Abstract
In this study we describe oxazolone colitis, a new form of experimental colitis. This model is induced in SJL/J mice by the rectal instillation of the haptenating agent, oxazolone, and is characterized by a rapidly developing colitis confined to the distal half of the colon; it consists of a mixed neutrophil/lymphocyte infiltration limited to the superficial layer of the mucosa which is associated with ulceration. Oxazolone colitis is a T helper cell type 2 (Th2)-mediated process since stimulated T cells from lesional tissue produce markedly increased amounts of interleukin (IL)-4 and IL-5; in addition, anti–IL-4 administration leads to a striking amelioration of disease, whereas anti–IL-12 administration either has no effect or exacerbates disease. Finally, this proinflammatory Th2 cytokine response is counterbalanced by a massive transforming growth factor-β (TGF-β) response which limits both the extent and duration of disease: lesional (distal) T cells manifest a 20–30-fold increase in TGF-β production, whereas nonlesional (proximal) T cells manifest an even greater 40–50-fold increase. In addition, anti–TGF-β administration leads to more severe inflammation which now involves the entire colon. The histologic features and distribution of oxazolone colitis have characteristics that resemble ulcerative colitis (UC) and thus sharply distinguish this model from most other models, which usually resemble Crohn's disease. This feature of oxazolone colitis as well as its cytokine profile have important implications to the pathogenesis and treatment of UC.
Keywords: hapten, inflammation, cytokine, T helper cell type 2, transforming growth factor-β
Hapten-induced experimental colitis in mice (i.e., TNBS1 colitis induced by the haptenating agent, 2,4,6-trinitrobenzene sulfonic acid) has proven to be an exceptionally useful model of certain forms of human inflammatory bowel disease. For example, the study of this model has led to the recognition that an IL-12–driven, Th1 T cell–mediated inflammation of the colon is not only prevented by the systemic administration of anti–IL-12 antibody, but can also be treated by such administration (1). This observation has provided the theoretical justification for the use of inhibitors of IL-12, including anti–IL-12 itself, in the treatment of Crohn's disease, an inflammation also dominated by a Th1 T cell response (2–5). Studies of the TNBS colitis model have also shown that administration of TNBS per rectum and per os have very different effects; rectal administration results in severe colitis whereas oral administration (either in the form of haptenated colonic protein or TNBS itself) leads to the induction of suppressor T cells producing TGF-β and the inhibition of colitis caused by TNBS given simultaneously by the rectal route (6, 7). These findings in concert with similar findings in other models establish that mucosal inflammation and/or its prevention depend at least in part on a balance between proinflammatory Th1 T cell responses and antiinflammatory TGF-β responses (1, 6–10).
While, as indicated above, TNBS has proven a useful agent in the induction of experimental colitis, its effects on the colon may be limited by the range of T cell responses it is capable of inducing. In this regard, previous studies imply that haptenating agents differ somewhat with respect to the cell populations they address and thus differ somewhat in the type of immune responses they induce (11–16). On this basis, it seemed possible that administration of other haptenating agents per rectum to mice might elicit a different type of colitis. An additional reason for exploring the colitogenic potential of a second haptenating agent arises from the fact that the feeding of a haptenating agent, as alluded to above, results in antigen nonspecific suppressor T cell responses which could potentially mediate bystander suppression of a colitis induced by an unrelated haptenating reagent (6, 7, 17–23). Thus, the identification of a second colitogenic haptenating reagent would allow one to test the possibility that the feeding of a haptenating agent could nonspecifically suppress (treat) colitis occurring in humans.
In this study we explored these possibilities by studying the colitogenic potential of the “classical” haptenating agent, oxazolone (24, 25). We found that oxazolone at the dose administered elicited a very different colitis than that obtained with TNBS administration in that it induced a colitis involving only the distal half of the colon and had histologic features resembling ulcerative colitis (UC) rather than Crohn's disease. In addition, oxazolone colitis is IL-4– driven rather than IL-12–driven, is prevented by the administration of anti–IL-4, and is exacerbated by the administration of anti–IL-12. Finally, we found that per rectal administration of oxazolone, in contrast to TNBS, induces a TGF-β response which plays an important role in limiting the inflammation both in extent and in time.
Materials and Methods
Induction of Colitis.
Colitis was studied in specific pathogen-free, 5–6-wk-old male SJL/J mice obtained from the National Cancer Institute (NCI, National Institutes of Health [NIH], Bethesda, MD) and maintained in the National Institute of Allergy and Infectious Diseases (NIAID) animal facility. For induction of colitis, mice were first lightly anesthetized with metofane (methoxyflurane; Pitman-Moore, Mundelein, IL) and then 6 mg of the haptenating agent, oxazolone (4-ethoxymethylene-2-phenyl-2-oxazolin-5-one) (Sigma Chemical Co., St. Louis, MO), was administered per rectum via a 3.5 F catheter equipped with a 1-ml syringe. The catheter was inserted so that the tip was 4 cm proximal to the anal verge and the oxazolone was injected with a total volume of 150 μl of a 1:1 H2O/ethanol mixture (50% ethanol). To ensure distribution of the oxazolone within the entire colon and cecum, mice were held in a vertical position for 30 s after the injection. Control mice were administered 50% ethanol alone using the same technique.
Histologic Assessment of Colitis.
Tissues obtained at indicated time points were fixed in 10% buffered formalin phosphate and then embedded in paraffin, cut into sections, and then stained with hematoxylin and eosin. Stained sections were examined for evidence of colitis using as criteria the presence of infiltration with lymphocyte, macrophages, or polymorphonuclear cells, elongation and/or distortion of crypts, crypt abscesses, reduction in goblet cell number, frank ulceration, and edema formation.
Isolation and Purification of Lamina Propria (LP) T Cells.
LP T cells were isolated from freshly obtained colonic specimens using a modification of the technique described by Van der Heijden and Stok (26). Using this technique, the colonic specimens were first washed in HBSS-calcium magnesium free and cut into 0.5-cm pieces. They were then incubated twice, each time for 15 min in HBSS containing EDTA (0.37 mg/ml) and dithiothreitol (0.145 mg/ml) at 37°C. The tissue was then digested further in RPMI containing collagenase D (400 U/ml) and DNase I (0.01 mg/ml) (both obtained from Boehringer Mannheim Biochemicals, Indianapolis, IN) in a shaking incubator at 37°C. The LP cells released from the tissue were then resuspended in 100% Percoll, layered under a 40% Percoll gradient (Pharmacia Biotech AB, Uppsala, Sweden), and spun at 1,800 rpm to obtain the lymphocyte-enriched population accumulating at the 40–100% interface. Finally, the lymphocyte-enriched population was further purified by negative selection using an Isocell mouse T cell isolation column (Pierce Chemical Co., Rockford, IL). The resultant T cell population, when analyzed by flow cytometry, using a FACScan® (Becton Dickinson, Sunnyvale, CA), was shown to be composed of >90% CD3+ T cells.
Isolation and Purification of Spleen T Cells.
For isolation of spleen T cells, spleens were aseptically removed. Cells were dispersed in 1× PBS by applied pressure to spleen tissue. The dispersed splenocytes were then filtered through a 100-μm filter and depleted of RBCs by hypotonic lysis with ACK lysing buffer (Biofluids Inc., Rockville, MD) using a standard technique (27). The cells were then layered on a 40–100% Percoll gradient and spun at 1,800 rpm to obtain the lymphocyte-rich cells at the 40–100% interface. The cells were further purified by negative selection using a mouse T cell isolation column as described above. The resultant T cell population when analyzed by flow cytometry was shown to be composed of >90% CD3+ T cells.
Culture of LP T Cells and Spleen T Cells for Assay of Cytokine Production.
Culture of LP T cells and spleen T cells was generally performed using complete medium consisting of RPMI 1640 (Whittaker M.A. Bioproducts, Inc., Walkersville, MD) supplemented with 3 mM l-glutamine, 10 mM Hepes buffer, 10 μg/ml gentamycin, 100 U/ml each of penicillin and streptomycin (Whittaker M.A. Bioproducts, Inc.), 0.05 mM 2-ME, and 10% by volume FCS (Sigma Chemical Co.). When cells were cultured for evaluation of TGF-β production, serum-free media supplemented with 1% nutridoma-SP (Boehringer Mannheim Biochemicals) were used. To measure cytokine production 106 LP T cells or spleen T cells purified as described above were loaded into uncoated culture wells (to measure production by unstimulated cells) or wells coated with murine anti-CD3ε antibody (clone 145-2C11; PharMingen, San Diego, CA) and 1 μg/ml soluble anti-CD28 (clone 37.51; PharMingen) (to measure production by stimulated cells) and cultured for 48 h (or 60 h in the case of TGF-β). The culture supernatants were then harvested and assayed for cytokine concentration by ELISA. The culture plates used in these studies were 24-well Costar plates (Costar Corp., Cambridge, MA); coating of these plates with anti-CD3 antibody was accomplished by exposing wells to a solution containing anti-CD3ε antibody (10 μg/ml) in carbonate buffer (pH 9.6) overnight at 4°C.
ELISA Assays.
Cytokine concentrations (except for TGF-β) were determined by commercially available specific ELISA assays using duo-paired murine cytokines as per the manufacturer's recommendations (Endogen Corp., Woburn, MA) on Immulon-4, 96-well microtiter plates (Dynatech Laboratories, Inc., Chantilly, VA). TGF-β concentrations were determined using the Predicta TGF-β ELISA assay (Genzyme Corp., Cambridge, MA). Optical densities were measured on a Dynatech MR 5000 ELISA reader at a wavelength of 490 nm.
Treatment of Mice with Anticytokine Antibodies.
Mice were administered various anticytokine antibodies via intraperitoneal injection at the time of disease induction with oxazolone. Rat anti– mouse IL-4 (3 mg per dose), murine anti–human TGF-β1,2,3 (1 mg per dose), and rat anti–mouse IL-12 (2 mg per dose) as well as isotype control (rat IgG2a) were used. Anti–IL-4 consisted of the monoclonal antibody produced from the 11B11 hybridoma cell line donated by Dr. William Paul (Laboratory Immuno-regulation, NIAID, NIH); this antibody was purified from ascites fluid produced in nude mice by precipitation in 50% saturated ammonium sulfate and then dialyzed overnight as per protocol (28). Anti–TGF-β consisted of the l.D11.6 monoclonal antibody purchased in purified form from the Genzyme Corp. Anti–IL-12 consisted of the monoclonal antibody produced by the C17.8 hybridoma cell line supplied by Dr. G. Trinchieri (Wistor Institute, Philadelphia, PA); this antibody was purified from ascites fluid produced in nude mice and was purified using E-Z-SEP purification kits (Middlesex Sciences, Inc., Foxborough, MA) according to manufacturer's protocol. Control rat IgG2a was obtained from Jackson ImmunoResearch (West Grove, PA).
Results
Intrarectal Administration of Oxazolone Induces a UC-like Colonic Inflammation in SJL/J Mice.
In previous studies we and others have shown that the administration of the haptenating agent TNBS induces an IL-12–driven, Th1 T cell colitis resembling Crohn's disease (1, 6, 7, 29, 30). In studies directed at exploring if similar disease is obtained with other haptenating agents, we subjected SJL/J mice to intrarectal administration of oxazolone (6 mg, dissolved in 50% ethanol), a haptenating agent that does not cross-react with TNBS (12, 13, 15, 23). As shown in Fig. 1, SJL/J mice so treated reproducibly developed a rapid onset colitis marked by weight loss and diarrhea peaking by day 2 after oxazolone administration and leading to death of 50% of the mice by day 4. Thereafter, surviving mice at days 4–7 after oxazolone administration slowly increased their weight and by days 10–12 the majority of the mice were free of diarrhea and appeared healthy.
Figure 1.
Wasting disease in mice with oxazolone colitis. Intrarectal administration of oxazolone induces rapid onset of severe bloody diarrhea and wasting disease. Shown are weight changes over a 10-d period occurring in normal SJL/J control mice treated with 50% ethanol alone, and SJL/J mice treated with oxazolone in 50% ethanol. Data are from one representative experiment (out of a total of three experiments). Each point represents average weight data pooled from five mice. Standard errors are indicated.
The above clinical picture of oxazolone colitis is vastly different from TNBS colitis, a colitis with a more gradual onset that peaks much later (7 d after induction) and is more persistent. The two diseases also differ on the macroscopic and microscopic levels. Thus, as shown in Fig. 2, on macroscopic examination of oxazolone colitis at 48 h after oxazolone administration, a severe hemorrhagic colitis which remarkably involves only the distal 50% of the bowel is observed; this is in contrast to TNBS colitis in which inflammation involving the entire length of the bowel is seen. In addition, as shown in Fig. 3, on microscopic examination of involved colon of oxazolone-treated mice (again at 48 h) a superficial inflammation characterized by the presence of epithelial cell loss and patchy ulceration, pronounced depletion of mucin producing–goblet cells, and reduction of the density of the tubular glands is present. In addition, in the LP, a mixed inflammatory cell infiltrate consisting of lymphocytes and granulocytes (the latter consisting mostly of neutrophils and, to a lesser extent, eosinophils) associated with an exudation of cells into the bowel lumen is observed. Finally, the submucosal layer displays marked edema with few inflammatory cells, while in the outer muscle layer one sees little or no evidence of inflammation at all. The foregoing changes in the involved areas of the colon are continuous, but end abruptly in mid-colon and in the noninvolved colon a normal microscopic appearance, i.e., no inflammation, is seen. These various changes are in obvious contrast to those found in TNBS colitis where one observes a transmural inflammation involving all layers of the bowel wall that is not associated with the presence of cellular exudation or the presence of significant numbers of neutrophils or eosinophils.
Figure 2.
Macroscopic changes of colons in hapten-treated mice. Photographs of dissected large intestine of (A) normal SJL/J control mouse treated with 50% ethanol, (B) SJL/J mouse treated with oxazolone in 50% ethanol 2 d after initial rectal administration, and (C) appearance of colons of mice with TNBS-induced colitis 7 d after intrarectal administration. The colons of oxazolone-treated mice display a hemorrhagic edematous colon limited to the distal half of the colon. This is in contrast to colons in TNBS-treated mice which display an inflammation of the entire colon.
Figure 3.

Histologic analysis of the colons from SJL/J mice with hapten-induced colitis and control mice. (A) Photomicrograph of hematoxylin and eosin– stained (H/E) paraffin section of distal colon (×100) from an oxazolone-treated mouse on day 2. Significant edema with inflammatory infiltrates localized to the superficial mucosal layer is present. (B) H/E-stained section of colon (×100) from a TNBS-treated mouse at 7 d after intrarectal administration: a severe transmural colitis with bowel wall thickening is seen. (C) Photomicrograph of H/E-stained section of colon (×150) from an oxazolone-treated mouse showing the presence of superficial hemorrhage, ulceration, distortion of the crypts, loss of goblet cells, and mucin depletion. (D) High-power micrograph of cross-section of H/E-stained section of colon (×400) of an oxazolone-treated mouse, showing a mixed lymphocytic infiltrate localized to the superficial layers of the mucosa and the presence of a luminal cellular exudate. (E) Photomicrograph of H/E-stained cross-section from the proximal colon (×100) of an oxazolone-treated mouse showing only a small amount of mucosal edema and lymphocytic infiltrate. (F) Photomicrograph of H/E-stained cross-section of normal control (×100) colon 2 d after ethanol administration.
The colons of mice killed after the inflammation had clinically subsided (at 10 d after intrarectal administration of oxazolone) showed some evidence of the earlier presence of inflammation. On microscopic analysis of the colonic tissue, one could see evidence of a resolving inflammatory process, including the presence of epithelial regeneration (mitotic figures), reappearance of goblet cells, and a residual inflammatory infiltrate consisting mainly of lymphocytes, but few if any granulocytes. Again, this inflammatory cell infiltrate was confined to the superficial layer of the mucosa and did not involve the outer, muscle layer (data not shown).
Taken together, these macroscopic and microscopic histologic features of oxazolone colitis are highly reminiscent of the features of the human inflammatory bowel disease, UC, and thus differ greatly from TNBS colitis, which more closely resembles Crohn's disease.
The Colonic Inflammation Characteristic of Oxazolone Colitis Is Associated with the Presence of T Cells Having a Th2 T Cell Profile.
To characterize the nature of the immune response in oxazolone colitis, we extracted T cells from inflamed LP and spleen specimens (at 48 h after oxazolone administration) and determined their capacity to secrete cytokines after stimulation with anti-CD3/anti-CD28 antibodies in vitro as described in Materials and Methods. As shown in Fig. 4 A, LP T cells (as well as spleen T cells) of mice with oxazolone colitis manifested an ∼10-fold increase in spontaneous (unstimulated) IL-4 production and a 5-fold increase in anti-CD3/anti-CD28–induced IL-4 production as compared with control mice (P < 0.01). In addition, as shown in Fig. 4 B, mice with oxazolone colitis demonstrated a >10-fold increase in unstimulated production of IL-5 and a 3-fold increase in anti-CD3/anti-CD28– induced production of IL-5 as compared with control mice (P < 0.05). In contrast, as shown in Fig. 4 C, LP T cells (as well as spleen T cells) of mice with oxazolone colitis manifested no increase in unstimulated or stimulated production of IFN-γ as compared with cells from control mice (P > 0.05). These data thus show that oxazolone colitis is associated with the presence of LP cells that are strongly skewed toward production of Th2 cytokines.
Figure 4.
Cytokine production of stimulated and unstimulated LP and spleen T cells in oxazolone-induced colitis. LP T and spleen T cells were isolated from oxazolone and control (ethanol-treated) mice on day 2 and cultured for 48 h in the presence or absence of anti-CD3 and anti-CD28 (see Materials and Methods). Culture supernatants were analyzed for concentrations of IL-4, IL-5, and IFN-γ secretion by specific ELISA. Data shown represent pooled values from three independent experiments. Standard errors are indicated.
In further studies we compared cytokine secretion exhibited by LP T cells isolated from inflamed distal colon with T cells isolated from noninflamed proximal colon. As shown in Fig. 5, whereas anti-CD3/CD28–induced T cells from inflamed areas produced increased amounts of IL-4 as shown above (P < 0.01), T cells from noninflamed areas produce levels of IL-4 similar to that of control T cells (P > 0.05). It should be noted, however, that the T cells from the uninflamed tissue were not completely normal in that they secreted an increased amount of IL-4, as compared with control colonic T cells when cultured in the absence of stimulants (P < 0.01).
Figure 5.
In vitro IL-4 secretion by LP T cells extracted from proximal (noninvolved) and distal (involved) colons of mice with oxazolone- induced colitis as compared with control (ethanol-treated) mice. Data shown represent pooled values from three independent experiments. Standard errors are indicated.
Oxazolone-induced Colitis Is Associated with Increased Production of the Suppressor Cytokine TGF-β.
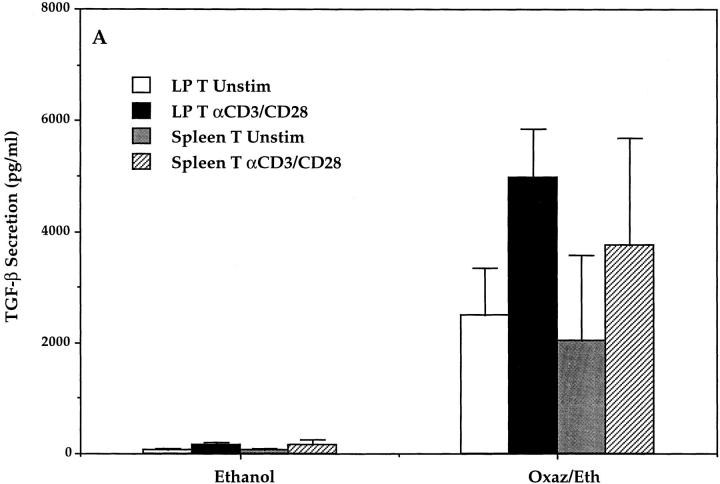
In previous studies of experimental colitis associated with a Th1 T cell response (such as TNBS colitis) it was shown that the T cell response and thus the colitis were counterregulated by the presence of T cells producing TGF-β (6–10). On this basis, we measured the capacity of purified LP T cells from whole colon and spleen specimens of mice with oxazolone colitis to produce TGF-β. As shown in Fig. 6 A, T cells of such mice (either LP T cells or spleen T cells) exhibited a 15-fold increase in TGF-β production when cultured without stimulation or a 30-fold increase in TGF-β production when cultured in the presence of anti-CD3/ CD28, as compared with cells from control mice (P < 0.01). In addition, as shown in Fig. 6 B, purified LP T cells isolated from the uninflamed proximal colonic tissue produced fourfold more TGF-β in the unstimulated state and almost twofold more TGF-β in the stimulated state than T cells isolated from inflamed distal colon tissue (P < 0.05).
Figure 6.
(A) TGF-β secretion of unstimulated and stimulated LP and spleen T cells (LP T cells isolated from whole colons of mice with oxazolone colitis). LP T and spleen T cells were isolated from oxazolone and control (ethanol-treated) mice on day 2, cultured for 48 h in the presence or absence of anti-CD3 and anti-CD28, and culture supernatants were analyzed for concentration of TGF-β secretion by specific ELISA. Data shown represent pooled values from three independent experiments. Standard errors are indicated. (B) In parallel experiments, purified LP T cells were extracted from proximal (noninvolved) and distal (involved) colons of mice with oxazolone colitis and compared with control (ethanol-treated) mice for TGF-β secretion. Data shown represent pooled values from three independent experiments. Standard errors are indicated.
Cytokine Production of LP T Cells of Mice with Oxazolone Colitis During the Resolution Phase of the Colitis.
In a further series of studies of cytokine production of T cells from mice with oxazolone colitis, we determined the cytokines produced by LP T cells obtained from mice who had survived oxazolone colitis and were in a resolution phase of the inflammation (at 10 d after oxazolone administration per rectum). As shown in Fig. 7, LP T cells from such mice, when cultured in vitro with anti-CD3/CD28, exhibited IL-4 production comparable with that of T cells from control mice (P > 0.05), but exhibited IL-5 production as high as that seen at the peak of the inflammation (i.e., at 48 h) (P < 0.01). In addition, while TGF-β production by T cells obtained from the distal colons of mice with resolving colitis compared with that by T cells from colons with acute colitis was diminished, it was still twofold higher than TGF-β production by control LP T cells when cultured in vitro with anti-CD3/CD28 (P < 0.05). Finally, T cells isolated from the proximal, noninvolved colons of mice with resolving colitis continued to secrete high levels of TGF-β (282 pg/ml in the unstimulated state, 5,316 pg/ml in the stimulated state).
Figure 7.
Cytokine production by LP T cells from resolving oxazolone colitis. LP T cells isolated from the distal colons of mice with oxazolone colitis and control ethanol-treated mice at 10 d after intrarectal administration were stimulated in vitro as indicated in the legend to Fig. 4. Culture supernatants were assayed for IL-4, IL-5, and TGF-β by specific ELISA. Data shown represent pooled values from three independent experiments. Standard errors are indicated.
Treatment of Oxazolone Colitis with Various Anticytokine Antibodies.
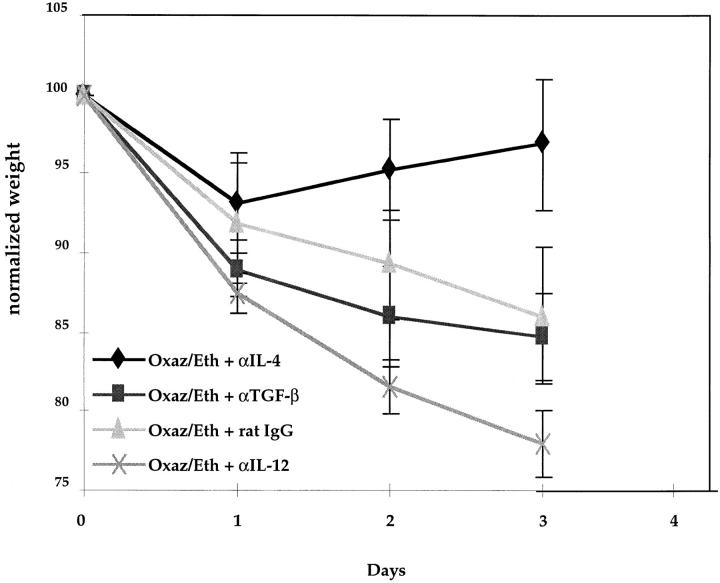
In a final series of studies we sought to evaluate the role of elevated IL-4 and TGF-β secretion in the induction and resolution of oxazolone colitis by the systemic coadministration of antibodies to these cytokines and to an inducer of Th1 responses, IL-12. Accordingly, we administered anti–IL-4 antibody (3 mg), anti–TGF-β (1 mg), anti–IL-12 (2 mg), or control rat IgG (1 mg) intraperitoneally to mice at the time of induction of oxazolone colitis with intrarectal oxazolone administration and then noted the effects of these treatments on the course of oxazolone colitis and on cytokine production. As shown in Fig. 8, while the mice that had received anti–IL-4 lost weight during the first 24 h after oxazolone administration, they quickly regained the lost weight and were near baseline weight within 4 d of oxazolone/antibody administration; in contrast, mice that had received anti–TGF-β or anti–IL-12 manifested a weight loss pattern similar to or more severe than that exhibited by the mice that had been administered control IgG.
Figure 8.
Anti–IL-4 antibodies can prevent the onset of oxazolone colitis. Weight changes in SJL/J mice who received intrarectal oxazolone administration and then treated with either rat IgG control Ab, anti– TGF-β mAb, or anti–IL-12 mAb. After initial reduction in body weight in all groups at day 1, the mice treated with anti–IL-4 showed a significant increase in body weight, whereas mice treated with either IgG control Ab, anti–TGF-β, or anti–IL-12 continued to lose body weight. Each point represents pooled data from five mice. Standard errors are indicated.
Macroscopic appearance of the colons of the mice in the various treatment groups is depicted in Fig. 9. While the colons from the anti–IL-4–treated mice were similar in appearance to that of the control ethanol-treated mice, the colons from the anti–TGF-β–treated mice revealed a severe colitis now involving the entire length of the colon, not just the distal half, as in untreated oxazolone colitis. The effects of anti–IL-12 varied; 50% of the mice manifested no change in the distal colitis seen after oxazolone administration whereas the other half displayed a colitis now involving the entire colon.
Figure 9.
Macroscopic appearance of colons obtained from (A) control (ethanol-treated) mice; SJL/J mice who received intrarectal oxazolone or intrarectal oxazolone plus (B) IgG control Ab; (C) anti–IL-4; (D) anti– TGF-β mAb; or (E) anti–IL-12 mAb (all antibodies were administered intraperitoneally). Mice treated with anti–IL-4 mAb showed a significant resolution in inflammation, whereas mice treated with anti–TGF-β reveal a severe colitis that now involves the entire colon. Colons from one representative experiment out of two are shown.
The above findings concerning the effects of antibody treatment on the development of oxazolone colitis correlated well with antibody treatment–induced changes in cytokine secretion. Thus, as shown in Fig. 10, administration of anti–IL-4 to mice undergoing induction of oxazolone colitis was, as expected, associated with a great reduction in IL-4 and TGF-β production by isolated colonic (lesional) T cells, as compared with cells obtained from untreated mice or mice administered control IgG. On the other hand, administration of anti–TGF-β to mice undergoing induction of oxazolone colitis was associated with an expected reduction in TGF-β production, but a continued increased level of IL-4 production. However, such treatment had no effect on IFN-γ production, i.e., such production remained undetectable (data not shown). Finally, administration of anti–IL-12 to mice undergoing induction of oxazolone colitis was also associated with enhanced production of IL-4, but no change in TGF-β secretion.
Figure 10.
Cytokine production of stimulated and unstimulated LP T cells in oxazolone-induced colitis mice treated with rat IgG control Ab, anti–IL-4, anti–TGF-β mAb, or anti–IL-12 mAb at the time of intrarectal oxazolone administration. LP T were isolated on day 3, cultured for 48 h in the presence or absence of anti-CD3 and anti-CD28, and culture supernatants were analyzed for concentration of (A) IL-4 and (B) TGF-β by specific ELISA. Data from one representative experiment out of two are shown.
The above “clinical” and cytokine data, taken together, strongly suggest that IL-4 has a primary proinflammatory role in the development of oxazolone colitis, whereas TGF-β has an important counterregulatory role in disease induction and the extent of colon involved with disease.
Discussion
Oxazolone colitis, the murine colitis described here, is a new form of experimental colitis that is easily distinguishable from the colitis produced by the intrarectal administration of another contactant, TNBS, or indeed most other forms of experimental colitis. First, it is a rapid onset inflammation that peaks within a few days of oxazolone administration and leads to wasting and bloody diarrhea resulting either in the death of the mouse or complete recovery. Thus, it is different from TNBS colitis, which is a more slowly developing intestinal inflammation that peaks 1 wk after TNBS administration and that tends to persist in surviving mice. Second, the macroscopic and microscopic changes observed in oxazolone colitis and TNBS colitis are very different; oxazolone colitis is marked by inflammation strictly limited to the distal half of the colon, whereas TNBS colitis is a pan-colitic. In addition, at the microscopic level the inflammation of oxazolone colitis is relatively superficial and is characterized by ulceration, a cellular exudate, and a mixed inflammatory infiltrate of lymphocytes, granulocytes, and eosinophils. TNBS colitis, on the other hand, is a full-thickness inflammation which is associated with a dense, lymphocyte/macrophage infiltration occasionally arrayed as a granuloma. Third, and perhaps most importantly, the nature of the immune response in the two colitides are different. In oxazolone colitis the T cell response is an IL-4–driven Th2 T cell response which is marked by elevated IL-4/IL-5 production and normal (low) IFN-γ production and which is prevented by the systemic coadministration of anti–IL-4. In contrast, in TNBS colitis an IL-12–driven Th1 T cell response prevails, marked by an elevated IFN-γ response and an inflammation that is treated by the administration of anti–IL-12 (1). These characteristics of oxazolone colitis and TNBS colitis can be compared with those of human UC and Crohn's disease, the two major forms of human inflammatory bowel disease. Thus, in previous studies conducted by ourselves and others, it has been shown that human UC is associated with elevated IL-5 secretion and normal IFN-γ secretion; nevertheless, it cannot as yet be called a Th2 inflammation because it is not associated with elevated IL-4 secretion, the usual driving force of Th2 responses. On the other hand, established Crohn's disease is quite definitely a Th1 inflammation since IL-12 and IFN-γ secretion is elevated and there is no increase in either IL-4 or IL-5 secretion (2–5, 31). On this basis, it seems reasonable to suggest that oxazolone colitis is a murine model related to but not identical to UC, whereas TNBS colitis is a model related to Crohn's disease.
Most experimental murine models of colitis studied thus far have been shown to be more like TNBS colitis than oxazolone colitis, i.e., IL-12–driven Th1 T cell–dependent inflammatory responses that, as mentioned, histopathologically resemble human Crohn's disease. This is true of colitis models with quite disparate immune defects such as the colitis occurring in SCID mice reconstituted with normal (naive) T cells, as well as IL-10 and IL-2 knockout mice (32– 34). In addition, it is also true of a recently described model of spontaneously occurring intestinal inflammation occurring in the SAMP1/YIT strain of mice that is unique because it involves the small intestine rather than the colon (35). The existence of these various models implies that a number of immunologic conditions occurring in both normal mice and mice with genetic defects of immune function can give rise to a final common pathway: the unregulated production of IL-12 and the resulting development of a Crohn's disease– like intestinal inflammation. Conversely, it implies that the skewed Th1 response occurring in the intestine of patients with Crohn's disease could result from any of several immunologic abnormalities whose only commonality is that they ultimately result in a dysregulated Th1 response.
An important and relevant exception to the above rule that experimental colidites in mice are usually the result of Th1 inflammatory responses is inherent in the characteristics of oxazolone colitis as well as the colitis developing in TCR-α chain knockout mice. Thus, as documented here and as reported previously, in both of these situations an IL-4–driven Th2 colitis develops that histopathologically more closely resembles UC than Crohn's disease (36–38). The existence of these models implies that a number of different immunologic conditions can also lead to another final common pathway, namely the unregulated induction of Th2 T cells. In addition, these two latter models, when considered in conjunction with the Th1 models, provide additional support for the view that immunologic conditions leading to Th2 and Th1 dysregulation are the basis of UC and Crohn's disease, respectively.
The colitis occurring in TCR-α chain knockout mice, in that it is immunologically similar to oxazolone colitis, bears further discussion. TCR-α chain knockout mice develop T cells with low expression of TCRs comprised of β-chain homodimers. Therefore, their tendency to develop Th2 responses may be related to necessarily aberrant interactions with APCs. The colitis that occurs in such mice is a slowly developing disease that appears to originate in the appendiceal tissue and thus initially involves the cecal area of the colon exclusively; ultimately, however, it involves the entire colon and then persists as a chronic inflammation (36–40). This pattern of inflammation differs from that seen in oxazolone colitis, since the latter is a distal rather than a proximal colitis and is an acute but ultimately self-limited disease (provided the mice survive the period of acute inflammation). The reason for these differences awaits further analysis of the cytokine milieu present in the two models, particularly after the inflammation becomes well established. One possibility, based on the fact that the locus and course of oxazolone colitis probably depend on the nature of the elicited TGF-β response, is that oxazolone colitis and the colitis of TCR-α chain knockout mice are associated with qualitatively and quantitatively different TGF-β responses.
One of the striking features of oxazolone colitis is that it is associated with high LP T cell production of TGF-β that is greater in proximal, uninvolved colons than in the distal, involved colon. This, plus the fact that anti–TGF-β administration to mice at the time of intrarectal oxazolone administration leads to pan-colitis (i.e., involvement of the normally uninvolved proximal colon), strongly suggests that the TGF-β response in the proximal colon prevents disease in this segment of bowel, whereas the response in the distal colon, while still relatively high, is not sufficient to prevent disease in this segment of the bowel; it is thus the colonic TGF-β gradient that explains the proximal distribution of disease in oxazolone colitis. In addition, it seems likely that the short-lived nature of the distal inflammation in oxazolone colitis is attributable to the still relatively high TGF-β response in this area of the colon and thus the ability of the latter response to eventually overcome the distal inflammation. On the basis of these findings in oxazolone colitis, it is reasonable to suggest that the generally distal distribution of inflammation in UC is also due to a TGF-β gradient in the human colons. However, this suggestion runs counter to recent findings showing that TGF-β synthesis is higher in the inflamed areas of the UC colon than in uninflamed areas (41). One possible resolution of this discrepancy lies in the fact that in the study cited TGF-β synthesis was measured at the mRNA level in whole colonic biopsies; thus TGF-β produced by all cells was measured and the gradient referred to above may only apply to T cell production of this cytokine.
The high TGF-β response in oxazolone colitis contrasts with the virtually nil TGF-β response in TNBS colitis (unless TNBS is concomitantly given by mouth in the form of haptenated protein) (6, 7). These very different TGF-β responses in oxazolone and TNBS colitis are at least partially explained by recent studies of the differentiation of naive T cells into TGF-β–producing T cells in primary and secondary cultures (42). In these studies it was shown that Th1 responses (i.e., IL-12 and IFN-γ production) inhibit the differentiation of TGF-β–producing cells whereas Th2 responses (IL-4 production) favor such differentiation. Whether or not these effects are completely independent is still somewhat unclear, but, in any case, they may explain the fact that a Th2 T cell–induced inflammation such as oxazolone colitis is associated with a high TGF-β response.
One question that remains to be answered concerning the high TGF-β response in oxazolone colitis is why this response did not prevent the inflammation from developing in the first place, in that much lower TGF-β responses induced by the feeding of TNP haptenated–protein to mice prevents the induction of TNBS colitis (6). The answer to this question may lie in the relative ability of TGF-β– producing T cells to suppress Th1 responses and Th2 responses: it is possible that Th2 responses are resistant to TGF-β–mediated suppression, whereas Th1 responses are susceptible to such suppression. Indirect evidence in favor of this possibility is that certain immune responses normally supported by Th2 cytokines such as humoral IgA responses actually require TGF-β and are not suppressed by the latter except at high TGF-β concentrations (43).
The induction of a Th2-mediated colitis by the rectal administration of oxazolone (at the doses used) and a Th1-mediated colitis by the rectal administration of TNBS raises the fundamental question as to the relation of the nature of the stimulating antigen to the course of T cell differentiation. At the moment this question can only be answered in general terms by the suggestion that the initial interaction between mucosal APCs presenting TNBS or oxazolone to T cells recognizing these haptens results in preferential excretion of IL-12 in the case of TNBS and IL-4 in the case of oxazolone. Whether this relates to antigen affinity for available T cell receptors and subsequent patterns of expression of CD40L and/or B7 is unknown and awaits further study. Nevertheless, this dichotomy does suggest that whether or not an individual susceptible to inflammatory bowel disease develops UC or Crohn's disease may to some extent depend on factors relating to the nature of the initial inducing antigen.
In summary, oxazolone colitis is a new mucosal model of colitis that is an IL-4–driven, Th2 inflammation that has features resembling the human disease, UC. This colitis is regulated in a unique manner by TGF-β production, an observation that may have significance to the factors that regulate its human counterpart.
Footnotes
M. Boirivant's current address is Laboratorio Di Immunologia, Istituto Superiore Di Sanita, Roma, Italy.
Abbreviations used in this paper: LP, lamina propria; TNBS, trinitrobenzene sulfonic acid; UC, ulcerative colitis.
M. Boirivant and I.J. Fuss contributed equally to this work.
References
- 1.Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to IL-12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuss IJ, Neurath MF, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 3.Parronchi P, Romagni P, Annunziato F, Sampognaro S, Becchio A, Giannarini L, Maggi E, Pupilli C, Tonelli F, Romagni S. Type 1 T-helper cell predominance and IL-12 expression in the gut of patients with Crohn's disease. Am J Pathol. 1997;150:823–832. [PMC free article] [PubMed] [Google Scholar]
- 4.Breese E, Braegger CP, Corrigan CJ, Walker-Smith JA, MacDonald TT. Interleukin-2 and interferon-γ secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993;78:127–131. [PMC free article] [PubMed] [Google Scholar]
- 5.Monteleone G, Biancone L, Marasco R, Marrone G, Marasco O, Luzza F, Pallone F. Interleukin-12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- 6.Neurath MF, Fuss I, Kelsall BL, Presky DH, Waegell W, Strober W. Experimental granulomatous colitis in mice is abrogated by induction of TGF-β-mediated oral tolerance. J Exp Med. 1996;183:2605–2616. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, Cong V, Black CA, Ridwan BW, McGhee JR. Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol. 1996;157:2174–2185. [PubMed] [Google Scholar]
- 8.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Reciprocal IFN-γ and TGF-β responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 9.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-β but not interleukin-4 in the suppression of T helper type 1-mediated colitis by CD45RB (low) CD4+T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludviksson BR, Ehrhardt RO, Strober W. TGF-β production regulates the development of the 2,4,6-trinitrophenol-conjugated keyhole limpet hemocyanin- induced colonic inflammation in IL-2-deficient mice. J Immunol. 1997;159:3622–3628. [PubMed] [Google Scholar]
- 11.Asherson GL, Perera MAC, Thomas WR. Contact sensitivity and the DNA response in mice to high and low doses of oxazalone: low dose unresponsiveness following painting and feeding and its prevention by treatment with cyclophosphamide. Immunology. 1979;36:449–459. [PMC free article] [PubMed] [Google Scholar]
- 12.Asherson GL, Zembala M. T suppressor cells and suppressor factor act at the efferent stage of the contact sensitivity skin reaction: their production by mice injected with water-soluble, chemically reactive derivatives of oxazalone and picyrl chloride. Immunology. 1980;42:1005–1013. [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira AM, Hurme M, Kaartinen M, Mäkelä O. Fine-specificity of the immune response to oxazalone. I. Contact sensitivity and early antibodies. J Immunol. 1981;127:2366–2371. [PubMed] [Google Scholar]
- 14.Kraal G, Verdan R, Twisk A. The influence of oxazalone on the recirculation capacity of B and T cells. Immunobiology. 1987;174:326–338. doi: 10.1016/S0171-2985(87)80007-4. [DOI] [PubMed] [Google Scholar]
- 15.Gautan SC, Chikkala NF, Battisto JR. Oral administration of the contact sensitizer trinitrochlorobenzene: initial sensitization and subsequent appearance of a suppressor population. Cell Immunol. 1990;125:437–448. doi: 10.1016/0008-8749(90)90097-b. [DOI] [PubMed] [Google Scholar]
- 16.Kimber I, Foster JR, Baker D, Turk JL. Selective impairment of T lymphocyte activation following contact sensitization with oxazalone. Int Arch Allergy Appl Immunol. 1991;95:142–148. doi: 10.1159/000235419. [DOI] [PubMed] [Google Scholar]
- 17.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor B after antigen-specific triggering. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and a differential upregulation of transforming growth factor-β, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner HL. Oral tolerance. Proc Natl Acad Sci USA. 1994;91:10764–10765. doi: 10.1073/pnas.91.23.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strober W, Kelsall B, Marth T. Oral tolerance. J Clin Immunol. 1998;18:1–30. doi: 10.1023/a:1023222003039. [DOI] [PubMed] [Google Scholar]
- 21.Asherson GL, Ptak W. Contact and delayed hypersensitivity in the mouse. III. Depression of contact sensitivity by pretreatment with antigen and the restoration of immune competence in tolerant mice by normal lymphoid and bone marrow cells. Immunology. 1970;18:99–106. [PMC free article] [PubMed] [Google Scholar]
- 22.Glaister JR. Some effects of oral administration of oxazalone to mice. Int Arch Allergy. 1973;45:828–843. doi: 10.1159/000231083. [DOI] [PubMed] [Google Scholar]
- 23.Asherson GL, Zembala M, Perera MAC, Mayhew B, Thomas WR. Production of immunity and unresponsiveness in the mouse by feeding contact sensitizing agents and the role of suppressor cells in the Peyer's patches, mesenteric lymph nodes and other lymphoid tissues. Cell Immunol. 1997;33:145–155. doi: 10.1016/0008-8749(77)90142-3. [DOI] [PubMed] [Google Scholar]
- 24.Asherson GL, Allwood GG, Mayhew B. Contact sensitivity in the mouse. XI. Movement of lymphoblasts in the draining lymph nodes to sites of inflammation. Immunology. 1973;25:485–492. [PMC free article] [PubMed] [Google Scholar]
- 25.Griswald DE, DiLorenzo JA, Calabresi P. Quantification and pharmacological dissection of oxazalone-induced contact sensitivity in the mouse. Cell Immunol. 1974;11:198–204. doi: 10.1016/0008-8749(74)90019-7. [DOI] [PubMed] [Google Scholar]
- 26.Van der Heijden PJ, Stok W. Improved procedure for the isolation of functionally active lymphoid cells from the murine intestine. J Immunol Methods. 1987;103:161–167. doi: 10.1016/0022-1759(87)90285-7. [DOI] [PubMed] [Google Scholar]
- 27.Kruisbeek, A.M. 1996. In vitro assays for mouse lymphocyte function. In Current Protocols in Immunology. Volume 1. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, and W. Strober, editors. John Wiley and Sons, Inc., New York. 3.1.2–3.1.5. [DOI] [PubMed]
- 28.Andrew, A.M., and J.A. Titus. 1997. Purification and fragmentation of antibodies. In Current Protocol in Immunology. Volume 1. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, and W. Strober, editors. John Wiley and Sons, Inc., New York. 2.7.1–2.7.11.
- 29.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of colonic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 30.Yamada T, Marshall S, Specian RD, Grisham MB. A comparative analysis of two models of colitis in rats. Gastroenterology. 1992;102:1524–1534. doi: 10.1016/0016-5085(92)91710-l. [DOI] [PubMed] [Google Scholar]
- 31.Mullin GE, Maycon ZR, Braun-Elwert L, Cerchia R, James SP, Katz S, Weissman GS, McKinley MJ, Fisher SE. Inflammatory bowel disease mucosal biopsies have specialized lymphokine mRNA profiles. Inflam Bowel Dis. 1996;2:16–26. [PubMed] [Google Scholar]
- 32.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in SCID mice reconstituted with CD45Rbhi CD4+T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 33.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 34.Ehrhardt RO, Ludviksson BR, Gray B, Neurath M, Strober W. Induction and prevention of colonic inflammation in IL-2-deficient mice. J Immunol. 1997;158:566–573. [PubMed] [Google Scholar]
- 35.Kosiewicz MM, Krishnan A, Shah M, Bentz M, Nast C, Matsumoto S, Cominelli F. Characterization of a new spontaneous murine model of inflammatory bowel disease. Gastroenterology. 1998;114:64143. . (Abstr.) [Google Scholar]
- 36.Mombaertz P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:275–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 37.Mizoguchi A, Mizoguchi E, Chiba C, Spiekermann GM, Tonegawa S, Nagler-Anderson C, Bhan AK. Cytokine imbalance and autoantibody production in T cell receptor-α mutant mice with inflammatory bowel disease. J Exp Med. 1996;183:847–856. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi I, Kiyono H, Hamada S. CD4+T-cell population mediates development of inflammatory bowel disease in T-cell receptor α chain-deficient mice. Gastroenterology. 1997;112:1876–1886. doi: 10.1053/gast.1997.v112.pm9178680. [DOI] [PubMed] [Google Scholar]
- 39.Mizoguchi A, Mizoguchi E, Chiba C, Bhan AK. Role of appendix in the development of inflammatory bowel disease in TCR-α mutant mice. J Exp Med. 1996;184:707–715. doi: 10.1084/jem.184.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizoguchi E, Mizoguchi A, Bhan AK. Role of cytokines in the early stages of chronic colitis in TCR α-mutant mice. Lab Investig. 1997;76:385–397. [PubMed] [Google Scholar]
- 41.Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975–984. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- 42.Marth T, Strober W, Seder RA, Kelsall BL. Regulation of transforming growth factor-β production by interleukin-12. Eur J Immunol. 1997;27:1213–1220. doi: 10.1002/eji.1830270524. [DOI] [PubMed] [Google Scholar]
- 43.McIntyre TM, Kehry MR, Snapper CM. Novel in vitro model for high rate IgA class switching. J Immunol. 1995;154:3156–3161. [PubMed] [Google Scholar]