Abstract
The molecular mechanisms underlying target recognition during natural killing are not well understood. One approach to dissect the complexities of natural killer (NK) cell recognition is through exploitation of genetic differences among inbred mouse strains. In this study, we determined that interleukin 2–activated BALB/c-derived NK cells could not lyse Chinese hamster ovary (CHO) cells as efficiently as C57BL/6-derived NK cells, despite equivalent capacity to kill other targets. This strain-determined difference was also exhibited by freshly isolated NK cells, and was determined to be independent of host major histocompatibility haplotype. Furthermore, CHO killing did not correlate with expression of NK1.1 or 2B4 activation molecules. Genetic mapping studies revealed linkage between the locus influencing CHO killing, termed Chok, and loci encoded within the NK gene complex (NKC), suggesting that Chok encodes an NK cell receptor specific for CHO cells. In vivo assays recapitulated the in vitro data, and both studies determined that Chok regulates an NK perforin–dependent cytotoxic process. These results may have implications for the role of NK cells in xenograft rejection. Our genetic analysis suggests Chok is a single locus that affects NK cell–mediated cytotoxicity similar to other NKC loci that also regulate the complex activity of NK cells.
Keywords: natural killer cell, natural killer gene complex, cytotoxicity, tumor
Natural killer (NK) cells are bone marrow–derived lymphocytes, distinct from T and B cells, that are capable of lysing certain tumor cells without prior sensitization (1, 2). Because of this capacity, termed natural killing, NK cells serve as a first line of defense against certain intracellular pathogens and perhaps developing tumor cells (3, 4). Although the phenomenon of natural killing has been studied intensely for many years and underlies the functional definition of NK cells, the molecular mechanisms underlying target recognition during natural killing are not well understood. Despite some descriptions of natural killing as being nonspecific, it is not random. In general, NK cells do not lyse normally dividing cells, and their susceptible targets are classically tumor cell lines, among which there is marked differential sensitivity (5). NK cells do not rearrange T cell antigen receptor genes nor do they express surface Ig (6). Therefore, NK cells must possess a recognition mechanism to discriminate between appropriate targets and normal cells that is distinct from the antigen-specific receptors of T and B cells.
Recent studies on the inverse correlation between target cell expression of MHC class I and target cell susceptibility to NK cell lysis have provided major insight into NK cell recognition. In the “missing-self hypothesis,” Kärre postulated that NK cells are chronically inhibited by MHC class I. Accordingly, if a cell lacks expression of MHC class I or expresses an aberrant form, the inhibitory influence is lost, permitting the NK cell to lyse the target (7–9). The identification of MHC class I–specific inhibitory receptors in rodents (Ly-49) and humans (killer cell inhibitory receptor [KIR]1 and CD94/NKG2) provides a molecular basis for this hypothesis. Receptors in all three of these families contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic domain, which have been shown to recruit cytoplasmic tyrosine phosphatases like SHP-1 upon receptor cross-linking and subsequent ITIM phosphorylation (10–15). Therefore, these MHC class I–specific inhibitory receptors on NK cells may explain the original observations regarding the inverse relationship between target cell expression of MHC class I and susceptibility to lysis that led to Kärre's hypothesis.
However, the known inhibitory receptors do not explain all aspects of NK cell specificity. For example, mouse NK cell clones have varying reactivity against a panel of tumor targets yet do not express members of the Ly-49 family (16). Moreover, in addition to the inhibitory receptors, NK cell recognition appears to involve activation receptors, giving rise to a two-receptor model for NK cell activation. This model predicts that the fate of a target cell is determined by the integration of signals transduced by inhibitory and activation receptors which are simultaneously expressed on a single NK cell (17, 18). Studies using mAbs specific for NK cell surface structures have led to the description of candidate activation receptors. Cross-linking these receptors with specific mAbs stimulates NK cells to kill targets that are otherwise inefficiently lysed. In rodents, these molecules include NKR-P1, CD69, Ly-6, 2B4, and Ly-49D (19–22). Although mAbs have helped identify candidate receptors, the role of these molecules in natural killing and in in vivo NK cell function remains unclear.
Recent studies by Ryan et al. took a mutagenesis approach in order to define the role of NKR-P1A in natural killing. An NKR-P1–deficient mutant of the rat RNK-16 line demonstrated natural killing of YAC-1 cells but not other mouse tumor targets (23). Transfection of the mutant RNK cells with rat NKR-P1A restored lysis of only the xenogeneic IC-21 target. Although these studies strongly suggest that NKR-P1A molecules are target-specific receptors that activate natural killing, the NKR-P1–deficient NK clones were able to lyse other targets, suggesting other routes for activation of natural killing. Hence, there must be other receptors, perhaps some yet to be described, that can influence NK cell recognition.
The current panoply of NK cell receptors is further complicated by the expression of multiple receptors on an individual NK cell and on overlapping NK cell subsets. The resultant heterogeneic NK cell receptor repertoire renders it difficult to analyze the contribution of individual receptors in natural killing. One approach to dissect the complexities of NK cell recognition is to exploit genetic differences among inbred strains of mice. Additionally, the availability of recombinant inbred and congenic strains of mice as well as the current wealth of informative genetic markers makes it possible to rapidly identify novel loci controlling NK cell function.
In the present study, we demonstrate that NK cells from two distinct inbred mouse strains possess differential cytotoxic capacity against Chinese hamster ovary (CHO) target cells. This is an intrinsic phenomenon, manifested in freshly isolated NK cells as well. We have exploited this differential cytotoxicity to identify a genetic locus, termed Chok, responsible for NK cell–mediated cytotoxicity of tumor cells. This locus is linked to the NK gene complex (NKC), and also influences the capacity to clear tumor cells in vivo through a perforin-dependent mechanism, consistent with the hypothesis that Chok encodes a recognition structure specific for certain targets.
Materials and Methods
Mice.
All mouse strains, except the BALB.B6-Cmv1r, B6.BALB-Cmv1s, and the intra-NKC recombinant strains, were obtained from The Jackson Laboratory (Bar Harbor, ME), including C57BL/6J (B6), BALB/cJ, C57BL/6ByJ, BALB/cByJ, CB6F1/J, C57BL10/J, B10.D2, BALB.B, NZB/B1NJ, NZW/ LacJ, NOD/LtJ, DBA/2J, 129/J, C3HeB/FeJ, AKR/J, A/J, SJL/ J, ST/J, CE/J, C57L/J, C57BR/cdJ, C58/J, C57BL/6-Pfptm1Sdz, and seven mice of the recombinant inbred panel derived from C57BL/6ByJ and BALB/cByJ parental strains, CXB-1 through -7. The BALB.B6-Cmv1r mouse is a congenic strain in which the murine cytomegalovirus (MCMV) resistance allele, Cmv1r, as well as other NKC-linked loci from C57BL/6J strain have been transferred onto the BALB/c genetic background as described previously (24). The B6.BALB-Cmv1s congenic strain supports high splenic MCMV titers since it carries the susceptible BALB/c allele, Cmv1s (25). This strain also contains other BALB/c-derived NKC loci on the B6 genetic background (25). All mouse strains were maintained in a pathogen-free facility at Washington University.
Cell Lines.
CHO cell lines (CHO as well as Lec1 and LEC11 mutants) were a gift from Dr. P. Stanley (Albert Einstein College of Medicine, Bronx, NY). The derivation of the mutants has been described previously (26). All three lines were cultured in MEM-α (GIBCO BRL, Gaithersburg, MD) supplemented with ribonucleosides, deoxyribonucleosides, and 10% FCS (Harlan Sprague Dawley, Inc., Indianapolis, IN) without any antibiotics. The BHK.vp16 hamster kidney line was provided by Dr. P. Hippenmeyer (Monsanto, St. Louis, MO [27]). All other cell lines were obtained from the American Type Culture Collection (Rockville, MD). Cells were grown in either RPMI 1640 medium (GIBCO BRL) supplemented with l-glutamine (300 μg/ml), penicillin (100 U/ml), streptomycin (100 μg/ml), 50 μM β-mercaptoethanol, and 10% FCS or in DME (GIBCO BRL) supplemented with l-glutamine (300 μg/ml), penicillin (100 U/ml), streptomycin (100 μg/ml), 50 μM β-mercaptoethanol, sodium pyruvate (1 mM), nonessential amino acids (0.1 mM), and 10% FCS.
IL-2 and Antibodies.
Human rIL-2 was purchased from Chiron Corp. (Emeryville, CA). mAbs used in this study include GK1.5 (rat IgG2b, anti–murine CD4), J11D (rat IgM, anti– murine B cells, neutrophils, erythrocytes, and immature thymocytes), M5/114.15.2 (rat IgG, anti–MHC II), MAR 18.5 (mouse IgG2a, anti–rat κ Ig), 53-6.72 (rat IgG2a, anti–murine CD8), H57-957.1 (hamster IgG, anti–murine TCR-α/β), 2.4G2 (rat IgG, anti–mouse FcγRII/III), PK136 (mouse IgG2a, anti-NK1.1), AF6-88.5.3 (mouse IgG2a, anti–H-2Kb), 145-2C11 (hamster IgG anti-CD3ε), anti-2B4 (mouse IgG2b, anti-2B4), 12A8 (rat IgG2a, anti–Ly-49D and –Ly-49A), 4E5 (rat IgG, anti– Ly-49D), 3D10 (mouse IgG1, anti–Ly-49H), and mouse IgG1 isotype control. All antibodies were either ascites preparations or purified mAb from hybridoma culture supernatants, except for anti-2B4 which was purchased from PharMingen (San Diego, CA). All mAb-producing hybridomas were obtained from American Type Culture Collection, with the exception of 12A8 and 4E5, a gift from L. Mason and J. Ortaldo (National Cancer Institute, Frederick, MD), and 3D10 developed in our lab.2 In addition, rabbit anti-asialo ganglio-N-tetraosylceramide (AGM1) antiserum was obtained from Wako Bioproducts (Richmond, VA), and FITC-conjugated and unconjugated goat F(ab′)2 fragments anti–mouse Ig as well as affinity-purified rabbit anti–mouse Ig were purchased from Cappel, Inc. (Malvern, PA).
IL-2–activated NK Cell Preparation.
Splenocyte suspensions were prepared in HBSS (GIBCO BRL) containing 10% FCS; RBCs were lysed using Tris-buffered ammonium chloride (0.14 M NH4Cl and 0.017 M Tris, pH 7.2). Washed cells were incubated on a nylon wool column for 1 h at 37°C. Nylon wool nonadherent cells were cultured in complete RPMI medium supplemented with 1,000 U/ml of rIL-2 at 2–4 × 106 cells/ml. On day 3 or 4, adherent cells were harvested with Ca2+/Mg2+–free PBS containing 0.2% EDTA (GIBCO BRL) and incubated (2 × 107 cells/ml) with the mAbs (0.5% ascites preparations) 53-6.72 and H57-957.2 on ice in HBSS plus 3% FCS for 30 min. The cells were washed and incubated (107 cells/ml) with 10 μg/ml affinity-purified rabbit anti–mouse Ig and 100 μg/ml rabbit complement (Cedarlane Labs, Inc., Westbury, NY) for 45 min at 37°C. Surviving cells were separated from dead cells using a Lympholyte-M density gradient (Cedarlane Labs, Inc.). These cells were then washed and expanded for 4–6 d in culture with IL-2– supplemented medium. Assays were performed with adherent cells harvested on days 7–9.
Freshly Isolated NK Cell Preparation.
Mouse spleens were harvested and splenocyte suspensions were depleted of RBCs as described above. Nylon wool nonadherent spleen cells (2 × 107/ ml) were incubated with ascites preparations (0.5% final dilution) of mAbs GK1.5, 53-6.72, J11D, and M5/114.15.2 on ice for 30 min. The cells were washed and plated onto T-75 flasks precoated with goat F(ab′)2 anti–mouse Ig at 10 μg/ml. After 1 h at 4°C, the nonadherent cells were resuspended at 107 cells/ml. Purified MAR 18.5 protein was added (10 μg/ml final) together with rabbit complement (100 μg/ml final; Cedarlane Labs, Inc.) and cells were incubated at 37°C for 45 min. The live cells were rescued using a Lympholyte-M density gradient. These cells were analyzed by FACS® and used in cytotoxicity assays.
Cell Surface Expression of Molecules.
Cell surface markers on IL-2–activated NK cells were evaluated using FACS® analysis for each cytotoxicity assay. Typical analysis examines the expression of several cell surface molecules, including CD16 (2.4G2, rat IgG anti-FcγRII/III), 2B4 (2B4, mouse IgG anti-2B4), NK1.1 (PK136, mouse IgG anti-NK1.1), and CD3ε (2C11, hamster IgG anti-CD3ε). Typically, our IL-2–activated NK cells constitute a cell population that is >90% CD3−CD16+ and 2B4+/−, NK1.1+/− (depending on the strain). Cells were incubated with saturating concentrations of the appropriate mAbs on ice for at least 30 min. The cells were then washed twice and incubated with FITC-conjugated goat F(ab′)2 anti–mouse Ig at 10 μg/ml final concentration. The FITC-conjugated goat F(ab′)2 anti–mouse Ig second step reagent cross-reacts with both the rat 2.4G2 and the hamster 2C11 mAbs. Stained cells were analyzed on FACScan® with CellQuest® software (Becton Dickinson, San Jose, CA). Dead cells were excluded by propidium iodide staining.
Natural Killing.
The standard 51Cr-release assay was used to establish NK lytic function. Tumor targets (2–4 × 106) were radiolabeled with 51Cr (50 or 100 μCi) in RPMI without FCS for 90 min. Effector cells were plated onto 96-well round-bottomed plates at various cell densities in order to achieve E/T ratios usually of 20:1, 6.7:1, and 2.2:1. Radiolabeled target cells (104 cells/ well) and effectors were incubated for 4 h in a 37°C humidified CO2 incubator. Subsequently, 100 μl of the supernatants was collected and assayed for 51Cr release. For antibody blocking experiments, mAbs were added at saturating levels to effectors and incubated for 15–30 min at room temperature before the addition of labeled target cells. The mAb 12A8 was used at 1:100 final concentration of an ascites preparation, and the purified mAb 4E5 at 10 μg/ml final concentration. Both the 3D10 (anti–Ly-49H) and its isotype control were used at a final concentration of 10 μg/ml. Specific cytotoxicity was calculated according to the standard formula:
% specific lysis = 100 × (exp − spont)/(max − spont),
where experimental (exp) release represents the radioactivity from the experimental wells, maximum (max) release represents counts from detergent-lysed targets, and spontaneous (spont) release represents background release from wells with targets alone.
Lung Clearance Assay.
Target cells (8 × 106) were incubated with 50 μg of 5-fluoro-2′-deoxyuridine (Sigma Chemical Co., St. Louis, MO) in 2 ml of complete DME for 15 min at 37°C. 125I-labeled 5-iodo-2′-deoxyuridine ([125I]dUrd; Amersham Pharmacia Biotech, Inc., Piscataway, NJ) was added (5 μCi), and cells were incubated for an additional 90–120 min at 37°C. Subsequently, the cells were washed in PBS. Mice were injected intravenously with 3 × 105 cells/200 μl PBS. 4 h after injection, the lungs were removed, rinsed with PBS, and soaked in 70% ethanol for at least 1 h. 125I activity in the lungs and in the inoculum (200 μl) was measured using a gamma counter. Results were determined by expressing the residual 125I activity measured in the lungs as a percentage of the total activity in the inoculum as follows:
percent retention in lung = (average lung cpm/average inocu- lum cpm) × 100.
Thus, percent retention is an indirect measure of number of surviving cells in the lungs. All experiments were done with groups of at least three mice. For in vivo NK cell depletion, 50 μl of anti-AGM1 antiserum diluted in 200 μl vol of PBS was injected intravenously (tail vein) 3 d before the lung clearance assay. In the case of PK136 (anti-NK1.1) or the isotype-matched AF6-88.5.3 (anti-Kb), 100 μg of purified mAb was injected intraperitoneally 2 d before the assay.
Results
Strain-determined Differences in Natural Killing.
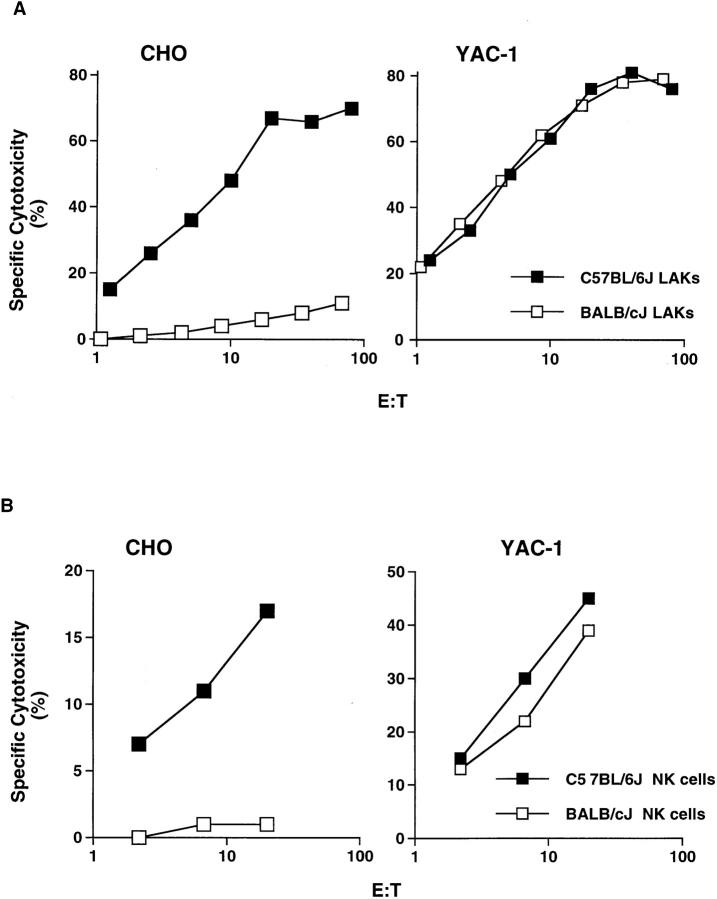
We evaluated the target cell repertoires of NK cells derived from two distinct inbred mouse strains, C57BL/6J (B6) and BALB/cJ, to screen for genetic loci that influence NK cell target specificity (Table 1). B6 IL-2–activated NK cells displayed efficient lysis of most murine tumor targets as well as several hamster cell lines, with the exception of BB88 and WR19L. For most targets, BALB/c-derived NK cells displayed a similar target specificity profile, suggesting that the intrinsic killing capacity of BALB/c NK cells was comparable to that of B6-derived effector cells. However, several targets were killed less efficiently by BALB/c-derived NK cells compared with B6-derived NK cells. This strain- determined difference in capacity for target lysis was most pronounced in killing of CHO targets as well as two variants of this line, Lec1 and LEC11. At E/T ratios >20:1, maximal YAC-1 killing by both effector populations is apparent, demonstrating that BALB/c effectors are potent killers for sensitive targets (Fig. 1 A). Despite this, the strain-determined difference in killing capacity of CHO targets was maintained at E/T ratios as high as 80:1, at which the BALB/c NK cells manifested only minimal lysis of CHO (Fig. 1 A). Furthermore, the difference in CHO cytotoxicity was not overcome by longer 51Cr-release assays. Similar differences were apparent in 4-, 8-, or 24-h assays (data not shown), suggesting that the observations were not due to varying kinetics of killing. The differential susceptibility of CHO targets was not dependent on differences in species origin of the target, since other hamster lines, BHK (Syrian or Golden) and AHL-1 (Armenian), as well as Chinese hamster–derived V79-4 and CHL/IU cells were lysed equivalently by both effector populations (Table 1). Thus, compared with B6-derived effector cells, IL-2-activated BALB/c NK cells manifest a qualitative difference in ability to kill CHO targets.
Table 1.
Strain-determined Lysis of a Panel of Tumor Targets
| Cell line | Type | n | C57BL/6J | BALB/cJ | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E/T ratios | ||||||||||||||||||
| 20 | 6.7 | 2.2 | 20 | 6.7 | 2.2 | |||||||||||||
| Hamster | Strain | |||||||||||||||||
| CHO | Fibroblast | Chinese | 4 | 76 | 56 | 29 | 8 | 3 | 2 | |||||||||
| Lec1 | Fibroblast | Chinese | 1 | 78 | 69 | 44 | 9 | 8 | 6 | |||||||||
| LEC11 | Fibroblast | Chinese | 1 | 86 | 70 | 54 | 8 | 8 | 6 | |||||||||
| V79-4 | Lung | Chinese | 1 | 71 | 61 | 42 | 45 | 32 | 18 | |||||||||
| CHL/IU | Lung | Chinese | 1 | 70 | 68 | 63 | 64 | 56 | 40 | |||||||||
| AHL-1 | Lung | Armenian | 1 | 50 | 40 | 24 | 35 | 33 | 12 | |||||||||
| BHK.vp16 | Kidney | Syrian or Golden | 1 | 62 | 59 | 41 | 68 | 55 | 32 | |||||||||
| Murine | H2 | |||||||||||||||||
| YAC-1 | Thymoma | a | 4 | 93 | 89 | 76 | 82 | 72 | 52 | |||||||||
| RAW264.7 | Macrophage | d | 1 | 100 | 100 | 26 | 82 | 75 | 39 | |||||||||
| SP2/0 | Myeloma | d | 1 | 100 | 93 | 74 | 90 | 78 | 56 | |||||||||
| PU51R | Macrophage | d | 1 | 93 | 82 | 49 | 87 | 66 | 40 | |||||||||
| MPC11 | Plasmacytoma | d | 1 | 56 | 31 | 19 | 52 | 31 | 15 | |||||||||
| MOPC315 | Plasmacytoma | d | 1 | 45 | 26 | 25 | 43 | 24 | 30 | |||||||||
| WEHI7.1 | Thymoma | d | 1 | 69 | 55 | 38 | 60 | 59 | 37 | |||||||||
| WR19L | Lymphoma | d | 1 | 30 | 12 | 2 | 20 | 13 | 2 | |||||||||
| BB88 | Leukemia | b | 1 | 9 | 1 | 0 | 15 | 0 | 0 | |||||||||
| IC-21 | Macrophage | b | 1 | 89 | 61 | 37 | 57 | 36 | 25 | |||||||||
| B16S | Melanoma | b | 1 | 71 | 69 | 28 | 54 | 54 | 39 | |||||||||
| BLKSVHD2 | Fibroblast | b | 1 | 60 | 47 | 31 | 50 | 41 | 30 | |||||||||
| EL-4 | Thymoma | b | 1 | 50 | 37 | 26 | 29 | 15 | 8 | |||||||||
| C1498 | Lymphoma | b | 1 | 72 | 65 | 49 | 71 | 47 | 25 | |||||||||
| RMA-S | Lymphoma | b | 1 | 65 | 50 | 29 | 48 | 35 | 20 | |||||||||
Lysis of various tumor targets by B6- and BALB/c-derived NK cells was determined in triplicate using the standard 51Cr-release cytotoxicity assays at E/T ratios of 20:1, 6.7:1, and 2.2:1. Tumor killing is expressed as percent specific cytotoxicity. For each target used the cell type is indicated. Additionally, for hamster targets, the strain is specified; for murine targets, the H2 haplotype is indicated.
Figure 1.
Strain-determined differences in CHO killing by NK cells. (A) Standard chromium-release assays were performed using B6 and BALB/c IL-2–activated NK cells (LAKs) against CHO and YAC-1 target cells at indicated E/T ratios. (B) Cytotoxicity assays against CHO or YAC-1 targets using freshly isolated NK cells from B6 and BALB/c.
In general, IL-2–activated NK cells display a broader target spectrum as well as higher lytic efficiency compared with freshly isolated NK cells (1, 28, 29). Therefore, it was possible that the differences in CHO killing were due to differential NK cell sensitivity to activation by IL-2. However, freshly isolated B6 NK cells killed CHO, whereas those from BALB/c did not (Fig. 1 B). Although less potent killers, freshly isolated effectors displayed the same differential killing capacity with regard to CHO cell targets. These results demonstrate that an intrinsic capacity unrelated to IL-2 activation controls NK cell–mediated CHO killing.
Inheritance of Strain Difference in CHO Cell Killing and Independence from Host MHC Haplotype.
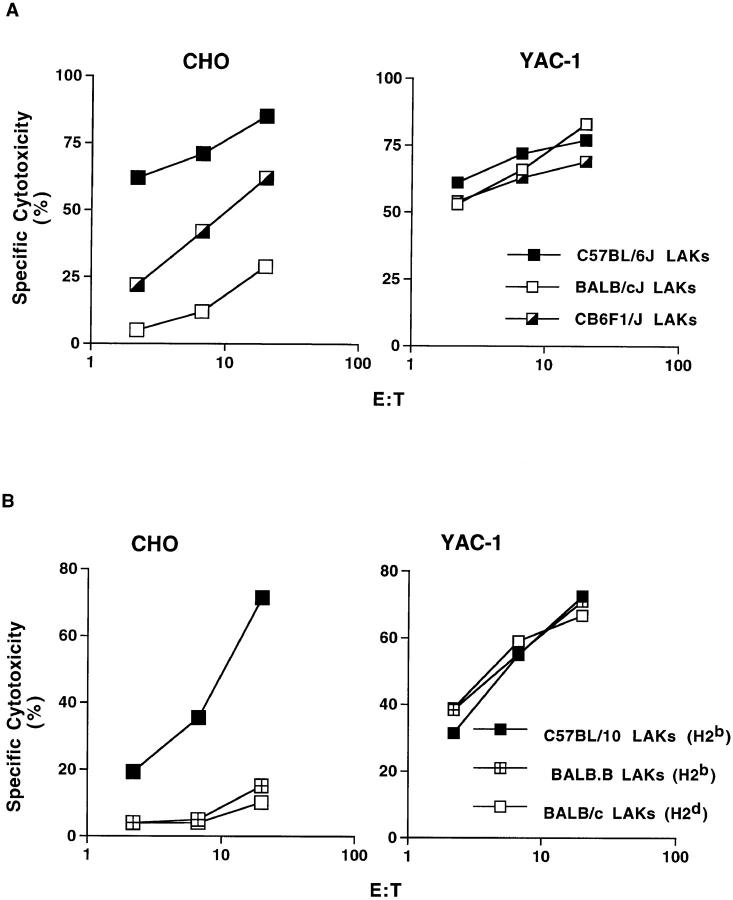
To characterize the inheritance pattern of this trait, cytotoxicity against CHO cells was compared using effector cells from (BALB/c × B6)F1 hybrid mice and the parental strains (Fig. 2 A). Interestingly, the F1 NK cells reproducibly exhibited an intermediate level of cytotoxicity against the CHO target, suggesting a gene dosage effect of an incomplete dominant or codominant inheritance pattern.
Figure 2.
Inheritance of strain difference in CHO cell killing and independence from host MHC haplotype. (A) Lytic capacity of IL-2–activated NK cells from the (BALB/c × C57BL/6)F1 strain was compared with that of parental B6 and BALB/c IL-2–activated NK cells against the CHO and YAC-1 targets. (B) No association between H2 haplotype and capacity to lyse CHO. IL-2–activated NK cells derived from C57BL/10, BALB/c, and BALB.B were compared for their capacity to lyse CHO and YAC-1 targets.
Previous studies on the Ly-49A NK cell receptor indicated that its expression and function are influenced by host MHC haplotype (30–33). Since BALB/c mice are H2d while B6 are H2b, we ascertained if CHO cell lysis is influenced by host MHC haplotype by using H2 congenic mouse strains on C57BL or BALB background. NK cells derived from C57BL background (C57BL/10, H2b; or B10.D2, H2d) exhibited significantly greater cytotoxicity against CHO cells than did NK cells from H2 congenic mouse strains with BALB/c background (BALB.B, H2b; or BALB/c, H2d) regardless of MHC haplotype (Fig. 2 B, and data not shown). These results argue against an influence of the host MHC on the capacity of effector cells to lyse CHO targets.
Panel of Inbred Mouse Strains Assessed for CHO Cytotoxicity.
We next assessed NK cells from several inbred mouse strains to determine if this differential CHO susceptibility is restricted to the B6 and BALB/c backgrounds (Table 2). For each strain tested, the cytotoxicity against the prototypical NK target, YAC-1, was determined in parallel to CHO. Effector NK cells always retained cytolytic activity versus YAC-1, and in no case was CHO killing more efficient than YAC-1 killing. Since there are differences in general capacity to mediate cytotoxicity as indicated by YAC-1 killing, we normalized for the strain differences in overall killing efficiency by determining lysis as a ratio of CHO to YAC-1 killing. We chose a minimum value of 0.5 as a measure of efficient (B6-like) CHO killing for each of the strains tested. Among the 17 additional strains examined, only 4, C57BL/10, NZB/B1NJ, NZW/LacJ, and NOD/ LtJ, possessed NK cells that displayed CHO cytotoxic capacity similar to B6 NK cells. NK cells derived from the remaining strains were not able to lyse CHO cells, thus displaying a phenotype similar to BALB/c.
Table 2.
CHO Cytotoxicity and Expression of 2B4 and NK1.1 Antigens by NK Cells Derived from a Panel of Inbred Mouse Strains
| Strain | H2 haplotype | NK1.1 expression | 2B4 expression | CHO percent lysis | YAC-1 percent lysis | CHO/YAC-1 ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C57BL/6J | b | + | + | 55 | 71 | 0.8 | ||||||
| C57BL/10SnJ | b | + | + | 36 | 55 | 0.7 | ||||||
| B10.D2 | d | + | + | 51 | 70 | 0.7 | ||||||
| NZB/B1NJ | d | + | − | 32 | 36 | 0.9 | ||||||
| NZW/LacJ | z | + | − | 45 | 67 | 0.7 | ||||||
| NOD/LtJ | g | − | − | 45 | 46 | 1.0 | ||||||
| BALB/cJ | d | − | − | 4 | 59 | 0.1 | ||||||
| BALB.B | b | − | − | 5 | 55 | 0.1 | ||||||
| DBA/2J | d | − | − | 16 | 77 | 0.2 | ||||||
| 129/J | b | − | − | 6 | 39 | 0.2 | ||||||
| C3HeB/FeJ | k | − | − | 1 | 58 | 0.0 | ||||||
| AKR/J | k | − | − | 17 | 69 | 0.2 | ||||||
| A/J | a | − | − | 8 | 53 | 0.2 | ||||||
| SJL/J | s | − | − | 2 | 30 | 0.1 | ||||||
| ST/bJ | k | + | − | 1 | 30 | 0.0 | ||||||
| CE/J | k | + | − | 4 | 27 | 0.1 | ||||||
| C57L/J | b | + | + | 10 | 52 | 0.2 | ||||||
| C57BR/cdJ | k | + | + | 7 | 24 | 0.3 | ||||||
| C58/J | k | + | + | 5 | 79 | 0.1 |
IL-2–activated NK cells derived from each indicated strain were assessed for their capacity to mediate CHO and YAC-1 lysis. Percent specific lysis values at a 6.7:1 E/T ratio are shown in the table; similar results were obained at other E/T ratios (data not shown). To normalize for strain differences in overall killing efficiencies, the CHO/YAC-1 ratio was determined. We arbitrarily set a value for CHO/YAC-1 ≥ 0.5 as an index of efficient CHO lysis. The IL-2–activated NK cells derived from each strain tested were also characterized (usually on the same day of the cytotoxicity assay) for their expression of NK1.1 and 2B4 antigens by flow cytometric analysis.
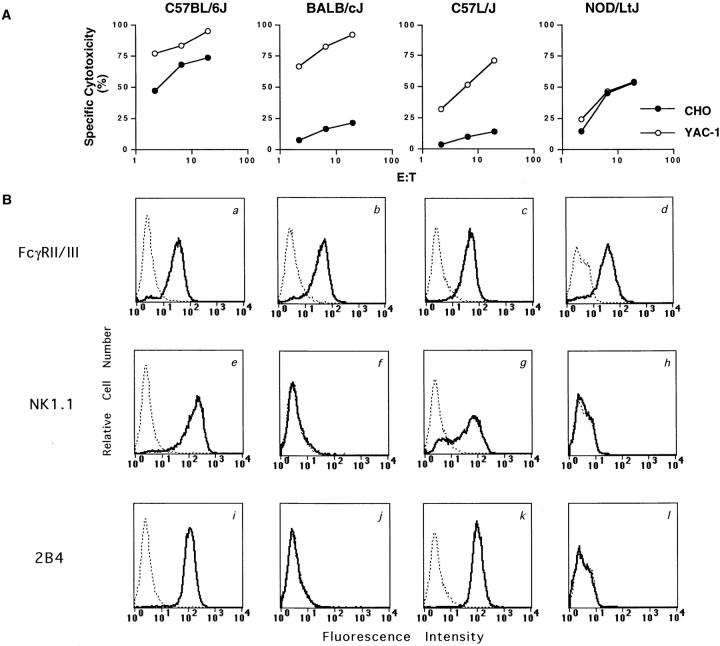
The NK1.1 and 2B4 molecules were both considered as candidates for mediating the differential CHO lysis because they are selectively expressed on NK cells, can activate NK cells (18, 20, 21, 34), and exhibit strain-specific expression on B6- but not BALB/c-derived NK cells (35–37). However, there was no correlation between expression of either epitope by FACS® analysis and the capacity to lyse CHO (Fig. 3, and Table 2). For example, NK cells from C57L/J, C57BR/cdJ, and C58/J strains, which express both NK1.1 and 2B4 epitopes, did not lyse CHO. Conversely, NK cells from NOD/LtJ strain that lack expression of either epitope displayed efficient lysis of CHO targets. Furthermore, murine NK cell clones generated in our laboratory from p53-deficient B6 mice (16) express NK1.1 and 2B4 but do not kill CHO cells despite efficient killing of YAC-1 targets (data not shown). Therefore, these data show that natural killing of CHO cell targets is not unique to the B6 strain and cannot be correlated with expression of the NK1.1 or 2B4 antigen. These data also independently confirm that MHC haplotype is not correlated with CHO killing.
Figure 3.
No correlation between expression of 2B4 and NK1.1 activation antigens and capacity to lyse CHO cells. (A) IL-2– activated NK cells from B6, BALB/cJ, C57L/J, and NOD/LtJ were analyzed for their capacity to lyse CHO and YAC-1 targets. (B) Flow cytometric analysis of IL-2– activated NK cells from B6, BALB/cJ, C57L/J, and NOD/LtJ strains. NK cells from each of the strains were incubated with mAbs specific for FcγRII/III (a–d), NK1.1 (e–h), or 2B4 (i–l) followed by FITC-conjugated goat F(ab′)2 anti–mouse Ig. Solid lines, specific staining; dotted lines, staining by secondary antibody alone. The 2.4G2 mAb is specific for both FcγRII and FcγRIII, even though mouse NK cells express only FcγRIII (reference 73).
Linkage of Chok to NKC-encoded Genes.
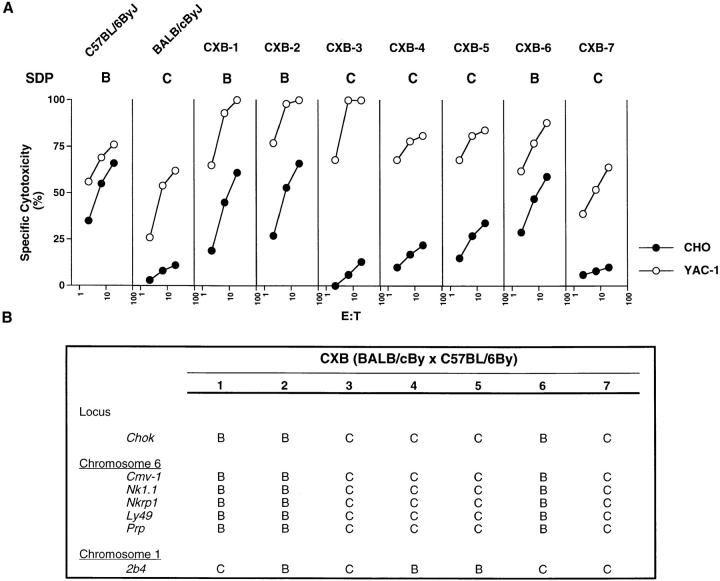
To further characterize the genetic basis for CHO cell killing, we used seven recombinant inbred (RI) mouse strains derived from BALB/cBy and C57BL/6By progenitor inbred strains (Fig. 4 A). Since each RI line is homozygous for either the BALB/c or B6 allele for CHO killing, we expected to see phenotypes that mirrored those of the progenitor strains. Indeed, NK cells derived from CXB-1, -2, and -6 were all B6-like (Fig. 4 B) since they lysed CHO cells efficiently. The remaining strains, CXB-3, -4, -5 and -7, were BALB/ c-like (Fig. 4 C) and did not lyse CHO despite efficient lysis of YAC-1. We observed a clear segregation of phenotypes into high or low levels of killing, and roughly 50% of the RI strains displayed either phenotype, consistent with the interpretation that a single chromosomal locus determines killing of CHO targets. We have termed this locus Chok for CHO killing.
Figure 4.
The SDP of CHO killing among the CXB RI mouse strains demonstrates linkage to the NKC. (A) IL-2–activated NK cells derived from seven CXB RI strains as well as the progenitor strains, C57BL/6ByJ and BALB/cByJ, were assessed for their capacity to mediate CHO and YAC-1 lysis. The SDP is summarized using the symbols B and C to indicate the alleles inherited from the C57BL/6By and BALB/cBy progenitor strains, respectively. (B) Linkage of Chok to NKC-encoded loci on chromosome 6. The SDP observed for the Chok locus is compared with SDPs of other genes previously typed using the same CXB-RI panel (references 35, 37, 47, 48, 74, and 75). The symbols B and C represent alleles inherited from the progenitor C57BL/6By and BALB/cBy strains, respectively.
A comparison of the strain distribution pattern (SDP) for Chok with SDPs for other genes previously typed in the CXB RI strains revealed complete concordance between Chok and Ly49a, Nkrp1, and Cmv1 loci, all of which have been mapped within the NKC on distal mouse chromosome 6 (Fig. 4 B). Although this analysis was limited to a small set of RI strains, these results strongly suggest that Chok is NKC linked.
To confirm the chromosomal location of Chok, we examined the BALB.B6-Cmv1r congenic strain that possesses the Cmv1rallele as well as other NKC loci from B6, backcrossed onto the BALB/c background (24). NK cells derived from BALB.B6-Cmv1r mice displayed efficient lysis against both YAC-1 and CHO cell targets (Fig. 5 A). We have recently established a reverse congenic strain, designated B6.BALB-Cmv1s, carrying the BALB/c Cmv1s allele and other BALB/c-derived NKC loci on the B6 genetic background (25). Consistent with the localization of Chok to the NKC, NK cells derived from B6.BALB-Cmv1s could not lyse CHO, indicating that these animals possess the BALB/c allele for Chok (Fig. 5 A). These results formally establish linkage of Chok to the NKC.
Figure 5.
NK cells from BALB.B6-Cmv1r as well as B6.BALB-Cmv1s confirm Chok linkage to the NKC, and Chok regulates NK-mediated in vivo elimination of tumor targets through a perforin-dependent pathway. (A) Cytotoxicity assays against CHO and YAC-1 targets were performed using IL-2–activated NK cells derived from B6, BALB/c, and the NKC-congenic, BALB.B6-Cmv1r, and B6.BALB-Cmv1s mouse strains. (B) 125I-radiolabeled CHO cells were injected into tail veins of perforin-deficient B6, BALB/c, or B6 mouse strains untreated or treated with anti-AGM1 antiserum 3 d before the assay. Each bar represents the mean percent retention of six mouse lungs except where # n = 5. (C) Lung clearance of [125I]UdR-labeled YAC-1 or CHO targets was assessed in BALB/c and BALB.B6- Cmv1r congenic mice, untreated or treated with 100 μg of either anti-NK1.1 mAb or an isotype-matched control anti-Kb mAb, intraperitoneally 2 d before the assay. Each bar represents the mean percent retention of four mouse lungs except where *n = 3. In all experiments, mice were killed and lungs were harvested 4 h after inoculation.
Antibodies against Ly-49D and Ly-49H Do Not Block CHO Lysis.
Since linkage of the Chok locus to the NKC suggests that Chok may encode an NK cell recognition receptor involved in tumor killing, we evaluated this region for genes that encode known receptors for which there are specific monoclonal reagents. Two members of the Ly-49 family of receptors, Ly-49D and Ly-49H, lack an ITIM in their cytoplasmic tail and have been shown to activate NK cells (22, 38).2 To determine if either of these receptors plays a role in CHO lysis, we examined the effect of antibodies against these molecules in our killing assays. There are two previously characterized anti–Ly-49D reagents: 12A8, which also recognizes Ly-49A, and 4E5, which is monospecific for Ly-49D (22, 39). Neither antibody had an effect on CHO lysis by either B6 or BALB/c NK cells (Fig. 6 A). To examine the role of Ly-49H, we made use of an antibody recently developed in our laboratory that is specific for the Ly-49H molecule.2 Inclusion of this reagent, mAb 3D10, in our assay also did not alter the levels of CHO lysis by B6 NK cells (Fig. 6 B). Thus, anti–Ly-49D and anti–Ly-49H do not block CHO killing.
Figure 6.
Antibodies against Ly-49D or Ly-49H do not block CHO lysis. (A) Cytotoxicity assays against CHO targets were performed using IL-2– activated NK cells derived from B6 and BALB/c in the presence or absence of anti–Ly-49D mAbs. (B) Neither mAb 3D10 (anti–Ly-49H) nor the isotype control had an effect on B6 NK–mediated CHO lysis.
Chok Regulates Rapid Elimination of CHO Cells In Vivo.
To determine if Chok could play a role in NK-mediated activity in vivo, we performed lung clearance assays in B6 and BALB/c hosts. Intravenously inoculated 125I-radiolabeled CHO cells were efficiently eliminated (within 4 h) from the lungs of B6 mice as demonstrated by the low residual radioactivity measured (5 ± 3%; Fig. 5 B). Treatment of B6 mice with anti-AGM1 antiserum 3 d before the assay resulted in an eightfold increase in radiolabel retention, consistent with a role for NK cells in tumor clearance from the lung. In contrast, untreated BALB/c mice retained high levels of radioactivity (39 ± 6%) similar to those detected in the lungs of anti-AGM1–treated B6 mice (41 ± 4%; Fig. 5 B). In BALB/c mice, anti-AGM1 treatment abolished the clearance of YAC-1 targets, but had no effect on CHO clearance, indicating that BALB/c NK cells were functionally intact yet incapable of recognizing the CHO targets (data not shown, and Fig. 5 B). Thus, the in vivo lung clearance assay results parallel the findings of the in vitro cytotoxicity assay.
To ascertain if this strain-determined difference in CHO elimination in vivo maps to the NKC, we compared the lung clearance capacity of BALB/c and the congenic BALB.B6-Cmv1r mouse strains. CHO cells were effectively eliminated in the congenic BALB.B6-Cmv1r strain but not in BALB/c mice despite efficient clearance of YAC-1 tumor cells in both strains (Fig. 5 C). The clearance of YAC-1 and of CHO cells was abrogated in the BALB.B6-Cmv1r mouse strain by prior injection of anti-NK1.1 mAb but not by injection of an isotype-matched control antibody, anti-Kb. It is noteworthy that the retention of radiolabeled CHO cell targets in the lungs of BALB/c mice (32.5 ± 6.5%) is about fivefold greater than the level retained in the BALB.B6-Cmv1r (7.1 ± 3.5%) lungs and is very similar to the retention detected in the lungs of NK-depleted BALB.B6-Cmv1r mice (33 ± 3.4%). These results strongly suggest that the B6 allele of Chok in the congenic strain also plays a role in NK-mediated tumor clearance of CHO targets in vivo.
Perforin Dependence of Chok Phenotype.
In addition to the membranolytic pathway involving granule exocytosis and release of pore-forming protein (perforin) and granzymes, NK cells are also capable of Fas ligand–mediated cytotoxicity (40, 41). To investigate the effector mechanisms used by B6 NK cells to kill CHO targets both in vitro and in vivo, we made use of the perforin-deficient B6 (B6-Pfp −/−) mouse strain. Importantly, in vitro analysis revealed that B6-Pfp −/− NK cells could not lyse either YAC-1 or CHO in the standard 4-h chromium-release assay (data not shown), in agreement with previous characterization of natural killing in these animals (42). Furthermore, we demonstrated that the B6-Pfp −/− mice were unable to clear the CHO cells as reflected in the high level of radiolabel retention (35 ± 4%; Fig. 5 C). This tumor retention is very similar to that observed in the B6 group treated with anti-AGM1 antiserum, and is about sevenfold greater than in wild-type B6 mice. Taken together, these results indicate that natural killing of CHO targets in vitro and NK-mediated elimination of CHO cells in vivo are perforin dependent, consistent with differential susceptibility to NK cell recognition and subsequent initiation of the granule exocytosis pathway.
Discussion
In this study, we determined that the capacity of NK cells from different strains of mice to kill CHO targets is due to a single genetic locus, Chok. Although resistance or susceptibility of CHO cells to NK lysis may have been due to differences in downstream signaling events or mediators of cytolysis, our observations strongly support a differential recognition event. BALB/c-derived NK cells were able to kill YAC-1 and most other targets as efficiently as B6- derived NK cells; however, they could not lyse CHO cell targets. Moreover, this differential capacity to kill CHO was extended to our in vivo tumor clearance model and was determined to be perforin dependent, suggesting that activation of granule exocytosis was required. Therefore, we postulate that this strain-specific cytotoxicity of CHO is due to significant differences in NK cell specificity of target recognition.
In support of the hypothesis that Chok encodes a phenotypically defined recognition structure, B6 and BALB/c NK cells express different alleles for several target recognition molecules, including 2B4, a putative activation receptor (37). Importantly, we have localized Chok to the NKC by assessment of RI strains, a finding verified by examination of the BALB.B6-Cmv1r and the B6.BALB-Cmv1s congenic mouse strains. The genetic analysis formally eliminates many known candidate genes, including 2B4. These studies highlight the power of genetic analysis and specifically, the utility of our RI and congenic mouse strains, in evaluation of candidate molecules in NK cell activation.
The known NKC genes encode NK receptors that have similar structure (type II integral membrane protein orientation, disulfide-linked dimers, and C-type lectin homology) and that regulate NK cell functions. The expression of these molecules is generally restricted to NK cells (18, 35) and includes the mouse NKR-P1 and Ly-49 families, as well as CD94 and NKG2, and CD69, which also have been described in humans and rats (43–46). The NKC also contains several other functional loci controlling NK cell activity, including Cmv1, which determines the NK cell– mediated clearance of MCMV, and Nka, which mediates lymphocyte alloreactivity (47–50). Inasmuch as several of these molecules are also known to influence NK cell specificity and recognition, we postulate that Chok encodes one of the known NKC-encoded molecules or represents a novel but structurally related molecule responsible for NK cell recognition of CHO target cells.
Molecules encoded in the NKC either activate or inhibit NK cells. With this dichotomy in mind, we propose two alternative hypotheses to account for the strain-determined difference in cytotoxicity against CHO. The B6-derived NK cells may possess an activating receptor, encoded by Chok, that interacts with the CHO target and mediates its lysis. BALB/c mice may either lack this gene (null) or express an allele that is incapable of CHO recognition. Alternatively, the BALB/c allele of an inhibitory receptor may recognize its ligand on the CHO cell, preventing NK cell activation. In this case, B6 NK cells may either lack this receptor or possess an allelic form that is incapable of CHO recognition. The intermediate killing of CHO targets by F1 hybrid mice provides some support for the activation receptor hypothesis, because NK cell inhibitory receptors tend to strongly dominate over NK cell activation. However, the available data do not permit unequivocal discrimination between these possibilities, and it remains possible that Chok is related to either type of NK cell receptor.
Cd69 and the Nkrp1 family of genes encode activation receptors on rodent NK cells (20, 23). However, CD69 can be excluded as a candidate for the gene product of Chok because it is not expressed on resting NK cells (51, 52). The Nkrp1 family includes Nkrp1c, which encodes the most specific serologic marker of NK cells in B6 mice (NK1.1), and which displays both strain-specific expression and allelic polymorphism between the B6 and BALB/c strains (36). However, there was discordance between NK1.1 expression on NK cells and capacity to lyse CHO targets (Table 2, and Fig. 3). Also, our B6-derived NK cell clones, which express NK1.1, were not able to lyse CHO targets (data not shown). Furthermore, attempts to block CHO killing by B6 IL-2–activated NK cells using the anti-NK1.1 mAb were not successful (data not shown). Thus, other known genes that reside in this region may potentially have a role in the Chok phenotype (e.g., the Ly-49 family).
The NKC-encoded Ly-49 family of inhibitory receptors displays several levels of polymorphism. At least nine distinct cDNAs (A–I) have been cloned from the B6 strain, and there is evidence implicating multiple alleles for each Ly49 gene among inbred strains. At least three members, Ly-49A, Ly-49C, and Ly-49G, are expressed on distinct but overlapping NK cell subsets and are inhibitory receptors specific for MHC class I molecules (53–57). In mouse strains expressing its MHC class I ligand, Ly-49A expression is downmodulated, thus establishing a mechanism whereby host MHC can influence the NK cell receptor repertoire, and presumably effector function (30–32, 58, 59). The level of Ly-49A expression appears to alter the capacity of the inhibitory effect, consistent with a role for host MHC in modulating NK cell cytotoxicity. Although our studies of H2 congenic strains demonstrated that CHO killing is independent of MHC haplotype, we cannot exclude the possibility that an inhibitory Ly-49 molecule is involved, since ligands for the majority of Ly-49 receptors have not been described. Similarly, NKR-P1B contains an ITIM and is probably inhibitory, but its ligand is not known. Furthermore, for most of these receptors, specific serologic reagents are not available.
On the other hand, both the Ly-49D and Ly-49H receptors lack ITIMs and can enhance NK cell lytic function in redirected lysis assays with an anti–Ly-49D or anti–Ly-49H mAbs (22, 38).2 However, anti–Ly-49D and anti–Ly-49H mAbs do not affect CHO killing by B6 NK cells (Fig. 6). Nonetheless, these results do not rule out involvement of either of these receptors, since mAbs used in our study may not bind epitopes necessary for target recognition. Finally, recent studies have identified the mouse orthologues of the human CD94 and NKG2 molecules. The genes for these molecules also reside in the mouse NKC near the Ly49 gene cluster (32), as predicted by the localization of the human genes to a syntenic region on human chromosome 12p13 (60, 61). To date, however, the expression patterns of these molecules and their functional role in NK cell activity in mice are unknown, and specific monoclonal reagents are lacking.
It is very possible that Chok may be the product of a novel gene, since the mouse NKC spans at least 2.1 million bases (50). Currently, there are cDNA cloning data on only 15 genes (9 Ly49, 3 NKRP1, CD69, NKG2D, and CD94) of an estimated 84 genes, assuming a conservative 1 gene per 25 kb. Thus, Chok could be the product of any one of the aforementioned candidate known genes, or the product of a novel gene. But whether the gene responsible for the Chok phenotype is a known or novel gene, allelic polymorphism of this NKC-encoded locus affects the natural killing of a tumor target and operates in vivo and in vitro through a perforin-dependent mechanism. Hence, the identification of the Chok gene product will further our insight of a known molecule or lead to the discovery of an important and as yet unidentified molecule.
If Chok encodes a recognition structure on NK cells, what is the nature of its putative ligand on CHO cells? Given that some NK cell receptors encoded in the NKC, such as Ly-49 inhibitory molecules, are known to interact with MHC class I molecules, hamster MHC class I molecules may be potential target ligands for the Chok gene product. However, V79-4 and CHL/IU are Chinese hamster cell lines that were killed efficiently by BALB/c effector populations. Since MHC polymorphism is comparatively limited in hamsters, it is unlikely that an MHC class I structure represents a ligand for Chok. Moreover, given that MHC ligands inhibit only a subset of NK cells bearing the cognate inhibitory receptor(s), it is unlikely that CHO cells would inhibit all BALB/c NK cells. Therefore, we currently favor the interpretation that the differential killing of CHO is not due to a direct inhibitory influence of target cell MHC class I molecules.
Since many of the known molecules encoded within the NKC are C-type lectins, it is possible that a carbohydrate moiety expressed by CHO targets may be involved in the interaction between CHO and NK cells. However, our studies using glycosylation mutant variants of the parental CHO cells strongly suggest that the ligand for Chok is independent of complex N-linked oligosaccharides. Lec1 mutants lack demonstrable N-acetylglucosaminyltransferase I activity and therefore have no detectable complex type N-linked oligosaccharides, whereas the LEC11 line expresses an α(1,3) fucosyltransferase not normally expressed by the CHO parental cells (26). Both mutant cell lines displayed susceptibility to B6 but not to BALB/c NK–mediated lysis, similar to what was observed for the parental CHO targets (Table 1). Although these results contradict studies using human NK cells, in which a role for N-linked oligosaccharides has been proposed (62–64), our data indicate that complex N-linked carbohydrates and fucose do not affect the susceptibility of CHO targets to lysis by murine NK cells. These data are consistent with the hypothesis that the product of Chok recognizes a noncarbohydrate ligand on CHO cells.
In addition to providing insight into the molecular basis for specific target recognition of CHO cells, our studies have important implications with regard to the general phenomena of natural killing of tumor targets. We examined a large panel of tumor targets, representing different MHC haplotypes, tissue origins, and species. Despite this, only CHO targets manifested a dramatic difference in killing by NK cells from the two mouse strains. If Chok influences natural killing through a putative activation receptor, and BALB/c lacks a functional Chok gene product, then BALB/c NK cells must utilize other recognition systems, since they can efficiently kill other targets. Therefore, on B6 NK cells, the putative receptor that triggers CHO killing may be coexpressed with receptor systems for other targets. Any or all of such receptors could be stimulated by a given target. Experimental strategies to dissect NK recognition that are based on a “one receptor per NK cell” model, such as those using specific mAb blockade of a single NK cell receptor, may be flawed because of the capacity of NK cells to be activated through other receptor pathways. This notion is supported by studies documenting the expression of more than one inhibitory receptor on an NK cell and on overlapping NK cell subsets (59). Yet, the Chok phenotype was revealed in studies of whole populations of heterogenous NK cells, indicating its dominant role in certain situations. Thus, the capacity to study the effect of a genetic locus that controls natural killing in isolation will aid in dissection of NK cell recognition.
Finally, xenotransplantation has received considerable interest in treating end organ failure in humans, particularly since there is a general shortage of suitable human tissue. The first major obstacle in xenotransplantation is hyperacute rejection, which is mediated by xenoreactive “natural antibodies” and complement (65). Blockade of this humoral response delays but does not abrogate rejection (65, 66). This delayed xenograft rejection is characterized by a cellular infiltrate consisting of mainly NK cells and macrophages (66, 67). Evidence is now mounting that human NK cells are active participants in delayed xenograft rejection (68). Furthermore, other studies revealed that depletion of rat NK cells led to prolonged hamster cardiac graft survival (67). Since these experiments were performed in the absence of xenoantibodies, they also demonstrated that NK cells may mediate xenograft rejection via direct cytotoxicity. In addition to xenogeneic solid tissue transplantation, NK cells play a role in xenogeneic bone marrow transplantation. Transplantation of rat bone marrow into supralethally irradiated mice revealed that B6 and (B6 × A)F1 hybrids are resistant, whereas A, BALB/c, CBA, DBA, and C3H mice are not (69). This phenomenon, termed xenogeneic resistance, is believed to be mediated by radioresistant NK cells and bears many physiologic and genetic similarities to allogeneic resistance, suggesting that the two types of incompatibility are similar, if not identical (70–72). Interestingly, the Chok distribution pattern among the mouse strains tested in this study is reminiscent of that for xenogeneic rat bone marrow rejection described above, suggesting that these may be different manifestations of a single mechanism. Importantly, therefore, allelic determinants of NK cell reactivity against xenogeneic cells, such as Chok, may affect xenotransplantation outcomes. Elucidation of the Chok gene product and its putative ligand on CHO target cells will provide insights into NK cell xenorecognition and facilitate potential clinical applications.
Acknowledgments
We are grateful to Drs. P. Stanley, J. Ortaldo, and P.J. Hippenmeyer for reagents. We thank S.J. Kim and N. Matsumoto for helpful discussions, and J. Heusel and M. Brown for critical reading of this manuscript.
This work was supported by grants from the National Institutes of Health and the Barnes-Jewish Hospital Research Foundation (to W.M. Yokoyama), and by the National Health and Medical Research Council of Australia (grant 961305 to A.A. Scalzo). K. Iizuka was supported by a fellowship from the Eastern Missouri Chapter of the Arthritis Foundation. A.A. Scalzo also received support from the Clive and Vera Ramaciotti Foundation and from the Department of Industry, Science and Technology under the auspices of the Bilateral Science and Technology Collaboration program. W.M. Yokoyama is an investigator of the Howard Hughes Medical Institute.
Abbreviations used in this paper
- B6
C57BL/6
- CHO
Chinese hamster ovary
- AGM1
asialo ganglio-N-tetraosylceramide
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- KIR
killer inhibitory receptor
- MCMV
murine cytomegalovirus
- NKC
NK gene complex
- RI
recombinant inbred
- SDP
strain distribution pattern
Footnotes
Smith, H.R.C., and W.M. Yokoyama, manuscript in preparation.
References
- 1.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G. Natural killer cells wear different hats: effector cells of innate resistance and regulatory cells of adaptive immunity and of hematopoiesis. Semin Immunol. 1995;7:83–88. doi: 10.1006/smim.1995.0012. [DOI] [PubMed] [Google Scholar]
- 4.Bancroft GJ. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 5.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 6.Lanier LL, Phillips JH, Hackett J, Jr, Tutt M, Kumar V. Natural killer cells: definition of a cell type rather than a function. J Immunol. 1986;137:2735–2739. [PubMed] [Google Scholar]
- 7.Kärre K, Ljunggren H-G, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 8.Piontek GE, Taniguchi K, Ljunggren HG, Gronberg A, Kiessling R, Klein G, Kärre K. YAC-1 MHC class I variants reveal an association between decreased NK sensitivity and increased H-2 expression after interferon treatment or in vivo passage. J Immunol. 1985;135:4281–4288. [PubMed] [Google Scholar]
- 9.Ljunggren HG, Kärre K. In search of the ‘missing self ': MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 10.Fry AM, Lanier LL, Weiss A. Phosphotyrosines in the killer cell inhibitory receptor motif of NKB1 are required for negative signaling and for association with protein tyrosine phosphatase 1C. J Exp Med. 1996;184:295–300. doi: 10.1084/jem.184.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura MC, Niemi EC, Fisher MJ, Shultz LD, Seaman WE, Ryan JC. Mouse Ly-49A interrupts early signaling events in natural killer cell cytotoxicity and functionally associates with the SHP-1 tyrosine phosphatase. J Exp Med. 1997;185:673–684. doi: 10.1084/jem.185.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet JP, Long EO. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason LH, Gosselin P, Anderson SK, Fogler WE, Ortaldo JR, McVicar DW. Differential tyrosine phosphorylation of inhibitory versus activating Ly-49 receptor proteins and their recruitment of SHP-1 phosphatase. J Immunol. 1997;159:4187–4196. [PubMed] [Google Scholar]
- 14.Olcese L, Lang P, Vely F, Cambiaggi A, Marguet D, Blery M, Hippen KL, Biassoni R, Moretta A, Moretta L, et al. Human and mouse killer-cell inhibitory receptors recruit PTP1C and PTP1D protein tyrosine phosphatases. J Immunol. 1996;156:4531–4534. [PubMed] [Google Scholar]
- 15.Carretero M, Palmieri G, Llano M, Tullio V, Santoni A, Geraghty DE, López-Botet M. Specific engagement of the CD94/NKG2-A killer inhibitory receptor by the HLA-E class Ib molecule induces SHP-1 phosphatase recruitment to tyrosine-phosphorylated NKG2-A: evidence for receptor function in heterologous transfectants. Eur J Immunol. 1998;28:1280–1291. doi: 10.1002/(SICI)1521-4141(199804)28:04<1280::AID-IMMU1280>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 16.Karlhofer FM, Orihuela MM, Yokoyama WM. Ly-49–independent natural killer (NK) cell specificity revealed by NK cell clones derived from p53-deficient mice. J Exp Med. 1995;181:1785–1795. doi: 10.1084/jem.181.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoyama WM. Recognition structures on natural killer cells. Curr Opin Immunol. 1993;5:67–73. doi: 10.1016/0952-7915(93)90083-5. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama WM, Seaman WE. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613–635. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]
- 19.Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, Hiserodt JC. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. J Exp Med. 1989;169:1373–1389. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlhofer FM, Yokoyama WM. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK1.1 antigen. IL-2-activated NK cells possess additional specific stimulation pathways. J Immunol. 1991;146:3662–3673. [PubMed] [Google Scholar]
- 21.Garni-Wagner BA, Purohit A, Mathew PA, Bennett M, Kumar V. A novel function-associated molecule related to non-MHC-restricted cytotoxicity mediated by activated natural killer cells and T cells. J Immunol. 1993;151:60–70. [PubMed] [Google Scholar]
- 22.Mason LH, Anderson SK, Yokoyama WM, Smith HRC, Winklerpickett R, Ortaldo JR. The Ly-49D receptor activates murine natural killer cells. J Exp Med. 1996;184:2119–2128. doi: 10.1084/jem.184.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan JC, Niemi EC, Nakamura MC, Seaman WE. NKR-P1A is a target-specific receptor that activates natural killer cell cytotoxicity. J Exp Med. 1995;181:1911–1915. doi: 10.1084/jem.181.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scalzo AA, Lyons PA, Fitzgerald NA, Forbes CA, Shellam GR. The BALB.B6-Cmv1 r mouse: a strain congenic for Cmv1and the NK gene complex. Immunogenetics. 1995;41:148–151. doi: 10.1007/BF00182328. [DOI] [PubMed] [Google Scholar]
- 25.Scalzo, A.A., M.G. Brown, D.T. Chu, J.W. Heusel, W.M. Yokoyama, and C.A. Forbes. 1998. Development of intra-natural killer complex (NKC) recombinant and congenic mouse strains for mapping and functional analysis of NK cell regulatory loci. Immunogenetics. In Press. [DOI] [PubMed]
- 26.Stanley P. Biochemical characterization of animal cell glycosylation mutants. Methods Enzymol. 1987;138:443–458. doi: 10.1016/0076-6879(87)38038-3. [DOI] [PubMed] [Google Scholar]
- 27.Hippenmeyer PJ, Highkin M. High level, stable production of recombinant proteins in mammalian cell culture using the herpesvirus VP16 transactivator. Biotechnology. 1993;11:1037–1041. doi: 10.1038/nbt0993-1037. [DOI] [PubMed] [Google Scholar]
- 28.Gunji Y, Vujanovic NL, Hiserodt JC, Herberman RB, Gorelik E. Generation and characterization of lymphokine-activated killer cells in mice. J Immunol. 1989;142:1748–1754. [PubMed] [Google Scholar]
- 29.Phillips JH, Lanier LL. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986;164:814–825. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlhofer FM, Hunziker R, Reichlin A, Margulies DH, Yokoyama WM. Host MHC class I molecules modulate in vivo expression of a NK cell receptor. J Immunol. 1994;153:2407–2416. [PubMed] [Google Scholar]
- 31.Olsson MY, Kärre K, Sentman CL. Altered phenotype and function of natural killer cells expressing the major histocompatibility complex receptor Ly-49 in mice transgenic for its ligand. Proc Natl Acad Sci USA. 1995;92:1649–1653. doi: 10.1073/pnas.92.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorfman JR, Raulet DH. Major histocompatibility complex genes determine natural killer cell tolerance. Eur J Immunol. 1996;26:151–155. doi: 10.1002/eji.1830260123. [DOI] [PubMed] [Google Scholar]
- 33.Sentman CL, Olsson MY, Kärre K. Missing self recognition by natural killer cells in MHC class I transgenic mice. A ‘receptor calibration' model for how effector cells adapt to self. Semin Immunol. 1995;7:109–119. doi: 10.1006/smim.1995.0015. [DOI] [PubMed] [Google Scholar]
- 34.Koo GC, Peppard JR, Mark WH. Natural killer cells generated from bone marrow culture. J Immunol. 1984;132:2300–2304. [PubMed] [Google Scholar]
- 35.Yokoyama WM, Ryan JC, Hunter JJ, Smith HR, Stark M, Seaman WE. cDNA cloning of mouse NKR-P1 and genetic linkage with Ly-49. Identification of a natural killer cell gene complex on mouse chromosome 6. J Immunol. 1991;147:3229–3236. [PubMed] [Google Scholar]
- 36.Giorda R, Weisberg EP, Ip TK, Trucco M. Genomic structure and strain-specific expression of the natural killer cell receptor NKR-P1. J Immunol. 1992;149:1957–1963. [PubMed] [Google Scholar]
- 37.Mathew PA, Garni-Wagner BA, Land K, Takashima A, Stoneman E, Bennett M, Kumar V. Cloning and characterization of the 2B4 gene encoding a molecule associated with non-MHC-restricted killing mediated by activated natural killer cells and T cells. J Immunol. 1993;151:5328–5337. [PubMed] [Google Scholar]
- 38.Smith KM, Wu J, Bakker AB, Phillips JH, Lanier LL. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J Immunol. 1998;161:7–10. [PubMed] [Google Scholar]
- 39.Mason LH, Willette-Brown J, Anderson SK, Gosselin P, Shores EW, Love PE, Ortaldo JR, McVicar DW. Characterization of an associated 16-kDa tyrosine phosphoprotein required for Ly-49D signal transduction. J Immunol. 1998;160:4148–4152. [PubMed] [Google Scholar]
- 40.Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: molecular and cellular aspects. Annu Rev Immunol. 1994;12:735–773. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- 41.Lee RK, Spielman J, Zhao DY, Olsen KJ, Podack ER. Perforin, Fas ligand, and tumor necrosis factor are the major cytotoxic molecules used by lymphokine-activated killer cells. J Immunol. 1996;157:1919–1925. [PubMed] [Google Scholar]
- 42.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 43.Yabe T, McSherry C, Bach FH, Fisch P, Schall RP, Sondel PM, Houchins JP. A multigene family on human chromosome 12 encodes natural killer-cell lectins. Immunogenetics. 1993;37:455–460. doi: 10.1007/BF00222470. [DOI] [PubMed] [Google Scholar]
- 44.Chang C, Rodriguez A, Carretero M, Lopez-Botet M, Phillips JH, Lanier LL. Molecular characterization of human CD94: a type II membrane glycoprotein related to the C-type lectin superfamily. Eur J Immunol. 1995;25:2433–2437. doi: 10.1002/eji.1830250904. [DOI] [PubMed] [Google Scholar]
- 45.Plougastel B, Jones T, Trowsdale J. Genomic structure, chromosome location, and alternative splicing of the human NKG2A gene. Immunogenetics. 1996;44:286–291. doi: 10.1007/BF02602558. [DOI] [PubMed] [Google Scholar]
- 46.Dissen E, Berg SF, Westgaard IH, Fossum S. Molecular characterization of a gene in the rat homologous to human CD94. Eur J Immunol. 1997;27:2080–2086. doi: 10.1002/eji.1830270836. [DOI] [PubMed] [Google Scholar]
- 47.Scalzo AA, Fitzgerald NA, Simmons A, La Vista AB, Shellam GR. Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J Exp Med. 1990;171:1469–1483. doi: 10.1084/jem.171.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scalzo AA, Fitzgerald NA, Wallace CR, Gibbons AE, Smart YC, Burton RC, Shellam GR. The effect of the Cmv-1 resistance gene, which is linked to the natural killer cell gene complex, is mediated by natural killer cells. J Immunol. 1992;149:581–589. [PubMed] [Google Scholar]
- 49.Dissen E, Ryan JC, Seaman WE, Fossum S. An autosomal dominant locus, Nka, mapping to the Ly-49 region of a rat natural killer (NK) gene complex, controls NK cell lysis of allogeneic lymphocytes. J Exp Med. 1996;183:2197–2207. doi: 10.1084/jem.183.5.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown MG, Fulmek S, Matsumoto K, Cho R, Lyons PA, Levy ER, Scalzo AA, Yokoyama WM. A 2-Mb YAC contig and physical map of the natural killer gene complex on mouse chromosome 6. Genomics. 1997;42:16–25. doi: 10.1006/geno.1997.4721. [DOI] [PubMed] [Google Scholar]
- 51.Lanier LL, Buck DW, Rhodes L, Ding A, Evans E, Barney C, Phillips JH. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J Exp Med. 1988;167:1572–1585. doi: 10.1084/jem.167.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Cabrera M, Munoz E, Blazquez MV, Ursa MA, Santis AG, Sanchez-Madrid F. Transcriptional regulation of the gene encoding the human C-type lectin leukocyte receptor AIM/CD69 and functional characterization of its tumor necrosis factor-alpha-responsive elements. J Biol Chem. 1995;270:21545–21551. doi: 10.1074/jbc.270.37.21545. [DOI] [PubMed] [Google Scholar]
- 53.Brennan J, Mager D, Jefferies W, Takei F. Expression of different members of the Ly-49 gene family defines distinct natural killer cell subsets and cell adhesion properties. J Exp Med. 1994;180:2287–2295. doi: 10.1084/jem.180.6.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daniels BF, Karlhofer FM, Seaman WE, Yokoyama WM. A natural killer cell receptor specific for a major histocompatibility complex class I molecule. J Exp Med. 1994;180:687–692. doi: 10.1084/jem.180.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2- activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 56.Stoneman ER, Bennett M, An J, Chesnut KA, Wakeland EK, Scheerer JB, Siciliano MJ, Kumar V, Mathew PA. Cloning and characterization of 5E6(Ly-49C), a receptor molecule expressed on a subset of murine natural killer cells. J Exp Med. 1995;182:305–313. doi: 10.1084/jem.182.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mason LH, Ortaldo JR, Young HA, Kumar V, Bennett M, Anderson SK. Cloning and functional characteristics of murine large granular lymphocyte-1: a member of the Ly-49 gene family (Ly-49G2) J Exp Med. 1995;182:293–303. doi: 10.1084/jem.182.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sykes M, Harty MW, Karlhofer FM, Pearson DA, Szot G, Yokoyama W. Hematopoietic cells and radioresistant host elements influence natural killer cell differentiation. J Exp Med. 1993;178:223–229. doi: 10.1084/jem.178.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Held W, Dorfman JR, Wu MF, Raulet DH. Major histocompatibility complex class I dependent skewing of the natural killer cell LY49 receptor repertoire. Eur J Immunol. 1996;26:2286–2292. doi: 10.1002/eji.1830261003. [DOI] [PubMed] [Google Scholar]
- 60.Ho EL, Heusel JW, Brown MG, Matsumoto K, Scalzo AA, Yokoyama WM. Murine Nkg2d and Cd94are clustered within the natural killer complex and are expressed independently in natural killer cells. Proc Natl Acad Sci USA. 1998;95:6320–6325. doi: 10.1073/pnas.95.11.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vance RE, Tanamachi DM, Hanke T, Raulet DH. Cloning of a mouse homolog of CD94 extends the family of C-type lectins on murine natural killer cells. Eur J Immunol. 1997;27:3236–3241. doi: 10.1002/eji.1830271222. [DOI] [PubMed] [Google Scholar]
- 62.Ahrens PB, Ankel H. The role of asparagine-linked carbohydrate in natural killer cell-mediated cytolysis. J Biol Chem. 1987;262:7575–7579. [PubMed] [Google Scholar]
- 63.Ahrens PB, Ankel H. Natural killer cells discriminate between high mannose- and complex-type asparagine-linked oligosaccharides. Biochimie. 1988;70:1619–1625. doi: 10.1016/0300-9084(88)90297-0. [DOI] [PubMed] [Google Scholar]
- 64.Ahrens PB. Role of target cell glycoproteins in sensitivity to natural killer cell lysis. J Biol Chem. 1993;268:385–391. [PubMed] [Google Scholar]
- 65.Kaufman CL, Gaines BA, Ildstad ST. Xenotransplantation. Annu Rev Immunol. 1995;13:339–367. doi: 10.1146/annurev.iy.13.040195.002011. [DOI] [PubMed] [Google Scholar]
- 66.Candinas D, Belliveau S, Koyamada N, Miyatake T, Hechenleitner P, Mark W, Bach FH, Hancock WW. T cell independence of macrophage and natural killer cell infiltration, cytokine production, and endothelial activation during delayed xenograft rejection. Transplantation (Baltimore) 1996;62:1920–1927. doi: 10.1097/00007890-199612270-00042. [DOI] [PubMed] [Google Scholar]
- 67.Lin Y, Vandeputte M, Waer M. Natural killer cell- and macrophage-mediated rejection of concordant xenografts in the absence of T and B cell responses. J Immunol. 1997;158:5658–5667. [PubMed] [Google Scholar]
- 68.Goodman DJ, Von Albertini M, Willson A, Millan MT, Bach FH. Direct activation of porcine endothelial cells by human natural killer cells. Transplantation (Baltimore) 1996;61:763–771. doi: 10.1097/00007890-199603150-00016. [DOI] [PubMed] [Google Scholar]
- 69.Rauchwerger JM, Gallagher MT, Trentin JJ. “Xenogeneic resistance” to rat bone marrow transplantation. I. The basic phenomenon. Proc Soc Exp Biol Med. 1973;143:145–146. doi: 10.3181/00379727-143-37272. [DOI] [PubMed] [Google Scholar]
- 70.Datta SK, Gallagher MT, Trentin JJ, Kiessling R, Wigzell H. Apparent identity of mechanisms of genetic resistance to marrow transplantation and natural killer cell activity. Biomedicine. 1979;31:62–66. [PubMed] [Google Scholar]
- 71.Lotzova E, Dicke KA, Trentin JJ, Gallagher MT. Genetic control of bone marrow transplantation in irradiated mice: classification of mouse strains according to their responsiveness to bone marrow allografts and xenografts. Transplant Proc. 1977;9:289–292. [PubMed] [Google Scholar]
- 72.Trentin JJ, Rauchwerger JM, Gallagher MT. Genetic resistance to marrow transplantation. Biomedicine. 1973;18:86–88. [PubMed] [Google Scholar]
- 73.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–491. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 74.Yokoyama WM, Kehn PJ, Cohen DI, Shevach EM. Chromosomal location of the Ly-49 (A1, YE1/ 48) multigene family. Genetic association with the NK 1.1 antigen. J Immunol. 1990;145:2353–2358. [PubMed] [Google Scholar]
- 75.Azen EA, Davisson MT, Cherry M, Taylor BA. Prp (Proline-rich protein) genes linked to markers Es-12 (Esterase-12), Ea-10(erythrocyte alloantigen), and loci on distal mouse chromosome 6. Genomics. 1989;5:415–422. doi: 10.1016/0888-7543(89)90004-9. [DOI] [PubMed] [Google Scholar]