal-regulated kinase (ERK), the c-Jun NH2-terminal kinase (JNK), and p38 MAP kinase pathways are triggered upon ligation of the antigen-specific T cell receptor (TCR). During the development of T cells in the thymus, the ERK pathway is required for differentiation of CD4−CD8− into CD4+CD8+ double positive (DP) thymocytes, positive selection of DP cells, and their maturation into CD4+ cells. However, the ERK pathway is not required for negative selection. Here, we show that JNK is activated in DP thymocytes in vivo in response to signals that initiate negative selection. The activation of JNK in these cells appears to be mediated by the MAP kinase kinase MKK7 since high levels of MKK7 and low levels of Sek-1/MKK4 gene expression were detected in thymocytes. Using dominant negative JNK transgenic mice, we show that inhibition of the JNK pathway reduces the in vivo deletion of DP thymocytes. In addition, the increased resistance of DP thymocytes to cell death in these mice produces an accelerated reconstitution of normal thymic populations upon in vivo DP elimination. Together, these data indicate that the JNK pathway contributes to the deletion of DP thymocytes by apoptosis in response to TCR-derived and other thymic environment– mediated signals.
Keywords: T lymphocyte, JNK, thymic development, apoptosis
The development of T cells occurs in the thymus where bone marrow precursor cells differentiate through multiple developmental stages involving maturation, proliferation, selection, and death. Early precursor cells that are initially CD4−CD8− double negative (DN)1 differentiate into CD4+CD8+ double positive (DP) cells. This process is controlled by a series of phenotypic changes, with the expression of the pre-TCR (at the DN stage) and the TCR (at the DP stage) being the most critical. DP thymocytes differentiate into mature single positive (SP) CD4+ and CD8+ thymocytes that are ready to leave the thymus and migrate to the periphery. However, before completing their maturation, DP and immature CD4+ and CD8+ thymocytes undergo positive and negative selection. Recognition of peptides by thymocytes in the context of the self-MHC leads to their rescue from apoptosis and their migration to the periphery (positive selection), while recognition of self-peptides induces death of the autoreactive T cells in the thymus by apoptosis (negative selection) (1–3). It remains unclear whether the signals mediated by the TCR during the selection of thymocytes and the activation of peripheral T cells are quantitatively or qualitatively different. Various models have been proposed to explain how recognition of each peptide-MHC complex results in a different final outcome. Quantitative models propose that the strength of the TCR signal determines whether a peptide causes positive or negative selection depending on the affinity of the TCR–MHC-peptide complexes. High intensity signals cause cell death, whereas low intensity signals rescue thymocytes from apoptosis. On the other hand, qualitative models consider that different signaling pathways control positive and negative selection of the immature thymocytes.
The MAP kinases play a key role in a variety of cellular responses. Several parallel MAP kinase signal transduction pathways have been defined in mammalian cells (4, 5). The extracellular signal-regulated kinase (ERK) family has been associated with the proliferation and differentiation of a number of different cell types, while the c-Jun NH2-terminal kinase (JNK) and the p38 families have been associated with apoptotic death in several systems (4, 5). During activation of T lymphocytes, these pathways are triggered upon ligation of the antigen-specific TCR, although JNK but not ERK activation needs a second signal provided by costimulatory molecules (6). During the development of T cells in the thymus, the Ras/Raf–ERK pathway is required for differentiation of immature thymocytes from the DN to the DP stage (7), positive selection of T cells (8–12), and T cell lineage commitment (13). However, negative selection of autoreactive T cells does not involve the ERK signaling pathway (8). In contrast to JNK, p38 MAP kinase is constitutively activated in DN and DP thymocytes, probably maintained by stimuli derived from the thymic environment (14). The role of the p38 MAP kinase pathway in positive and negative selection remains to be addressed.
The JNK MAP kinase group consists of three different members (JNK1, JNK2, and JNK3) and at least 10 different isoforms which result from alternative splicing of these three genes (15). JNK1 and JNK2 are widely expressed in several tissues, whereas JNK3 is more selectively expressed in brain, testis, and heart. The specific contribution of these JNK isoforms to T cell development remains unknown. In correlation with previous studies indicating a role of the JNK signaling pathway in cell death (5), we have shown recently that disruption of the JNK3 gene results in the abrogation of neuronal apoptosis in response to exocitotoxic stress (16). JNK family members are regulated and activated by MAPK kinases. Two MAPK kinases, MKK4 and MKK7, have been found to be the primary activators of JNK (17–20).
Here we have examined the regulation and function of the JNK pathway in the deletion of DP thymocytes in response to signals that initiate negative selection. TCR ligands activate JNK in vivo, and this process is associated with cell death and DP thymocyte deletion. Using transgenic mice overexpressing a dominant negative mutant of JNK1 (dnJNK1), we demonstrate that inhibition of the JNK signaling pathway results in increased resistance of DP thymocytes to cell death in response to TCR- and thymic microenvironment–mediated signals. Thus, this study indicates that JNK is activated and contributes to the negative selection of DP thymocytes and supports the qualitative signaling model to distinguish positive and negative selection.
Materials and Methods
Generation of dnJNK1 Transgenic Mice.
A cDNA encoding the dominant negative form of JNK1, where Thr183 was replaced by Ala and Tyr185 was replaced by Phe (21), was subcloned downstream of the distal promoter of lck (22). A 2-kb fragment from the human growth hormone (hGH) was used to provide the polyadenylation and intron sequences (23). The DNA fragment containing the distal lck promoter, the dnJNK1 cDNA, and the hGH region was injected in (C57BL/6 × C3H)F2 eggs to generate the dnJNK1 transgenic mice, as previously described (24). Three expression-positive founder lines were established and backcrossed onto B10.BR (The Jackson Laboratory, Bar Harbor, ME) to obtain the progeny for these studies.
Cell Preparation and Staining.
CD4+ T cell populations were isolated from spleen and lymph nodes from wild-type and transgenic mice by negative selection as previously described (25, 26). The DP thymocytes used for the in vivo activation of JNK1 were obtained by staining with a FITC-conjugated anti-CD8 mAb (PharMingen, San Diego, CA) and a PE-conjugated anti-CD4 mAb (PharMingen) and cell sorting using a flow cytometer (EPICS; The Coulter Corp., Miami, FL).
Expression of CD4 and CD8 was analyzed by cell surface staining using a FITC-conjugated anti-CD8 mAb and Red613-conjugated anti-CD4 mAb (GIBCO BRL, Gaithersburg, MD). A biotinylated anti-Vβ3 mAb (PharMingen) was used in combination with PE-conjugated streptavidin to determine the expression of Vβ3 in the different populations by three color staining.
Reverse Transcription-PCR.
Cytoplasmic RNA was obtained from different tissues, as previously described (27). First strand cDNA was synthesized from 5 μg of RNA by using reverse transcriptase (GIBCO BRL) for 1 h at 37°C (50 μl final volume). 1/5 of the cDNA reaction was used for the PCR reaction to amplify the dnJNK1 transcript or the endogenous JNK1; 1/25 of the cDNA was used for detection transcripts of the γ-actin and hypoxanthine-guanine phosphoribosyl transferase (HPRT) housekeeping genes. After 35 cycles, 15 μl of the reaction (50 μl) was analyzed by gel electrophoresis and detected by ethidium bromide staining. Two specific primers for mouse JNK1 were used to detect endogenous JNK1, a 5′ primer (5′ GTGTGCAGCTTATGATGCTATTCTTGAA 3′) and a 3′ primer (5′ TTTGGATAACAAAT-CTCTTGCC 3′). A primer located in human JNK1 (5′ primer, 5′ GCCCTCTCCTTTAGCACAG 3′) and a second primer corresponding to the hGH region used to provide the polyadenylation and intron sequences (23) for the transgene (3′ primer, 5′ CAGAA-CCCCCAGACCTCCCTC 3′) were used to analyze the expression of the dnJNK1 transgene.
JNK1 Activity.
JNK activity in cell lysates was performed by immunoprecipitation of JNK1 kinase and incubation with the substrate glutathione S-transferase (GST)–c-Jun immobilized on GSH-agarose beads, as previously described (21, 26, 28). After 12 h at 4°C, the beads were washed extensively in lysis buffer followed by kinase assay buffer (25 mM Hepes, pH 7.4, 25 mM β-glycerophosphate, 25 mM MgCl2, 0.1 mM sodium orthovanadate, 0.5 mM dithiothreitol) and the activity of the bound JNK was detected by the addition of [γ-32P]ATP for 30 min at 30°C. The reaction products were resolved by SDS-PAGE and the incorporation of [32P]phosphate was quantitated by PhosphorImager analysis (Molecular Dynamics, Sunnyvale, CA).
AP-1 Transcriptional Activity.
AP-1 transcriptional activity was determined by analysis of the luciferase activity in the cell extracts from AP-1–luciferase reporter transgenic mice (25) using the Luciferase Assay Kit (Promega Corp., Madison, WI).
Reagents.
PMA (Sigma Chemical Co., St. Louis, MO), ionomycin (Sigma Chemical Co.), Con A (Boehringer Mannheim, Mannheim, Germany), anti-CD3 mAb (145-2C11), synthetic moth cytochrome c (Cyt c) peptide (Peptide Synthesis Facility, Yale University, New Haven, CT), and Staphylococcus enterotoxin B (Sigma Chemical Co.) were obtained from the suppliers indicated; GST–c-Jun (residues 1–79) was described previously (21).
Western Blot Analysis.
MKK4 and JNK protein levels were assayed by immunoblot analysis using anti–human MKK4 and anti–human JNK mAb (PharMingen). Immune complexes were detected by enhanced chemiluminescence as instructed by the manufacturer (Amersham International, Little Chalfont, UK).
RNA Extraction and Northern Assay.
Total RNA from brain or thymus was extracted using the Ultraspec RNA isolation system (Biotex Laboratories, Inc., Houston, TX) as recommended by the manufacturer. Total RNA (8 μg) was analyzed by Northern blot as previously described (29). A 500-bp EcoRV MKK4 cDNA fragment and a 1.3-kb EcoRI-XbaI MKK7 cDNA fragment were used as specific probes labeled by random primer using the Random Primer Kit (Stratagene Inc., La Jolla, CA) using [α-32P]dCTP.
Results
Regulation of JNK Activity in CD4+CD8+ Thymocytes.
The ERK MAP kinase pathway is required for positive selection, but not for negative selection in the thymus (8– 11). JNK, another member of the MAP kinase family, has been reported to be activated in total thymocytes in response to TCR-independent stimulus (6). However, the regulation of JNK activity by TCR-mediated signals in immature CD4+CD8+ (DP) has not yet been examined. Since activation of JNK has also been associated with cell death in other cell types (4, 5), we examined whether the JNK pathway could also be involved in the deletion of DP cells during negative selection in the thymus. We examined the regulation of JNK in this population upon ligation of the TCR. The cross-linking of the TCR by an immobilized anti-CD3 mAb did not upregulate JNK activity in isolated DP thymocytes (Fig. 1 A). Previous studies indicate that the activation of JNK in the Jurkat T cell line appears to require signals mediated by both the TCR and costimulatory molecules such as the CD28 (6). Therefore, we also examined the regulation of JNK in the presence of costimulatory signals. However, stimulation with anti-CD3 mAb in combination with anti-CD28 mAb did not cause a significant increase of JNK activity in DP thymocytes (Fig. 1 A). These results suggested that TCR-mediated JNK activation may require costimulatory signals other than those mediated by CD28 in DP thymocytes.
Figure 1.
Regulation of JNK activation in DP thymocytes. (A) JNK activity in stimulated DP thymocytes in vitro. DP thymocytes were obtained by staining of total thymocytes with an anti-CD4 and anti-CD8 mAb and cell sorting (FACS®). Purified DP thymocytes (5 × 105 cells) were incubated in the presence of medium (−), immobilized anti-CD3 mAb (10 μg/ml) (anti-CD3), or immobilized anti-CD3 (10 μg/ml) plus anti-CD28 (10 μg/ml) mAbs (anti-CD3/CD28) for 3 h. JNK1 was immunopurified from DP extracts and assayed for kinase activity in vitro using the GST-cJun as substrate. The radioactivity incorporated into GST–c-Jun was quantitated after SDS-PAGE by PhosphorImager analysis. (B) Activation of JNK1 upon in vivo anti-CD3 mAb injection. Wild-type mice were injected with PBS (−) or anti-CD3 mAb (2C11, 50 μg/200 μl PBS) for 6 or 12 h. Total thymocytes were stained for CD4 and CD8, and DP thymocytes (4 × 105 cells) were isolated by cell sorting. JNK1 was immunopurified from DP extracts and assayed for kinase activity, as described in A. (C) Activation of JNK1 upon Con A stimulation in vitro. DP thymocytes (5 × 105 cells) were obtained as described in A and treated with medium alone (−) or Con A (2.5 μg/ml) for 2 or 4 h. JNK1 activity was determined as described in A.
To examine the regulation of JNK in DP thymocytes upon ligation of the TCR in the context of other thymic environmental signals in vivo, we examined JNK activity induced by injection with anti-CD3 mAb. JNK activity was significantly increased in DP thymocytes isolated from mice injected with anti-CD3 mAb (Fig. 1 B) before the deletion of these cells. These results indicate that activation of the JNK pathway can be triggered in DP thymocytes by TCR ligation in combination with costimulatory signals other than CD28 that are provided by the thymic environment.
The additional thymic environmental signals that are required for TCR-mediated JNK activation could be provided by soluble factors or cell surface molecules. To address this question further, we examined the regulation of JNK activity in DP thymocytes upon stimulation with Con A in vitro. It is known that Con A activates mature T cells by ligation of the TCR, but also activates other, additional molecules (30). Purified DP thymocytes were stimulated with Con A alone. Interestingly, in contrast to the combination of anti-CD3 and anti-CD28 mAbs, Con A significantly upregulated JNK activity in DP thymocytes (Fig. 1 C). These results supported the hypothesis that activation of JNK by TCR ligation requires signals mediated by costimulatory molecules different from CD28 molecule in DP thymocytes.
It is well accepted that negative selection of DP thymocytes also requires costimulation in addition to TCR-mediated signals (31–33). However, the nature of the costimulatory signal provided by the thymic environment and the role of CD28 as a costimulator in the thymus remain unclear. In correlation with previous studies (34), we found that in vitro treatment of total thymocytes with immobilized anti-CD3 and anti-CD28 mAbs did not cause significant elimination of the DP cells, but resulted in reduced expression of CD4 and CD8 on the surface of DP thymocytes (Fig. 2 A). The downmodulation of CD4 and CD8 molecules has been associated with the induction of apoptosis in thymocytes (35). No substantial reduction of the number of total thymocytes was observed upon treatment with anti-CD3/anti-CD28 mAbs compared with untreated thymocytes (data not shown). The deletion of the DP population was not increased even after extended periods of activation (data not shown). In contrast, the in vivo administration of anti-CD3 mAb or specific antigen causes deletion of the majority of the DP thymocytes by inducing apoptotic cell death through a mechanism that mimics negative selection (36–40). In correlation with these studies, a pronounced deletion of the DP thymocyte population was detected 48 h after treatment with anti-CD3 mAb in vivo (Fig. 2 A). These results support the hypothesis that the elimination of immature DP thymocytes requires signals additional to the TCR provided by the thymic environment. Unlike anti-CD3 plus anti-CD28, stimulation with Con A in vitro also caused a rapid and dramatic thymocyte cell death. A massive loss of the number of DP thymocytes was observed (data not shown), and the percentage of DP cells in the live population was also reduced (Fig. 2 B) upon Con A treatment in vitro. Thus, the signal requirement for deletion of DP thymocytes correlates with the signals needed for TCR-mediated JNK activation.
Figure 2.
TCR-mediated deletion of DP thymocytes in vivo and in vitro. (A) Total thymocytes from untreated mice were incubated for 24 h in the presence of medium alone (Control) or immobilized anti-CD3 (10 μg/ml) plus anti-CD28 (10 μg/ml) mAbs (In vitro anti-CD3/anti-CD28); total thymocytes were isolated from mice injected with anti-CD3 mAb (2C11, 50 μg/200 μl PBS) for 2 d (In vivo anti-CD3). The cells were stained with anti-CD4 and anti-CD8 mAbs and analyzed by flow cytometry. Profiles for CD4 and CD8 expression in gated live cells are presented. Numbers represent the percentage of each population (DP, CD4+, and CD8+). (B) Total thymocytes were incubated for 24 h in the presence of medium alone (Control) or Con A (2.5 μg/ml) (ConA). The cells were then examined as described in A.
JNK activity is regulated by phosphorylation by an upstream MAPK kinase. Two different MAPK kinases have been identified that phosphorylate and activate JNK, MKK4/Sek1 and MKK7 (17–20). The specific contribution of these two protein kinases to the control of JNK activity in different cell types remains unclear. In addition, the relative expression of the two JNK activators in the murine thymus has not yet been addressed. Thus, we examined the expression of both the MKK4 and MKK7 genes in total thymocytes. High levels of MKK7 mRNA were detected in the thymus (Fig. 3 A), similar to those detected in the brain. Interestingly, although MKK4 was expressed in the brain as previously described (17, 18, 20), no significant level of MKK4 gene expression was detected in thymocytes (Fig. 3 A). To investigate further the lack of expression of MKK4 in the thymus, we examined MKK4 protein expression by Western blotting. High levels of MKK4 protein were observed in the brain, but almost undetectable levels of MKK4 were found in the thymus (Fig. 3 B). We have also examined the expression of JNK protein in these tissues and found similar expression of JNK1 and JNK2 proteins in the brain and thymus. Since DP thymocytes constitute the most abundant population (80– 85%) in the thymus, these results suggested that MKK7, not MKK4, is the primary regulator of JNK in DP thymocytes.
Figure 3.
MKK4 and MKK7 expression in thymocytes. (A) MKK4 and MKK7 gene expression. Total RNA was isolated from the thymus or brain from wild-type mice and analyzed by Northern blot as described in Materials and Methods, using specific 32P-labeled cDNA probes for MKK7, MKK4, and HPRT genes. (B) MKK4 and JNK protein expression. Whole extracts were obtained from the thymus and brain from wild-type mice and analyzed by Western blot as described in Materials and Methods using an anti-MKK4 specific mAb or anti-JNK polyclonal antibody.
Generation of Dominant Negative JNK Transgenic Mice.
Activation of JNK in vivo (6–12 h; Fig. 1 B) appears to precede deletion of DP thymocytes (24–48 h) in response to anti-CD3 mAb treatment (Fig. 2 A). To determine whether JNK activation was a cause of DP thymocyte deletion we generated transgenic mice that overexpressed a dominant negative form of JNK1 (dnJNK1; T 183 A and Y 185 F) (21) under the control of the distal lck promoter (22) (Fig. 4 A). Three founder animals were obtained, each of which expressed dnJNK1 mRNA in the thymus (Fig. 4 B). In addition to the thymus, the dnJNK1 transgene was also expressed in the spleen and purified T cells at levels substantially higher than the endogenous JNK1 gene (Fig. 4 C). Semi-quantitative analysis showed that the mRNA levels for the dnJNK1 transgene in the thymus were at least fivefold higher than the levels of endogenous JNK1 mRNA (Fig. 4 D).
Figure 4.
Generation and characterization of lck-dnJNK1 transgenic mice. (A) Schematic representation of the dnJNK1 transgene. The dnJNK1 cDNA in which Thr183 and Tyr185 were replaced by Ala and Phe, respectively, was subcloned downstream of the distal lck promoter and upstream of the hGH polyadenylation signals and intron sequences. (B) Expression of the dnJNK1 transgene. The presence of the dnJNK1 mRNA was analyzed in total thymocytes from positive mice from three independent transgenic lines (Tg+; lines 37, 44 and 45) or from negative littermate control mice (NLC) by reverse transcriptase-PCR using specific primers for the dnJNK1 transgene. As positive control, γ-actin primers were used. (C) Comparative expression of the endogenous JNK1 gene and the dnJNK1 transgene. cDNA from spleen or purified T cells from a dnJNK1 transgenic mouse (line 44) was analyzed by PCR using specific primers for endogenous mouse JNK1, the dnJNK1 transgene, or HPRT. (D) Semiquantitative PCR analysis for the expression of the endogenous JNK1 gene and dnJNK1 transgene using different amounts of cDNA (0.5, 2.5, and 5 μl in the left, center, and right lanes, respectively) prepared from the thymus of a dnJNK1 mouse. (E) JNK1 activity is reduced in dnJNK1 mice. Total thymocytes (5 × 105) from dnJNK1 (Tg+) or negative littermate control mice were unstimulated or stimulated with PMA (5 ng/ml) plus ionomycin (250 ng/ml) (P/I) for 30 min, harvested, and lysed. Whole extracts were assayed for JNK activity using the substrate GST–c-Jun (26). (F) AP-1 transcriptional activity is inhibited in dnJNK1 transgenic mice. T cells (5 × 105 cells) were purified from AP-1–luciferase reporter transgenic mice (NLC) or double AP1-luciferase × dnJNK1 transgenic mice (Tg+) and were incubated with medium alone (−) or with PMA (5 ng/ml) plus ionomycin (250 ng/ml) (P/I). After 24 h, the cells were harvested and luciferase activity was measured. The data shown are representative of two independent experiments.
No significant JNK activity was detected in unstimulated thymocytes from either wild-type or dnJNK1 mice (Fig. 4 E). Stimulation with PMA and ionomycin activated JNK in thymocytes from negative littermate control mice (Fig. 4 E). However, the activation of JNK was significantly compromised in dnJNK1 thymocytes (Fig. 4 E), indicating that the overexpression of dnJNK1 inhibits endogenous JNK.
The AP-1 transcription factor is activated by the JNK pathway at least in part by phosphorylation of the transactivation domain of c-Jun (4, 5). Although substrates other than AP-1 may be regulated by JNK in the DP thymocytes, AP-1 transcriptional activity cannot be induced in DP cells even in response to TCR-independent signals (41). In contrast, AP-1 transcriptional activity is induced in peripheral T cells in response to protein kinase C and calcium signals (25). To further demonstrate the functionality of the dominant negative JNK1 in the dnJNK1 mice, we crossed these transgenic mice with AP-1–luciferase reporter transgenic mice in which the expression of a luciferase reporter gene is driven by AP-1 DNA regulatory elements (16, 25, 26, 41, 42). Since AP-1 activity cannot be induced in DP thymocytes, we isolated peripheral CD4+ T cells from AP-1–luciferase transgenic mice or from AP-1–luciferase × dnJNK1 double transgenic mice and stimulated them with PMA and ionomycin. AP-1 transcriptional activity was induced in T cells from negative littermates but was significantly reduced in the dnJNK1 transgenic mice, establishing that the dominant negative transgene could indeed antagonize signal transduction by JNK (Fig. 4 F).
T Cell Proliferative Response in dnJNK1 Mice.
We examined the CD4+ and CD8+ T cell subsets in peripheral lymphoid organs. The frequency of CD4+ and CD8+ T cells in lymph nodes (Fig. 5 A) and spleen (data not shown) was indistinguishable between negative littermate control and dnJNK1 transgenic mice. The TCR expression levels were also normal in T cells from dnJNK1 transgenic mice (data not shown).
Figure 5.
Proliferative T cell response in dnJNK1 mice. (A) Cell surface staining for CD4 and CD8 in total lymph node cells from dnJNK1 transgenic (line 44) (Tg+) or negative littermate control (NLC) mice was analyzed by flow cytometry. The numbers represent the percentage of cells in each quadrant. (B) Spleen cells (2 × 105 cells/well) from dnJNK1 (Tg+) or negative littermates control (NLC) mice were stimulated with Con A (2.5 μg/ml), anti-CD3 mAb (1 μg/ml), or PMA (5 ng/ml) plus ionomycin (250 ng/ml) (P/I) and proliferation was determined by [3H]thymidine incorporation. Results are representative of three independent experiments. (C) Purified CD4+ T cells (5 × 104 cells/well) from dnJNK1 (Tg+) and negative littermate control (NLC) mice were stimulated with Con A (2.5 μg/ml) in the presence of the indicated numbers of mitomycin C (50 μg/ml) treated splenocytes from wild-type mice as the source of APC. Numbers (%) represent the percentage of proliferation of dnJNK1 CD4+ T cells compared with the proliferation of CD4+ T cells from NLC mice.
We have demonstrated previously that TCR-mediated signals in combination with costimulatory signals induce AP-1 transcriptional activity and JNK in peripheral CD4+ T cells (25, 26). To determine the role of JNK in T cell activation we examined the proliferative response of total spleen cells induced by Con A, anti-CD3 mAb, or PMA plus ionomycin. A small reduction in proliferation was reproducibly observed in the dnJNK1 mice (Fig. 5 B). However, the proliferative response of purified peripheral CD4+ T cells in the presence of a limiting number of wild-type APCs was significantly reduced (67%) in the dnJNK1 mice (Fig. 5 C). The extent of inhibition of the proliferation of dnJNK1 CD4+ T cells decreased as the number of APCs was increased. This dependence on APC number may account for the weak effect of the dnJNK1 transgene in the cultures using total spleen cells where the ratio of APCs to T cells is relatively high.
JNK Does Not Affect Positive Selection of Thymocytes.
To examine the effect of the overexpression of the dnJNK1 transgene on the development of T cells in the thymus, we analyzed the distribution of thymic populations. The ratio of DN, DP, and SP CD4+ and CD8+ thymocytes was not significantly affected in the dnJNK1 transgenic mice (Fig. 6 A). The number of total thymocytes was slightly increased (10–20%) in adult (>8 wk) dnJNK1 transgenic mice compared with negative littermate control mice, although a greater difference between dnJNK and wild-type thymocyte numbers (twofold) was observed in very young dnJNK1 transgenic mice (4–6 wk).
Figure 6.
Normal positive selection in dnJNK1 transgenic mice. (A) Cell surface staining for CD4 and CD8 in total thymocytes from dnJNK1 transgenic (line 44) (Tg+) or negative littermate control (NLC) mice was analyzed by flow cytometry. The numbers represent the percentage of cells in each quadrant. (B) Total thymocytes from Cyt c TCR transgenic mice (NLC) or Cyt c TCR × dnJNK1 double transgenic mice (Tg+) were analyzed for CD4 and CD8 expression in the total thymus (top) and Vβ3 expression in the DP or single CD4+ gates (bottom). The numbers indicate the percentage of DP CD4+CD8+ and SP CD4 + cells in the total thymus.
To test the effect of the dnJNK1 transgene expression on positive selection, we crossed these mice with transgenic mice expressing a TCR from a CD4+ clone specific for moth Cyt c (43). This TCR is strongly positively selected in the H-2k MHC background, as shown by the pronounced accumulation of the single CD4+ TCR Vβ3+ cells, together with a reduction in the DP cells (Fig. 6 B). No differences were observed in thymic populations and the levels of specific TCR (Vβ3) between transgene-positive and transgene-negative mice showing the accumulation of SP CD4+ TCR Vβ3+ cells in both cases. These results suggest that positive selection is unaffected by dominant negative JNK1. Thus, the ERK pathway (8, 11), but not the JNK pathway, appears to be required for positive selection.
JNK Signaling Pathway Contributes to the Deletion of DP Thymocytes In Vivo.
To examine negative selection in the dnJNK1 transgenic mice, we first analyzed the deletion of DP thymocytes induced by anti-CD3 mAb treatment in vivo. As shown above, 2 d after injection with anti-CD3 mAb in vivo, the DP population was almost completely eliminated in negative littermate control mice (Fig. 7 A). In contrast, a higher proportion (three- to fivefold) of DP cells remained viable in dnJNK1 transgenic mice (line 44) (Fig. 7 A). The same results were obtained with transgenic mice derived from a second transgenic line (line 45) (Fig. 7 B). The fact that the inhibition mediated by this transgene is incomplete is not surprising since similar partial inhibition phenotypes were obtained with other dominant negative transgene models (8, 9, 11). Moreover, it is possible that the dnJNK1 transgene may not ablate signals mediated by other members of the JNK family, for example JNK2. Nevertheless, the percentage and absolute number of DP thymocytes recovered after 2 d of anti-CD3 mAb injection in dnJNK1 transgenic mice were consistently higher than those in negative littermate control mice (Fig. 7 C). Thus, inhibition of the JNK signaling pathway partially protected DP thymocytes from deletion in vivo.
Figure 7.
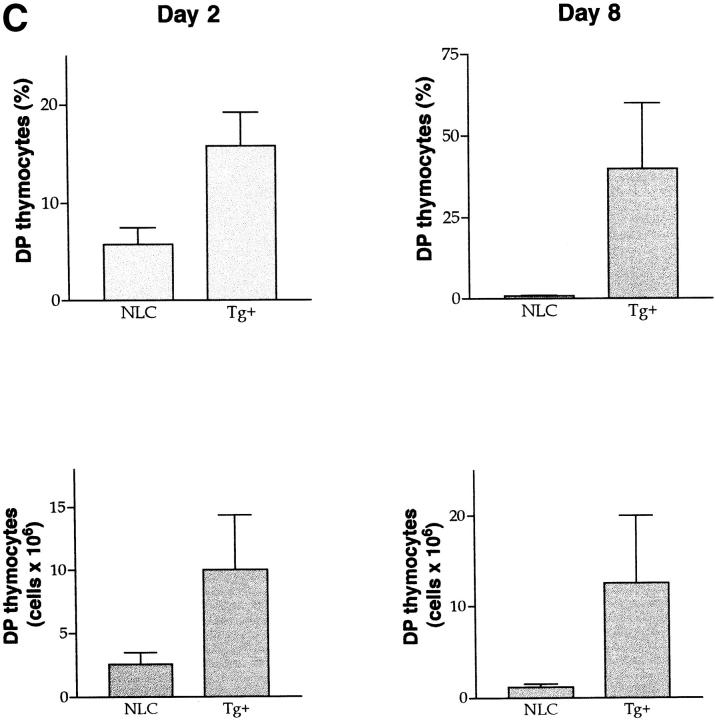
Deletion of DP cells by anti-CD3 mAb in vivo administration is impaired in dnJNK1 transgenic mice. (A) dnJNK1 transgenic (Tg+) and negative littermate control (NLC) mice (from line 44) were interperitoneally injected with purified anti-CD3 mAb (50 μg/200 μl PBS). 2, 4, or 8 d after injection total thymocytes were stained and CD4 and CD8 expression in the live population was analyzed. The time at 0 d shows the thymocyte distribution in uninjected mice. Numbers represent the percentage of live cells in each gate, CD4+CD8+ (upper gate) and single CD4+ (lower gate). Data shown are from one representative experiment out of three carried out. (B) dnJNK1 transgenic (Tg+) and negative littermate control (NLC) mice from line 45 were injected with anti-CD3 mAb (50 μg/200 μl PBS) and thymocytes were analyzed 2 d later as described in A. (C) Percentage of DP thymocytes (top) and absolute number of DP thymocytes (bottom) in the thymus from negative littermate control (NLC) and dnJNK1 transgenic (Tg+) mice 2 d (left) or 8 d (right) after anti-CD3 mAb in vivo administration. The mean and standard deviations from four (day 2) and three (day 8) independent experiments are presented. (D) dnJNK1 transgenic (Tg+) and negative littermate control (NLC) mice were injected with purified anti-CD3 mAb. The thymus was isolated 2, 4, or 8 d after injection and from control mice (0 d). Total thymocytes were analyzed by forward scatter (FSC) and side scatter (SSC) flow cytometry parameters. Numbers express the percentage of cells in each gate, live cells (right gates) and dead cells (left gates). Data represent one experiment of three performed and represent an independent experiment from the data presented in A.
4 d after administration of anti-CD3 mAb, most of the DP thymocytes were also deleted in dnJNK1 mice, although the percentage of this population was still higher than in negative littermates (Fig. 7 A). Interestingly, 8 d after injection of anti-CD3, the DP population was dramatically reconstituted in the dnJNK1 transgenic mice (Fig. 7, A and C). In contrast, this recovery was not observed in negative littermate control mice (Fig. 7, A and C). Moreover, the number of total thymocytes (data not shown) and the number of DP thymocytes recovered after 8 d of injection (Fig. 7 C) were substantially increased (7–14-fold) in the dnJNK1 transgenic mice. Therefore, the dnJNK1 transgenic mice showed delayed elimination and accelerated reconstitution of the DP population in response to anti-CD3 mAb administration in vivo, likely due to an increased resistance to cell death.
To demonstrate that the reduced deletion of the DP population was associated with an inhibition of apoptosis in the thymus, we examined the morphology of the thymocytes by FACS® analysis using side scatter and forward scatter as parameters for the complexity (e.g., granularity) and the size of cells, respectively. Thymocytes were isolated and analyzed at different time points after anti-CD3 mAb administration. It has been shown previously (44) that apoptotic cells present reduced forward scatter (reduced size) and higher side scatter (higher complexity) compared with live cells (which exhibit higher forward scatter and lower side scatter). 2 d after anti-CD3 mAb injection, the percentage of the apoptotic population was reduced (three- to fivefold), while the percentage of the live population was increased in the dnJNK1 transgenic mice compared with wild-type mice (Fig. 7 D). Although smaller differences were observed at day 4, after 8 d a significantly higher proportion of apoptotic cells remained in the negative littermate control mice. In contrast, only a few apoptotic cells were observed in the dnJNK1 transgenic mice and the live thymocyte population had almost fully recovered (Fig. 7 D). Taken together, these data indicate that JNK is required for the complete deletion of DP thymocytes by apoptosis in vivo.
To further confirm that TCR-mediated negative selection in the thymus is regulated by JNK, we examined the deletion of autoreactive thymocytes induced by specific self-antigens. The dnJNK1 transgenic mice were crossed with Cyt c–reactive TCR transgenic mice. As described above, the expression of the dnJNK1 transgene did not affect positive selection of Cyt c-bearing thymocytes (Fig. 6 B). A specific Cyt c peptide was administrated to the Cyt c TCR transgenic mice and the Cyt c–specific TCR × dnJNK1 double transgenic mice. After 20 h, the thymic populations were examined. The percentage of apoptotic thymocytes was increased and the live population was reduced in the Cyt c TCR transgenic mice upon peptide administration (Fig. 8 A). In contrast, no significant increase of thymocyte cell death was observed in the Cyt c TCR × dnJNK1 double transgenic mice in response to the peptide (Fig. 8 A). The percentage of DP and mature CD4+ thymocytes recovered upon peptide injection was only slightly increased in the dnJNK1 × Cyt c double transgenic mice (Fig. 8 B). However, the absolute numbers of DP and CD4+ thymocytes were higher (two- to threefold) in the Cyt c TCR × dnJNK1 double transgenic mice than in the Cyt c TCR transgenic mice (Fig. 8 C). These results indicated that deletion of autoreactive thymocytes by specific antigens is impaired in the dnJNK1 mice.
Figure 8.
Antigen-specific induced apoptosis in thymus is impaired in dnJNK1 transgenic mice. (A) Cyt c TCR transgenic mice (NLC) or Cyt c TCR × dnJNK1 double transgenic mice (Tg+) were injected with PBS or a synthetic moth Cyt c peptide (250 μM/200 μl/mouse) for 24 h. Thymocytes were then isolated, stained, and analyzed by flow cytometry for side scatter (SSC) and forward scatter (FSC) flow cytometry. Numbers express the percentage of cells in each gate, live cells (right gates) and dead cells (left gates). (B) CD4 and CD8 expression in live cells from Cyt c TCR transgenic mice (NLC) or Cyt c TCR × dnJNK1 double transgenic mice (Tg+) injected with PBS or a synthetic moth Cyt c peptide for 24 h. Numbers represent the percentage of cells in each gate (DP, CD4+, and DN cells). (C) Absolute number of DP, CD4+, and DN cells in Cyt c TCR transgenic mice (NLC) or Cyt c TCR × dnJNK1 double transgenic mice (Tg+) 24 h after injection with a synthetic moth Cyt c peptide. A total of 40–60 × 106 cells was found in thymuses from both Cyt c TCR transgenic mice and Cyt c TCR × dnJNK1 double transgenic mice before peptide injection. The results are representative of two independent experiments.
Regulation of In Vitro DP Thymocyte Deletion by JNK.
We have shown that JNK activity was not significantly induced in DP thymocytes in response to a combination of anti-CD3 and anti-CD28 mAbs (Fig. 1 A). In correlation with these results, we did not observe significant differences in the percentage (Fig. 9 A) or absolute number (data not shown) of DP thymocytes recovered after 16 h of treatment with anti-CD3 and anti-CD28 mAbs in vitro between the dnJNK1 transgenic and wild-type mice.
Figure 9.
TCR-mediated deletion of DP thymocytes in vitro from dnJNK1 mice. (A) Total thymocytes from dnJNK1 transgenic (Tg+) and negative littermate control (NLC) mice were incubated in the presence of medium (−), or immobilized anti-CD3 (10 μg/ml) plus anti-CD28 (10 μg/ml) mAbs (anti-CD3/ anti-CD28) or Con A (2.5 μg/ ml). After 24 h, the cells were harvested, stained with anti-CD4 and anti-CD8 mAbs, and analyzed by FACS®. The profiles represent CD4 and CD8 expression in live cells. Numbers represent the percentage in each gated population. (B) Total thymocytes from dnJNK1 transgenic (Tg+) and negative littermate control (NLC) mice were incubated in the presence of medium (−) or Con A (2.5 μg/ml). Cell viability was analyzed by trypan blue staining at the indicated times. The results presented are representative of three independent experiments.
We also examined thymic deletion induced by Con A treatment in vitro, which significantly activated JNK in DP thymocytes (Fig. 1 C). Con A–mediated signals rapidly induced pronounced thymocyte death and loss of the absolute number of DP cells (data not shown). However, thymocytes from dnJNK1 mice were more resistant to Con A–induced cell death, as shown by increased viable total thymocytes (Fig. 9 B) and the percentage of live DP cells (Fig. 9 A). Together, these data indicate that JNK is also important to induce cell death in DP thymocytes by signals mediated by the TCR and costimulatory molecules in vitro.
Discussion
The balance of survival signals (positive selection) and death signals (negative selection) is critical for the development of T cells with an appropriate repertoire in the thymus. A predominance of survival signals could lead to the presence of autoreactive cells in the periphery that have escaped negative selection in the thymus. Similarly, excessive cell death may cause immunodeficiencies due to underrepresentation of the complete repertoire. Thus, it is important to dissect the molecular mechanisms utilized by the TCR as well as other molecules that lead to a survival or death signal in developing thymocytes. Moreover, it is critical to determine whether these mechanisms are common to the activation signals in mature T cells in the periphery.
The MAP kinase family is widely implicated in the control of survival and death in several cellular systems (4, 5). The ERK-MAP kinase pathway is primarily associated with cell survival and proliferation. In contrast, both the p38 and the JNK pathways have often been related to induction of apoptosis or cell death.
Inhibition of the ERK pathway by retroviral-mediated overexpression of a dominant negative MEK (an upstream activator of ERK) in fetal thymic organ culture causes impaired differentiation of DN into DP thymocytes (7). In correlation, inhibition of the ERK pathway by the PD98059 drug also causes a partial reduction of the expansion and differentiation of DN into DP cells (13). In contrast, overexpression of dnMEK1 in transgenic mouse models did not significantly affect this stage of thymic development (8, 11). The role of p38 MAP kinase in thymic development has not yet been addressed, although the high level of constitutive p38 activity detected in DN and DP thymocytes (14) suggests that p38 may also contribute to the differentiation of DN to DP thymocytes. Here, we show that inhibition of the JNK pathway in dnJNK1 transgenic mice does not affect the development of DP from DN thymocytes. However, the distal lck promoter (22) used to generate these mice does not drive high levels of expression in DN thymocytes. Thus, based on our studies, we cannot conclude that the JNK pathway is not involved in the control of DN differentiation.
The Ras/Raf–ERK signaling pathway is required for positive selection of DP thymocytes, as shown by the effects of overexpression of dominant interfering forms of Ras, Raf, or MEK (8, 9, 11, 12). In contrast, no effect on negative selection of this pathway has been observed in these studies, suggesting that the ERK pathway is not required for negative selection of DP thymocytes. Here, we show that inhibition of the JNK pathway does not affect positive selection of DP thymocytes, but instead results in defective deletion of DP thymocytes in response to negative selection signals. These results correlate with previous studies in other cellular systems that have demonstrated an association of the ERK and JNK pathways with survival and death signals, respectively (4, 5, 45).
In contrast to the high p38 activity that we (46) and others (14) have detected in unstimulated thymocytes, only very low JNK activity was detected in thymocytes before activation. Here, for the first time, we show that the JNK pathway is activated in vivo in DP thymocytes in response to TCR-mediated signals, probably in combination with additional signals provided by the thymic environment (see below). The activation of JNK in DP thymocytes precedes the induction of apoptosis and deletion of these cells, suggesting that the activation of JNK could be required for the initiation of the apoptotic cascade. The phenotype of the dnJNK1 transgenic mice support this model. First, we have demonstrated that DP thymocytes from the dnJNK1 transgenic mice are more resistant to deletion induced by anti-CD3 mAb in vivo. Injection with anti-CD3 has been widely used to study negative selection in non-TCR transgenic mouse models (36–40). The strong signal induced by the ligation of the TCR with an anti-CD3 mAb may mimic the signals that are clonally induced by high signal intensity TCR ligands, such as negative selecting peptides (47–50). In addition, similar results were obtained when a specific peptide was used to induce antigen-dependent deletion of DP thymocytes. Therefore, we have shown that thymocytes from Cyt c TCR transgenic mice are more resistant to in vivo cell death in response to the more relevant signal delivered by a specific Cyt c peptide when the JNK pathway is impaired. Moreover, deletion of DP thymocytes upon injection of Staphylococcus enterotoxin B superantigen in vivo was also reduced in the dnJNK1 transgenic mice, although no significant differences were observed in the deletion of Vβ5 and Vβ11 mature thymocytes by endogenous viral superantigens (data not shown). Thymocytes from dnJNK1 transgenic mice are also more resistant to cell death induced by Con A in vitro. The four approaches together provide strong support for the requirement of JNK in the deletion of DP thymocytes. Further work will be required to determine the role of JNK in deletion mediated by endogenous superantigens.
The reduced deletion of dnJNK1 DP thymocytes appears to be caused by increased resistance to apoptosis, as shown by the reduction of the number of apoptotic cells and the increase of live thymocytes after 2 d of anti-CD3 mAb treatment in vivo. Furthermore, the resistance of DP thymocytes from the dnJNK1 transgenic mice to cell death is consistent with the accelerated reconstitution of the DP population in these mice after the deletion caused by anti-CD3 mAb administration. After 8 d of anti-CD3 mAb injection, almost no thymocytes were found in the wild-type thymus, whereas the number of thymocytes from dnJNK1 mice was substantially increased. Analysis of thymic populations at this time demonstrated that the DP population is recovered in dnJNK1 mice, whereas no DP thymocytes were detected in wild-type mice. Together, these data indicate that inhibition of the JNK pathway renders DP thymocytes more resistant to death signal and they can expand and repopulate the thymus more rapidly.
Activation of the protein kinase C or Ras pathways by PMA is not sufficient to induce JNK activity in Jurkat T cells and thymocytes; an additional calcium signal is also required to activate JNK (6), indicating the importance of the calcium pathway in the regulation of JNK. A role for calcium signals in thymocyte negative selection has been suggested by a number of studies (50–53). Defective negative selection associated with reduced calcium flux in response to TCR stimulation has been found in mice deficient for the protooncogene Vav (54) which functions as a guanine nucleotide exchange factor for the Rho/Rac/cdc42 family of GTPases (55–57). Interestingly, Vav can transduce a signal that leads to the activation of the JNK pathway in a Rac-1–dependent manner (58, 59). Thus, the calcium requirement for negative selection and for JNK activation correlates with the requirement of JNK activation for negative selection that we propose in this study and has been previously suggested by others (54).
The ERK pathway is required not only for positive selection, but it is also involved in T cell lineage commitment. It has been shown that the overexpression of a dominant gain-of-function mutant of ERK2 favored differentiation of DP thymocytes to the CD4+ lineage (13) and that pharmacological inhibition of this pathway favors differentiation to the CD8+ lineage. In contrast to the study by Sharp et al. (13), the inhibition of the ERK pathway in mice overexpressing a dominant negative Mek-1 did not affect CD8+ T cell development (8, 11). We did not observe significant differences in CD4+ and CD8+ lineages in the dnJNK1 transgenic mice, but we cannot eliminate the possibility that JNK may also be involved in lineage commitment.
The JNK-MAP kinase group is formed by three genes: JNK1, JNK2, and JNK3. Each of these genes expresses several JNK isoforms that are generated by alternative splicing (15). JNK family members are phosphorylated and activated by members of the MAPK kinase family. Sek1/ MKK4 was the first activator to be identified (17–19). We and others have shown that JNK activation is not completely abrogated in Sek1/MKK4 deficient cells and that the extent of inhibition depends on the stimulus used (60, 61). More recently, a new activator of JNK has been identified (20). This activator, MKK7, may also contribute to JNK regulation in thymocytes. The contribution of these two activators, MKK4 and MKK7, in the regulation of JNK activity in vivo remains to be determined.
It has been reported that DP thymocytes from Rag2−/− chimeric Sek1/MKK4 deficient mice are more susceptible to cell death induced in vitro by anti-Fas or anti-CD3 mAbs, but not by anti-CD3/anti-CD28 mAbs (60). The deletion of DP cells in vivo was not examined in Sek1/ MKK4 deficient mice (60). A role for the JNK signaling pathway in the inhibition of apoptosis during T cell development was suggested in this study. However, more recently, similar studies by Swat et al. (62) have shown that thymic development is normal in Rag2−/− chimeric Sek1/MKK4 deficient mice, but the mice develop lymphadenopathy. Thus, these results indicate that MKK4 is not relevant for development of T cells in the thymus. In correlation, we have found that MKK4 is expressed only at very low levels in thymocytes. Thus, it is surprising that deletion of a gene (MKK4) which is not expressed (neither as RNA nor as protein) in the thymus could result in a substantial phenotype, as observed by Nishina et al. (60). However, in contrast to MKK4, we have shown that MKK7 is highly expressed in the thymus, suggesting that MKK7, and not MKK4, is the major regulator on the JNK pathway in thymocytes. Therefore, it is likely that MKK7 mediates the activation of JNK in DP thymocytes in response to anti-CD3 mAb treatment in vivo. Moreover, studies by Swat et al. (62) indicate that MKK4 is required for maintaining peripheral lymphoid homeostasis. Our data indicate that the activation of JNK probably by MKK7 is required for complete elimination of DP cells in vivo in the thymus. Together, these results suggest a differential regulation of JNK in immature thymocytes and mature T cells. The specific role of MKK7 in T cell development and T cell activation remains to be addressed.
It is well accepted that the negative selection of autoreactive cells in the thymus requires costimulatory signals in addition to the TCR-mediated signal. However, the nature of the costimulatory signals remains unclear (31–33). CD28 is able to provide the signal requirement for negative selection of immature heat stable antigen, HSAlow, CD4+ thymocytes (34). In contrast, the CD28 costimulatory signal does not appear to be sufficient, even in conjunction with TCR ligation, to induce deletion of DP thymocytes. Thus, in vitro stimulation of thymocytes with immobilized anti-CD3 mAb in combination with anti-CD28 mAb caused downmodulation of CD4 and CD8 molecules on the surface of DP thymocytes. However, this stimulation does not induce complete deletion of the DP population, although the CD4lowCD8low subpopulation has been characterized as apoptotic cells (35). Moreover, the specific role of B7.1 and B7.2 molecules in the deletion of DP thymocytes remains controversial (33, 63, 64). In contrast to these in vitro studies, the presence of anti-CD3 mAb or other TCR ligands (e.g., superantigens, specific antigens) alone induces significant deletion of DP thymocytes in vivo. Together, this evidence suggests that thymic environmental signals other than CD28 may play an important role in negative selection of autoreactive T cells. Thus, CD30 deficient mice contain elevated numbers of thymocytes, and negative selection, but not positive selection, is defective in these mice, suggesting that CD30 is an important coreceptor in deletion of autoreactive T cells (65). CD40 ligand has also been reported to play a role in negative selection (66).
In correlation with the requirements for negative selection, we show here that JNK activity is not upregulated in DP thymocytes stimulated with immobilized anti-CD3 and anti-CD28 mAbs in vitro (Fig. 1 A). In contrast, Con A stimulation induced JNK activity in DP thymocytes in vitro. Since Con A is a lectin that can bind molecules other than the TCR, it is possible that these other accessory molecules provide the costimulatory signals required for the activation of JNK by TCR ligation. In addition, the administration of anti-CD3 mAb in vivo also induced JNK activation in DP thymocytes, suggesting a requirement of additional thymic environmental components for the activation of JNK through the TCR. The studies performed in the dnJNK1 mice confirm these results. Deletion of DP thymocytes by either Con A treatment in vitro or anti-CD3 mAb administration in vivo is impaired in the dnJNK1 mice. In contrast, cell death induced by anti-CD3/anti-CD28 mAb treatment in vitro was not affected in these mice.
In our in vivo models, we cannot distinguish whether the inhibition of JNK directly affects TCR-mediated negative signals or whether it affects any other signals delivered by the thymic environment (e.g., cytokines, accessory molecules). Nevertheless, it has not yet been established that TCR signal delivered during negative selection directly causes apoptosis. Therefore, it is also possible that TCR-mediated signals only sensitize DP thymocytes to apoptotic stimuli provided by other environmental factors, such as members of the TNF receptor (TNFR) families (including Fas, CD30, CD40, and TNFR), as has been suggested previously (67).
We have shown for the first time that JNK is activated in DP thymocytes in vivo, and this activation is likely mediated by MKK7, rather than MKK4. Deletion of DP thymocytes in vivo by apoptosis is impaired in dnJNK1 transgenic mice, indicating that signaling via JNK contributes to negative selection in the thymus. We propose that a downstream target of JNK contributes to the apoptotic response in thymocytes during negative selection, as described for the apoptotic responses in other cell types (5, 16, 45, 68, 69). Thus, during thymocyte development the positive selection of CD4+ T cells is mediated through the ERK pathway, as shown by Perlmutter and colleagues (8, 11), while JNK contributes to negative selection. Together, these results support a qualitative rather than quantitative model to explain how recognition of each peptide-MHC complex results in a different final outcome, positive versus negative selecting signals, via the ERK and JNK pathways, respectively.
Acknowledgments
We thank C. Hughes and D. Butkus for generating the transgenic mice, T. Barrett, I.-H. Wu, D.T. Zapton, and C. Merrit for technical assistance, C. Charland for cell sorting and S. Hedrick for providing the Cyt c transgenic mice, R. Perlmutter for the distal Ick-promoter construct, and F. Manzo for secretarial assistance.
This work was supported in part by Howard Hughes Medical Institute Research Resources Program (to M. Rincón), and National Institutes of Health grants AI29902 and AI36529 (to R.A. Flavell) and CA65861 (to R.J. Davis). R.A. Flavell and R.J. Davis are Investigators and D.D. Yang was an Associate of the Howard Hughes Medical Institute.
Footnotes
D.D. Yang's present address is Lilly Research Laboratories, Lilly Corporate Center, Indianapolis, IN 46285.
Abbreviations used in this paper: Cyt c, cytochrome c; DN, double negative; DP, double positive; ERK, extracellular signal-regulated kinase; GST, glutathione S-transferase; hGH, human growth hormone; JNK, c-Jun NH2-terminal kinase; SP, single positive.
References
- 1.Fink PJ, Bevan MJ. Positive selection of thymocytes. Adv Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- 2.Kisielow P, von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- 3.Nossal GJV. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 4.Whitmarsh AJ, Davis RJ. Transcription factor AP-1: regulation by mitogen activated protein kinases signal transduction pathways. J Mol Med. 1996;17:2360–2371. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 5.Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK): from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 6.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 7.Crompton T, Gilmour KC, Owen MJ. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocytes. Cell. 1996;86:243–251. doi: 10.1016/s0092-8674(00)80096-3. [DOI] [PubMed] [Google Scholar]
- 8.Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 9.Swan KA, Alberola-Ila J, Gross JA, Appleby MW, Forbush KA, Thomas JF, Perlmutter RM. Involvement of p21rasdistinguishes positive and negative selection in thymocytes. EMBO (Eur Mol Biol Organ) J. 1995;14:276–285. doi: 10.1002/j.1460-2075.1995.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Shea CC, Crompton T, Rosewell IR, Hayday AC, Owen MJ. Raf regulates positive selection. Eur J Immunol. 1996;26:2350–2355. doi: 10.1002/eji.1830261012. [DOI] [PubMed] [Google Scholar]
- 11.Alberola-Ila J, Hogquist KA, Swan KA, Bevan MJ, Perlmutter RM. Positive and negative selection invoke distinct signaling pathways. J Exp Med. 1996;184:9–18. doi: 10.1084/jem.184.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swat W, Shinkai Y, Cheng H-L, Davidson L, Alt FW. Activated Ras signals differentiation and expansion of CD4+CD8+thymocytes. Proc Natl Acad Sci USA. 1996;93:4683–4687. doi: 10.1073/pnas.93.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp LL, Schwarz DA, Bott CM, Marshall CJ, Hedrick SM. The influence of the MAPK pathway on T cell lineage commitment. Immunity. 1997;7:609–618. doi: 10.1016/s1074-7613(00)80382-9. [DOI] [PubMed] [Google Scholar]
- 14.Sen J, Kapeller R, Fragoso R, Sen R, Zon LI, Burakoff SJ. Intrathymic signals in thymocytes are mediated by p38 mitogen-activated protein kinase. J Immunol. 1996;156:4535–4538. [PubMed] [Google Scholar]
- 15.Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Dérijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO (Eur Mol Biol Organ) J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 16.Yang DD, Kuan C-Y, Whitmarsh AJ, Rincón M, Zheng TS, Davis RJ, Rakic P, Flavell RA. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 17.Dérijard B, Raingeaud J, Barret T, Wu I-H, Han J, Ulevitch RJ, Davis RJ. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:683–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis JM, Zon LI. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 19.Lin A, Minden A, Martinetto H, Claret F-X, Lange-Carter C, Mercurio F, Johnson GL, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 20.Tournier C, Whitmarsh AJ, Cavanagh J, Barrett T, Davis RJ. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci USA. 1997;94:7337–7342. doi: 10.1073/pnas.94.14.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dérijard B, Hibi M, Wu I-H, Barret T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 22.Wildin RS, Garvin AM, Pawar S, Lewis DB, Abraham KM, Forbush KA, Ziegler SF, Allen JM, Perlmutter RM. Developmental regulation of lckgene expression in T lymphocytes. J Exp Med. 1991;173:383–393. doi: 10.1084/jem.173.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinster RL, Allen JM, Behringer RR, Gelinas RE, Palmiter RD. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci USA. 1988;85:836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogan, B., F. Constantini, and E. Lacy. 1986. Manipulating the Mouse Embryo. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 332 pp.
- 25.Rincón M, Flavell RA. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO (Eur Mol Biol Organ) J. 1994;13:4370–4381. doi: 10.1002/j.1460-2075.1994.tb06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rincón M, Dérijard B, Chow C-W, Davis RJ, Flavell RA. Reprogramming the signaling requirement for AP-1 (activator protein-1) activation during differentiation of precursor CD4+T cells to effector Th1 and Th2 cells. Genes Funct. 1997;1:51–68. doi: 10.1046/j.1365-4624.1997.00007.x. [DOI] [PubMed] [Google Scholar]
- 27.Favaloro J, Freisman R, Kamen R. Transcription maps of polyoma virus specific mRNA. Analysis by two dimensional nucleases S1 gel mapping. Methods Enzymol. 1980;65:718. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- 28.Raingeaud J, Gupta S, Roger J, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 MAP kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 29.Rincón M, Tugores A, López-Rivas A, Silva A, Alonso M, de Lándazuri MO, López-Botet M. Prostaglandin E2and the increase of intracellular cAMP inhibit the expression of interleukin 2 receptors in human T cells. Eur J Immunol. 1988;18:1791–1796. doi: 10.1002/eji.1830181121. [DOI] [PubMed] [Google Scholar]
- 30.Liener, I.E., N. Sharon, and I.J. Goldstein. 1986. The Lectins: Properties, Functions and Applications in Biology and Medicine. Academic Press LTD, London. 600 pp.
- 31.Page DM, Kane LP, Allison JP, Hedrick SM. Two signals are required for negative selection of CD4+CD8+thymocytes. J Immunol. 1993;151:1868–1880. [PubMed] [Google Scholar]
- 32.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishimoto H, Cai ZC, Brunmark A, Jackson MR, Peterson PA, Sprent J. Differing roles for B7 and intercellular adhesion molecules-1 in negative selection of thymocytes. J Exp Med. 1996;184:531–537. doi: 10.1084/jem.184.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185:263–271. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kishimoto H, Surh CD, Sprent J. Upregulation of surface markers on dying thymocytes. J Exp Med. 1995;181:649–655. doi: 10.1084/jem.181.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith CA, Williams GT, Kingston R, Jenkinson EJ, Owen JJ. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989;337:181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 37.Shi YF, Bissonnette RP, Parfrey N, Szalay M, Kubo RT, Green DR. In vivo administration of monoclonal antibodies to the CD3 T cells receptor complex induces cell death (apoptosis) in immature thymocytes. J Immunol. 1991;146:3340–3346. [PubMed] [Google Scholar]
- 38.Owen JJ, Owen MJ, Williams GT, Kingston R, Jenkinson EJ. The effects of anti-CD3 antibodies on the development of T-cell receptor αβ+lymphocytes in embryonic thymus organ cultures. Immunology. 1988;63:639–642. [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, Milbrandt J. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nurr77) Science. 1995;269:532–534. doi: 10.1126/science.7624775. [DOI] [PubMed] [Google Scholar]
- 40.Field SJ, Tsai F-Y, Kuo F, Livingston DM, Orkin SH, Greenberg ME. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 41.Rincón M, Flavell RA. Regulation of AP-1 and NFAT transcription factors during thymic selection of T cells. Mol Cell Biol. 1996;16:1074–1084. doi: 10.1128/mcb.16.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C, Ma W-Y, Dawson M, Rincón M, Flavell RA, Dong Z. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc Natl Acad Sci USA. 1997;94:5826–5830. doi: 10.1073/pnas.94.11.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaye J, Hsu M-L, Sauron M-E, Jameson SC, Gascoigne NRJ, Hedrick SM. Selective development of CD4+T cells in transgenic mice expressing a class II MHC–restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 44.Renno T, Hahne M, Tschopp J, MacDonald R. Peripheral T cells undergoing superantigen-induced apoptosis in vivo express B22O and upregulate Fas and Fas ligand. J Exp Med. 1996;183:431–437. doi: 10.1084/jem.183.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinase on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 46.Rincón M, Enslen H, Raingeaud J, Recht M, Zapton T, Su MS-S, Penix LA, Davis RJ, Flavell RA. Interferon-γ expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO (Eur Mol Biol Organ) J. 1998;17:2817–2829. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohashi PS, Pircher H, Burki K, Zinkernagel RM, Hengartner H. Distinct sequence of negative or positive selection implied by thymocytes T cell-receptor densities. Nature. 1990;346:861–863. doi: 10.1038/346861a0. [DOI] [PubMed] [Google Scholar]
- 48.Allen PM. Peptides in positive and negative selection: a delicate balance. Cell. 1994;76:593–596. doi: 10.1016/0092-8674(94)90497-9. [DOI] [PubMed] [Google Scholar]
- 49.Page DM, Alexander J, Snoke E, Appella A, Sette A, Hedrick SM, Grey HM. Negative selection of CD4+CD8+thymocytes by T-cell receptor peptide antagonists. Proc Natl Acad Sci USA. 1994;91:4057–4061. doi: 10.1073/pnas.91.9.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vasquez NJ, Kane LP, Hedrick SM. Intracellular signals that mediate thymic negative selection. Immunity. 1994;1:45–56. doi: 10.1016/1074-7613(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 51.Kane LP, Hedrick SM. A role for calcium influx in setting the threshold for CD4+CD8+thymocytes negative selection. J Immunol. 1996;156:4594–4601. [PubMed] [Google Scholar]
- 52.Wang C-R, Hashimoto K, Kubo S, Yokochi T, Kubo M, Suzuki M, Suzuki K, Tada T, Nakayama T. T cell receptor–mediated signaling events in CD4+CD8+thymocytes undergoing thymic selection: requirement of calcineurin activation for thymic positive selection but not negative selection. J Exp Med. 1995;181:927–941. doi: 10.1084/jem.181.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Page DM, Kane LP, Onami TM, Hedrick SM. Cellular and biochemical requirements for thymocyte negative selection. Semin Immunol. 1996;8:69–82. doi: 10.1006/smim.1996.0010. [DOI] [PubMed] [Google Scholar]
- 54.Turner M, Mee J, Walters AE, Quinn ME, Mellor AL, Zamoyska R, Tybulewicz VLJ. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–460. doi: 10.1016/s1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- 55.Katzav LP, Martin-Zanca D, Barbacid M. Vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO (Eur Mol Biol Organ) J. 1989;8:2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams JM, Houston H, Allen J, Lints T, Harvey R. The hematopoietically expressed vav proto-oncogene shares homology with the dbl GDP-GTP exchange factor, the bcr gene and a yeast gene (CDC42) involved in cytoskeletal organization. Oncogene. 1992;7:611–618. [PubMed] [Google Scholar]
- 57.Boguski MS, Bairoch A, Attwood TK, Michaels GS. Proto-vav and gene expression. Nature. 1992;358:113. doi: 10.1038/358113a0. [DOI] [PubMed] [Google Scholar]
- 58.Crespo P, Bustelo XR, Aaronson DS, Coso OA, López-Barahona M, Barbacid M, Gutkind JS. Rac-1 dependent stimulation of the JNK/SAPK signaling pathway by Vav. Oncogene. 1996;13:455–460. [PubMed] [Google Scholar]
- 59.Teramoto H, Salem P, Robbins KC, Bustelo XR, Gutkind JS. Tyrosine phosphorylation of the vav proto-oncogene product links FcReRI to the Rac1-JNK pathway. J Biol Chem. 1997;272:10751–10755. doi: 10.1074/jbc.272.16.10751. [DOI] [PubMed] [Google Scholar]
- 60.Nishina H, Fischer KD, Radvanyl L, Shahinian A, Haken R, Rubie EA, Bernstein A, Mak TW, Woodgett JR, Penninger JM. Stress-signaling kinase Sek 1protects thymocytes from apoptosis mediated by CD95 and CD3. Nature. 1997;385:350–353. doi: 10.1038/385350a0. [DOI] [PubMed] [Google Scholar]
- 61.Yang D, Tournier C, Wysk M, Lu H-T, Jie X, Davis RJ, Flavell RA. Targeted disruption of the MKK4 gene causes embryonic death inhibition of c-Jun NH2terminal kinase activation, and defects in AP-1 transcriptional activity. Proc Natl Acad Sci USA. 1997;94:3004–3009. doi: 10.1073/pnas.94.7.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swat W, Fujikawa K, Ganiatsas S, Yang DD, Xavier RJ, Harris NL, Davidson L, Ferrini R, Davis RJ, Labow MA, et al. SEK1/MKK4 is required for maintenance of a normal peripheral lymphoid compartment but not for T lymphocyte development. Immunity. 1998;8:625–634. doi: 10.1016/s1074-7613(00)80567-1. [DOI] [PubMed] [Google Scholar]
- 63.Jones LA, Izon DJ, Nieland JD, Linsley PS, Kruisbeek AM. CD28-B7 interactions are not required for intrathymic clonal deletion. Int Immunol. 1993;5:503–512. doi: 10.1093/intimm/5.5.503. [DOI] [PubMed] [Google Scholar]
- 64.Tan R, Teh S-J, Ledbetter JA, Linsley PS, Teh H-S. B7 costimulates proliferation of CD4−8+ T lymphocytes but is not required for the deletion of immature CD4+8+thymocytes. J Immunol. 1992;149:3217–3224. [PubMed] [Google Scholar]
- 65.Amakawa R, Hakem A, Kundig T, Matsuyama T, Simard JJ, Timms E, Wakeham A, Mittruecker HW, Griesser H, Takimoto H, et al. Impaired negative selection of T cells in Hodgkin's disease antigen CD30-deficient mice. Cell. 1996;84:551–562. doi: 10.1016/s0092-8674(00)81031-4. [DOI] [PubMed] [Google Scholar]
- 66.Foy TM, Page DM, Waldschmidt TJ, Schoneveld A, Laman JD, Masters SR, Tygrett L, Ledbetter JA, Aruffo A, Claassen E, et al. An essential role for gp39, the ligand for CD40 in thymic selection. J Exp Med. 1995;182:1377–1388. doi: 10.1084/jem.182.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castro JE, Listman JA, Jacabson BA, Wang Y, López PA, Ju S, Finn PW, Perkins DL. Fas modulation of apoptosis during negative selection of thymocytes. Immunity. 1996;5:617–627. doi: 10.1016/s1074-7613(00)80275-7. [DOI] [PubMed] [Google Scholar]
- 68.Verheij M, Bose R, Lin X-H, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, et al. Requirement for ceramide-initiated SAPK/JNK signaling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 69.Chen YR, Meyer CF, Tan T-H. Persistent activation of c-Jun N-terminal kinase JNK1 in γ-radiation-induced apoptosis. J Biol Chem. 1996;271:631–634. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]