Abstract
A system to innocuously visualize T cell lineage commitment is described. Using a “knock-in” approach, we have generated mice expressing a β-galactosidase reporter in place of CD4; expression of β-galactosidase in these animals appears to be an accurate and early indicator of CD4 gene transcription. We have exploited this knock-in line to trace CD4/CD8 lineage commitment in the thymus, avoiding important pitfalls of past experimental approaches. Our results argue in favor of a selective model of thymocyte commitment, demonstrating a fundamentally symmetrical process: engagement of either class of major histocompatibility complex (MHC) molecule by a differentiating CD4+CD8+ cell can give rise to T cell antigen receptor (TCR)hi thymocytes of either lineage. Key findings include (a) direct demonstration of a substantial number of CD4-committed, receptor/coreceptor-mismatched cells in MHC class II– deficient mice, a critical prediction of the selective model; (b) highly efficient rescue of such “mismatched” intermediates by forced expression of CD8 in a TCR transgenic line, and an explanation of why previous experiments of this nature were less successful—a major past criticism of the selective model; (c) direct demonstration of an analogous, though smaller, population of CD8-committed mismatched intermediates in class I–deficient animals. Finally, we found no evidence of a CD4 default pathway.
Keywords: homologous recombination, transgenesis, positive selection, CD4, β-galactosidase
Most conventional T lymphocytes fall into two classes— MHC class II–restricted, CD4+ helper, and MHC class I–restricted, CD8+ cytotoxic cells. Precursors of both classes differentiate in the thymus, according to an elaborate program classically visualized by following changes in expression of cell surface markers, in particular the CD4 and CD8 coreceptors (1, 2). The most immature thymocytes express no (or very little) CD4 or CD8 and are thus termed double-negative (CD4−CD8−; DN)1 cells; they also display no α/β TCR on the surface. DN thymocytes differentiate into double-positive (CD4+CD8+; DP) cells, most of which express low levels of surface TCR. Only 3–5% of DP thymocytes survive the transition to end-stage single-positive (SP) cells (3, 4) committed to either the CD4+ or CD8+ lineage and expressing high levels of TCR.
Survival and commitment at the DP stage of thymocyte differentiation are dictated by positive selection events that depend critically on specific interaction between the thymocyte's TCR and MHC molecules expressed on thymic stromal cells. Experiments involving TCR transgenic (tg) mice have demonstrated that DP thymocytes expressing class I–reactive TCRs are selected to become CD8+ cells (5–7), whereas those displaying class II–reactive receptors differentiate into CD4+ cells (8, 9). Thus, lineage commitment is an inseparable by-product of the positive selection process, the specificity of the TCR being matched to a particular class of MHC molecule and thereby to a particular coreceptor.
The mechanism by which this match is achieved and the nature of the signals directing CD4/CD8 lineage commitment remain unclear. For several years, discussions have crystallized around the issue of whether lineage choice is primarily instructive (directed by the TCR–MHC interaction) or selective (decided after an initially noncommittal TCR–MHC interaction), or is some combination of the two. A series of studies on MHC-deficient and TCR tg mice had concluded that DP thymocytes expressing a class I–reactive TCR can commit to either the CD4+ or CD8+ lineage (10–14); the same appeared true of DP cells with a class II–reactive receptor (10, 15–17). These conclusions, in line with a selective mechanism of lineage commitment, were based mainly on two kinds of observations. First, intermediate thymocyte populations (CD4+CD8int, CD4intCD8+) were described, apparently composed of cells in transit between the DP and SP compartments and including a fraction with mismatched receptors and coreceptors (10, 15, 16). Second, it was possible to “rescue” a population of mature lymphocytes seemingly derived from these “mistaken” thymocytes by forcing expression of the appropriate coreceptor (14, 15). In most of these studies, lineage commitment was assessed by one of two criteria: reduced surface expression of one of the coreceptors after positive selection, and helper or cytotoxic function of mature lymphocyte descendents in cases where terminal differentiation was (or was made) possible.
Unfortunately, results from more recent experiments have seriously undermined these conclusions, in particular the reliance on surface levels of coreceptor to identify intermediate populations and to serve as a marker of lineage commitment. For example, when the class I–reactive population of CD4+ CD8int thymocytes from MHC class II–deficient mice was injected into wild-type thymi, only CD8+ thymocytes were eventually recovered (18–20). Furthermore, when the same population was analyzed via an assay based on protease stripping and coreceptor reexpression, it appeared that many CD4+CD8int cells had turned off CD4 but not CD8 transcription (21, 22). These findings argued for greater complexity during the positive selection process, and demonstrated that surface coreceptor levels can be tardy and potentially misleading indicators of gene activity and lineage commitment.
However, intrathymic cell transfers and coreceptor reexpression assays are themselves potentially artifactual, as they entail manipulation of surface molecules (i.e., cross-linking, proteolysis) that could easily alter experimental outcome. Thus, the mechanism of lineage commitment is still a contentious issue, but it remains an important one as a framework for understanding the positive selection decision-making process at the molecular level. Therefore, we sought to develop a system for visualizing T cell commitment ex vivo in an innocuous manner. We searched for a reporter of gene activity that could be detected easily without perturbing manipulations and whose expression would be independent of transport or stability of the coreceptors. We report here the generation of mice with the LacZ gene targeted into the CD4 locus and experiments exploiting them to study CD4/CD8 lineage commitment.
Materials and Methods
Construction of the Recombinant Vector and Production of CD4+ /L Mice.
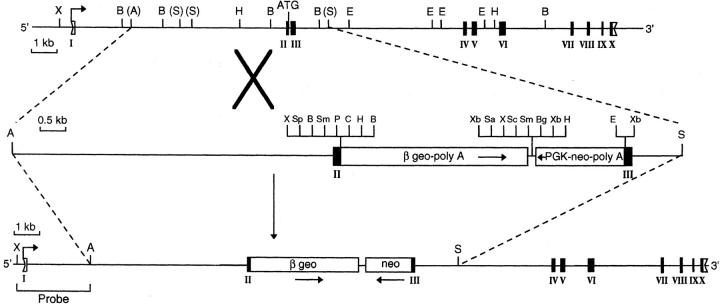
To obtain CD4+/L mice, we constructed a targeting vector as illustrated in Fig. 1. In brief, a 7.8-kb AvrII-SphI genomic fragment from the CD4 locus was subcloned. A 323-bp fragment containing most of exons 2 and 3 (including a 30-bp intron) was deleted, and three cloning sites (XhoI, EcoRI, and XbaI) were inserted by single-strand mutagenesis. The fragment's coding potential was interrupted by insertion of the 4.3-kb βgeo cassette (with its splice acceptor, Kozak ATG, and polyA tail, and multiple cloning sites [23]) into the XhoI site of exon 2 immediately before the CD4 ATG site, so that exon 2 begins and ends as follows: 5′-GCTCAGATTCCCAACCAACAAGAGCTC-3′. The 1.8-kb phosphoglycerate kinase (PGK)-neo cassette (containing the PGK promoter, a polyA tail, and cloning sites [24]) was inserted in reverse orientation immediately downstream of the βgeo cassette between XhoI and EcoRI sites. The PGK-neo cassette was thus fused to the remainder of exon 3: 5′-XbaI-TCTGGGGCAGCATGGCAAAGGTGTATTAATTAGAG-3′.
Figure 1.
Production of CD4+/L mice. A 326-bp fragment containing most of exons 2 and 3 (including a 30-bp intron) was deleted and replaced by the βgeo and neo cassettes. The 5′ external probe, an XhoI-AvrII fragment, detects an endogenous 15-kb XhoI band on Southern blots of DNA from wild-type D3 ES cells and a smaller 9.6-kb band on blots of DNA from cells carrying the knock-in mutation. Exons are marked with roman numerals, untranslated regions by open boxes, and translated regions by filled boxes. Arrows below the cassettes represent their 5′ to 3′ orientation. Bent arrows mark the transcription initiation sites. Restriction enzyme sites are abbreviated as follows: X (XhoI), B (BamHI), A (AvrII), S (SphI), H (HindIII), E (EcoRI), Sp (SpeI), Sm (SmaI), P (PstI), C (ClaI), Xb (XbaI), Sa (SalI), Sc (SacI), and Bg (BglII).
The resulting vector was linearized and electroporated into D3 embryonic stem (ES) cells as described (25). Of the 100 G418- resistant D3 clones analyzed, 6 carrying the predicted integration by homologous recombination were identified. Using an XhoI-SphI 5′ external probe, we considered a clone positive if it contained the normal 15-kb band plus an additional 9.6-kb band after XhoI digestion and Southern blotting. The positive clones were secondarily confirmed using an internal neo probe. Clone VE34 was expanded and injected into C57Bl/6 (B6) blastocysts, which were reimplanted into pseudopregnant females. Chimeras were crossed onto B6 mice to obtain germline transmission, and offspring carrying the mutation were maintained in a conventional animal facility.
“Homozygous” CD40/L mice were produced by crossing the CD4+/L line with heterozygous CD4-deficient (CD4+/0) mice (26).
Antibodies, Cytofluorimetric Analyses, and Cell Sorting.
The following antibodies were used: FITC-labeled anti-CD8α, biotinylated anti-CD8α, PE-labeled anti-CD4 (Caltag Laboratories, Inc., Burlingame, CA); KT3, specific for CD3 (27); T3.70, specific for the α chain of the transgene-encoded HY TCR (gift of H. von Boehmer, Institut Necker, Paris, France [5]); and B-20.1, specific for Vα2 (28). Texas Red–conjugated anti–rat antibodies and streptavidin-Cy5 were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) or Caltag Laboratories, Inc. Staining of thymocyte and lymph node cell suspensions was performed as described (25).
Three- to five-color flow cytometric analyses were performed on a cytometer (Elite; Coulter Corp., Hialeah, FL) equipped with 4 decade logarithmic amplifiers. List mode data were collected on 5 × 104 or 105 cells.
Fluorescein Digalactopyranoside Staining.
Fluorescein digalactopyranoside (FDG) staining was adapted from Nolan et al. (29). In brief, 4–6 × 106 cells were stained, washed, and resuspended in 120 μl PBS. FDG (Molecular Probes, Inc., Eugene, OR, or Sigma Chemical Co., St. Louis, MO) at the stock concentration of 100 mM in H2O/DMSO/ethanol, 1:1:1 (vol/vol), was diluted to a working condition of 7.5 mM with H2O. Both cells and diluted FDG were warmed to 37°C for 5 min. 80 μl of warmed FDG was added to cells while gently vortexing. Cells were incubated for 5 min at 37°C. FDG loading was stopped by adding 2 ml of ice-cold PBS. Cells were kept on ice for 5 min, then transferred to a 15°C water bath for 15–30 min to enhance β-galactosidase (βgal) activity. Cells were then ready to analyze, or were kept on ice until analysis by flow cytometry.
Immunoprecipitation Experiments.
Thymocyte suspensions were washed three times with methionine-free DME (GIBCO BRL, Gaithersburg, MD) supplemented with 5% FCS. Cells were labeled with [35S]methionine (∼1,200 Ci/mmol; Amersham Pharmacia Biotech, Inc., Piscataway, NJ) at 107 cells/ml/1 mCi. To begin the chase, thymocytes were washed with DME containing 10× cold methionine, and then recultured in DME (plus 5% FCS, 10× cold methionine) for the different time points. At each time point, aliquots (107) of cells were washed twice with PBS and frozen as pellets in liquid nitrogen. Frozen pellets were lysed with 1 ml lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, plus protease inhibitors) on ice for 30 min. After lysis, supernatants were cleared of cellular debris by centrifugation at 13,000 rpm for 15 min. Cell lysates were precleared twice for 30 min with 25 ml (50%) rat serum–coupled protein G–Sepharose 4FF (Amersham Pharmacia Biotech, Inc.). The first incubation was usually overnight. The precleared lysate was then precipitated for 1 h with protein G coupled to GK1.5 (specific for CD4 [30]) or anti-βgal (Promega Corp., Madison, WI). As a control, precipitations were prepared with rat serum–coupled beads. Immunoprecipitations were washed three times with 1 ml lysis buffer (at 0.2% Triton X-100), then once with PBS.
25 μl of Laemmli buffer (60 mM Tris, pH 6.8, 2% SDS, 10% glycerol, 5% 2-ME, 0.001% bromophenol blue) was added to each immunoprecipitation. The samples were heated for 5 min at 100°C and then loaded and run on a 10% SDS-PAGE gel. Signals on the gel were enhanced using Enlightning (NEN Life Science Products, Boston, MA) after running and were quantified with a Bio Imaging Analyzer (Fuji Photo Film Co., Tokyo, Japan).
Reverse Transcriptase PCR.
RNA was isolated from sorted cells by standard techniques. In brief, RNA was prepared by NP-40 lysis from 1–5 × 104 sorted cells to which 106 HeLa cells were added as carrier. cDNA was synthesized by reverse transcriptase. Serial dilutions of the cDNA were then used as a template for PCR amplification with specific primers for CD8 (5′-ATGGACGCCGAACTTGGTCAGAAGGTG-3′ and 5′-CCACGTTATCTTGTTGTGGGATGAAGCC-3′) and TCR-β (5′-GGCAAGGAGGTCCACAGTGGGGTCAGC-3′ and 5′-GGCCACTGACCAGCACAGCATATAGGGTGG-3′). After amplification (5 min at 94°C; 30 cycles of 94°C 30 s, 50°C 30 s, 72°C 1 min; 10 min at 72°C), the products were run on agarose gels, transferred, and detected by hybridization using CD8- or TCR-β– specific oligonucleotides (5′-GGTGGGCTGGGGGAGTTTGGAGCTGG-3′ and 5′-GAACATCAGTGCAGAGGCCTGGGGCCGAGC-3′, respectively). Signals were quantified using a Bio Imaging Analyzer. Amplification of both CD8 and TCR-β were performed in the same PCR tube.
Results
Generation of CD4+ /L Mice.
To provide a convenient and harmless means of monitoring CD4 gene activity, we set out to confer a protein reporter with an identical pattern of expression. βgal seemed a good reporter candidate because it is easy to visualize, is inert, and, being cytoplasmic, possesses no signaling capacity, a disadvantage of most cell-surface reporters (31, 32). We chose a “knock-in” approach rather than transgenesis of a promoter–reporter construct in order to mimic as closely as possible the complex transcriptional controls on the native CD4 gene (33–37). Thus, mice expressing βgal in place of CD4 were generated by targeting a βgal coding region into the CD4 locus via homologous recombination in ES cells (Fig. 1). To ensure faithful expression of the βgal reporter (and to inactivate the CD4 gene at the same time), we introduced the βgal coding sequences exactly at the CD4 initiation codon in exon 2. The inserted sequence directs the translation of a fusion protein comprised of βgal and the neomycin (neo) resistance domain (βgeo, chosen because it is known to be expressed in normal T cells [23]); the insert also contains an expressible neo gene under an independent PGK promoter for selection of ES cell transfectants (Fig. 1).
The final construct was electroporated into ES cells, and G418-resistant clones carrying the specific homologous recombination event were selected. Chimeras generated from the injection of a positive clone into B6 blastocysts were mated with B6 females to obtain germline transmission of the knock-in mutation. The resulting mouse line was generally kept in heterozygous form (CD4+/L; hereafter “+” designates the wild-type CD4 locus, “L” our targeted CD4-βgal insertion, and “0” the original CD4 knockout mutation of Killeen et al. [26] used in some of the crosses). In the heterozygotes, both βgal and CD4 (at half normal levels; data not shown) should be simultaneously expressed under the influence of elements that control the CD4 locus.
It was first necessary to determine just how closely the expression of βgal matched that of CD4. Lymph node cells and thymocytes were stained with FDG to detect cytoplasmic βgal expression (29) in addition to antibodies against CD4, CD8, and CD3, and were analyzed by flow cytometry (Fig. 2). βgal expression was essentially superimposable with that of CD4 in both primary and secondary lymphoid organs. In the lymph nodes, CD4+ cells expressed high levels of βgal, whereas CD8+ and non-T cells expressed low to background levels (Fig. 2 A). Similarly in the thymus, CD4 SP and CD4+CD8+ DP cells were βgal+, whereas mature CD8 (CD3hi) SP and immature DN cells were not (Fig. 2 B). βgal did not appear in only a select subset of CD4+ T cells: for example, the minor (and peculiar) NK1.1+CD4+ subset of α/β T cells (38) was also βgal+ in thymic and spleen cell suspensions of CD4+/L mice (not shown). Importantly, βgal expression did not detectably alter thymus cellularity or population distributions, as assessed with a panel of markers including CD3, CD4, CD8, CD69, CD24, CD5, CD44 and peanut agglutinin– binding polysaccharides (not shown).
Figure 2.
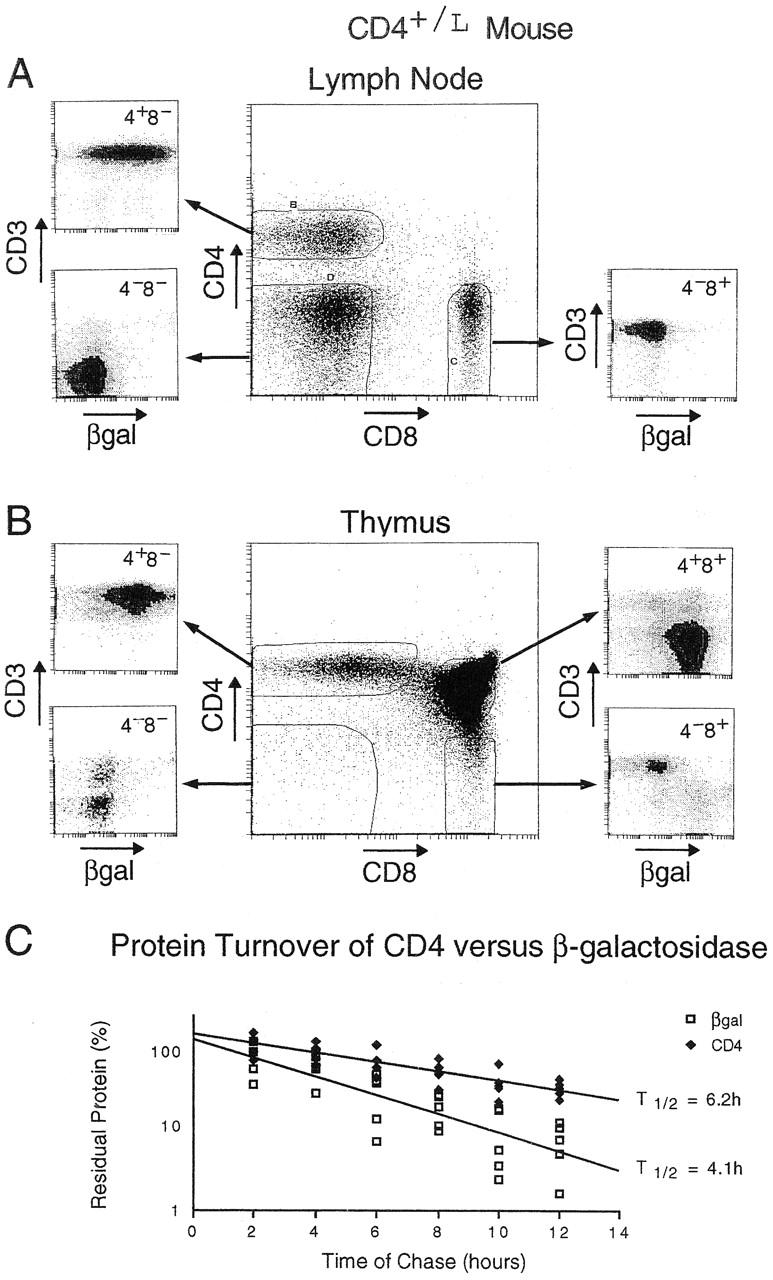
Characteristics of the βgal reporter. (A and B) Profile of the βgal marker in CD4+/L mice. Lymph node cells (A) or thymocytes (B) were analyzed by four-color flow cytometry. Anti-CD4 and anti-CD8 antibodies were used to define and set the analysis gates for the major populations. The smaller cytograms represent the CD3 and βgal intensities within each of the CD4/CD8 gates. (C) Turnover of βgal versus CD4 protein. Thymocytes were labeled in vitro for 1 h with [35S]methionine and chased for the indicated times, and the proteins were precipitated using anti-CD4 or anti-βgal antibodies. 100% was defined as the amount of labeled protein precipitated after 2 h of chase. Half-life was calculated as follows: t 1/2 = (1/a)log10(1/2) + 2 h, where a = slope. The half-life of βgal is 3.64 < t 1/2 = 4.07 < 4.84; the half-life of CD4 is 5.38 < t 1/2 = 6.18 < 7.57 at 95% confidence. The combined results of five experiments are shown.
Upon closer examination, we noticed some interesting features of the expression of the βgal reporter. First, CD3− thymocytes within the CD8 SP population, the immediate precursors of DP cells, were βgal+, implying that enzymatic activity was detectable earlier than surface expression of CD4 (Fig. 2 B). Second, a proportion of the CD4+ CD8+CD3hi thymocytes expressed low levels of βgal, suggesting that reporter activity was downregulated earlier than surface CD4 upon positive selection. These observations argued that βgal levels responded faster to changes in transcriptional activity at the CD4 locus than the level of CD4 protein itself. To test this hypothesis, we compared the turnover of CD4 and βgal proteins (Fig. 2 C). In vitro metabolic labeling with [35S]methionine and precipitation with antibodies against CD4 and βgal revealed that the half-life of βgal (4.1 h) was significantly shorter than that of CD4 (6.2 h).
Thus, not only was βgal a faithful reporter of CD4 gene expression, it also appeared to be an early indicator.
βgal as a Marker for CD4 and CD8 Lineage Commitment in the Thymus.
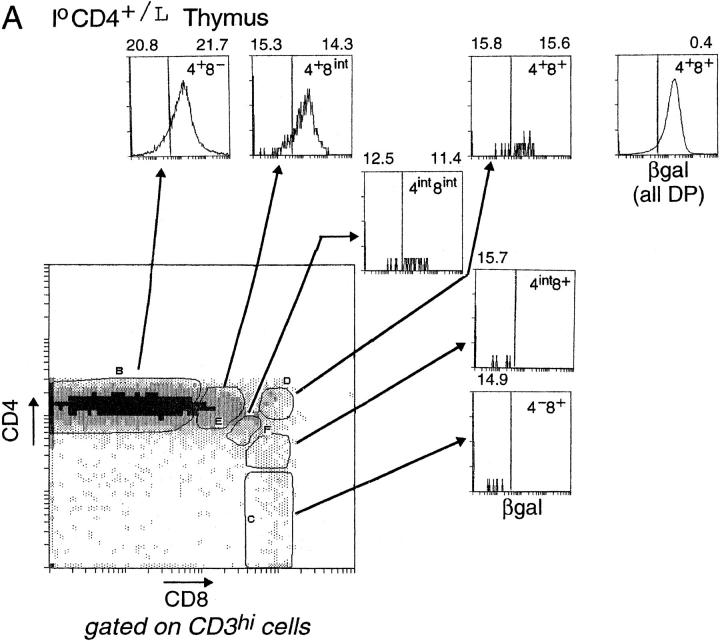
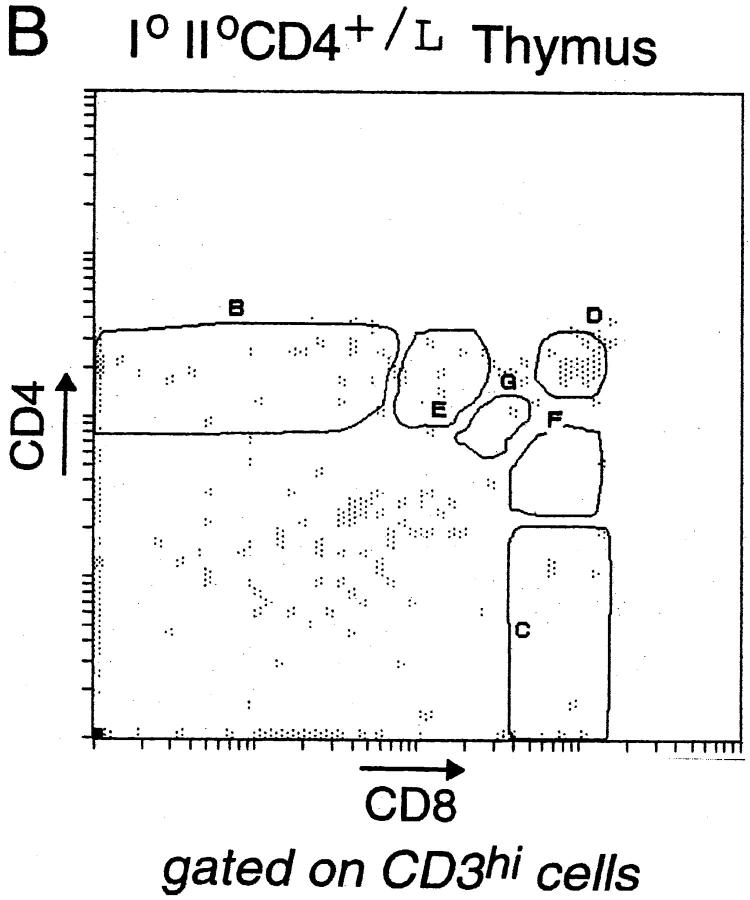
Since βgal appeared to be an early indicator of CD4 gene transcription, it represented a valuable marker of lineage commitment during the initial stages of T cell selection in the thymus. Thus, we stained CD4+/L thymocytes simultaneously for CD4, CD8, CD3, and βgal. In Fig. 3 A, CD3hi thymocytes are displayed in a CD4/CD8 plot, and show the customary boomerang-shaped distribution extending from the mature CD4 SP to the mature CD8 SP compartments. Several distinct gates were set within this boomerang to permit us to investigate the activity of the CD4 gene, as reflected by βgal staining, within different populations. The terminally differentiated populations (gates B and C) showed essentially homogeneous patterns of βgal staining: CD4 SP thymocytes were βgal+, CD8 SP cells βgal−. In contrast, most of the intermediate populations (gates E, G, and F) contained cells of both phenotypes, even the small CD3hi subpopulation which displayed high surface levels of CD4 and CD8 (gate D). These profiles confirmed the notion that surface coreceptor levels do not fully and immediately reflect lineage commitment (18, 21), and encouraged us to exploit the βgal marker for investigating lineage commitment ex vivo under unmanipulated conditions. However, it should be kept in mind that, although the βgal− phenotype unambiguously denotes a shut-off of CD4 transcriptional activity in this context, and thus commitment to the CD8 lineage, βgal+ populations cannot be interpreted as readily, potentially including cells that have not yet committed to or those that are already committed to the CD4 lineage.
Figure 3.
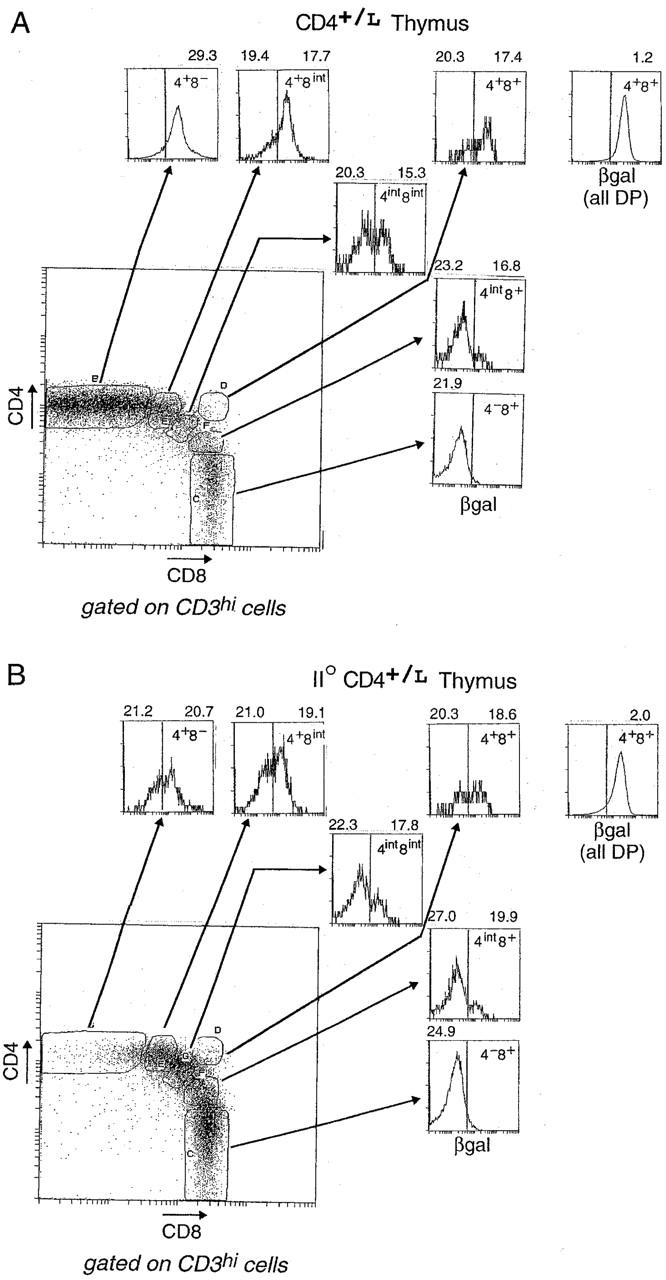
Expression of the βgal reporter in thymocyte populations. (A) βgal expression in CD4+/L mice. CD3hi thymocytes were gated according to their CD4/CD8 expression into distinct populations: CD4 SP cells (4+8−), transitional CD4+CD8int cells (4+8int), DP cells (4+8+), CD4intCD8int cells (4int8int), intermediate CD4intCD8+ cells (4int8+), and CD8 SP cells (4−8+). Histograms represent the intensity of βgal expression within each of these CD4/CD8 gates. The mean fluorescence intensity (MFI) values for CD3 expression within the respective βgallo and βgalhi subsets are shown above each histogram, except in cases where there are few or no cells within the βgal gates. The separate histogram at the upper right hand corner represents the intensity of βgal expression for total DP thymocytes. (B) βgal expression in II0 CD4+/L mice. βgal expression within CD4/CD8 thymocyte subsets of CD4+/L mice on an MHC class II–negative background. Thymocytes were analyzed as in A.
CD4+/L mice were crossed with MHC class II–negative (II0) animals (25) to allow us to evaluate the commitment status of MHC class I–reactive CD4+CD8int thymocytes, cells proposed to be transitory intermediates committed to the CD4 lineage (10). Thymocytes from II0 CD4+/L mice were analyzed as above (Fig. 3 B). The CD4+CD8int population (gate E) split clearly into two subsets, βgal+ and βgal−; there was a significant increase in the βgal− subset compared with the same transitional population in the class II–positive mouse. This result is consistent with the notion that at least some CD8-committed thymocytes transiently downmodulate surface CD8 levels after positive selection (18, 21), but also suggests that a sizeable contingent of CD4-committed cells can be selected in the absence of MHC class II molecules.
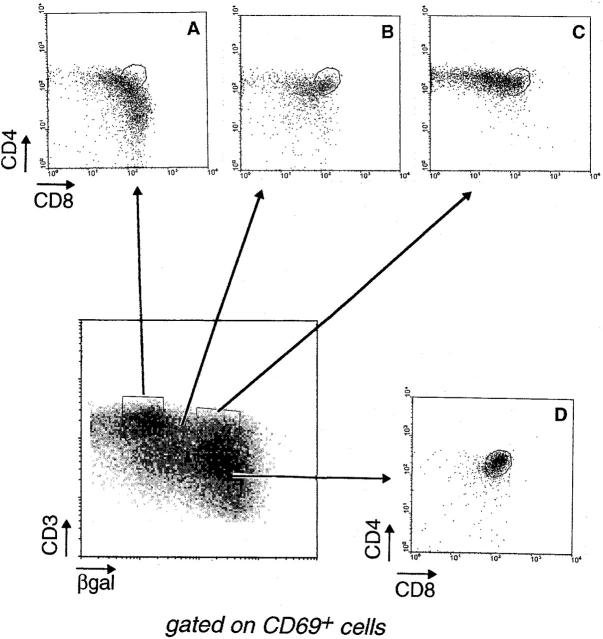
That CD4-committed thymocytes were selected on MHC class I (in the absence of class II) molecules is substantiated in Fig. 4, a five-color cytofluorimetric analysis of the expression of CD4, CD8, CD3, βgal, and CD69, an early and transient marker of positive selection (14, 39–41). When CD69+ thymocytes from II0 mice were displayed in CD3/βgal plots, it seemed that CD4- and CD8-committed thymocytes could be traced by virtue of their very positions within this plot. The CD8-committed cells appeared to reside chiefly within the CD3hiβgallo and βgal− gates; as they lost βgal expression, they also began to switch from the transitional CD4+CD8int phenotype to the definitive CD4int CD8+ and CD8 SP phenotypes (compare the CD4/CD8 profiles, gates A and B of Fig. 4). In contrast, only CD4+ thymocytes were found within the CD3hiβgalhi population (gate C), well separated from cells along the CD8 pathway.
Figure 4.
Multiparameter visualization of lineage commitment. Thymocytes from a II0 CD4+/L mouse were analyzed after gating on CD69+ cells. The lower left panel displays the βgal/CD3 profile of these cells, from which four populations can be distinguished: cells that express the highest levels of CD3 but have downregulated βgal expression (A); others that also express the highest levels of CD3, but have maintained CD4 gene activity, as evidenced by full βgal staining (C); intermediates in the process of downregulating βgal (B); and immature thymocytes that express low levels of CD3 and high levels of βgal (D). The CD4/CD8 profiles of these populations are shown in the smaller panels.
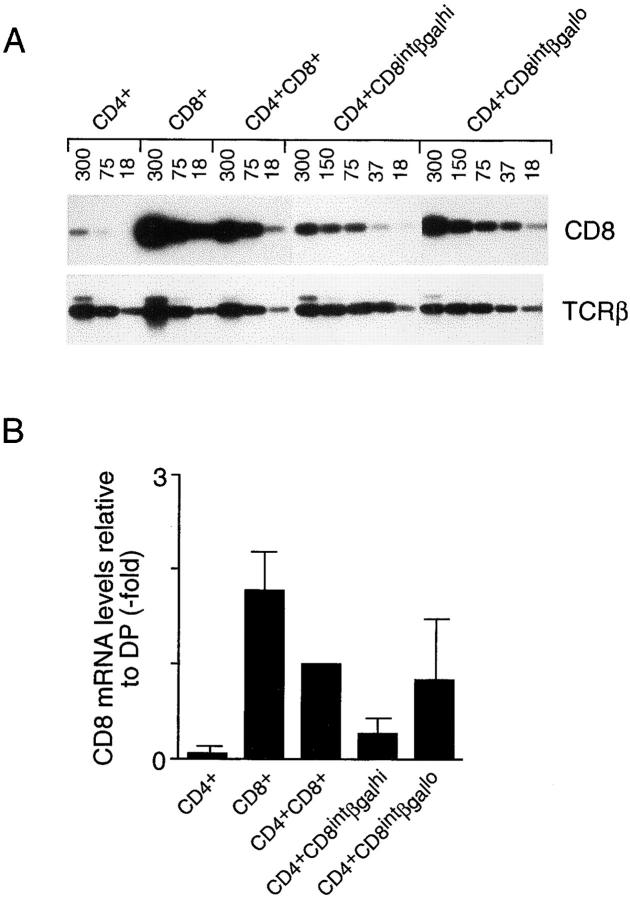
Although rendered unlikely by the very appearance of the profiles in Fig. 4, a caveat to these interpretations is that CD4+CD8int cells with high βgal expression may still have been destined for the CD8 lineage, but just had not yet downregulated βgal expression. To address this point, we purified CD4+CD8intCD3hiβgalhi and CD4+CD8intCD3hi βgallo thymocytes, and compared their CD8 mRNA content by reverse transcriptase (RT)-PCR (Fig. 5). If the CD4+CD8intβgalhi cells were CD8-committed or even uncommitted, their CD8 mRNA levels should be as high as those detected in the CD4+CD8intβgallo or DP populations. However, if the CD4+CD8intβgalhi thymocytes were CD4-committed, they should have downregulated CD8 transcription at least to some degree. The latter scenario appears to be the correct one: CD4+CD8intβgalhi thymocytes expressed on average two- to threefold less CD8 mRNA than their βgallo counterparts, suggesting that many, if not all, of the CD4+CD8intβgalhi thymocytes in II0 mice were CD4 committed.
Figure 5.
CD8 mRNA levels in transitional intermediates. (A) CD3hi thymocytes from CD4+/L mice (CD4+ and CD8+ populations) and II0 CD4+/L mice (CD4+CD8intβgalhi, CD4+CD8intβgallo), as well as total CD4+CD8+ thymocytes from II0 CD4+/L animals, were purified and analyzed by RT-PCR for their expression of CD8 mRNA. Cytoplasmic RNA was prepared, cDNA synthesized, and serial dilutions of the different cDNA samples (amount indicated above each lane as cell equivalents) were used as template for simultaneous PCR amplification using CD8 and TCR-β–specific primers. PCR products were detected by hybridization. (B) Results from five RT-PCR experiments where the amount of PCR product hybridizing to a CD8 probe was quantified, standardized with the internal TCR-β control, and normalized as fold over DP cells (defined as 1). The CD8 mRNA ratio of CD4+CD8intβgallo to CD4+ CD8intβgalhi thymocytes is 2.76 ± 0.94.
Our original results (10) and those of van Meerwijk and Germain (16) suggested that a mirror-image population, committed to the CD8 lineage upon selection on MHC class II molecules, also exists. However, this interpretation was questioned in subsequent reports (21, 22, 42). Therefore, we analyzed β2-microglobulin (β2m)-negative (I0) mice (43) in which the CD4-βgal reporter had been introduced, allowing a distinction between true CD8-committed cells and other populations (i.e., DPs, CD4-committed). The cytometric analysis in Fig. 6 A illustrates that CD4intCD8+CD3hi cells did exist in class I–deficient mice, albeit at a much lower frequency than their counterparts in class II–deficient animals, and that they displayed the βgallo phenotype expected of the CD8 lineage. The low expression of βgal established that these cells were not contaminants from the neighboring CD4intCD8intCD3hi population, the great majority of which were βgalhi. Our results also indicated that the CD4intCD8+CD3hi cells were a homogeneous set, as we did not detect CD4-committed cells within this population, a result consistent with recent findings from other groups (18, 21, 44, 45).
Figure 6.
Lineage commitment in MHC class I–deficient and MHC-deficient mice. (A) βgal expression within defined CD4/CD8 thymocyte subsets of CD4+/L mice on a β2m− background. Thymocytes were analyzed as described in the legend to Fig. 3. (B) CD3hi thymocytes in MHC-deficient mice. CD3hi thymocytes were stained and gated according to their CD4/ CD8 expression.
In the same series of experiments, MHC double-deficient (I0II0) mice with a CD4+/L genotype were analyzed (Fig. 6 B): the CD3hi CD4+CD8int and CD4intCD8+ thymocyte populations present in II0 and I0 mice, respectively, were not observed in MHC-deficient animals, in agreement with our previous findings (10); the rare CD3hi cells were all βgalhi and probably represent nonstandard T lineages (46). This result confirms that the intermediate populations discussed above require MHC engagement in order to be positively selected, indicating that a CD4 lineage default pathway in the absence of MHC recognition does not exist in vivo.
Two Modes of CD8 Commitment.
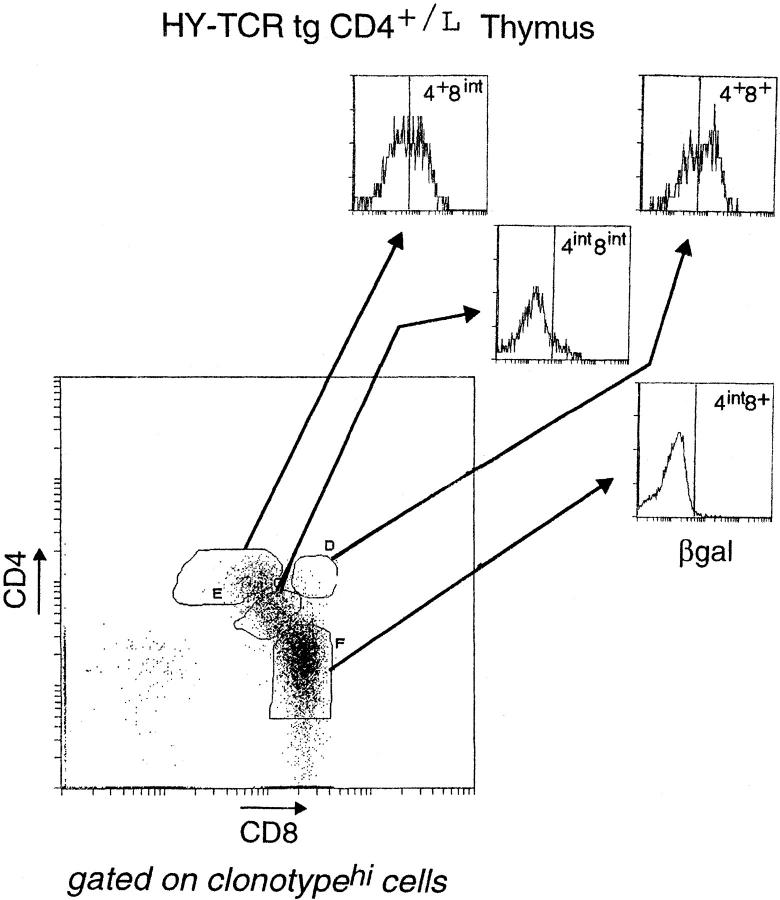
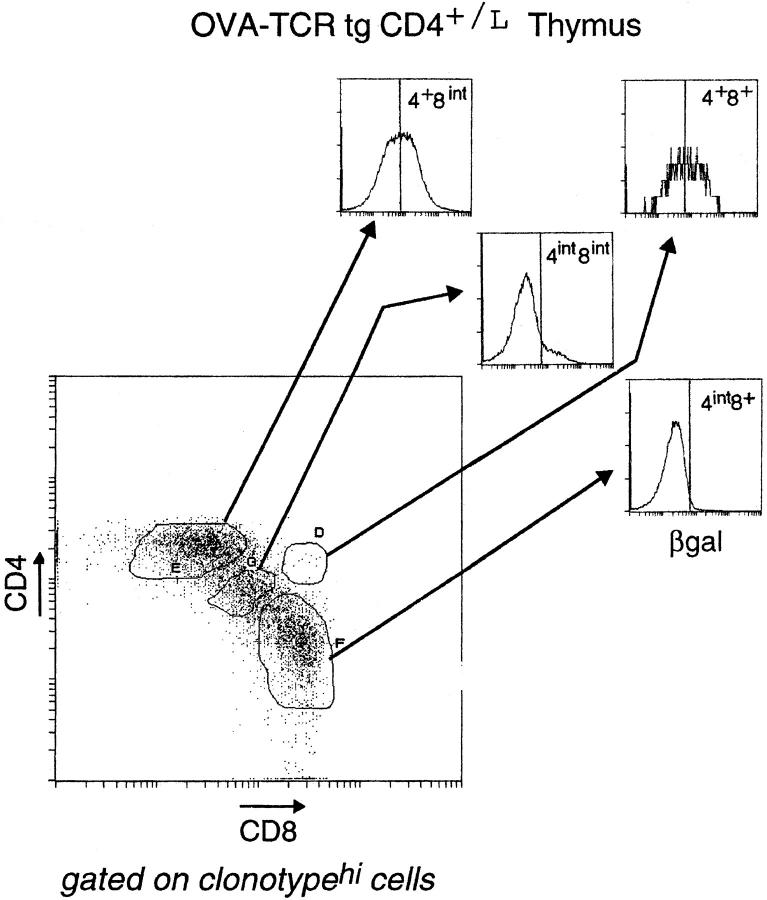
We wondered whether all T cells destined for the CD8 lineage transit through the CD4+CD8int stage, and just how early CD8-committed cells switch off CD4 gene activity. Therefore, we crossed the CD4+/L line with two TCR tg lines expressing different MHC class I–restricted receptors: the “HY” TCR (selected on Db) and the “OVA” TCR (selected on Kb) (47, 48). Thymocytes from HY+CD4+/L and OVA+CD4+/L mice were stained for CD4, CD8, and βgal in addition to the appropriate V regions (Fig. 7; panels are gated on clonotypehi cells, thereby focusing the analysis only on cells expressing the transgene-encoded receptor). In terms of βgal expression, the patterns were quite similar in the two types of animals: downregulation of the βgal marker began in the DP compartment, and the CD4+CD8int population contained both βgalhi and βgallo cells; from the CD4int CD8int stage onwards, βgal expression was essentially shut off. Furthermore, both the timing and levels of TCR expression, as reflected by CD3 expression (not shown), were also similar for the two tg lines, not surprising since expression of both transgenes are driven by the same TCR Vβ promoter (Correia-Neves, M., unpublished results). However, the HY and OVA mice were strikingly different in their numbers of intermediate CD4+CD8int thymocytes. Consistent with a previous report (18), there were three to four times more transitional CD4+CD8int thymocytes in OVA than in HY mice. (Note that the OVA line used here expresses the same receptor as the OVA–tcr-1 line reported previously [48], but was generated using a different vector for transgenesis; that the two behave identically in having a large CD4+CD8int population indicates that this phenotype is not an artifact of transgene vectors or sites of integration.) Thus, in the HY system, differentiation towards the CD8 lineage seems to proceed quite directly, from DP to CD4int CD8int to CD4−CD8+ cells; with the OVA line, it appears more convoluted, generating large numbers of CD4+ CD8int cells. This also appears to be true when endogenous TCR gene rearrangements in both transgenic lines are prevented by the SCID, RAG, or TCR Cα mutation (10; Heath, W., personal communication; Correia-Neves, M., unpublished results). Such a dichotomy suggests that there must be at least two modes (or a continuum) of class I–restricted CD8 lineage differentiation.
Figure 7.
βgal expression in thymocyte populations of HY TCR tg and OVA TCR tg mice. Thymocytes from HY-TCRtgCD4+/L and OVA-TCRtgCD4+/L mice were analyzed by four-color flow cytometry. CD4/CD8 profiles of thymocytes expressing the transgene-encoded TCR (as distinguished by T3.70 for the HY TCR and anti-Vα2 for the OVA TCR) are shown. Thymocyte subpopulations were further defined (see the legend to Fig. 3) and analyzed for βgal expression.
With both class I–restricted TCR tg lines, the CD4+ CD8int populations contained βgallo and βgalhi cells. βgallo cells were most likely CD8-committed, in keeping with results from cell transfer experiments (18). A key question was whether the βgalhi cells were just precursors of the CD8-committed cells or whether they really included a CD4-committed population as we suspected. If there were CD4-committed cells, destined to die because they bear mismatched T cell receptors and coreceptors, it should be possible to rescue them by restoring surface CD8 expression. Furthermore, the efficiency of the rescue should correlate with the ability of the TCR to generate the transitional CD4+ CD8intβgalhi phenotype. Thus, one would predict that such a rescue experiment should be more successful in the OVA than the HY animals, which had previously yielded few rescued cells (49–51). We tested this prediction by crossing the HY and OVA lines with a transgenic mouse line expressing near-physiological levels of the CD8 α and β chains (CD8ααβ [42]). Representative CD4/CD8 profiles of thymocytes and lymph node cells from these crosses are shown in Fig. 8. As observed previously (49–51), rescue of CD4+(tgCD8+) T cells was very limited in the HY mouse; in striking contrast, close to half of the peripheral clonotypehi lymphocytes in the OVA cross were of the CD4 lineage. Thus, there was a direct relationship between the number of CD4+CD8intβgalhi thymocytes and the number of rescued class I–restricted CD4+(tgCD8+) cells. We also observed a significant decrease in the positive selection of OVAhi thymocytes into the CD8 SP compartment. As the affinity of the OVA receptor may be quite high for its selecting ligand (18), it is possible that the CD8-committed cells were deleted due to the higher levels of CD8 expression.
Figure 8.
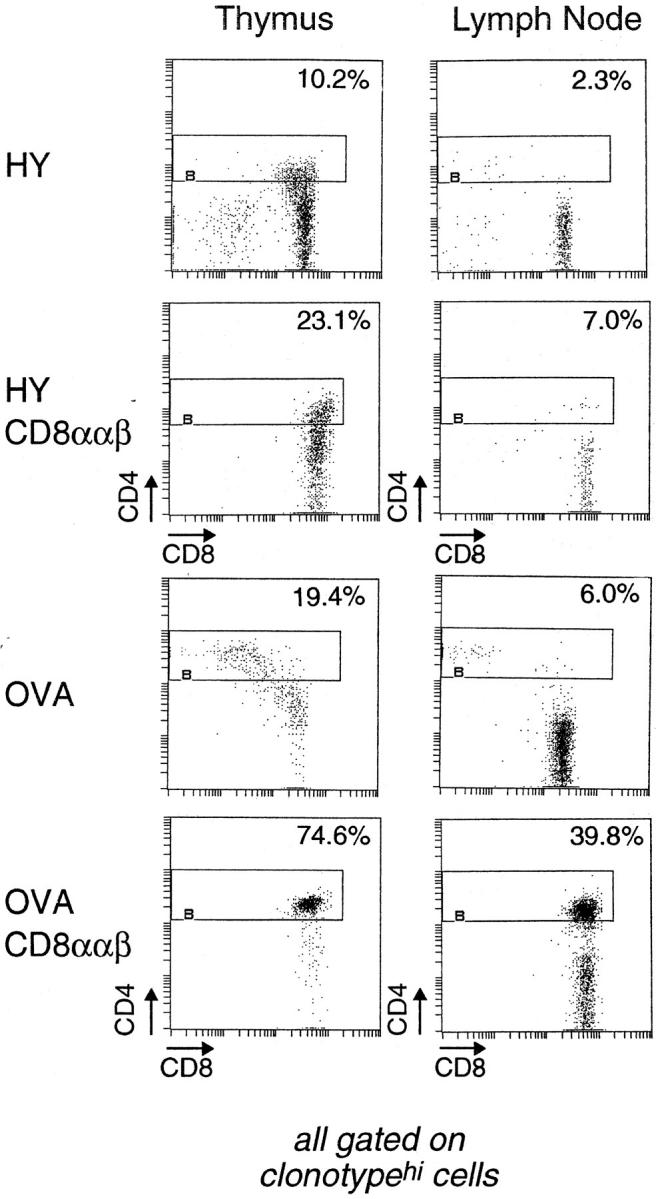
Rescue of CD4 lineage–committed cells in OVA TCR tg, but not HY TCR tg, mice upon forced expression of CD8. Thymocytes and lymph node cells from HY TCR, HY TCR/CD8ααβ, OVA TCR, and OVA TCR/CD8ααβ tg mice were analyzed by three-color flow cytometry using the antibodies anti-CD4, anti-CD8, T3.70 (for the HY TCR), and anti-Vα2 (for the OVA TCR). The CD4/CD8 profiles are gated on the transgenic TCRhi cells. The percentages are indicated for CD4-expressing cells within the clonotypehi population.
CD4 Lineage Cells in the Absence of CD4.
Finally, we made use of the βgal reporter mice to examine the issue of CD4 lineage commitment in the absence of CD4. Previous studies had demonstrated a population of MHC class II– restricted, CD4−CD8−TCRhi Th cells in CD4-deficient mice (26, 52–54). Although it was never formally demonstrated, these cells were quite logically considered to belong to the CD4 lineage.
We readdressed this issue by analyzing offspring from crosses of CD4-βgal knock-in mice with one of the original CD4 knockout lines (26), comparing profiles of βgal+ cells in heterozygous CD4+/L animals with those in fully deficient CD40/L animals. In a four-color cytofluorimetric analysis using FDG and antibodies against CD4, CD8, and CD3, the usual CD4/CD8 profile can be substituted with a βgal/CD8 profile (Fig. 9). In CD4+ mice, the βgal+CD8− cells in the thymus and lymph nodes were essentially all CD4+CD3hi, as expected; on average, they amounted to 4.0 and 15.1% of total thymocytes and lymphocytes, respectively. When the same gate was applied to βgal+ CD8− cells from CD4-negative CD40/L mice, the same populations of CD3+ T cells were present (now CD4−). The thymocyte population was only moderately diminished in number, whereas the reduction was more marked in the lymph nodes. The βgal+CD8− cells were analyzed for various surface markers and for their TCR Vβ region usage. Preliminary results indicated that these cells were not particularly different in CD4+ and CD4− mice, implying that the cells selected in the absence of CD4 were not a special subset of Th cells (not shown).
Figure 9.
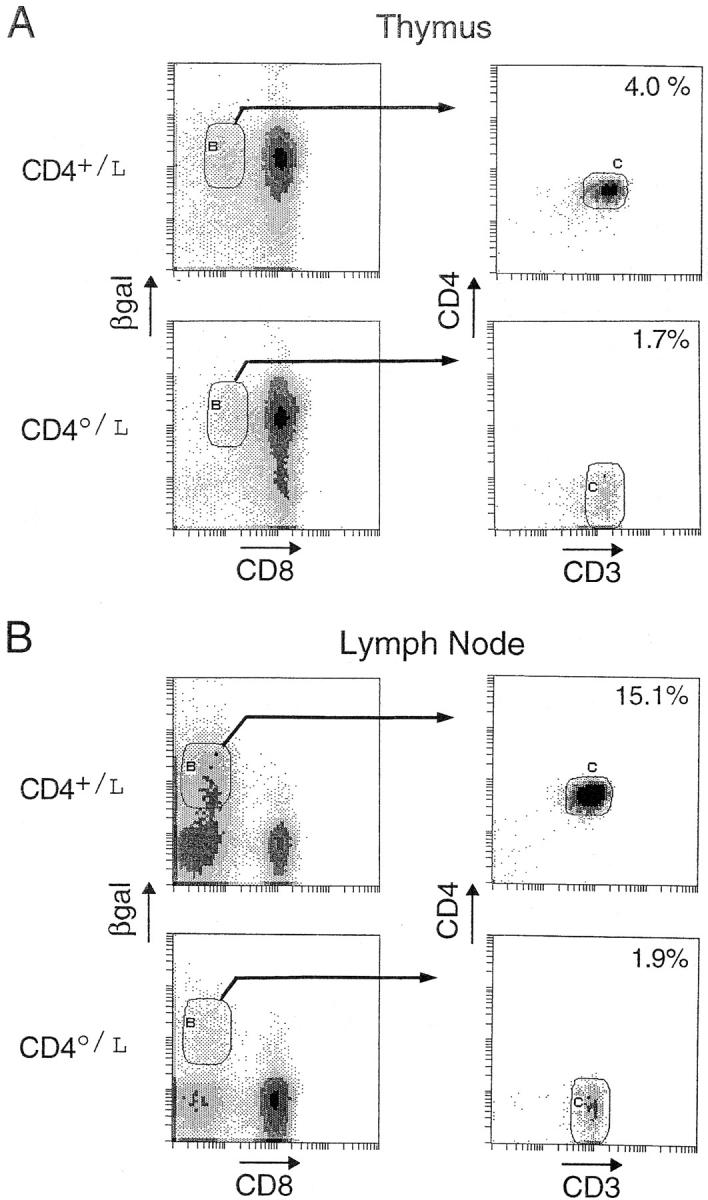
βgal+ DN cells in CD4-deficient mice. Thymocytes and lymph node cells from homozygous (CD40/L) and heterozygous (CD4+/L) mice were compared using four-color flow cytometry. Cells were first plotted for their expression of βgal and CD8. CD8+βgal+ cells were gated and further evaluated for their expression of CD4 and CD3. Percentages are of total thymocytes.
These results formally demonstrate that thymocytes can be selected into the CD4 lineage in the absence of cell surface CD4.
Discussion
The CD4-βgal knock-in mouse line provides an accurate, convenient, and harmless means of monitoring CD4 gene activity directly ex vivo. Expression of the βgal reporter faithfully mimicked CD4 expression, was readily detectable, and had no detectable immunological consequences. The reporter could be used whether or not the CD4 molecule itself was present. The choice of reporter also proved advantageous because the half-life of the βgeo chimeric protein was significantly shorter than that of CD4, meaning that βgal activity more closely approximated CD4 gene transcription than surface display of CD4 itself. In this report, we have exploited the CD4-βgal line to study CD4/CD8 lineage commitment.
CD4-committed Intermediates in the Absence of MHC Class II Molecules.
For some years, debate on the mechanism of CD4/CD8 lineage commitment has centered around the issue of whether commitment is essentially instructive or selective (49, 55, 56). A crucial distinguishing feature of these two models is the possibility of transitional populations, with mismatched receptors and coreceptors in the latter but not the former. Evidence for such populations was provided by studies on MHC-deficient and TCR tg mice and by rescue experiments based on forced expression of coreceptors (11, 12, 14, 15). However, a key finding in several of these studies was brought into question because of results from transfer experiments (18, 19) and coreceptor reexpression assays (21). In particular, these data were considered to invalidate the support given the selective model of lineage commitment by the demonstration of transitional intermediates in class II– and class I–deficient mice. We now exploit the βgal marker to establish that the CD4+CD8int population from class II–deficient mice includes cells committed to both the CD4 and CD8 lineages. CD4-committed cells maintain active CD4 gene transcription, whereas CD8-committed cells have turned off the CD4 gene, as reflected by expression of the βgal reporter (Figs. 3 B and 5).
It might be argued that the CD4+CD8intβgalhi thymocytes seen in class II–deficient mice are just precursors of the βgallo cells, having not yet shut down transcription of the CD4 gene. Several observations are inconsistent with this contention: the low level of CD8 mRNA in CD4+CD8intβgalhi cells compared with their βgallo counterparts, as predicted if they are of the CD4 lineage; the very position of the βgalhi cells on the βgal/CD3 plot, visibly on a different branch than that of the βgallo cells (Fig. 4); the correlation between the numbers of CD4+CD8int βgalhi cells and the ability of the CD8 transgenes to rescue the CD4-committed cells in OVA and HY transgenic mice (Figs. 7 and 8). Finally, we have recently found that altering the avidity of TCR signaling by introducing a CD5 knockout mutation (57) increases the numbers of CD4+ CD8intβgalhi cells in II0 mice, without affecting CD4+CD8int βgallo numbers, a change in ratio incompatible with a simple precursor/product relationship (Chan, S., manuscript in preparation).
The data presented here do not really contradict those from the transfer and coreceptor reexpression studies (18, 19, 21, 22). The transfer experiments (18, 19, 58) demonstrated that the CD4+CD8int population from II0 mice contained CD8-committed thymocytes, but did not rule out that it also includes CD4-committed cells because, having mismatched receptors and coreceptors, these cells should not have survived in the host wild-type thymus any better than in the II0 donor. As for the coreceptor reexpression assays, the data actually did show that cells committed to both lineages were present within the CD4+CD8int population of II0 mice (Fig. 4 A in reference 21). The existence of CD4-committed cells was discounted by these authors in favor of a CD4 “default selection” pathway, hypothesized to occur independently of MHC engagement. However, no evidence for such a pathway has been observed in several studies (22, 41; Fig. 6 B), so that it appears reasonable to equate the CD4-committed CD4+CD8int cells described in this report with those that reexpressed only CD4 after pronase treatment.
The existence of CD4-committed, but class I–reactive, intermediates is consistent with results from the older experiments forcing expression of coreceptors via transgenesis (11, 12, 14). Our data (Figs. 7 and 8) extend the older data by fulfilling, in two TCR transgenic systems, the prediction that the numbers of intermediates with mismatched receptors and coreceptors should correlate with their ability to be rescued to full maturity by artificially expressing the appropriate coreceptor. Our results also explain the range in observations made in previous rescue experiments, relatively efficient rescue being obtained in some cases (11, 14, 15, 59) but not in others (49, 50). Inefficient rescue has been cited repeatedly as evidence for instructive or hemi-instructive models (11, 12, 21). It is clear now that rescue experiments are not inherently inefficient but one must choose an appropriate TCR tg system that provides enough intermediates to be rescued.
The Asymmetry of Commitment.
The present data reaffirm the existence of CD4 lineage–committed transitional intermediates that were nudged down the differentiation pathway by engagement of their TCRs by MHC class I molecules in the absence of class II molecules. An analogous population of CD8 lineage–committed cells was hypothesized when CD4intCD8+ cells were found in class I–deficient mice (10, 16), but their existence has been questioned (20–22, 42). Our findings confirm that transitional intermediates committed to the CD8 lineage in the absence of MHC class I molecules do exist, as evidenced by the absence of βgal expression in CD4+CD8intCD3hi cells in I0 mice (Fig. 6 A). This result is consistent with reports that transgenic mice expressing class II–restricted TCRs (17, 60) and MHC-deficient mice complemented with MHC class II genes delivered by an adenovirus vector injected intrathymically (Rooke, R., manuscript in preparation) can give rise to mature bona fide CD8+ cells. However, the numbers of CD8-committed transitional intermediates in class I–deficient mice are low: 2.3 ± 0.9% of the numbers in normal mice (n = 5), compared with 20 ± 5% the normal numbers for CD4-committed intermediates in class II–negative animals. The rarity of the CD4intCD8+ cells may explain why they were not detected by other groups—either because three-color bromodeoxyuridine labeling experiments are inherently maladapted for the detection of small populations (42), or because the analysis/ sorting gates that were used included DP cells, thereby masking the transitional intermediates (20, 21). Thus, CD4/ CD8 lineage commitment seems to exhibit qualitative symmetry in that receptor/coreceptor-mismatched commitment occurs in both pathways, but there is quantitative asymmetry in that such “mistaken” commitment to the CD4 lineage is far more frequent than that to the CD8 lineage—actually somewhat in line with the earlier assertions of asymmetry in the positive selection process (21, 61).
Asymmetry is also evident in the fact that no CD4-committed equivalent of CD8-committed CD4+CD8int thymocytes (waltzers) has been described. Here, we detected no βgalhi cells in the CD4intCD8+ population, in good agreement with findings from several groups (18, 21, 22). Thus, while commitment to the CD8 lineage can launch at least two different modes of differentiation, either direct progression to the CD4−CD8+ phenotype as seen for cells in the HY system, or transit through the CD4+CD8int stage as seen for OVA T cells, commitment to the CD4 lineage seems to provoke a quite direct progression to the mature SP stage.
Pathways.
What explains the diverse modes of differentiation exhibited by the CD8 lineage? A hint may come from the results of recent analyses of cis-control elements of the CD8α gene (62, 63) and past studies on CD4 gene control elements (35, 36, 64). Distinct enhancer elements promote expression of CD8, and perhaps CD4, in DP versus mature SP cells; regulation is further complicated by a silencer located in the CD4 gene. It may be that, concomitant with positive selection, transcriptional activity driven by the DP cell–specific enhancer is shut off, and then whether the cells destined to become CD8 SP proceed directly or transit through the CD4+CD8int stage may simply reflect the speed with which the transcription factors binding to the “mature” CD8 SP–specific enhancer and/or the CD4 silencer become operational.
Differential mobilization or demobilization of transcription factors could result from different TCR affinities/avidities. It was suggested some time ago that the affinity/avidity of a DP thymocyte's TCR for the positively selecting ligand or stromal cells might play a role in determining its pathway of differentiation (14, 49), and recent data support this notion (60, 65). It seems likely that the divergent behaviors of OVA and HY TCR tg thymocytes reflect different strengths of interaction between the two TCRs and their positively selecting ligands (18). Pircher and colleagues have also attributed the differing numbers of CD4+CD8int cells found in the P14 TCR tg line on the H-2b and H-2bm13 backgrounds to different avidities for the selecting ligands (13). At present, it is not clear whether stronger signals promote differentiation along the CD4 or CD8 pathway, as arguments have been presented on both sides (14, 49, 60, 66). Thymocyte-extrinsic factors within the thymus milieu as well as intrinsic factors of a more generalized nature also play a role in determining cell fate. Concerning the former, it should be kept in mind that the thymic stroma is very heterogeneous, containing niches where the expression of MHC molecules can vary markedly (4, 67); concerning the latter, recent results on Notch are intriguing (68).
CD4 Lineage Cells in the Absence of CD4.
Finally, we confirmed that the population of MHC class II–restricted CD4−CD8− T lymphocytes previously observed in CD4-deficient mice (26, 52–54) do indeed belong to the CD4 lineage. In CD4− mice (CD40/L), a subset of DN α/β T cells in both the thymus and the peripheral lymphoid organs expresses high levels of βgal (Fig. 9). Thus, commitment to the CD4 lineage does not require the CD4 molecule; in fact, it is quite efficient in its absence. Interestingly, although the numbers of DN CD3+βgal+ thymocytes generated after selection were quite high (∼30% the numbers in normal mice), their relative abundance was lower in the periphery (∼12%). A possible explanation for this diminution is that signals from the CD4 molecule contribute (albeit in a dispensable fashion) both to the thymic selection of CD4+ T cells and to their later export and persistence in the peripheral lymphoid organs. This would be consistent with the idea that maintenance of naive CD4+ T cells in the periphery requires continued “tickling” by MHC molecules (69, 70).
Conclusions.
The results presented here argue that CD4/CD8 lineage commitment is fundamentally symmetrical, in that engagement of either class of MHC molecule by a differentiating DP thymocyte can give rise to transitional intermediates committed to either lineage. There does not appear to be a CD4 lineage default pathway, nor any special requirements to provoke commitment to the CD8 lineage. Along both routes of differentiation, the initial lineage choice is validated at a later stage, when only those cells expressing appropriately matched T cell receptors and coreceptors are permitted to survive.
Acknowledgments
We thank S. Gilfillan for the D4 EcoRI genomic library; P. Soriano for the βgeo cassette; D. Littman for the CD4-deficient mice; H. von Boehmer and P. Kisielow for the HY TCR tg mice; R. Dubridge and D. Capon for the β2m− mice; A. Baron for the CD8 transgenics; P. Kastner for critically reading the manuscript; C. Waltzinger for performing the cytofluorimetric analyses and cell sorting; P. Bohn-Marchal, C. Ebel, P. Gerber, J. Hergueux, and Corinne Bronn for excellent assistance; and P. Michel, F. Fischer, and V. Louerat for maintaining the mice.
This work was supported by institute funds from the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, the Hôpital Universitaire de Strasbourg, and Bristol-Myers Squibb, and by grants to D. Mathis and C. Benoist from the Human Frontier Science Program and the Ministère de la Recherche. S. Chan was supported by the Association Nationale pour la Recherche sur le SIDA, the American Cancer Society, and the Ligue National contre le Cancer. M. Correia-Neves received fellowships from the Programa Gulbenkian de Doutoramento em Biologia e Medicina and the Junta Nacional de Investigaçao Cientifica e Tecnologica.
Abbreviations used in this paper
- β2m
β2-microglobulin
- B6
C57Bl/6
- βgal
β-galactosidase
- DN
double-negative
- DP
double-positive
- ES
embryonic stem
- FDG
fluorescein digalactopyranoside
- I0
MHC class I-negative
- II0
MHC class II–negative
- I0II0
MHC double-deficient
- PGK
phosphoglycerate kinase
- RT
reverse transcriptase
- SP
single-positive
- tg
transgenic
References
- 1.Fowlkes BJ, Pardoll DM. Molecular and cellular events of T cell development. Adv Immunol. 1989;44:207–264. doi: 10.1016/s0065-2776(08)60643-4. [DOI] [PubMed] [Google Scholar]
- 2.Robey E, Fowlkes BJ. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K, Vremec D, Egerton M. The kinetics of T cell antigen receptor expression by subgroups of CD4+8+ thymocytes: delineation of CD4+8+3(2+) thymocytes as post-selection intermediates leading to mature T cells. J Exp Med. 1991;173:323–332. doi: 10.1084/jem.173.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huesmann M, Scott B, Kisielow P, von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991;66:533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 5.Teh HS, Kisielow P, Scott B, Kishi H, Uematsu Y, Bluthmann H, von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988;335:229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- 6.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 7.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 8.Berg LJ, Pullen AM, Fazekas de St B, Groth, Mathis D, Benoist C, Davis MM. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- 9.Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 10.Chan SH, Cosgrove D, Waltzinger C, Benoist C, Mathis D. Another view of the selective model of thymocyte selection. Cell. 1993;73:225–236. doi: 10.1016/0092-8674(93)90225-f. [DOI] [PubMed] [Google Scholar]
- 11.Robey E, Itano A, Fanslow WC, Fowlkes BJ. Constitutive CD8 expression allows inefficient maturation of CD4+helper T cells in class II major histocompatibility complex mutant mice. J Exp Med. 1994;179:1997–2004. doi: 10.1084/jem.179.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itano A, Kioussis D, Robey E. Stochastic component to development of class I major histocompatibility complex-specific T cells. Proc Natl Acad Sci USA. 1994;91:220–224. doi: 10.1073/pnas.91.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pircher H, Ohashi PS, Boyd RL, Hengartner H, Brduscha K. Evidence for a selective and multi-step model of T cell differentiation: CD4+CD8lowthymocytes selected by a transgenic T cell receptor on major histocompatibility complex class I molecules. Eur J Immunol. 1994;24:1982–1987. doi: 10.1002/eji.1830240907. [DOI] [PubMed] [Google Scholar]
- 14.Chan SH, Waltzinger C, Baron A, Benoist C, Mathis D. Role of coreceptors in positive selection and lineage commitment. EMBO (Eur Mol Biol Organ) J. 1994;13:4482–4489. doi: 10.1002/j.1460-2075.1994.tb06770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis CB, Killeen N, Crooks ME, Raulet D, Littman DR. Evidence for a stochastic mechanism in the differentiation of mature subsets of T lymphocytes. Cell. 1993;73:237–247. doi: 10.1016/0092-8674(93)90226-g. [DOI] [PubMed] [Google Scholar]
- 16.van Meerwijk JP, Germain RN. Development of mature CD8+ thymocytes: selection rather than instruction? . Science. 1993;261:911–915. doi: 10.1126/science.8102208. [DOI] [PubMed] [Google Scholar]
- 17.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+single positive cells with a class II major histocompatibility complex–restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundberg K, Heath W, Kontgen F, Carbone FR, Shortman K. Intermediate steps in positive selection: differentiation of CD4+8int thymocytes into CD4−8+ TCRhithymocytes. J Exp Med. 1995;181:1643–1651. doi: 10.1084/jem.181.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kydd R, Lundberg K, Vremec D, Harris AW, Shortman K. Intermediate steps in thymic positive selection. Generation of CD4-8+ T cells in culture from CD4+8+, CD4int8+, and CD4+8intthymocytes with up-regulated levels of TCR-CD3. J Immunol. 1995;155:3806–3814. [PubMed] [Google Scholar]
- 20.Benveniste P, Knowles G, Cohen A. CD8/ CD4 lineage commitment occurs by an instructional/default process followed by positive selection. Eur J Immunol. 1996;26:461–471. doi: 10.1002/eji.1830260229. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H, Punt JA, Granger LG, Singer A. Asymmetric signaling requirements for thymocyte commitment to the CD4+ versus CD8+T cell lineages: a new perspective on thymic commitment and selection. Immunity. 1995;2:413–425. doi: 10.1016/1074-7613(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 22.Lucas B, Germain RN. Unexpectedly complex regulation of CD4/CD8 coreceptor expression supports a revised model for CD4+CD8+thymocyte differentiation. Immunity. 1996;5:461–477. doi: 10.1016/s1074-7613(00)80502-6. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Friedrich GA, Soriano P. Transcriptional enhancer factor 1 disruption by a retroviral gene trap leads to heart defects and embryonic lethality in mice. Genes Dev. 1994;8:2293–2301. doi: 10.1101/gad.8.19.2293. [DOI] [PubMed] [Google Scholar]
- 24.Adra CN, Boer PH, McBurney MW. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60:65–74. doi: 10.1016/0378-1119(87)90214-9. [DOI] [PubMed] [Google Scholar]
- 25.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 26.Killeen N, Sawada S, Littman DR. Regulated expression of human CD4 rescues helper T cell development in mice lacking expression of endogenous CD4. EMBO (Eur Mol Biol Organ) J. 1993;12:1547–1553. doi: 10.1002/j.1460-2075.1993.tb05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomonari K. A rat antibody against a structure functionally related to the mouse T-cell receptor/T3 complex. Immunogen. 1988;28:455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- 28.Pircher H, Rebai N, Groettrup M, Grégoire C, Speiser DE, Happ MP, Palmer E, Zinkernagel RM, Hengartner H, Malissen B. Preferential positive selection of Vα2+CD8+ T cells in mouse strains expressing both H-2k and T cell receptor Vαahaplotypes: determination with a Vα2-specific monoclonal antibody. Eur J Immunol. 1992;22:399–404. doi: 10.1002/eji.1830220217. [DOI] [PubMed] [Google Scholar]
- 29.Nolan GP, Fiering S, Nicolas JF, Herzenberg LA. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-d-galactosidase activity after transduction of Escherichia colilacZ. Proc Natl Acad Sci USA. 1988;85:2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dialynas DP, Quan ZS, Wall KA, Pierres A, Quintans J, Loken MR, Pierres M, Fitch FW. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 31.Le Mouellic H, Lallemand Y, Brulet P. Targeted replacement of the homeobox gene Hox-3.1 by the Escherichia colilacZ in mouse chimeric embryos. Proc Natl Acad Sci USA. 1990;87:4712–4716. doi: 10.1073/pnas.87.12.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansour SL, Thomas KR, Deng CX, Capecchi MR. Introduction of a lacZ reporter gene into the mouse int-2 locus by homologous recombination. Proc Natl Acad Sci USA. 1990;87:7688–7692. doi: 10.1073/pnas.87.19.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uematsu Y, Ryser S, Dembic Z, Borgulya P, Krimpenfort P, Berns A, von Boehmer H, Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988;52:831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- 34.Adlam M, Duncan DD, Ng DK, Siu G. Positive selection induces CD4 promoter and enhancer function. Int Immunol. 1997;9:877–887. doi: 10.1093/intimm/9.6.877. [DOI] [PubMed] [Google Scholar]
- 35.Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 36.Siu G, Wurster AL, Duncan DD, Soliman TM, Hedrick SM. A transcriptional silencer controls the developmental expression of the CD4 gene. EMBO (Eur Mol Biol Organ) J. 1994;13:3570–3579. doi: 10.1002/j.1460-2075.1994.tb06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tutt Landolfi, M.M., R. Scollay, and J.R. Parnes. Specific demethylation of the CD4 gene during CD4 T lymphocyte differentiation. Mol Immunol. 1997;34:53–61. doi: 10.1016/s0161-5890(96)00104-6. [DOI] [PubMed] [Google Scholar]
- 38.Bendelac A, Rivera MN, Park S-H, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 39.Bendelac A, Matzinger P, Seder RA, Paul WE, Schwartz RH. Activation events during thymic selection. J Exp Med. 1992;175:731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swat W, Dessing M, von Boehmer H, Kisielow P. CD69 expression during selection and maturation of CD4+8+thymocytes. Eur J Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- 41.Punt JA, Suzuki H, Granger LG, Sharrow SO, Singer A. Lineage commitment in the thymus: only the most differentiated (TCRhibcl-2hi) subset of CD4+CD8+thymocytes has selectively terminated CD4 or CD8 synthesis. J Exp Med. 1996;184:2091–2099. doi: 10.1084/jem.184.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas B, Vasseur F, Penit C. Stochastic coreceptor shut-off is restricted to the CD4 lineage maturation pathway. J Exp Med. 1995;181:1623–1633. doi: 10.1084/jem.181.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+T cells. Science. 1990;248:1227–1229. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 44.Akashi K, Weissman IL. The c-kit+maturation pathway in mouse thymic T cell development: lineages and selection. Immunity. 1996;5:147–161. doi: 10.1016/s1074-7613(00)80491-4. [DOI] [PubMed] [Google Scholar]
- 45.Akashi K, Kondo M, Weissman IL. Two distinct pathways of positive selection for thymocytes. Proc Natl Acad Sci USA. 1998;95:2486–2491. doi: 10.1073/pnas.95.5.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+T cells in major histocompatibility complex class II–deficient mice. J Exp Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 48.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 49.Robey EA, Fowlkes BJ, Gordon JW, Kioussis D, von Boehmer H, Ramsdell F, Axel R. Thymic selection in CD8 transgenic mice supports an instructive model for commitment to a CD4 or CD8 lineage. Cell. 1991;64:99–107. doi: 10.1016/0092-8674(91)90212-h. [DOI] [PubMed] [Google Scholar]
- 50.Borgulya P, Kishi H, Muller U, Kirberg J, von Boehmer H. Development of the CD4 and CD8 lineage of T cells: instruction versus selection. EMBO (Eur Mol Biol Organ) J. 1991;10:913–918. doi: 10.1002/j.1460-2075.1991.tb08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seong RH, Chamberlain JW, Parnes JR. Signal for T-cell differentiation to a CD4 cell lineage is delivered by CD4 transmembrane region and/or cytoplasmic tail. Nature. 1992;356:718–720. doi: 10.1038/356718a0. [DOI] [PubMed] [Google Scholar]
- 52.Rahemtulla A, Fung-Leung WP, Schilham MW, Kündig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, et al. Normal development and function of CD8+cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 53.Rahemtulla A, Kündig TM, Narendran A, Bachmann MF, Julius M, Paige CJ, Ohashi PS, Zinkernagel RM, Mak TW. Class II major histocompatibility complex-restricted T cell function in CD4-deficient mice. Eur J Immunol. 1994;24:2213–2218. doi: 10.1002/eji.1830240942. [DOI] [PubMed] [Google Scholar]
- 54.Locksley RM, Reiner SL, Hatam F, Littman DR, Killeen N. Helper T cells without CD4: control of Leishmaniasis in CD4-deficient mice. Science. 1993;261:1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- 55.von Boehmer H. The selection of the α, β heterodimeric T cell receptor for antigen. Immunol Today. 1986;7:333–336. doi: 10.1016/0167-5699(86)90139-8. [DOI] [PubMed] [Google Scholar]
- 56.Chan SH, Benoist C, Mathis D. A challenge to the instructive model of positive selection. Immunol Rev. 1993;135:119–131. doi: 10.1111/j.1600-065x.1993.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 57.Tarakhovsky A, Muller W, Rajewsky K. Lymphocyte populations and immune responses in CD5-deficient mice. Eur J Immunol. 1994;24:1678–1684. doi: 10.1002/eji.1830240733. [DOI] [PubMed] [Google Scholar]
- 58.Barthlott T, Kohler H, Pircher H, Eichmann K. Differentiation of CD4highCD8lowcoreceptor-skewed thymocytes into mature CD8 single-positive cells independent of MHC class I recognition. Eur J Immunol. 1997;27:2024–2032. doi: 10.1002/eji.1830270829. [DOI] [PubMed] [Google Scholar]
- 59.Baron A, Hafen K, von Boehmer H. A human CD4 transgene rescues CD4−CD8+cells in beta 2-microglobulin-deficient mice. Eur J Immunol. 1994;24:1933–1936. doi: 10.1002/eji.1830240834. [DOI] [PubMed] [Google Scholar]
- 60.Matechak EO, Killeen N, Hedrick SM, Fowlkes BJ. MHC class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity. 1996;4:337–347. doi: 10.1016/s1074-7613(00)80247-2. [DOI] [PubMed] [Google Scholar]
- 61.Marodon G, Rocha B. Generation of mature T cell populations in the thymus: CD4 or CD8 down-regulation occurs at different stages of thymocyte differentiation. Eur J Immunol. 1994;24:196–204. doi: 10.1002/eji.1830240131. [DOI] [PubMed] [Google Scholar]
- 62.Hostert A, Tolaini M, Roderick K, Harker N, Norton T, Kioussis D. A region in the CD8 gene locus that directs expression to the mature CD8 T cell subset in transgenic mice. Immunity. 1997;7:525–536. doi: 10.1016/s1074-7613(00)80374-x. [DOI] [PubMed] [Google Scholar]
- 63.Ellmeier W, Sunshine MJ, Losos K, Hatam F, Littman D. An enhancer that directs lineage-specific expression of CD8 in positively selected thymocytes and mature T cells. Immunity. 1997;7:537–547. doi: 10.1016/s1074-7613(00)80375-1. [DOI] [PubMed] [Google Scholar]
- 64.Uematsu Y, Donda A, De Libero G. Thymocytes control the CD4 gene differently from mature T lymphocytes. Int Immunol. 1997;9:179–187. doi: 10.1093/intimm/9.1.179. [DOI] [PubMed] [Google Scholar]
- 65.Sharp LL, Schwarz DA, Bott CM, Marshall CJ, Hedrick SM. The influence of the MAPK pathway on T cell lineage commitment. Immunity. 1997;7:609–618. doi: 10.1016/s1074-7613(00)80382-9. [DOI] [PubMed] [Google Scholar]
- 66.Bommhardt U, Cole MS, Tso JY, Zamoyska R. Signals through CD8 or CD4 can induce commitment to the CD4 lineage in the thymus. Eur J Immunol. 1997;27:1152–1163. doi: 10.1002/eji.1830270516. [DOI] [PubMed] [Google Scholar]
- 67.Merkenschlager M, Benoist C, Mathis D. Evidence for a single-niche model of positive selection. Proc Natl Acad Sci USA. 1994;91:11694–11698. doi: 10.1073/pnas.91.24.11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robey E, Chang D, Itano A, Cado D, Alexander H, Lans D, Weinmaster G, Salmon P. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- 69.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+T cells, but affect their long-term life span. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 70.Rooke R, Waltzinger C, Benoist C, Mathis D. Targeted complementation of MHC class II deficiency by intrathymic delivery of recombinant adenoviruses. Immunity. 1997;7:123–134. doi: 10.1016/s1074-7613(00)80515-4. [DOI] [PubMed] [Google Scholar]