Abstract
Oxidized low-density lipoprotein (oxLDL) is considered one of the principal effectors of atherogenesis. To explore mechanisms by which oxLDL affects human mononuclear phagocytes, we incubated these cells in medium containing oxLDL, acetylated LDL (acLDL), or native LDL, or on surfaces coated with these native and modified lipoproteins. The presence of soluble oxLDL, acLDL, or native LDL in the medium did not stimulate H2O2 secretion by macrophages. In contrast, macrophages adherent to surfaces coated with oxLDL secreted three- to fourfold more H2O2 than macrophages adherent to surfaces coated with acLDL or native LDL. Freshly isolated blood monocytes secreted little H2O2 regardless of the substrate on which they were plated. H2O2 secretion was maximal in cells maintained for 4–6 d in culture before plating on oxLDL-coated surfaces. Fucoidan, a known ligand of class A macrophage scavenger receptors (MSR-A), significantly reduced macrophage adhesion to surfaces coated with oxLDL or acLDL. Monoclonal antibody SMO, which blocks oxLDL binding to CD36, did not inhibit adhesion of macrophages to oxLDL-coated surfaces but markedly reduced H2O2 secretion by these cells. These studies show that MSR-A is primarily responsible for adhesion of macrophages to oxLDL-coated surfaces, that CD36 signals H2O2 secretion by macrophages adherent to these surfaces, and that substrate-bound, but not soluble, oxLDL stimulates H2O2 secretion by macrophages.
Keywords: macrophages, scavenger receptors, CD36, oxidized low-density lipoproteins, H2O2 secretion
Elevated plasma levels of low-density lipoprotein (LDL) predispose to atherosclerosis (1). Since native LDL is not atherogenic, it is thought that atherogenesis results from the formation and accumulation of oxidized LDL (oxLDL)1 in the arterial intima (2). Indeed, accumulation of oxLDL (2) and of lipoprotein degradation products (3, 4) are among the first morphologically detectable changes in developing atherosclerotic lesions. Products of modified lipoproteins stimulate endothelial cells to produce monocyte chemoattractants, such as monocyte chemoattractant protein 1 (MCP-1) and Gro-1α, and these chemokines stimulate monocytes to adhere to, and to enter, the subendothelial space (5, 6). There, the monocytes mature into macrophages, take up lipids, and become foam cells. Monocytes, macrophages, and foam cells together comprise a majority of cells in fatty streaks, which are thought to be the precursors of atherosclerotic plaques (7).
OxLDL interacts with monocytes and macrophages via class A and class B scavenger receptors (MSR-A and MSR-B [8–11]). MSR-A are homotrimeric proteins. There are two types of MSR-A. Type I receptors contain a cysteine-rich domain at their COOH (exofacial) terminus, whereas type II receptors do not. Both type I and II class A receptors mediate endocytosis of acetylated LDL (acLDL) and oxLDL (8).
Macrophages also express MSR-B. MSR-B are a structurally heterogeneous family of proteins that includes CD36 (11). In addition to macrophages, CD36 is found on platelets (12) and microvascular endothelial cells in some, but not all, organs (13, 14). CD36 mediates endocytosis of oxLDL but not of acLDL (15).
Monocytes from humans genetically deficient in CD36 have a decreased capacity to take up oxLDL (16). Mice with targeted disruption of MSR-A and of apolipoprotein E genes develop smaller atherosclerotic lesions than wild-type mice (17). These findings implicate both MSR-A and MSR-B in the metabolism of modified lipoproteins and link MSR-A to the pathogenesis of atherosclerosis.
Monocytes and macrophages secrete reactive oxygen species when they adhere via MSR-A to surfaces bearing fibrillar β-amyloid, a ligand for these receptors (18). H2O2 secreted by these cells could oxidize LDL trapped in the subendothelium, and both oxLDL and H2O2 have been shown to affect gene expression in macrophages (19, 20), smooth muscle cells (21), and endothelial cells (22). We have examined the effects of soluble and substrate-bound oxLDL, acLDL, and native LDL on monocyte/macrophage adhesion, chemotaxis through Matrigel, and secretion of H2O2, and the effects of antibodies that block the ligand-binding domain of CD36 on macrophage H2O2 secretion. Our findings extend those reported previously (23) by comparing the effects of soluble versus substrate-bound oxLDL, acLDL, and native LDL on macrophage H2O2 secretion.
Materials and Methods
Materials.
Krebs Ringer buffer with glucose (KRB-G) contained 145 mM NaCl, 4.86 mM KCl, 0.45 mM CaCl2, 1.22 mM MgSO4, 5.7 mM Na2HPO4, and 5.5 mM glucose, pH 7.35. KRBG-A was KRB-G with 1 mg/ml BSA (Sigma Chemical Co., St. Louis, MO). PD buffer was as described (24). 5 mM EDTA (Sigma Chemical Co.) was added to buffers in adhesion experiments. Catalase and anti–human CD36 mAb (SMO) were from Sigma Chemical Co. Control antibody, MOPC104E (mouse IgM γ light chains), was from Organon Teknika Corp., Cappel Research Products (Durham, NC). Human LDL (density = 1.019–1.063 g/ml), DiI-labeled LDL, acLDL, and DiI-labeled acLDL were from Intracel, Inc. (Issaquah, WA). OxLDL and DiI-labeled oxLDL were prepared by incubating equal volumes of EDTA-free LDL or DiI-labeled LDL in 50 μM CuSO4 for 12 h at 37°C. OxLDL and DiI-oxLDL were dialyzed for 2 or more hours versus sterile H2O to remove CuSO4, and stored in the dark at 4°C for up to 4 wk before use. Calcein/AM and Pluronic F-127 were from Molecular Probes, Inc. (Eugene, OR). Fetal bovine serum was from GIBCO BRL (Gaithersburg, MD).
Macrophages.
Fresh buffy coats from healthy human volunteers were obtained from the New York Blood Center. The buffy coat was centrifuged on Ficoll-Hypaque (density 1.077; Sigma Chemical Co.) at 400 g for 30 min at room temperature. The major band, containing the mononuclear cells, was harvested, and the mononuclear cells were washed by centrifugation three times using RPMI 1640 supplemented with l-glutamine (Fisher Scientific Co., Springfield, NJ), then resuspended in RPMI complete medium (RPMI 1640 supplemented with 30% heat-inactivated [56°C for 30 min] human ultra serum [Gemini Bio-Products, Inc., Calabasas, CA], 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate [Celox Laboratories, St. Paul, MN]). 2 × 107 cells were placed into each 75-cm2 tissue culture flask (Corning Inc., Corning, NY) and incubated for 2 h at 37°C in an atmosphere containing 5% CO2 and 95% air. Nonadherent cells were removed by washing, and the cell layer was incubated in RPMI complete medium for 10 h at 37°C, at which time the medium was removed and the macrophages were detached by washing with PD buffer (24) supplemented with 5 mM EDTA. The cells were centrifuged, resuspended in RPMI complete medium, and incubated in teflon beakers at 37°C in a 5% CO2/95% air atmosphere until used for experiments. Cell viability was checked before each experiment by trypan blue exclusion and was always >95%.
Preparation of Lipoprotein-coated Surfaces.
Wells of a 96-well flat-bottomed microtiter plate were coated with either oxLDL, acLDL, or native LDL by adding 50 μl of H2O containing these lipoproteins in amounts indicated in the figure legends, and allowing the water to evaporate at room temperature for ∼12 h in a sterile hood. In all experiments, at least three wells were coated with the indicated lipoprotein preparation for each time point assayed. To determine the stability of adhesion of these lipoproteins to the surface of the wells, multiple wells of a 96-well microtiter plate were precoated with 100 μl of H2O containing 10 μg of DiI- oxLDL, DiI-acLDL, or DiI-LDL, and allowed to dry for ∼12 h. Each well was filled with 100 μl of KRBG-A, the plates were incubated for 10 min to 4 h at 37°C, and the KRBG-A in each well was collected and assayed fluorometrically on a Cytofluor II cell plate reader (25) to determine their content of DiI-oxLDL, DiI-acLDL, or DiI-LDL. No significant DiI fluorescence above background was observed in any of these samples, indicating that the DiI-labeled lipoproteins did not elute from the surface of the wells.
Chemotaxis.
Chemotaxis was assayed as described (26).
Adhesion Assay.
Macrophages, cultured for the periods indicated in the figure legends, were washed, labeled with calcein/ AM for 40 min as described (27), washed three times with KRBG-A, and resuspended at 2.5 × 105 cells/ml in KRBG-A containing 5 mM EDTA, and 100 μl of this suspension was added to each well of a 96-well flat-bottomed microtiter plate (Falcon; Becton Dickinson Labware, Lincoln Park, NJ) which had been precoated with 5 μg oxLDL, acLDL, or native LDL per well, as described above. Cells were allowed to adhere for 90 min at 37°C, washed twice gently with KRBG-A, and lysed by incubation in 100 μl KRBG-A plus 10 μl of saponin solution (25 mg/ ml; Sigma Chemical Co.) for 40 min at room temperature. Calcein fluorescence was read using a Cytofluor II cell plate reader as described (27). In each experiment, we developed a standard curve relating the number of adherent cells to calcein fluorescence by plating varying numbers of calcein-labeled macrophages in triplicate on uncoated 96-well microtiter plates for 90 min at 37°C. The values obtained from these standard curves were used to calculate the number of cells that adhered under each experimental condition. Control experiments showed that 17% of the calcein was released from monocytes/macrophages into the medium after 90 min at 37°C. This percentage of dye release was taken into account in calculating the total number of adherent cells.
H2O2 Secretion.
We used the method of De la Harpe and Nathan (25) to measure H2O2 secretion. Scopoletin (Sigma Chemical Co.) was dissolved at 1 mM in KRB and stored at 4°C. Horseradish peroxidase (HRP; Sigma Chemical Co.) was dissolved in PD at 1,000 U/ml and stored at −20°C. Immediately before use, KRBG-A containing 20 μM scopoletin and 2 U/ml HRP (KRBG-A, scopoletin/HRP) was prepared. Macrophages were pelleted by centrifugation, resuspended at 2 × 106 macrophages per ml in KRBG-A, scopoletin/HRP, and 100 μl of this suspension was placed into each well of a 96-well microtiter plate precoated as described above with oxLDL, acLDL, or native LDL. For experiments using control or anti-CD36 antibodies, macrophages were preincubated for 10 min at room temperature with the indicated antibody concentration in KRBG-A. The cells were then added to wells coated with the indicated lipoprotein preparation, and a sufficient volume of the scopoletin and HRP stock solutions was added to bring the final concentrations of these substances to 20 μM and 2 U/ml, respectively. In experiments comparing effects of soluble versus surface-bound lipoproteins, cells were added to uncoated wells ∼5 min before adding the soluble lipoproteins. All experiments were done in triplicate and repeated the number of times indicated in the figure legends using macrophages from different donors.
Results
Effects of Unmodified and Chemically Modified LDL on Adhesion and Migration of Monocyte-derived Macrophages.
In general, monocytes that enter the subendothelial space of the arterial intima do not become resident there. Rather, they continue to migrate through the vessel wall. Thus, the accumulation of monocytes in the subendothelium, a distinguishing feature of nascent atherosclerotic lesions, reflects a change in the usual behavior of these cells. Schmidt et al. (28) and El Khoury et al. (18) reported that monocytes become sessile when they encounter extracellular matrix proteins bearing ligands (e.g., glycation products [28] and β-amyloid fibrils [18], respectively) that bind to monocyte plasma membrane receptors. Since oxLDL is a ligand for both MSR-A and CD36, we reasoned that the presence of oxLDL particles in the subendothelium might trap mononuclear phagocytes at these sites. To test this idea, we plated macrophages on surfaces coated with native LDL, acLDL, or oxLDL and measured the number of cells that adhered to these surfaces 90 min later.
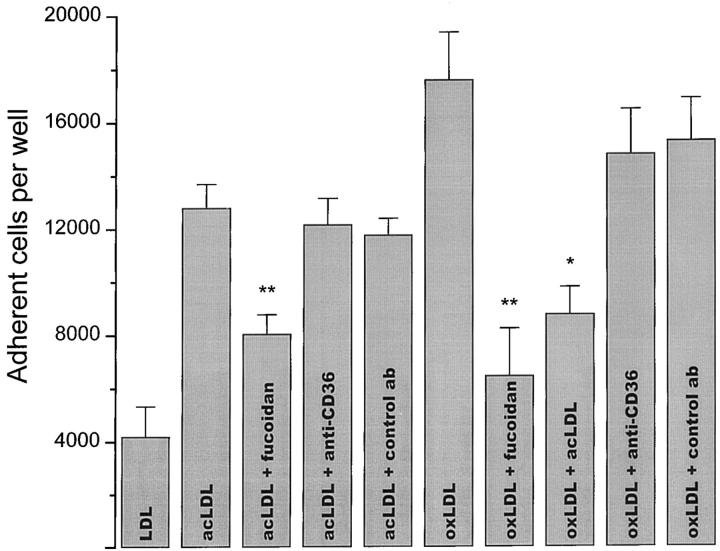
Unlike integrins, scavenger receptors do not require divalent cations to bind their ligands. Therefore, to distinguish integrin receptor–mediated adhesion from scavenger receptor– mediated adhesion, we plated macrophages on lipoprotein-coated surfaces in divalent cation–deficient medium containing 5 mM EDTA (18). Monocyte-derived macrophages adhered most efficiently to surfaces coated with oxLDL. About 25 and 75% fewer macrophages adhered to surfaces coated with acLDL or native LDL, respectively (Fig. 1). The presence of divalent cations did not significantly enhance macrophage adhesion to oxLDL- or acLDL-coated surfaces (data not shown). These experiments indicate that as observed previously with fibrillar β-amyloid–coated surfaces (18), integrins are not required for macrophage adhesion to oxLDL- or acLDL-coated surfaces. In contrast, addition of fucoidan, a polysaccharide ligand that competes for the ligand-binding domain of class A scavenger receptors (9, 18), blocked adhesion of macrophages to oxLDL- or acLDL-coated surfaces by ∼62 and ∼38%, respectively (Fig. 1). The addition of 50 μg/ml of soluble acLDL, a ligand for class A scavenger receptors (9), reduced adhesion of macrophages to oxLDL-coated surfaces by ∼50% (Fig. 1). Macrophage adhesion to surfaces coated with either oxLDL or acLDL was unaffected by mAb SMO, which specifically blocks the ligand-binding domain of CD36 (29), or by a control murine antibody of the same immunoglobulin class (IgM) as SMO. These results show that integrins and CD36 play an insignificant role in adhesion of macrophages to oxLDL- or acLDL-coated surfaces in the absence of divalent cations. They strongly suggest that class A scavenger receptors are the principal receptors responsible for adhesion of macrophages to oxLDL- or acLDL-coated surfaces.
Figure 1.
Effects of surface-bound native or modified LDL on macrophage adhesion. Human monocyte–derived macrophages (2.5 × 104 cells in 100 μl KRBG-A) that had been cultured for 5 d were added to each well of a 96-well plate, which had been precoated with 5 μg of native, acLDL, or oxLDL per well as described in Materials and Methods. Cells were allowed to adhere for 90 min at 37°C in the presence of KRBG-A plus 5 mM EDTA alone or in KRBG-A plus 5 mM EDTA containing anti-CD36 mAb SMO (20 μg/ml), control antibody MOPC104E (20 μg/ml), or fucoidan (500 μg/ml). The number of adherent cells was determined as a function of fluorescence intensity as described in Materials and Methods. The data presented are the average ± SEM of six experiments done in quadruplicate. Using a Student's two-paired t test, statistical significance at the level of **P < 0.001 was obtained for fucoidan inhibition of adhesion to acLDL- or oxLDL-coated surfaces. Statistical significance at the level of *P < 0.05 was obtained for acLDL inhibition of adhesion to acLDL- and oxLDL-coated surfaces. Macrophage plating efficiency on native LDL–, acLDL-, and oxLDL-coated surfaces was 16, 50, and 70%, respectively.
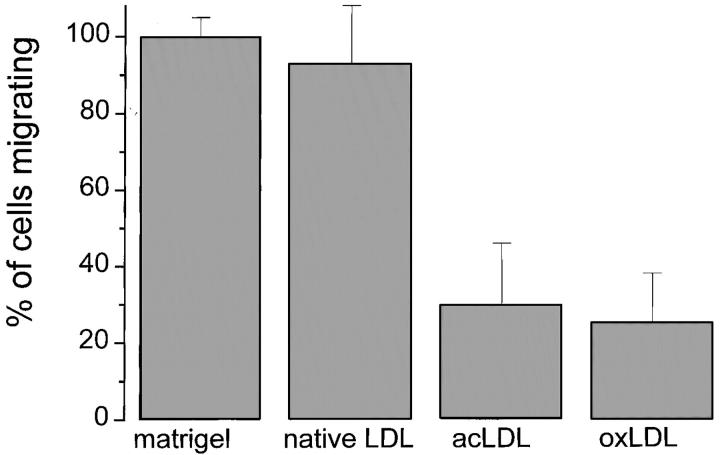
To determine whether the presence of oxLDL or acLDL affects chemotaxis of mononuclear phagocytes, we used chemotaxis chambers coated with Matrigel (26, 30) containing oxLDL, acLDL, or native LDL. Monocytes, cultured for 24 h, were placed in each chamber's upper compartment, the chemoattractant FMLP was added to each chamber's lower compartment, and the chambers were incubated at 37°C for 24 h. We then measured the number of cells that migrated into the lower compartment. Only 30% as many macrophages migrated into the lower chamber through Matrigel containing oxLDL or acLDL as through Matrigel containing native LDL. Roughly equal numbers of mononuclear phagocytes migrated into the lower chamber through Matrigel and through Matrigel containing native LDL (Fig. 2). Thus, the presence of oxLDL or acLDL markedly inhibited macrophage chemotaxis through a three- dimensional matrix containing basement membrane proteins.
Figure 2.
Effects of matrix-bound native LDL, acLDL, or oxLDL on chemotaxis of mononuclear phagocytes. Mononuclear phagocytes, isolated and maintained in culture for 24 h as described in Materials and Methods, were suspended at 2.5 × 106/ml in RPMI containing 0.1% human serum albumin (RPMI-A). 500 μl of this suspension was placed into the upper chamber of each chemotaxis insert precoated with Matrigel and, where indicated, 50 μg of native LDL, oxLDL, or acLDL. The bottom chamber was filled with RPMI-A containing the chemoattractant FMLP (10−6 M), and the chambers were incubated at 37°C for 24 h. The number of cells in the bottom chamber was assayed as described (reference 26). The data presented are the average ± SEM of two experiments done in triplicate.
Quinn et al. (31) reported that lipids, extracted from oxLDL, block monocyte chemotaxis. Since acLDL was as effective as oxLDL in blocking chemotaxis (Fig. 2), it seems unlikely that oxidized lipids were responsible for the inhibition of chemotaxis we observed in these experiments.
Effect of Soluble versus Surface-bound Native and Modified LDL on H2O2 Secretion by Monocyte-derived Macrophages.
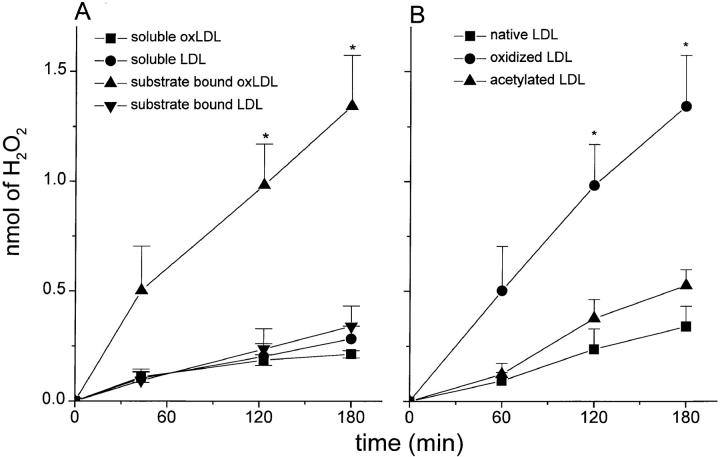
Products of oxidatively modified LDL have been identified in the matrix and within foam cells in atherosclerotic lesions (32, 33). Although several pathways for LDL oxidation in vivo have been suggested (34–39), the precise mechanisms by which LDL becomes oxidized are unresolved. Montgomery et al. (23) reported that neither soluble acLDL nor soluble oxLDL stimulate H2O2 secretion by macrophages. We have confirmed this result (Fig. 3 A [soluble oxLDL], and data not shown [acLDL]). To test whether the presence of native or modified lipoproteins on surfaces has a different effect than soluble lipoproteins on H2O2 secretion by macrophages, we compared H2O2 secretion by macrophages plated on surfaces coated with oxLDL, acLDL, or native LDL versus macrophages adherent to albumin-coated surfaces and incubated in medium containing the same amount of soluble oxLDL, acLDL, or native LDL as contained on the substrate. Macrophages adherent to surfaces coated with 10 μg oxLDL secreted approximately fourfold more H2O2 than macrophages adherent to surfaces coated with albumin and incubated in medium containing 10 μg of soluble oxLDL (Fig. 3 A). Macrophages plated on surfaces coated with native LDL (Fig. 3, A and B) or with acLDL (Fig. 3 B) produced only 25% of the amount of H2O2 as macrophages plated on surfaces coated with oxLDL (Fig. 3 B). There were no significant differences in the rates or amounts of H2O2 secreted by macrophages plated on acLDL-coated surfaces versus native LDL-coated surfaces (Fig. 3 B), even though approximately threefold more macrophages adhered to surfaces coated with acLDL than with native LDL (Fig. 1). We derive two conclusions from these experiments: first, surface-bound acLDL does not stimulate macrophages to secrete H2O2; and second, surface-bound, but not soluble, oxLDL stimulates macrophages to secrete H2O2.
Figure 3.
Surface-bound, but not soluble, oxLDL stimulates H2O2 secretion by macrophages. (A) Macrophages (2 × 105 cells in 100 μl KRBG-A), 4–7 d in culture, were plated and maintained in each well of 96-well microtiter plates for up to 3 h at 37°C in KRBG-A containing scopoletin/HRP. Each well was precoated with 10 μg of oxLDL or native LDL, or the medium contained 10 μg/100 μl oxLDL or native LDL. H2O2 was measured as described in Materials and Methods. Data reported are the average ± SEM for H2O2 secreted in three experiments done in triplicate. Statistical significance at the levels of *P < 0.05 using Student's two-paired t test in comparing substrate-bound oxLDL with soluble LDL. (B) Macrophages (2 × 105 cells in 100 μl KRBG-A), 3–7 d in culture, were plated and maintained for up to 3 h at 37°C in each well of 96-well microtiter plates in KRBG-A containing scopoletin/HRP. Wells were precoated as indicated with 10 μg of native LDL (squares), oxLDL (circles), or acLDL (triangles), as described in Materials and Methods. H2O2 was measured as in A and is the average ± SEM of three experiments, each done in triplicate. Statistical significance at the level of *P < 0.05 using Student's two-paired t test in comparing oxLDL with either native LDL or acLDL.
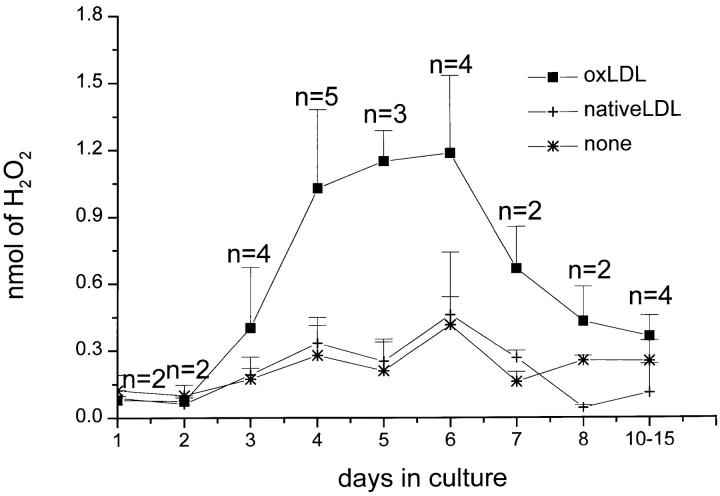
Effects of Amounts of Substrate-adherent OxLDL and of Monocyte Maturation on H2O2 Secretion.
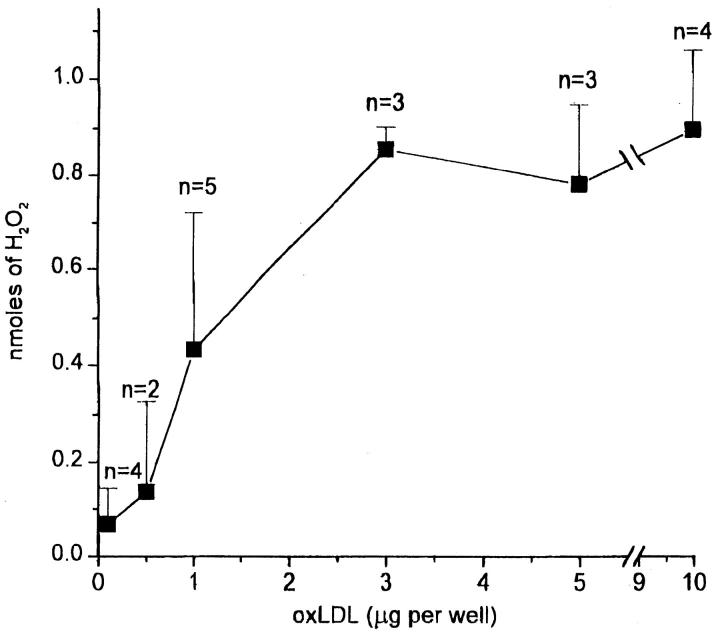
To determine whether the amount of oxLDL on the substrate affected H2O2 secretion, we plated macrophages on surfaces incubated with 0.1–10 μg oxLDL. Surfaces coated with >3 μg oxLDL induced maximal H2O2 secretion (Fig. 4). Surfaces coated with ∼1 μg oxLDL stimulated ∼50% maximal H2O2 secretion (Fig. 4). The amount of H2O2 secreted by macrophages plated on surfaces incubated with <0.5 μg oxLDL was not significantly different from the amount secreted by macrophages plated on surfaces coated with native LDL or acLDL (compare Fig. 4 with Fig. 3, A and B, and Fig. 5).
Figure 4.
Effect of amount of surface-bound oxLDL on H2O2 secretion by macrophages. Macrophages (2 × 105 in 100 μl KRBG-A), 4–7 d in culture, were plated and incubated for 2 h at 37°C in each well of 96-well microtiter plates precoated as indicated with 0.1–10 μg oxLDL. H2O2 secretion was measured using scopoletin/HRP, as described for Fig. 3 A. Each point is the average ± SEM nmoles H2O2 secreted in two to five experiments, each experiment done in triplicate.
Figure 5.
Effect of in vitro maturation of mononuclear phagocytes on H2O2 secretion stimulated by substrate-bound oxLDL or native LDL. Macrophages, 0–15 d in culture, were plated and incubated for 2 h at 37°C in 96-well flat-bottomed microtiter plates precoated with 5–10 μg of oxLDL or native LDL, and H2O2 secretion was measured, all as described for Fig. 3 A. Data are the average ± SEM of two to five experiments done in triplicate.
Monocytes plated on acLDL- (not shown), oxLDL-, or native LDL–coated surfaces immediately after isolation, and macrophages derived from monocytes maintained in culture for 1–2 d, secreted very little H2O2 when plated on native LDL– or oxLDL-coated surfaces (Fig. 5). As expected (18, 40), monocytes cultured for 1 d secreted substantial H2O2 in response to zymosan or phorbol esters (data not shown), confirming that these cells were capable of H2O2 secretion when suitably stimulated.
Monocytes that had matured in culture for 4–6 d before plating secreted approximately three- to fourfold more H2O2 when plated on surfaces coated with oxLDL than on uncoated surfaces or on surfaces coated with native LDL (Fig. 5). Macrophages cultured for 7 d or more before plating on oxLDL-coated surfaces secreted less H2O2 according to the length of time beyond 7 d they were maintained in culture before plating (Fig. 5).
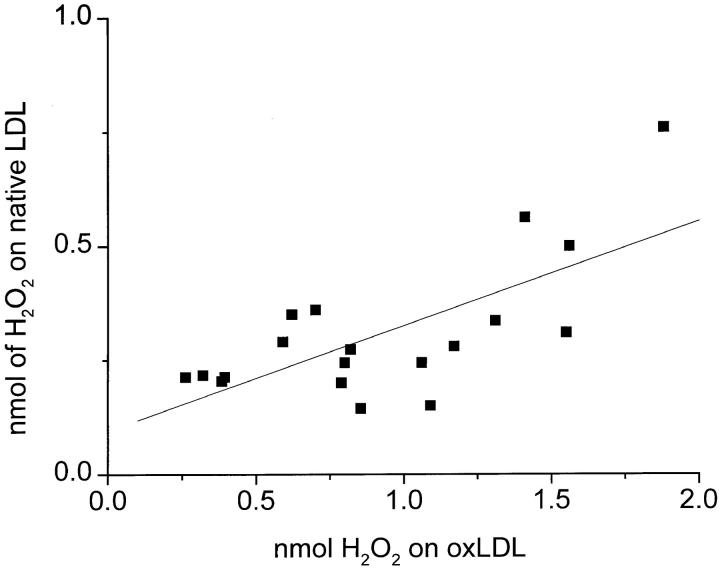
Variations in the Amounts of H2O2 Secreted by Macrophages from Different Donors.
As noted by Nakagawara et al. (40), there was considerable variation in the absolute amount of H2O2 secreted by mononuclear phagocytes from different donors. To confirm that the results reported above were indeed due to the effects of oxLDL-coated surfaces and not to chance variations in H2O2 secretory capacity of cells from different donors, we compared H2O2 secreted by macrophages from 19 different donors when plated on surfaces coated with native LDL versus oxLDL. As shown in Fig. 6, regardless of the absolute amount of H2O2 secreted by macrophages from a specific donor, macrophages from that donor consistently secreted approximately threefold more H2O2 when plated on surfaces coated with oxLDL than on surfaces coated with native LDL. Thus, the variation in absolute amounts of H2O2 secreted by cells from different donors appears to reflect intrinsic differences in the H2O2 secretory capacity of these cells and does not affect the conclusion that surfaces coated with oxLDL stimulate significantly greater macrophage H2O2 secretion than surfaces coated with native LDL.
Figure 6.
Variation in H2O2 secretion of macrophages from different donors plated on surfaces coated with oxLDL versus native LDL. Macrophages (2 × 105 in KRBG-A), 3–7 d in culture, were plated and incubated for 2 h at 37°C in 96-well microtiter plates precoated with 10 μg of oxLDL or native LDL. Each point is the average nmoles H2O2 secreted in 19 experiments done in triplicate. The correlation line was calculated using the Origin 4.10 linear regression program.
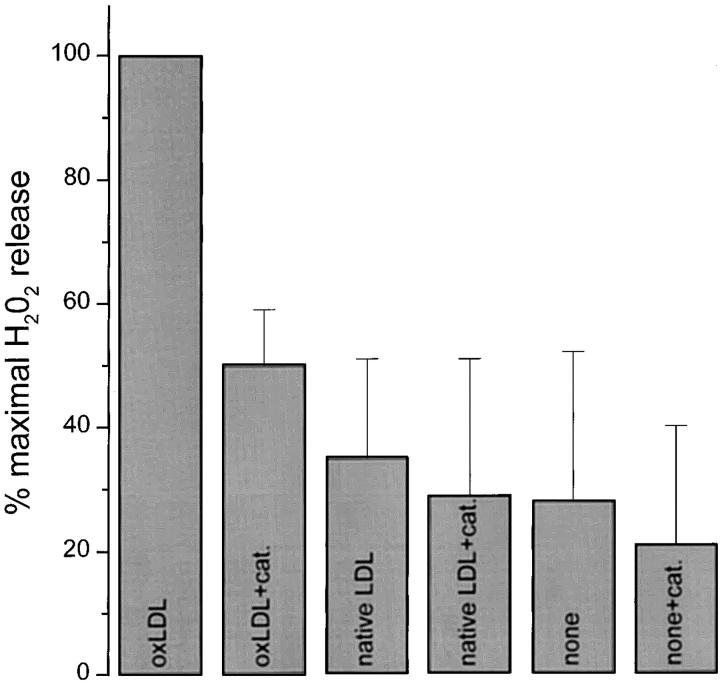
Effects of Catalase and Anti-CD36 on Scopoletin Oxidation by H2O2 Secreted by Macrophages Adherent to OxLDL-coated Surfaces.
Addition of catalase to the medium of macrophages plated on surfaces coated with oxLDL reduced scopoletin oxidation by ∼50% (Fig. 7). Catalase lowered by ∼20% the small amount of scopoletin oxidized by macrophages plated on uncoated surfaces, native LDL–coated (Fig. 7), or acLDL-coated surfaces (not shown). In other control experiments, catalase inhibited scopoletin oxidation by PMA-stimulated macrophages by ∼50% (not shown). Thus, catalase inhibited scopoletin oxidation to a similar extent, regardless of whether the initiating stimulus was surface-bound oxLDL or phorbol ester. These experiments confirm that the major proportion of scopoletin oxidized by macrophages plated on oxLDL-coated surfaces is due to H2O2 secreted by these cells.
Figure 7.
Effect of catalase on detection of H2O2 secreted by macrophages on oxLDL-coated surfaces. Macrophages (2 × 105 cells in 100 μl KRBG-A), cultured for 3–5 d, were plated on precoated surfaces and incubated in KRBG-A containing scopoletin/HRP and 1,800 U/ml catalase (cat.) for 2 h at 37°C. Each well of a 96-well microtiter plate was precoated with 10 μg of native LDL or oxLDL, as indicated. H2O2 was measured as described for Fig. 3 A. Each point is the mean ± SEM nmoles H2O2 from four experiments, each done in triplicate.
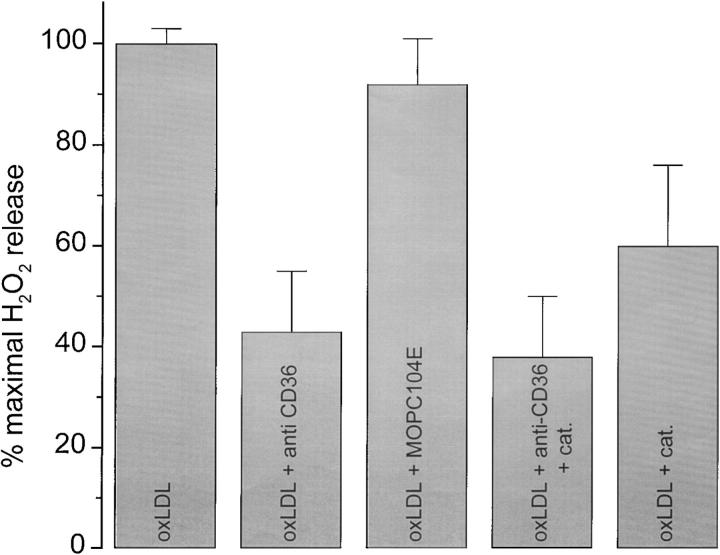
MSR-A is expressed by freshly explanted monocytes, and continues to be expressed as they differentiate into macrophages (41). This is consistent with the finding that MSR-A is the primary receptor responsible for adhesion of monocytes (data not shown) and macrophages (Fig. 1) to oxLDL-coated surfaces. However, the findings that mononuclear phagocytes adherent to oxLDL-coated surfaces did not secrete significant amounts of H2O2 above background until day 3 of culture (Fig. 5) and that H2O2 secretion by these cells diminished progressively after day 7 of culture seemed inconsistent with a primary role for MSR-A in signaling H2O2 secretion. CD36, a scavenger receptor known to bind oxLDL and to signal H2O2 secretion in macrophages (42), is first expressed on day 2 of monocyte culture and wanes after day 7 (11). Thus, the reported time course of CD36 expression appeared to be more closely correlated with the onset and decline of H2O2 secretion by macrophages plated on oxLDL-coated surfaces (Fig. 5) than the reported time course of MSR-A expression. Therefore, we tested the effect of SMO, an antibody that blocks the interaction of oxLDL with CD36, on H2O2 secretion by macrophages adherent to oxLDL-coated surfaces. Anti-CD36 antibody inhibited scopoletin oxidation to about the same extent as catalase (Fig. 8), whereas an isotype-matched control antibody (MOPC104E) was without effect (Fig. 8). The combination of anti-CD36 and catalase was no more effective in blocking scopoletin oxidation by macrophages plated on oxLDL-coated surfaces than anti-CD36 or catalase alone (Fig. 8). As expected, fucoidan, which blocks macrophage adhesion to oxLDL-coated surfaces by ∼58% (Fig. 1), also inhibited by 75–80% H2O2 secretion by macrophages plated on oxLDL-coated surfaces (data not shown). We conclude that signals initiated by the interaction of CD36 with substrate-bound oxLDL are responsible for the catalase-sensitive H2O2 secreted by macrophages adherent to this substrate.
Figure 8.
Effect of antibody directed against CD36 on H2O2 secretion by macrophages on oxLDL-coated surfaces. Macrophages (2 × 105 cells in KRBG-A), 6–7 d in culture, were preincubated with anti-CD36 mAb (SMO, 10 μg/ml) or control antibody (MOPC104E, 10 μg/ml) for 10 min at room temperature before plating. Each point is the mean ± SEM nmoles H2O2 secreted in three experiments done in triplicate.
Discussion
The evidence that MSR-A play a central role in chronic degenerative diseases such as atherosclerosis and Alzheimer's disease has been strengthened considerably by the reports of Suzuki et al. (16) and El Khoury et al. (18). Suzuki et al. (16) showed that apolipoprotein E–deficient mice whose MSR-A had been genetically disrupted developed smaller atherosclerotic lesions than mice with normal MSR-A function. Similarly, El Khoury et al. (18) showed that MSR-A mediates adhesion of microglial cells to fibrillar β-amyloid, and that this adhesive interaction leads to the secretion of reactive oxygen species by the microglial cells. The findings reported here extend these reports (16, 18), and provide new insights regarding three aspects of the behavior and physiology of monocytes and macrophages in atherogenesis.
Proposed Mechanism for the Retention of Mononuclear Phagocytes in the Arterial Intima at Sites of Nascent Atherogenesis.
The experiments reported here show that macrophages adhere much more avidly to surfaces coated with acLDL or oxLDL than to surfaces coated with native LDL (Fig. 1), and that matrices containing acLDL or oxLDL markedly inhibit macrophage chemotaxis. That macrophage adhesion to oxLDL-coated surfaces was significantly inhibited by fucoidan (Fig. 1), a ligand for scavenger receptors, but not by an mAb that blocks CD36 (Fig. 1), suggests that the macrophages adhere to the substrate via interactions of MSR-A with matrix-bound acLDL or oxLDL. The capacity of acLDL or oxLDL, but not of native LDL, to reduce by ∼70% macrophage chemotaxis through Matrigel (Fig. 2) is consistent with previous observations of El Khoury et al. (18) that ligands for MSR-A block both spontaneous and chemoattractant-stimulated migration of mononuclear phagocytes.
Deposition of antibodies directed against basement membrane proteins in the glomerular basement membrane (43) leads to retention of mononuclear phagocytes in the glomerulus. Presumably, this occurs because monocytes, attracted to the glomerular basement membrane by complement components (e.g., C5a), adhere via their Fc and complement receptors to antibodies and complement components deposited on the glomerular basement membrane, and thereby become trapped at this site.
The findings reported here suggest that a similar adhesive event, albeit mediated by MSR-A, leads to the trapping of monocytes in the subendothelial space in nascent atherosclerotic lesions. Oxidized and degraded lipoproteins are deposited in the subendothelium before the accumulation of monocytes at these sites (4). Oxidized lipoproteins stimulate endothelial cells to express adhesion receptors for monocytes (e.g., vascular cell adhesion molecule 1 [VCAM-1; reference 42]) and to secrete chemokines (5, 6), which stimulate monocytes to adhere to and migrate into the subendothelial space. Deposition of oxidized and degraded lipoproteins in the subendothelial matrix of arteries creates ligands for MSR-A at these sites. We suggest that interactions of MSR-A with these matrix-associated ligands lead to trapping of mononuclear phagocytes at sites of nascent atheroma formation, just as interactions of MSR-A with ligands on matrix-bound β-amyloid fibrils inhibit monocyte migration (18).
Matrix-associated OxLDL Stimulates H2O2 Secretion by Macrophages.
Substrate-adherent phagocytes secrete H2O2 in larger amounts and for a more sustained period than phagocytes in suspension (44). However, differences in cell adhesion do not explain the differences in H2O2 secretion we have observed. 75 and 25% as many macrophages adhered to acLDL- or native LDL–coated surfaces, respectively, as to oxLDL-coated surfaces (Fig. 1), yet macrophages adherent to oxLDL-coated surfaces secreted ∼2.5–3-fold more H2O2 than macrophages adherent to acLDL- or native LDL–coated surfaces (Fig. 3 A). Moreover, macrophages plated on surfaces coated with native LDL or acLDL secreted approximately equivalent amounts of H2O2 (Fig. 3 B), yet adhered in much larger numbers to surfaces coated with acLDL. Thus, efficiency of adhesion cannot account for the observed differences in H2O2 secretion by macrophages adherent to oxLDL- versus acLDL-coated surfaces.
Several investigators have tested the effects of soluble native LDL and soluble oxLDL on H2O2 secretion by monocytes and macrophages (23, 42). Those who used scopoletin/HRP to measure H2O2, such as Montgomery et al. (23), reported that neither native nor modified LDL in solution stimulated H2O2 production by these cells. We have confirmed and extended their findings, as described above.
OxLDL, but not acLDL, is a ligand for CD36 (15). Our studies show that mAb SMO, which is directed against CD36, has no effect on macrophage adhesion to surfaces coated with oxLDL (Fig. 1), but inhibits ∼55% of H2O2 production by macrophages plated on oxLDL-coated surfaces (Fig. 8). Thus, while MSR-A appears to be the principal macrophage plasma membrane receptor that mediates adhesion of macrophages to oxLDL-coated surfaces (Fig. 1), the interaction of CD36 with oxLDL on these surfaces is required for H2O2 secretion by these cells.
Agents that cross-link CD36 are reported to stimulate macrophages to secrete H2O2 (42). mAb SMO is an IgM, and might be expected to cross-link CD36, thereby signaling H2O2 secretion. But SMO does not stimulate H2O2 secretion, it inhibits it (Fig. 8). Nonetheless, it is evident that substrate-bound oxLDL is a powerful stimulus for H2O2 secretion, whereas soluble oxLDL is not.
Like SMO antibody, soluble oxLDL should be a polyvalent ligand for CD36. We suspect soluble oxLDL and SMO antibody are ineffective in stimulating H2O2 secretion because they do not cause them to aggregate in the appropriate orientation or together with other receptors (e.g., MSR-A). mAbs directed against CD36 stimulate H2O2 secretion by macrophages most efficiently when cross-linked by a secondary antibody (42). Assuming that adsorption of oxLDL to the substrate produces multiple CD36 ligands in close apposition to one another, substrate-adherent oxLDL may be much more efficient than soluble oxLDL in cross-linking CD36 with itself or with other scavenger-type receptors.
The Capacity of Macrophages Matured in Culture for 4–6 d to Secrete H2O2 in Response to Substrate-bound OxLDL Is Correlated with CD36 Expression.
Freshly isolated monocytes, and monocytes cultured for 1–2 d, secrete very little H2O2 when plated on oxLDL-coated substrates (Fig. 5). This very low level of H2O2 secretion cannot be ascribed to the inability of these immature cells to synthesize or secrete H2O2. Nakagawara et al. (40) reported that freshly isolated blood monocytes secrete nearly as much H2O2 in response to PMA stimulation as monocyte-derived macrophages maintained in culture for 3–5 d. Similarly, monocytes maintained in culture for 1 d secrete substantial amounts of H2O2 in response to zymosan (data not shown, and reference 18). The studies of Huh et al. (11) provide a possible explanation for the lack of H2O2 secretion by freshly explanted monocytes plated on substrates coated with oxLDL. They found little or no CD36 mRNA or protein in freshly isolated blood monocytes, but noted markedly increased CD36 mRNA expression beginning on day 2 of culture. They showed that CD36 mRNA and protein levels were maximal from days 3 to 6 of culture, and were substantially reduced in cells cultured for 7 d or more. Thus, the chronology of CD36 expression parallels very closely the one we observed for H2O2 secretion by macrophages plated on oxLDL-coated surfaces (Fig. 5).
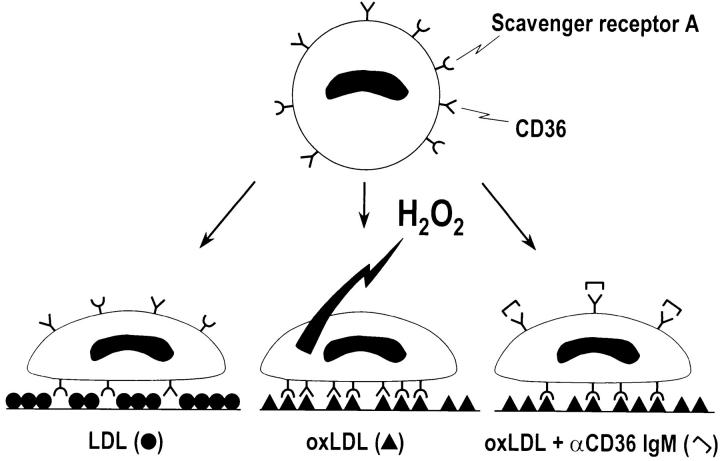
Ligands that block MSR-A, such as fucoidan, block macrophage adhesion to oxLDL-coated surfaces. mAb SMO, which inhibits the interaction of CD36 with oxLDL, blocks H2O2 secretion (Fig. 8) without affecting macrophage adhesion to oxLDL-coated surfaces (Fig. 1). These findings suggest that CD36 is the principal receptor that mediates H2O2 secretion by macrophages adherent to oxLDL-coated surfaces, and that MSR-A's principal function is to promote cell adhesion to these surfaces, as shown diagrammatically in Fig. 9.
Figure 9.
Diagram summarizing proposed effects of CD36 on H2O2 secretion by macrophages adherent to surfaces coated with native LDL or oxLDL. Macrophages adhere inefficiently to surfaces coated with native LDL (Fig. 1). Native LDL does not engage CD36 or MSR-A (macrophage on left). Macrophages adhere efficiently to surfaces coated with oxLDL (Fig. 1, and macrophages illustrated in center and on right). MSR-A is the principal surface receptor that mediates macrophage adhesion to oxLDL-coated surfaces (Fig. 1, and macrophage illustrated on right). Engagement of CD36 by substrate-bound oxLDL signals H2O2 secretion by macrophages (Fig. 8, and macrophage illustrated in center). Antibodies that block interaction between CD36 and substrate-bound oxLDL inhibit H2O2 secretion by macrophages adherent to oxLDL-coated surfaces (Fig. 8, and macrophage illustrated on right).
Atherosclerotic lesions generally take decades to form and the better portion of a human lifetime to reach clinical significance. However, repetitive episodes of H2O2 secretion by macrophages adherent to foci of matrix-bound oxLDL, even at the leisurely pace signaled by CD36, may over many years be sufficient to cause considerable tissue damage. The system we describe here provides a model for exploring the ways MSR-A and CD36 may cooperate in signaling H2O2 secretion by mononuclear phagocytes, and for assessing the roles of other secretory products of mononuclear phagocytes (e.g., myeloperoxidase [36, 45]) in damaging extracellular matrix proteins.
Acknowledgments
Supported by National Institutes of Health grant R37-AI20516 (to S.C. Silverstein); an Investigatorship from Arthur N. Saydman Trust Fund, New York, NY, for Research in Septicemia in honor of Dr. Harold C. Neu at Columbia University (to C.A. Thomas); and a Research Fellowship from the Lucille P. Markey Charitable Trust, Miami, FL (to C.A. Thomas).
Abbreviations used in this paper
- acLDL
acetylated low-density lipoprotein
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- HRP
horseradish peroxidase
- KRBG-A
Krebs Ringer buffer with glucose and albumin
- MSR
macrophage scavenger receptors
- oxLDL
oxidized low-density lipoprotein
References
- 1.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990's. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Witztum JL, Steinberg D. Role of oxidized LDL in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenfeld ME, Palinski W, Yla-Herttuala S, Butler S, Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990;10:336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- 4.Simionescu N, Mora R, Vasile E, Lupu F, Filip DA, Simionescu M. Prelesional modifications in the vessel wall in hyperlipidemic atherogenesis: extracellular accumulation of modified and reassembled lipoproteins. Ann NY Acad Sci. 1990;598:1–16. doi: 10.1111/j.1749-6632.1990.tb42271.x. [DOI] [PubMed] [Google Scholar]
- 5.Takahara N, Kashiwagi A, Nishio Y, Harada N, Kojima H, Maegawa H, Hidaka H, Kikkawa R. Oxidized lipoproteins found in patients with NIDDM stimulate radical-induced monocyte chemoattractant protein-1 mRNA expression in cultured human endothelial cells. Diabetologia. 1997;40:662–670. doi: 10.1007/s001250050731. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz D, Andalibi A, Chalverri-Almada L, Berliner JA, Kirchgesser T, Tang ZT, Tekamp-Olson P, Galegos C, Fogelman AM. Role of the GRO family of chemokines in monocyte adhesion to MM-LDL–stimulated endothelium. J Clin Invest. 1994;94:1968–1973. doi: 10.1172/JCI117548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faggioto A, Ross R, Harker L. Studies of hypercholesterolemia in the non-human primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984;4:323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- 8.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 9.Krieger M. Molecular flypaper and atherosclerosis: structure of the macrophage scavenger receptor. Trends Biochem Sci. 1992;17:141–146. doi: 10.1016/0968-0004(92)90322-z. [DOI] [PubMed] [Google Scholar]
- 10.Calvo D, Gomez-Coronado D, Lasuncion MA, Vega MA. CLA-1 is an 85-kD plasma membrane glycoprotein that acts as a high affinity receptor for both native (HDL, LDL, and VLDL) and modified (oxLDL and acLDL) lipoproteins. Arterioscler Thromb Vasc Biol. 1997;7:2341–2349. doi: 10.1161/01.atv.17.11.2341. [DOI] [PubMed] [Google Scholar]
- 11.Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood. 1996;87:2020–2028. [PubMed] [Google Scholar]
- 12.Tandon NN, Lipsky PH, Burgess WH, Jamieson GA. Isolation and characterization of platelet glycoprotein IV (CD36) J Biol Chem. 1988;264:7570–7575. [PubMed] [Google Scholar]
- 13.Knowles DM, II, Tolidjian B, Marboe C, D'Agati V, Grimes M, Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including reactivity with vascular endothelium. J Immunol. 1984;132:2170–2173. [PubMed] [Google Scholar]
- 14.Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992;141:673–683. [PMC free article] [PubMed] [Google Scholar]
- 15.Endemann G, Stanton LW, Madden KS, Bryan CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 16.Nozaki S, Kashiwagi H, Yamashita S, Nakagawa T, Kostner B, Tomiyama Y, Nakata A, Ishigami M, Miyagawa J, Kameda-Takemura K. Reduced uptake of oxidized low density lipoproteins in monocyte-derived macrophages from CD36-deficient subjects. J Clin Invest. 1995;96:1859–1865. doi: 10.1172/JCI118231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;368:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 18.El Khoury, J., S.E. Hickman, C.A. Thomas, L. Cao, S.C. Silverstein, and J.D. Loike. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 19.Hong YH, Peng HB, La Fata V, Liao JK. Hydrogen peroxide-mediated transcriptional induction of macrophage colony-stimulating factor by TGF-β1. J Immunol. 1997;159:2418–2423. [PubMed] [Google Scholar]
- 20.Brand K, Eisele T, Kreusel U, Page M, Page S, Haas M, Gerling A, Kaltschmidt C, Neumann FJ, Mackman N, et al. Dysregulation of monocytic nuclear factor-κB by oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol. 1997;17:1901–1909. doi: 10.1161/01.atv.17.10.1901. [DOI] [PubMed] [Google Scholar]
- 21.Ares MP, Kallin B, Eriksson P, Nilsson J. Oxidized LDL induces transcription factor protein-1 but inhibits activation of nuclear factor-κB in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1995;15:1584–1590. doi: 10.1161/01.atv.15.10.1584. [DOI] [PubMed] [Google Scholar]
- 22.Khan BV, Parthasarathy SS, Alexander RW, Medford RM. Modified low density lipoprotein and its constituents augment cytokine-activated vascular adhesion molecule-1 gene expression in human vascular endothelial cells. J Clin Invest. 1995;95:1262–1270. doi: 10.1172/JCI117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery RR, Nathan CF, Cohn ZA. Effects of reagent and cell-generated hydrogen peroxide on the properties of low density lipoprotein. Proc Natl Acad Sci USA. 1986;83:6631–6635. doi: 10.1073/pnas.83.17.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dulbecco R, Vogt M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954;99:167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De la Harpe J, Nathan CF. A semi-automated micro-assay for H2O2release by human blood monocytes and mouse peritoneal macrophages. J Immunol Methods. 1985;78:323–336. doi: 10.1016/0022-1759(85)90089-4. [DOI] [PubMed] [Google Scholar]
- 26.Loike JD, El J, Khoury, Cao L, Richards CP, Rascoff H, Manderville JTH, Maxfield FR, Silverstein SC. Fibrin regulates neutrophil migration in response to interleukin 8, leukotriene B4, tumor necrosis factor, and formyl-methionyl-leucyl-phenylalanine. J Exp Med. 1995;181:1763–1772. doi: 10.1084/jem.181.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braut-Boucher F, Pichon J, Rat P, Adolphe M, Aubery M, Font J. A non-isotopic, highly sensitive, fluorimetric, cell-cell adhesion microplate assay using calcein AM-labeled lymphocytes. J Immunol Methods. 1995;178:41–51. doi: 10.1016/0022-1759(94)00239-s. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt AM, Yan SD, Brett J, Mora R, Noygrod R, Stern D. Regulation of human mononuclear phagocyte migration by cell surface–binding proteins for advanced glycation end products. J Clin Invest. 1993;91:2155–2168. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogg N, MacDonald S, Slusarenko M, Beverley PC. Monoclonal antibodies specific for human monocytes, granulocytes, and endothelium. Immunology. 1984;53:753–767. [PMC free article] [PubMed] [Google Scholar]
- 30.Emonard H, Grimaud JA, Nusgens B, Lapiere CM, Foidart JM. Reconstituted basement-membrane matrix modulates fibroblast activities in vitro. . J Cell Physiol. 1987;133:95–102. doi: 10.1002/jcp.1041330112. [DOI] [PubMed] [Google Scholar]
- 31.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci USA. 1987;84:2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. . Proc Natl Acad Sci USA. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenfeld ME, Khoo JC, Miller E, Parthasarathy S, Palinski W, Witztum JL. Macrophage-derived foam cells freshly isolated from rabbit atherosclerotic lesions degrade modified lipoproteins, promote oxidation of low-density lipoproteins, and contain oxidation-specific lipid-protein adducts. J Clin Invest. 1991;87:90–99. doi: 10.1172/JCI115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 35.Leeuwenburgh C, Hardy MM, Hazen SL, Wagner P, Oh-ishi S, Steinbrecher UP, Heinecke JW. Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J Biol Chem. 1997;272:1433–1436. doi: 10.1074/jbc.272.3.1433. [DOI] [PubMed] [Google Scholar]
- 36.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinecke JW. Mechanisms of oxidative damage of low density lipoprotein in human atherosclerosis. Curr Opin Lipidol. 1997;8:268–274. doi: 10.1097/00041433-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Folcik VA, Nivar-Aristy RA, Krajewski LP, Cathcart MK. Lipoxygenase contributes to the oxidation of lipids in human atherosclerotic plaques. J Clin Invest. 1995;96:504–510. doi: 10.1172/JCI118062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20965. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawara A, Nathan CF, Cohn ZA. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981;68:1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ando M, Lundkvist I, Bergstrom J, Lindholm B. Enhanced scavenger receptor expression in monocyte-macrophages in dialysis patients. Kidney Int. 1996;49:773–780. doi: 10.1038/ki.1996.107. [DOI] [PubMed] [Google Scholar]
- 42.Schuepp BJ, Pfister H, Clemetson KJ, Silverstein RL, Jungi TW. CD36-mediated signal transduction in human monocytes by anti-CD36 antibodies but not by anti-thrombospondin antibodies recognizing cell membrane-bound thrombospondin. Biochem Biophys Res Commun. 1991;175:263–270. doi: 10.1016/s0006-291x(05)81229-x. [DOI] [PubMed] [Google Scholar]
- 43.Schreiner GF, Cotran RS, Pardo V, Unanue ER. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978;147:369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuroiwa A, Igisu K, Yano T, Okada N, Okada H. Fibronectin enhances respiratory burst of phagocytes stimulated by zymosan and immune complexes. Immunology. 1988;65:177–180. [PMC free article] [PubMed] [Google Scholar]
- 45.Hazen SL, Gaut JP, Hsu FF, Crowley RR, d'Avignon A, Heinecke JW. p-Hydroxyphenylacetaldehyde, the major product of l-tyrosine oxidation by the myeloperoxidase-H2O2-chloride system of phagocytes, covalently modifies epsilon-amino groups of protein lysine residues. J Biol Chem. 1997;272:16990–16998. doi: 10.1074/jbc.272.27.16990. [DOI] [PubMed] [Google Scholar]