Abstract
Mouse germinal center (GC) B cells have been shown to undergo secondary V(D)J (V, variable; D, diversity; J, joining) recombination (receptor editing) mediated by the reexpressed products of recombination activating gene (RAG)-1 and RAG-2. We show here that interleukin (IL)-7 as well as IL-4 was effective in inducing functional RAG products in mouse IgD+ B cells activated via CD40 in vitro. Blocking of the IL-7 receptor (IL-7R) by injecting an anti– IL-7R monoclonal antibody resulted in a marked suppression of the reexpression of RAG-2 and subsequent V(D)J recombination in the draining lymph node of immunized mice, whereas RAG-2 expression was not impaired in immunized IL-4–deficient mice. Further, these peripheral B cells activated in vitro or in vivo were found to express IL-7R. These findings indicate a novel role for IL-7 and IL-7R in inducing receptor editing in GC B cells.
Keywords: recombination activating genes, mature B cells, germinal center, interleukin 7, interleukin 7 receptor
Bcell antigen receptor diversity is generated by the recombination of three classes of germline DNA segments: variable (V),1 diversity (D), and joining (J) (1). V(D)J recombination is mediated by the products of recombination activating gene (RAG)-1 and RAG-2 during the early stages of B cell development in the bone marrow (2–5). In some cases, it has been reported that a V(D)J combination thus established can be replaced by a secondary rearrangement during immature stages. For instance, transgenes encoding autoantibodies were frequently replaced by new endogenously rearranged L and/or H chain genes, leading to the formation of a nonautoreactive antigen receptor (6–9). IgM+IgD− immature B cells isolated from bone marrow have been shown to express RAG genes and to undergo further rearrangement of L chain genes in in vitro cultures (10–12). This secondary V(D)J rearrangement is termed receptor editing, and is thought to be involved in the maintenance of self-tolerance in primary lymphoid tissue (9). However, an established V(D)J combination in a given mature B cell clone has been thought to be stable during differentiation in the periphery, since RAG expression has been believed to be shut down in peripheral mature B cells (13, 14).
Recently, we and another group of investigators have shown that RAG-1 and RAG-2 proteins are reexpressed in germinal center (GC) B cells in secondary lymphoid tissues of immunized mice (15–17). The reexpressed RAG products are considered to be functional as V(D)J recombinase because recombination signal sequence breaks reflecting the rearrangement of κ–L chain and H chain gene loci (18, 19) and circular DNA excision product derived from λ–L chain gene rearrangement (20) have been detected in GC B cells of immunized mice. Further, V(D)J rearrangement was also observed in mature mouse B cells stimulated in vitro with LPS plus IL-4 (19, 20), which is an effective stimulus for inducing RAG expression in IgD+ mature mouse B cells (15). These findings strongly suggest that GC B cells undergo receptor editing by using reexpressed RAG-1 and RAG-2.
GC is a microenvironment in which somatic hypermutation, isotype switching, and affinity maturation of Abs are undertaken. The somatic mutation is basically a random process, and thus considered to result in the generation of B cell clones with unwanted (autoreactive or low affinity) as well as high affinity antigen receptors, the former of which may be selected negatively by apoptosis or rendered unresponsive (5, 21). The discovery of receptor editing in GC B cells implies the existence of a novel pathway for the revision of unwanted B cell antigen receptors in peripheral lymphoid tissues (22). However, the overall profile of the pathway, including what really triggers receptor editing and how RAG reexpression is regulated, remains to be elucidated.
To examine these issues, we analyzed cytokines that are responsible for reexpression of RAG-1 and RAG-2 in GC B cells. As reported previously, IL-4 is effective as a cofactor in inducing RAG expression in anti-CD40–stimulated mouse splenic B cells in vitro (15). However, our preliminary experiments using IL-4–deficient mutant mice indicated that IL-4 deficiency did not impair RAG expression in draining LNs after immunization, suggesting that cytokine(s) other than IL-4 are more important in the gene expression in vivo. In the present work, we show that IL-7, a critical cofactor in bone marrow B cell development (11, 23, 24), also plays a pivotal role in inducing RAG expression and the subsequent V(D)J recombination in mouse splenic B cells stimulated in vitro with an anti-CD40 mAb and in GC B cells in immunized mice.
Another important finding of this study is that IL-7R was induced in these peripheral B cells activated both in vitro and in vivo, since IL-7R has been reported to be present in pre-B cells, whereas B cells in later developmental stages, including pre–B-II, immature B, and mature B cells, do not express a detectable level of IL-7R (24–26). IL-7 has been regarded as a crucial cytokine involved in B cell development at premature stages (24), but its function in mature B cells has not been fully investigated. Our findings suggest a novel role for IL-7 and IL-7R in the diversification and selection of B cells in GCs. These results are discussed in terms of the striking analogy between GCs and bone marrow as microenvironments for B cell differentiation.
Materials and Methods
Cell Culture.
B cells were prepared by treating spleen cells from male C3H/HeN mice (7-9 wk of age; Charles River Japan, Kanagawa, Japan) with anti-Thy 1.2 mAb (Serotec Ltd., Kidlington, UK) followed by incubation with low-toxic rabbit complement (Cedarlane Labs., Ontario, Canada), as described previously (27). The B cells (3 × 106/ml) were cultured with 1 μg/ml rat anti–mouse CD40 mAb (LB429; Seikagaku Kogyo Co. Ltd., Tokyo, Japan) and 10 ng/ml mouse recombinant IL-4 or IL-7 (PeproTech, Inc., Princeton, NJ) for 2–3 d in 1 ml of RPMI 1640 medium containing 10% fetal bovine serum (GIBCO BRL, Gaithersburg, MD), 10−5 M 2-ME, 100 U/ml penicillin G, and 50 μg/ml streptomycin.
Other cytokines were added to the culture as follows: human recombinant IL-2 (donated by Takeda Chemical Industry, Osaka, Japan) at 200 U/ml; mouse recombinant IL-3, IL-6, and IL-10 and human recombinant IL-13 (PeproTech, Inc.) at 10 ng/ml; and mouse recombinant IFN-γ and IL-5 (Genzyme Corp., Cambridge, MA) at 100 U/ml and 50 U/ml, respectively. The level of each cytokine tested was sufficient for exerting its typical biological activity.
In some experiments, cultures were performed using purified IgD+ splenic B cells. The IgD+ B cells were prepared by panning splenic B cells on anti–mouse IgD–coated culture plates as reported previously (15).
Reverse Transcriptase–dependent PCR.
Total RNA was extracted from 106 cells by the RNAZol B method, as described (15). The extracted RNA preparations were reverse transcribed, and the resultant cDNA was amplified by PCR using sense and antisense primers 5′-CACATCCACAAGCAGGAAGTACAC-3′ and 5′-GGTTCAGGGACATCTCCTACTAAG-3′ for RAG-2, and 5′-CCATCACCATCTTCCAGGAG-3′ and 5′-CCTGCTTCACCACCTTCCTTG-3′ for GAPDH, respectively. Both primer pairs spanned an intron, and did not amplify the contaminating genomic DNA. Time-release PCR, which heightens the specificity of the amplification, was performed using AmpliTaq Gold (Perkin-Elmer Corp., Foster City, CA) as polymerase according to the manufacturer's protocol. PCR was carried out for RAG-2 at 94°C for 12 min, followed by 40 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min; and for GAPDH, 26 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s. These PCR cycles were confirmed to be in the exponential phase of the amplification. The amplified products were electrophoresed on 7.5% polyacrylamide gel and visualized by staining with SYBER Green I (FMC Bioproducts, Rockland, ME). In some experiments, RAG-2 expression was estimated by reverse transcriptase–dependent (RT)-PCR followed by Southern blotting as described previously (15, 17). The PstI-HindI 124-bp internal fragment of RAG-2 cDNA was used as a probe. RAG-2 cDNA was provided by Dr. K. Hamatani (Radiation Effects Research Foundation, Hiroshima, Japan).
RT-PCR analysis of other gene expression was carried out in a similar fashion, using the following primers: 5′-GCCTGTCACATCATCTGAGTGCC-3′ and 5′-CAGGAGGCATCCAGGAACTTCTG-3′ for IL-7, 5′-GAGATCATCGGCATTTTGAAC-3′ and 5 ′-CTTGGACTCATTCATGGTGCA-3′ for IL-4, 5′-CGAGTGAAATGCCTAACTC-3′ and 5′-GCGTCCAGTTGCTTTCAC-3′ for IL-7Rα, and 5′-GGAGCTCCAAGGTCCTCATGT-3′ and 5 ′-TAGCTTCTGTACAGCTCGCCT-3′ for the γ common chain of IL-7R (γc).
Detection of DNA Excision Product Derived from Vλ1-Jλ1 Rearrangement.
106 cells that had been cultured with anti-CD40 mAb/IL-7 for 4 d or isolated from the draining LN on day 7 after immunization were suspended in 200 μl of the lysis buffer containing 10 mM Tris-HCl (pH 8.0), 10 mM KCl, 1.5 mM MgCl2, 0.5% Tween 20, and 0.1 mg/ml Proteinase K. The suspension was incubated at 56°C for 45 min and 95°C for 10 min. The supernatant was then directly analyzed for circular Vλ1-Jλ1 excision product by PCR. PCR amplification (28–32 cycles) was performed using AmpliTaq Gold as polymerase and primers which span the signal joint (6), 5′-GCTGCATACATCACAGATGC-3′ and 5′-CAATGATTCTATGTTGTGCC-3′. Each cycle consisted of 94°C for 30 s, 62°C for 30 s, and 72°C for 90 s. The amplified products were electrophoresed and visualized by Southern blotting using a 32P-labeled probe, which is the HindIII-NspI 183-bp fragment derived from a DNA segment of the Vλ1-Jλ1 excision product (provided by Dr. D. Nemazee, National Jewish Medical and Research Center, Denver, CO).
Exon 4 of the γ-actin gene was amplified as an internal control using the primer pair 5′-ATGTTTGAAACCTTCAATAC-3′ and 5′-GGAAGGAAGGCTGGAAGAGT-3′. The amplified product was visualized by staining with SYBER Green I.
Flow Cytometric Analysis.
Cultured B cells (106) were incubated with rabbit anti–mouse IL-7Rα (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and rat anti–mouse IgD mAb (Cosmo Bio Co. Ltd., Tokyo, Japan) at 4°C for 30 min, followed by staining with FITC-labeled goat anti–rabbit IgG (Sigma Chemical Co., St. Louis, MO), PE-labeled goat anti–rat IgG (PharMingen, San Diego, CA), and cychrome-labeled mAb to B220 (PharMingen). Each Ab was added at 10 μg/ml. Flow cytometric analysis was performed by FACSCalibur® (Becton Dickinson, San Jose, CA). An irrelevant rabbit IgG did not give positive results.
Immunization and Administration of IL-7R–blocking Ab.
IL-4– deficient BALB/c mice (28) were maintained in the facility at Kitasato University. Male C3H/HeN mice or female wild-type or IL-4–deficient BALB/c mice were immunized in each hind footpad with 20 μg of TNP-KLH and 0.45 mg of alum in 20 μl saline. On days 6–8 after immunization, popliteal LN cells were examined for expression of various genes. For blocking IL-7Rα in vivo, 1 mg of rat anti–mouse IL-7Rα mAb derived from a hybridoma A7R34 established by S.-I. Nishikawa and colleagues (29) was injected intraperitoneally on days 0, 2, and 4 after immunization. It was confirmed in an in vitro experiment that 1:1,000 diluted A7R34 ascites completely suppressed RAG-2 expression in anti-CD40–stimulated splenic B cells in the presence of 10 ng/ml IL-7. Control mice received the same amount of rat IgG purified from normal rat serum.
Immunohistochemical Analysis of Draining LNs.
On day 8 after immunization, popliteal LNs were excised and subjected to immunohistochemical analysis. For immunofluorescent staining of LN sections, 8-μm-thick cryosections mounted onto slides were allowed to air-dry for 20 min and were fixed in ice-cold acetone for 10 min. Rehydration and preblocking were performed as described previously (15). A buffer containing 10 mM Tris (pH 8.0), 150 mM NaCl, 0.05% Tween 20, and 1% BSA (30) was used for diluting Abs and for washing. The slides were treated for 1 h with the following three combinations of first Abs: (a) biotinylated rabbit anti–mouse IL-7 (Santa Cruz Biotechnology, Inc.) and rat anti–mouse follicular dendritic cell (FDC) mAb, FDC-M1; (b) biotinylated rat mAb GL-7 and rabbit anti–mouse IL-7Rα; and (c) FITC-conjugated rabbit anti–mouse IL-7Rα and biotinylated rabbit anti–RAG-1 (PharMingen). After washing thoroughly, a and b slides were reacted for 40 min with rhodaminated avidin (Sigma Chemical Co.), and FITC-conjugated goat anti–rat IgG (Organon Teknika-Cappel, Durham, NC) and rhodaminated avidin and FITC-conjugated goat anti–rabbit IgG, respectively. A c slide was treated with rhodaminated avidin alone. Each Ab was added at 5 μg/ml. Rhodaminated avidin was used at 2 μg/ml. FDC-M1 and GL-7 mAbs were provided by Dr. M.H. Kosco-Vilbois (Geneva Biomedical Research Institute, Geneva, Switzerland) and Dr. K.S. Hathcock (National Institutes of Health, Bethesda, MD), respectively. The slides were finally mounted with low fluorescent glycerol and coverslip protection, and were observed with a confocal laser scanning microscope (Bio-Rad Laboratories, Hercules, CA). Specificity of each immunolabeling was confirmed using irrelevant control Abs.
Results and Discussion
IL-7 as a Cofactor for the Expression of RAG Genes in Anti-CD40–stimulated Mouse Splenic B Cells In Vitro.
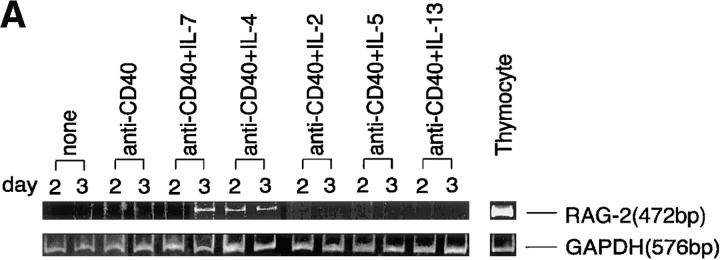
To analyze cytokines involved in the reexpression of RAG genes in peripheral B cells, a series of cytokines was tested for their potency to induce gene expression in mouse splenic B cells cultured with an anti-CD40 mAb in vitro. The expression of RAG-2 transcripts was assessed by RT-PCR after culture for 2 or 3 d. As reported previously (15), IL-4 was effective in inducing the expression of RAG-2 in this culture system (Fig. 1 A). Among cytokines tested other than IL-4, IL-7 was also an effective cofactor for RAG-2 expression. This is worth noting because IL-7 has been reported to be critical for B cell development in bone marrow (24), but its function in peripheral B cells has not been fully elucidated. IL-7–dependent RAG-2 expression was reproducible in at least seven repeated experiments, in which RAG-2 expression usually peaked on day 3 of culture. IL-2, IL-5, and IL-13 were not effective under the same experimental conditions (Fig. 1 A), and neither were IL-3, IL-6, IL-10, and IFN-γ (data not shown).
Figure 1.
IL-7 as a cofactor for reexpression of RAG-2 in mouse splenic B cells stimulated with anti-CD40 mAb in vitro. (A) Splenic B cells from C3H/HeN mice (3 × 106/ml) were cultured with 1 μg/ml anti-CD40 mAb (LB429) in the presence or absence of IL-7 (10 ng/ml), IL-4 (10 ng/ml), and other indicated cytokines for 2 or 3 d. RAG-2 expression was assessed by RT-PCR. Mouse thymocytes were used as a positive control. (B) Dependence of RAG-2 expression on both IL-7 and anti-CD40. The B cells were cultured as indicated for 3 d.
A combination of IL-7 and anti-CD40 was required to induce RAG-2 expression, since neither IL-7 nor anti-CD40 alone was active (Fig. 1 B). It was also confirmed (data not shown) that (a) the concentrations of IL-4 and IL-7 used in the experiment shown in Fig. 1 were optimal for RAG-2 expression; (b) a combination of IL-7 and IL-4 at their optimal doses did not further enhance RAG-2 expression; and (c) RAG-1 was also expressed in parallel with RAG-2 in B cells stimulated with anti-CD40/IL-7.
IL-7–induced RAG Products Are Functional as V(D)J Recombinase.
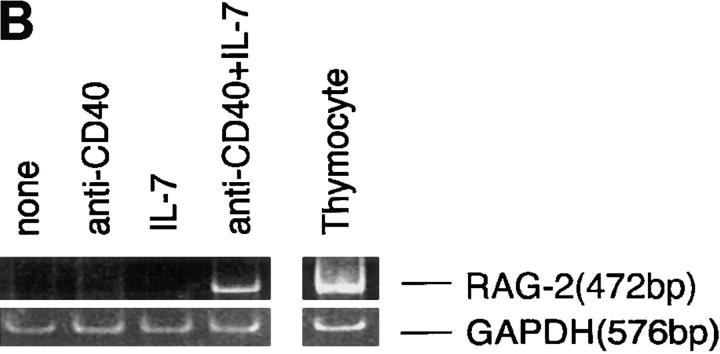
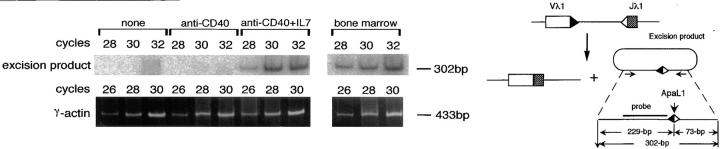
In a previous report, we showed that RAG gene products expressed in LPS/IL-4–stimulated splenic B cells mediated de novo Vλ1-Jλ1 rearrangement in λ–L chain genes (20). Therefore, it was investigated whether IL-7–induced RAG products are also functional as a V(D)J recombinase. Circular DNA excision product derived from Vλ1-Jλ1 rearrangement was amplified by PCR using primers spanning the signal joint, and the amplified product was detected by Southern blotting (Fig. 2). The excision product was observed in splenic B cells stimulated with anti-CD40/IL-7, but was negligible in unstimulated cells and in cells stimulated with anti-CD40 alone. Thus, RAG gene products induced by anti-CD40/IL-7 are thought to have potency to mediate V(D)J recombination.
Figure 2.
Vλ1-Jλ1 rearrangement in mouse splenic B cells stimulated with anti-CD40 mAb plus IL-7. B cells were cultured by themselves or with anti-CD40 alone or anti-CD40 plus IL-7 for 4 d. Circular DNA excision product derived from Vλ1-Jλ1 rearrangement was amplified by PCR using the depicted primers for 28, 30, or 32 reaction cycles, and visualized by Southern blotting.
It has been reported that signal joint formation in the excision product generates a restriction site cleavable with ApaLI (Fig. 2; reference 31). ApaLI was found to cleave the PCR-amplified product (302 bp) into two fragments at the expected site, thus confirming the identity of the amplified product (data not shown). It has been reported that signal joint formation is a relatively slow process and requires cell cycle progression (32). It is possible that the circular DNA excision product observed in anti-CD40/IL-7–stimulated B cells is not generated by the mere joining of the signal breaks produced during premature stages, since stimulation with LPS alone, which causes the cell cycle to progress but fails to induce RAG expression, did not produce the excision product in these B cells, as reported previously (20).
Expression of RAG-2 and IL-7R in IgD+ Mature B Cells Stimulated with Anti-CD40/IL-7 In Vitro.
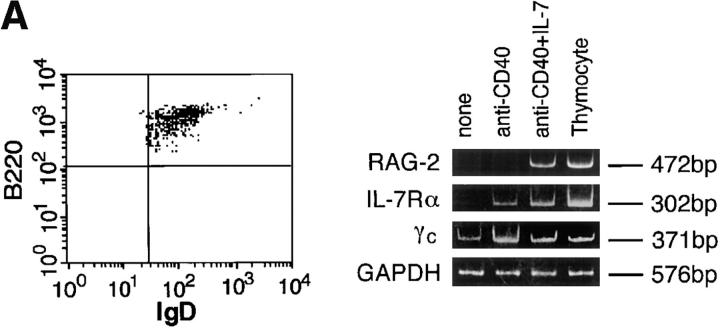
The effectiveness of IL-7 in inducing RAG expression in mouse splenic B cells is surprising because it has been reported that B cells in later developmental stages, including late pre–B-II, immature, and mature B cells, lose responsiveness to IL-7 due to downregulation of IL-7R (24, 26). We have verified that surface IgD+ mature mouse B cells express RAG genes when they are stimulated with LPS/IL-4 in vitro (15). Since IL-7 is a growth factor of premature B cells (24, 25), it was important to determine whether the IL-7–induced RAG-2 expression and Vλ1-Jλ1 rearrangement observed in splenic B cells (Figs. 1 and 2) occurred in mature B cells, or whether RAG expression was due to the outgrowth of premature B cells that might have been present in the B cell preparation. For this purpose, similar experiments to those shown in Fig. 1 were performed using purified IgD+ mature B cells. The IgD+ B cells (>99% pure as assessed by flow cytometry) prepared by panning splenic B cells on anti-IgD–coated culture plates were found to express RAG-2 when stimulated with anti–CD40/IL-7 in vitro (Fig. 3 A), indicating that activated mature mouse B cells can express the RAG gene in response to IL-7.
Figure 3.
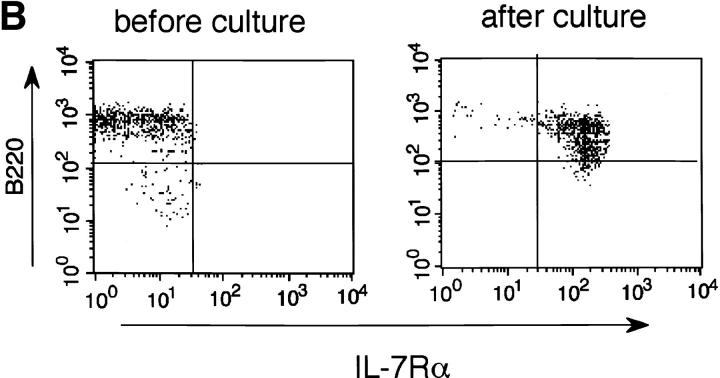
Expression of RAG-2, IL-7Rα, and γc in purified IgD+ mature B cells. (A) Purified IgD+ B cells prepared by panning splenic B cells on anti-IgD– coated culture plates were stimulated with anti-CD40/IL-7 for 3 d, followed by assessment of the expression of the indicated genes by RT-PCR. Purity of the IgD+ B cell preparation was confirmed by flow cytometry. (B) The purified IgD+ B cells were analyzed for expression of immunoreactive IL-7Rα before and after stimulation with anti-CD40/IL-7 for 3 d.
Since mature B cells have been reported to express no detectable level of IL-7R (25, 26), IgD+ B cells may have to regain IL-7R to express RAG-2 transcripts in response to exogenous IL-7. Interestingly, the following findings show that this is the case. RT-PCR analysis showed that the purified IgD+ B cells cultured without stimuli did not express a detectable level of the α chain of IL-7R (IL-7Rα), whereas stimulation with anti-CD40 plus IL-7 resulted in the induction of an appreciable level of IL-7Rα (Fig. 3 A). A slight expression of IL-7Rα was observed when cells were stimulated with anti-CD40 alone. γc, another subunit of IL-7R (24), was also found to be expressed in the IL-7Rα+ cells, suggesting the formation of functional IL-7R. Further, consistent with the RT-PCR analysis shown in Fig. 3 A, flow cytometric analysis revealed that a majority of the IgD+ B cells became positive for IL-7Rα after stimulation with anti-CD40/IL-7 (Fig. 3 B). Taken together, our observations indicate that IL-7 can act as a potent cofactor for inducing RAG expression via IL-7R in activated mature B cells.
Role of IL-7 in RAG Expression in GC B Cells In Vivo.
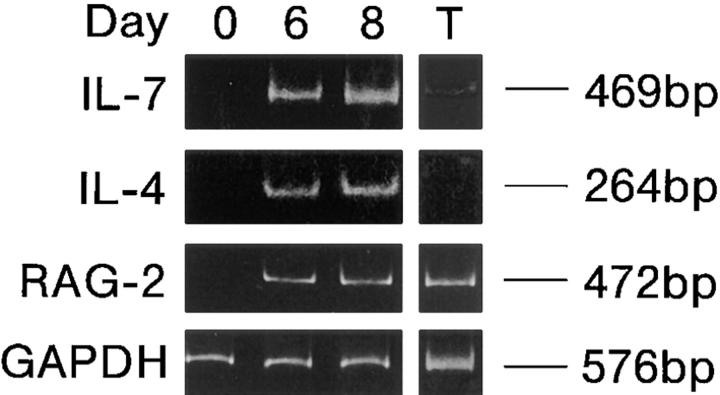
Does IL-7 also mediate RAG expression in vivo in a similar fashion? As shown in Fig. 4, transcripts of IL-7, IL-4, and RAG-2 were induced on days 6 and 8 after immunization in popliteal LN cells from mice immunized in the footpad, whereas these transcripts were not detected in LN cells on day 0. Under the same experimental conditions, the DNA excision product derived from Vλ1-Jλ1 rearrangement was found to be generated in GCs in parallel with RAG-2 expression, as reported previously (20).
Figure 4.
Expression of IL-7, IL-4, and RAG-2 transcripts in draining LN cells in immunized mice. C3H/HeN mice were immunized with TNP-KLH/alum in their footpads. Expression of IL-7, IL-4, and RAG-2 transcripts was assessed by RT-PCR in popliteal LN cells on days 0, 6, and 8 after immunization. Mouse thymocytes (T) were used as a positive control for RAG-2 expression.
Immunohistochemical analysis of popliteal LNs on day 8 after immunization showed that the majority of IL-7–containing cells costained with an mAb to FDC, suggesting that FDCs are a major source of IL-7 in the LN (Fig. 5 A). Another interesting observation is that a population of GL-7 mAb–reactive cells that represent GC B cells (33) was positive for immunoreactive IL-7Rα (Fig. 5 B). It has been reported that CD4+ T cells are also present in the light zone of GCs (33). Thus, GC B cells may be able to receive the signals necessary for RAG expression from FDCs and/or CD4+ T cells.
Figure 5.
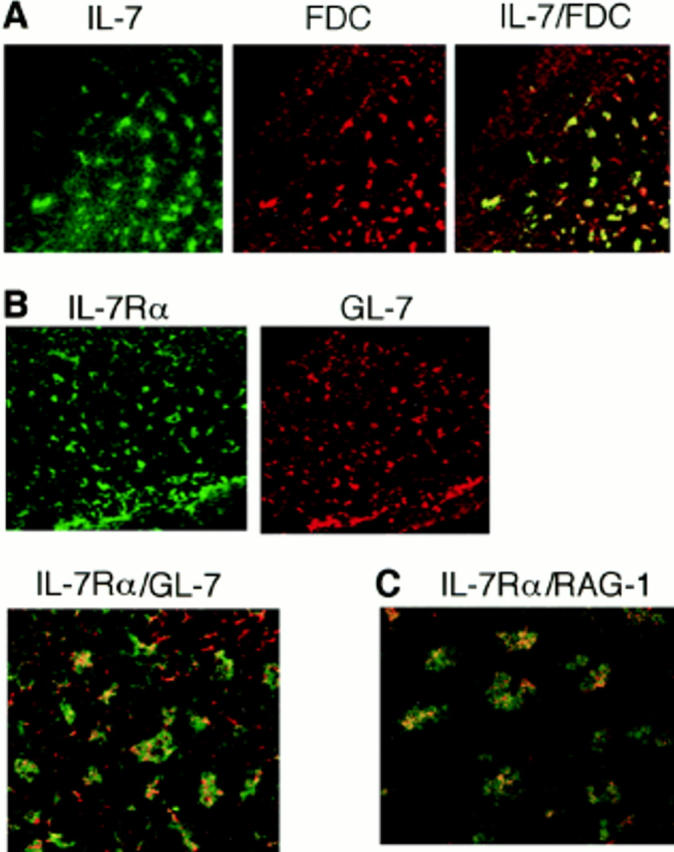
Immunohistochemical analysis of the expression of IL-7, IL-7Rα, and RAG-1 in GCs of draining LNs. C3H/HeN mice were immunized in each hind footpad with TNP-KLH/alum, and cryosections of popliteal LNs were prepared on day 8 after immunization. They were double-labeled with (A) FDC-specific mAb and polyclonal anti–IL-7, (B) polyclonal anti–IL-7Rα and GL-7 mAb, or (C) polyclonal anti– IL-7Rα and anti–RAG-1. FDC and IL-7Rα were visualized with FITC (green), and IL-7, GL-7, and RAG-1 were visualized with rhodamine (red) as described in Materials and Methods. Each FITC and rhodamine image and superimposition of the two are shown in A and B. C, A superimposed image of IL-7Rα and RAG-1. Costaining with FITC and rhodamine gave orange-yellow fluorescence. Original magnification ×100.
We have shown by immunohistochemical analysis that RAG-1 and RAG-2 proteins are expressed in GC B cells in popliteal LNs after immunization (15, 17). Other investigators have detected RAG-mediated generation of recombination signal sequence breaks in GL-7+ GC B cells (18, 19). Expression of RAG-1 was again confirmed in the present experiment. An important observation was that the RAG-1 molecules visualized with rhodamine (red fluorescence) were present in IL-7Rα+ cells labeled with FITC (green fluorescence; Fig. 5 C), suggesting that a population of GL-7+ GC B cells regains IL-7R and subsequently expresses RAG proteins in response to IL-7, the majority of which may be supplied from FDCs. It has been reported that IL-7 was detected in human tonsillar FDCs, and tonsillar B cells have been shown to proliferate in response to IL-7 (34). However, expression of IL-7R on these B cells has not been directly demonstrated. Although IL-7R has not been detected in immature and mature mouse B cells (24–26), IgD+ mature B cells stimulated in vitro with anti-CD40/ IL-7 were shown to express IL-7R (Fig. 3). To our knowledge, this is the first documented report describing reexpression of IL-7R on peripheral B cells in vitro and in vivo.
IL-7R–dependent Receptor Editing in GCs.
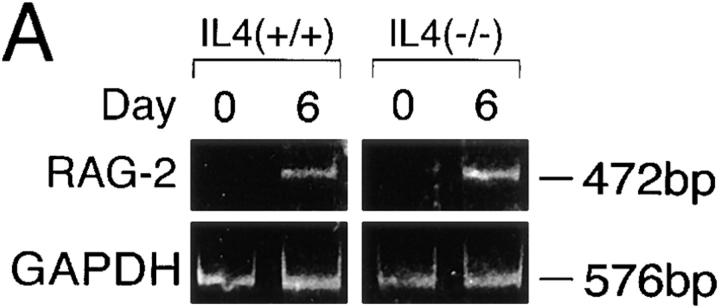
As described above, both IL-4 and IL-7 proved effective for inducing RAG-2 expression in vitro (Fig. 1). Are IL-7 and IL-4 equally responsible for RAG expression in vivo? Experiments using mutant mice with a disrupted IL-4 gene revealed that IL-4 depletion did not impair expression of RAG-2 in draining LNs of immunized mutant mice (Fig. 6 A), suggesting that the IL-4 deficiency was compensated for by other cofactor(s), including IL-7. In addition, IL-4 deficiency did not affect GC formation in draining LNs after immunization (data not shown). However, an auxiliary or compensatory role for IL-4 cannot be ruled out. For instance, we observed that IL-4 was as effective as IL-7 in upregulating IL-7Rα in B cells activated in vitro via CD40, suggesting that IL-4 enhances responsiveness of GC B cells to IL-7 (our unpublished observations).
Figure 6.
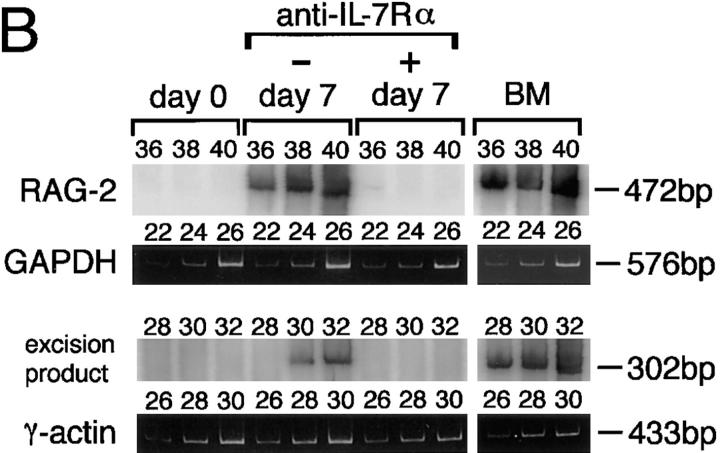
Analysis of cytokines involved in RAG-2 expression in vivo in draining LNs of immunized mice. (A) RAG-2 expression was not impaired in IL-4–deficient mice. Wild-type or IL-4–deficient BALB/c mice (n = 3) were immunized in their footpads with TNP-KLH/alum. On day 7 after immunization, pooled popliteal LN cells from each group were assessed for expression of RAG-2 by RT-PCR. (B) Suppression of RAG-2 expression and Vλ1-Jλ1 rearrangement in draining LNs of immunized mice by injection of anti–IL-7Rα mAb (A7R34). C3H/ HeN mice (n = 2) were immunized in their footpads with TNP-KLH/alum, then injected intraperitoneally with 1 mg/d anti–IL-7Rα mAb or rat IgG on days 0, 2, and 4 after immunization. The pooled popliteal LN cells were examined for expression of RAG-2 and excision product derived from Vλ1-Jλ1 rearrangement on day 7 after immunization, by RT-PCR/Southern blotting and PCR/Southern blotting, respectively. PCR was performed with the varying reaction cycles indicated. Bone marrow cells (BM) were used as a positive control.
IL-7– and IL-7R–deficient mice are inappropriate for analyzing the role of IL-7 in RAG expression in vivo, since the generation of mature B cells is impaired in these mice (24). Therefore, we used an anti–IL-7Rα mAb (A7R34) that has been shown to block signaling by IL-7 (29). A group of mice immunized with TNP-KLH/alum in their footpads was injected intraperitoneally with the anti–IL-7Rα mAb on days 0, 2, and 4 after immunization, and the popliteal LN cells were examined for expression of RAG-2 on day 7. Interestingly, RAG-2 expression and subsequent Vλ1-Jλ1 rearrangement in popliteal LN cells were markedly suppressed in mice treated with anti–IL-7Rα mAb compared with those receiving control Ab (Fig. 6 B). These results and the observations made in the in vitro experiments (Figs. 1–3) strongly suggest that IL-7 plays a pivotal role in inducing receptor editing in GC B cells. However, the participation of other IL-7R ligands cannot be ruled out, since it has been reported that the IL-7Rα chain binds another ligand, thymic stroma–derived lymphopoietin (35).
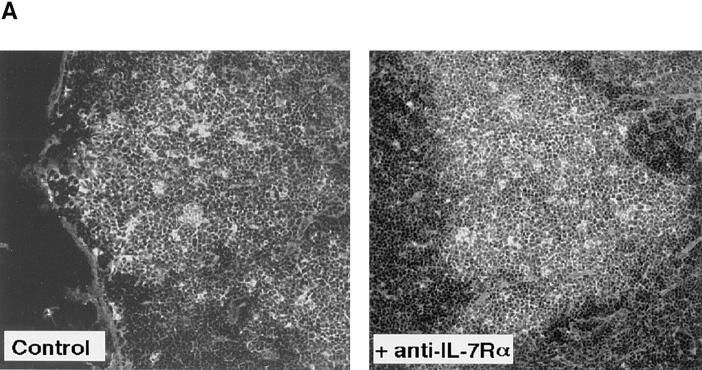
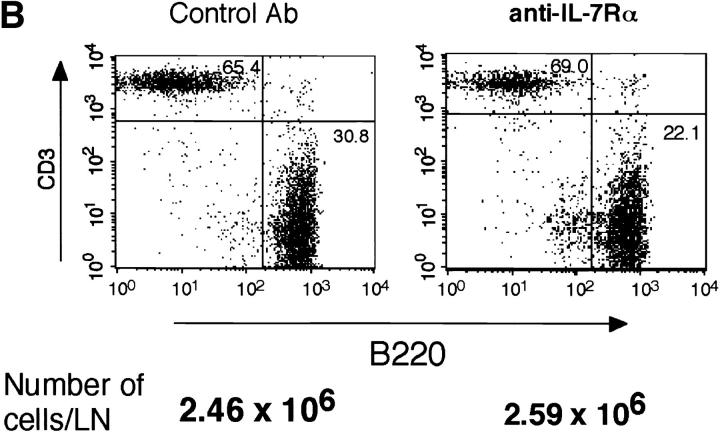
The following experiments were performed to confirm that inhibition of RAG-2 expression by anti–IL-7Rα treatment is a specific result of suppressed lymphopoiesis (Fig. 7). The size and number of GCs in the LNs were histochemically comparable between control and anti–IL-7Rα– treated mice examined in Fig. 6 B (data not shown). A representative microscopic image of a GC from each group of mice is shown (Fig. 7 A). The number of cells in the popliteal LNs usually increased by 8–10-fold on day 7 after immunization in the footpad. Treatment of mice with anti–IL-7Rα mAb resulted in neither a reduction in the number of viable cells nor a significant change in T to B cell ratio in LNs on day 7 after immunization (Fig. 7 B). Further, anti-TNP IgM and IgG1 Ab responses in anti–IL-7Rα–treated mice were at least 80% as high as those observed in control mice (data not shown). Thus, blockade of IL-7R by mAb may specifically suppress the GC reaction under our experimental conditions. Taking these data together, the use of anti–IL-7Rα mAb can be considered advantageous for analyzing the physiological role of reexpressed RAG products.
Figure 7.
GC formation in draining LNs of anti–IL-7Rα–treated immunized mice. Control and anti–IL-7Rα–treated C3H/HeN mice (n = 2) used in Fig. 6 B were analyzed for GC formation in popliteal LNs. (A) GC formation was assessed by staining fixed cryosections of LNs with FITC–peanut agglutinin as described previously (reference 17). A representative image of a GC from each group of mice is shown (original magnification ×100). (B) The number of viable cells per LN (an average of three LNs) and the T to B cell ratio in the LN cells are shown. Flow cytometric analysis was performed by staining the LN cells with PE-labeled anti-B220 and FITC-labeled anti-CD3 (Sigma Chemical Co.).
It has been shown that IL-7 enhances the rearrangement of the T cell receptor β locus in cultured mouse thymocytes by sustaining RAG expression (36), whereas the involvement of IL-7 in B cell development appears to be more complicated. IL-7 has been shown to stimulate the rearrangement at the H chain locus in cultured pre-B cells in the initial phase (24, 25, 37). However, the persistent presence of IL-7 in culture inhibited the subsequent rearrangement of the L chain locus. Conversely, removing IL-7 from the culture resulted in an increase in RAG expression that allowed the cells to undergo L chain gene rearrangement (11, 25). In contrast, we found that IL-7 was effective in inducing RAG expression and the subsequent rearrangement at the λ–L chain locus in mature B cells, suggesting that IL-7 acts on premature and mature B cells in a different way.
It has been pointed out that GCs as primary lymphoid tissue resemble bone marrow, in that molecules characteristically expressed in bone marrow B cells, including RAG proteins (15, 16), λ5 surrogate L chain (18), the heat-stable antigen, Fas, and IL-7, are shared by GC B cells (21, 33). The observation that B cells restore responsiveness to IL-7 in GCs further supports the striking analogy between GCs and bone marrow as microenvironments for B cell development.
In conclusion, these findings and those of previous reports (18–20) show that a V(D)J combination can be secondarily rearranged by reexpressed RAG-1 and RAG-2 proteins in GCs. IL-7 and IL-7R are considered to play a pivotal role in inducing RAG expression. A plausible role for reexpressed RAG products is to revise unwanted antigen receptors that may be generated during somatic hypermutations in GC B cells. Such GC B cell clones may move the differentiation process upstream to an IL-7–responsive premature phenotype and enter the receptor editing pathway.
Acknowledgments
This work was supported in part by a grant-in-aid from The Ministry of Education, Science and Culture of Japan, and by grants from the Nagase Science and Technology Foundation and the Suzuken Memorial Foundation (to H. Ohmori).
Abbreviations used in this paper
- D
diversity
- FDC
follicular dendritic cell
- γc
the γ common chain of IL-7R
- GC
germinal center
- J
joining
- RAG or RAG
recombination activating gene
- RT-PCR
reverse transcriptase– dependent PCR
- V
variable
References
- 1.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 2.Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- 3.Schatz DG, Oettinger MA, Schlissel MS. V(D)J recombination: molecular biology and regulation. Annu Rev Immunol. 1992;10:359–383. doi: 10.1146/annurev.iy.10.040192.002043. [DOI] [PubMed] [Google Scholar]
- 4.van Gent DC, Ramsden DA, Gellert M. The RAG1 and RAG2 proteins establish the 12/23 rule in V(D)J recombination. Cell. 1996;85:107–113. doi: 10.1016/s0092-8674(00)81086-7. [DOI] [PubMed] [Google Scholar]
- 5.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 6.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J Exp Med. 1996;184:1685–1697. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Prak EL, Weigert M. Editing disease-associated autoantibodies. Immunity. 1997;6:97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- 9.Radic M, Zouali M. Receptor editing, immune diversification, and self tolerance. Immunity. 1996;5:505–511. doi: 10.1016/s1074-7613(00)80266-6. [DOI] [PubMed] [Google Scholar]
- 10.Ghia P, Gratwohl A, Singer E, Winkler TH, Melchers F, Rolink AG. Immature B cells from human and mouse bone marrow can change their surface light chain expression. Eur J Immunol. 1995;25:3108–3114. doi: 10.1002/eji.1830251118. [DOI] [PubMed] [Google Scholar]
- 11.Rolink A, Grawunder U, Haasner D, Strasser A, Melchers F. Immature surface Ig+B cells can continue to rearrange kappa and lambda L chain gene loci. J Exp Med. 1993;178:1263–1270. doi: 10.1084/jem.178.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herz M, Nemazee D. BCR ligation induces receptor editing in IgM+IgD−bone marrow B cells in vitro. Immunity. 1997;6:429–436. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 13.Grawunder U, Leu TMJ, Schatz DG, Werner A, Rolink AG, Melchers F, Winkler TH. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 14.Hagman J, Grosschedl R. Regulation of gene expression at early stages of B-cell differentiation. Curr Opin Immunol. 1994;6:222–230. doi: 10.1016/0952-7915(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 15.Hikida M, Mori M, Takai T, Tomochika K, Hamatani K, Ohmori H. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 1996;274:2092–2094. doi: 10.1126/science.274.5295.2092. [DOI] [PubMed] [Google Scholar]
- 16.Han S, Zheng B, Schatz DG, Spanopolou E, Kelsoe G. Neoteny in lymphocytes: RAG1 and RAG2 expression in germinal center B cells. Science. 1996;274:2094–2097. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- 17.Hikida M, Mori M, Kawabata T, Takai T, Ohmori H. Characterization of B cells expressing recombination activating genes in germinal centers of immunized mouse lymph nodes. J Immunol. 1997;158:2509–2512. [PubMed] [Google Scholar]
- 18.Han S, Dillon SR, Zheng B, Shimoda M, Schlissel MS, Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–305. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 19.Papavasiliou F, Casellas R, Suh H, Qin X-F, Besmer E, Pelanda R, Nemazee D, Rajewsky K, Nussenzweig MC. V(D)J recombination in mature B cells: a mechanism for altering antibody responses. Science. 1997;278:298–301. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- 20.Hikida M, Ohmori H. Rearrangement of λ light chain genes in mature B cells in vitro and in vivo. Function of reexpressed recombination-activating gene (RAG) products. J Exp Med. 1998;187:795–799. doi: 10.1084/jem.187.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulendran B, van Driel R, Nossal GJV. Immunological tolerance in germinal centers. Immunol Today. 1997;18:27–32. doi: 10.1016/s0167-5699(97)80011-4. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y-J. Reuse of B lymphocytes in germinal centers. Science. 1997;278:238–239. doi: 10.1126/science.278.5336.238. [DOI] [PubMed] [Google Scholar]
- 23.Grabstein KH, Waldschmidt TJ, Finkelman FD, Hess BW, Alpert AR, Boiani NE, Namen AE, Morrisey PJ. Inhibition of murine B and T lymphopoiesis in vivo by an anti–interleukin 7 monoclonal antibody. J Exp Med. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Candéias S, Muegge K, Durum SK. IL-7 receptor and VDJ recombination: trophic versus mechanistic actions. Immunity. 1997;6:501–508. doi: 10.1016/s1074-7613(00)80338-6. [DOI] [PubMed] [Google Scholar]
- 25.Melamed D, Kench JA, Grabstein K, Rolink A, Nemazee D. A functional B cell receptor transgene allows efficient IL-7-independent maturation of B cell precursors. J Immunol. 1997;159:1233–1239. [PubMed] [Google Scholar]
- 26.Henderson AJ, Narayanan R, Collins L, Dorshkind K. Status of κ L chain gene rearrangements and c-kit and IL-7 receptor expression in stromal cell-dependent pre-B cells. J Immunol. 1992;149:1973–1979. [PubMed] [Google Scholar]
- 27.Hikida M, Takai T, Ohmori H. Requirements of a costimulus for IL-4-induced IgE class switching in murine B cells activated via antigen receptors. Effectiveness of 8-mercaptoguanosine. J Immunol. 1996;156:2730–2736. [PubMed] [Google Scholar]
- 28.Noben-Trauth N, Köhler G, Burki K, Ledermann B. Efficient targeting of the IL-4 gene in a BALB/c embryonic stem cell line. Transgenic Res. 1996;5:487–491. doi: 10.1007/BF01980214. [DOI] [PubMed] [Google Scholar]
- 29.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H, Nishikawa S-I. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci USA. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spanopoulou E, Cortes P, Shin C, Huang C-M, Silver DP, Svec P, Baltimore D. Localization, interaction, and RNA binding properties of the V(D)J recombination-activating proteins RAG1 and RAG2. Immunity. 1995;3:715–726. doi: 10.1016/1074-7613(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 31.van Gent DC, McBlane JF, Ramsden DA, Sadofsky MJ, Hesse JE, Gellert M. Initiation of V(D)J recombination in a cell-free system. Cell. 1995;81:925–934. doi: 10.1016/0092-8674(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 32.Ramsden DA, Gellert M. Formation and resolution of double-strand break intermediates in V(D)J rearrangement. Genes Dev. 1995;9:2409–2420. doi: 10.1101/gad.9.19.2409. [DOI] [PubMed] [Google Scholar]
- 33.Kelsoe G. Life and death in germinal centers (redux) Immunity. 1996;4:107–111. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- 34.Kröncke R, Loppnow H, Flad HD, Gerdes J. Human follicular dendritic cells and vascular cells produce interleukin-7: a potential role for interleukin-7 in the germinal center reaction. Eur J Immunol. 1996;26:2541–2544. doi: 10.1002/eji.1830261040. [DOI] [PubMed] [Google Scholar]
- 35.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 36.Muegge K, Vila MP, Durum SK. Interleukin-7: a cofactor for V(D)J rearrangement of the T cell receptor β gene. Science. 1993;261:93–95. doi: 10.1126/science.7686307. [DOI] [PubMed] [Google Scholar]
- 37.Corcoran AE, Smart FM, Cowling RJ, Crompton T, Owen MJ, Venkitaraman AR. The interleukin-7 receptor α chain transmits distinct signals for proliferation and differentiation during B lymphopoiesis. EMBO (Eur Mol Biol Organ) J. 1996;15:1924–1932. [PMC free article] [PubMed] [Google Scholar]