Abstract
VDJ recombination of T cell receptor and immunoglobulin loci occurs in immature lymphoid cells. Although the molecular mechanisms of DNA cleavage and ligation have become more clear, it is not understood what controls which target loci undergo rearrangement. In interleukin 7 receptor (IL-7R)α−/− murine thymocytes, it has been shown that rearrangement of the T cell receptor (TCR)-γ locus is virtually abrogated, whereas other rearranging loci are less severely affected. By examining different strains of mice with targeted mutations, we now observe that the signaling pathway leading from IL-7Rα to rearrangement of the TCR-γ locus requires the γc receptor chain and the γc-associated Janus kinase Jak3. Production of sterile transcripts from the TCR-γ locus, a process that generally precedes rearrangement of a locus, was greatly repressed in IL-7Rα−/− thymocytes. The repressed transcription was not due to a lack in transcription factors since the three transcription factors known to regulate this locus were readily detected in IL-7Rα−/− thymocytes. Instead, the TCR-γ locus was shown to be methylated in IL-7Rα−/− thymocytes. Treatment of IL-7Rα−/− precursor T cells with the specific histone deacetylase inhibitor trichostatin A released the block of TCR-γ gene rearrangement. This data supports the model that IL-7R promotes TCR-γ gene rearrangement by regulating accessibility of the locus via demethylation and histone acetylation of the locus.
Keywords: T cell receptor, thymus, VDJ recombination, interleukin 7, T cell receptor γ locus, γ/δ T cells
Signals from the IL-7 receptor are critical for early stages in development of several of the lymphoid lineages (1, 2). Thus, IL-7Rα−/− mice produce very few α/β T cells and B cells, and the γ/δ T cell lineage is even more severely repressed (3, 4). The IL-7Rα signal triggers at least two types of responses in lymphoid precursor cells (for review see reference 5). One signal is for survival of pro-T cells and is associated with the level of bcl-2 in the cells (6); thus, the α/β T cell deficiency in IL-7Rα−/− mice can be partly ameliorated by a bcl-2 transgene (7, 8). The second signal from IL-7 receptor promotes VDJ recombination at several loci, including TCR-β (9, 10), IgH (11, 12), and the TCR-γ locus (13, 14); of these loci, rearrangement of the TCR-γ locus is the most severely repressed in IL-7Rα−/− mice.
VDJ recombination is a stringently regulated event that is restricted (with few exceptions) to certain early stages in lymphoid development. The mechanisms of this strict regulation are not fully understood. However, several types of controls are identified or presumed. One level of control is expression of the recombinase components, RAG1 and -2, which mediate cleavage of target gene segments (15). Thus, expression of the RAG genes is restricted to early lymphoid cells. On the other hand, religation of the target locus involves components that are not restricted to the lymphoid lineage; these include Ku, p350 kinase catalytic subunit, XRCC4, and DNA ligase (for review see references 16, 17). IL-7 has been shown to promote the expression of RAG1 and -2 in pro-T cells (9, 18). Moreover, IL-7Rα−/− mice showed suppressed expression of the Rag genes in pro-T cells, whereas the later stage (CD4+CD8+) expressed Rag genes normally, indicating that after the pro-T cell stage expression of the Rag genes becomes IL-7R independent (10).
A second control of VDJ recombination governs whether a locus is accessible to cleavage by the Rag proteins (for review see references 5, 16). This control is necessary because the motifs recognized by the Rag proteins are similar in all rearranging loci. Since different cell types rearrange different loci, there is presumed to be a mechanism governing accessibility of the locus. For example, pro-T cells rearrange the TCR-β, -γ, and -δ loci at about the same time, do not fully rearrange the immunoglobulin loci, and the TCR-α locus is rearranged at a later stage. Little is understood of the process rendering a locus accessible to recombination. The enhancer of a locus, normally defined based on its ability to promote transcription, also can be involved in promoting rearrangement of a locus. This is thought to account for the observation that a given locus generally produces sterile transcripts before it undergoes rearrangement. Deletion of the respective enhancers abolishes rearrangement of the TCR-β locus (19, 20), and greatly suppresses rearrangement of the IgH locus (21), the Igκ locus (22), and the TCR-α locus (23). Transcription of a gene is not required for its rearrangement (24), so the enhancer is presumed to play a role in remodeling chromatin structure, rendering nearby regions accessible to both recombination and transcriptional machinery.
In this study, we investigated the signaling mechanism by which IL-7Rα promotes rearrangement of the TCR-γ locus. There are two ligands for the murine IL-7Rα chain, IL-7 and thymic stromal-derived lymphopoietin (TSLP)1 (25). IL-7 signaling involves pairing of the IL-7Rα chain with the γc chain (26, 27), whereas TSLP signaling is thought to involve pairing of the IL-7Rα chain with a different chain (2); for this reason we examined the role of γc in signaling rearrangement of the TCR-γ locus. There are several tyrosine kinases activated by IL-7R (28–30); we examined the role of one of these, the Janus kinase Jak3 (31, 32), which is associated with γc (33–35). In B cells, it has also been shown that phosphatidylinositol 3 (PI3) kinase is activated by IL-7 and is involved in triggering proliferation; however, we have not found a requirement for PI3 kinase in IL-7 signaling in pro-T cells (6). We also examined whether the TCR-γ locus produces sterile transcripts and test for several transcription factors implicated in activating the TCR-γ enhancer. We examined whether IL-7R signals could induce demethylation of the TCR-γ locus, which could influence actetylation of chromatin. Finally, we circumvented the need for the IL-7R signal by the use of the specific deacetylase inhibitor trichostatin A (TSA), showing that it promotes TCR-γ gene rearrangement in vitro.
Materials and Methods
Mice.
Embryonic thymus was obtained by performing timed breeding of C57BL/6 mice maintained at Animal Production (Frederick, MD). IL-7Rα−/− mice (1) and Rag2−/− mice (36) were produced in the NCI facility (Frederick, MD) from breeders purchased from The Jackson Laboratory (Bar Harbor, ME). γc −/− mice (37) were bred in the National Institutes of Health (NIH) facility (Bethesda, MD). Jak3−/− mice (38) were bred at the University of Massachusetts Medical Center (Worcester, MA). Animal care was provided in accordance with the procedures outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 86-23, 1985).
PCR Analysis.
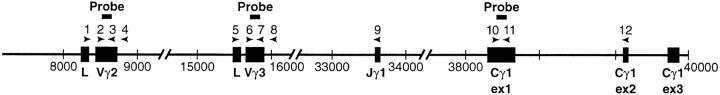
DNA was extracted (39) from adult thymi of the indicated strain or fetal C57Bl/6 thymus from day 15 of gestation. In the case of fetal Rag2−/− or IL-7Rα−/−, several thymi were pooled, whereas all other thymi were individually treated. Approximately 1 μg of DNA was used for each PCR reaction in 50 μl reaction buffer (PE Applied Biosystems, Inc., Foster City, CA) with 0.2 mM of each NTP, 1 μM of each primer, and 1 U of Taq polymerase. The PCR reaction was performed with a hot start: (94°C for 3 min) 94°C for 1 min, 55°C for 1 min, 72°C for 1 min for 30 cycles (extension, 10 min at 72°C). RNA was extracted with RNAzol according to the manufacturer's protocol and reverse transcribed as described with oligo(dT) primers (9). PCR was performed as for genomic DNA with the exemption that the annealing time was 2 min at 55°C. PCR products were separated on a 1.4% agarose gel, blotted, and pre-hybridized (Nycomed Amersham, Arlington Heights, IL) for 1 h before addition of the γ-ATP 5′ end-labeled probe. After 12–16 h of hybridization at 42°C, blots were washed according to the manufacturer's protocol (Nycomed Amersham) and visualized by autoradiography. In Fig. 3 (see Results), the PCR product has not been blotted, but rather is the negative image of an original ethidium bromide–stained agarose gel. The location of primers within the TCR-γ locus are shown in Fig. 1 (see Results). The primers for detection of DNA gene rearrangement were derived from sequences as previously published (40). The primers for detection of sterile transcripts have been published previously (41), as have the primers for detection of GAPDH mRNA (42). The following oligonucleotides were used (the bp number in parentheses indicates the precise position in the locus as reported in EMBL/GenBank/DDBJ under accession No. AF037352). No. 1, Vγ2 leader peptide sense (8,280 bp): 5′-CTGGGAATTCAACCTGGCAGATG-3′; No. 2, Vγ2 sense (8,460 bp): 5′-CATGGGAAGTTGGAGCAACCTGAAATATC-3′; No. 3, Vγ2 antisense (8,734 bp): 5′-GCTTCGTCTTCTTCCTCCAAGGAATA-3′; No. 4, Vγ2-3a′antisense (8,846 bp): 5′-GCTAAGAAGGATGTGGGTTG-3′, probe for Vγ2 (8,586 bp): 5′-AACCAAGGTTTAGAGTTTCTATTA-3′; No. 5, Vγ3 leader peptide sense (15,508 bp): 5′-CCAGCAGCCACTAAAATGTC-3′; No. 6, Vγ3 sense (15,678 bp): 5′-GACTCCTGGATATCTCAGGATCAGC-3′; No. 7, Vγ3 antisense (15,947 bp): 5′-CGTGGCTTCATCCGATGTGACAAC-3′; No. 8, Vγ3-3a′ antisense (16,059 bp): 5′-TGGAGGATCCTTGGTGGGTTCA-3′, probe for Vγ3 (15,795 bp): 5′-CTGAAAGAAGGGGAGCCCCTGAGACG-3′; No. 9, Jγ1 antisense (33,661 bp): 5′-CGGGATCCCAGAGGGAATTACTATGAGC-3′; No. 10, TCR-γ constant first exon sense (37,363 bp): 5′-AGGCTTGATGCAGACATTTCCCCC-3′; No. 11, TCR-γ constant first exon antisense (37,657 bp): 5′-GATCTGCTCCTCCTTTGTTGTTCTC-3′; No. 12, TCR-γ constant second exon antisense (39,154 bp): 5′-CTTGCCAGCAAGTTGTAGGCTTGG-3′, probe for TCR-γ constant (37,504 bp): 5′-GATGGCAATACTATCCTGGACTCC-3′.
Figure 3.
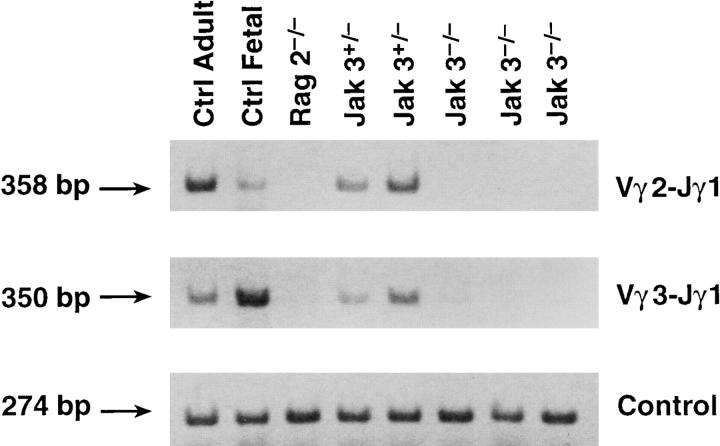
Defective rearrangement of the TCR-γ locus in Jak3−/− thymocytes. Thymocytes from Jak3−/− mice were compared with normal adult C57BL/6, fetal (day 15), and RAG2−/− mice for rearrangements of the TCR-γ locus as in Fig. 2. Control lanes represent a pool of five individuals, whereas individual mice are shown from the Jak3−/− and Jak3+/− strains.
Figure 1.
Location of primers and probes within the TCR-γ1 locus.
Gel Mobility Shift Assay.
Nuclear extracts were prepared (43) from ∼107 fetal thymocytes (day 15 of gestation) from normal C57BL/6 mice, or from adult mice (4–6-wk-old) of Rag2−/− or IL7Rα−/− strains. The binding reaction was conducted at room temperature for 20 min with 1 to 3 μg of protein in 25 μl of binding buffer (25 mM hepes, pH 7.5, 10% glycerol, 1 μg poly[d(I-C)], 50 mM NaCl, 0.05% NP-40, 1 mM dithiothreitol) and 10,000 cpm of γ-[32P]ATP 5′ end-labeled oligonucleotide. The reaction mixture was separated on a 7% polyacrylamide gel at 4°C, dried, and visualized by autoradiography. The nuclear extracts were tested for equal binding to the control nucleotide of the housekeeping factor Sp-1. For competitive inhibition of the gel shift, 50-fold excess of the specific oligonucleotide or a nonspecific competitor was used (the nuclear factor [NF]γ2 site for binding to NFγ3 or NFγ4, and Sp-1 for binding to NFγ2). For inhibition with antibodies, the extracts were incubated with 1 μl of specific antisera against Stat5a and Stat5b (R&D Systems, Minneapolis, MN) or control rabbit serum at 8°C for 2 h before the addition of the radiolabeled oligonucleotides. The following double-stranded oligonucleotides were used for detection of nuclear proteins that bind to the murine TCR-γ enhancer (52). Nfγ3: 5′-CATAGGAGCAGTTAAACCACAGCCAG-3′, 5′-CTGGCTGTGGTTTAACTGCTCCTATG-3′; Nfγ2: 5′-GTTGCTTCCTGGAAAATGGTTAAAG-3′, 5′-CTTTAACCATTTTCCAGGAAGCAAC-3′; and Nfγ4: 5′-CTTTCGAAAGACCACAGCTATTAG-3′, 5′-CTAATAGCTGTGGTCTTTCGAAAG-3′.
Assay for Methylation of DNA.
To analyze the methylation status of the TCR-γ locus, 15 μg of DNA was digested overnight with MspI or HpaII (which cannot cleave at methylated residues). The product was fractionated on a 0.8% agarose gel, blotted, and then prehybridized overnight. The blot was hybridized with a 294-bp probe generated by PCR from the first exon of the constant region of TCR-γ1 using primer Nos. 10 and 11 (as described above; see Fig. 1). The probe was random primed, then hybridized with the blot overnight in hybridization buffer containing 5× SSC, 10× Denhardt's solution, salmon sperm DNA (0.5 mg/ml), and 1% SDS. Blots were then washed 10 min in 3× SSC and 0.5% SDS at room temperature followed by washing 45 min in 0.1% SSC and 0.5% SDS at 60°C. The location of the MspI/HpaII sites in the TCR-γ locus is shown in Fig. 6.
Figure 6.
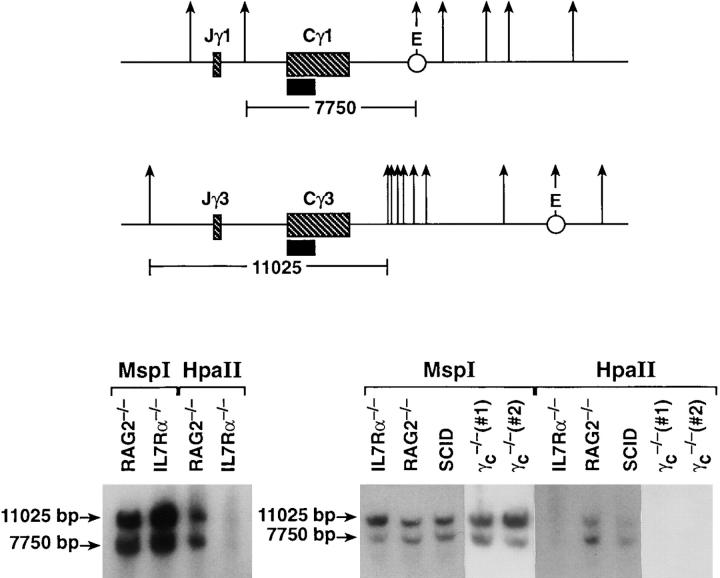
Methylation of the TCR-γ locus in IL-7Rα−/− and γc −/− thymocytes. Thymocyte cell suspensions were prepared from IL-7Rα−/−, γc −/−, RAG2−/−, or SCID mice. DNA was extracted and digested with MspI or HpaII. Southern blot analysis was performed using a probe for the TCR-γ constant region. Each lane represents a pool of two to five individuals except γc −/− representing one individual per lane. Position of MspI/HpaII cleavage sites within the TCR-γ locus are indicated with an arrow. Position of the probe used for hybridization is indicated with a closed bar.
Reconstitution and Culture of Thymic Lobes Containing IL-7R− /− Thymocytes.
Embryonic thymic lobes were obtained from C57Bl/6 embryos at day 14 of gestation. Lobes were depleted of endogenous thymocytes by irradiation (30 Gy), then reconstituted by placing in hanging drops of 35 μl of medium together with IL-7R−/− bone marrow cells (5 × 104/lobe) for 24 h. Reconstituted lobes were then washed and cultured in microtiter wells, one lobe per well, in 50 μl of medium for 96 h together with the specific histone deacetylase inhibitor TSA (Wako Chemicals).
Results
Role of the γc Chain.
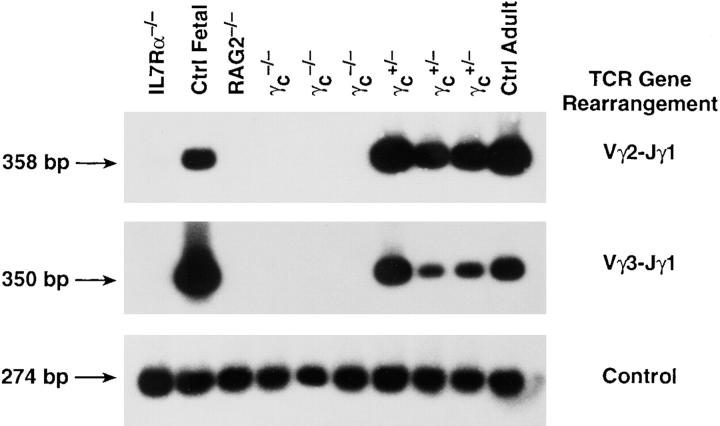
It was previously shown that IL-7Rα−/− thymocytes from two independent lines were defective in rearrangement of the TCR-γ locus (13, 14). A second chain, γc, serves as part of the receptor for IL-7 (26, 27), whereas it has been speculated that TSLP (25), a homologue of IL-7, signals through IL-7Rα together with a different chain. We therefore tested whether γc was required for rearrangement of the TCR-γ locus. Several rearrangements of this locus were examined using PCR, which generates a product if the locus is rearranged, bringing two gene segments in sufficiently close proximity to permit the polymerization reaction (Fig. 1). Using this method, thymocytes from various strains of mice were analyzed for rearrangement of Vγ3 and Vγ2, as shown in Fig. 2, and Vγ4 (data not shown), which showed the same pattern. As shown in Fig. 2, γc −/− thymocytes were as deficient as IL-7Rα−/− thymocytes in the rearrangement of this locus. Rag2−/− thymocytes are shown as a control since they are unable to initiate VDJ recombination at any locus. We conclude that both IL-7Rα and γc chains are essential parts of the receptor complex that signals the rearrangement of the TCR-γ locus.
Figure 2.
Defective rearrangement of the TCR-γ locus in IL-7R−/− and γc −/− thymocytes. Thymocyte suspensions were prepared from normal adult C57BL/6 or fetal (day 15) mice or from mice deficient in IL-7Rα, Rag2, or γc. DNA was extracted and PCR reactions were performed to detect the indicated rearrangements of the TCR-γ locus. The control PCR reaction uses internal primers for Vγ2 (which detects both germ line and rearranged DNA). Each lane represents a pool of five individuals except for γc −/− and +/−, for which three individuals are shown.
Role of Jak3 Kinase.
Several kinases are activated by IL-7R cross-linking, including those of the src (28, 30) and Janus families (29). One of the Janus kinases, Jak3, was examined for a possible role in signaling TCR-γ locus rearrangement because Jak3 is physically associated with γc and has been shown to be essential for normal lymphoid development (38, 44, 45). As shown in Fig. 3, a deficiency in TCR-γ rearrangement was noted in these Jak3−/− mice that was comparable to the deficiency observed in thymocytes deficient in either IL-7Rα or γc chains. Thus, Jak3 is an essential component of the signaling pathway leading from IL-7R cross-linking to rearrangement of the TCR-γ locus.
Role of Expression of the Rag Genes.
Expression of Rag genes was previously shown to be sustained by IL-7 (9, 18, 46, 47) and it has been observed previously that transcripts for RAG1 and -2 are deficient in IL-7R−/− thymocytes (10). We examined IL-7Rα−/− thymocytes for expression of Rag1 and -2 and observed considerable variability (data not shown): different batches of thymocytes (pooled from five individuals) showed levels ranging from barely detectable to normal levels for both RAG1 and -2. Some IL-7Rα−/− mice have been shown to display a “leaky” phenotype in that they develop small numbers of CD4+CD8+ cells (2); this cell population has been shown to express Rag messages in IL-7Rα−/− thymocytes (10), whereas the same study observed suppressed Rag expression in the pro-T cells from the same mice. However, in our IL-7Rα−/− mouse colony, some pools of thymocytes from “non-leaky” mice (with minimal numbers of CD4+CD8+ cells) also expressed high levels of Rag messages, so we presume that even among pro-T1 cells (CD44+CD25−) there is variability among individual mice. Thus, the deficiency in rearrangement of the TCR-γ locus, which is observed in all our IL-7R−/− mice, could not be explained purely by a deficiency in expression of Rag genes. Moreover, rearrangement of the TCR-γ locus is more repressed than the other loci that undergo VDJ recombination (14), strongly suggesting that locus-specific influences are signaled by the IL-7Rα (5). We therefore sought additional mechanisms that could be involved in the severe repression of rearrangement of the TCR-γ locus in these mice.
Production of Sterile Transcripts.
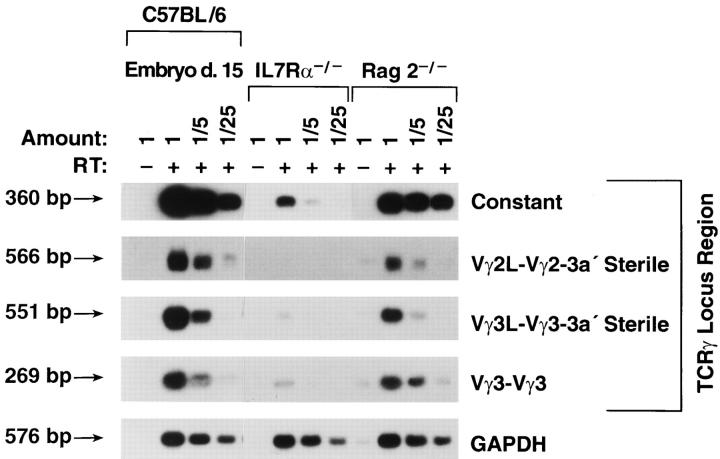
A mechanism that is thought to control VDJ recombination is whether or not the locus is accessible to the RAG proteins (for review see reference 16). One indicator that the chromatin is open around a rearranging gene is the production of sterile transcripts emanating from that locus before its rearrangement, a phenomenon that has been reported for most of the loci that undergo VDJ recombination (48, 49), including the TCR-γ locus (50). Sterile transcripts from the TCR-γ locus were greatly reduced in IL-7Rα−/− thymocytes as shown in Fig. 4. Transcription from both constant and variable regions were affected, and several different regions of the locus were repressed to a similar degree. Positive controls for production of sterile transcripts are day 15 embryonic thymus and RAG2−/− thymus, both of which contain a high proportion of pro-T cells. The deficiency in sterile transcripts in IL-7Rα−/− thymocytes suggests that signals from the IL-7R may control the accessibility of the TCR-γ locus, which would in turn affect its ability to be cleaved by the RAG proteins. However, an alternative explanation is that the locus is open before the IL-7R signal, which then induces its transcription, for example by inducing transcription factors that bind the enhancer and upregulate expression of the gene. We therefore performed additional assays to distinguish whether the transcriptional defect in IL-7Rα−/− thymocytes was due to a lack of transcription factors or to inaccessibility of chromatin to those transcription factors.
Figure 4.
Defective expression of sterile TCR-γ transcripts and constant region transcripts in IL-7Rα−/− thymocytes. Thymocyte suspensions were prepared from normal C57BL/6 mice (embryonic day 15), IL-7R−/−, or RAG2−/− mice. RNA was extracted and cDNA was synthesized. PCR reactions were performed for unrearranged transcripts from the TCR-γ locus, including the constant region and the indicated V regions. GAPDH transcripts were analyzed as a positive control for RNA loading. Three dilutions of each cDNA were tested to reflect quantitation.
Role of Transcription Factors.
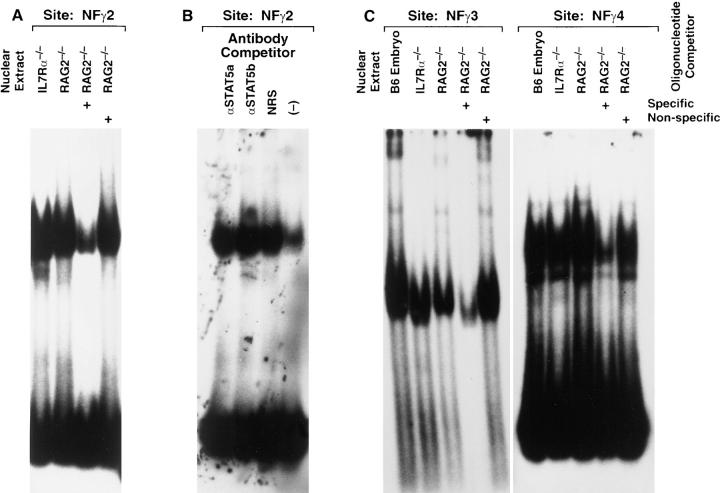
To test for defects in transcription factors in IL-7Rα−/− thymocytes, we examined nuclear proteins that bind to the enhancer regions which are located 3′ to three of the four constant regions of the TCR-γ locus. The enhancer confers lymphoid-specific transcription if coupled to a heterologous promoter (51, 52). Within the enhancer, three sites (NFγ2, 3, and 4) have been identified based on footprinting studies. The NFγ2 site resembles the consensus motif for binding of STAT5, which has been previously shown to be activated by IL-7 (29, 53). The proteins binding the NFγ3 site were shown to contain c myb and core-binding factor (54). The components of the complex that bind the NFγ4 site have not been identified. In Fig. 5, nuclear extracts from IL-7Rα−/− thymocytes are compared with those from RAG2−/− thymocytes, since thymic development in both of these strains is arrested at pro-T cell stages. The IL-7Rα−/− thymocytes did not show a deficiency of complexes that bound any of the three enhancer sites. In some nuclear extract preparations from IL-7Rα−/− thymocytes, an additional band was observed that migrated faster than the band in the control RAG2−/− extract; we do not know whether this smaller complex is functionally significant, but suspect it represents a degradation product. In any case, we observed no clear deficiency in any complex in IL-7Rα−/− thymocytes. Since STAT5 is activated by IL-7, and since the NFγ2 site resembles a STAT5 consensus site (see Materials and Methods for sequence), we tested whether the complex binding to that site contained STAT5a or -b using antibodies directed against these proteins. No effect of these antibodies was observed on the complexes, nor was complex formation blocked, nor was an increase in mass observed (as a positive control for the antisera, they were shown to supershift the STAT5 complex induced in the YT line by IL-2 stimulation; data not shown). Hence, the complex that binds the NFγ2 site does not appear to contain STAT5. In conclusion, we found no evidence that IL-7Rα−/− thymocytes lacked any of the nuclear proteins previously implicated in activating the TCR-γ enhancer. However, some transcription factors can bind their motif in an “inactive” state, then phosphorylation allows them to interact with the transcriptional machinery (AP-1 is an example of such a transcription factor). Thus, it is possible that one of these three transcriptional complexes, although it binds DNA, is inactive in these thymocytes. It is also possible, since these sites were identified in lines of mature T cells, that pro-T cells use different sites to regulate transcription.
Figure 5.
Nuclear proteins from IL-7Rα−/− thymocytes binding to regions of the TCR-γ enhancer. Thymocyte cell suspensions were prepared from normal C57BL/6 embryos (day 15 of gestation), IL-7Rα−/−, or Rag2−/− mice. Nuclear extracts were prepared. Gel mobility shift assays were performed using labeled oligonucleotides from three sites in the enhancer region of the TCR-γ locus. To assess specificity of binding, unlabeled competitors were added in 10-fold excess to the labeled oligonucleotide (Specific denotes the same sequence used in the labeled probe; Non-specific is an unrelated sequence of the same size). Antibodies were added to determine whether the NFγ2-binding protein complex contained STAT5a or -b proteins.
Methylation Status of the TCR-γ Locus.
To determine whether signals from the IL-7R could control chromatin structure of the TCR-γ locus, we examined the methylation of this gene. Methylation of the cytosine residues in the sequence 5′ CpG are often associated with silencing of genes (for review see reference 55). This silencing is thought to be based on the binding of proteins to these methylated sites, thereby altering the access of transcriptional machinery (see below). Methylation is also able to block VDJ recombination of minichromosomes after replication (56). We previously noted that during T cell development, the TCR-β locus was demethylated just before undergoing VDJ recombination (Muegge, K., unpublished data). We therefore tested whether IL-7R signals controlled the methylation of the TCR-γ locus. This was indeed the case as shown by Southern blot analysis in Fig. 6. This assay is based on the properties of two restriction enzymes, MspI and HpaII, both of which cleave at the same restriction site, whereas methylation of the cytosine in that site interferes with cleavage by HpaII but not MspI. As shown, HpaII was unable to cleave sites in the TCR-γ locus in thymocytes from IL-7Rα−/− or γc −/−, whereas thymocytes from RAG2−/− or SCID mice were cleaved by HpaII. Fig. 6 also illustrates the position of the methylation-sensitive cleavage sites within TCR-γ clusters 1 and 2 (the probe used for hybridization is 100% identical with cluster 1 and 95% identical with cluster 2). One site in cluster 1 generating the 7.7-kb fragment is located between the joining region and the constant region, whereas the other site is located within the first enhancer. This indicates that signals from the IL-7R induce demethylation of the TCR-γ locus, which may in turn keep the locus “open” for either transcription (production of sterile transcripts) or rearrangement. We have also examined the methylation status of the TCR-β locus (data not shown) and found that, in distinction to the TCR-γ locus, it appears to be equally demethylated in IL-7Rα−/− and Rag2−/− thymocytes. Thus, the requirement for IL-7R signals in rearrangement of the TCR-γ locus is consistent with an effect on accessibility.
Treatment of T Cell Precursors with a Specific Inhibitor of Histone Deacetylase Overcomes the Need for the IL-7R Signal.
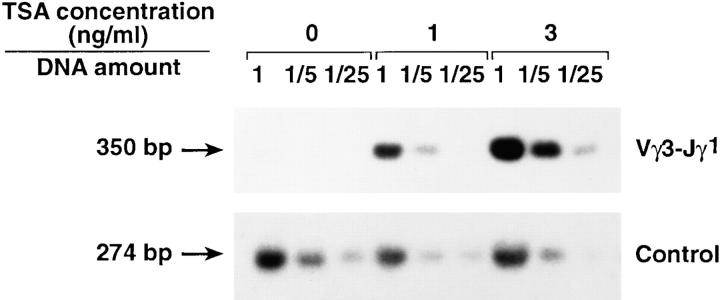
Methylation of DNA is thought to obstruct accessibility by altering chromatin structure. The methyl-CpG binding protein MeCP2 interacts specifically with methylated DNA and mediates repression of transcription. It has been shown that MeCP2 complexes with transcriptional repressors including mSin3A and histone deacetylases (57). The specific histone deacetylase inhibitor TSA can relieve repressed transcription, indicating that histone acetylation is an important feature of accessible chromatin (57, 58). Thus, we tested whether the need for IL-7R could be bypassed by enhancing histone acetylation with the use of TSA. Bone marrow cells from IL-7Rα−/− mice were allowed to reconstitute an irradiated fetal thymus in a hanging drop culture. The reconstituted thymus was then treated for 96 h with different concentrations of TSA. As shown in Fig. 7, TSA induced TCR-γ gene rearrangement in IL-Rα−/− thymocytes. Fetal thymus that had not been reconstituted with bone marrow cells showed no TCR-γ rearrangement in the presence of TSA (data not shown). These data are consistent with a model that IL-7R signals control rearrangement of the TCR-γ locus via histone acetylation.
Figure 7.
TSA restores rearrangement of the TCR-γ locus in IL-7Rα−/− thymocytes. Bone marrow from IL-7R−/− mice was used to reconstitute irradiated embryonic thymic lobes (from C57BL/6 mice) in hanging drops. Reconstituted lobes were then cultured in the indicated concentrations of TSA for 96 h and analyzed for rearrangement of the TCR-γ locus.
Discussion
IL-7Rα−/− mice show a severe deficiency in rearrangement of the TCR-γ locus. In this study we examine the mechanism by which IL-7Rα signals VDJ recombination of the TCR-γ locus. Based on studies in knockout mice, we observed that the γc component of the receptor and the γc-associated kinase Jak3 are required to deliver the signal from IL-7Rα to the TCR-γ locus. The production of sterile transcripts from the TCR-γ locus was greatly impaired in IL-7Rα−/− thymocytes. This transcriptional defect could either reflect a deficiency in transcription factors or an inaccessibility of the TCR-γ locus. The latter hypothesis is favored by three observations in IL-7Rα−/− thymocytes: (a) we readily detected the transcription factors binding to regions that have been shown to be important for expression of the TCR-γ genes; (b) the TCR-γ locus was methylated, which may obstruct its accessibility to both transcriptional and recombinational proteins; and (c) the need for IL-7Rα signals could be overcome in vitro by the use of the specific histone deacetylase inhibitor TSA.
The IL-7Rα effect on rearrangement of the TCR-γ locus is unlikely to be simply the result of the trophic effect of promoting the survival of cells undergoing the rearrangement. First, the TCR-γ locus rearranges in pro-T cells at about the same time as do the TCR-δ and -β loci. Thus, death of the pro-T cell would be expected to equally repress rearrangement of all three loci, yet the latter two loci show detectable rearrangements in IL-7Rα−/− mice, whereas rearrangement of the TCR-γ locus is almost abrogated. Even though pro-T2 cells are virtually absent in these mice, and this is the stage in which TCR gene rearrangements normally begin, only rearrangement of the TCR-γ locus is eliminated. Second, a bcl-2 transgene was shown to promote development of the α/β lineage but not the γ/δ lineage (7, 8), so although the IL-7Rα signal also provides a trophic effect, there is no evidence that this is sufficient to sustain TCR-γ locus rearrangement. On the other hand, introducing a rearranged TCR-γ transgene only partially restored γ/δ T cell development in γc −/− mice (59), reflecting the requirement for a second trophic signal from the IL-7 receptor.
The IL-7Rα chain is reported to be a component of the receptor for TSLP, a homologue of IL-7. Knockout of IL-7 (60, 61) has a less severe phenotype than knockout of IL-7Rα, in that more α/β T cells develop and γ/δ T cells are at least detectable. This has led to the suggestion that TSLP can partly compensate for the absence of IL-7 in IL-7 knockout mice, and implies that TSLP and IL-7 have similar activities. There has also been some speculation that the receptor for TSLP does not use the γc chain (2). Thus, if TSLP were capable of inducing T cell development, it might be independent of γc and Jak3, which could explain why knockout mice for γc and Jak3 have a less severe phenotype, more like IL-7– than IL-7Rα–deficient mice. In any case, our data show that both IL-7Rα and γc chains are required for the signal to rearrange the TCR-γ locus. To test whether receptors from another cytokine family could induce rearrangement of the TCR-γ locus, we have injected IL-7R−/− mice with oncostatin M. However, we have not detected rearrangement of the locus as of the writing of this paper.
To determine whether the TCR-γ enhancer is the target of IL-7R stimulation, we examined nuclear proteins from IL-7R−/− thymocytes. We did not find a deficiency in DNA binding based on gel mobility shift assays. However, the binding of a transcription factor to DNA does not necessarily indicate that it is transcriptionally active. Cross-linking of the IL-7R has been shown to activate the transcription factor STAT5, inducing its translocation to the nucleus (29), and it has been shown that there is a deficiency in nuclear STAT5 in IL-7R−/− thymocytes (62). We have confirmed the latter finding that IL-7R−/− thymocytes were deficient in nuclear proteins that bind a STAT5 motif (data not shown). STAT5 seemed a likely candidate for the relevant IL-7–induced transcription factor, since one of the enhancer sites resembles a consensus binding site for STAT5. However, we observed that the complex that actually binds this enhancer site in vivo does not contain STAT5a or -b based on a failure of specific antibodies to block binding in vitro. These findings argue against the STAT5 pathway being involved in rearrangement of the TCR-γ locus, which is also consistent with the observation of the relatively normal T cell development that occurs in STAT5a−/−b−/− mice (63).
It has recently been suggested that the IL-7 effect on rearrangement of the IgH locus may be via the transcription factor Pax5 (12) based on several findings: (a) Pax5 binds a site in the IgH locus; (b) Pax5−/− mice resemble IL-7Rα−/− mice in that the IgH locus successfully rearranges D to J, but fails to normally rearrange V to D; (c) IL-7Rα−/− B cells showed reduced expression of Pax5. However, we detected normal levels of Pax5 transcripts in pro-T cells from IL-7Rα−/− mice (data not shown), suggesting that its regulation is dependent on IL-7R in the B but not the T lineage, and arguing against it mediating the signal to rearrange the TCR-γ locus. That study parallels ours in noting that IL-7Rα−/− mice were defective in producing sterile transcripts from the IgH locus and proposing that IL-7R regulated accessibility of that locus. Since thymocytes normally perform DH to JH but not VH to DH rearrangements, we tested whether TSA treatment would overcome the latter block. TSA did not induce VH to DH rearrangements in normal thymocytes when used under similar conditions to those in Fig. 7 (data not shown), indicating that histone deacetylation is not sufficient to account for the suppression of this rearrangement in the T cell lineage.
Our data favor the hypothesis that IL-7R signals an opening of the TCR-γ locus accompanied by locus-specific demethylation, with one site being in the enhancer, which could be a major controlling region. This idea is supported by the observations that in cell lines, methylated minichromosomes show repressed VDJ recombination (56) and that in transgenic mice, methylation of the transgene represses its rearrangement (64). A number of molecular mechanisms have been suggested to participate in transcriptional repression by DNA methylation (for review see reference 55). Methylated DNA binds proteins such as MeCP2 (which is essential for embryogenesis), which can silence chromatin over a considerable distance; for example MeCP2 separated by 1,774 bp from a transcriptional start site reduced transcription >70% (65). MeCP2 is thought to mediate this repression by attracting histone deacetylase to the region (57). Our data supports the idea that IL-7 controls the acetylation status of histones resulting in “active” chromatin. To determine how rapidly IL-7 induced demethylation of the TCR-γ locus, it would be useful to treat IL-7−/− mice with IL-7, then monitor the methylation status of the locus. The alteration of chromatin by IL-7R signals may then allow access for transcription as well as cleavage by the Rag proteins. It remains to be determined which cis-acting elements in the TCR-γ locus respond to the IL-7 receptor signal, how they induce demethylation, whether this can cause specific histone acetylation of the TCR-γ locus, and whether this causes a direct increase of locus accessibility.
Acknowledgments
We are grateful to R. Wiles for technical assistance and to J.J. Oppenheim for comments on the manuscript.
Abbreviations used in this paper
- NF
nuclear factor
- TSA
trichostatin A
- TSLP
thymic stromal-derived lymphopoietin
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
A.M. Baird is a Leukemia Society Fellow for L.J. Berg, and L.J. Berg is supported by grants from the NIH and the American Cancer Society. This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. N01-C0-56000.
S. Candèias' present address is CEA-Grenoble, DBMS/ICH, INSERM U238, 17 rue des Martyrs, 38054 Grenoble cedex 9, France.
References
- 1.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peschon, J.J., B.C. Gliniak, P. Morrissey, and E. Maraskovsky. 1998. Lymphoid development and function in IL-7R deficient mice. In Cytokine Knockouts. S.K. Durum and K. Muegge, editors. Humana Press, Totowa, NJ. 37–52.
- 3.Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki J, Ikuta K. Interleukin 7 receptor-deficient mice lack γδ T cells. Proc Natl Acad Sci USA. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He YW, Malek TR. Interleukin-7 receptor α is essential for the development of γδ+T cells, but not natural killer cells. J Exp Med. 1996;184:289–293. doi: 10.1084/jem.184.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CandPias S, Muegge K, Durum SK. IL-7 receptor and VDJ recombination: trophic versus mechanistic actions. Immunity. 1997;6:501–508. doi: 10.1016/s1074-7613(00)80338-6. [DOI] [PubMed] [Google Scholar]
- 6.Kim K, Lee C-K, Sayers TJ, Muegge K, Durum SK. The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of pro-T1, T2 and T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J Immunol. 1998;160:5735–5741. [PubMed] [Google Scholar]
- 7.Maraskovsky E, O'Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/−mice. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 8.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 9.Muegge K, Vila MP, Durum SK. Interleukin-7: a cofactor for V(D)J rearrangement of the T cell receptor β gene. Science. 1993;261:93–95. doi: 10.1126/science.7686307. [DOI] [PubMed] [Google Scholar]
- 10.Crompton T, Outram SV, Buckland J, Owen MJ. A transgenic T cell receptor restores thymocyte differentiation in interleukin-7 receptor α chain-deficient mice. Eur J Immunol. 1997;27:100–104. doi: 10.1002/eji.1830270115. [DOI] [PubMed] [Google Scholar]
- 11.Corcoran AE, Smart FM, Cowling RJ, Crompton T, Owen MJ, Venkitaraman AR. The interleukin-7 receptor α chain transmits distinct signals for proliferation and differentiation during B lymphopoiesis. EMBO (Eur Mol Biol Organ) J. 1996;15:1924–1932. [PMC free article] [PubMed] [Google Scholar]
- 12.Corcoran AE, Riddell A, Krooshoop D, Venkitaraman AR. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature. 1998;391:904–907. doi: 10.1038/36122. [DOI] [PubMed] [Google Scholar]
- 13.Maki K, Sunaga S, Ikuta K. The V-J recombination of T cell receptor-γ genes is blocked in interleukin-7 receptor–deficient mice. J Exp Med. 1996;184:2423–2427. doi: 10.1084/jem.184.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CandPias S, Peschon JJ, Muegge K, Durum SK. Defective T-cell receptor γ gene rearrangement in interleukin 7 receptor knockout mice. Immunol Lett. 1997;57:9–14. doi: 10.1016/s0165-2478(97)00062-x. [DOI] [PubMed] [Google Scholar]
- 15.McBlane JF, van Gent DC, Ramsden DA, Romeo C, Cuomo CA, Gellert M, Oettinger MA. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995;83:387–395. doi: 10.1016/0092-8674(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 16.Schlissel MS, Stanhope-Baker P. Accessibility and the developmental regulation of V(D)J recombination. Semin Immunol. 1997;9:161–170. doi: 10.1006/smim.1997.0066. [DOI] [PubMed] [Google Scholar]
- 17.Lewis SM, Wu GE. The origins of V(D)J recombination. Cell. 1997;88:159–162. doi: 10.1016/s0092-8674(00)81833-4. [DOI] [PubMed] [Google Scholar]
- 18.Appasamy PM, Kenniston TW, Weng Y, Holt EC, Kost J, Chambers WH. Interleukin 7–induced expression of specific T cell receptor γ variable region genes in murine fetal liver cultures. J Exp Med. 1993;178:2201–2206. doi: 10.1084/jem.178.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bories J-C, Demengeot J, Davidson L, Alt FW. Gene targeted deletion and replacement mutations of the T-cell receptor β-chain enhancer: the role of enhancer elements in controlling V(D)J recombination accessibility. Proc Natl Acad Sci USA. 1996;93:7871–7876. doi: 10.1073/pnas.93.15.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouvier G, Watrin F, Naspetti M, Verthuy C, Naquet P, Ferrier P. Deletion of the mouse T-cell receptor β gene enhancer blocks αβ T-cell development. Proc Natl Acad Sci USA. 1996;93:7877–7881. doi: 10.1073/pnas.93.15.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serve M, Sablitzky F. V(D)J recombination in B cells is impaired but not blocked by targeted deletion of the immunoglobulin heavy chain intron enhancer. EMBO (Eur Mol Biol Organ) J. 1993;12:2321–2327. doi: 10.1002/j.1460-2075.1993.tb05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda S, Zou Y-R, Bluethmann H, Kitamura D, Muller U, Rajewsky K. Deletion of the immunoglobulin kappa chain intron enhancer abolishes kappa chain gene rearrangement in cis but not lambda chain gene rearrangement in trans. EMBO (Eur Mol Biol Organ) J. 1993;12:2329–2336. doi: 10.1002/j.1460-2075.1993.tb05887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleckman BP, Bardon CG, Ferrini R, Davidson L, Alt FW. Function of the TCR alpha enhancer in αβ and γδ T cells. Immunity. 1997;7:505–515. doi: 10.1016/s1074-7613(00)80372-6. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez JD, Anderson SJ, Loh DY. V(D)J recombination and allelic exclusion of a TCR beta-chain minilocus occurs in the absence of a functional promoter. J Immunol. 1995;155:1191–1202. [PubMed] [Google Scholar]
- 25.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 26.Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X, Leonard WJ. Interleukin-2 receptor γ chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 27.Kondo M, Takeshita T, Higuchi M, Nakamura M, Sudo T, Nishikawa S, Sugamura K. Functional participation of the IL-2 receptor gamma chain in IL-7 receptor complexes. Science. 1994;263:1453–1454. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- 28.Seckinger P, Fougereau M. Activation of src family kinases in human pre-B cells by IL-7. J Immunol. 1994;153:97–109. [PubMed] [Google Scholar]
- 29.Foxwell BM, Beadling C, Guschin D, Kerr I, Cantrell D. Interleukin-7 can induce the activation of Jak 1, Jak 3 and STAT 5 proteins in murine T cells. Eur J Immunol. 1995;25:3041–3046. doi: 10.1002/eji.1830251109. [DOI] [PubMed] [Google Scholar]
- 30.Page TH, Lali FV, Foxwell BM. Interleukin-7 activates p56lck and p59fyn, two tyrosine kinases associated with the p90 interleukin-7 receptor in primary human T cells. Eur J Immunol. 1995;25:2956–2960. doi: 10.1002/eji.1830251036. [DOI] [PubMed] [Google Scholar]
- 31.Johnston JA, Kawamura M, Kirken RA, Chen YQ, Blake TB, Shibuya K, Ortaldo JR, McVicar DW, O'Shea JJ. Phosphorylation and activation of the Jak-3 kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 32.Witthuhn BA, Silvennoinen O, Miura O, Lai KS, Cwik C, Liu ET, Ihle JN. Involvement of the Jak-3 janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 33.Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, et al. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 34.Boussiotis VA, Barber DL, Nakarai T, Freeman GJ, Gribben JG, Bernstein GM, D'Andrea AD, Ritz J, Nadler LM. Prevention of T cell anergy by signalling through the gamma c chain of the IL-2 receptor. Science. 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu ZJ, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, et al. Functional activation of Jak1 and Jak3 by selective association with the IL-2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 36.Shinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart B, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. Rag-2–deficient mice lack mature lymphocytes owing to inability to initiate V(D)J recombination. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 37.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 38.Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 40.Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986;45:733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- 41.Goldman JP, Spencer DM, Raulet DH. Ordered rearrangement of variable region genes of the T cell receptor gamma locus correlates with transcription of the unrearranged genes. J Exp Med. 1993;177:729–739. doi: 10.1084/jem.177.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hikida M, Mori M, Takai T, Tomochika K, Hamatani K, Ohmori H. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 1996;274:2092–2094. doi: 10.1126/science.274.5295.2092. [DOI] [PubMed] [Google Scholar]
- 43.Muegge K, Vila M, Gusella GL, Musso T, Herrlich P, Stein B, Durum SK. Interleukin 1 induction of the c-jun promoter. Proc Natl Acad Sci USA. 1993;90:7054–7058. doi: 10.1073/pnas.90.15.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nosaka T, van Deursen JM, Tripp RA, Thierfelder WE, Witthuhn BA, McMickle AP, Doherty PC, Grosveld GC, Ihle JN. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 45.Macci P, Villa A, Gillani S, Sacco MG, Frattini A, Porta F, Ugazio AG, Johnston JA, Candotti F, O'Shea JJ, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immunodeficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 46.Tagoh H, Kishi H, Okumura A, Kitagawa T, Nagata T, Mori K, Muraguchi A. Induction of recombination activating gene expression in a human lymphoid progenitor cell line: requirement of two separate signals from stromal cells and cytokines. Blood. 1996;89:4463–4473. [PubMed] [Google Scholar]
- 47.Hikida M, Nakayama Y, Yamashita Y, Kumazawa Y, Nishikawa SI, Ohmori H. Expression of recombination activating genes in germinal center B cells: involvement of Interleukin-7 (IL-7) and the IL-7 receptor. J Exp Med. 1998;188:365–372. doi: 10.1084/jem.188.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlissel MS, Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of kappa gene transcription. Cell. 1989;58:1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- 49.Fondell JD, Marcu KB. Transcription of germ line Vα segments correlates with ongoing T-cell receptor α-chain rearrangement. Mol Cell Biol. 1992;12:1480–1489. doi: 10.1128/mcb.12.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gotlieb WJ, Bristol LA, Weissman AM, Durum SK, Takacs L. Upregulation of T cell receptor γ chain transcription by interleukin-2. Cell Immunol. 1993;151:345–355. doi: 10.1006/cimm.1993.1244. [DOI] [PubMed] [Google Scholar]
- 51.Kappes DJ, Browne CP, Tonegawa S. Identification of a T-cell-specific enhancer at the locus encoding T-cell antigen receptor gamma chain. Proc Natl Acad Sci USA. 1991;88:2204–2208. doi: 10.1073/pnas.88.6.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer DM, Hsiang Y, Goldman JP, Raulet DH. Identification of a T-cell–specific transcriptional enhancer located 3′ of Cγ1 in the murine T-cell receptor γ locus. Proc Natl Acad Sci USA. 1991;88:800–804. doi: 10.1073/pnas.88.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 54.Hsiang YH, Goldman JP, Raulet DH. The role of c-myb or a related factor in regulating the T cell receptor γ gene enhancer. J Immunol. 1995;154:5195–5204. [PubMed] [Google Scholar]
- 55.Siegfried Z, Cedar H. DNA methylation: a molecular lock. Curr Biol. 1997;7:R305–307. doi: 10.1016/s0960-9822(06)00144-8. [DOI] [PubMed] [Google Scholar]
- 56.Hsieh C-L, Lieber MR. CpG methylated minichromosomes become inaccessible for V(D)J recombination after undergoing replication. EMBO (Eur Mol Biol Organ) J. 1992;11:315–325. doi: 10.1002/j.1460-2075.1992.tb05054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 59.Malissen, M., P. Pereira, D.J. Gerber, B. Malissen, and J. diSanto. 1997. The common cytokine receptor gamma chain controls survival of γ/δ T cells. J. Exp. Med. 186:1277–1285. [DOI] [PMC free article] [PubMed]
- 60.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene–deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Freeden-Jeffry, U., T.A. Moore, A. Zplotnik, and R. Murray. 1998. IL-7 knockout mice and the generation of lymphocytes. In Cytokine Knockouts. S.K. Durum and K. Muegge, editors. Humana Press, Totowa, NJ. 21–36.
- 62.Perumal NB, Kenniston TW, Tweardy DJ, Dyer KF, Hoffman R, Peschon J, Appasamy PM. TCR-gamma genes are rearranged but not transcribed in IL-7R alpha-deficient mice. J Immunol. 1997;158:5744–5750. [PubMed] [Google Scholar]
- 63.Teglund S, McKay C, Schuetz E, van Duersen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 64.Engler P, Weng A, Storb U. Influence of CpG methylation and target spacing on V(D)J recombination in a transgenic substrate. Mol Cell Biol. 1993;13:571–577. doi: 10.1128/mcb.13.1.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nan X, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–481. doi: 10.1016/s0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]