Abstract
An intact chemotactic response is vital for leukocyte trafficking and host defense. Opiates are known to exert a number of immunomodulating effects in vitro and in vivo, and we sought to determine whether they were capable of inhibiting chemokine-induced directional migration of human leukocytes, and if so, to ascertain the mechanism involved. The endogenous opioid met-enkephalin induced monocyte chemotaxis in a pertussis toxin–sensitive manner. Met-enkephalin, as well as morphine, inhibited IL-8–induced chemotaxis of human neutrophils and macrophage inflammatory protein (MIP)-1α, regulated upon activation, normal T expressed and secreted (RANTES), and monocyte chemoattractant protein 1, but not MIP-1β–induced chemotaxis of human monocytes. This inhibition of chemotaxis was mediated by δ and μ but not κ G protein–coupled opiate receptors. Calcium flux induced by chemokines was unaffected by met-enkephalin pretreatment. Unlike other opiate-induced changes in leukocyte function, the inhibition of chemotaxis was not mediated by nitric oxide. Opiates induced phosphorylation of the chemokine receptors CXCR1 and CXCR2, but neither induced internalization of chemokine receptors nor perturbed chemokine binding. Thus, inhibition of chemokine-induced chemotaxis by opiates is due to heterologous desensitization through phosphorylation of chemokine receptors. This may contribute to the defects in host defense seen with opiate abuse and has important implications for immunomodulation induced by several endogenous neuropeptides which act through G protein–coupled receptors.
Keywords: chemokine, chemokine receptor, opioid receptor, desensitization, neuropeptide
Long-term abuse of opiates results in immune defects, including impaired NK cell activity and altered CD4+ and CD8+ T cell numbers (1), and it has been implicated as a contributing factor in HIV infection (2). In animal models, parenteral use of opiates has been demonstrated to inhibit mitogenic and effector cell responses in B and T cells (3, 4), cellular immunity (5), phagocytosis by neutrophils and macrophages (6, 7), tumor cell suppression (6, 7), and cytokine production (8). Morphine inhibits monocyte and neutrophil functions through the physiologically defined μ3 opiate receptor (9, 10); indeed, binding sites and protein expression for δ and κ subclasses of G protein–coupled opiate receptors (11, 12), in addition to gene expression of δ, κ, and μ subclasses (13–15), have been described in leukocytes.
Chemokines are a large family of chemotactic cytokines that mediate their effects on leukocytes through a number of G protein–coupled, seven transmembrane–spanning (STM)1 receptors (16). Although there is apparent redundancy in the ligand binding characteristics of these chemokine receptors, specificity is provided by patterns of receptor and G protein expression, ligand potency, and levels of receptor desensitization. Interactions among STM receptors are complex (17): in a process known as receptor cross-regulation, or heterologous desensitization, thrombin receptor activation has been shown to cause phosphorylation of several chemoattractant receptors, including the IL-8 receptor CXCR1, the C5a receptor, and the receptor for platelet-activating factor, but not that for FMLP (18). Such cross-regulation does not occur between all classes of STM receptors; for example, α1-adrenergic receptors are unable to desensitize chemoattractant receptors (19). We have shown recently that homologous desensitization through phosphorylation of the IL-8 receptor CXCR2 occurs in response to its native ligands IL-8 and neutrophil-activating peptide (NAP)-2 (20). Together, these findings led us to the hypothesis that the impairment of leukocyte functions mediated by opiates may involve inhibition of chemokine effects based on cross-regulation, resulting in phosphorylation and hence to heterologous desensitization of chemokine receptors. The studies reported here show that opiates, acting through δ and μ subclasses of opiate receptors expressed on human monocytes and neutrophils, are capable of inhibiting subsequent migratory responses to chemokines, and that this process of heterologous desensitization or transdeactivation is associated with phosphorylation of chemokine receptors.
Materials and Methods
Cells.
Human polymorphonuclear neutrophils (21) and monocytes were obtained from healthy donor blood packs. Neutrophils were isolated from human blood by 3% dextran in PBS sedimentation of Ficoll-Hypaque–generated pellets followed by hypotonic lysis (0.2% NaCl) of contaminating red cells. Neutrophils were >95% pure by morphological criteria. Monocytes were purified from Ficoll-Hypaque–generated mononuclear cells using MACS® CD14 microbeads (Miltenyi Biotec Inc., Auburn, CA) as per the manufacturer's instructions. The monocytes were >90% pure by nonspecific esterase staining.
Chemotaxis.
In vitro chemotaxis was performed as described previously (22). In brief, cells were incubated at 37°C with medium, pertussis toxin (Sigma Chemical Co., St. Louis, MO) 500 ng/ml for 90 min, met-enkephalin (Sigma Chemical Co.) in varying concentrations for 60 min, naloxone (Sigma Chemical Co.) 10−8 M for 10 min before met-enkephalin, or naloxone alone. In other experiments, cells were preincubated with opiate receptor-specific agonists or antagonists, or opiate incubation was preceded by pretreatment with the nitric oxide (NO) synthase inhibitor, l-NMMA (N G-monomethyl-l-arginine monoacetate [Sigma Chemical Co.]). Chemotaxis was assessed in 48-well microchemotaxis chambers, using polyvinylpyrrolidone-free 5-μm pore size membranes (Nucleopore; NeuroProbe, Cabin John, MD). Migration in response to met-enkephalin, morphine, chemokines, or FMLP was allowed to continue for 90 min at 37°C in 5% CO2. The membrane was removed, and the upper surface was washed with PBS, scraped, fixed, and stained. The results are expressed as chemotaxis index (mean number of cells per high power field for chemoattractant dilution/mean number of cells per high power field for medium) ± SE from at least three separate experiments.
Intracellular Calcium Flux.
Human peripheral blood monocytes and neutrophils were incubated at 107/ml for 30 min at room temperature in HBSS containing 0.1% BSA and 1 μM Fura-2 (23). Cells were then washed in PBS and incubated with met-enkephalin (10−9 M) or morphine (10−6 M) for 60 min, with or without preincubation for 10 min in naloxone (10−8 M). The cells were washed and resuspended, and Fura-2 excitation was assessed at 340 and 380 nm with detection at 510 nm in a luminescence spectrometer using FL WinLab software (Perkin-Elmer Corp., Norwalk, CT), after stimulation with opiate or chemokine.
Assessment of Receptor Internalization Using Confocal Laser Microscopy.
Rat basophil leukemia cells (RBL2H3) stably transfected to express CXCR1 were derived as described previously (24). Transfected cells were untreated or incubated with IL-8 1 μg/ml for 30 min or with met-enkephalin 10−9 M for 3 h, fixed in 4% paraformaldehyde, and centrifuged onto gelatin-coated slides. Cells were permeabilized in 0.15% saponin before incubation for 60 min with an antiepitope mAb (12CA5; Boehringer Mannheim Biochemicals, Indianapolis, IN). After washing and incubation with FITC-labeled goat anti–mouse IgG, the cells were incubated with DAPI (Sigma Chemical Co.) for 10 min. Slides were examined using a confocal laser scanning microscope (model 310; Carl Zeiss, Inc., Thornwood, NY). Nomarski, FITC (488 nm, green), and DAPI (UV 364 nm, blue) images were prepared for each specimen. Blue and green images were superimposed onto the Nomarski image.
Binding Assays.
Binding of chemokines to their receptors was assessed using 1 ng/ml of 125I-labeled chemokine (NEN Research Products, Boston, MA) in the presence of various concentrations of unlabeled ligands or met-enkephalin (from equimolar to 1,000-fold excess), or after preincubation of the cells with met-enkephalin for 60 min with or without pretreatment for 10 min with naloxone. Cells at 107/ml in RPMI 1640/1% BSA/25 mM Hepes were incubated with ligands for 45 min at room temperature and pelleted through 10% sucrose; cell pellet–associated radioactivity was then determined in a γ-counter. Binding data were analyzed using the computer program LIGAND.
Chemokine Receptor Phosphorylation.
Epitope-tagged FMLP receptor–transfected (25) and CXCR1-transfected RBL2H3 cells were washed, then incubated in phosphate-free media for 3 h. The cells were then labeled with 150 μCi/7.5 × 106 cells of [32P]orthophosphate (NEN Research Products) for 90 min at 37°C before stimulation with native ligands for 5 min, met-enkephalin 10−9 M for 60 min, or naloxone 10−8 M for 10 min before met-enkephalin. Equivalent numbers of cells for each treatment were lysed in RIPA buffer containing protease and phosphatase inhibitors. Epitope-tagged FMLP receptors and CXCR1 were immunoprecipitated as described previously (18) and run in 10% SDS-polyacrylamide gels. The gels were dried and exposed to radiographic film.
Results
Opiate-mediated Inhibition of Chemokine Chemotaxis.
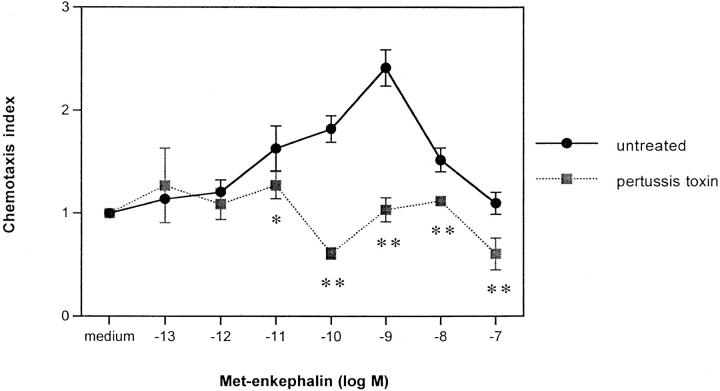
We sought initially to confirm previous reports (26, 27) that opiate compounds, including met-enkephalin, are chemotactic for monocytes and neutrophils. Preliminary studies confirmed that phagocytes respond chemotactically, with a chemotaxis index 2–2.5-fold higher than controls, to met-enkephalin and morphine, with optimal activity at 10−9 and 10−6 M, respectively. This chemotaxis was inhibited by the opiate receptor antagonist naloxone (data not shown). To investigate the mechanism of chemotaxis, monocytes were incubated for 90 min with pertussis toxin 500 ng/ml before exposure to met-enkephalin in microchemotaxis chambers. Fig. 1 demonstrates that the chemotactic activity of met-enkephalin for monocytes was abolished completely by pertussis toxin, suggesting association with the Giα subclass of G proteins.
Figure 1.
Chemotactic response of human peripheral blood monocytes to met-enkephalin and effect of pertussis toxin pretreatment. The results are expressed as chemotaxis index (mean number of cells per high power field for met-enkephalin dilution/mean number of cells per high power field for medium) ± SE from two separate experiments. *P <0.05; **P <0.01.
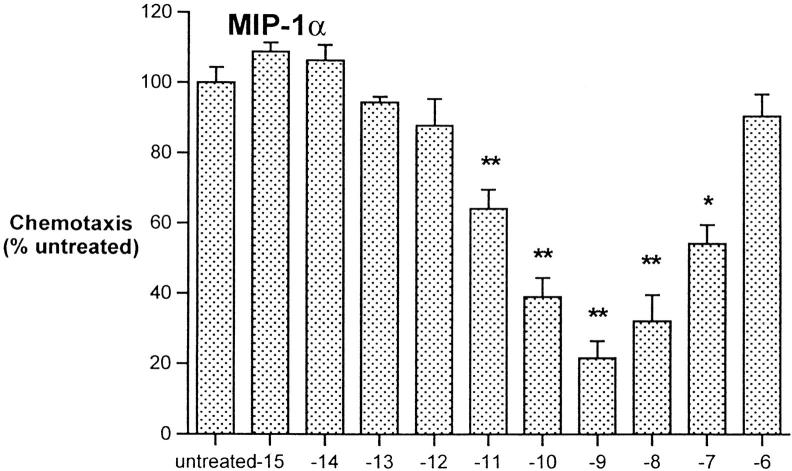
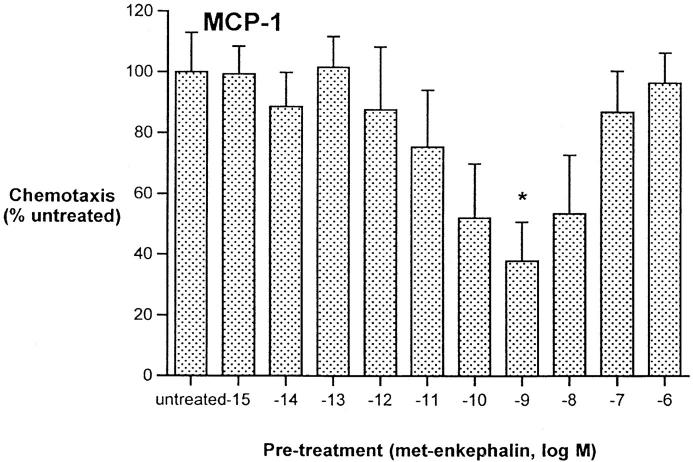
The immunosuppressive and antiinflammatory effects of opiates led us to assess the ability of met-enkephalin to downregulate chemotaxis of monocytes in response to macrophage-inflammatory protein (MIP)-1α and monocyte chemoattractant protein (MCP)-1. Preincubation of freshly isolated human monocytes for 60 min with met-enkephalin at concentrations of 10−15–10−6 M demonstrated that the optimal concentration for inhibition of MIP-1α and MCP-1–induced chemotaxis was equivalent to the optimal chemotactic dose, 10−9 M (Fig. 2). This concentration, as well as the peak chemotactic concentration of 10−6 M for morphine, was used in subsequent inhibition experiments.
Figure 2.
Inhibition of monocyte chemotaxis in response to MIP-1α 100 ng/ml and MCP-1 100 ng/ml, after pretreatment using met-enkephalin. Results are expressed as percentage of chemotactic response of untreated cells. Data from two experiments. *P <0.05; **P <0.01.
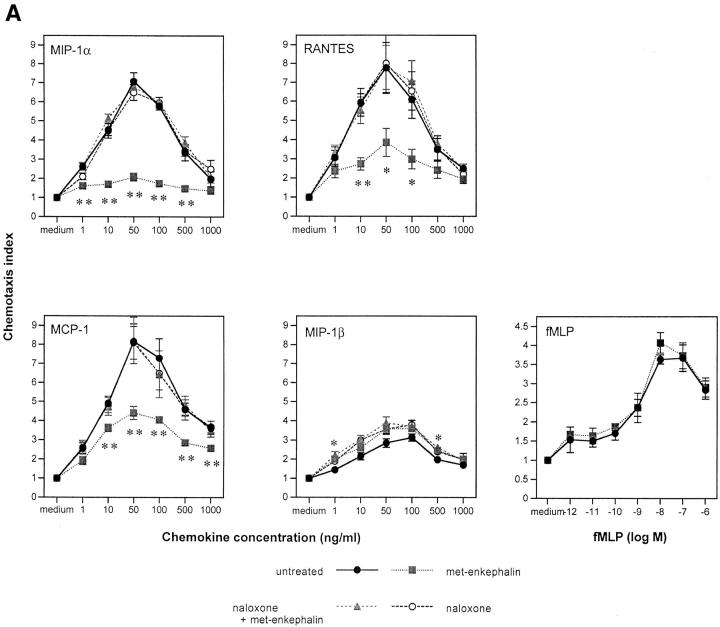
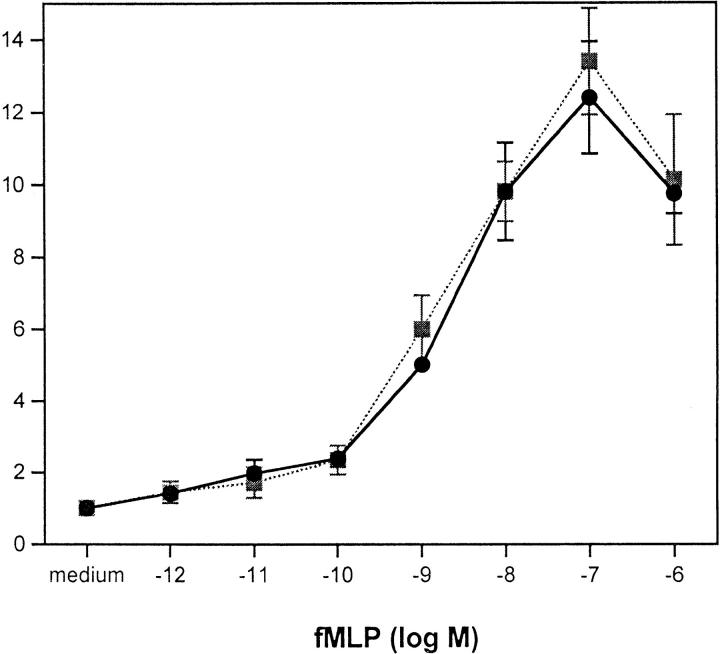
We next assessed the effect of preincubation of monocytes with met-enkephalin on chemotaxis in response to a number of CC chemokines. MIP-1α, regulated upon activation, normal T expressed and secreted (RANTES), and MCP-1 at their optimal concentrations of 50 ng/ml were potent inducers of monocyte chemotaxis but were inhibited by 82, 58, and 52%, respectively, by met-enkephalin (Fig. 3 A). These inhibitory effects were prevented by preincubation with 10−8 M naloxone. In contrast, chemotaxis in response to MIP-1β was not inhibited by met-enkephalin (Fig. 3 A). Although MIP-1α and RANTES act through a number of CC chemokine receptors, including CCR1, CCR3, and CCR5, MIP-1β acts principally through CCR5 (28). These data suggest that some but not all CC chemokine receptors can be inhibited by met-enkephalin, and that the degree of inhibition varies between receptors. Preincubation of monocytes with met-enkephalin also had no effect on chemotaxis induced by FMLP (Fig. 3 A), indicating that the monocyte FMLP receptor, like its neutrophil counterpart, is spared.
Figure 3.
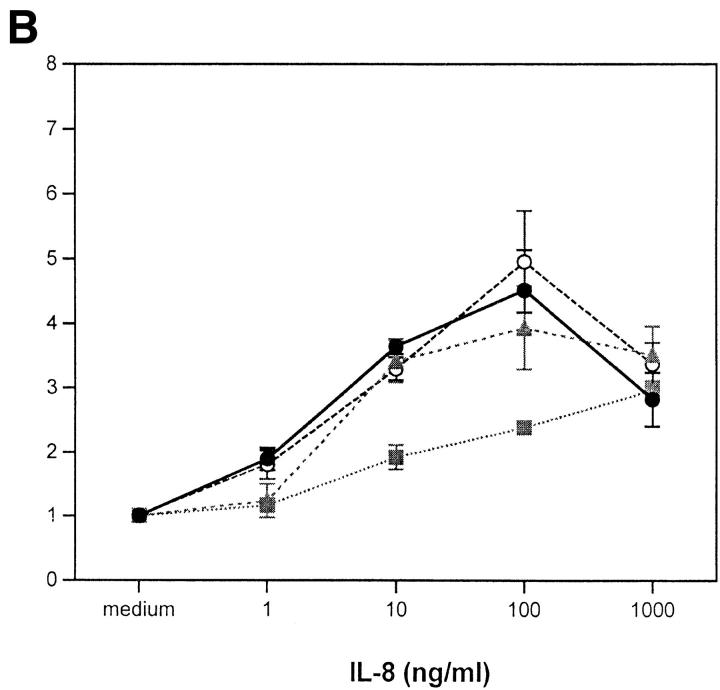
(A) Effects of met-enkephalin on FMLP (fMLP) and CC chemokine–induced monocyte chemotaxis. (B) Effects of met-enkephalin on IL-8– and FMLP (fMLP)-induced neutrophil chemotaxis. The results are expressed as chemotaxis index (mean number of cells per high power field for chemokine dilution/mean number of cells per high power field for medium) ± SE from at least three separate experiments. *P <0.05; **P <0.01.
Preincubation of human neutrophils with met-enkephalin 10−9 M resulted in 61% inhibition of chemotaxis induced by 100 ng/ml IL-8 (Fig. 3 B). Inhibition was reversed by the presence of 10−8 M naloxone during the incubation period, suggesting that this enkephalin-induced effect was opiate receptor–mediated. Naloxone alone, although thought to act as a partial agonist for opiate receptors (29), was ineffective at inhibiting chemotaxis in response to IL-8 (Fig. 3 B). Importantly, FMLP-induced chemotaxis of neutrophils was not inhibited by preincubation with met-enkephalin (Fig. 3 B), indicating that the opiate effect does not apply to all chemoattractant STM receptors.
Opiate Receptor Specificity.
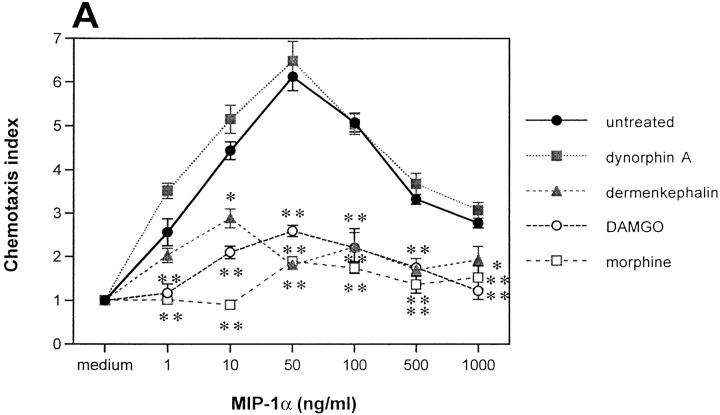
To determine the potential role of morphine in inhibiting chemokine effects and to dissect opiate receptor specificity, monocytes were preincubated with morphine and certain opiate receptor–specific agonists, then tested for their chemotactic response to MIP-1α. Like met-enkephalin, morphine caused significant inhibition (86%) of MIP-1α–induced chemotaxis of monocytes (Fig. 4 A), which was reversed by naloxone. Since morphine is thought to mediate its effects in monocytes primarily through μ3 receptors (30), we examined the response of monocytes to the μ opiate receptor–selective peptide DAMGO ([d-ala2,N-me-phe-gly5(ol)]-enkephalin). Pretreatment with this compound at 10−9 M also resulted in significant inhibition of MIP-1α chemotaxis (76%; Fig. 4 A). Moreover, the δ-selective agonist dermenkephalin at 5 × 10−8 M inhibited MIP-1α chemotaxis by 52%, whereas the κ-selective agonist dynorphin A at 10−8 M caused no significant inhibition (Fig. 4 A). Similar results were observed for neutrophils: DAMGO and dermenkephalin inhibited IL-8–induced chemotaxis by 50 and 34%, respectively, whereas dynorphin A had no effect (data not shown). Together, these results suggest that opiate-mediated inhibition of chemokine-induced chemotaxis occurs through activation of μ and δ but not κ opiate receptors expressed on monocytes and neutrophils.
Figure 4.
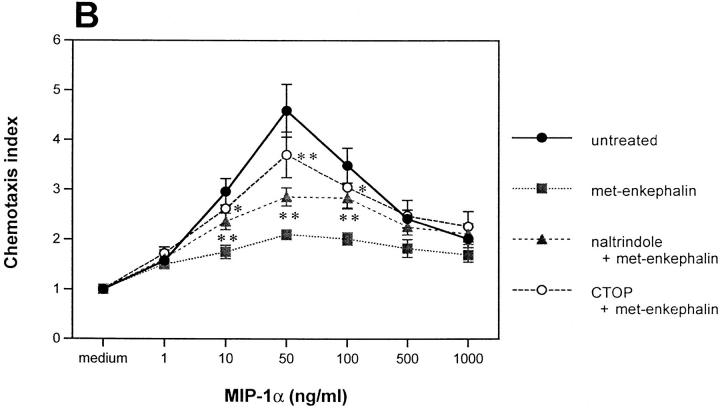
Effects of opiate receptor–specific agonists and antagonists on chemokine-induced monocyte chemotaxis. (A) Effect on MIP-1α–induced chemotaxis of monocytes, of preincubation with the κ-specific agonist dynorphin A, the δ-specific agonist dermenkephalin, the μ-specific agonist DAMGO, and morphine. (B) Effect on MIP-1α–induced chemotaxis of monocytes, of pretreating before met-enkephalin with the δ-specific antagonist naltrindole and the μ-specific antagonist CTOP. *P <0.05; **P <0.01.
To clarify opiate receptor specificity further, monocytes and neutrophils were incubated with the μ-specific antagonist CTOP (cys2, tyr3, orn5, pen7 amide) at 10−7 M or the δ-selective antagonist naltrindole at 2 × 10−8 M before met-enkephalin. CTOP reversed 67% of the met-enkephalin–induced inhibition of MIP-1α–induced monocyte chemotaxis, whereas naltrindole reversed 30% (Fig. 4 B), indicating a greater effect through μ than through δ opiate receptors on monocytes. Combining these antagonists before met-enkephalin treatment reversed completely the met-enkephalin effect. Similarly, preincubation of neutrophils with the μ receptor antagonist CTOP and with the δ antagonist naltrindole reversed 66 and 46% of the met-enkephalin–mediated inhibition of IL-8–induced chemotaxis, respectively (data not shown).
Intracellular Calcium Flux.
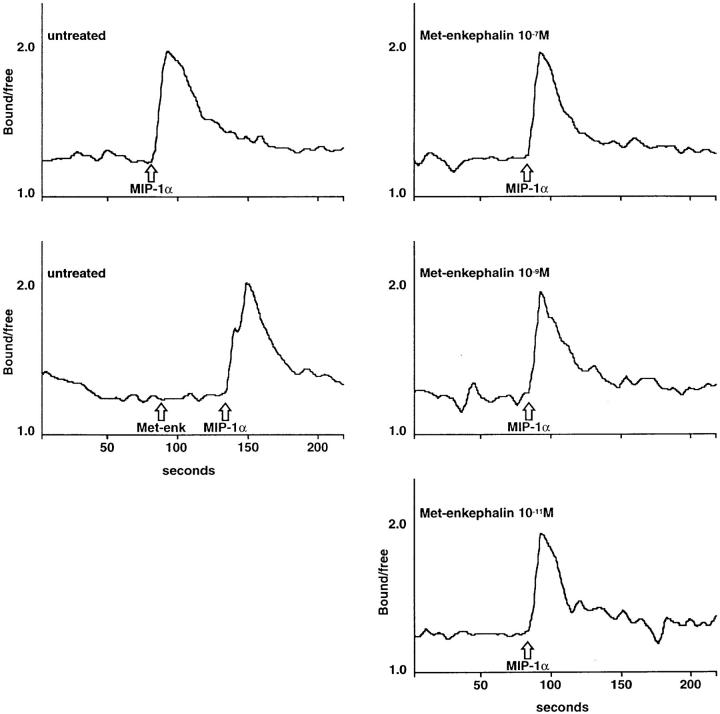
In addition to their ability to generate chemotaxis of leukocytes, chemokines induce intracellular Ca2+ fluxes in many responding cells. Met-enkephalin and morphine were assessed for their ability to induce changes in intracellular Ca2+ concentration, and for any activity in heterologously reducing chemokine–induced Ca2+ mobilization. There was no evidence of direct induction of Ca2+ flux by met-enkephalin in monocytes, nor did preincubation with varying concentrations of met-enkephalin alter the Ca2+ flux induced by activating doses of MIP-1α (Fig. 5). Analysis of the effect of met-enkephalin on the EC50 of MIP-1α demonstrated a Ca2+ flux response at low doses. Peak Ca2+ flux occurred using 10 ng/ml MIP-1α, and the EC50 was ∼2 ng/ml, with no significant difference between untreated and met-enkephalin–pretreated monocytes (data not shown). The chemokines IL-8, NAP-2, MCP-1, MCP-3, and RANTES, and the chemoattractant FMLP, were also assessed in neutrophils and monocytes, as appropriate, and their capacity to induce Ca2+ flux was similarly unaffected by met-enkephalin or morphine pretreatment (data not shown).
Figure 5.
Effect on Ca2+ flux induced by MIP-1α 1 μg/ml in untreated monocytes or monocytes pretreated with met-enkephalin 10−11 M, 10−9 M, and 10−7 M. Met-enkephalin 10−9 M did not induce a Ca2+ flux in these cells. Representative experiment of four similar experiments.
CXCR1 Internalization.
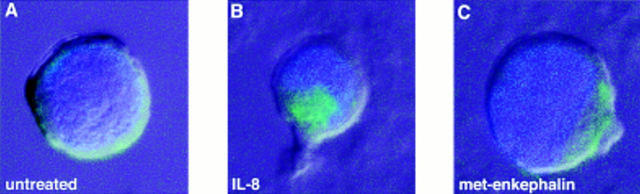
To determine if the ability of opiates to inhibit chemokine-induced chemotaxis arises through chemokine receptor internalization, epitope-tagged CXCR1-transfected RBL2H3 cells (24) were examined by confocal laser microscopy. Initial experiments demonstrated that these cells responded chemotactically to IL-8, and that this chemotactic effect was inhibited in a naloxone-sensitive manner by met-enkephalin pretreatment, confirming the presence of functional opiate receptors (data not shown). After preincubation with IL-8 1 μg/ ml for 30 min, the transfected cells showed prominent internalization of CXCR1, but no internalization was seen in response to preincubation for 60 min using met-enkephalin 10−9 M (Fig. 6). Similar observations were made for CXCR2 on human neutrophils and CCR5 on human monocytes (data not shown).
Figure 6.
Absence of CXCR1 internalization in response to pretreatment of epitope-tagged CXCR1-transfected RBL2H3 cells with met-enkephalin. Transfected cells were (A) untreated, (B) incubated with IL-8 1 μg/ml for 30 min, or with (C) met- enkephalin 10-9 M for 3 h. Representative micrographs from >10 experiments are shown.
Chemokine Binding after Opiate Treatment.
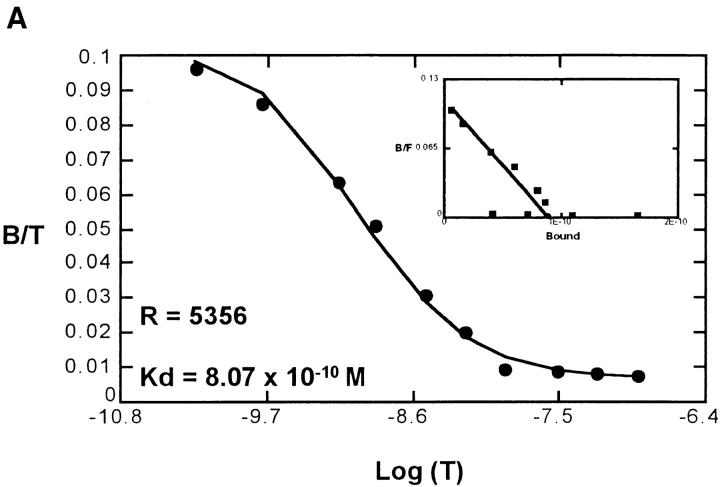
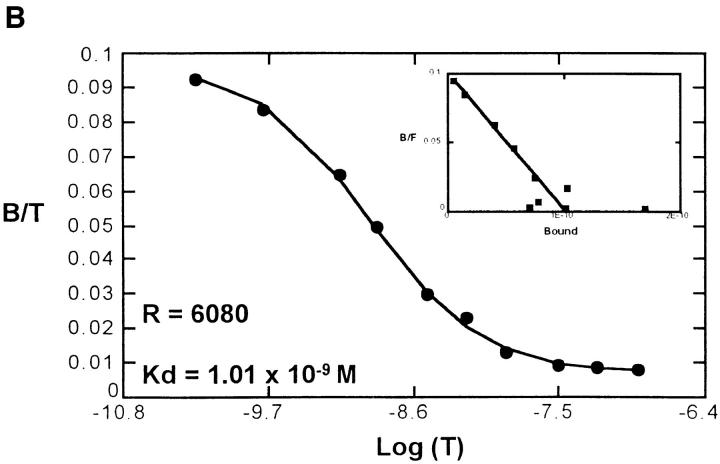
To confirm the lack of receptor internalization in response to opiates, we examined the ability of met-enkephalin to inhibit binding of CXC and CC chemokines to their specific receptors. Using 125I-labeled chemokines and met-enkephalin on fresh human neutrophils and monocytes, there was no evidence in direct competition assays of binding of met-enkephalin to receptors for IL-8, NAP-2, MCP-1, MCP-2, MCP-3, MIP-1α, MIP-1β, or RANTES. Similarly, after preincubation of cells with met-enkephalin for 60 min, there was no impairment of chemokine binding (Fig. 7), suggesting that chemokine receptor affinity and number were not modulated by this duration of met-enkephalin treatment. Therefore, the observed met-enkephalin effects on chemotactic responses were not occurring at the level of chemokine receptor binding of ligands.
Figure 7.
(A) Scatchard analysis of MIP-1α binding to untreated monocytes, showing 5,356 binding sites per cell. After pretreatment of monocytes with met-enkephalin 10-9 M for 60 min (B), the binding characteristics of MIP-1α were unchanged, with 6,080 binding sites per cell detected. Representative plots of two experiments. B/T, Bound/total. B/F, Bound/free.
Involvement of NO.
Recent data suggest that morphine-mediated effects in monocytes and neutrophils may be due to the generation of NO (30). Monocytes treated with the NO synthase inhibitor l-NMMA (N G-monomethyl-l-arginine monoacetate) before incubation with opiates showed no change in the opiate-induced inhibition of MIP-1α chemotaxis, nor did the NO donor diethylamine dinitric oxide (31) at 500 μM, which generates NO at a concentration ∼104-fold higher than the inhibitory concentration thought to be produced by morphine-stimulated monocytes (30), reproduce the opiate effect (data not shown). Thus, opiate-induced inhibition of chemokine effects is not mediated by NO.
Phosphorylation of Chemoattractant Receptors.
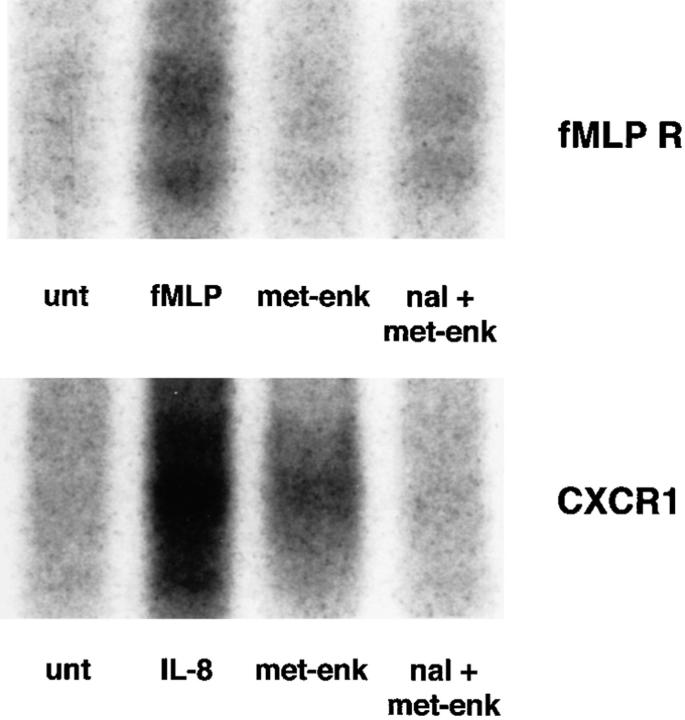
Since opiate molecules, like chemokines, mediate their actions through STM receptors, the possibility that inhibition of chemokines occurred by a process of heterologous desensitization was next examined. For technical reasons, analysis of chemokine receptor phosphorylation in neutrophils and monocytes was unsuccessful; in view of this, phosphorylation was examined in a receptor transfected cell system. Epitope-tagged FMLP receptor–expressing and epitope-tagged CXCR1-expressing RBL2H3 cells were incubated in phosphate-free medium, then with [32P]orthophosphate. They were then stimulated with FMLP 10−6 M (for the FMLP receptor–expressing cells), IL-8 500 ng/ml (for the CXCR1-expressing cells), or met-enkephalin 10−9 M. After whole cell lysis, chemoattractant receptors were immunoprecipitated and examined on SDS-polyacrylamide gels exposed to radiographic film (18). The results demonstrate that in addition to the expected phosphorylation of the chemoattractant receptors induced by the native ligands, CXCR1 was phosphorylated in response to met-enkephalin (Fig. 8). This phosphorylation was inhibited by naloxone (Fig. 8). CXCR2 immunoprecipitated from retinoic acid–differentiated U937 cells also was found to be phosphorylated after exposure of the cells to met-enkephalin (data not shown). The FMLP receptor was not phosphorylated in response to met-enkephalin treatment (Fig. 8).
Figure 8.
Phosphorylation of chemoattractant receptors in response to native ligands, met-enkephalin, or naloxone and met-enkephalin. Equivalent numbers of cells for each treatment were analyzed. Lane 1, Untreated (unt); lane 2, native ligand (FMLP [fMLP] 10-7 M or IL-8 1 μg/ml); lane 3, met-enkephalin (met-enk); lane 4, naloxone plus met-enkephalin (nal + met-enk). Representative of three experiments.
Discussion
This paper describes for the first time a plausible and intriguing mechanism by which opiates may mediate the impaired leukocyte function observed in drug abusers and in animal models of opiate abuse. It also suggests the possibility of an immunomodulatory role for endogenous opiates in controlling leukocyte recruitment. It confirms earlier studies (26, 27) showing that opiate molecules can act directly, albeit weakly, as chemoattractants, and demonstrates that this functional effect is pertussis toxin–sensitive, suggesting coupling of opiate receptors in monocytes to the Giα subclass of G proteins. Although this has been shown for opiate responses in hippocampal slices (32) and μ receptor–transfected Jurkat cells (33), to our knowledge this is the first analysis of G protein use by opiate receptors on native leukocytes.
The heterologous desensitization of chemokine responses observed in these experiments has definite selectivity, since the FMLP receptor is unaffected and CCR5 may also be relatively spared, indicating that there is no generalized impairment of cell motility. The intracellular controls that generate this apparent selectivity remain to be clearly defined, although sparing of the FMLP receptor in heterologous phosphorylation processes has been observed previously and may reflect the absence of protein kinase C phosphorylation sites in intracellular portions of the receptor (18). A further clue to molecular mechanisms of opiate-induced desensitization is suggested by the lack of inhibition of chemokine-induced Ca2+ flux and absence of chemokine receptor internalization by opiates, despite both impairment of the chemotactic response and enhancement of receptor phosphorylation. These features imply a lower requirement for G protein activation by chemokine receptors for Ca2+ mobilization than for chemotaxis. Alternatively, by analogy with studies examining cross-regulation among chemoattractant receptors (34), there may be levels in addition to receptor phosphorylation at which desensitization occurs. Studies are planned to identify the roles of various receptor kinases and intracellular receptor residues involved in the divergent desensitization responses observed here.
The effects of met-enkephalin observed in monocytes and neutrophils are mediated through μ and δ but not κ opiate receptors. All three receptor types are known to be expressed by leukocytes (11–15), and their differential ability to interact with chemokine receptors suggests the possibility of using specific receptor agonists as potential antiinflammatory molecules. The lack of efficacy of κ agonists in inhibiting chemokine effects contrasts with the ability of κ receptor activation to inhibit replication of HIV-1 in microglial cells, the central nervous system equivalent of resident macrophages (15), suggesting divergence between blood monocytes and tissue macrophages in opiate receptor expression or responsiveness. The molecular mechanisms of opiate receptor selectivity in chemokine receptor desensitization remain to be elucidated. Although some opiate-mediated, and specifically morphine-mediated, effects on leukocytes appear to involve NO-dependent devices (30), it is clear from our results that desensitization of chemokine responses by μ and δ opiate receptor activation occurs independently of NO generation.
This study has broad implications for the inhibition of leukocyte responses by opiate molecules, and indeed by other apparently nonimmune mediators acting through STM receptors. The data suggest a mechanism by which opiates function as antiinflammatory or immunosuppressive agents, by interfering with chemokine-induced directional migration of inflammatory cells. That inhibition or absence of chemokines can markedly suppress inflammatory reactions has been demonstrated in several animal models of disease by administration of neutralizing antibodies (35, 36), as well as in virally infected chemokine deletion mutants (37). Therefore, exposure of leukocytes to opiates in vivo may result in profoundly impaired host responses. This may have direct clinical relevance, such as in the setting of analgesia given in the presence of infectious or surgical insults or in the intravenous drug user exposed to infectious agents. However, this study may also have relevance in the potential modulation of immune and inflammatory responses due to endogenous opiate molecules, which among other neuropeptides are known to be released by cells of the immune system (38), and which may act as normal regulators of chemotaxis. These molecules also may play a role in altering inflammatory responses in pathophysiological states such as septic shock (39). Moreover, the finding of opiate-induced neuroimmunomodulation of chemokine function raises the prospect that other neuroendocrine mediators, acting through STM receptors, might operate through similar mechanisms, a possibility we are currently investigating.
Acknowledgments
The authors are grateful to Dr. S.-B. Su for assisting with calcium mobilization experiments; to Drs. P.M. Murphy (National Institute of Allergy and Infectious Diseases, Bethesda, MD), A. Richmond (Vanderbilt University, Nashville, TN), and C. Hébert (Genentech Inc., San Francisco, CA) for supplying antibodies; to Dr. G. Cox of Science Applications International Corp., Frederick, and Dr. R. Shen of ABL-Basic Research Program, National Cancer Institute – Frederick Cancer Research and Development Center, for their helpful advice; and to Drs. R. Neta and S. Durum for reviewing the manuscript.
Abbreviations used in this paper
- CCR
CC chemokine receptor
- CXCR
CXC chemokine receptor
- CTOP
cys2, tyr3, orn5, pen7 amide
- DAMGO
[d-ala2,N-me-phe-gly5(ol)]-enkephalin
- MCP
monocyte chemoattractant protein
- MIP
macrophage-inflammatory protein
- NAP
neutrophil-activating peptide
- NO
nitric oxide
- RANTES
regulated upon activation, normal T expressed and secreted
- STM
seven transmembrane–spanning
Footnotes
This research is sponsored in part by the National Cancer Institute, Department of Health and Human Services, under contract with ABL. The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
References
- 1.Novick DM, Ochshorn M, Ghali V, Croxson TS, Mercer WD, Chiorazzi N, Kreek MJ. Natural killer cell activity and lymphocyte subsets in parenteral heroin abusers and long-term methadone maintenance patients. J Pharmacol Exp Ther. 1989;250:606–610. [PubMed] [Google Scholar]
- 2.Donahoe, R.M., and A. Falek. 1988. Neuroimmunomodulation by opiates and other drugs of abuse: relationship to HIV infection and AIDS. In Psychological, Neuropsychiatric, and Substance Abuse Aspects of AIDS. T.P. Bridge, A.F. Mirsky, and F.K. Goodwin, editors. Raven Press, Ltd., New York. 145–158. [PubMed]
- 3.Bryant HU, Bernton EW, Holaday JW. Immunosuppressive effects of chronic morphine treatment in mice. Life Sci. 1987;41:1731–1738. doi: 10.1016/0024-3205(87)90601-1. [DOI] [PubMed] [Google Scholar]
- 4.Taub DD, Eisenstein TK, Geller EB, Adler MW, Rogers TJ. Immunomodulatory activity of μ- and κ-selective opioid agonists. Proc Natl Acad Sci USA. 1991;88:360–364. doi: 10.1073/pnas.88.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellis NR, Harper C, Dafny N. Suppression of the induction of delayed hypersensitivity in rats by repetitive morphine treatments. Exp Neurol. 1986;93:92–97. doi: 10.1016/0014-4886(86)90148-2. [DOI] [PubMed] [Google Scholar]
- 6.Tubaro E, Borelli G, Croce C, Cavallo G, Santiangeli C. Effect of morphine on resistance to infection. J Infect Dis. 1983;148:656–666. doi: 10.1093/infdis/148.4.656. [DOI] [PubMed] [Google Scholar]
- 7.Pacifici R, Patrini G, Venier I, Parolaro D, Zuccaro P, Gori E. Effect of morphine and methadone acute treatment on immunological activity in mice: pharmacokinetic and pharmacodynamic correlates. J Pharmacol Exp Ther. 1994;269:1112–1116. [PubMed] [Google Scholar]
- 8.Bhargava HN, Thomas PT, Thorat S, House RV. Effects of morphine tolerance and abstinence on cellular immune function. Brain Res. 1994;642:1–10. doi: 10.1016/0006-8993(94)90899-0. [DOI] [PubMed] [Google Scholar]
- 9.Stefano GB, Digenis A, Spector S, Leung MK, Bilfinger TV, Makman MH, Scharrer B, Abumrad NN. Opiate-like substances in an invertebrate, an opiate receptor on invertebrate and human immunocytes, and a role in immunosuppression. Proc Natl Acad Sci USA. 1993;90:11099–11103. doi: 10.1073/pnas.90.23.11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makman MH, Bilfinger TV, Stefano GB. Human granulocytes contain an opiate alkaloid-selective receptor mediating inhibition of cytokine-induced activation and chemotaxis. J Immunol. 1995;154:1323–1330. [PubMed] [Google Scholar]
- 11.Carr DJ, Kim CH, deCosta B, Jacobson AE, Rice KC, Blalock JE. Evidence for a δ-class opioid receptor on cells of the immune system. Cell Immunol. 1988;116:44–51. doi: 10.1016/0008-8749(88)90208-0. [DOI] [PubMed] [Google Scholar]
- 12.Carr DJ, deCosta BR, Kim CH, Jacobson AE, Guarcello V, Rice KC, Blalock JE. Opioid receptors on cells of the immune system: evidence for δ- and κ-classes. J Endocrinol. 1989;122:161–168. doi: 10.1677/joe.0.1220161. [DOI] [PubMed] [Google Scholar]
- 13.Chuang LF, Chuang TK, Killam KF, Jr, Chuang AJ, Kung HF, Yu L, Chuang RY. Delta opioid receptor gene expression in lymphocytes. Biochem Biophys Res Commun. 1994;202:1291–1299. doi: 10.1006/bbrc.1994.2071. [DOI] [PubMed] [Google Scholar]
- 14.Chuang TK, Killam KF, Jr, Chuang LF, Kung HF, Sheng WS, Chao CC, Yu L, Chuang RY. Mu opioid receptor gene expression in immune cells. Biochem Biophys Res Commun. 1995;216:922–930. doi: 10.1006/bbrc.1995.2709. [DOI] [PubMed] [Google Scholar]
- 15.Chao CC, Gekker G, Hu S, Sheng WS, Shark KB, Bu D-F, Archer S, Bidlack JM, Peterson PK. κ opioid receptors in human microglia downregulate human immunodeficiency virus 1 expression. Proc Natl Acad Sci USA. 1996;93:8051–8056. doi: 10.1073/pnas.93.15.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy PM. Chemokine receptors: structure, function and role in microbial pathogenesis. Cytokine Growth Factor Rev. 1996;7:47–64. doi: 10.1016/1359-6101(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 17.Snyderman, R., and R.J. Uhing. 1992. Chemoattractant stimulus-response coupling. In Inflammation: Basic Principles and Clinical Correlates. 2nd ed. J.I. Gallin, I.M. Goldstein, and R. Snyderman, editors. Raven Press, Ltd., New York. 421–439.
- 18.Ali H, Tomhave ED, Richardson RM, Haribabu B, Snyderman R. Thrombin primes responsiveness of selective chemoattractant receptors at a site distal to G protein activation. J Biol Chem. 1996;271:3200–3206. doi: 10.1074/jbc.271.6.3200. [DOI] [PubMed] [Google Scholar]
- 19.Didsbury JR, Uhing RJ, Tomhave E, Gerard C, Gerard N, Snyderman R. Receptor class desensitization of leukocyte chemoattractant receptors. Proc Natl Acad Sci USA. 1991;88:11564–11568. doi: 10.1073/pnas.88.24.11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Baruch A, Grimm M, Bengali K, Evans GA, Chertov O, Wang JM, Howard OMZ, Mukaida N, Matsushima K, Oppenheim JJ. The differential ability of IL-8 and NAP-2 to induce attenuation of chemotaxis is mediated by their divergent capabilities to phosphorylate CXCR2 (IL-8RB) J Immunol. 1997;158:5927–5933. [PubMed] [Google Scholar]
- 21.Johnston JA, Ferris DK, Wang JM, Longo DL, Oppenheim JJ, Kelvin DJ. Staurosporine restores signaling and inhibits interleukin-8-induced chemotactic desensitization. Eur J Immunol. 1994;24:2556–2562. doi: 10.1002/eji.1830241044. [DOI] [PubMed] [Google Scholar]
- 22.Wang JM, McVicar DW, Oppenheim JJ, Kelvin DJ. Identification of RANTES receptors on human monocytic cells: competition for binding and desensitization by homologous chemotactic cytokines. J Exp Med. 1993;177:699–705. doi: 10.1084/jem.177.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badolato R, Johnston JA, Wang JM, McVicar D, Xu LL, Oppenheim JJ, Kelvin DJ. Serum amyloid A induces calcium mobilization and chemotaxis of human monocytes by activating a pertussis toxin-sensitive signaling pathway. J Immunol. 1995;155:4004–4010. [PubMed] [Google Scholar]
- 24.Richardson RM, DuBose RA, Ali H, Tomhave ED, Haribabu B, Snyderman R. Regulation of human interleukin-8 receptor A: identification of a phosphorylation site involved in modulating receptor functions. Biochemistry. 1995;34:14193–14201. doi: 10.1021/bi00043a025. [DOI] [PubMed] [Google Scholar]
- 25.Ali H, Richardson RM, Tomhave ED, Didsbury JR, Snyderman R. Differences in phosphorylation of formylpeptide and C5a chemoattractant receptors correlate with differences in desensitization. J Biol Chem. 1993;268:24247–24254. [PubMed] [Google Scholar]
- 26.Simpkins CO, Dickey CA, Fink MP. Human neutrophil migration is enhanced by beta-endorphin. Life Sci. 1984;34:2251–2255. doi: 10.1016/0024-3205(84)90213-3. [DOI] [PubMed] [Google Scholar]
- 27.Van Epps DE, Saland L. β-Endorphin and Met-enkephalin stimulate human peripheral blood mononuclear cell chemotaxis. J Immunol. 1984;132:3046–3053. [PubMed] [Google Scholar]
- 28.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein A. Interactions of narcotic antagonists with receptor sites. Adv Biochem Psychopharmacol. 1973;8:471–481. [PubMed] [Google Scholar]
- 30.Magazine HI, Liu Y, Bilfinger TV, Fricchione GL, Stefano GB. Morphine-induced conformational changes in human monocytes, granulocytes, and endothelial cells and in invertebrate immunocytes and microglia are mediated by nitric oxide. J Immunol. 1996;156:4845–4850. [PubMed] [Google Scholar]
- 31.Sheffler LA, Wink DA, Melillo G, Cox GW. Characterization of nitric oxide-stimulated ADP-ribosylation of various proteins from the mouse macrophage cell line ANA-1 using sodium nitroprusside and the novel nitric oxide-donating compound diethylamine dinitric oxide. J Leukocyte Biol. 1995;57:152–159. doi: 10.1002/jlb.57.1.152. [DOI] [PubMed] [Google Scholar]
- 32.Dunwiddie TV, Su MT. Pertussis toxin pretreatment antagonizes the actions of μ- and δ-opiate agonists in hippocampal slices. Neurosci Lett. 1988;95:329–334. doi: 10.1016/0304-3940(88)90680-5. [DOI] [PubMed] [Google Scholar]
- 33.Sharp BM, Shahabi NA, Heagy W, McAllen K, Bell M, Huntoon C, McKean DJ. Dual signal transduction through δ opioid receptors in a transfected human T-cell line. Proc Natl Acad Sci USA. 1996;93:8294–8299. doi: 10.1073/pnas.93.16.8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson RM, Ali H, Tomhave ED, Haribabu B, Snyderman R. Cross-desensitization of chemoattractant receptors occurs at multiple levels—evidence for a role for inhibition of phospholipase C activity. J Biol Chem. 1995;270:27829–27833. doi: 10.1074/jbc.270.46.27829. [DOI] [PubMed] [Google Scholar]
- 35.Broaddus VC, Boylan AM, Hoeffel JM, Kim KJ, Sadick M, Chuntharapai A, Hébert CA. Neutralization of IL-8 inhibits neutrophil influx in a rabbit model of endotoxin-induced pleurisy. J Immunol. 1994;152:2960–2967. [PubMed] [Google Scholar]
- 36.Wada T, Tomosugi N, Naito T, Yokoyama H, Kobayashi K, Harada A, Mukaida N, Matsushima K. Prevention of proteinuria by the administration of anti-interleukin 8 antibody in experimental immune complex–induced glomerulonephritis. J Exp Med. 1994;180:1135–1140. doi: 10.1084/jem.180.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O. Requirement of MIP-1α for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 38.Blalock JE. The syntax of immune-neuroendocrine communication. Immunol Today. 1994;15:504–511. doi: 10.1016/0167-5699(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 39.Harbour DV, Galin FS, Hughes TK, Smith EM, Blalock JE. Role of leukocyte-derived pro-opiomelanocortin peptides in endotoxic shock. Circ Shock. 1991;35:181–191. [PubMed] [Google Scholar]