Abstract
Mice rendered deficient for interleukin (IL) 6 by gene targeting were evaluated for their response to T cell–dependent antigens. Antigen-specific immunoglobulin (Ig)M levels were unaffected whereas all IgG isotypes showed varying degrees of alteration. Germinal center reactions occurred but remained physically smaller in comparison to those in the wild-type mice. This concurred with the observations that molecules involved in initial signaling events leading to germinal center formation were not altered (e.g., B7.2, CD40 and tumor necrosis factor R1). T cell priming was not impaired nor was a gross imbalance of T helper cell (Th) 1 versus Th2 cytokines observed. However, B7.1 molecules, absent from wild-type counterparts, were detected on germinal center B cells isolated from the deficient mice suggesting a modification of costimulatory signaling. A second alteration involved impaired de novo synthesis of C3 both in serum and germinal center cells from IL-6–deficient mice. Indeed, C3 provided an essential stimulatory signal for wild-type germinal center cells as both monoclonal antibodies that interrupted C3-CD21 interactions and sheep anti–mouse C3 antibodies caused a significant decrease in antigen-specific antibody production. In addition, germinal center cells isolated from C3–deficient mice produced a similar defect in isotype production. Low density cells with dendritic morphology were the local source of IL-6 and not the germinal center lymphocytes. Adding IL-6 in vitro to IL-6–deficient germinal center cells stimulated cell cycle progression and increased levels of antibody production. These findings reveal that the germinal center produces and uses molecules of the innate immune system, evolutionarily pirating them in order to optimally generate high affinity antibody responses.
Keywords: germinal center, interleukin 6, complement, antibody, follicular dendritic cells
Interleukin 6 exerts its effects on many biological systems (1, 2). It is responsible for mediating the production of acute phase proteins (3, 4), fever (5), and release of hormones (6) in response to injury or infection. In hematopoiesis, IL-6 appears to potentiate the action of other factors such as GM-CSF and M-CSF (7, 8). As a growth and differentiation factor, IL-6 has been used for optimal production of B cell hybridomas and mouse plasmacytomas (9–11). This wide spectrum of activities is largely due to the numerous cell types that produce IL-6 including, but not limited to, fibroblasts (12, 13) endothelial cells (13), macrophages (11), T cells (11, 14), and B cells (15). Its production is regulated by several cytokines including IL-1, IL-2, IL-3, IL-4, IL-13, γ-IFN, TNF and platelet-derived growth factor as well as LPS, calcium ionophore, and viruses (1, 16). IL-6 exerts its effect through a receptor composed of the restricted IL-6 receptor chain (IL-6R) and a common signal transducer, gp130 (17).
Dysregulation of IL-6 is associated with several pathological states. These include cachexia, lymphomas, multiple myeloma, psoriasis, osteoporosis, rheumatoid arthritis, and Castleman's disease (18). In many of these conditions, increased levels of IL-6 cause an overproduction of antibody contributing significantly to the clinical pathology. This relationship is supported by two transgenic models in which overproduction of IL-6 leads to splenomegaly and hypergammaglobulinemia (19, 20). Similarly, transferring preB cells derived from IL-6 transgenic mice into Rag 2-deficient or SCID mice results in significantly more IgG and IgA production than with wild-type preB cells (21).
Previously, it has been reported that IL-6–deficient mice are impaired in their ability to mount an acute phase response as well as control the infections of vaccinia virus and Listeria monocytogenes (4). To further investigate the role of IL-6 in humoral immunity, deficient mice were immunized with a T cell dependent antigen. During antibody responses, naive antigen-specific B cells are initially activated in the outer T cell zones or follicular borders via interactions with dendritic cell primed T cells (22–26). Some then enter follicular dendritic cell (FDC)1 networks where they acquire the ability to effectively process and present antigen (27–29). To date, the various gene-targeted mice have shown that this initial interaction between B cells and FDC must occur in order to initiate germinal center formation (30, 31). In combination with costimulatory molecules, the subsequent presentation of peptide to local antigen-specific T cells results in the delivery of signals producing a germinal center (25, 30, 32). Expansion, hypermutation and immunoglobulin switch mechanisms are activated (33, 34). Selection of high affinity B cells presumably occurs while noncompetitive low affinity cells are left to die by apoptosis (35, 36). The consequences of these events are the generation of high affinity and immunoglobulin switched memory B and preplasma cells (37).
The importance of complement during T cell–dependent antibody responses was first demonstrated long before the advent of gene-targeted mice (38, 39). The use of depleting agents identified a role for C3 in follicular localization of antigen as well as induction of T-dependent antibody production (38, 40–42) and the local synthesis of C3 was documented in lymphoid tissues (43, 44). Antibodies to mouse C3 were found to inhibit T cell–dependent antibody production in vitro (44) and furthermore complement dependent mixed aggregation of different lymphoid cell types was reported (45). Much more recently, studies in genetically deficient mice have provided further detailed information about the role of C3 as these mice have a reduced but not totally impaired ability to form germinal centers and mount antigen-specific antibody responses (46, 47). In addition, using these mice, Carroll and colleagues have shown that wild-type bone marrow–derived macrophages corrected the knock out phenotype by providing local C3 production (48).
These observations are significant because as we show here, in addition to several more subtle effects, IL-6–deficient mice have impaired local production of C3. Furthermore, germinal center cells isolated from IL-6– and from C3-deficient mice have a comparable defect in IgG2a and IgG2b antibody production. We propose that the production of IL-6 and of C3 is linked as part of the highly coordinated events occurring locally within germinal centers to insure the generation of high affinity antibodies.
Materials and Methods
Mice, Antigen, and Immunization.
IL-6–deficient mice were generated by homologous recombination as described elsewhere (4). C3-deficient mice were obtained from M.C. Carroll (Harvard Medical School, Boston, MA; reference 47). All mice were housed under specific pathogen-free conditions. Wild-type (i.e., littermate) control, IL-6–deficient (129sv × C57BL/6 or C57BL/6), or C3-deficient (C57BL/6) mice were used between 8 and 16 wk of age. Mice were immunized with either OVA or DNP-OVA both precipitated in alum (49).
For ascertaining serum antibody titers, mice were immunized with 100 μg/ml DNP-OVA intraperitoneally (0.2 ml), subcutaneously in each of the two rear limbs (0.05 ml/site) and intranuchally (0.1 ml). 14 d later, blood samples were collected. For a secondary response, at day 14 after a primary injection, the mice were given the same immunization protocol and blood samples were collected 10 d later. For the isolation of antigen-specific T cells or germinal center cells, mice were immunized as above with OVA and the cells isolated from the draining lymph nodes on day 7.
Measurement of Antibody Titers by ELISA.
DNP-specific antibodies were detected by an ELISA using standard procedures. Goat anti–mouse IgG1, IgG2a, IgG2b, IgG3, IgM antibodies (Southern Biotechnology Associates, Birmingham, AL), and the rat anti–mouse IgE antibody, EM95.3, (provided by Z. Eshhar, The Weizmann Institute of Science, Rehovot, Isreal; reference 50) were used for revealing isotype-specific serum antibodies. The relative antibody concentrations in the serum and supernatant samples of Figs. 1 and 7 B, respectively, correspond to the dilution at OD 50%.
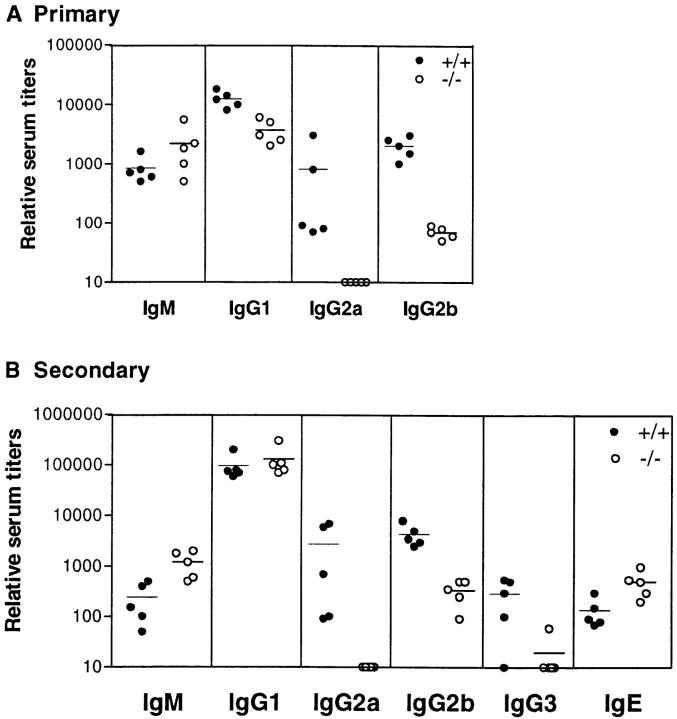
Figure 1.
Antigen-specific serum antibody titers are lowered in the absence of IL-6. Wild-type (closed circles) and IL-6–deficient (open circles) 129 × C57BL/6 mice were bled 14 d after the primary (A) and 10 d after the secondary (B) immunization with DNP-OVA. The DNP- (shown here) and OVA- (not shown) specific isotype titers were determined using standard ELISA techniques. Each symbol represents an individual mouse.
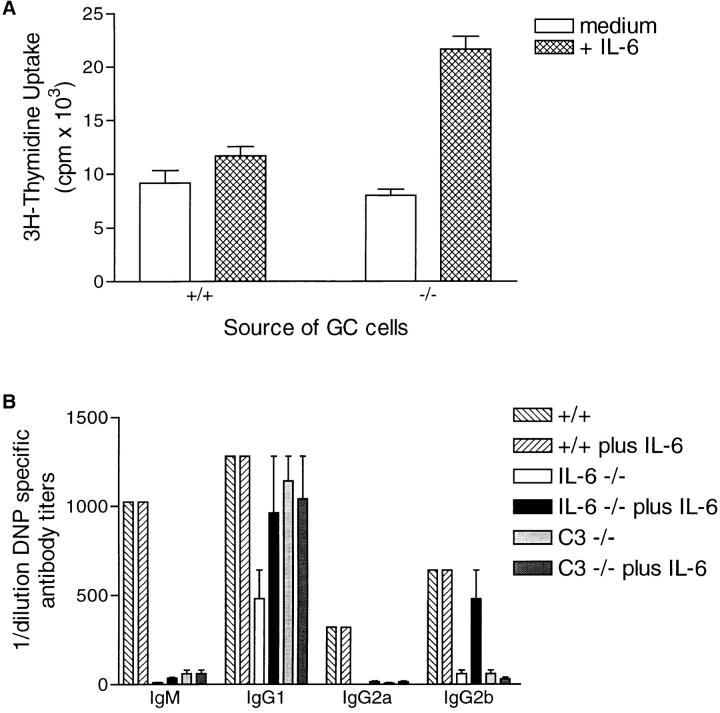
Figure 7.
IL-6 augments proliferation and antibody production by germinal center cells in vitro. (A) germinal center cells were isolated from wild-type and IL-6–deficient mice 7 d after primary immunization. The germinal center cells were cultured in the presence (hatched areas) or absence (open areas) of IL-6 for 72 h and 3[H]thymidine added for the final 24 h. The data represent triplicates ± SD. (B) germinal center cells isolated from wild-type, IL-6–deficient, or C3-deficient mice were cultured in the presence or absence of IL-6 (as indicated on the figure) for 7 d and the supernatants then assayed for antigen-specific antibody titers using standard isotype ELISA procedures. The figure shows representative data from 1 of 3 experiments in which 3–5 mice per group were pooled.
Immunohistology and Morphometric Analysis.
Spleens and lymph nodes were take from mice before and at various times after immunization. Serial cryosections were prepared, fixed in acetone for 10 min stored in airtight containers at −20°C. For determination of germinal center volume, the lymph nodes were entirely sectioned (e.g., 30–40 serial sections) from 3 mice per time point (i.e., day 8, 12 and 15 after primary immunization) and then incubated with the plant lectin, peanut agglutinin (PNA), a reagent that localizes germinal center cells in situ (51). The PNA was obtained biotinylated (Vector Laboratories Inc., Burlingame, CA) and revealed using streptavidin-peroxidase (Vectastain Elite ABC; Vector Laboratories, Inc.). All peroxidase reactions were developed using diaminobenzidine (1 mg/ml; Sigma Chemical Co., St. Louis, MO) containing 0.1% H2O2.
Once serial sections were processed, 8–12 germinal centers of each time point were photographed from beginning to end using a Zeiss axiophot microscope. Using the photographs, the longest axis of the x and y plane were measured and the volume calculated using the equation: volume of an ellipse = 4/3πabc (where a and b are the two-dimensional measurements taken from the photos and c is the value obtained by calculating the number of photographs the germinal center was present in and multiplying this by the section thickness). The values obtained for the wild-type were in agreement for those previously published (52).
T Cell Proliferation and Cytokine Production.
T cells were obtained from spleens of mice 7 d after a primary immunization with OVA. After lysis of red blood cells with a hypertonic solution, cells were layered into a discontinuous gradient. The cells in bands corresponding to densities of >1.080 g/ml were harvested. The preparation was further enriched for CD4+ cells by depleting other cells types with antibody coated magnetic beads (Dynabeads; Dynal, Oslo, Norway). Specifically, the MHC class II+ cells were negatively selected using the mAb, M5/114 (American Tissue Culture Collection, Rockville, MD), and the CD8+ cells using the mAb, 53.6.78 (PharMingen, San Diego, CA). APC were obtained from spleens after lysis of red blood cells and depletion of CD4+ and CD8+ cells using GK1.5 (PharMingen) or 53.6.78 mAb-coated magnetic beads, respectively. The APC were irradiated with 30Gy then placed at 2 × 105 cells per well in round-bottom 96-well plates. T cells were added at a concentration of 2 × 105 cells per well in the presence or absence of OVA as indicated in Fig. 3 A or at 10 μg/ml for Fig. 3 B. For the proliferation assay, cells were cultured for 4 d and 3[H]thymidine added for the final 18 h. For the detection of cytokines, cells were cultured for 48 h, the supernatants harvested and the levels of γ-IFN and IL-5 assessed using cytokine ELISA kits (Genzyme Corp., Boston, MA).
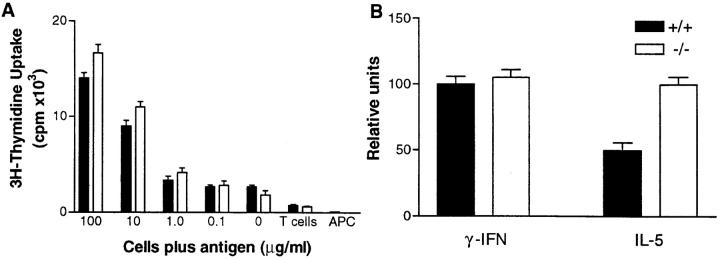
Figure 3.
Analysis of T cell priming and cytokine production in IL-6– deficient mice. T cells were isolated from wild-type (solid bars) or IL-6– deficient (open bars) mice at day 7 after primary immunization with OVA. (A) the T cells were culture with irradiated APC in the presence or absence of various OVA concentrations. 3[H]thymidine was added on the third day of culture for 8 h. The data are representative of triplicate wells ± SD. (B) The T cells were restimulated with irradiated APC in the presence of OVA for 48 h. The supernatants were collected and the cytokine levels determined by standard ELISA procedures. Triplicate wells were assessed and the data is expressed as relative units between deficient and the control wild-type mice ± SD.
Isolation of Germinal Center Cells.
Germinal center cells were isolated from the lymph nodes of mice 7 d after a primary immunization with OVA or DNP-OVA according to the procedure described elsewhere (53). In brief, the low density, nonadherent cells were obtained using: (a) an enzyme cocktail to digest the collagen, allowing embedded cell types including FDC to be released from the stroma (collagenase, No. 4188; Worthington Biomedical Corp., Freehold, NJ; deoxyribonuclease I; Sigma Chemical Co.); (b) a continuous Percoll gradient to obtain the 1.060–1.065 g/ml density cells; and (c) a 1-h adherence step at 37°C to remove the adherent cells such as macrophages and dendritic cells. The resulting population has previously been shown to have the following features: contains >75% B cells, 10% T cells, 10% FDC, and <5% TBM; both antigen-specific proliferation and antibody production of IgG and IgE isotypes occurs; and within 24 h of culture, the B cells form clusters around FDC that are dependent on both the presence of FDC and T cells (53).
Flow Cytometry.
The level of CD80 (B7.1) and CD86 (B7.2) expression on germinal center B cells was determined using flow cytometry. For this, isolated germinal center cells were incubated with the PE-labeled CD45RA antibody (B220; clone 14.8; PharMingen) and either the biotinylated hamster anti–mouse CD80 (clone 16-10A1; PharMingen) or CD86 mAb (clone GL1; PharMingen) revealed using FITC-Streptavidin (Southern Biotechnology Associates, Inc.). A gate was established for the B220 positive cells and then the amount of CD80 and CD86 determined.
C3 Measurement.
Mouse serum C3 concentrations were measured by electroimmunoassay calibrated with standards based on isolated pure murine C3, as previously described (54).
Reverse Transcription PCR for C3.
Total cellular RNA was prepared from isolated germinal center cells above using TrizolTM (GIBCO BRL, Gaithersburg, MD). Random hexamer-primed reverse transcription (RT) was performed with Superscript-RT (GIBCO BRL) using one quarter of total RNA obtained in a 25-μl reaction volume containing 0.04 u (1.2 ng) random hexamers (Pharmacia Biotech, Inc., Piscataway, NJ), 0.4 mM dNTP (Promega Corp., Madison, WI) 50 mM Tris-HCl, pH 8.3, 75 mM KCL, 3 mM MgCl2, and 0.5 units RNAsin (Promega Corp.). After 90 min of incubation at 37°C, samples were heated at 94°C and then quickly chilled on ice. cDNA samples were diluted to 100 μl with H2O and 1 to 2 μl used for PCR. Cycling conditions for PCR amplification of the complement 3 alpha chain using specific oligonucleotides C3for2257:TGCGTGAACAA CACAGAAGAG and C3rev2764: AAGGGGACAATGACATACGG were 94°C for 1 min before 35 cycles of 94°C for 20 s, 58°C for 30 s, and 72°C for 30 s, followed by a final extension at 72°C for 5 min. cDNA samples were standardized by competitive PCR for expression of β2-microglobulin using a mimic control vector essentially as described (55).
Antibody Inhibition Assay.
Germinal center cells were isolated at day 7 after a primary injection of OVA. Cells were placed in round-bottom 96-well plates at 3 × 105 cells per well in the presence or absence of 10 μg/ml 7G6 (rat IgG2b anti–mouse complement receptors 1 and 2 mAb; provided by T. Kinoshita, Osaka University Medical School, Osaka, Japan; reference 56) or 10 μg/ml FDC-M1 (rat IgG2b anti–mouse isotype control mAb; reference 53). In addition, the cells were also incubated in the presence of 10 μg/ml affinity-purified F(ab′)2 fragments of the IgG1 fraction of a monospecific polyclonal sheep anti–mouse C3 antiserum (W23); reference 44) or sheep IgG1 (as the isotype control). After 7 d, the supernatants were harvested and the levels of OVA-specific IgG1 and IgG2a determined as stated above.
RT-PCR for IL-6.
Semiquantitative RT-PCR was performed as described elsewhere (57, 58). cDNA was quantified by PCR using HPRT (hypoxanthine phosphoribosyl-transferase) as a cDNA reference. A Biometra TRIO-thermoblock thermal cycler was used for all PCRs, operating on a regime of 96°C for 6 s, 45°C (for IL-6) or 55°C (for HPRT) for 15 s, 72°C for 60 s, for 31 cycles, followed by 72°C for 10 min. PCR reactions were of 15 μl, using cDNA derived from an estimated 20 cells for HPRT standardization and 100 cell equivalents for IL-6 (57, 58).
The cDNA from the following cell populations were assessed for IL-6 mRNA expression: positive control, concanavalin A stimulated splenocytes; clusters, germinal center cells cultured for 24 h to form aggregates and then the aggregates collected using sedimentation over a 30% FCS gradient for 1 h; FDC sorted, isolated germinal center cells were labeled with the rat anti–mouse FDC mAb, FDC-M1, followed by a mouse anti–rat IgG F(ab′)2 conjugated to fluorescein (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) then FDC-M1 positive cells were obtained by sorting on a FACScan®. Because of the fragile nature of their elongated dendritic processes, FDC tend to not do well passing through the FACScan®. As such, in order to obtain enough material, the positive cells were directly sorted into a solution of 5.5 M guanidinium thiocyanate, 25 mM sodium citrate and 0.5% sodium lauroyl sarcosine (Pharmacia Biotech, Inc.); FDC irrad, a second way to enrich for FDC is to irradiate mice 2 d after the secondary challenge with 6Gy (Cesium source; reference 59). This exposes dividing cells to damaging amounts of irradiation, significantly decreasing lymphocytes in the preparation (49). The FDC were obtained from the irradiated mice using the same protocol for germinal center cells, yielding a purity of 50% FDC; T cell– and B cell–sorted, isolated germinal center cells were labeled with the rat anti–mouse thy1 T24) or rat anti–mouse B220 (14.8) mAbs followed by a mouse anti–rat IgG F(ab′)2 conjugated to FITC (Jackson ImmunoResearch Laboratories, Inc.) in order to obtain germinal center T cells or B cells, respectively, by sorting on a FACScan®.
Immunohistochemistry for IL-6.
IL-6 protein was localized on acetone fixed cryosections of immunized lymph nodes. For this, the tissue was pretreated in PBS containing 0.05% saponin and all washing steps and dilutions of reagents were carried out using this solution. Endogenous peroxidase activity was blocked using 0.01% H2O2 in PBS. The sections were then subjected to the avidin-biotin blocking kit according to the manufacturer's specifications (No. SP-2001; Vector Laboratories Inc.). The anti-IL-6– specific mAb, clone MP5-20F3 (PharMingen), was applied to the sections for 2 h followed by a biotinylated rabbit anti–rat IgG (mouse absorbed; No. BA-4001; Vector Laboratories Inc.) for 30 min. On a serial section, the FDC network was localized using the rat anti–mouse FDC mAb, FDC-M1, followed by a mouse anti–rat IgG F(ab′)2 conjugated to biotin (Jackson ImmunoResearch Laboratories, Inc.). Both the anti-IL-6 and anti-FDC mAbs were revealed with the Vector ABC kit in conjunction with diaminobenzidine (1 mg/ml) containing 0.1% H2O2. The sections were counterstained using methyl green.
Proliferation and Antigen-specific Antibody Production.
Germinal center cells were isolated 7 d after a primary immunization and placed in round-bottom 96-well plates at a density of 3 × 105 cells per well. Recombinant IL-6 was added (200 U/ml; PharMingen) at the initiation of the cultures. For the proliferation assay, 3[H]thymidine was added after 48 h of culture and the plates harvested 24 h later. For determination of antibody production, the supernatants were harvested after 7 d of culture. The supernatants were then assayed for antigen-specific antibody isotypes using the ELISA methods outlined above.
Results
Antigen-specific Serum Antibody Titers Are Altered in IL-6– deficient Mice.
The role of IL-6 during humoral responses was initially evaluated by testing the level of various isotypes produced after injection of the T cell–dependent antigen, DNP-OVA. As shown in Fig. 1 A, whereas the amount of IgM produced was comparable to wild-type controls after a primary injection, the level of IgG was impaired. IgG1 was least affected being reduced only two- to threefold whereas IgG2a and IgG2b were more dramatically influenced. For the 129sv × C57BL/6 background, IgG2a levels were decreased between 10- and 1,000-fold while in the C57BL/6 background, the decrease was limited to 10–20-fold. IgG2b was less strain dependent, decreasing between 10–50-fold independent of the genetic background. The large range in antigen-specific IgG2a titers using the mixed 129sv × C57BL/6 background was also observed in the wild-type littermate controls (Fig. 1, A and B).
After secondary antigen challenge, the IL-6–deficient mice presented yet another profile. IgM, IgG1, and IgE titers were comparable or slightly higher than the wild-type controls (Fig. 1 B). The levels of IgG2a and IgG2b remained low and IgG3 was also minimal. In addition, after both the primary and secondary immunizations, the total amount of specific immunoglobulin produced was 10-fold less in the deficient mice as assessed using anti-kappa reagents demonstrating that the inability to produce normal levels of antibody was not compensated by other isotypes (data not shown).
Absence of IL-6 Results in Impaired Germinal Center Development.
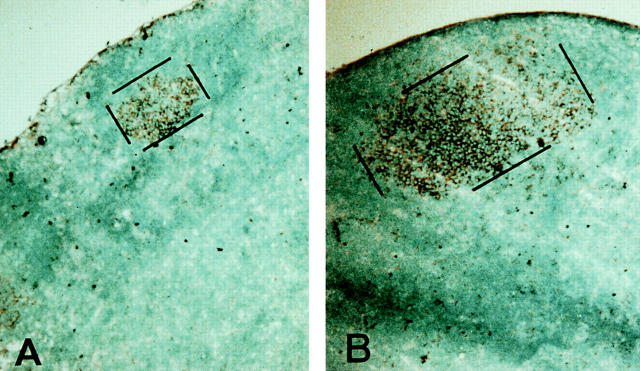
Since the serum titers for the high affinity isotypes were the most dramatically affected, the ability to form germinal centers was evaluated. Preparing lymph nodes from mice 5, 7, 9, 12, and 15 d after a primary immunization with DNP-OVA, a reduction in the size of the area labeled with PNA was observed in IL-6–deficient mice (Fig. 2 A) as compared with the controls (Fig. 2 B). Morphometric analysis of these tissues revealed that while the average volume of the PNA positive foci was similar at day 7 (wild-type = 2.1 × 10−6 μm3 versus IL-6–deficient mice = 2.0 × 10−6 μm3), by day 9, the average volume of the germinal centers was diminished (Fig. 2 C). In the absence of IL-6, the cells did not create germinal centers large enough to easily observe the classical light and dark zones. Labeling with the rabbit anti–mouse polyclonal antibody, Ki67 (31), revealed that the germinal center cells were in cycle (data not shown). In addition, although tingible body macrophages and apoptosis were still observed in these areas, the absolute number of TBM were reduced similar to the decrease in germinal center volume (data not shown). As such, it did not appear that increased apoptosis or an inability to proliferate were directly affected by the lack of IL-6. Instead, it could be argued that the cells exited the germinal center prematurely possibly due to a lack of responsiveness to events mediated downstream of an IL-6 signal.
Figure 2.
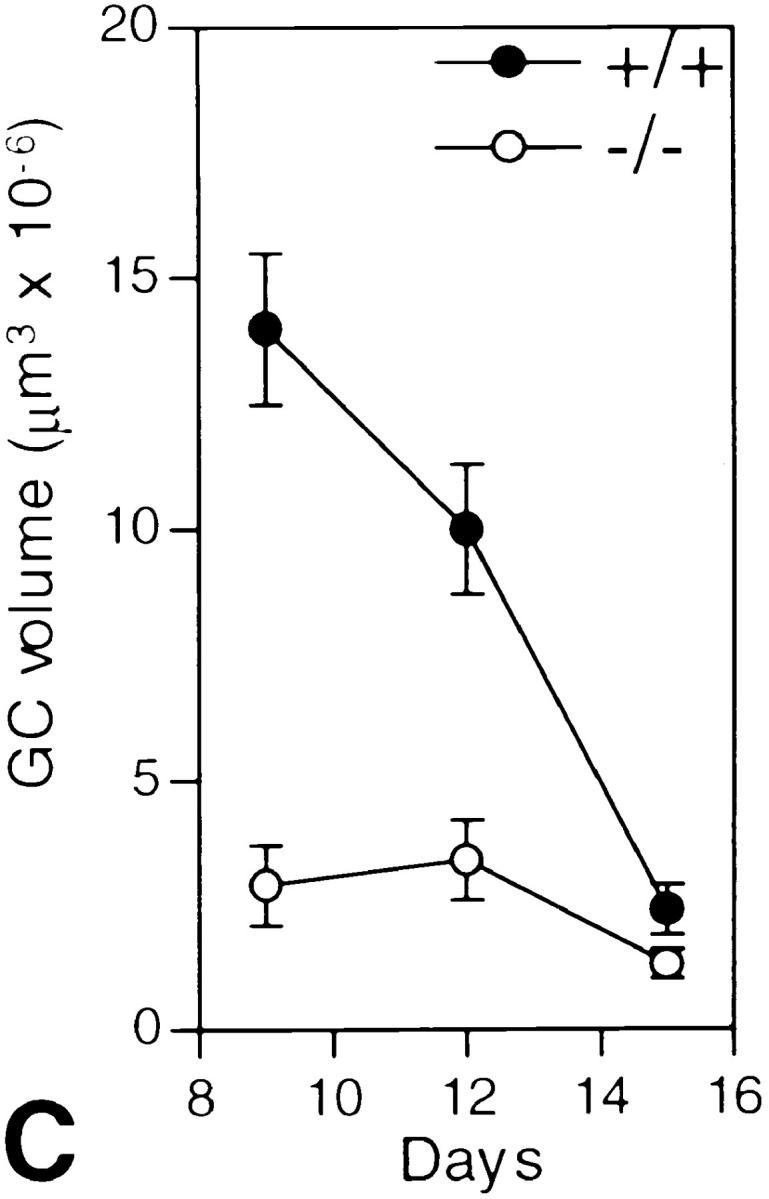
Germinal center size is limited in IL-6–deficient mice. Lymph nodes from wild-type and IL-6–deficient mice were isolated at day 9, 12, and 15 after primary immunization with DNP-OVA and prepared for immunohistochemistry. Using PNA as the marker for germinal centers, 30–40 serial sections were labeled (brown) and single germinal centers morphometrically analyzed to determine their volume. A and B show the average size of a germinal center in the IL-6– deficient (A) versus the wild-type (B) mice at day 9 after immunization (PNA labeled area outlined with black lines). Magnification ×250. C shows the cumulative data of the analysis (open circles, deficient mice; solid circles, wild-type mice). The data represents the analysis of 6–8 complete germinal centers from a total of 3 mice for each time point. Error bars represent the standard deviation.
As it was observed that at day 7 after antigen injection the histology of germinal centers in lymph nodes of wild-type and IL-6–deficient were similar, subsequent experiments used cells isolated at day 7 to attempt to dissect the processes involved for the phenotype produced.
T Cell Priming Is Not Affected in IL-6–deficient Mice.
To rule out the possibility that a direct effect within the T cell compartment occurred in IL-6–deficient mice, the number of antigen-specific T cells was determined. Using a bulk culture assay (i.e., T cells isolated 7 d after a primary immunization cultured with APC in the presence of antigen), similar amounts of 3[H]thymidine were incorporated by the T cells isolated from the deficient and wild-type mice (Fig. 3 A). This result was confirmed using a limiting dilution assay showing that the number of antigen-specific IL-3 producing cells was 110 versus 125 per 106 cells in deficient and wild-type control mice, respectively (data not shown).
To address T cell cytokine profiles, T cells were also cultured for 48 h with APC plus antigen and the supernatant used to determine levels of γ-IFN and IL-5 (Th1 versus Th2 profile, respectively). As can be seen in Fig. 3 B, no gross difference in levels of cytokines produced by the activated T cells was observed although the IL-5 (and IL-4, data not shown) levels were consistently elevated by two-fold in the IL-6–deficient mice.
Alteration of Cell Surface B7.1 but not B7.2 Expression Occurs in the Absence of IL-6.
To determine the role of IL-6 specifically on germinal center cells, the low buoyant density, nonadherent population of lymph node cells were obtained 7 d after a primary immunization with DNP-OVA. As reported previously (53), this population of cells mimics many of the characteristics attributed to the germinal center cells in vivo. It consists of 5–10% antigen bearing FDCs, 75–80% B cells, 5–10% T cells and <5% TBM. They spontaneously form clusters within 24 h of culture and exhibit antigen-specific B cell proliferation and antibody production.
RNA species encoding proteins that appear to be essential for the formation of germinal centers such as B7.2 (60, 61), CD40 (62), and TNF-R1 (63) were present in the freshly isolated day 7 populations correlating with the ability to initiate the formation of PNA+ foci (data not shown). However, the mRNA levels for B7.1 were slightly higher in the IL-6–deficient mice derived cells than the wild-type (data not shown). This led to the investigation of protein production for these costimulatory molecules. As can be seen in Fig. 4, whereas both germinal center B cell populations expressed similar amounts of B7.2, only those obtained from the IL-6–deficient mice expressed B7.1 molecules. These data suggest that under normal conditions, the signals which exist (at day 7) within the germinal center support a T cell phenotype promoted by B7.2. However, in the absence of IL-6, B7.1 is also expressed, potentially causing a premature signal for downregulating the response (64).
Figure 4.
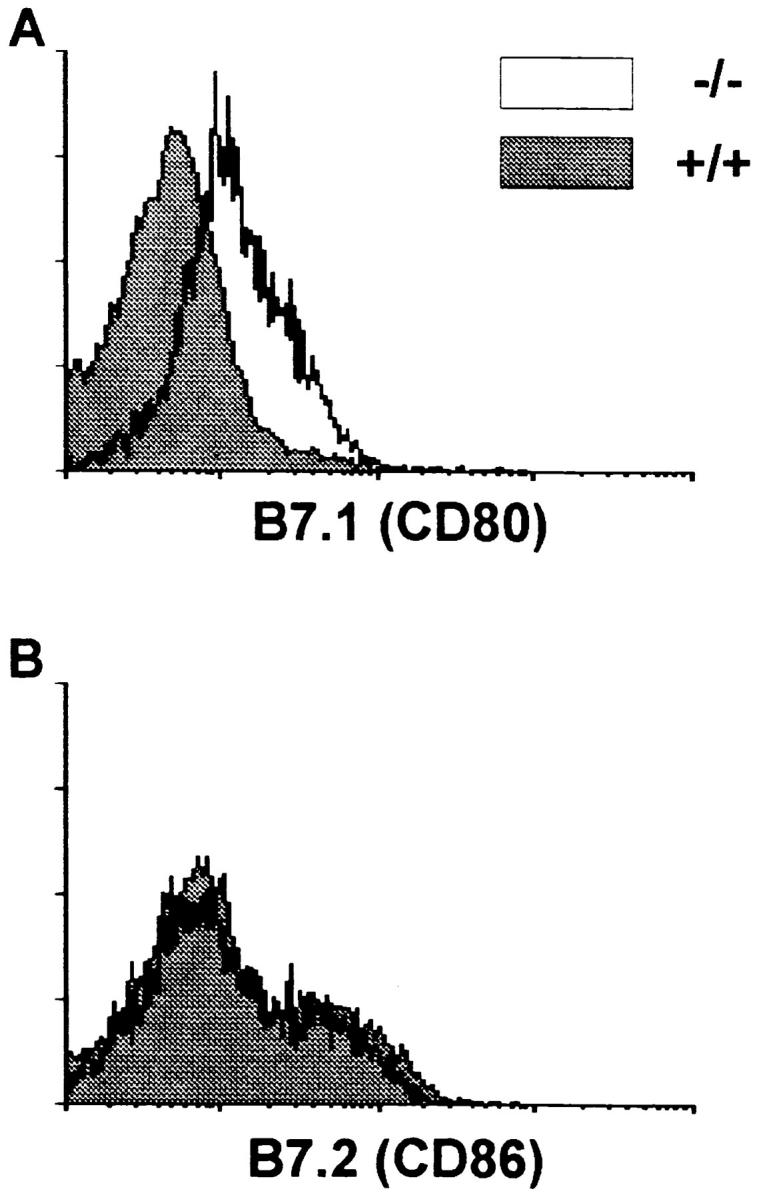
Expression of B7.2 is similar while B7.1 is upregulated on germinal center B cells of IL-6–deficient as compared with wild-type mice. Germinal center cells were isolated as described in the Materials and Methods section. The B cells were gated on using B220 conjugated to PE and then the level of B7.1 (A) and B7.2 (B) determined using biotinylated reagents plus avidin-FITC. The results are presented as levels on the wild-type derived (solid histograms) versus germinal center B cells obtained from the deficient mice (open histograms). Isotype controls were similar to the level seen with B7.1 on wild-type derived cells.
IL-6–deficient Mice Demonstrate Altered C3 Responses After Antigenic Stimulation.
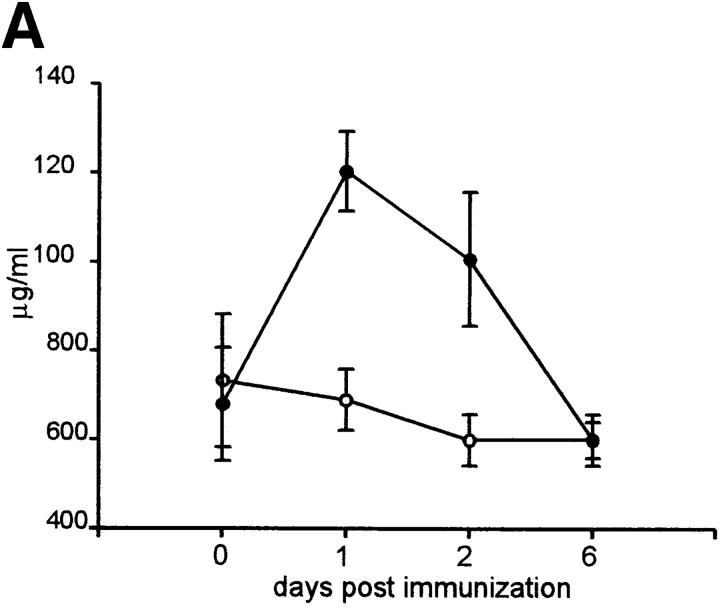
Activation products of C3 are known to interact with complement receptor 2 (CR2; CD21) molecules and augment B cell activation in conjunction with the coligation of the B cell antigen receptor (BCR; references 65, 66). As IL-6 plays a central role in the induction of acute phase protein synthesis, including C3, C3 levels were determined in serum taken before and at 1, 2 and 6 d after antigen injection. The results demonstrated that C3 levels were significantly elevated in wild-type mice when responding to an immunogen (Fig. 5 A). However, IL-6–deficient mice did not show a deviation from steady state levels.
Figure 5.
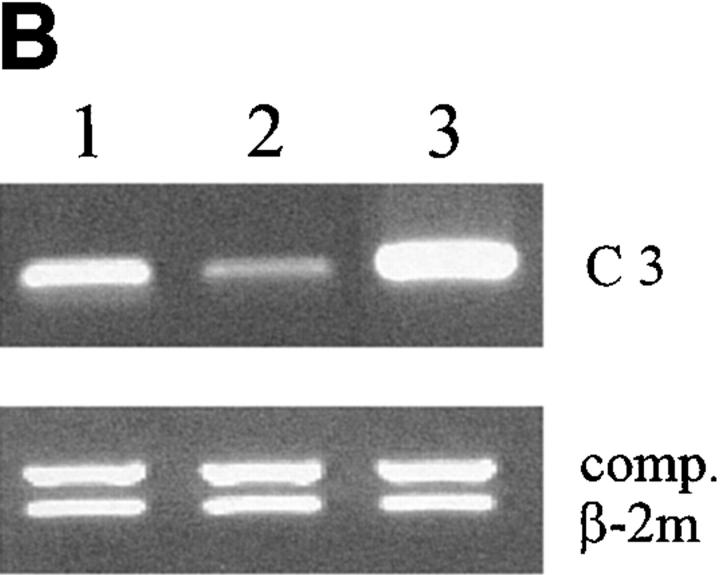
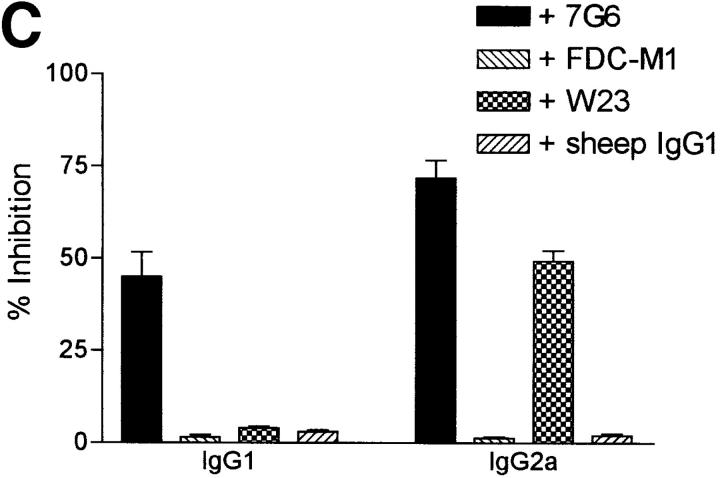
C3 production and mRNA levels are altered in response to antigen challenge in IL-6–deficient mice and blocking C3-CD21 interactions between germinal center cells results in decreased antibody production. (A) Sera were obtained from wild-type (solid circles) and IL-6–deficient (open circles) mice at various times before and after immunization. The C3 levels were determined and the data representative of three mice per group ± SD. (B) Germinal center cells were purified from wild-type or IL-6–deficient groups of mice (n = 4) at day 7 after immunization as described in Materials and Methods and C3 mRNA expression was analyzed by semiquantitative RT-PCR. Samples were standardized for the expression of β2 microglobulin (β2 m) using a competitor plasmid (55). As a positive control for C3 expression, liver RNA of wild-type mice was obtained 24 h after injection of silver nitrate for induction of an acute phase response. Lane 1, wild-type germinal center cells; lane 2, IL-6–deficient germinal center cells; lane 3, positive control from liver. (C) Germinal center cells were isolated from wild-type mice at 7 d after primary immunization with OVA and incubated for 7 d in the presence or absence of 7G6 (anti-CD21), FDC-M1 (isotype control), W23 (anti-C3), or sheep IgG1 (isotype control). OVA specific antibody titers were determined for IgG1 and IgG2a using standard ELISA procedures. The data is represented as percent inhibition as compared with wells with medium alone, done in triplicates ± SD.
Although failure to upregulate serum C3 levels correlated with impaired humoral immunity in IL-6–deficient mice, the circulating C3 concentration was still normal and certainly sufficient for any complement-dependent function taking place in the plasma. This suggested that if C3 was involved it must be C3 within the lymphoid tissue microenvironment, and specifically the germinal centers in which the interactions productive of the T dependent immune response take place between antigen, presenting cells and lymphocytes. The original studies of immunosuppression produced by cobra venom factor and its abrogation by passively administered anti-cobra factor antibodies, provided evidence for just this important local role of C3 within responding lymphoid tissue (40, 41). Indeed, analyzing the levels of mRNA for C3 in the germinal center cells isolated at day 7 revealed a strong band from the wild-type mice (Fig. 5 B). In contrast, the germinal center cells from the IL-6– deficient mice showed a reduction of C3 specific transcripts.
Next, to investigate specifically what a lack of C3 would cause in our system, wild-type germinal center cells were cultured in the presence or absence of an anti-CD21 mAb (7G6) or F(ab′)2 fragments of an anti-mouse C3 Ab (W23). Supernatants obtained from these cultures demonstrated that both abs inhibited antigen-specific IgG2a and IgG2b titers by 50–80% (Fig. 5 C and data not shown). Thus, local C3 is at least part of the mechanism for optimal germinal center function.
IL-6 Production Lies within the Nonlymphocytic Cells of the Germinal Center.
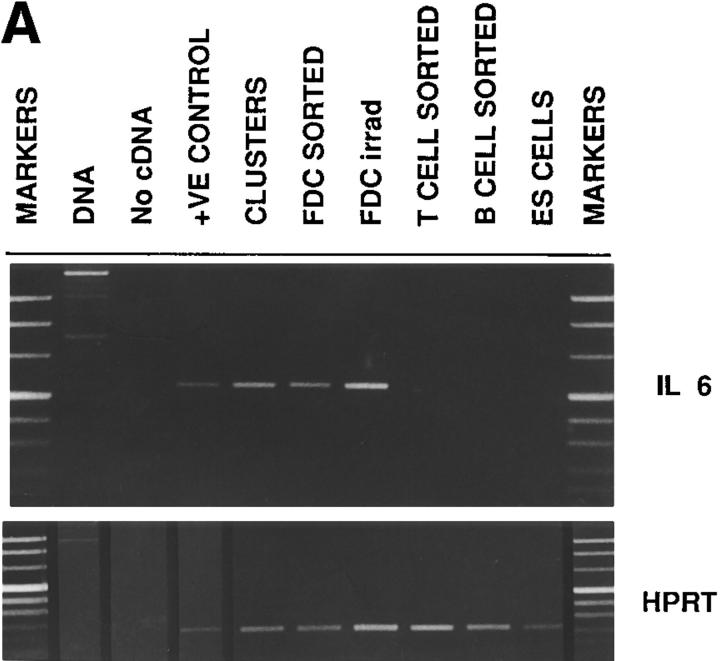
To determine which cells produce IL-6 in germinal centers, extracts from various wild-type germinal center cell populations were obtained and analyzed for levels of IL-6 mRNA. The low buoyant density, nonadherent fraction was obtained from the lymph nodes of immune mice and either immediately FACS® sorted to obtain highly purified B or T cells for RNA (Fig 6, B CELL SORTED and T CELL SORTED), or placed in culture to allow the formation of FDC-B cell-T cell clusters. After 24 h, the clusters were separated from the single cells in the cultures then prepared for RNA (Fig. 6, CLUSTERS). As FDC are difficult to obtain in a highly purified form, the following strategy was used. As previously reported, in order to enrich for FDC, 48 h after a secondary immunization mice were subjected to 6Gy of gamma irradiation (49). The lymph nodes were then excised 48 h later and the low buoyant density, nonadherent fraction now containing <5% lymphocytes (as compared with 80–90% without irradiation treatment) and ≤50% FDC were obtained and the RNA extracted (Fig. 6, FDC IRRAD). To further enrich for FDC, this preparation was double labeled with an anti-Ig reagent and the anti-FDC mAb, FDC-M1. The double positive cells consisting of >80% FDC were FACS® sorted and the RNA extracted (Fig. 6, FDC SORTED).
Figure 6.
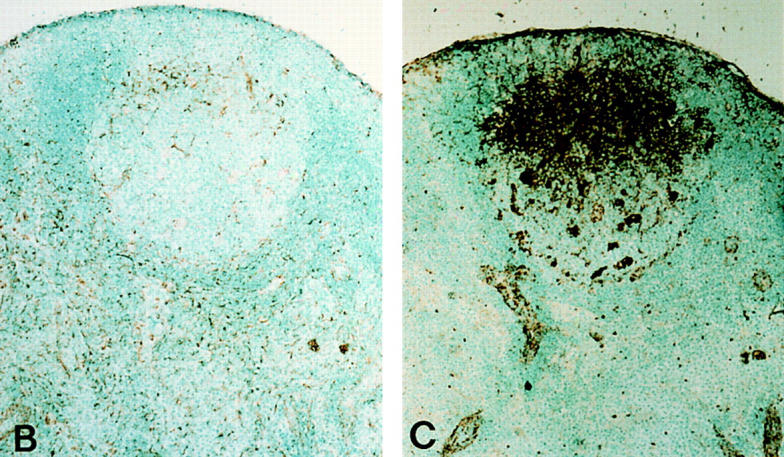
FDC produce IL-6 in germinal centers. (A) mRNA. Germinal center cells were isolated from wild-type mice as specified in Materials and Methods. T cells, B cells and FDC were either further separated by flow cytometry using the markers, Thy-1, B220, and FDC-M1, respectively (T CELL SORTED, B CELL SORTED and FDC SORTED) or cultured overnight in order to form clusters (CLUSTERS). FDC were also isolated from immune mice 2 d after receiving 6Gy of irradiation (FDC irrad). A Th2 cell line was used as the positive control (+VE CONTROL). Embryonic stem cells were used as a negative control (ES CELLS). RNA was prepared and the level of message for IL-6 determined using RT-PCR. HPRT was used as control for sample loading. (B and C) Cryosections were processed from lymph nodes of wild-type mice at day 10 after primary immunization for immunoperoxidase labeling (brown). The germinal center localization of IL-6–producing cells (B) was revealed in the light zone using the anti-mouse IL-6 mAb, MP5-20F3, whereas the adjacent sections demonstrate the extent of the FDC network in this corresponding area using the anti-mouse FDC labeling mAb, FDC-M1 (C).
The results demonstrate that IL-6 mRNA is produced by cells within the germinal center clusters (Fig. 6 A). The dissection of the various fractions revealed it was not the lymphocytes, but the FDC-enriched populations that produced the IL-6 mRNA. Furthermore, using a bioassay for IL-6 extended these results demonstrating that IL-6 was present only in cultures containing either clusters or the FDC irradiated population but not in the sorted T or B lymphocytes (data not shown). Immunohistochemistry performed on cryosections from immune wild-type mice confirmed these results revealing that a subset of cells with dendritic morphology (Fig. 6 B) within the FDC network (Fig. 6 C) produced IL-6. This production was limited to active germinal centers and not detected in primary follicles (data not shown).
Exogenous IL-6 Augments Proliferation and Antibody Production by Germinal Center B Cells from IL-6–deficient Mice.
When germinal center cells were isolated from wild-type mice and cultured with additional IL-6, no effect was observed (Fig. 7). This most likely reflects that optimal stimulation already exists within the wild-type derived population. In contrast, germinal center cells isolated from IL-6–deficient mice responded significantly to exogenous IL-6 with both increases in DNA synthesis (Fig. 7 A) and antigen-specific antibody production (Fig. 7 B). These observations suggest that IL-6 plays a role in cell cycle progression that influences the ability to differentiate along the plasmacytoid pathway (67).
Germinal Center Cells Isolated from C3-deficient or IL-6– deficient Mice Have Similar Defects in Antibody Production.
It has been previously shown that C3-deficient mice have impaired germinal center formation and defects in antigen-specific antibody production (47). To further correlate the phenotype observed in the IL-6–deficient germinal center cells with a role for C3, the antigen-specific antibody isotypes were compared between IL-6–deficient and C3-deficient mice. As shown in Fig. 7 B, whereas C3-deficient mice can mount an IgG1 response, IgG2a and IgG2b titers were equally affected in both targeted mice.
Discussion
IL-6 signaling via its two subunit receptor (the IL-6 receptor chain and gp130) mediates numerous biological activities. To understand the role of IL-6 in high affinity antibody responses, we concentrated on the cells of the germinal center. Our initial observations demonstrated that in IL-6–deficient mice antigen-specific antibody titers and the development of germinal centers were impaired. These effects are consistent with a report concerning mice overexpressing a dominant negative form of gp130, the signal-transducing element for IL-6 as well as other cytokines such as IL-11, oncostatin M and leukemia inhibitory factor (68, 69). These mice have severely lowered antibody titers. However, in IL-6–deficient mice, the IgG2a and IgG2b responses were most affected and IgE responses were slightly enhanced rather than decreased revealing more specifically the influence of IL-6 itself on antibody responses. The IgE levels also suggested that IL-4 production was not impaired. Indeed, T cell activation and T helper cell subset differentiation appeared not to be significantly altered in IL-6–deficient mice. Interestingly, IgE antibody formation is suppressed by in vivo complement depletion (70), indicating the existence of differing pathways for induction of the different T-dependent isotypes. In vitro restimulation of primed T cells with irradiated APCs in the presence of antigen showed similar levels of antigen-specific T cells and Th1 responses (γ-IFN production), whereas Th2 responses (IL-4, IL-5) were slightly elevated. Our results imply that in vivo Th1 and Th2 development does not depend on IL-6, although in vitro IL-6 may favor the induction of a Th2 phenotype (reference 71; Kopf, M., unpublished observations).
During humoral responses, our data demonstrates that IL-6 appears to act on germinal center B cells directly via the IL-6R and gp130, and indirectly by promoting the production of C3 in the local environment. Germinal center B cells are known to express IL-6 receptors but, unlike other B cells, lack the ability to make the cytokine (Fig. 6 A; reference 15). It appears that this is the role of a subset of low density cells with dendritic morphology. As critical events occur in this microenvironment to produce a high affinity immunoglobulin molecule, a stepwise series of events ensures checkpoints to minimize autoreactivity (72). Certainly, the sensitivity of germinal center reactions to deficiencies in numerous molecules supports this view of multistep and multicellular mechanisms (30, 31). It is interesting then that the expression of B7.1 is detected at day 7 on cells from the deficient mice and not on those of the wild-type. Although the phenotype of B7.1 and B7.2 single and double deficient mice would suggest these molecules have some overlapping effects (61), clearly overexpressing B7.1 demonstrated a negative regulatory modification of humoral responses (64). Hence, a more specific evaluation of the role of these two molecules in germinal center responses using these sets of mice would be of interest.
C3 contributes to the germinal center response by (a) forming complexes with antibody and antigen that are trapped on the plasma membrane of FDC (73, 74); and (b) providing optimal B cell stimulation via ligation of CD21 (the complement receptor type 2 [CR2], a part of the CD19-CD21-CD81 complex; references 75–77). It has long been known that spleen cells produce C3 and that anti-C3 antibodies can inhibit T-dependent antibody production (39, 44). Carroll and colleagues have recently shown that myeloid cells within lymphoid tissues are responsible for this local C3 production and that it is regulated by responses to antigen (48). Our data concerning C3 mRNA expression in germinal center cells and its regulation by IL-6 are novel and for the first time link these events. Further evidence of this relationship is found by comparing the phenotype observed in the IL-6– and C3-deficient germinal center cells (Fig. 7). Another possible candidate for regulation of local C3 levels could be γ-IFN (78, 79). However, as we did not find any difference in γ-IFN production by T cells isolated from wild-type versus IL-6–deficient mice, this appears not to be the mechanism. Thus, C3 plays a central role in generating high affinity antibody responses and our data would suggest that IL-6 plays a role in initiating these events.
It is relevant to also compare the IL-6–deficient mice with the phenotypes published for the C3- and CD21-deficient mice (46, 47). They are the only genetically engineered mice published to date that show a reduced but not totally ablated germinal center development (30, 59). Unfortunately, in these studies, individual IgG isotypes were not reported. In our study, both anti-C3 and anti-CD21 neutralizing antibodies inhibited IgG2a and IgG2b production by the germinal center cell cultures, whereas only anti-CD21 inhibited IgG1. This may reflect the difference in blocking a receptor that has multiple ligands versus neutralizing a single ligand. In addition, the more diverse effect could be related to interfering with the CD19-CD21-CD81 complex (75). Our results using the antibodies were confirmed using the C3-deficient mice (Fig. 7) and thus show the utility of analyzing immunoglobulin isotypes in order to define more precisely the role of molecules during humoral responses.
It is well known that the effects of the complement system can be overcome by altering the dose of antigen, the site of the immunization and the type of adjuvant (39, 56, 80, 81). Similarly, the requirement of IL-6 for humoral responses appears not to be an absolute. For instance, IL-6– deficient mice infected with M. tuberculosis show slightly enhanced (twofold) rather than reduced specific serum antibody titers 3 mo after infection (82). Furthermore, IL-6– deficient mice with systemic candidiasis produce elevated levels of Candida-specific IgG2a antibody compared with control IL-6 wild-type mice (83). However, IL-6–deficient mice are highly susceptible to infection with both M. tuberculosis and C. albicans. Numbers of yeast cells or mycobacteria in infected organs are ≤100-fold increased in the absence of IL-6. In contrast, IgG2a responses after infection with Plasmodium chabaudi were strongly impaired in IL-6–deficient mice (Langhorne, J., and M. Kopf, unpublished data).
Studies of mucosal responses in IL-6–deficient mice have revealed what appeared to be conflicting results regarding the role of IL-6 in the regulation of IgA. IL-6–deficient mice infected intranasally with recombinant vaccinia virus expressing the hemagglutinin (HA) glycoprotein of influenza virus have strongly reduced numbers of HA-specific IgA and IgG antibody-producing cells (84). Likewise, intraduodenal injection of ovalbumin resulted in impaired IgA responses (84). In contrast, normal mucosal IgA and IgG responses were observed in IL-6–deficient mice after either inoculation with Helicobacter felis or repeated peroral immunization with soluble protein (ovalbumin) in the presence of cholera toxin (CT; reference 85). Our present results may help to resolve this conflict. The defective primary response against HA expressed by attenuated vaccinia virus probably reflects complement dependence, whereas the response against repeated antigen immunization in the presence of the strong adjuvant such as CT, or a high infection dose with H. felis, does not require complement. Our results show that the primary response against ovalbumin was more affected than after the secondary challenge (Fig. 1), and that a tertiary response eventually showed little differences, again suggesting that with multiple insults, pathways exist to circumvent the loss of one pathway.
A model incorporating these and the recent findings of others would involve the early induction of de novo C3 synthesis by IL-6 in order to permit attachment of immune complexes to FDC and possibly also to activate naive B cells with low affinity immunoglobulin receptors, both intrinsic steps of germinal center development. One potential source for early IL-6 production could be the antigen stimulated dendritic cells (86). As they migrate through macrophage rich zones in order to reach the T cell areas (87), these cells may induce local myeloid C3 production. Later, during the dynamic processes occurring in germinal centers, FDC would receive appropriate signals to produce IL-6 that may amplify local C3 production by tingible body macrophages as well as directly act on germinal center B cells via IL-6–sensitive transcription factors. These scenarios clearly demonstrate the complicated nature of the events occurring to generate high affinity antibody responses.
Signaling of CD21 expressed by B cells via C3 components is essential then for normal germinal center reactions (88). It has also been demonstrated that C3 must be in a form that is able to cross-link CD21 (e.g., on the surface of FDC), as soluble C3 stimulates the proliferation of activated, but not resting, B cells (89). Interestingly, a novel C3 mRNA transcript has been identified that enhances B cell proliferation (90). The mechanism of action then for IL-6 concerning the increased thymidine uptake in our germinal center B cell-T cell-FDC cultures could be the generation of this C3 protein locally. The experiments addressing these issues are ongoing. In addition, the consequences of IL-6 production locally within the germinal center may be to act on centoblasts directly via transcription factors and cyclin-dependent kinases (2, 67). Indeed the increase of antigen-specific antibody that occurred when exogenous IL-6 was added to the IL-6–deficient germinal center cell cultures exemplifies the role of IL-6 in differentiation and cell cycle control of late stage B cells (20, 89, 91).
Acknowledgments
The authors would like to thank D. Scheidegger, B. Johansson, C. Lefrang, and J. Herbert for their excellent technical assistance, and M.C. Carroll for valuable discussions as well as C3-deficient mice. We would also like to thank T. Rolink, J. Poudrier, and G. Dasic for critical reading of the manuscript.
Abbreviations used in this paper
- BCR
B cell antigen receptor
- FDC
follicular dendritic cell
- HA
hemagglutinin
- HPRT
hypoxanthine phosphoribosyltransferase
- PNA
peanut agglutinin
- RT
reverse transcription
Footnotes
M.B. Pepys is supported by Programme Grant G97900510. The Basel Institute for Immunology was founded and is supported by F. Hoffmann-La Roche, Basel, Switzerland.
Address all correspondence to M.H. Kosco-Vilbois at her present address: Serono Pharmaceutical Research Institute, 14, chemin des Aulx, CH-1228 Geneva, Switzerland. Phone: 41-22-706-9708. Fax: 41-22-794-6965. E-mail: marie.kosco-vilbois@serono.com
S. Herren's present address is Serono Pharmaceutical Research Institute, CH-1228 Geneva, Switzerland.
References
- 1.van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 2.Kishimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992;258:593–597. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- 3.Geiger T, Andus T, Klapproth J, Hirano T, Kishimoto T, Heinrich PC. Induction of rat acute-phase proteins by interleukin 6 in vivo. Eur J Immunol. 1988;18:717–721. doi: 10.1002/eji.1830180510. [DOI] [PubMed] [Google Scholar]
- 4.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 5.Helle M, Brakenhoff JP, de Groot ER, Aarden LA. Interleukin 6 is involved in interleukin 1-induced activities. Eur J Immunol. 1988;18:957–959. doi: 10.1002/eji.1830180619. [DOI] [PubMed] [Google Scholar]
- 6.Marinkovic S, Jahreis GP, Wong GG, Baumann H. Il-6 modulates the synthesis of a specific set of acute phase plasma proteins in vivo. J Immunol. 1989;142:808–812. [PubMed] [Google Scholar]
- 7.Hoang T, Haman A, Goncalves O, Wong GG, Clark SC. Interleukin-6 enhances growth factor-dependent proliferation of the blast cells of acute myeloblastic leukemia. Blood. 1988;72:823–826. [PubMed] [Google Scholar]
- 8.Bot FJ, van Eijk L, Broeders L, Aarden LA, Lowenberg B. Interleukin-6 synergizes with M-CSF in the formation of macrophage colonies from purified human marrow progenitor cells. Blood. 1989;73:435–437. [PubMed] [Google Scholar]
- 9.Nordan RP, Potter M. A macrophage-derived factor required by plasmacytomas for survival and proliferation in vitro. Science. 1986;233:566–569. doi: 10.1126/science.3726549. [DOI] [PubMed] [Google Scholar]
- 10.van Snick J, Vink A, Cayphas S, Uyttenhove C. Interleukin-hp1, a T cell-derived hybridoma growth factor that supports the in vitro growth of murine plasmacytomas. J Exp Med. 1987;165:641–649. doi: 10.1084/jem.165.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Snick J, Cayphas S, Vink A, Uyttenhove C, Coulie PG, Rubira MR, Simpson RJ. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci USA. 1986;83:9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissenbach J, Chernajovsky Y, Zeevi M, Shulman L, Soreq H, Nir U, Wallach D, Perricaudet M, Tiollais P, Revel M. Two interferon mRNAs in human fibroblasts: in vitro translation and Escherichia colicloning studies. Proc Natl Acad Sci USA. 1980;77:7152–7156. doi: 10.1073/pnas.77.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbel C, Melchers F. The synergism of accessory cells and of soluble alpha-factors derived from them in the activation of Bb cells to proliferation. Immunol Rev. 1984;78:51–74. doi: 10.1111/j.1600-065x.1984.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 14.Hirano T, Taga T, Nakano N, Yasukawa K, Kashiwamura S, Shimizu K, Nakajima K, Pyun KH, Kishimoto T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFP-2) Proc Natl Acad Sci USA. 1985;82:5490–5494. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdin N, Galibert L, Garrone P, Durand I, Banchereau J, Rousset F. Inability to produce IL-6 is a functional feature of human germinal center B lymphocytes. J Immunol. 1996;156:4107–4113. [PubMed] [Google Scholar]
- 16.de Vries JE, Zurawski G. Immunoregulatory properties of IL-13: its potential role in atopic disease. Int Arch Allergy Immunol. 1995;106:175–179. doi: 10.1159/000236842. [DOI] [PubMed] [Google Scholar]
- 17.Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 19.Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman's disease in mice. J Clin Invest. 1990;86:592–599. doi: 10.1172/JCI114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1989;86:7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oka Y, Rolink AG, Suematsu S, Kishimoto T, Melchers F. An interleukin-6 transgene expressed in B lymphocyte lineage cells overcomes the T cell-dependent establishment of normal levels of switched immunoglobulin isotypes. Eur J Immunol. 1995;25:1332–1337. doi: 10.1002/eji.1830250530. [DOI] [PubMed] [Google Scholar]
- 22.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacob J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3- nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med. 1992;176:679–687. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelsoe G. In situ studies of the germinal center reaction. Adv Immunol. 1995;60:267–288. doi: 10.1016/s0065-2776(08)60587-8. [DOI] [PubMed] [Google Scholar]
- 25.MacLennan ICM, Gulbranson-Judge A, Toellner KM, Casamayor-Palleja M, Chan E, Sze DMY, Luther SA, Acha H, Orbea The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 26.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 27.Kosco MH, Szakal AK, Tew JG. In vivo obtained antigen presented by germinal center B cells to T cells in vitro. J Immunol. 1988;140:354–360. [PubMed] [Google Scholar]
- 28.Kosco-Vilbois MH, Gray D, Scheidegger D, Julius M. Follicular dendritic cells help resting B cells to become effective antigen-presenting cells: induction of B7/bb1 and upregulation of major histocompatibility complex class II molecules. J Exp Med. 1993;178:2055–2066. doi: 10.1084/jem.178.6.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guery JC, Ria F, Adorini L. Dendritic cells but not B cells present antigenic complexes to class II-restricted T cells after administration of protein in adjuvant. J Exp Med. 1996;183:751–757. doi: 10.1084/jem.183.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosco-Vilbois MH, Bonnefoy JY, Chvatchko Y. The physiology of murine germinal center reactions. Immunol Rev. 1997;156:127–136. doi: 10.1111/j.1600-065x.1997.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 31.Kosco-Vilbois MH, Zentgraf H, Gerdes J, Bonnefoy JY. To ‘b' or not to ‘b' a genal center. Immunol Today. 1997;18:225–230. doi: 10.1016/s0167-5699(97)01048-7. [DOI] [PubMed] [Google Scholar]
- 32.Tew JG, Wu J, Qin D, Helm S, Burton GF, Szakal AK. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol Rev. 1997;156:39–52. doi: 10.1111/j.1600-065x.1997.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 33.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 34.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 35.MacLennan IC, Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 36.Smith KG, Nossal GJ, Tarlinton DM. FAS is highly expressed in the germinal center but is not required for regulation of the B-cell response to antigen. Proc Natl Acad Sci USA. 1995;92:11628–11632. doi: 10.1073/pnas.92.25.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tew JG, DiLosa RM, Burton GF, Kosco MH, Kupp LI, Masuda A, Szakal AK. Germinal centers and antibody production in bone marrow. Immunol Rev. 1992;126:99–112. doi: 10.1111/j.1600-065x.1992.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 38.Pepys MB. Role of complement in induction of the allergic response. Nat New Biol. 1972;237:157–159. doi: 10.1038/newbio237157a0. [DOI] [PubMed] [Google Scholar]
- 39.Pepys MB. Role of complement in the induction of immunological responses. Transplant Rev. 1976;32:93–120. doi: 10.1111/j.1600-065x.1976.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 40.Pepys MB. Role of complement in induction of antibody production in vivo. J Exp Med. 1974;140:126–145. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pepys MB. Studies in vivo of cobra venom factor and murine C3. Immunology. 1975;28:369–377. [PMC free article] [PubMed] [Google Scholar]
- 42.Papamichail M, Gutierrez C, Embling P, Johnson P, Holborow J, Pepys M. Complement dependence of germinal center localization of aggregated γ-globulin. Scand J Immunol. 1975;4:343–347. doi: 10.1111/j.1365-3083.1975.tb02635.x. [DOI] [PubMed] [Google Scholar]
- 43.Phillips ME, Thorbecke GJ. Serum protein formation of donor type in rat into mouse chimeras. Nature. 1965;207:376–378. doi: 10.1038/207376a0. [DOI] [PubMed] [Google Scholar]
- 44.Feldmann M, Pepys MB. Role of C3 in in vitro lymphocyte cooperation. Nature. 1974;249:159–161. doi: 10.1038/249159a0. [DOI] [PubMed] [Google Scholar]
- 45.Pepys M. Complement-mediated mixed aggregation of murine spleen cells. Nature. 1974;249:51–53. doi: 10.1038/249051a0. [DOI] [PubMed] [Google Scholar]
- 46.Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, Carroll MC. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 47.Fischer MB, Ma M, Goerg S, Zhou X, Xia J, Finco O, Han S, Kelsoe G, Howard RG, Rothstein TL, et al. Regulation of the B cell response to T-dependent antigen by classical pathway complement. J Immunol. 1996;157:549–556. [PubMed] [Google Scholar]
- 48.Fischer MB, Ma M, Hsu NC, Carroll MC. Local synthesis of C3 within the splenic lymphoid compartment can reconstitute the impaired immune response in C3 deficient mice. J Immunol. 1998;160:2619–2625. [PubMed] [Google Scholar]
- 49.Gray D, Kosco M, Stockinger B. Novel pathways of antigen presentation for the maintenance of memory. Int Immunol. 1991;3:141–148. doi: 10.1093/intimm/3.2.141. [DOI] [PubMed] [Google Scholar]
- 50.Baniyash M, Eshhar Z. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur J Immunol. 1984;14:799–807. doi: 10.1002/eji.1830140907. [DOI] [PubMed] [Google Scholar]
- 51.Coico RF, Bhogal BS, Thorbecke GJ. Relationship of germinal centers in lymphoid tissue to immunological memory. VI. Transfer of B cell memory with lymph node cells fractionalted according to their receptors for peanut agglutinin. J Immunol. 1983;131:2254–2257. [PubMed] [Google Scholar]
- 52.Szakal AK, Taylor JK, Smith JP, Kosco MH, Burton GF, Tew JJ. Kinetics of germinal center development in lymph nodes of young and aging immune mice. Anat Rec. 1990;227:475–485. doi: 10.1002/ar.1092270411. [DOI] [PubMed] [Google Scholar]
- 53.Kosco MH, Pflugfelder E, Gray D. Follicular dendritic cell-dependent adhesion and proliferation of B cells in vitro. J Immunol. 1992;148:2331–2339. [PubMed] [Google Scholar]
- 54.Pepys MB, Dash AC, Fielder AH, Mirjah DD. Isolation and study of murine c3. Immunology. 1977;33:491–499. [PMC free article] [PubMed] [Google Scholar]
- 55.Kopf M, Brombacher F, Kohler G, Kienzle G, Widmann KH, Lefrang K, Humborg C, Ledermann B, Solbach W. IL-4-deficient BALB/c mice resist infection with Leishmania major. J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heyman B, Wiersma EJ, Kinoshita T. In vivo inhibition of the antibody response by a complement receptor-specific monoclonal antibody. J Exp Med. 1990;172:665–668. doi: 10.1084/jem.172.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johansson BM, Wiles MV. Evidence for involvement of activin a and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol Cell Biol. 1995;15:141–151. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kosco-Vilbois, M.H. 1997. Isolation and enrichment of follicular dendritic cells from murine lymphoid tissue. In Immunology Methods Manual. I. Lefkovits, editor. Academic Press, London, UK. 1468–1473.
- 60.Lane P, Burdet C, Hubele S, Scheidegger D, Muller U, McConnell F, Kosco-Vilbois M. B cell function in mice transgenic for mCTLA4-Hγ1: lack of germinal centers correlated with poor affinity maturation and class switching despite normal priming of CD4+T cells. J Exp Med. 1994;179:819–830. doi: 10.1084/jem.179.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 62.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 63.Le Hir M, Bluethmann H, Kosco-Vilbois MH, Muller M, di Padova F, Moore M, Ryffel B, Eugster HP. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J Exp Med. 1996;183:2367–2372. doi: 10.1084/jem.183.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sethna MP, van Parijs L, Sharpe AH, Abbus AK, Freeman GJ. A negative regulatory function of B7 revealed in B7-1 transgenic mice. Immunity. 1994;1:415–421. doi: 10.1016/1074-7613(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 65.Carter RH, Spycher MO, Ng YC, Hoffman R, Fearon DT. Synergistic interaction between complement receptor type 2 and membrane igm on B lymphocytes. J Immunol. 1988;141:457–463. [PubMed] [Google Scholar]
- 66.Carter RH, Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256:105–107. [PubMed] [Google Scholar]
- 67.Morse L, Chen D, Franklin D, Xiong Y, Chen-Kiang S. Induction of cell cycle arrest and B cell terminal differentiation by cdk inhibitor p18(ink4c) and IL-6. Immunity. 1997;6:47–56. doi: 10.1016/s1074-7613(00)80241-1. [DOI] [PubMed] [Google Scholar]
- 68.Kumanogoh A, Marukawa S, Kumanogoh T, Hirota H, Yoshida K, Lee IS, Yasui T, Taga T, Kishimoto T. Impairment of antigen-specific antibody production in transgenic mice expressing a dominant-negative form of gp130. Proc Natl Acad Sci USA. 1997;94:2478–2482. doi: 10.1073/pnas.94.6.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akira S, Yoshida K, Tanaka T, Taga T, Kishimoto T. Targeted disruption of the IL-6 related genes: gp130 and nf-IL-6. Immunol Rev. 1995;148:221–253. doi: 10.1111/j.1600-065x.1995.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 70.Pepys M, Brighton W, Hewitt B, Bryant D, Pepys J. Complement in the induction of IgE antibody formation. Clin Exp Immunol. 1977;27:397–400. [PMC free article] [PubMed] [Google Scholar]
- 71.Rincón M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4–producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pulendran B, van Driel R, Nossal GJ. Immunological tolerance in germinal centres. Immunol Today. 1997;18:27–32. doi: 10.1016/s0167-5699(97)80011-4. [DOI] [PubMed] [Google Scholar]
- 73.Tew JG, Kosco MH, Burton GF, Szakal AK. Follicular dendritic cells as accessory cells. Immunol Rev. 1990;117:185–211. doi: 10.1111/j.1600-065x.1990.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 74.Fang Y, Xu C, Fu YX, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]
- 75.Fearon DT, Carter RH. The CD19/CR2/ Tapa-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 76.Tedder TF, Inaoki M, Sato S. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–118. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 77.Fischer MB, Goerg S, Shen L, Prodeus AP, Goodnow CC, Kelsoe G, Carroll MC. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 1998;280:582–585. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- 78.Celada A, Klemsz MJ, Maki RA. Interferon-γ activates multiple pathways to regulate the expression of the genes for major histocompatibility class II I-Aβ, tumor necrosis factor and complement component C3 in mouse macrophages. Eur J Immunol. 1989;19:1103–1110. doi: 10.1002/eji.1830190621. [DOI] [PubMed] [Google Scholar]
- 79.Mitchell TJ, Naughton M, Norsworthy P, Davies KA, Walport MJ, Morley BJ. IFNγ up-regulates expression of complement components C3 and C4 by stabilization of mRNA. J Immunol. 1996;156:4429–4438. [PubMed] [Google Scholar]
- 80.Boettger EC, Bitter-Suermann D. Complement and the regulation of humoral immune responses. Immunol Today. 1987;8:261–265. doi: 10.1016/0167-5699(87)90184-8. [DOI] [PubMed] [Google Scholar]
- 81.O'Neil KM, Ochs HD, Heller SR, Cork LC, Morris JM, Winkelstein JA. Role of C3 in humoral immunity. Defective antibody production in C3-deficient dogs. J Immunol. 1988;140:1939–1945. [PubMed] [Google Scholar]
- 82.Ladel CH, Blum C, Dreher A, Reifenburg K, Kopf M, Kaufmann SHE. Lethal tuberculosis in IL-6 deficient mice. Infect Immun. 1997;65:4843–4849. doi: 10.1128/iai.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. . J Exp Med. 1996;183:1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramsay AJ, Husband AJ, Ramshaw IA, Bao S, Matthaei KI, Koehler G, Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264:561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- 85.Bromander AK, Ekman L, Kopf M, Nedrud JG, Lycke NY. IL-6-deficient mice exhibit normal mucosal IgA responses to local immunizations and helicobacter felis infection. J Immunol. 1996;156:4290–4297. [PubMed] [Google Scholar]
- 86.Cumberbatch M, Dearman RJ, Kimber I. Constitutive and inducible expression of interleukin-6 by Llangerhans cells and lymph node dendritic cells. Immunology. 1996;87:513–518. doi: 10.1046/j.1365-2567.1996.504577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steinman RM, Pack M, Inaba K. Dendritic cells in the T cell areas of lymphoid organs. Immunol Rev. 1997;156:25–38. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 88.Croix DA, Ahearn JM, Rosengard AM, Han S, Kelsoe G, Ma M, Carroll MC. Antibody response to a T dependent antigen requires B cell expression of complement receptors. J Exp Med. 1996;183:1857–1864. doi: 10.1084/jem.183.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melchers F, Erdei A, Schulz T, Dierich MP. Growth control of activated, synchronized murine B cells by the C3d fragment of human complement. Nature. 1985;317:264–267. doi: 10.1038/317264a0. [DOI] [PubMed] [Google Scholar]
- 90.Cahen-Kramer Y, Martensson IL, Melchers F. The structure of an alternate form of complement C3 that displays costimulatory growth factor activity for B lymphocytes. J Exp Med. 1994;180:2079–2088. doi: 10.1084/jem.180.6.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1992;89:232–235. doi: 10.1073/pnas.89.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]