Abstract
The α1,3-fucosyltransferase, FucT-VII, is crucial for the formation of ligands for all three selectins, and its expression regulates the synthesis of these ligands. Short-term polarized T helper (Th)1, but not Th2 or naive CD4+ T cells, can home to sites of inflammation, but the molecular basis for this difference has remained unclear. Here we show that naive CD4+ T cells do not express FucT-VII and fail to bind vascular selectins. We also show that when CD4+ T cells are activated in the presence of the Th1 polarizing cytokine interleukin (IL)-12, levels of FucT-VII mRNA and binding to E- and P-selectin are significantly augmented. In contrast, activation of CD4+ T cells in the presence of IL-4, a Th2 polarizing cytokine, inhibited FucT-VII expression and binding to vascular selectins. T cell activation upregulated expression of the Core2 transferase, C2GnT, equivalently regardless of the presence or absence of polarizing cytokines. These data indicate that the selective ability of Th1 cells, as opposed to Th2 cells or naive CD4+ T cells, to recognize vascular selectins and home to sites of inflammation is controlled principally by the expression of a single gene, FucT-VII.
Keywords: selectin, T helper cell type 1 and T helper cell type 2, fucosyltransferase, cytokine, gene expression
The selectins are a family of carbohydrate binding adhesion molecules which are essential in the regulated recruitment of leukocytes to inflammatory lesions and in the recirculation of naive lymphocytes through secondary lymphoid organs (1). Controlled expression of vascular selectins and their ligands is critical for proper and efficient recruitment of leukocytes to sites of tissue injury and infection. All physiologic selectin ligands appear to be sialylated, fucosylated, and sometimes sulfated carbohydrate structures, the formation of which depends critically on expression of a particular α1,3-fucosyltransferase (FucT-VII)1 (2, 3). The crucial role of FucT-VII in the construction of selectin ligands is highlighted by the severely impaired selectin-dependent adhesion and in vivo leukocyte rolling of FucT-VII–deficient animals (2). Enforced expression of FucT-VII is sufficient for the generation of fully functional E-selectin ligands, and is required for binding to P-selectin (3–6). In activated T lymphocytes, expression of FucT-VII appears to directly regulate binding to E-selectin (3).
During T cell activation, differences in the cytokine environment where T cells become activated lead to differences in their differentiation program. In the presence of IL-12, T cells differentiate into Th1 effector cells, whereas in the presence of IL-4 they become Th2 cells. Recent work has suggested that Th1 and Th2 cells exhibit distinct trafficking patterns (7). T cells which were polarized in vitro into Th1 effector cells showed preferential recruitment to cutaneous inflammatory lesions, whereas Th2 cells were not recruited to these sites. This differential trafficking appeared to result from differences in expression of ligands for the vascular selectins (7, 8). However, the molecular basis for this difference has remained unknown.
Here, we provide evidence for the molecular mechanism underlying the differential migration patterns of Th1 versus Th2 and naive T cells. Naive CD4+ T cells, which do not bind to vascular selectins (9, and this report) expressed no detectable FucT-VII mRNA. Activation of T cells in the presence of IL-12, which promotes Th1 cell development, induced increased levels of FucT-VII mRNA and enhanced interactions with both E- and P-selectin. In contrast, activation of T cells in the presence of IL-4, which promotes Th2 cell development, inhibited expression of FucT-VII and binding to vascular selectins. Expression of the glycosyltransferase, core 2 β1,6-N-acetylglucosaminyltransferase (C2GnT), which is required for PSGL-1 binding to P-selectin (10, 11), was easily detectable in naive T cells and was upregulated equivalently in activated T cells regardless of which cytokines were present. These studies provide the first molecular explanation for the differences in selectin binding by Th1 and Th2 cells, and give significant insight into the mechanisms of regulation of T cell trafficking.
Materials and Methods
mAb and Cell Lines.
The mAb used in these studies were as follows: S3.1 (anti-CD4, hybridoma supernatant or FITC conjugate; produced in this laboratory); TS2/9.3.4.1 (anti–LFA-3, hybridoma supernatant; American Type Culture Collection, Rockville, MD); UCHL-1 (anti-CD45RO, purified mAb); 2H4 (anti-CD45RA, FITC conjugate; Coulter Immunology, Hialeah, FL); OKT3 (anti-CD3, purified or FITC conjugate), anti-CD28 (purified; R&D Systems Inc., Minneapolis, MN), MECA-79 (rat IgM negative control, hybridoma supernatant; American Type Culture Collection, or purified mAb; L. Picker, University of Texas, Dallas, TX); HECA-452 (anti-CLA, hybridoma supernatant; American Type Culture Collection, or purified or biotinylated mAb; L. Picker); goat anti–mouse IgM (FITC conjugate; Biosource, Camarillo, CA); goat anti–mouse IgG (FITC conjugate; Biosource International); goat anti–human IgM (PE conjugate; Sigma Chemical Co., St. Louis, MO). Chinese hamster ovary (CHO)/E-selectin and CHO/P-selectin transfectants were generated by transfection of CHO-K1 cells with linearized E- or P-selectin cDNA by electroporation as described previously (5). Stable lines expressing each selectin were selected by growth in 8 μg/ml puromycin, cloned by limiting dilution, and identified by FACS® analysis. Cloned stable transfectants were maintained in Ham's F12 medium supplemented with 10% FCS, 1% penicillin/ streptomycin, and 2 mM l-glutamine.
Isolation of Normal Human CD4+ T Cells.
Cells were isolated by standard protocols from buffy coat leukocytes obtained from healthy volunteers. PBMCs were isolated by Ficoll-Hypaque centrifugation and recovery of cells at the interface. CD4+ T cells were isolated from PBMCs by first rosetting with neuraminidase-treated sheep erythrocytes and recovering rosetted cells after red cell lysis. This T cell–enriched population was then used to isolate CD4+ T lymphocytes by positive panning with mAb against CD4. The purity of the recovered cells was assessed by flow cytometry, and was >95% for all experiments. Naive and memory CD4+ T cells were isolated by FACS® sorting of the purified CD4+ T cells after staining with FITC-conjugated UCHL-1 (anti-CD45RO), with gates set to exclude CD45RO-low cells from both populations. Alternatively, naive CD4+ T cells were isolated by positive selection on LS+ Magnetic Separation Columns (Miltenyi Biotec Inc., Auburn, CA) of purified CD4+ T cells after staining with MACS CD45RA Microbeads (Miltenyi Biotec Inc.), according to the manufacturer's protocol. Naive and memory CD4+ T cell subpopulations were >99% pure for all experiments, as confirmed by FACS® staining with FITC-conjugated 2H4 mAb (anti-CD45RA).
T Cell Activation Cultures.
For T cell activation, CD4+ T cells were activated using either 2 μg/ml PHA-P (Sigma Chemical Co.) or 100 ng/ml toxic shock syndrome toxin-1 (TSST-1) (Sigma Chemical Co.). For these experiments, cultures were initiated at 106 cells/ml, 5 ml/flask, in complete media (CM) containing 2% irradiated (1,500 rad) nonrosetting cells and 10 U/ml rhIL-2 (Biosource). Where indicated, cultures also contained 100 ng/ml rhIL-4 or 0.2 ng/ml rhIL-12. As described in the text, cells were removed at indicated time points for analysis, and media containing cytokines and stimulants were replenished as needed.
Alternatively, 24-well plates were precoated overnight at 4°C with 10 μg/ml anti-CD3 + 10 μg/ml anti-CD28 mAb in PBS. Cultures were initiated with CD4+ T cells at 2 × 106/ml (2 ml/ well) in RPMI 1640 (GIBCO BRL, Gaithersburg, MD) containing 5% FCS, 1% penicillin/streptomycin, and 2 mM l-glutamine (CM). T cells were incubated in mAb-coated wells for 48 h at 37°C (cycle 1). Where indicated, cultures also contained 100 ng/ ml rhIL-4 (Biosource) or 2 ng/ml rhIL-12 (R&D Systems), and these cytokines were present throughout the activation protocol; however, IL-2 was not present during mAb activation, and was included only during the expansion phase of the protocol, when cells were incubated without mAb (see below). After 48 h, cells were removed from mAb-coated wells, washed, and diluted to 5 × 105 cells/ml in CM containing 10 U/ml IL-2 (Biosource). IL-4 or IL-12 was also included, as indicated. Cells were expanded for 5–6 d, and the cell concentration was maintained at 5 × 105 cells/ml by diluting the cultures daily with fresh cytokine-containing media. After 5–6 d, cells were washed and reactivated for 18 h with plate-bound anti-CD3 + anti-CD28 (cycle 2), again in the absence of IL-2. After 18 h, cells were removed from plates, washed, and diluted to 5–10 × 105 cells/ml in CM containing IL-2 and IL-4 or IL-12, as indicated. Cells were again expanded in cytokine-containing media for 2–5 d, maintaining the cell concentration between 5 and 10 × 105. At the indicated time points, cells were removed from cultures for FACS® analysis, reverse transcriptase (RT)-PCR analysis, or rolling assays.
Semiquantitative RT-PCR.
Detection of FucT-VII, C2GnT, and phosphoglycerate kinase 1 (PGK1) mRNA by RT-PCR was performed as described elsewhere (5). In brief, total cellular RNA was isolated from 107 cells, and 1 μg total RNA was used as template in a 20-μl RT reaction. PCR amplification of cDNA was carried out with cycle numbers previously titered to be well below the plateau phase of amplification to give an accurate reflection of the relative starting concentration of mRNA. For PGK1 detection 25 cycles were used, for C2GnT detection 28 cycles were used, and for FucT-VII detection 33 cycles were used. Each cycle consisted of 30 s at 94°C, 30 s at 68°C, and 30 s at 72°C. For detection of FucT-VII mRNA, the sense primer 5′-CCC ACC GTG GCC CAG TAC CGC TTC T-3′ and the antisense primer 5′-CTG ACC TCT GTG CCC AGC CTC CCG T-3′ were used. For C2GnT detection, the sense primer 5′-TTT TCW GGC AGT GCC TAC TTC GTG GTC-3′ and the antisense primer 5′-ATG CTC ATC CAA ACA CTG GAT GGC AAA-3′ were used, where W = 50% A, 50% T. For PGK1 detection, the sense primer 5′-ATG ATT ATT GGT GGT GGA ATG GCT-3′ and the antisense primer 5′-TCA TCC ATG AGA GCT TTG GTT CC-3′ were used. To enhance comparisons between different groups of activated CD4+ T cells, some PCR reactions were carried out as above using twofold serial dilutions of the input cDNA. PCR products were detected by agarose gel electrophoresis and Southern blotting, as described previously (5).
Flow Cytometry.
FACS® analysis was performed as described elsewhere (5). mAbs for FACS® were used at empirically determined optimal dilutions. For detection of E- and P-selectin ligands by flow cytometry, E- and P-selectin IgM chimeras (2) were obtained from Dr. John Lowe (Howard Hughes Medical Institute, University of Michigan, Ann Arbor, MI). Selectin-IgM (RIgM) chimeras were collected from the media of COS cells transfected with chimera expression vectors, and used to detect selectin ligands on resting and activated CD4+ T cells. 5 × 105 cells were incubated in 100 μl E-RIgM supernatant (undiluted) or 5 μg/ml purified P-RIgM protein for 15 min at 4°C. Cells were washed once with 100 μl DME (GIBCO BRL) containing 1% serum and 0.1% NaN3. Pellets were resuspended in 100 μl of PE-conjugated goat anti–human IgM (Sigma Chemical Co.) diluted 1:50 in DME/1% serum, and incubated for 15 min at 4°C. Cells were again washed with 100 μl DME/1% serum/0.1% NaN3, and resuspended in this media for FACS® analysis. For all analyses, data were collected on a FACScan® or FACSCalibur® flow cytometer (Becton Dickinson, Mountain View, CA) and analyzed with CellQuest software. Histograms of relative fluorescence intensity were analyzed and data are presented as the percentage of cells staining for the indicated epitope, as determined by the position of the negative control histogram.
Low Shear Adhesion Assays.
Binding of cells to E- or P-selectin under low shear conditions was determined as described previously (4, 5). In brief, cells were allowed to adhere to COS cells transiently transfected with either E- or P-selectin with low shear generated by constant rocking. The data are presented as the mean number of cells bound per COS cell ± SEM.
In Vitro Rolling Assays.
Adhesion of cells to E- or P-selectin was determined in a parallel plate flow chamber (CytoDyne Inc., San Diego, CA) at a shear stress of 1.7 dyn/cm2. Shear stress in the chamber was controlled by regulating the fluid flow rate by adjusting the height of a raised reservoir connected to the inlet port. The required flow rate was calculated for a given shear stress by the following equation: SS = 6Qμ/bh 2, where SS = shear stress (dyn/cm2), Q = flow rate (cm3/s), μ = fluid viscosity (dyn × s/cm2), b = channel width (cm), and h = channel height (cm). Activated CD4+ T lymphocytes were perfused through the chamber at 5 × 104 cells/ml in RPMI 1640 containing 0.1% serum. Phase–contrast images of T cells rolling on monolayers of CHO cell transfectants expressing either E-selectin or P-selectin were obtained using a Nikon Diaphot 300 microscope equipped with a 10× objective and a Hamamatsu Argus 20 CCD camera and image processor, and recorded on videotape for offline analysis. Data are expressed as numbers of interacting cells/field/min, and are averaged for at least 10 different fields of view.
Statistical Analysis.
For those data which satisfied the normality test, adhesion data were analyzed by one-way ANOVA with the Bonferroni correction. For data which failed the normality test, the nonparametric Kruskall-Wallis one-way ANOVA was used, with Dunn's method for pair-wise analysis. Differences were considered statistically significant with P < 0.05.
Results and Discussion
Naive CD4+ T Cells Do Not Express Detectable FucT-VII mRNA and Fail to Bind Vascular Selectins.
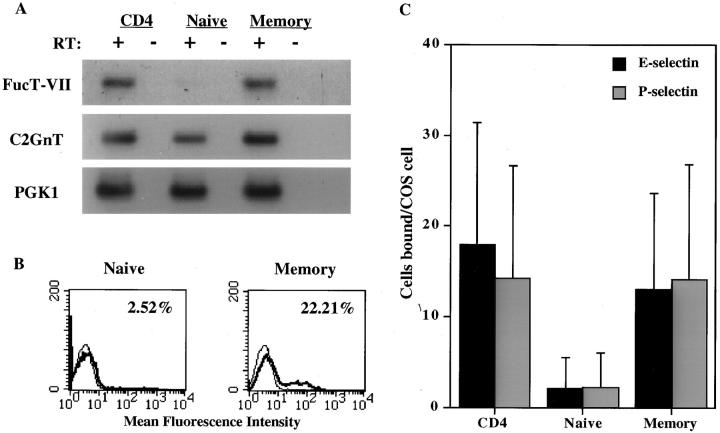
The critical role of FucT-VII in the generation of selectin ligands (2) suggested that the absence of this enzyme may represent the molecular basis for the failure of naive cells to bind to vascular selectins. Therefore, we analyzed purified unfractionated, naive (CD45RO-negative), and memory (CD45RO-high) CD4+ T cells for expression of FucT-VII mRNA by semiquantitative RT-PCR. Naive CD4+ T cells expressed no detectable FucT-VII mRNA, whereas unfractionated CD4 cells and memory T cells expressed FucT-VII mRNA at significant levels (Fig. 1 A). To further confirm the lack of FucT-VII activity in naive CD4+ T cells, we analyzed purified naive and memory cells by flow cytometry for expression of HECA-452 antigen (CLA), which identifies FucT-VII reporter epitopes whose level of expression above a certain threshold corresponds well to the level of FucT-VII expression (3, 5, 6). Analysis with HECA-452 mAb also allows estimation of FucT-VII activity on a per cell basis, whereas RT-PCR analysis represents the average of expression across an entire population. As described previously (12, 13), naive CD4+ cells failed to stain appreciably with HECA-452 mAb, whereas ∼15–20% of CD4+ memory cells were positive for this epitope (Fig. 1 B). Consistent with previous reports (9) and the absence of detectable FucT-VII (Fig. 1 A), naive CD4+ T cells also failed to interact appreciably with either E-selectin or P-selectin in a sensitive low shear assay (Fig. 1 C). In contrast, both unfractionated CD4+ cells and purified memory T cells exhibited significantly higher (P < 0.05) levels of binding to both E- and P-selectin. Thus, naive T cells do not express FucT-VII, which is sufficient to explain their inability to bind vascular selectins. These data indicate that upregulation of FucT-VII expression may be directly responsible for the acquisition of E- and P-selectin binding ability by activated and memory CD4+ T cells.
Figure 1.
Naive CD4+ T cells do not express FucT-VII and do not bind to vascular selectins. (A) Expression of FucT-VII and C2GnT mRNA by unfractionated, naive, and memory CD4+ T lymphocytes was determined by semiquantitative RT-PCR analysis, as described in Materials and Methods. RT indicates reverse transcriptase; + indicates reactions in which RT was included; − indicates control reactions in which RT was omitted. PGK1 is a housekeeping gene used as an internal control for the RT reaction. (B) Expression of HECA-452 mAb epitope by purified naive and memory CD4+ T lymphocytes was determined by FACS® analysis. Numbers in the upper right corner of each histogram indicate the percent HECA-452 positive cells in each population. (C) Binding of unfractionated, naive, and memory CD4+ T lymphocytes to COS cells transiently transfected with E-selectin (solid bars) or P-selectin (gray bars). Data are presented as mean numbers of lymphocytes bound per COS cell ± SEM.
Th1 Cells Express Much Higher Levels of FucT-VII mRNA Than Th2 Cells.
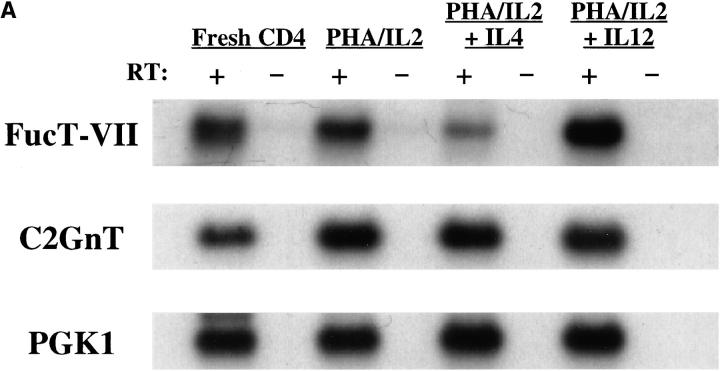
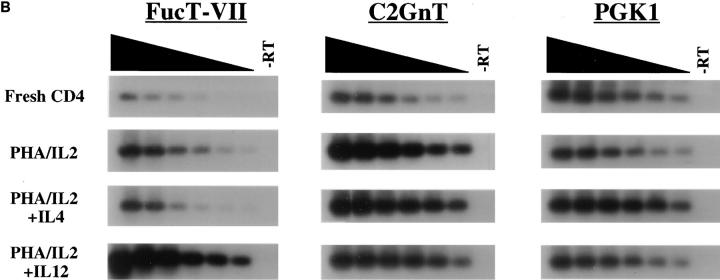
Austrup et al. recently identified a striking difference in the ability of short-term polarized Th1 versus Th2 cells to migrate to inflammatory sites in vivo (7), which they have attributed to differences in expression of selectin ligands by these cells (8). To investigate whether differences in FucT-VII expression were responsible for these differences, purified CD4+ T cells were activated with PHA in the presence of either IL-2 alone (nonpolarizing), IL-2 + IL-4 (Th2-polarizing), or IL-2 + IL-12 (Th1-polarizing), and the relative level of expression of FucT-VII mRNA was determined for each condition (Fig. 2). FucT-VII mRNA levels were higher in Th1-type cells than in freshly isolated CD4+ T cells or T cells activated with IL-2 alone. In contrast, the level of FucT-VII mRNA expressed by Th2-type cells was reduced sharply when compared with levels of FucT-VII expressed by unstimulated or IL-2 activated cells (Fig. 2 A). Detailed analysis of these samples by twofold serial dilution of the input cDNA (Fig. 2 B) indicated that Th1 cells exhibited >30-fold higher amounts of steady-state FucT-VII mRNA than Th2 cells, and >30-fold higher levels than fresh CD4+ T cells. CD4+ T cells activated with IL-2 alone showed approximately fourfold higher levels of FucT-VII than Th2 cells, and approximately four- to eightfold lower levels than Th1 cells, and reproducibly exhibited higher levels of FucT-VII mRNA than fresh, resting CD4 cells. A similar analysis after activation of CD4+ T cells with plate-bound anti-CD3/anti-CD28 revealed a similar pattern of induction of FucT-VII in Th1 cells and inhibition of FucT-VII in Th2 cells (data not shown). Thus, these data strongly support the hypothesis that IL-12 induces higher levels of FucT-VII mRNA in activated T cells, whereas IL-4 downregulates FucT-VII expression, probably at the level of transcription.
Figure 2.
Effect of Th1 or Th2 polarizing conditions on expression of FucT-VII and C2GnT mRNA. RNA was isolated from fresh CD4+ T lymphocytes or after activation with PHA and culture with IL-2 alone, IL-2 + IL-4, or IL-2 + IL-12. (A) Expression of FucT-VII and C2GnT mRNA was determined by semiquantitative RT-PCR, as in Fig. 1. (B) A more detailed analysis of FucT-VII and C2GnT expression was carried out using twofold serial dilutions of input cDNA, using the same amount of cDNA in the first (left) lane as in A. Amounts of input cDNA decrease to the left, as indicated above the blots. The lanes on the right (−RT) are the negative controls in which the RT is omitted from the RT reaction.
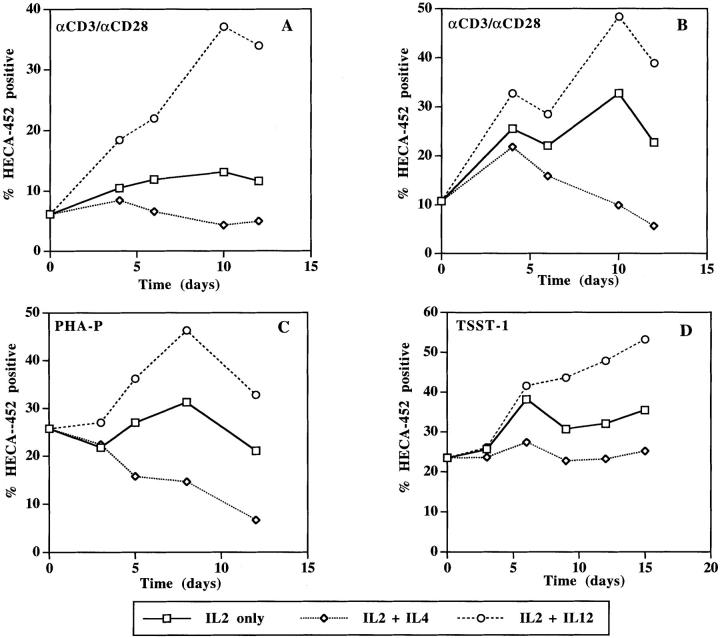
To investigate the time course of FucT-VII induction or inhibition in Th1 and Th2 polarized T cells, purified naive CD4+ T cells or unfractionated CD4+ T cells were activated in the presence of either IL-4 or IL-12, and expression of FucT-VII reporter epitopes, defined by HECA-452, was used to monitor FucT-VII activity. After activation with anti-CD3/anti-CD28, naive CD4+ T cells cultured with IL-2 alone showed little induction of HECA-452 reactivity; however, addition of IL-12 to the cultures induced approximately threefold more HECA-452 staining (Fig. 3 A). In contrast, naive CD4+ T cells cultured in the presence of IL-4 showed reduced staining with HECA-452 mAb. These Th2-type cells showed approximately two- to threefold decreased reactivity with HECA-452 as compared with cells activated in the presence of IL-2 alone, and approximately ninefold less HECA-452 staining as compared with Th1-type T cells. Th1- and Th2-polarizing cytokines had similar effects on FucT-VII activity in cultures of unfractionated CD4+ T cells, which would be expected to contain both naive and memory/activated T cell subsets. As in cultures of naive cells, CD4+ T cells activated in the presence of IL-2 alone showed some induction of HECA-452 reactivity (Fig. 3 B). Addition of IL-12 induced two- to threefold more HECA-452 staining, whereas culture with IL-4 clearly inhibited HECA-452 reactivity (approximately fourfold as compared with cells cultured with IL-2 alone). Ultimately, the opposite effects of IL-4 and IL-12 on T cell FucT-VII activity led to an approximately five- to sevenfold difference in HECA-452 reactivity in Th1- versus Th2-type T cells. Importantly, the observed differences in HECA-452 reactivity were not dependent on the type of stimulus, as a similar pattern was observed when CD4+ T cells were activated with PHA (Fig. 3 C) or with the superantigen TSST-1 (Fig. 3 D).
Figure 3.
HECA-452 reactivity of CD4+ T cells in response to varying culture conditions. HECA-452 staining of FucT-VII reporter epitopes (5) was used to assess FucT-VII activity. Naive CD4+ T cells (A) or unfractionated CD4+ T cells (B–D) were activated with either anti-CD3/anti-CD28 (A and B), PHA-P (C), or TSST-1 (D). In all panels, squares denote cultures containing IL-2 alone, diamonds denote cultures containing IL-2 + IL-4, and circles denote cultures containing IL-2 + IL-12. Data are presented as percent HECA-452 positive lymphocytes for each culture condition at the indicated time point.
C2GnT is required for leukocyte adhesion to P-selectin (10, 11). In contrast with FucT-VII, naive cells expressed easily detectable C2GnT mRNA (Fig. 1 A), and the levels of C2GnT mRNA increased equivalently upon activation, independent of which cytokines were present (Fig. 2 A). Twofold serial dilution of input cDNA revealed little if any difference in C2GnT mRNA levels between Th1, Th2, and unpolarized CD4+ T cells, but C2GnT levels were generally at least 8–16-fold higher in activated cells than in fresh CD4+ T cells (Fig. 2 B). Increased expression of C2GnT could represent a secondary mechanism for regulation of P-selectin binding in activated or memory CD4+ T cells. However, the lack of differential regulation of C2GnT expression during Th1 or Th2 differentiation underscores the critical role of FucT-VII in determining the differences in selectin binding by Th1- and Th2-type cells.
Expression of FucT-VII Directly Correlates with Binding to E- and P-selectin.
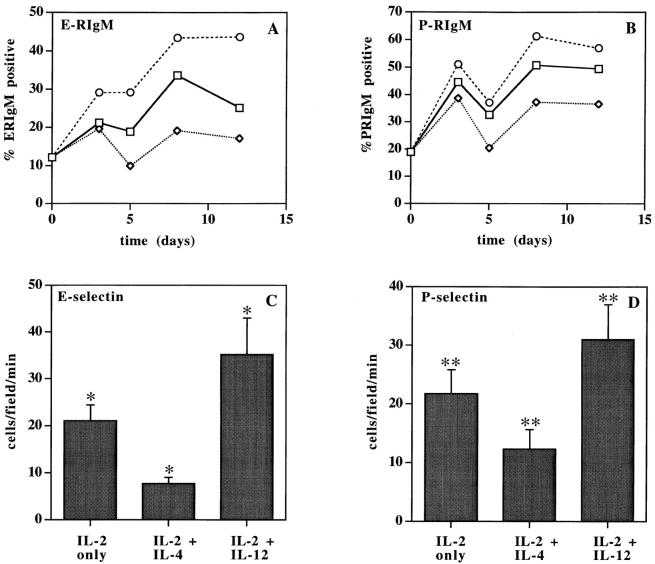
We analyzed the ability of anti-CD3/ anti-CD28 activated CD4+ T cells to interact with E- and P-selectin by FACS® analysis with RIgM chimera proteins (Fig. 4, A and B) and in a parallel plate flow chamber under defined laminar shear stress (Fig. 4, C and D). The data show a direct correlation of FucT-VII expression with the ability to bind E- and P-selectin. By FACS® analysis, staining with E-RIgM and P-RIgM chimeras paralleled HECA-452 reactivity, as Th1-type cells showed increased reactivity with both E- and P-RIgM, whereas Th2-type cells showed decreased staining with the chimera proteins (Fig. 4, A and B). Similarly, in in vitro flow assays, Th1-type cells showed significantly (P < 0.05) greater numbers of interactions with E-selectin than T cells cultured with IL-2 alone, whereas Th2-type cells showed significantly (P < 0.05) diminished rolling interactions (Fig. 4 C). Cells activated with IL-2 + IL-12 exhibited ∼4.5-fold more rolling interactions than cells activated with IL-2 + IL-4 (P < 0.05). Similar differences were observed for rolling on P-selectin (Fig. 4 D), where the differences were smaller (∼2.5-fold) but still significant (P < 0.05), consistent with the much greater sensitivity of binding to E-selectin to levels of FucT-VII (6, 14). Similar results were obtained when T cells were activated with PHA-P (data not shown). These data indicate that cytokine-directed changes in FucT-VII expression underlie the distinct adhesive capacities of Th1 and Th2 cells.
Figure 4.
Binding of Th1- or Th2-type cells to E- and P-selectin. (A and B) Expression of selectin ligands by anti-CD3/anti-CD28 activated CD4+ T lymphocytes was determined by flow cytometry using RIgM chimera proteins. Data are presented as percentage of cells staining with E-RIgM (A) or P-RIgM (B) chimera for each culture condition at the indicated time point. Squares denote cultures containing IL-2 alone, diamonds denote cultures containing IL-2 + IL-4, and circles denote cultures containing IL-2 + IL-12. (C and D) Binding of activated CD4+ T cells to vascular selectins was assessed in in vitro rolling assays at 1.7 dyn/ cm2. Cells were activated with anti-CD3/anti-CD28 and analyzed 2 d after the second activation. HECA-452 reactivity of the analyzed cells was as follows: IL-2 only, 32.7% positive; IL-2 + IL-4, 9.9% positive; IL-2 + IL-12, 48.3% positive. Data are expressed as the mean number of rolling cells/field/min ± SD for at least 10 different fields of view. (C) Rolling interactions of CD4+ T lymphocytes on CHO/E-selectin monolayers. (D) Rolling interactions of CD4+ T lymphocytes on CHO/P-selectin monolayers. (*, **) For each selectin, differences in rolling among unpolarized, Th1-, and Th2-type CD4+ T cell groups were statistically significant (P < 0.05).
Taken together, these data demonstrate a direct and significant effect of Th1 and Th2 polarizing cytokines on binding to E- and P-selectin, and indicate that this effect is mediated through changes in FucT-VII gene expression. Our findings provide a molecular basis for the ability of short-term polarized Th1 but not Th2 or naive CD4+ T cells to interact with vascular selectins and to home to sites of inflammation in vivo. Thus, IL-12, in addition to promoting the differentiation of CD4+ T cells into Th1 cells, also induces expression of FucT-VII. In contrast, IL-4, while initiating differentiation into a Th2 phenotype, inhibits expression of FucT-VII. These changes in FucT-VII coordinately affect the ability of the T cell to synthesize and express ligands for vascular selectins, and thus underlie the differences observed in Th1 and Th2 cells in binding to E- and P-selectin. Importantly, other glycosyltransferases important for selectin ligand construction, particularly C2GnT (Fig. 2, A and B) and FucT-IV (data not shown), do not appear to be differentially regulated by IL-12 and IL-4, further emphasizing the key regulatory role of FucT-VII in expression of functional selectin ligands.
Several investigators have examined the regulation of the migratory capacities of T lymphocytes as a function of activation and differentiation. In addition to differential interactions of newly polarized Th1 versus Th2 cells with E- and P-selectin (7, 8), expression of receptors for several chemokines appears to be differentially regulated among Th1 and Th2 cells (15–18). Thus, the accumulating evidence suggests that there will be several control points in determining the trafficking patterns of different subtypes of CD4+ T lymphocytes, reflecting defined steps in the multistep cascade of leukocyte–endothelial cell interactions (19). Recent evidence suggests that the effects of IL-12 in upregulating FucT-VII may be important in the regulation of selectin binding in multiple classes of leukocytes, as similar effects of IL-12 are seen in CD8+ T lymphocytes (our unpublished observations) and in NK cells (20). The requirement for FucT-VII expression in construction of selectin ligands, and thus in efficient leukocyte recruitment to peripheral inflammatory sites, makes downregulation of this enzyme's activity an attractive target for therapeutic intervention aimed at blockade of chronic inflammatory disease.
Acknowledgments
We gratefully acknowledge J. Lowe for provision of FucT-VII cDNA, M. Fukuda for provision of C2GnT cDNA, and L. Picker for provision of HECA-452 mAb.
Footnotes
G.S. Kansas is an Established Investigator of the American Heart Association; A.J. Wagers is a predoctoral fellow of the National Science Foundation. This study was supported by grants from the American Cancer Society and the National Institutes of Health.
Abbreviations used in this paper: C2GnT, core 2 β1,6-N-acetylglucosaminyltransferase; CHO, Chinese hamster ovary; CLA, HECA-452 antigen; CM, complete media; FucT-VII, α1,3-fucosyltransferase VII; PGK1, phosphoglycerate kinase 1; RIgM, selectin-IgM; TSST-1, toxic shock syndrome toxin-1.
References
- 1.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3286. [PubMed] [Google Scholar]
- 2.Maly P, Thall AD, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, et al. The α(1,3) fucosyltransferase FucT-VII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 3.Knibbs RN, Craig RA, Natsuka S, Chang A, Cameron M, Lowe JB, Stoolman LM. The fucosyltransferase FucT-VII regulates E-selectin ligand synthesis in human T cells. J Cell Biol. 1996;133:911–920. doi: 10.1083/jcb.133.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagers AJ, Lowe JB, Kansas GS. An important role for the α1,3-fucosyltransferase FucT-VII in leukocyte adhesion to E-selectin. Blood. 1996;88:2125–2132. [PubMed] [Google Scholar]
- 5.Wagers AJ, Stoolman LM, Kannagi R, Craig R, Kansas GS. Expression of leukocyte fucosyltransferases regulates binding to E-selectin. Relationship to previously implicated carbohydrate epitopes. J Immunol. 1997;159:1917–1929. [PubMed] [Google Scholar]
- 6.Snapp KR, Wagers AJ, Craig R, Stoolman LM, Kansas GS. P-selectin glycoprotein ligand-1 (PSGL-1) is essential for adhesion to P-selectin but not E-selectin in stably transfected hematopoietic cell lines. Blood. 1996;89:896–901. [PubMed] [Google Scholar]
- 7.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T helper 1 but not T helper 2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 8.Borges E, Tietz W, Steegmaier M, Moll T, Hallmann R, Hamann A, Vestweber D. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med. 1997;185:573–578. doi: 10.1084/jem.185.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtman AH, Ding H, Henault L, Vachino G, Camphausen R, Cumming D, Luscinskas FW. CD45RA− R0+ (memory) but not CD45RA+R0−(naive) T cells roll efficiently on E- and P-selectin and VCAM-1 under flow. J Immunol. 1997;158:3640–3650. [PubMed] [Google Scholar]
- 10.Li F, Wilkins PP, Crawley S, Weinstein J, Cummings RD, McEver RP. Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J Biol Chem. 1996;271:3255–3264. [PubMed] [Google Scholar]
- 11.Kumar R, Camphausen RT, Sullivan FX, Cumming DA. Core 2 β-1,6-N-acetylglucosaminyltransferase enzyme activity is critical for P-selectin glycoprotein ligand-1 binding to P-selectin. Blood. 1996;88:3872–3879. [PubMed] [Google Scholar]
- 12.Picker LJ, Kishimoto TK, Smith CW, Warnock RA, Butcher EC. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991;349:796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- 13.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LW. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte–associated antigen, a tissue- selective homing receptor for skin-homing T cells. J Immunol. 1993;150:1122–1136. [PubMed] [Google Scholar]
- 14.Knibbs, R.N., R.A. Craig, P. Maly, P.L. Smith, F.M. Wolber, N.E. Faulkner, J.B. Lowe, and L.M. Stoolman. 1998. α1,3-fucosyltransferase VII dependent synthesis of P- and E-selectin ligands on cultured T lymphoblasts. J. Immunol. In press. [PubMed]
- 15.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 16.Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D'Ambrosio D, O'Garra A, Robinson D, Rocchi M, et al. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]
- 17.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siveke JT, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160:550–554. [PubMed] [Google Scholar]
- 19.Butcher EC. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 20.Yago T, Tsukuda M, Fukushima H, Yamaoka H, Kurata-Miura K, Nishi T, Minami M. IL-12 promotes the adhesion of NK cells to endothelial selectins under flow. J Immunol. 1998;161:1140–1145. [PubMed] [Google Scholar]