Abstract
We have developed a model of cutaneous leishmaniasis due to Leishmania major that seeks to mimic the natural conditions of infection. 1,000 metacyclic promastigotes were coinoculated with a salivary gland sonicate (SGS) obtained from a natural vector, Phlebotomus papatasii, into the ear dermis of naive mice or of mice preexposed to SGS. The studies reveal a dramatic exacerbating effect of SGS on lesion development in the dermal site, and a complete abrogation of this effect in mice preexposed to salivary components. In both BALB/c and C57Bl/6 (B/6) mice, the dermal lesions appeared earlier, were more destructive, and contained greater numbers of parasites after infection in the presence of SGS. Furthermore, coinoculation of SGS converted B/6 mice into a nonhealing phenotype. No effect of SGS was seen in either IL-4– deficient or in SCID mice. Disease exacerbation in both BALB/c and B/6 mice was associated with an early (6 h) increase in the frequency of epidermal cells producing type 2 cytokines. SGS did not elicit type 2 cytokines in the epidermis of mice previously injected with SGS. These mice made antisaliva antibodies that were able to neutralize the ability of SGS to enhance infection and to elicit IL-4 and IL-5 responses in the epidermis. These results are the first to suggest that for individuals at risk of vector-borne infections, history of exposure to vector saliva might influence the outcome of exposure to transmitted parasites.
Keywords: Leishmania major, sand flies, saliva, skin, cytokines
Experimental models of leishmaniasis have only rarely attempted to reproduce the biology of natural transmission, including a consideration of (a) dose—sand flies inoculate low numbers of parasites, variably estimated at 10–1,000 metacyclic promastigotes (1); (b) saliva—parasites are coinoculated with small amounts of sand fly saliva that contain antihemostatic (2) and potentially immunomodulatory (3, 4) molecules that facilitate blood feeding; and (c) site of inoculation—infection is initiated in the skin, which is organized as specialized dermal and epidermal compartments containing unique cells such as keratinocytes, dendritic epidermal T cells (DETC),1 and Langerhans' cells that enable it to deal with the hostile external environment to which it is frequently exposed (5). When attention has been paid to one or more of these variables, exceptional outcomes have been observed. With respect to dose, infection with 100–1,000 parasites, as opposed to the more usual experimental dose range of 105–107, has been reported to convert genetically susceptible BALB/c mice to a resistant phenotype (6, 7). The discovery that small amounts of sand fly saliva could enhance infection when coinoculated with Leishmania promastigotes (8) was the first to formally invalidate the notion that bloodsucking arthropod vectors are merely “flying syringes.” So far as we are aware, there is only a single published report in which the outcome of cutaneous leishmaniasis initiated in a strictly dermal site has been described (9). In this study, the lesions in BALB/c mice inoculated intradermally versus subcutaneously at the base of the tail were contained in the former and progressive in the later. While L. major infection has also been initiated in the ear dermis, this site was primarily used to study the interaction of Leishmania and dendritic cells (10). Only recently has the ear dermis been used to follow actual lesion development and immune response (Belkaid, Y., M. Lebastard, V. Leclercq, and G. Milon, manuscript submitted for publication). Finally, one potentially important aspect of natural transmission has never been addressed, so far as we are aware; individuals who live in endemic areas will generally be exposed to the bites of uninfected sand flies before the bite that transmits infection. The possibility that preexposure to vector saliva might modulate infection has not been explored.
This report describes a model of cutaneous leishmaniasis that takes into account each of these conditions of natural infection. It describes the outcome of cutaneous leishmaniasis in BALB/c and C57Bl/6 (B/6) mice inoculated with low numbers of metacyclic promastigotes, with and without salivary gland sonicate (SGS), into the ear dermis of both naive mice and mice previously exposed to SGS. The skin, in addition to being the natural site of parasite and saliva deposition, offers the advantage that the lymphomyeloid cells within the inflammatory dermal and epidermal compartments can be recovered, enumerated, and identified (11). In these studies, the function of these cells with respect to cytokine response was characterized for the first time at the single cell level using intracellular staining in combination with flow cytometry. The data reveal a dramatic effect of salivary components on the course and severity of L. major infection in the ear dermis that is associated with a rapid increase in the frequency of epidermal cells producing type 2 cytokines, and a complete reversal of these effects due to the presence of antisaliva antibodies in mice preexposed to SGS.
Materials and Methods
Mice.
Female 8–12-wk-old, BALB/c, C57Bl/6N (B/6) mice were purchased from the National Cancer Institute, Department of Cancer Treatment (Frederick, MD). The IL-4–deficient BALB/c and C57Bl/6 mice were generated from either BALB/c or C57Bl/6 embryonic stem cell lines (12, 13). IL-12p40 −/− mice on the C57Bl/6 genetic background were generated by Magram et al. (14) and were provided by B. Kelsall and S. Gurunathan (National Institute of Allergy and Infectious Diseases [NIAID], Bethesda, MD). SCID mice (C.B.17 scid/scid, H-2d) were provided by K. Tirumalai (NIAID). All mice were kept in the NIAID animal care facility under pathogen-free conditions.
Parasite.
L. major clone V1 (MHOM/IL/80/Friedlin) was cultured in medium 199 supplemented with 20% HI-FCS (Hyclone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 40 mM Hepes, 0.1 mM adenine (in 50 mM Hepes), 5 mg/ml hemin (in 50% triethanolamine), and 1 mg/ml 6-biotin (M199/S). Infective stage metacyclic promastigotes of L. major were isolated from stationary culture (5–6-d old) by negative selection using peanut agglutinin (Vector Laboratories Inc., Burlingame, CA).
Sand Fly Salivary Glands.
SGS were prepared from 7–10-d-old laboratory-bred, female P. papatasii isolated from the Jordan Valley (Israel) as described previously (8). Briefly, 20 pairs of salivary glands were dissected, placed in 20 μl of ice cold Hepes buffer, and stored at −70°C. Immediately before use, the glands were sonicated, microfuged at 8,000 g, and the supernatant was collected. Protein was quantified using the BCA protein assay reagent A (Pierce Chemical Co., Rockford, IL) and comprised 2–3 μg per pair.
Intradermal Infection and Sensitization.
1,000 metacyclic L. major promastigotes with or without 0.2 pair of SGS were inoculated intradermally into the ear dermis using a 27.5 G needle in a volume of 10 μl PBS. Some mice were injected intradermally in one ear two times with 0.2 pair of SGS at 2-wk intervals, and challenged in the opposite ear 2 wk after the last injection. The evolution of the infection was monitored by measuring the diameter of the induration of the ear lesion with a direct reading vernier caliper.
Quantification of Parasites.
The two dermal sheets of the infected ears were separated, deposited dermal side down in DMEM containing 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mg/ml collagenase A (Sigma Chemical Co., St. Louis, MO), and incubated for 2 h at 37°C. The dermal sheets were then homogenized using a Teflon-coated microtissue grinder in a microfuge tube containing 100 μl of M199/S. The tissue homogenates were serially diluted in 96-well flat bottom microtiter plates containing biphasic medium prepared using 50 μl NNN medium with 30% of defibrinated rabbit blood and overlaid with 50 μl M199/S. The number of viable parasites in each tissue was determined from the highest dilution at which promastigotes could be grown out after up to 7 d of incubation at 26°C.
Analysis of the Inflammatory Dermal Site.
The cells in the inflammatory ear dermis were recovered as described previously (11). Briefly, at different time points after intradermal inoculation the ears were collected, rinsed in 70% ethanol with vigorous shaking, and allowed to dry. Using a pair of fine forceps, the ventral and dorsal dermal sheets were separated and immediately processed. The two leaflets were transferred on culture medium dermal side down into a 6-well plate (non–tissue culture-treated; Greiner Labortechnik, Kremsmuenster, Austria) for 6 h. Each well contained 4 ml of RPMI 1640, NaHCO3 with 25 mM Hepes, 10% HIFBS and penicillin/streptomycin. The cell populations spontaneously emigrating out of the dermal layers, containing nonadherent and loosely adherent cells, were pooled. The loosely adherent cells were recovered by further incubation for 20 min at 37°C in PBS without calcium and magnesium, containing 2 mg/ml glucose. The pooled cells were filtered through a 70-μm nylon cell strainer (Becton Dickinson, Mountain View, CA) and washed twice in HBSS without phenol red.
Epidermal Cell Preparation.
At different time points after intradermal inoculation, mice were killed and their ears were collected. The ears were rinsed in 70% ethanol with vigorous shaking and allowed to dry. Using a pair of fine forceps, the ventral and dorsal dermal sheets were separated and transferred dermal side down on DMEM-penicillin/streptomycin with 0.5% trypsin (USB Biologicals, Cleveland, OH) for the dorsal face and 1% trypsin for the ventral face. The sheets were incubated for 30 min at 37°C. The epidermal cells were recovered as described previously (15) with modifications. In brief, the epidermis was separated from the dermis and deposited on a 70-μm nylon cell strainer (Becton Dickinson), which was placed in a petri dish containing DMEM plus 20% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.05% DNase (Sigma Chemical Co.). The filter was shaken gently for 2 min, the cells passing through the filter were washed, and 3 × 106 cells in 5 ml were distributed in 6-well plates. After incubation at 37°C for 6 h in the presence of 10 μg/ml Brefeldin A (Sigma Chemical Co.), the cells were collected, fixed with 4% paraformaldehyde in PBS for 20 min, and washed with cold PBS containing 0.1% BSA before staining.
Immunolabeling and Flow Cytometry.
Each labeling was carried out on 106 cells for 30 min on ice in a 100 μl volume in U bottom 96-well microtiter plates. The dermal cells were incubated with 10% normal mouse serum in PBS containing 0.1% BSA and 0.01% NaN3 before being incubated with anti-Fc receptor antibody (2.4G2; PharMingen Co., San Diego, CA). The double staining was done by using directly conjugated antibodies incubated simultaneously. The dermal inflammatory cells were identified by characteristic size (FSC) and granulosity (SSC) combined with two-color analysis, as described previously (11). Briefly, the dendritic leukocytes were identified as large cells, MHC class II (25-9-17; PharMingen) bright, and F4/80 positive (A3-1; Caltag Lab, Burlingame, CA). The mononuclear phagocytes were identified as F4/80 positive and MHC class II low or negative. The neutrophils were identified as small cells, Ly-6G bright (RB6-8C5; PharMingen), and negative for F4/80 (or MHC class II); the eosinophils by their granulosity associated with F4/80 lightly positive and MHC class II negative. The CD4 lymphocytes were identified by their small size and by CD4 (RM4-5; PharMingen) and CD3 (145-2C11; PharMingen) expression. The isotype controls used were rat IgG2b (A95-1; PharMingen) and rat IgG2a (R35-95; PharMingen). After staining, the cells were fixed again with 1% paraformaldehyde and washed. For each sample, 104 cells were analyzed. Each sample was cytospun in parallel and stained with Diff-Quik solution (Dade Behring Inc., Düdingen, Switzerland); the percentages of neutrophils, eosinophils, and mononuclear phagocytes estimated by microscopic examination of stained cells were similar to those obtained by flow cytometry.
For surface staining of epidermal cells, the cells were incubated, after trypsin treatment, in complete DMEM overnight to allow reexpression of surface markers, and identified as either γδ T cells by staining with anti-γδ T cell receptor antibody (GL3; PharMingen), or as Langerhans' cells by two-color staining using anti–MHC class II (25-9-17; PharMingen) and anti-B7.1 (1G10; PharMingen). For intracellular staining of cytokines, the fixed epidermal cells were washed twice with cold PBS-BSA 0.1%, then incubated with anti-Fc receptor antibody in PBS containing 10% normal mouse serum, 0.1% saponin (Fisher Scientific Co., Pittsburgh, PA), 0.1% BSA, 1 mM CaCl, 1 mM MgSO4, and 40 mM Hepes (permeabilization buffer). After washing, the cells were incubated with the anticytokine antibodies and their corresponding isotype control. All of the anticytokine antibodies were obtained from PharMingen and were directly conjugated to R-PE: anti–TNF-α, (MP6-XT22), anti–IL-3 (MP2-8F8), anti–IL-5 (TRFK5), anti–IL-6 (MP5-20F3), anti–IL-12 (C15.6), anti–IFN-γ (XMG1.2), and isotype control, rat IgG1 (R3-34); anti–IL-2 (JE56-5H4), anti–IL-4 (BVD4-1D11), anti–IL-10, (JES5-16E3), and their isotype control, rat IgG2b (R35-38); anti–GM-CSF-PE (PI-22E9) and its isotype control, rat IgG2a (R35-95), anti– MCP-1 (2H5), and its isotype control hamster IgG (G235-2356). After staining, the cells were fixed again with 1% paraformaldehyde and washed. For each sample, 2 × 104 cells were analyzed. The data were collected and analyzed using CELLQuest™ software and a FACScalibur® flow cytometer (Becton Dickinson Immunocytometry System, San Jose, CA).
Lymphocyte Culture and Cytokine Assays.
For measurement of in vitro cytokine production, single-cell suspensions of retromaxillar lymph nodes draining the infected ears were prepared aseptically at various times after infection. The cells were diluted to 8 × 106 cells/ml, and dispensed into 96-well plates without antigen or with soluble L. major antigen (SLA) (25 μg/ml) or Con A (10 μg/ml) in 100 μl of complete RPMI containing 0.05 mM β-mercaptoethanol. Cultures were incubated at 37°C in 5% CO2. Supernatant fluids were harvested at 72 h and assayed for IL-4 and IFN-γ by ELISA as described previously (16).
Analysis of Antisaliva Antibodies by ELISA and Western Blotting.
The IgG fraction of control mouse serum and serum from mice injected intradermally twice with SGS were purified by Immunopure Plus (A) IgG purification kit (Pierce Chemical Co.). The presence of antisaliva antibodies in the IgG fraction or in whole sera was determined by ELISA. 96-well microplates were coated overnight at 4°C with 50 μl SGS diluted to 10 pair/ml in the coating buffer (0.1 M Na2HCO3). After washing with PBS-Tween, and saturation with PBS-FBS 20%, serial dilutions of sera from control or immunized mice were incubated overnight at 4°C. After washing, plates were incubated with peroxidase-conjugated goat anti–mouse IgG (heavy and light chain specific) antibody in PBS-BSA 1%, followed by TMB peroxidase substrate (Kirkegaard and Perry Laboratories Inc., Gaithersburg, MD). The absorbance was recorded at 405 nm. Western blotting was performed by electrophoresis of SGS (1 gland per lane) under reducing conditions. After transfer to nitrocellulose using the Novex Xcell Blot II module (Novex, San Diego, CA) the membranes were cut into strips, blocked with 5% dried skim milk in PBS-0.05% Tween 20 for 1 h at room temperature, then incubated with serum (1:200 dilution) in blocking buffer for 1 h, followed by the anti–mouse IgG peroxidase conjugate (1:1,000). Bands were visualized using the TMB membrane peroxidase substrate (Kirkegaard and Perry Laboratories Inc.).
Results
Effect of Sand Fly Saliva on the Course of L. major Infection in the Mouse Ear Dermis.
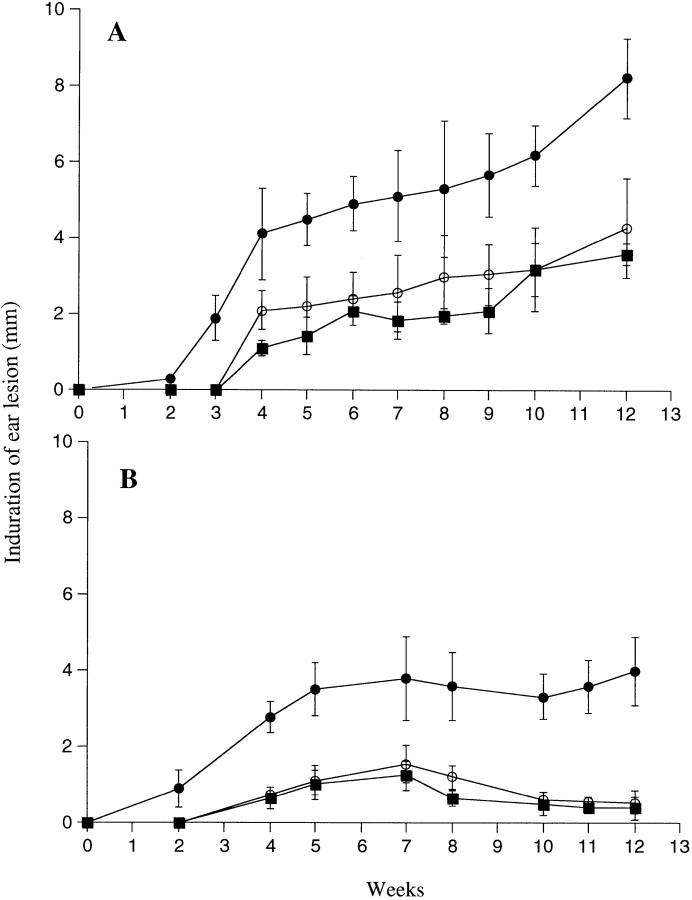
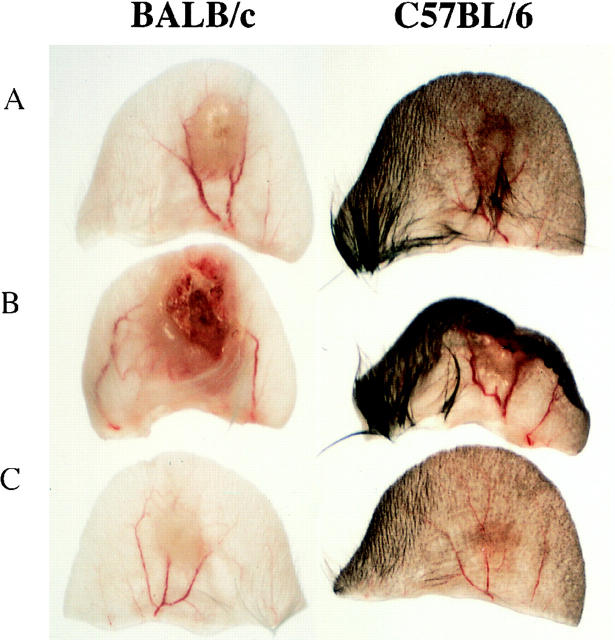
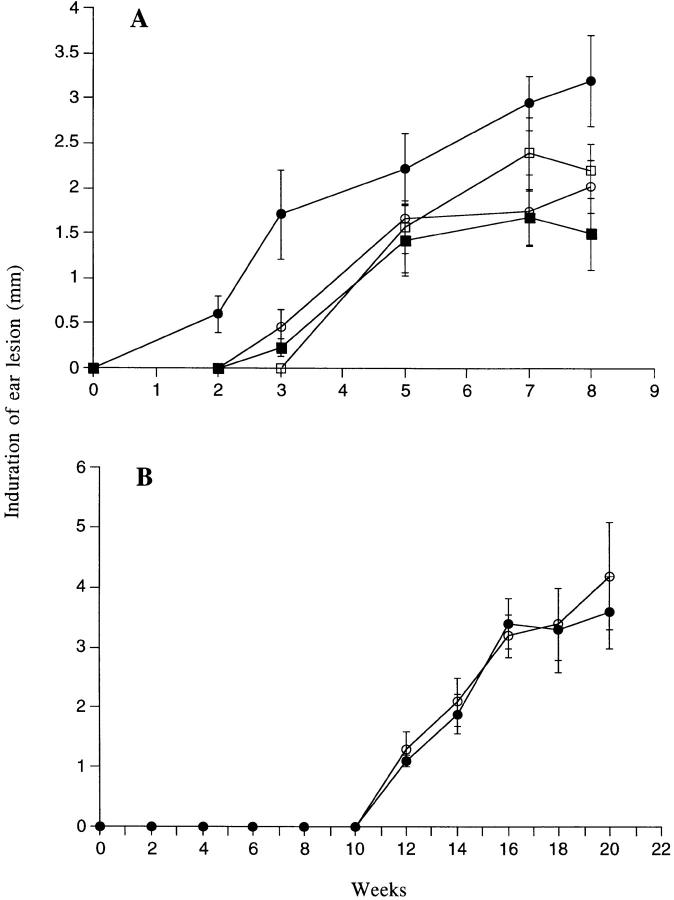
It has been shown previously that the coinoculation of sand fly saliva with L. major into the mouse footpad increases the infectivity of the parasite (8, 17–19). We attempted to extend these observations using a more physiologic model of infection in which a low number (1,000) of L. major metacyclics promastigotes was inoculated into the ear dermis with or without 0.2 pair of SGS. In BALB/c mice infected with parasites alone (Fig. 1 A), the dermal lesions were first detectable around week 4; they increased slowly in size, accompanied by ulceration and tissue necrosis after 2–3 mo (Fig. 2 A). The lesions continued to progress for up to 7 mo, at which time extensive necrosis and dermal erosion were observed. In contrast, the lesions in the B6 mice initiated using parasites alone appeared around week 4, peaked in size at 7 wk, then began to resolve so that by 10 wk only a small degree of induration (<1 mm) was measurable, persisting for up to 7 mo (Fig. 1 B). Even the maximum size lesions in B/6 mice remained nonulcerative and nonnecrotic (Fig. 2 A). Thus the genetically controlled nonhealing and healing phenotypes of BALB/c and B/6 mice, respectively established using higher numbers of parasites inoculated in a subcutaneous site, are maintained in a dermal model using 1,000 metacyclic promastigotes. When the same number of parasites was coinoculated with 0.2 pair SGS from P. papatasii, the sizes of the lesions in both mouse strains were significantly larger (P < 0.01) at virtually all time points examined, beginning at 3 wk for BALB/c and at 2 wk for B/6 mice. The dermal lesions in BALB/c mice appeared 1–2 wk earlier, expanded rapidly for the first 4 wk, then progressed at a slower rate until they began to ulcerate and necrose by 6–8 wk (Fig. 2 B). By 12 wk extensive dermal erosion had occurred. For B/6 mice, the dermal lesions progressed to approximately three times the maximum size seen in the controls, they became ulcerative and necrotic (Fig. 2 B), and while their growth after 7 wk appeared to be contained, the lesions did not diminish in size or severity for up to 8 mo after infection.
Figure 1.
Size of induration of the ear lesion for (A) BALB/c or (B) C57BL/6 mice after intradermal inoculation of 1,000 L. major metacyclic promastigotes alone (○) or with 0.2 pair of SGS to naive mice (•) or to SGS presensitized mice (▪). Mean induration in mm ± 1 SD, 8–10 mice per group.
Figure 2.
Photomicrograph of BALB/c or C57BL/6 ears 10 wk after intradermal inoculation of 1,000 L. major metacyclic promastigotes alone (A), or with 0.2 pair of SGS to naive (B), or to SGS presensitized mice (C).
The size of the dermal lesions correlated well with numbers of viable parasites contained within the dermal site. When evaluated 8 wk after infection, BALB/c and B/6 mice coinoculated with SGS had a 1,000- and a 100-fold increase, respectively, in the number of lesion amastigotes (Table 1). The table also includes the dermal parasite quantitation of B/6 mice 8 mo after infection, in which the mice treated with SGS still had a 100-fold increase in the number of lesion amastigotes compared with mice infected with parasites alone. It is important to note that even the control mice continued to harbor parasites in the skin.
Table 1.
Effect of SGS and SGS Presensitization on the Number of Tissue Amastigotes in Mouse Ears 8 wk or 8 mo after Infection
| L. major | L. major+ SGS | L. major+ SGS-sensitized mice | ||||
|---|---|---|---|---|---|---|
| BALB/c 8 wk | 1.3 × 103 ± 4.2 × 102 | * 1.2 × 106 ± 1.1 × 105 | 9.8 × 103 ± 7.5 × 102 | |||
| C57BL/6 8 wk | 1.4 × 103 ± 6.2 × 102 | * 1.2 × 105 ± 2.3 × 104 | 1.6 × 103 ± 5.1 × 102 | |||
| C57BL/6 8 mo | 3.8 × 102 ± 1.5 × 102 | ‡ 1.4 × 104 ± 1.7 × 103 | 1.6 × 103 ± 4.3 × 102 |
Values are mean number of amastigotes per ear ± 1 SD, 8 ears per group. Values are significantly greater (
P < 0.001 and
P < 0.05) than the parasite numbers in the two other groups.
The influence of SGS on the outcome of L. major infection was reflected in the levels of L. major–specific IFN-γ and IL-4 produced in vitro by cells from the draining lymph node 8 wk after infection (Table 2). In control mice, the amount of IL-4 produced by lymph node cells from BALB/c mice was roughly 10-fold higher than B/6, consistent with the nonhealing status of BALB/c mice after infection in the dermal site. These levels of IL-4 were increased by 1.5- and 3-fold, respectively, in BALB/c and B/6 mice infected in the presence of saliva. The IFN-γ response of SGS coinoculated B/6 mice was also significantly reduced. Thus, vector saliva shifted the adaptive immune response in the direction of Th2 responsiveness in both BALB/c and B/6 mice.
Table 2.
Effect of SGS and SGS Presensitization on Leishmania–specific Cytokine Production by Lymph Node Cells Draining the Ear 8 wk after Infection
| L. major | L. major + SGS | L. major + SGS-sensitized mice | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-4 | IFN-γ | IL-4 | IFN-γ | IL-4 | IFN-γ | |||||||
| pg/ml | pg/ml | pg/ml | pg/ml | pg/ml | pg/ml | |||||||
| BALB/c | 2,942 ± 215* | 8,981 ± 925 | ‡ 4,263 ± 620 | 6,937 ± 850 | 544 ± 155 | 2,510 ± 620 | ||||||
| C57BL/6 | 172 ± 49 | 7,896 ± 850 | ‡ 529 ± 133 | § 3,127 ± 666 | 100 ± 66 | 8,999 ± 450 | ||||||
Mean cytokine concentration ± 1 SD produced by lymph node cells (four lymph nodes per group) assayed 72 h after stimulation with 25 μg/ml SLA.
Significantly greater than the IL-4 produced by cells of the same mouse strain in the two other groups; P < 0.01.
Significantly less than the IFN-γ produced by cells of the same mouse strain in the two other groups; P < 0.02.
The Effect of Preexposure to SGS on the Course of L. major Infection in the Mouse Ear Dermis.
Because of the likelihood that individuals who are at risk of L. major infection are preexposed to the bites of uninfected sand flies, we investigated whether this exposure might modify the host response to parasites plus SGS. Mice were sensitized twice, 2 wk apart, by intradermal inoculation in the left ear using the equivalent of 0.2 pair of SGS prepared from 10-d-old sand flies. They were challenged in the right ear 2 wk later with parasites plus SGS. As shown in Fig. 1, preexposure to SGS completely abrogated the effect of the saliva on the size of dermal lesions for both BALB/c and B/6 mice. In each case, the SGS-sensitized mice behaved like the control mice injected with parasites alone in terms of lesion size and tissue pathology. Their tissue parasite loads at 8 wk were reduced ∼100-fold compared with SGS naive mice infected in the presence of saliva (Table 1). Finally, the abrogation of the salivary effect on infection was associated with a reduced level of L. major–specific IL-4 production by lymph node cells 8 wk after infection (Table 2). In fact, for both BALB/c and B/6 mice, the IL-4 produced was less than even control mice infected in the absence of SGS. The IFN-γ response in SGS-sensitized BALB/c mice was also significantly reduced, although the ratio of IFN-γ and IL-4 still indicated a shift toward Th1 responsiveness in these mice compared with the response observed in the SGS naive animals after parasite delivery in the presence of saliva.
Analysis of the Early Dermal and Epidermal Reactivity after Challenge in Naive Mice and in Mice Sensitized to SGS.
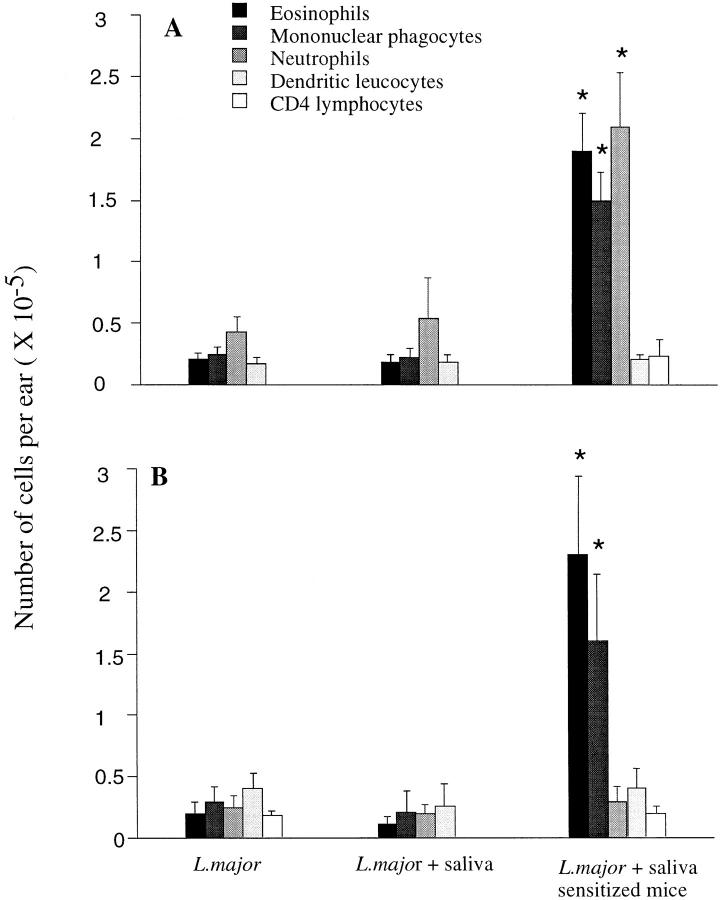
The mechanism by which vector saliva enhances leishmanial infection remains to be clarified. One possibility that has not been addressed previously is that the antihemostatic activities of saliva promote cellular recruitment into the skin, including mononuclear phagocytes, allowing a higher proportion of parasites to find appropriate cells for infection within the inoculation site. Since the dermis is the site of cellular recruitment into the skin, which can occur from blood and the overlying epidermis, we analyzed the leukocyte trafficking within the dermis after intradermal inoculation of the parasite alone or in the presence of SGS at 2, 4, 6, 12, 18, and 48 h after injection. The cells emigrating from the L. major–loaded dermis were recovered and identified by flow cytometry using a combination of antibodies, as described previously (11). The number of red blood cells present in the dermis was approximately five times higher in ears coinoculated with saliva. Surprisingly, when the numbers of leukocytes were compared, no significant difference between the control ears and the SGS coinoculated ears was seen during any of the early time points examined (Fig. 3, A and B). Even when the dermal sheets were treated with collagenase to recover immobilized cells, there was no indication that SGS elicited an influx of leukocytes into the dermis (data not shown). By comparison, in the mice preexposed to SGS, the dermal inflammatory response to parasites plus SGS was significantly altered. The number of leukocytes recruited into the dermis at 18 h was four- to fivefold greater, and was composed mainly of mononuclear phagocytes, eosinophils, and neutrophils in BALB/c mice, and of mononuclear phagocytes and eosinophils in B/6 mice. The kinetics of this response, with an accumulation of mononuclear phagocytes and eosinophils beginning at 6 h and peaking at 48 h, plus the fact that this response was abrogated after in vivo depletion of CD4+ cells using GK1.5 antibody (data not shown), indicated a delayed type hypersensitivity (DTH) response to salivary components in SGS-sensitized mice. The influx of both mononuclear phagocytes and eosinophils suggests that this response is not especially polarized in terms of the CD4+ subsets that mediate the DTH.
Figure 3.
Leukocytes present in the dermal compartment after intradermal inoculation of L. major into the ear of (A) BALB/c or (B) C57BL/6 mice. Mice were injected with 1,000 of L. major metacyclic promastigotes alone or with 0.2 pair of SGS to naive or to SGS presensitized mice. The ears were processed 18 h after inoculation, the cells obtained from four to five ears per group were pooled, and the different populations of leukocytes were identified by staining and flow cytometry as described in Materials and Methods. The data shown are the mean ± 1 SD of three separate experiments. * Values significantly greater (P < 0.001) than the values in the comparable cell populations of the two other groups.
The first line of defense of the body is the epidermis, which in the mouse contains three major reactive cell populations: keratinocytes, DETC, and Langerhans' cells. The number of epidermal cells recovered was high (∼2.5 × 106 cells/ear for BALB/c and 2.9 × 106 cells/ear for B/6). Approximately 5–7% of the cells stained bright using anti-γδ T cell receptor antibody, and 5–10% were Langerhans' cells, based on their strong dual staining with anti–MHC class II and anti-B7.1 (data not shown). The remaining cells, presumably keratinocytes, were not distinguishable by surface staining. Each of these populations secretes a distinct set of cytokines (20), which we attempted to analyze at the single cell level by intracellular staining and flow cytometry. The epidermal cells were collected 6 h after inoculation and incubated overnight in the presence of Brefeldin A to allow intracellular accumulation of produced cytokines. The frequency of cells staining positive for each cytokine was determined by two-color analysis of cells singly stained with PE-conjugated anticytokine antibody to compensate for the considerable autofluorescence that is characteristic of the epidermal cells. The steady state frequencies of cytokine-positive cells were determined using epidermal cells recovered from uninjected ears. Of these, only a small proportion stained positive for IL-5 (0.5% for BALB/c) or IL-3 (0.7% for BALB/c and 0.9% for B/6, data not shown). The steady state cells were negative for the remainder of the cytokines examined. Fig. 4 shows the dot plot analyses from one of two separate experiments in which a complete analysis of eight different cytokines was carried out, showing the frequency of epidermal cells producing each of the cytokines after intradermal injection of PBS, 1,000 metacyclic promastigotes, or metacyclics plus SGS. The needle injection of 10 μl of PBS induced production of cytokines in response to tissue injury: primarily IL-5, IL-3, TNF-α, and IFN-γ. The especially strong TNF-α response produced by B/6 mice was observed in both experiments, as was the small increase in the frequency of epidermal cells producing IFN-γ and MCP-1. Whereas the strong TNF-α response in BALB/c seemed to be induced by the parasites, this was not observed in each analysis, and it is more likely that in this experiment we failed to detect the TNF-α made in response to the control needle injection. Changes in the frequency of cells producing the other cytokines examined were not observed. When the parasite was inoculated with 0.2 pair SGS, there was an increase in the frequency of epidermal cells producing Th2 cytokines, including IL-4 (1.9%), IL-5 (8.4%), and GM-CSF (1.6%) for BALB/c, and IL-4 (4.3%) and IL-5 (5.5%) for B/6 mice. The effect of SGS preexposure was to virtually eliminate the early IL-4 response in the epidermis after injection of parasites plus SGS (1.9 vs. 0.1% in BALB/c mice; 4.3 vs. 0.7% in B/6 mice). In BALB/c mice, the frequencies of cells staining for IL-5 and GM-CSF were also reduced in SGS-sensitized mice. These results indicate that SGS induces rapid production of type 2 cytokines by epidermal cells, and that prior exposure to SGS abolishes this host response.
Figure 4.
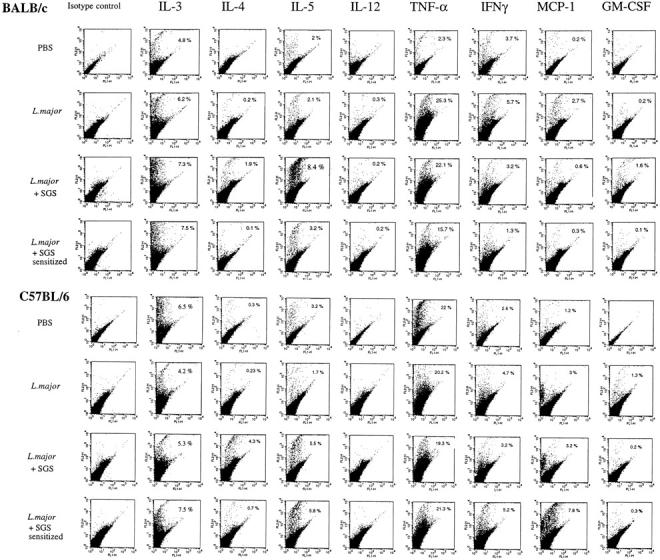
Two-parameter dot plots of epidermal cells of BALB/c and C57BL/6 mice stained for intracellular cytokines produced at 6 h in response to the intradermal inoculation of PBS, or 1,000 L. major metacyclic promastigotes alone, or 1,000 metacyclics plus 0.2 pair of SGS, to naive or to SGS presensitized mice. After recovery, the epidermal cells from six ears per group, were pooled and preincubated with Brefeldin A for 6 h before being stained. Numbers represent the percentage of cells with FL-2 signals for the particular cytokine that were greater than the background signals established using the PE-isotype control (IgG1). The data shown are from a single experiment, representative of two separate experiments.
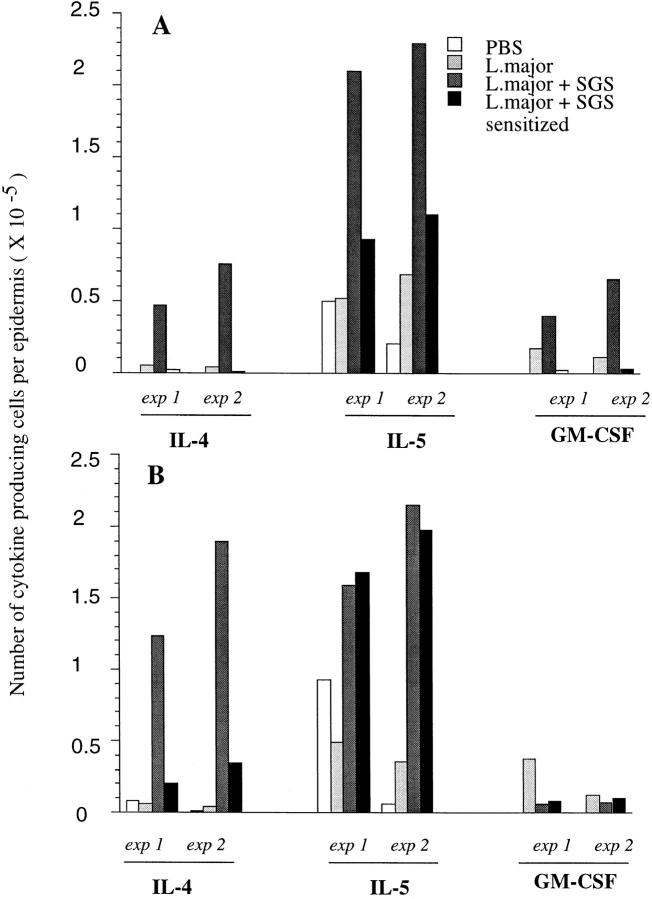
In Fig. 5, the data from the flow analyses for the two experiments were replotted in order to compare the absolute number of cytokine producing cells per epidermis. Only the data for IL-4, IL-5, and GM-CSF are shown, since these were the only cytokines affected by coinoculation of SGS. Considering the large number of epidermal cells recovered per ear, the absolute number of cells producing these cytokines in response to SGS is remarkably high (e.g., 1.24–1.88 × 105 cells/ear producing IL-4 in B/6 mice). The inability of SGS to elicit these responses in SGS presensitized animals is clear; for BALB/c mice the numbers of IL-4, IL-5, and GM-CSF producing cells in the two experiments were reduced by 94 or 98%, 56 or 58%, and 94 or 96%, respectively, whereas for B/6 mice the number of IL-4–producing cells was reduced by 89 or 82%, and the IL-5 response appeared unaffected.
Figure 5.
Total number of epidermal cells per ear producing type 2 cytokines in (A) BALB/c and (B) C57Bl/6 mice, 6 h after intradermal inoculation of PBS, L. major, L. major + SGS, and L. major + SGS in SGS-sensitized mice. Data from two separate experiments are shown, determined from the two-color FACS® analysis of epidermal cells pooled from 6 ears per group, and calculated as the average number of epidermal cells per ear × the percentage of cells staining positive for each cytokine.
Effect of SGS on the Course of L. major Infection in IL-4– deficient, IL-12p40–deficient, and SCID mice.
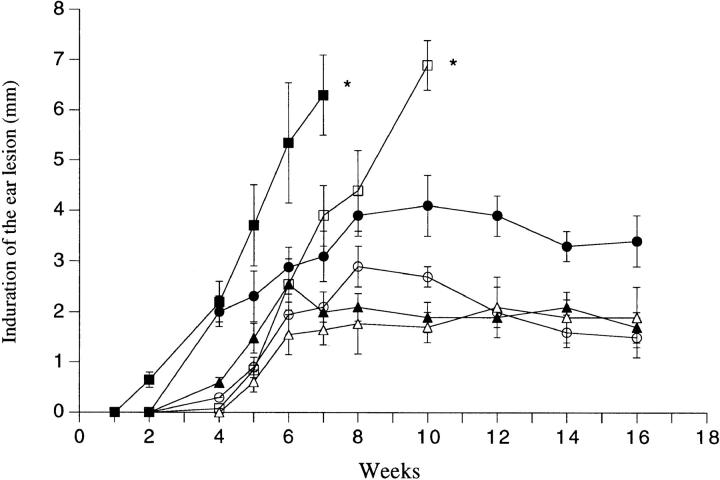
The ability of SGS to elicit an early type 2 response in the epidermis, in association with its ability to exacerbate disease, led us to investigate the effect of SGS on disease exacerbation in IL-4– deficient mice. While IL-4 deletion can itself, depending on the dose and strain of L. major used, convert BALB/c mice into a more resistant phenotype (21), the development of nonhealing dermal lesions initiated with 1,000 Friedlin strain metacyclics was unaffected by the IL-4 deficiency in BALB/c mice (Fig. 6 A). However, the IL-4 deficiency did eliminate any effect of SGS on disease exacerbation in these mice. In IL-4–deficient B/6 mice, SGS produced a slight enhancement of lesion size during the early stage of development, but the effect was much less than that observed in the wild-type mice, which again developed large, nonhealing, necrotic dermal lesions (Fig. 7). Thus, on both L. major–susceptible and –resistant genetic backgrounds, IL-4 deficiency abrogates the ability of SGS to promote infection in a dermal site. In contrast, dermal infections in IL-12p40 knockout B/6 mice, which as expected were nonhealing and uncontrolled even when infected with parasites alone, were exacerbated even further by the inclusion of SGS in the inoculum (Fig. 7). Whereas in the absence of SGS the dermal lesions first appeared in the IL-12p40 knockout mice at week 5 and progressed rapidly with extensive dermal erosion present at week 10, in the SGS coinoculated IL-12p40 knockout mice, each of these events were advanced by 3–4 wk. Lastly, the ability of SGS to exacerbate infection was investigated in SCID mice. Lesion appearance was delayed until week 10 for mice infected with or without SGS, after which the development of progressive, uncontained dermal lesions was identical in each group (Fig. 6 B). These data indicate that both the early onset of lesion development and the exacerbative effect of saliva require functional lymphocytes.
Figure 6.
Size of the induration of the ear lesion in (A) BALB/c wild-type mice (○, •) or in IL-4–deficient BALB/c mice (□, ▪) after intradermal inoculation of 1,000 metacyclic promastigotes alone (open symbols) or with 0.2 pair of SGS (closed symbols) and in (B) BALB/c SCID mice inoculated with the parasite alone (○) or with 0.2 pair of SGS (•). Mean induration in mm ± 1 SD, six to eight mice per group.
Figure 7.
Size of the induration of the ear lesion in C57Bl/6 wild-type mice (○, •); C57Bl/6 IL-4–deficient mice (▵, ▴); or C57Bl/6 IL-12p40–deficient mice (□, ▪) after intradermal inoculation of 1,000 metacyclic promastigotes alone (open symbols) or with 0.2 pair of SGS (closed symbols). Mean induration in mm ± 1 SD, 6–10 mice per group.
Analysis of Antisaliva Antibodies in SGS-sensitized Mice and Their Role in Neutralizing the Effects of SGS on L. major Infection and Epidermal Cytokine Responses.
One explanation for the inability of SGS to enhance infection in mice presensitized to SGS is that these animals make antisaliva antibodies that neutralize its bioactivity. Anti-SGS antibodies in mice immunized twice with 0.2 salivary gland equivalents were readily detected by ELISA (data not shown). A Western blot analysis of the serum showed four major bands recognized by the serum of the immunized BALB/c and B/6 mice (Fig. 8).
Figure 8.
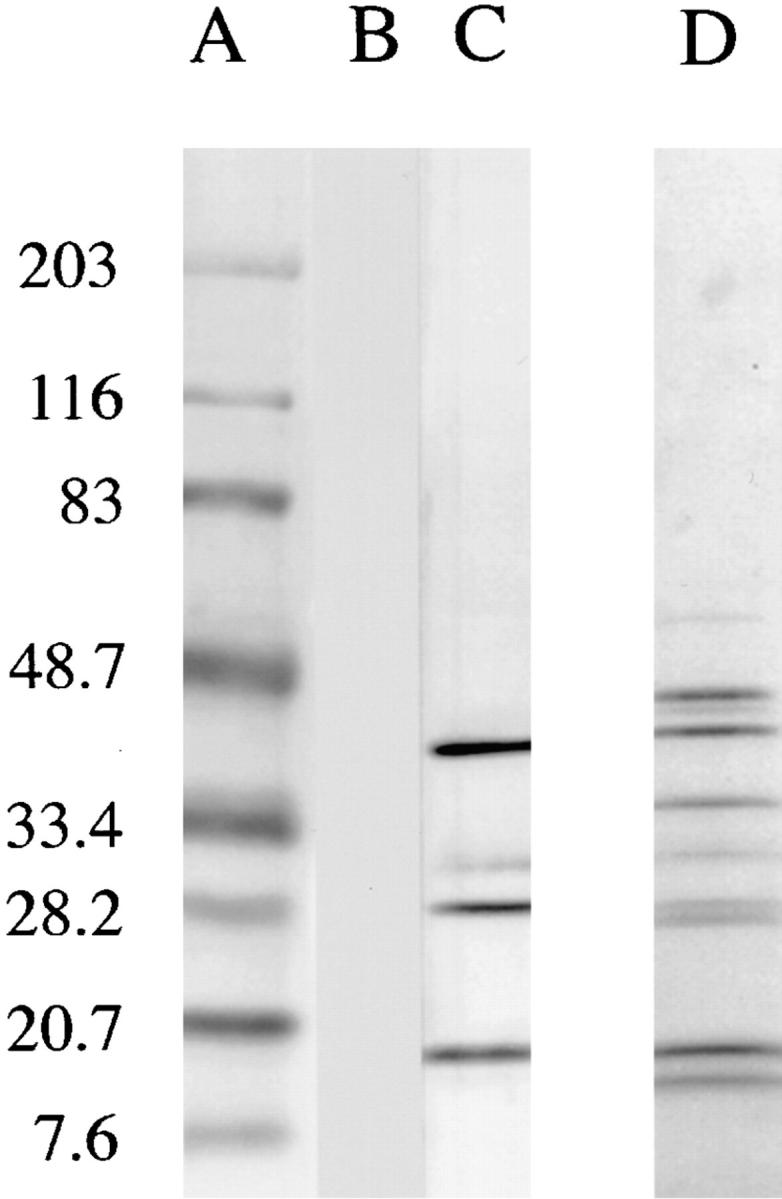
Western blot analysis of salivary glands (1 gland per lane) using pooled sera from unimmunized BALB/c mice (lane B), BALB/c mice injected intradermally two times with 0.2 pair SGS (lane C); Coomassie blue– stained gel before transfer is shown in lane D; molecular weight markers are shown in lane A.
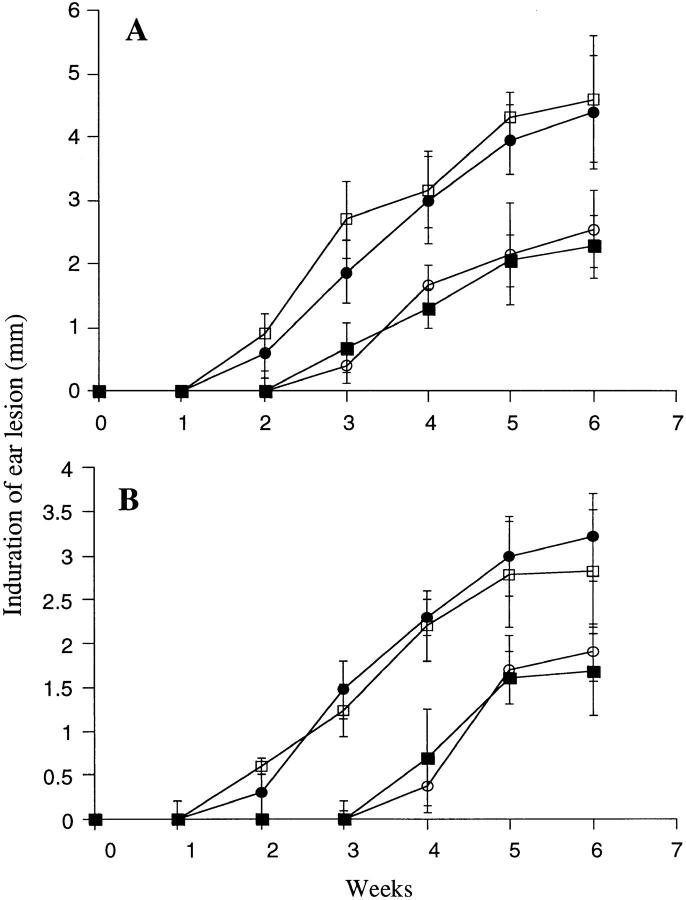
To determine if the anti-SGS antibodies could neutralize the ability of SGS to enhance infection, an IgG fraction was prepared from immune and control sera, which were then incubated with SGS for 10 min before addition of parasites and intradermal injection. The antibodies present in the SGS-sensitized mice completely abrogated the effect of SGS on disease exacerbation in both BALB/c and B/6 mice (Fig. 9). Beginning at 2 wk, the lesions in the ears coinoculated with either SGS or SGS preincubated with control IgG were significantly larger (P < 0.01) than the ears receiving either parasites alone or parasites plus SGS preincubated with IgG from sensitized mice. When the SGS that had been preincubated with IgG from immune sera was used to elicit cytokine responses in the epidermis of naive mice, the frequency of cells staining positive for type 2 cytokines displayed a similar profile as that seen when SGS was used to elicit epidermal responses in SGS presensitized mice. These frequencies, along with the absolute number of IL-4– and IL-5–producing cells per ear, are shown for two separate experiments in Fig. 10. The antibodies from the SGS-sensitized mice neutralized the ability of SGS to induce IL-4 and IL-5 responses in BALB/c mice, whereas in B/6 mice only the IL-4 response was neutralized.
Figure 9.
Size of the induration of the ear lesion in (A) BALB/c and (B) C57BL/6 mice after intradermal inoculation of 1,000 metacyclic promastigotes alone (○) or with 0.2 pair of SGS saliva (•), with 0.2 pair of SGS preincubated with serum IgG from normal mice (□), or with 0.2 pair of SGS preincubated with serum IgG from BALB/c or C57Bl/6 mice injected twice with SGS (▪). Mean induration in mm ± 1 SD, six mice per group.
Figure 10.
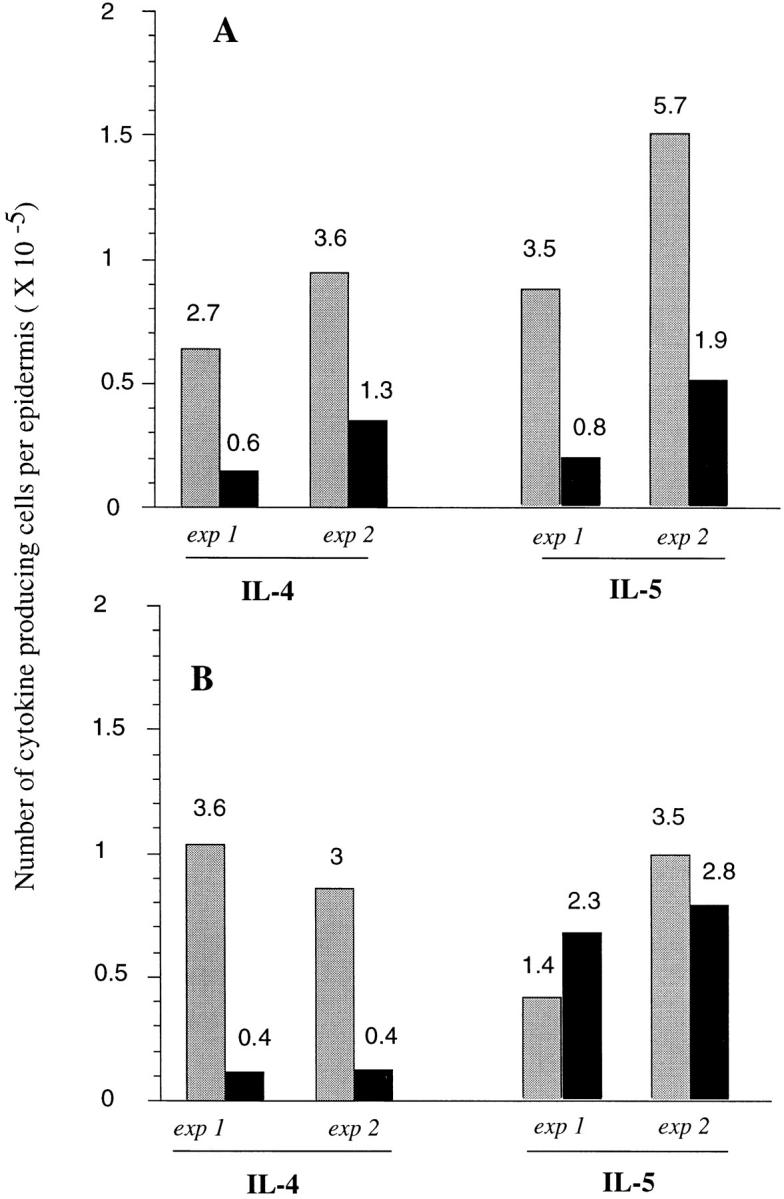
Total number of epidermal cells per ear staining for selected cytokines in (A) BALB/c and (B) C57Bl/6 mice, 6 h after intradermal inoculation of 1,000 metacyclic promastigotes plus 0.2 gland SGS preincubated with control serum IgG (gray bars) or with serum IgG from SGS presensitized mice (solid bars). Data from two separate experiments are shown, determined from the two-color FACS® analysis of epidermal cells pooled from six ears per group, and calculated as the average number of epidermal cells per ear × the percentage of cells staining positive for each cytokine (numbers above each bar).
Discussion
In attempts to mimic the biology of natural transmission, we have established a model of cutaneous leishmaniasis in the mouse that includes inoculation of low numbers of L. major metacyclic promastigotes plus salivary gland lysates from a natural vector, P. papatasii, into the mouse ear dermis. Blood sucking arthropods, including infected sand flies capable of egesting Leishmania, will salivate into the skin in order to locate blood and maintain its flow during ingestion (2). The importance of taking into account this component of natural transmission was first addressed by Titus and Ribeiro (8), who showed that in addition to its powerful antihemostatic activities, sand fly saliva will enhance the infectivity of Leishmania. These results have since been reinforced using diverse species of Leishmania (L. major, L. braziliensis, and L. amazonensis) coinoculated with saliva from two vector species (L. longipalpis and P. papatasii) (17– 19). In each case, the infection model was confined to the evaluation of footpad lesions after subcutaneous inoculation of parasites plus saliva. The rationale for extending these observations to the skin is that in addition to being the tissue environment in which these encounters normally occur, the skin is comprised of specialized structures and cells that may be better adapted to respond to these insults in a host-protective rather than a disease-promoting manner. On the contrary, we found that the ability of SGS from P. papatasii to exacerbate L. major infection is especially pronounced in the skin. The nonhealing versus healing phenotypes of BALB/c versus B/6 mice, established using subcutaneous sites of infection, were each maintained in the dermal models. However, the effects of P. papatasii saliva, which for L. major were relatively modest in the subcutaneous footpad infections (17), were severe in the ear dermis as assessed by the time course of lesion appearance, the maximal size of lesion growth, and the tissue destruction associated with lesion progression. The effect in B/6 mice was especially dramatic in that a large proportion of the dermal lesions failed to heal for up to 8 mo after infection, an outcome that was not observed when infections were initiated in the footpad.
We have used this model of natural infection to introduce an additional variable into the interplay between vector, parasite, and host. Since in endemic areas it is likely that individuals at risk of exposure to infected sand flies will have prior exposure to the bites of uninfected flies, we investigated whether preexposure to vector saliva via intradermal inoculation of SGS could modify the course of L. major infection in mice challenged with parasites plus SGS. In both BALB/c and B/6 mice, preexposure to salivary lysate completely abrogated the exacerbative effect of SGS on the development of the dermal lesions. Two intradermal injections of SGS elicited both anti-SGS antibodies and DTH responses in these mice. Thus, there is no indication from these studies that components in vector saliva suppress the development of immune responses to salivary antigens, as has been proposed (4). We were able to formally demonstrate a role for the antisaliva antibodies in the reversal of the salivary effect since preincubation of SGS with serum IgG from sensitized animals completely neutralized its ability to enhance infection in both mouse strains. The results suggest that in endemic areas, the intensity of exposure to uninfected sand fly bites might influence the epidemiology of cutaneous leishmaniasis. Thus, young children or individuals coming from nonendemic regions might be at greater risk of developing more serious disease because they are immunologically naive with respect to sand fly saliva, not just Leishmania. So far as we are aware, no clinical studies have been undertaken to demonstrate the presence of anti–sand fly saliva antibodies in people living in sand fly– infested areas. Repeated bites of mosquitoes have been shown to result in the production of antibodies against saliva (22–24). Circulating antibodies against tick saliva are produced in mice and cattle that augment complement-mediated host resistance, expressed by reduced engorgement weights and increased tick mortality, and this response has been exploited as a means of vector control (25). The ability of antisaliva antibodies to neutralize the bioactivity of salivary components has also been demonstrated in mice immunized by repeated bites of Anopheles stephensi mosquitoes, which developed antibodies to apyrase that inhibited its catalytic activity (26). Apart from this study, only one other experimental study has examined the presence and effect of antisaliva antibodies in animals exposed to sand fly bites. Hamsters exposed to Phlebotomus argentipes bites developed high antibody titers to saliva, which appeared to result in the increased mortality of flies fed on sensitized animals (27). We have not noted any increased mortality of sand flies fed on animals used as a repeated source of blood meals (data not shown).
The molecule(s) present in vector saliva that are responsible for the enhancement of infectivity have not been identified. However, the finding that antibodies from sensitized mice neutralize this effect should facilitate the screening of native or recombinant salivary proteins. Nonetheless, the present studies have clarified the mechanism(s) by which saliva might exacerbate infection. By using recently developed methods that permit recovery of cells from the inflammatory ear dermis, and by using intracellular staining and flow cytometry for the first time to analyze cytokine production by epidermal cells at the single cell level, a consistent pattern of host response to sand fly saliva has emerged. The analysis of the dermal explant cultures during the first 48 h indicated that the steady state of the dermal compartment was relatively unperturbed by the introduction of 1,000 parasites or parasites plus saliva. Only in the mice presensitized with SGS did coinoculation of SGS plus parasites produce a significant change in the recruitment of cells into the dermis. The response was characteristic of a DTH response; it was comprised of an influx of eosinophils and macrophages, and it was eliminated by in vivo treatment with anti-CD4 antibodies (data not shown). In contrast to the dermal compartment, the epidermal response to SGS in naive mice was early and dynamic. Flow cytometric analyses of epidermal cells stained for intracellular cytokines in response to the tissue injury associated with needle injection of PBS revealed a significant increase in the frequency of cells producing IL-3, IL-5, TNF-α, IFN-γ, and MCP-1. The ability of keratinocytes, DETC, and Langerhans' cells to produce a wide range of cytokines in response to tissue injury is well described (for review see reference 20). In particular, the extremely high frequency of cells staining for TNF-α is consistent with the ability of keratinocytes to produce this cytokine in response to different epidermal exposures, including barrier disruption, burns, UV irradiation, and TNF-α itself (28, 29). Needle injection of 1,000 metacyclic promastigotes produced little change in the early epidermal response profile over that seen with PBS. In contrast, the inclusion of SGS in the low dose inoculum elicited a consistent increase in the frequency of IL-4, IL-5, and in the case of BALB/c mice, GM-CSF–producing cells. In the only other study that has examined the dermal cytokine response to vector saliva, in situ hybridization was used to detect IFN-γ, IL-2, and IL-4 mRNA in mouse skin at feeding sites of Ixodes ricinus ticks (30). Tick bites have also been shown to upregulate IL-4 production by mitogen-stimulated lymph node and spleen cells from exposed mice (31, 32), although the systemic effects were not observed until 6–8 d after tick infestation.
In this study, the kinetics of the type 2 cytokine response in the epidermis (6 h) suggest that components in sand fly saliva are able to activate innate responses by DETC, which in the mouse skin bear primarily the γδ form of the antigen receptor (33). Confirmation that these cytokines were indeed made by γδ T cells using FACS® analysis of doubly labeled cells has thus far been compromised by the poor staining of their cell surface markers, due presumably to the use of trypsin to recover the epidermal cells. In contrast to acquired immunity, innate responses are not dependent on clonally distributed receptors and MHC-restricted antigen recognition (for review see reference 34). They can be elicited instead by pattern recognition of certain types of molecules common to microbial pathogens (35) and damaged or stressed cells (36, 37). Sand fly saliva may possess a similar class of molecules. Alternatively, the activation might be mediated by a pharmacologically active salivary component (e.g., apyrase) that generates increased concentration of some extracellular signaling molecule (e.g., AMP or adenosine).
A role for innate γδ T cell responses in the regulation of subsequent adaptive immune responses by CD4+ T cells is well described (38–40). In addition, an early burst of IL-4 produced by CD4+, L. major–specific T cells controls, at least in part, L. major susceptibility in BALB/c mice (41). Thus, it was reasonable to suspect that the powerful effects of saliva on disease exacerbation in the skin might be related to the early epidermal IL-4 that favored the development of Th2 cells in the adaptive phase of the immune response. In both BALB/c and B/6 mice, the L. major antigen-specific IL-4 response of lymph node cells 8 wk after infection was significantly enhanced in mice infected in the presence of SGS. Furthermore, the 6-h epidermal response to parasites plus SGS in the mice presensitized to saliva was altered primarily in the loss of cells staining for IL-4, and their antigen-specific IL-4 response in draining nodes 8 wk after challenge returned to the levels seen in control mice. Finally, preincubation of the SGS with antibodies from sensitized mice, in addition to neutralizing its disease exacerbating activity, also neutralized its ability to elicit a rapid IL-4 response in the epidermis of naive mice.
Direct evidence that IL-4 elicited by SGS was at least partially responsible for the enhancement of infection was provided by the studies in IL-4–deficient BALB/c and B/6 mice, in which SGS failed in each case to significantly alter the severity of the dermal lesions. This is consistent with a recent report regarding the abrogation of the salivary effect by anti–IL-4 antibodies in BALB/c mice infected with L. braziliensis (19). Since in this L. braziliensis model lesion development appeared to be strictly IL-4 dependent, it is possible that the saliva elicited other responses (e.g., IL-10, TGF-β) that enhanced infection but could not be revealed in the absence of IL-4. In contrast, IL-4 seems not to be required for the Friedlin strain of L. major to produce dermal lesions in B/6 mice, or even for the evolution of nonhealing dermal lesions in BALB/c mice, consistent with the absence of an effect of the IL-4 deletion on the growth of certain other L. major strains in BALB/c mice (42). Nonetheless, our data suggest that IL-4 upregulation by SGS can produce an additive effect on disease exacerbation. The absence of the salivary effect in SCID mice reinforces the likely role of T cells in mediating the exacerbation. The finding that SGS could still exacerbate disease in the IL-12p40 knockout mouse suggests that the effect is not strictly due to the downregulation of a host protective Th1 response or to an ability to directly suppress IFN-γ–mediated activation of macrophages, as has been suggested by in vitro experiments (3). The continued effects seen in mice with a IFN-γ–inducing defect, in addition to the sustained effects seen in the skin long after saliva has presumably disappeared from the inoculation site, emphasize the more likely role of saliva in enhancing an adaptive Th2 response rather than directly suppressing macrophage effector functions. The Th2 cytokines elicited by SGS might further exacerbate infection in mice that already have a defective capacity for killing by promoting the recruitment of inflammatory cells available for infection.
In conclusion, infection of a natural tissue site, the ear dermis, reveals an especially powerful effect on enhancement of L. major infection by a key component of natural transmission, sand fly saliva. The effect seems attributable to the ability of saliva to elicit innate immune responses in the skin, in particular the production of type 2 cytokines by epidermal cells. The finding that the ability of SGS to promote infection is completely neutralized by antisaliva antibodies present in mice that were previously exposed to SGS has important implications regarding the epidemiology of leishmanial disease, and validates the prior suggestion (17) that salivary molecules might be effective components of an anti–L. major vaccine.
Acknowledgments
We thank Sandra Cooper for help with the mouse care and breeding, Drs. S. Gurunathan and K. Tirumalai for kindly providing the gene knockout and SCID mice, and Drs. Genevieve Milon and Mark Udey for critical review of the manuscript.
Abbreviations used in this paper
- DETC
dendritic epidermal T cells
- DTH
delayed type hypersensitivity
- SGS
salivary gland sonicate
- SLA
soluble L. major antigen
References
- 1.Warburg A, Schlein Y. The effect of post-bloodmeal nutrition of Phlebotomus papatasii on the transmission of Leishmania major. . Am J Trop Med Hyg. 1986;35:926–930. doi: 10.4269/ajtmh.1986.35.926. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro JMC. Role of saliva in blood-feeding by arthropods. Annu Rev Entomol. 1987;32:463–478. doi: 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- 3.Hall LR, Titus RG. Sand fly saliva selectively modulates macrophage functions that inhibit killing of Leishmania majorand nitric oxide production. J Immunol. 1995;155:3501–3506. [PubMed] [Google Scholar]
- 4.Titus RG. Salivary gland lysate from the Sand fly Lutzomia longipalpissuppresses the immune response of mice to sheep red blood cells in vivo and concanavalin A in vitro. Exp Parasitol. 1998;89:133–136. doi: 10.1006/expr.1998.4272. [DOI] [PubMed] [Google Scholar]
- 5.Wikel, S.K. 1996. Immunology of the skin. In The Immunology of Host-Ectoparasitic Arthropod Relationships. S.K. Wikel, editor. CAB International, Wallingford, U.K. 1–29.
- 6.Bretscher PA, Wei G, Menon JN, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. . Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 7.Doherty TM, Coffman RL. Leishmania major: effect of infectious dose on T cell subset development in BALB/c mice. Exp Parasitol. 1996;84:124–135. doi: 10.1006/expr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 8.Titus RG, Ribeiro JMC. Salivary gland lysates from the sand fly Lutzomia longipalpis enhance Leishmaniainfectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF, Curtis J, Handman E. Resistance to cutaneous leishmaniasis in genetically susceptible BALB/c mice. Aust J Exp Biol Med Sci. 1981;59:555–565. doi: 10.1038/icb.1981.48. [DOI] [PubMed] [Google Scholar]
- 10.Blank C, Fuchs H, Rappersberger K, Rollinghoff M, Moll H. Parasitism of epidermal Langerhans' cells in experimental cutaneous leishmaniasis with Leishmania major. . J Infect Dis. 1993;167:418–425. doi: 10.1093/infdis/167.2.418. [DOI] [PubMed] [Google Scholar]
- 11.Belkaid Y, Jouin H, Milon G. A method to recover, enumerate, and identify lymphomyeloid cells present in an inflammatory dermal site. A study in laboratory mice. J Immunol Methods. 1996;199:5–25. doi: 10.1016/s0022-1759(96)00117-2. [DOI] [PubMed] [Google Scholar]
- 12.Noben-Trauth N, Kohler G, Burki K, Ledermann B. Efficient gene targeting of the IL-4 gene in a BALB/c embryonic stem cell line. Transgenic Res. 1996;5:487–491. doi: 10.1007/BF01980214. [DOI] [PubMed] [Google Scholar]
- 13.Ledermann B, Burki K. Establishment of a germ-line competent C57BL/6 embryonic stem cell line. Exp Cell Res. 1991;197:254–258. doi: 10.1016/0014-4827(91)90430-3. [DOI] [PubMed] [Google Scholar]
- 14.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu C, Ferrante J, Stewart C, Sarmiento U, Faherty D, Gately M. IL-12 deficient mice are defective in IFN-gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 15.Schuler, G., and F. Koch. 1990. Enrichment of epidermal Langerhans' cells. In Epidermal Langerhans' Cells. G. Schuler and F. Koch, editors. CRC Press, Inc., Boca Raton, FL. 139–157.
- 16.Gurunathan S, Sacks DL, Brown DR, Reiner SC, Charest H, Glaichenhaus N, Seder RA. Vaccination with DNA encoding the immunodominant Lack parasite antigen confers protective immunity to mice infected with Leishmania major. . J Exp Med. 1997;186:1137–1147. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theodos CM, Ribeiro JMC, Titus RG. Analysis of enhancing effect of sand fly saliva on Leishmaniainfection in mice. Infect Immun. 1991;59:1592–1598. doi: 10.1128/iai.59.5.1592-1598.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuelson J, Lerner E, Tesh R, Titus R. A model of Leishmania braziliensisinfection produced by coinjection with sand fly saliva. J Exp Med. 1991;173:49–54. doi: 10.1084/jem.173.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima HC, Titus RG. Effect of sand fly vector saliva on development of cutaneous lesions and the immune response to Leishmania braziliensisin BALB/c mice. Infect Immun. 1996;64:5442–5445. doi: 10.1128/iai.64.12.5442-5445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takashima A, Bergstresser PL. Cytokine-mediated communication by keratinocytes and Langerhans' cells with dendritic epidermal T cells. Semin Immunol. 1996;8:333–339. doi: 10.1006/smim.1996.0044. [DOI] [PubMed] [Google Scholar]
- 21.Kopf M, Brombacher F, Kohler G, Kienzle G, Widmann KH, Lefrang K, Humborg C, Ledermann B, Solbach W. IL-4–deficient BALB/c mice resist infection with Leishmania major. . J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reunala T, Brummer-Korvenkontio H, Palosuo T. Are we really allergic to mosquito bites? . Ann Med. 1994;26:301–306. doi: 10.3109/07853899409147906. [DOI] [PubMed] [Google Scholar]
- 23.Peng Z, Simons FER. Cross-reactivity of skin and serum specific IgE response and allergen analysis for three mosquito species with worldwide distribution. J Allergy Clin Immunol. 1997;100:192–198. doi: 10.1016/s0091-6749(97)70224-0. [DOI] [PubMed] [Google Scholar]
- 24.Peng Z, Simons FER. A prospective study of naturally acquired sensitization and subsequent desensitization to mosquito bites and concurrent antibody responses. J Allergy Clin Immunol. 1998;2:284–286. doi: 10.1016/s0091-6749(98)70395-1. [DOI] [PubMed] [Google Scholar]
- 25.Wikel SK, Bergman D. Tick-host immunology: significant advances and challenging opportunities. Parasitol Today. 1997;13:383–389. doi: 10.1016/s0169-4758(97)01126-5. [DOI] [PubMed] [Google Scholar]
- 26.Mathews GV, Sidjanski S, Vanderberg JP. Inhibition of mosquito salivary gland apyrase activity by antibodies produced in mice immunized by bites of Anopheles stephensimosquitoes. Am J Trop Med Hyg. 1996;55:417–423. doi: 10.4269/ajtmh.1996.55.417. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh KN, Mukhopadhyay J. The effect of anti-sand fly saliva antibodies on Phlebotomus argentipes and Leishmania donovani. . Int J Parasitol. 1998;28:275–281. doi: 10.1016/s0020-7519(97)00152-5. [DOI] [PubMed] [Google Scholar]
- 28.Piguet PF. Keratinocyte-derived tumor necrosis factor and the physiopathology of the skin. Springer Semin Immunopathol. 1992;13:345–354. doi: 10.1007/BF00200533. [DOI] [PubMed] [Google Scholar]
- 29.Wood LC, Stalder AK, Liou A, Campbell IL, Grunfeld C, Elias PM, Feingold KR. Barrier disruption increases gene expression of cytokines and the 55kD TNF receptor in murine skin. Exp Dermatol. 1997;6:98–104. doi: 10.1111/j.1600-0625.1997.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 30.Mbow ML, Rutti B, Brossard M. IFN-gamma IL-2, and IL-4 mRNA expression in the skin and draining lymph nodes of BALB/c mice repeatedly infested with nymphal Ixodes ricinusticks. Cell Immunol. 1994;156:254–261. doi: 10.1006/cimm.1994.1170. [DOI] [PubMed] [Google Scholar]
- 31.Ganapamo F, Rutti B, Brossard M. In vivo production of IL-4 and interferon-gamma by lymph node cells from BALB/c mice infested with Ixodes ricinusticks. Immunology. 1995;85:120–124. [PMC free article] [PubMed] [Google Scholar]
- 32.Zeidner N, Mbow M, Dolan M, Massung R, Baca E, Piesman J. Effects of Ixodes scapularis and Borrelia burgdorferion modulation of the host immune response: induction of Th2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect Immun. 1997;65:3100–3106. doi: 10.1128/iai.65.8.3100-3106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergstresser PR, Tigelaar RE, Dees JH, Streilein JW. Thy-1 antigen-bearing dendritic cells populate murine epidermis. J Investig Dermatol. 1983;81:286–288. doi: 10.1111/1523-1747.ep12518332. [DOI] [PubMed] [Google Scholar]
- 34.Boismenu R, Havran WL. An innate view of gamma-delta T cells. Curr Opin Immunol. 1997;9:57–63. doi: 10.1016/s0952-7915(97)80159-8. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka Y, Morita GT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma-delta T cells. Nature. 1995;375:155–157. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 36.Huber H, Descossy P, van Brandwijk R, Knop J. Activation of murine epidermal TCR gamma-delta T cells by keratinocytes treated with contact sensitizers. J Immunol. 1995;155:2888–2894. [PubMed] [Google Scholar]
- 37.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gamma-delta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 38.Ferrick DA, Schrenzel M, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon gamma and interleukin 4 in response to Th1- and Th2-stimulating pathogens by gamma-delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh B, Schrenzel M, Mulvania T, Lepper H, DiMolfetto-Landon L, Ferrick DA. In vivo cytokine production in murine listeriosis. Evidence for immunoregulation by gamma-delta T cells. J Immunol. 1996;156:232–237. [PubMed] [Google Scholar]
- 40.Huber S, Mortensen A, Moulton G. Modulation of cytokine expression by CD4+T cells during coxsackievirus B3 infections of BALB/c mice initiated by cells expressing the gamma-delta T cell receptor. J Virol. 1996;70:3039–3044. doi: 10.1128/jvi.70.5.3039-3044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Launois P, Maillard I, Pingel S, Swihart KG, Xenarios I, Acha H, Orbea, Diggelmann H, Locksley RM, MacDonald HR, Louis JA. IL-4 rapidly produced by Vα4Vβ8 CD4 T cells instructs TH2 development and susceptibility to Leishmania majorin BALB/c mice. Immunity. 1997;6:541–550. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 42.Noben-Trauth N, Kropf F, Muller I. Susceptibility to Leishmania majorinfection in interleukin-4-deficient mice. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]