Abstract
Mast cells are thought to contribute significantly to the pathology and mortality associated with anaphylaxis and other allergic disorders. However, studies using genetically mast cell–deficient WBB6F1-KitW/KitW-v and congenic wild-type (WBB6F1-+/+) mice indicate that mast cells can also promote health, by participating in natural immune responses to bacterial infection. We previously reported that repetitive administration of the c-kit ligand, stem cell factor (SCF), can increase mast cell numbers in normal mice in vivo. In vitro studies have indicated that SCF can also modulate mast cell effector function. We now report that treatment with SCF can significantly improve the survival of normal C57BL/6 mice in a model of acute bacterial peritonitis, cecal ligation and puncture (CLP). Experiments in mast cell–reconstituted WBB6F1-KitW/KitW-v mice indicate that this effect of SCF treatment reflects, at least in part, the actions of SCF on mast cells. Repetitive administration of SCF also can enhance survival in mice that genetically lack tumor necrosis factor (TNF)-α, demonstrating that the ability of SCF treatment to improve survival after CLP does not solely reflect effects of SCF on mast cell– dependent (or –independent) production of TNF-α. These findings identify c-kit and mast cells as potential therapeutic targets for enhancing innate immune responses.
Keywords: c-kit, innate immunity, mast cells, stem cell factor, tumor necrosis factor-α
Mast cells are thought of primarily as key effector cells in IgE-dependent immune responses, such as those involved in the pathogenesis of allergic disorders or in certain examples of immunity to parasites (1). However, recent work has identified another facet of mast cell effector function, the promotion of innate, or “natural”, immunity to bacterial infection (2, 3). For example, Echtenacher et al. (2) reported that genetically mast cell–deficient KitW/KitW-v mice exhibited greatly increased mortality after cecal ligation and puncture (CLP)1 compared with wild-type mice, and that the survival of KitW/KitW-v mice in this model of septic peritonitis was improved if the KitW/KitW-v mice had undergone adoptive repair of their peritoneal mast cell deficiency before CLP. The same study also showed that the mast cell–dependent protective response to CLP could be greatly diminished in mice treated with antibodies to TNF-α (2). Prodeus et al. (4) later reported that normal levels of mast cell activation and TNF-α production in this CLP model required an intact complement system, and that complement C3 or C4 knockout mice had greatly increased mortality after CLP compared with wild-type mice.
These findings indicated that a lack of mast cells, or deficits in other components of innate defense mechanisms (e.g., TNF-α, complement), can result in impaired natural immunity to bacterial infection (2–5). However, these studies did not evaluate whether, in normal animals, manipulations that can increase mast cell numbers and/or enhance mast cell function might improve the animals' ability to express innate immunity.
We therefore investigated whether repetitive administration of the c-kit ligand, stem cell factor (SCF [references 6, 7]; also known as kit ligand [reference 8], mast cell growth factor [MGF, reference 9], or steel factor [reference 10]), could influence the survival of mice subjected to CLP. By acting synergistically with other growth factors, SCF can promote the proliferation and further differentiation of hematopoietic progenitor cells; SCF is also critical for the normal development of germ cells, melanocytes, and interstitial cells of Cajal (6, 7). However, interactions between SCF and c-kit are especially important in promoting mast cell survival (11–13), proliferation (14, 15), and maturation (14, 15), and can also enhance certain mast cell effector functions (16–19). We previously reported that the daily subcutaneous administration of E. coli–derived recombinant rat SCF164 (rrSCF164) to normal mice or rats can increase mast cell numbers in many anatomical sites, including the peritoneal cavity (13, 15, 20). We now report that repetitive treatment of mice with SCF can markedly improve their survival after CLP, and that this effect of SCF treatment reflects, at least in part, its actions on mast cells.
Materials and Methods
Animals.
C57BL/6 mice, genetically mast cell–deficient WBB6F1-KitW/KitW-v (KitW/KitW-v) mice, and the congenic normal WBB6F1-+/+ (Kit +/+) mice were purchased from The Jackson Laboratory, Bar Harbor, ME. Adult KitW/KitW-v mice ordinarily contain <1.0% of the number of dermal mast cells present in the skin of the congenic normal (+/+) mice, and have no detectable mature mast cells in the gastrointestinal tract or peritoneal cavity (21–23). TNF-α−/− mice were generated by gene targeting, and TNF-α−/− and +/+ mice were maintained on a mixed 129/Sv × C57BL/6 genetic background (24). Mice were kept in community cages at the Animal Care Facilities of the Beth Israel Deaconess Medical Center or, for TNF-α−/− and +/+ mice, the GSF-Forschungszentrum, at light periods of 12 h and were fed water and mouse chow ad libitum. All animal care and experimentation was conducted in accord with current National Institutes of Health and Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee guidelines or under official permission from the Regierung der Oberpfalz.
Treatment with SCF.
Mice received 21 daily subcutaneous injections into the same area of back skin of vehicle alone (sterile 0.9% NaCl containing 0.1% BSA, fraction V, fatty acid-free [ICN Immunobiologicals, Lisle, IL]), Escherichia coli-derived recombinant rat SCF164 (rrSCF164) at 50, 100, or 200 μg/kg per day in 150–250 μl of vehicle, or rrSCF164 that had been modified by the covalent attachment of polyethylene glycol (rrSCF164-peg) to increase the biological half-life of the cytokine, at 30 or 100 μg/kg per day in 150–250 μl of vehicle (13, 15, 25). rrSCF164 and rrSCF164-peg were from AMGEN Inc. (Thousand Oaks, CA).
Peritoneal Lavage.
Mice were killed by CO2 inhalation, then the abdominal skin was washed with 70% ethanol, the peritoneum was exposed by a 1–2-cm midline abdominal incision, and 2.0 ml of sterile, pyrogen-free 0.9% NaCl and 8.0 ml of air were injected into the peritoneal cavity via a 25-gauge needle. The abdomen was massaged gently for ∼3 min and the peritoneal fluid was recovered via a 22-gauge needle, stained for mast cells by Kimura stain, and counted in a Neubauer chamber; the lavage fluid was then cytospun and stained by May Grünwald-Giemsa stain (26).
Cecal Ligation and Puncture.
CLP was performed as previously described (2, 27). In brief, mice were deeply anesthetized and the cecum was exposed by a 1–2-cm midline incision on the anterior abdomen and subjected to ligation of the distal half followed by a single puncture with a 0.7- or 0.9-mm (for TNF-α−/− or +/+ mice) needle. The cecum was then replaced into the abdomen and the wound was closed using 9-mm steel wound clips. Mice were observed for mortality at least four times daily over a period of 14 d. Mice that clearly were moribund were killed by CO2 inhalation. Some TNF-α−/− and +/+ mice were subjected to ligation of 80% of the distal cecum followed by two punctures with a 0.9-mm needle in order to induce a more severe bacterial peritonitis.
Selective Mast Cell Reconstitution of KitW/KitW-v Mice.
Kit W /KitW-v mice (male, 4–6 wk old) were repaired of their mast cell deficiency selectively and locally by the injection of growth factor– dependent bone marrow–derived cultured mast cells (BMCMCs) into the peritoneal cavity (2, 23). In brief, femoral bone marrow cells from Kit +/+ mice were maintained in vitro for ∼4 wk in IL-3–containing, Con A–stimulated mouse spleen cell–conditioned medium until mast cells represented >95% of the total cells according to staining by Giemsa (6, 16, 22). Mast cells (106 in 200 μl of Hanks' MEM containing 0.47 g/liter Pipes instead of NaHCo3 [HMEM-Pipes]) or HMEM-Pipes alone were injected intraperitoneally and mice were used for experiments, together with gender- and age-matched mast cell–deficient KitW/KitW-v and Kit +/+ mice, 4 wk after adoptive transfer of cultured mast cells. The selectivity of the repair of the mast cell deficiency of the KitW/KitW-v mice was assessed before using the mice in experiments by confirming that the adoptive transfer of BMCMCs failed to improve the recipients' anemia (6, 22, 23).
Histologic Studies.
After the mice had been killed, biopsy specimens of the back skin at SCF or vehicle injection sites were fixed in Carnoy's fixative, processed into paraffin-embedded, Alcian blue–stained sections, coded so that the observer was not aware of the identity of the individual specimens, and then examined at 400× by light microscopy to quantify mast cells per squared millimeter of dermis (15).
Statistical Analysis.
The significance of differences in the survival rates after CLP was assessed using the Mantel-Cox Logrank test. All other data were tested for statistical significance using the unpaired two-tailed Student's t test. Unless otherwise specified, all data are presented as the mean ± SEM.
Results and Discussion
Repetitive Treatment with SCF Increases Peritoneal Mast Cell Numbers and Enhances Survival after CLP in C57BL/6 Mice.
We first administered various doses of non–peg-derivatized rrSCF164 (rrSCF) or peg-derivatized rrSCF164 (rrSCF-peg), or vehicle alone subcutaneously to C57BL/6 mice daily for 21 d, then killed some of the mice for quantification of mast cells in the peritoneal cavity and in the rrSCF or vehicle cutaneous injection sites, whereas other, identically treated, mice underwent CLP; the CLP-treated mice continued to receive daily subcutaneous injections of rrSCF, rrSCF-peg, or vehicle for as long as they survived.
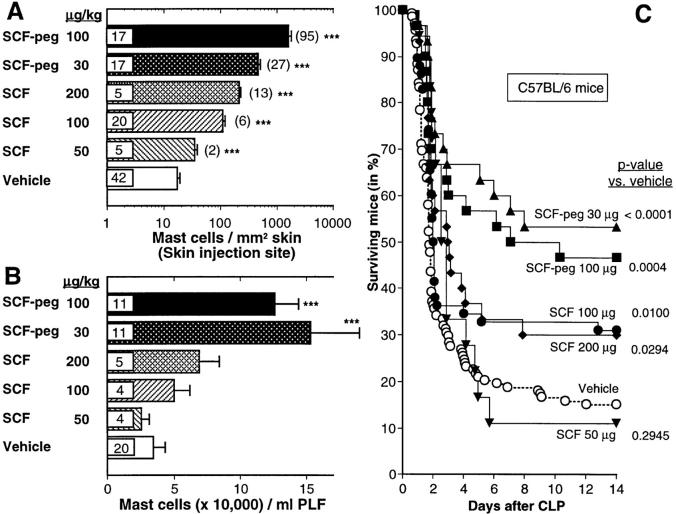
We found that rrSCF-peg was more effective than rrSCF in increasing numbers of mast cells at the cutaneous injection sites (Fig. 1 A) or in the peritoneal cavity (Fig. 1 B). Both rrSCF-peg and rrSCF exhibited a positive dose– response effect on mast cell numbers at the skin injection sites (Fig. 1 A), whereas rrSCF-peg gave high, but statistically indistinguishable, enhancement of numbers of peritoneal mast cells (PMCs) at either 30 or 100 μg/kg per day (Fig. 1 B).
Figure 1.
Long-term subcutaneous treatment of C57BL/6 mice (male, 6–9 wk old) with SCF results in increased numbers of dermal and peritoneal mast cells and enhanced survival after CLP. (A and B) Some mice were killed after the last of 21 daily subcutaneous injections with vehicle, rrSCF (50, 100, or 200 μg/kg per day) or rrSCF-peg (30 or 100 μg/kg per day) for assessment of (A) the numbers of mast cells/mm2 of dermis at the site of injections and (B) the numbers of mast cells in peritoneal lavage fluid; data in A also include values for some mice in C that died within 2 d of CLP. Numbers of mice per group are indicated inside the bars; in A, fold change (i.e., value for SCF-treated group/value for vehicle-treated group) is given in parentheses. ***P < 0.005 versus values for mice treated with vehicle alone. (C) Some mice (male, 6–9 wk old) were treated daily for 21 d before, and for 14 d or until death after, CLP (∼50% ligation, single puncture with a 0.7-mm needle); mice received subcutaneous injections of vehicle, 50, 100, or 200 μg/kg per day rrSCF, or 30 or 100 μg/kg per day rrSCF-peg (n = 138, 18, 58, 30, 30, and 30, respectively). Data were pooled from at least three independent experiments per treatment group. PLF, peritoneal lavage fluid.
Survival after CLP was significantly better in all SCF treatment groups (compared with that in the vehicle-treated group) except for the one that had been treated with rrSCF at 50 μg/kg per day (Fig. 1 C). Even mice treated with rrSCF at 100 μg/kg per day, a dose that had little or no effect on numbers (Fig. 1 A) or percentages (data not shown) of PMCs, exhibited ∼2× the survival after CLP as did vehicle-treated mice (P = 0.01). However, the best survival after CLP (∼2.5× the level in the vehicle-treated group, i.e., 53 vs. 15% survival at 14 d, P < 0.0001) was observed in mice treated with rrSCF-peg at 30 μg/kg per day (Fig. 1 C). This was also the treatment protocol that had the greatest effect on numbers of PMCs (Fig. 1 B). Accordingly, we used this dose of rrSCF-peg in the rest of our studies.
Repetitive Treatment with SCF Improves Survival after CLP in Mast Cell–reconstituted WBB6F1-KitW/KitW-v Mice, but Not in Mast Cell–deficient KitW/KitW-v Mice.
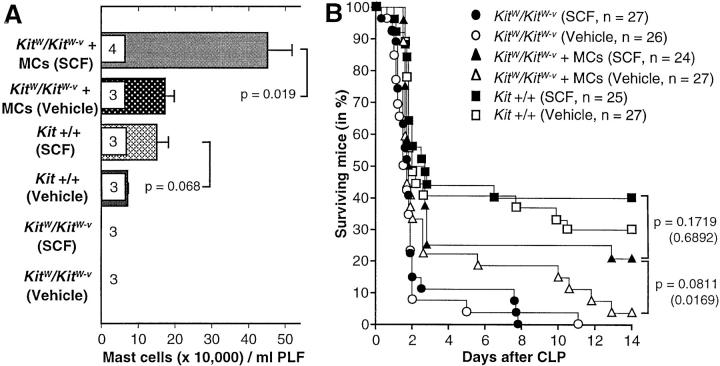
Mast cells are distinct from virtually all other hematopoietic lineages in that mature cells continue to express relatively high levels of c-kit (6, 7). Moreover, at the doses tested in this report, rrSCF has modest or no effects on numbers of circulating leukocytes in normal mice (6). Nevertheless, it is possible that some of the protective effects of SCF treatment in CLP in normal mice might reflect actions of SCF on the numbers or function of c-kit+ cell types other than mast cells. To address this possibility, we performed two experiments in which rrSCF-peg-(30 μg/kg per day) or vehicle-treated mast cell-deficient KitW/KitW-v mice were subjected to CLP after some of the mice had undergone adoptive repair of their peritoneal mast cell deficiency (i.e., +/+ BMCMC → KitW/KitW-v mice) by the intraperitoneal transfer of BMCMCs of Kit +/+ origin. The data from these two experiments, which gave very similar results, are pooled in Fig. 2.
Figure 2.
Long-term subcutaneous treatment with SCF increases the numbers of mast cells in the peritoneal lavage fluid, and survival after CLP, in wild-type Kit +/+ mice and in mast cell–reconstituted KitW/KitW-v mice, but not in mast cell–deficient KitW/KitW-v mice. (A) Some mice (male, 11–13 wk old at time of death or CLP) were killed after the last of 21 daily subcutaneous injections with vehicle or rrSCF-peg (30 μg/kg per day) for assessment of the numbers of mast cells in peritoneal lavage fluid. Numbers of mice per group are indicated inside the bars. (B) Some mice were treated daily for 21 d before, and for 14 d or until death after, CLP (∼50% ligation, single puncture with a 0.7-mm needle). P values in parentheses are for survival after day 3. Data were pooled from two independent experiments per treatment group. MC, mast cell; PLF, peritoneal lavage fluid.
SCF treatment had no significant effects on PMC numbers (Fig. 2 A) nor on overall or late (after day 3) survival after CLP (Fig. 2 B and Table 1) in KitW/KitW-v mice; these mice express kit derived from the KitW-v c-kit allele, which exhibits markedly reduced tyrosine kinase activity upon binding of SCF (28). By contrast, both overall and late survival were significantly better in SCF-treated +/+ BMCMC → KitW/KitW-v mice than in either SCF- or vehicle-treated mast cell–deficient KitW/KitW-v mice (Fig. 2 B and Table 1). Moreover, SCF treatment not only increased PMC numbers in +/+ BMCMC → KitW/KitW-v mice (by ∼160%, P = 0.019; Fig. 2 A), but also increased CLP survival in these mice (from ∼4 to ∼21% at day 14, P = 0.0811 for overall survival, P = 0.0169 for survival after day 3, versus the corresponding values in the vehicle-treated +/+ BMCMC → KitW/KitW-v mice (Fig. 2 B and Table 1).
Table 1.
Significance (P Values, Mantel-Cox Logrank Test) of Differences in Overall or Late-phase (>3 d after CLP) Survival in the Mice Shown in Fig. 2 B
| Overall survival | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KitW/KitW-v(vehicle) | KitW/KitW-v(SCF) | KitW/KitW-v+ MCs (vehicle) | KitW/KitW-v+ MCs (SCF) | Kit +/+ (vehicle) | Kit +/+ (SCF) | |||||||
| KitW/KitW-v (vehicle) | – | 0.7113 | 0.0325 | 0.0015 | 0.0001 | <0.0001 | ||||||
| KitW/KitW-v (SCF) | 0.6104* | – | 0.0634 | 0.0076 | 0.0005 | 0.0001 | ||||||
| KitW/KitW-v + MCs (vehicle) | 0.5151* | 0.0397* | – | 0.0811 | 0.0124 | 0.0029 | ||||||
| KitW/KitW-v + MCs (SCF) | 0.0084* | 0.0018* | 0.0169* | – | 0.4274 | 0.1719 | ||||||
| Kit +/+ (vehicle) | 0.0846* | 0.0015* | 0.0515* | 0.5675* | – | 0.4648 | ||||||
| Kit +/+ (SCF) | 0.0019* | 0.0023* | 0.0023* | 0.6892* | 0.3158* | – | ||||||
MCs, mast cells.
Late-phase survival (>3 d after CLP).
Because the repair of the mast cell deficiency in +/+ BMCMC → KitW/KitW-v mice is selective (2, 22, 23), the adoptively transferred mast cells are the only cellular lineage in these mice that express the wild-type kit. Accordingly, the ability of SCF treatment to enhance survival after CLP in +/+ BMCMC → KitW/KitW-v mice must reflect actions of SCF treatment on mast cells. Indeed, CLP survival in SCF-treated +/+ BMCMC → KitW/KitW-v mice was not significantly different (albeit somewhat lower) than that in SCF-treated wild-type mice (Fig. 2 B and Table 1). However, compared with results in C57BL/6 mice, treatment of Kit +/+ mice with rrSCF-peg at 30 μg/kg per day had more modest effects on both PMC numbers (compare Fig. 2 A with Fig. 1 B) and survival after CLP (compare Fig. 2 B with Fig. 1 C), perhaps reflecting strain differences in these responses to SCF treatment. Note also that despite having higher numbers of PMCs (Fig. 2 A), vehicle-treated +/+ BMCMC → KitW/KitW-v mice had significantly poorer survival 14 d after CLP than did vehicle-treated Kit +/+ mice (∼4 vs. 30%, P < 0.0001 for overall survival and P = 0.0846 for after day 3 survival). A number of factors may have contributed to this finding, including phenotypic/functional differences between the endogenous PMCs in Kit +/+ mice and the adoptively transferred, in vitro–derived mast cells in the +/+ BMCMC → KitW/ KitW-v mice (15, 23).
Repetitive Treatment with SCF Improves Survival after CLP in TNF-α− /− Mice.
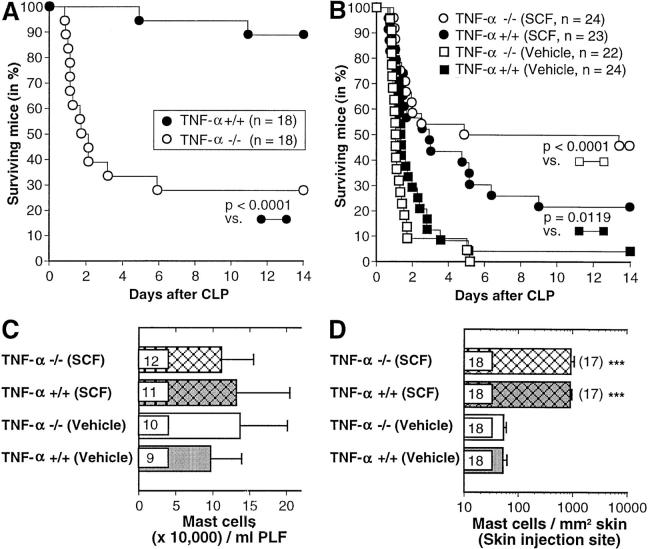
Several lines of evidence indicate that TNF-α represents one important mediator of mast cell– dependent host resistance in CLP and other models of innate immunity to bacteria (2–4). In support of this hypothesis, we found that TNF-α−/− mice exhibited significantly impaired survival after the standard CLP procedure (50% ligation, one needle puncture), in comparison to the corresponding wild-type mice (Fig. 3 A). To assess the extent to which the effects of SCF treatment on CLP survival might be TNF-α dependent, we performed two experiments in which survival after CLP was compared in vehicle- or rrSCF-peg– (30 μg/kg per day) treated TNF-α−/− or +/+ mice. Both experiments gave very similar results, which are pooled in Fig. 3, B–D.
Figure 3.
(A) Survival after CLP (∼50% ligation, single puncture with a 0.9-mm needle) in TNF-α−/− and TNF-α+/+ mice. The data were pooled from two independent experiments with male and female (1:1) mice that gave similar results, one with 9–10-wk-old mice and the other with 17–18-wk-old mice. (B) Survival after CLP (80% ligation, two punctures with a 0.9-mm needle) in TNF-α−/− and TNF-α+/+ mice that received daily subcutaneous treatment (for 21 d before CLP and then daily after CLP) with rrSCF-peg 30 μg/kg per day or vehicle. The data were pooled from two independent experiments with male and female (1:1) mice that gave similar results, one with 26–27-wk-old mice and the other with 17–18-wk-old mice. (C and D) Some TNF-α−/− and TNF-α+/+ mice were killed after the last of 21 daily injections with vehicle or rrSCF-peg (30 μg/kg per day) for assessment of (C) the numbers of mast cells in peritoneal lavage fluid or (D) the numbers of mast cells/mm2 of dermis at the site of subcutaneous injections; data in D also include values for some mice in B that died within 2 d of CLP. Numbers of mice per group (in C and D) are indicated inside the bars and, in D, the fold change versus values for the vehicle-treated group of the same genotype is given in parentheses. ***P < 0.0001 versus values for mice of the same genotype treated with vehicle alone. PLF, peritoneal lavage fluid.
These experiments used a more severe CLP procedure (80% ligation, two punctures with a 0.9-mm needle) in order to observe better any favorable effect of SCF treatment on survival. In these experiments, in contrast to those shown in Fig. 3 A, vehicle-treated TNF-α+/+ mice exhibited only marginally enhanced overall survival (P = 0.0655) compared with vehicle-treated TNF-α−/− mice (Fig. 3 B). However, SCF treatment resulted in improved survival after CLP in both TNF-α−/− mice (P < 0.0001 versus vehicle-treated TNF-α−/− mice) and TNF-α+/+ mice (P = 0.0119 versus vehicle-treated TNF-α+/+ mice). Indeed, SCF treatment had an even more striking effect on survival after CLP in TNF-α−/− mice than in the wild-type controls (Fig. 3 B). Notably, treatment with rrSCF-peg at 30 μg/kg per day did not significantly increase numbers of PMCs in TNF-α−/− or +/+ mice, possibly in part because “baseline” levels of PMCs (e.g., in vehicle-treated mice) were already substantially higher in TNF-α−/− or +/+ mice than in C57BL/6 mice (compare Fig. 3 C with Fig. 1 B). On the other hand, SCF treatment did greatly increase mast cell numbers at skin injection sites in TNF-α−/− and +/+ mice (Fig. 3 D).
Conclusions.
In C57BL/6 mice, repetitive treatment with SCF significantly enhanced survival after CLP roughly in parallel with the ability of such treatment to increase numbers of PMCs. However, improved survival after CLP was also seen in C57BL/6 mice treated with 100 μg/kg per day of non–peg-derivatized rrSCF, and in TNF-α−/− or TNF-α+/+ mice treated with 30 μg/kg per day of rrSCF-peg, even though these SCF-treated mice did not exhibit significantly increased numbers of PMCs. The latter findings strongly suggest that actions of SCF treatment other than simply the expansion of PMC numbers can contribute to the ability of this agent to enhance survival in CLP. These alternative consequences of SCF treatment in this model of innate immunity may include effects on mast cell effector function (16–20); they also may include actions of SCF on c-kit+ lineages other than mast cells. For example, the CD56bright subset of human natural killer cells expresses c-kit and can exhibit enhanced IFN-γ production in response to stimulation with SCF (29, 30); however, we have no data that would permit us to speculate about the relevance of these in vitro findings in human cells to our in vivo study in mice.
On the other hand, the experiments with +/+ BMCMC → KitW/KitW-v mice indicate that at least some of the critical effects of SCF on CLP survival can reflect actions of SCF on mast cells. Thus, KitW/KitW-v mice exhibited no protective effect of SCF treatment on survival after CLP unless the animals had first been repaired of their PMC deficiency; in this setting, only the adoptively transferred mast cells of wild-type origin expressed normal kit, and therefore could have responded normally to SCF treatment.
Our studies also indicate that treatment with SCF can enhance survival after CLP even in mice that genetically lack TNF-α, indicating that SCF treatment must be able to augment mechanisms of host defense in innate immunity that can be mobilized independently of TNF-α. Finally, in confirmation of the results of our earlier experiments with WCB6F1-+/+ mice (20), we found that mice treated with rrSCF-peg (30 μg/kg per day for 21 d) did not appear to be at substantially increased risk (versus vehicle-treated mice) for death when IgE-dependent systemic anaphylaxis was induced by intraperitoneal challenge with specific antigen (our unpublished data).
These findings are the first to show that survival in a model of innate immunity can be enhanced by treatment with SCF, a cytokine with diverse effects on mast cells, as well as many other cell types. These data are also the first to show that normal animals that have been treated to develop higher than baseline levels of mast cells can exhibit enhanced resistance to bacterial infection. Although great caution must be exercised when extrapolating from mouse studies to human medicine, our findings suggest a new approach for attempting to manage patients at risk for bacterial infection. It may be of particular interest to evaluate SCF treatment in patients with congenital or acquired immunodeficiency disorders, since such individuals have been reported to have greatly decreased numbers of mast cells in the gastrointestinal mucosa (31).
Acknowledgments
We thank Robert Parker of the Biometrics Center of the Beth Israel Deaconess Medical Center for consultation regarding the statistical analysis of the data; S. Fish, Z.-s. Wang, and H. Broszeit for technical assistance; and F.-T. Liu (La Jolla Institute of Allergy and Immunology, San Diego, CA) and D.H. Katz (Medical Biology Institute, La Jolla, CA) for H 1 DNP-ε-26 hybridoma cells.
This work was supported by United States Public Health Science Grants CA/AI72074, AI/GM23990, and 5 U19 AI41995 (Project 1) (to S.J. Galli), by a grant of the Deutsche Forschungsgemeinschaft (to M. Maurer), and by AMGEN Inc. S.J. Galli performs research funded by AMGEN Inc., and consults for AMGEN Inc., under terms that are in accord with Beth Israel Deaconess Medical Center and Harvard Medical School conflict-of-interest policies.
Abbreviations used in this paper
- BMCMCs
mouse bone marrow-derived cultured mast cells
- CLP
cecal ligation and puncture
- PMCs
peritoneal mast cells
- rrSCF
E. coli-derived recombinant rat stem cell factor164
- rrSCF-peg
polyethylene glycol-derivatized rrSCF
- SCF
stem cell factor
Footnotes
B. Echtenacher and L. Hültner contributed equally to this paper.
References
- 1.Galli, S.J., and C.S. Lantz. 1999. Allergy. In Fundamental Immunology, 4th edition. W.E. Paul, editor. Lippincott-Raven Press, Philadelphia. 1137–1184.
- 2.Echtenacher B, Männel DN, Hültner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 3.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 4.Prodeus AP, Zhou X, Maurer M, Galli SJ, Carroll MC. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 5.Galli SJ, Wershil BK. The two faces of the mast cell. Nature. 1996;381:21–22. doi: 10.1038/381021a0. [DOI] [PubMed] [Google Scholar]
- 6.Galli SJ, Zsebo KM, Geissler EN. The kit ligand, stem cell factor. Adv Immunol. 1994;55:1–96. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- 7.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 8.Huang E, Nocka K, Beier DR, Chu T-Y, Buck J, Lahm H-W, Wellner D, Leder P, Besmer P. The hematopoietic growth factor KL is encoded at the Sl locus and is the ligand of the c-kitreceptor, the gene product of W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Raugh C, March CJ, Boswell HS, Gimpel SD, Cosman D, Williams DE. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990;63:235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- 10.Witte ON. Steel locus defines new multipotent growth factor. Cell. 1990;63:5–6. doi: 10.1016/0092-8674(90)90280-r. [DOI] [PubMed] [Google Scholar]
- 11.Yee ND, Paek I, Besmer P. Role of kit-ligand in proliferation and suppression of apoptosis in mast cells: basis for radiosensitivity of White spotting and Steelmutant mice. J Exp Med. 1994;179:1777–1787. doi: 10.1084/jem.179.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mekori YA, Oh CK, Metcalfe DD. IL-3-dependent murine mast cells undergo apoptosis on removal of IL-3. Prevention of apoptosis by c-kitligand. J Immunol. 1993;151:3775–3784. [PubMed] [Google Scholar]
- 13.Iemura A, Tsai M, Ando A, Wershil BK, Galli SJ. The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am J Pathol. 1994;144:321–328. [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ. Induction of mast cell proliferation, maturation and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai M, Shih L-S, Newlands GFJ, Takeishi T, Langley KE, Zsebo KM, Miller HRP, Geissler EN, Galli SJ. The rat c-kitligand, stem cell factor, induces the development of connective tissue-type and mucosal mast cells in vivo. Analysis by anatomical distribution, histochemistry and protease phenotype. J Exp Med. 1991;174:125–131. doi: 10.1084/jem.174.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wershil BK, Tsai M, Geissler EN, Zsebo KM, Galli SJ. The rat c-kit ligand, stem cell factor, induces c-kit receptor-dependent mouse mast cell activation in vivo. Evidence that signaling through the c-kitreceptor can induce expression of cellular function. J Exp Med. 1992;175:245–255. doi: 10.1084/jem.175.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami M, Austen KF, Arm JP. The immediate phase of c-kit ligand stimulation of mouse bone marrow-derived mast cells elicits rapid leukotriene C4 generation through posttranslational activation of cytosolic phospholipase A2 and 5-lipoxygenase. J Exp Med. 1995;182:197–206. doi: 10.1084/jem.182.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu-Kuo JM, Austen KF, Katz HR. Post-transcriptional stabilization by interleukin-1β of interleukin-6 mRNA induced by c-kit ligand and interleukin-10 in mouse bone marrow-derived mast cells. J Biol Chem. 1996;271:22169–22174. doi: 10.1074/jbc.271.36.22169. [DOI] [PubMed] [Google Scholar]
- 19.Gagari E, Tsai M, Lantz CS, Fox LG, Galli SJ. Differential release of mast cell interleukin-6 via c-kit. Blood. 1997;89:2654–2663. [PubMed] [Google Scholar]
- 20.Ando A, Martin TR, Galli SJ. Effects of chronic treatment with the c-kit ligand, stem cell factor, on immunoglobulin E-dependent anaphylaxis in mice: genetically mast cell–deficient Sl/Sldmice acquire anaphylactic responsiveness, but the congenic normal mice do not exhibit augmented responses. J Clin Invest. 1993;92:1639–1649. doi: 10.1172/JCI116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura Y, Go S, Hatanaka S. Decrease of mast cells in W/Wvmice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 22.Galli SJ, Kitamura Y. Animal model of human disease. Genetically mast cell-deficient W/Wv and Sl/Sld mice: their value for the analysis of the roles of mast cells in biological responses in vivo. . Am J Pathol. 1987;127:191–198. [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, Asai H, Yonezawa T, Kitamura Y, Galli SJ. Fate of bone marrow–derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell–deficient W/Wvmice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162:1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF-α– deficient mice: a critical requirement for TNF-α in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zsebo, K.M., D.A. Williams, E.N. Geissler, V.C. Broudy, F.H. Martin, H.L. Atkins, R.-Y. Hsu, N.C. Birkett, K.H. Okino, D.C. Murdock, et al. Stem cell factor (SCF) is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 63:213–224. [DOI] [PubMed]
- 26.Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ. Mast cells can secrete VPF/VEGF and exhibit enhanced release after IgE-dependent upregulation of FcεRI expression. J Exp Med. 1998;188:1135–1146. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Echtenacher B, Falk W, Männel DN, Krammer PH. Requirement of endogenous tumor necrosis factor/ cachectin for recovery from experimental peritonitis. J Immunol. 1990;145:3762–3766. [PubMed] [Google Scholar]
- 28.Nocka K, Tan J, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and . W EMBO (Eur Mol Biol Organ) J. 1990;9:1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matos ME, Schnier GS, Beecher MS, Ashman LK, Williams DE, Caligiuri MA. Expression of a functional c-kitreceptor on a subset of natural killer cells. J Exp Med. 1993;178:1079–1084. doi: 10.1084/jem.178.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carson WE, Fehniger TS, Caligiuri MA. CD56brightnatural killer cell subsets: characterization of distinct functional responses to interleukin-2 and the c-kit ligand. Eur J Immunol. 1997;27:354–360. doi: 10.1002/eji.1830270203. [DOI] [PubMed] [Google Scholar]
- 31.Irani AA, Golzar N, DeBlois G, Elson CO, Schechter NM, Schwartz LB. Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J Immunol. 1987;138:4381–4386. [PubMed] [Google Scholar]