Abstract
The incidence of septic shock caused by gram-positive bacteria has risen markedly in the last few years. It is largely unclear how gram-positive bacteria (which do not contain endotoxin) cause shock and multiple organ failure. We have discovered recently that two cell wall fragments of the pathogenic gram-positive bacterium Staphylococcus aureus, lipoteichoic acid (LTA) and peptidoglycan (PepG), synergize to cause the induction of nitric oxide (NO) formation, shock, and organ injury in the rat. We report here that a specific fragment of PepG, N-acetylglucosamine-β-[1→ 4]-N-acetylmuramyl-l-alanine–d-isoglutamine, is the moiety within the PepG polymer responsible for the synergism with LTA (or the cytokine interferon γ) to induce NO formation in the murine macrophage cell line J774.2. However, this moiety is also present in the PepG of the nonpathogenic bacterium Bacillus subtilis. We have discovered subsequently that S. aureus LTA synergizes with PepG from either bacterium to cause enhanced NO formation, shock, and organ injury in the rat, whereas the LTA from B. subtilis does not synergize with PepG of either bacterium. Thus, we propose that the structure of LTA determines the ability of a particular bacterium to cause shock and multiple organ failure (pathogenicity), while PepG acts to amplify any response induced by LTA.
Keywords: gram-positive shock, nitric oxide, peptidoglycan, lipoteichoic acid
Aresurgence of nosocomial gram-positive infections was first noticed in the early 1980s. Today, between one third and half of all cases of sepsis are caused by gram-positive organisms, and it is likely that the incidence of gram-positive sepsis will continue to rise and predominate in the years to come (1, 2). Gram-positive organisms do not contain LPS (or endotoxin), which is the cell wall component of gram-negative bacteria responsible for the initiation of gram-negative septic shock. However, gram-positive bacteria can cause septic shock and multiple organ failure without causing endotoxemia (3, 4), and endotoxin is not always found in the serum of patients with septic shock (1, 2). The cell wall of gram-positive bacteria contains lipoteichoic acid (LTA)1 and peptidoglycan (PepG), which themselves can activate leukocytes, stimulate the generation of proinflammatory cytokines, and hence, cause a moderate systemic inflammatory response syndrome (5–8).
The endogenous vasodilator autacoid nitric oxide (NO) is generated by three different isoforms of NO synthase (NOS), two of which are expressed constitutively (eNOS in endothelium, nNOS in brain), the third (iNOS) induced by endotoxin (LPS) or cytokines (9). There is now substantial evidence that an enhanced formation of NO by iNOS contributes to the circulatory failure (hypotension, and vascular hyporeactivity to vasoconstrictors) and possibly the organ injury associated with endotoxemia (10–12).
LTA is a macroamphiphile, equivalent to LPS in gram-negative bacteria, containing a substituted poly-glycerophosphate backbone attached to a glycolipid (for a review, see reference 13). The glycolipid content in LTA resembles the bacterial membrane composition, which usually varies in a genus-specific manner (13, 14). LTA from Staphylococcus aureus can cause a moderate induction of iNOS (15–17) which (in murine macrophages) requires the activation of tyrosine kinases and NF-κB (17). Although LTA from S. aureus can cause moderate hypotension in the rat, LTA (unlike S. aureus itself) does not cause multiple organ failure or death in this species (16). However, we have discovered recently that LTA and PepG act in synergy to release TNF-α and IFN-γ, to induce iNOS, and to cause shock and multiple organ failure in anesthetized rats (18, 19). The mechanism by which LTA and PepG act in synergy is not clear. In addition, it is not known which of the structural components of PepG (or LTA) is essential for the observed synergism.
PepG is a large polymer that provides stress resistance and shape-determining properties to bacterial cell walls. This polymer contains long sugar chains of two alternating sugar derivatives, N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM), which are highly cross-linked by peptide subunits and bridges (see Fig. 1). The peptide subunit (or stem peptide) consists of alternating l– and d–amino acids, up to four or five in length, and is connected to the COOH group of NAM. Among different bacterial species, the structure of the sugar chains is highly preserved, while the composition of the peptide subunits varies.
Figure 1.
Structure of S. aureus PepG. The shaded area on the PepG monomer shows the minimal component that accounts for the ability of PepG to synergize with LTA (or IFN-γ) to induce NO formation in J774.2 macrophages. The points at which enzymes lyse PepG are identified by markers ( filled circle, mutanolysin or M1; open square, lysostaphin; filled triangle, SALE). Numbers, Amino acid position on the stem peptide.
This study was designed to investigate which of the structural elements of PepG are essential to synergize with LTA to cause the induction of iNOS in macrophages, and shock and multiple organ failure in vivo. Two distinct gram-positive organisms were used: S. aureus, a bacterium most commonly implicated in gram-positive sepsis (1), and Bacillus subtilis, a nonpathogenic bacterium. We have identified a structural moiety present in the PepG polymer of both S. aureus and B. subtilis that is essential to synergize with LTA or IFN-γ to induce iNOS in macrophages. Furthermore, we provide evidence that the origin of LTA but not PepG determines whether the cell wall components from a gram-positive organism synergize to induce iNOS and multiple organ failure.
Materials and Methods
Bacterial Cell Wall Components.
Cell walls from vegetative cells of B. subtilis and S. aureus were prepared as described previously (20). Secondary polymers (teichoic and teichiuronic acids) were removed from the PepG by treatment of cell walls (10 mg dry wt/ml) with 48% vol/vol hydrofluoric acid at 4°C for 24 h. The pure PepG was then washed six times, by centrifugation (14,000 g, 5 min) and resuspension in distilled water, until the pH was neutral before storage at −20°C. In one study, the PepG polymer of S. aureus was digested by various enzymes to further investigate the structural requirements of PepG for the induction of iNOS. The PepG polymer (10 μg/ml) and the coinducer IFN-γ (10 IU/ml) were incubated in DMEM for 1 h at 37°C with various enzymes (at 10 μg/ml) which hydrolyze the PepG polymer at specific points (Fig. 1). Previous studies by Timmerman et al. (21) have shown that this procedure is sufficient to completely digest the PepG polymer.
In one experiment, the LTA extract of S. aureus (obtained from Sigma Chemical Co., Poole, Dorset, UK) was further purified using an octyl–sepharose column (CL-4B, 1.5 × 11 cm). LTA dissolved in equilibration buffer consisting of 0.1 M sodium acetate (pH 4.7) and 15% vol/vol propan-1-ol was loaded on the column. The column was then washed using equilibration buffer. Subsequently, bound components from the LTA extract were eluted using a linear gradient of propan-1-ol (15–100% vol/vol in equilibration buffer), and fractions of 2 ml vol were collected. The fractions were freeze dried and resuspended in distilled water. Phosphate concentration in the samples was determined as described by Kusunoki et al. (22).
Cell Culture Experiments.
Murine macrophages (J774.2; The European Collection of Animal Cell Cultures, Salisbury, UK) were cultured in 96-well plates with DME (200 μl/well) containing 10% FCS (GIBCO BRL, Paisley, UK) and l-glutamine (4 mM). Rat aortic smooth muscle cells (RASMC, A10 cell line; The European Collection of Animal Cell Cultures) were cultured in 96-well plates with RPMI 1640 medium (200 μl/well) containing 10% FCS and l-glutamine (4 mM). The 96-well plates containing the cells were then incubated at 37°C in a humidified incubator until the cells reached confluence. Cultured cells were activated by the isolated bacterial cell wall components or fragments, and after 24 h, the accumulation of nitrite in the supernatant was measured as an indication of iNOS activity. Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamide in 5% phosphoric acid) was added to the culture medium, and the difference in OD between 550 and 650 nm was measured. The nitrite concentrations were calculated using standard solutions of sodium nitrite prepared in culture medium (23).
Cell viability was assessed by the mitochondrial reduction of 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan (23). After removal of supernatant for nitrite measurements, cells were incubated with MTT (0.4 mg/ml) for 30 min at 37°C. Medium was removed by aspiration, cells were solubilized in DMSO, and the reduction of MTT was measured by spectrometry at 550 nm. Unless otherwise stated, there were no significant changes in the cell viability after various treatments of either macrophages or RASMC.
Immunoblot (Western Blot) Analysis for iNOS.
Western blot analysis was used to determine the expression of iNOS protein. After exposure of J774.2 macrophages to the relevant cell wall components, cells were suspended in homogenization buffer comprised of Tris-HCl (50 mM), EDTA (10 mM), Triton X-100 (1% vol/ vol), and the protease inhibitors pepstatin A (50 μM), leupeptin (0.2 mM), and PMSF (1 mM). The homogenate was boiled for 5 min with gel loading buffer (Tris 20 mM, EDTA 2 mM, SDS 2% wt/vol, glycerol 20% vol/vol, 2-ME 10% vol/vol, and bromphenol blue 2 mg/ml, pH 6.8) in a ratio of 1:1 (vol/vol). Total protein equivalents for each sample were resolved on SDS/7.5% vol/ vol polyacrylamide gels and transferred to nitrocellulose. The membranes were incubated for 20 h at 4°C with a rabbit polyclonal antibody raised to murine macrophage iNOS (24). Bands were detected using a peroxidase-conjugated anti–rabbit IgG and 3,3'-diaminobenzidine or enhanced chemiluminescence (Amersham International, Little Chalfont, Buckinghamshire, UK). The expression of immunoreactive iNOS was quantified by scanning densitometry (imaging densitometer model GS-700; Bio-Rad Laboratories, Hemel Hempstead, Hertfordshire, UK).
In Vivo Experiments.
Male Wistar rats (240–300 g; Glaxo Wellcome PLC, Greenford, Middlesex, UK) were anesthetized with thiopentone sodium (120 mg/kg, i.p.). The trachea was cannulated to facilitate respiration, and the rectal temperature was maintained at 37°C using a homeothermic blanket system (Harvard Apparatus Ltd., Edenbridge, Kent, UK). The right carotid artery was cannulated and connected to a pressure transducer (SensoNor 840; SensoNor A.S., Horten, Norway) for the measurement of mean arterial blood pressure and heart rate (MacLab 8; AD Instruments, Hastings, UK). The jugular vein was cannulated for the administration of compounds. Cardiovascular parameters were allowed to stabilize, and at time 0, animals received (a) S. aureus LTA (3 mg/ kg), (b) B. subtilis LTA (3 mg/kg), (c) B. subtilis PepG (1 mg/kg), (d) S. aureus LTA (3 mg/kg) and B. subtilis PepG (1 mg/kg), (e) B. subtilis LTA (3 mg/kg) and B. subtilis PepG (1 mg/kg), or (f) saline (0.3 ml 0.9% NaCl). Hemodynamic parameters were measured for 6 h. The pressor response to norepinephrine was assessed 15 min before and every hour after the administration of the bacterial cell wall components. At the end of the experiment at 6 h, a blood sample was obtained for the measurement of markers of organ failure. Serum concentrations of creatinine, urea (indicators of the development of renal dysfunction), and alanine aminotransferase (ALT, an indicator of the development of liver injury) (25, 26) were analyzed by a contract laboratory for veterinary clinical chemistry (Vetlab Services, West Sussex, UK). Lungs were collected and stored (−80°C) for the determination of iNOS activity.
Lung iNOS Activity Assay.
The activity of iNOS in lung homogenates was determined by measuring the conversion of [3H]l-arginine to [3H]l-citrulline in the absence of free calcium. Frozen lungs were homogenized on ice in a buffer composed of Tris-HCl (50 mM), EDTA (0.1 mM), EGTA (0.1 mM), 2-ME (12 mM), and PMSF (1 mM), pH 7.4. Lung homogenates (∼100 μg protein) were incubated in the presence of [3H]l-arginine (7.5 kBq per tube of 100 μl), l-arginine (10 μM), NADPH (1 mM), calmodulin (300 IU/ml), tetrahydrobiopterin (5 μM), l-valine (50 mM), and EDTA (1 mM) for 30 min at 25°C in Tris-buffer. Reactions were stopped by addition of 1 ml ice-cold Hepes buffer (pH 5.5) containing EGTA (2 mM) and EDTA (2 mM). After separation using Dowex 50W (sodium form) columns, the eluted [3H]l-citrulline activity was measured by scintillation counting. Experiments performed in the absence of NADPH determined the extent of [3H]l-citrulline formation independent of iNOS activity.
Materials and Drug Solutions.
Unless otherwise stated, all compounds were obtained from Sigma Chemical Co. l-[2,3,4,5-3H]arginine hydrochloride was obtained from Amersham International, and tetrahydrobiopterin (6R-l-erythro-5,6,7,8-tetra-hydrobiopterin) was obtained from Schircks Laboratoire (Jona, Switzerland). Thiopentone sodium was obtained from Rhône Merieux Ltd. (Harlow, Essex, UK). The PepG fragments were prepared by Dr. S.J. Foster (University of Sheffield). S. aureus lytic enzyme (SALE) was obtained from Dr. L. De Graaf (University of Utrecht, Utrecht, The Netherlands). Murine iNOS antibody was a gift from Dr. Claire Bryant (William Harvey Research Institute). All stock solutions were prepared in nonpyrogenic saline (0.9% NaCl; Baxter Healthcare Ltd., Thetford, Norfolk, UK), and care was taken to prevent endotoxin contamination. For cell culture experiments, all solutions of agents were sterilized by filtration through a filter (pore size 0.22 μm).
Statistical Analysis.
All data are presented as means ± SE of n observations. For the cell culture experiments, at least three independent experiments were performed in triplicate. In anesthetized rats, the pressor response to norepinephrine was calculated as the area under the response and expressed as mmHg·min. Statistical analysis was performed either by one-way analysis of variance for multiple comparison or by Student's t test as appropriate. If the analysis of variance yielded significance (P <0.05), Dunnett's test for multiple comparisons with one control mean or Bonferroni's test for multiple comparison on selected single means was performed (27).
Results
Structural Requirement of PepG to Synergize with IFN-γ or LTA to Induce iNOS.
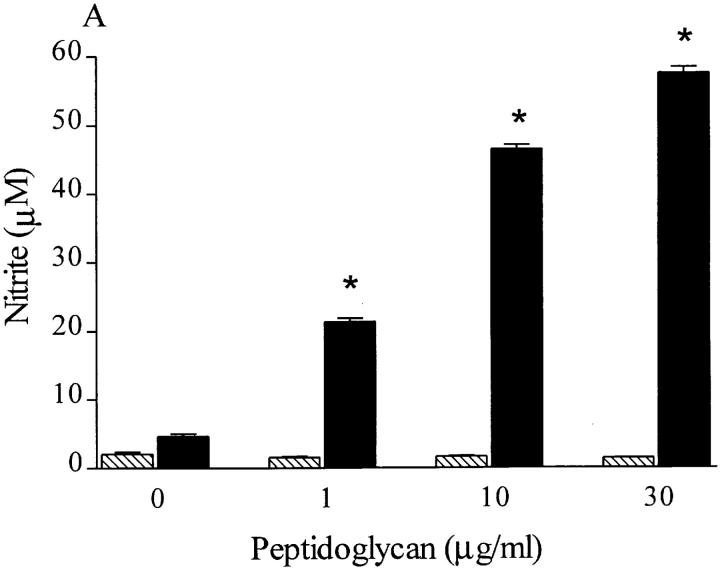
Incubation of J774.2 macrophages with PepG from S. aureus (1–30 μg/ml) in the presence of IFN-γ (10 IU/ml) resulted in the accumulation of nitrite after 24 h (Fig. 2 A). PepG alone (up to 100 μg/ml) did not cause an increase in nitrite accumulation (Fig. 2 A; 2.0 ± 0.1 M nitrite for 100 μg/ml PepG). The enhanced formation of nitrite caused by PepG (10 μg/ml) in the presence of IFN-γ (10 IU/ml) was prevented, in a concentration-dependent manner, by the NOS inhibitors N G-methyl- l-arginine (L-NMMA; IC50 = 80 μM) or aminoethyl-isothiourea (AE-ITU; IC50 = 15 μM) (Fig. 2 B). Western blot analysis revealed an increase in the expression of an immunoreactive iNOS protein of ∼130 kD in J774 macrophages challenged with PepG (10 μg/ml) and IFN-γ (10 IU/ml) for 24 h (Fig. 2 C). In contrast, neither PepG nor IFN-γ alone caused the expression of iNOS protein. Additionally, the expression of iNOS protein in cells treated with LTA (0.1 μg/ml) and PepG (10 μg/ml) was greater than the level of iNOS expression caused by these components alone (Fig. 2 C).
Figure 2.
(A) S. aureus PepG concentration-dependently increases the nitrite accumulation over 24 h in the presence (black bars) but not the absence (hatched bars) of IFN-γ. (B) The increase in nitrite formation caused by PepG (10 μg/ml) plus IFN-γ (10 IU/ml) was concentration-dependently prevented by either L-NMMA (hatched bars) or AE-ITU (black bars). (C) Western (immuno-)blot analysis of macrophage extracts using an iNOS-specific antibody revealed a significant expression of iNOS protein induced by PepG (10 μg/ml) plus IFN-γ (10 IU/ml) (lane d). In contrast, the expression of iNOS protein in untreated macrophages (lane a) or in macrophages treated with IFN-γ (10 IU/ml; lane b) or PepG (10 μg/ml; lane c) was much less. There was a significantly higher expression of iNOS protein in cells treated with LTA (0.1 μg/ml) and PepG (10 μg/ml) (lane f) compared with LTA alone (lane e). Data are expressed as means ± SE of 12 wells from four independent experiments. *P <0.05 versus (A) untreated cells and (B) PepG plus IFN-γ control response. The Western blot is representative of four separate experiments.
To further elucidate the structural elements of PepG that are essential to induce iNOS activity in the presence of IFN-γ, PepG from S. aureus was digested for 1 h with various enzymes before addition to macrophages. Preincubation with the N-acetylmuramidases mutanolysin or M1, which hydrolyze the PepG backbone between NAM and NAG (see Fig. 1), significantly attenuated the nitrite accumulation caused by S. aureus PepG in the presence of IFN-γ (Fig. 3). Lysozyme, a muramidase which is unable to lyse PepG derived from S. aureus, did not affect the increase in nitrite caused by PepG plus IFN-γ (Fig. 3). The endopeptidase lysostaphin, which breaks the pentaglycine interpeptide cross-bridge, markedly enhanced the formation of nitrite (Fig. 3). In contrast, SALE, which has both endopeptidase and amidase activity (i.e., hydrolyzes the bond NAM– L-ala, which connects the sugar chain and stem peptide), prevented the ability of PepG to synergize with IFN-γ to induce nitrite formation (Fig. 3). These effects were specific for PepG, as the induction of nitrite accumulation by S. aureus LTA (1 μg/ml) was not affected by pretreatment with these enzymes (results not shown).
Figure 3.

Effects of various enzymes that lyse PepG on the nitrite formation induced by PepG (10 μg/ml) plus IFN-γ (10 IU/ml) in macrophages at 24 h. The muramidases, mutanolysin and M1, attenuated significantly the nitrite formation caused by PepG plus IFN-γ. In contrast, lysozyme, a muramidase that does not lyse the PepG from S. aureus, did not affect this response. The endopeptidase lysostaphin significantly enhanced the nitrite formation, whereas SALE, which has both endopeptidase and amidase activities, completely prevented the nitrite formation. Data are expressed as means ± SE of 9–12 wells from three to four independent experiments. *P <0.05 versus PepG plus IFN-γ control response.
Hydrolysis of the PepG polymer affected the induction of iNOS by PepG in the presence of IFN-γ, suggesting that a small moiety within PepG is responsible for the synergism with IFN-γ to cause induction of iNOS. To further elucidate which fragment of PepG is involved, macrophages were incubated with muropeptides, PepG-constituent amino sugars, peptides, and related molecules. A concentration-dependent increase in nitrite formation by macrophages was observed 24 h after the addition of either the NAG-NAM-l-ala-d-isoglutamine fragment (EC50 = 40 nM) or the NAM-l-ala-d-isoglutamine fragment lacking the NAG residue (EC50 = 100 nM) in the presence of IFN-γ (Fig. 4 A). To investigate the specificity of these effects, two stereoisomers of NAM-l-ala-d-isoglutamine were tested: NAM- l-ala-L-isoglutamine and NAM-D-ala-d-isoglutamine. The maximal induction of nitrite formation was decreased significantly with NAM-l-ala-L-isoglutamine and nearly abrogated with NAM-D-ala-d-isoglutamine (Fig. 4 A). Other fragments lacking the NAG-l-ala-d-isoglutamine moiety, including NAM, NAG, stem peptide (l-ala-d-isoglutaminyl-l-lys-d-ala-d-ala), triglycine, and pentaglycine, did not cause an increase in nitrite formation up to a concentration of 100 μg/ml when given either alone or in combination with IFN-γ (results not shown). As with the PepG polymer, NAG-NAM-l-ala-d-isoglutamine or NAM-l-ala- d-isoglutamine alone did not induce nitrite formation in macrophages (2 ± 0.5 and 3 ± 1 μM, respectively, at 100 μg/ml).
Figure 4.
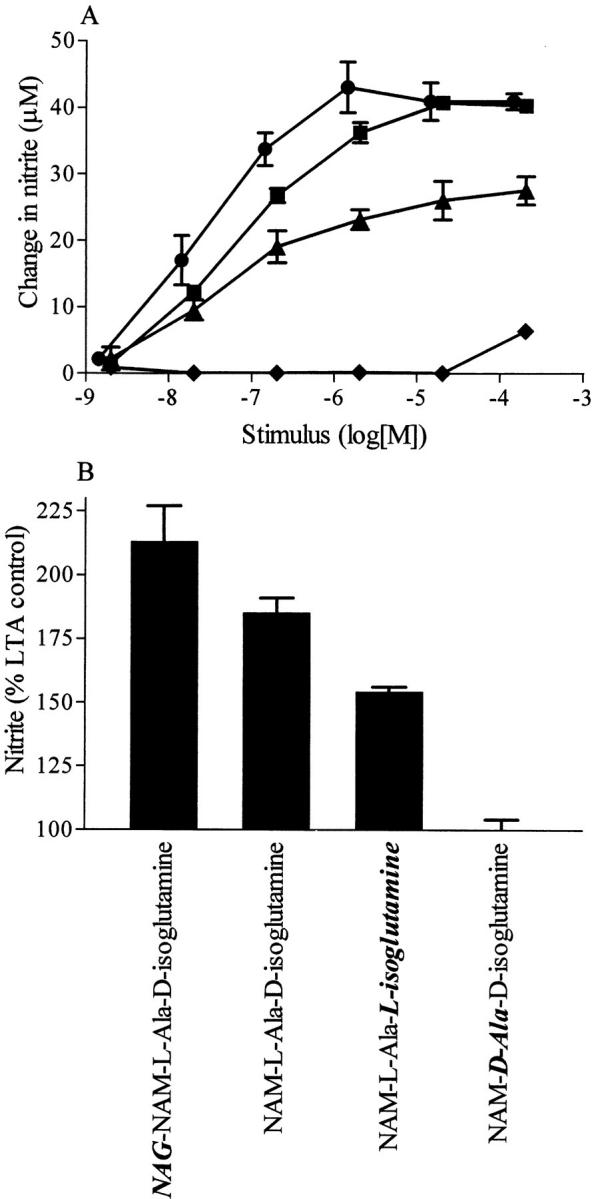
Effects of various fragments of PepG in the presence of (A) IFN-γ or (B) LTA (from S. aureus) on the nitrite accumulation in the supernatant of macrophages. The PepG fragments NAG-NAM-l-ala-d-isoglutamine (A, circles; B, 1 μg/ml) and NAM-l-ala-d-isoglutamine (A, squares; B, 1 μg/ml) concentration-dependently synergized with IFN-γ (10 IU/ml) or LTA (0.1 μg/ml) to cause nitrite accumulation. The NAM-l-ala-L-isoglutamine fragment (A, triangles; B, 1 μg/ml) was less effective, whereas the fragment NAM-D-ala-d-isoglutamine (A, diamonds; B, 100 μg/ml) was inactive in synergizing with IFN-γ or LTA. Data are expressed as means ± SE of 9–12 wells from three to four independent experiments. LTA (0.1 μg/ml) produced 15 ± 1 μM at 24 h.
We have demonstrated previously that the S. aureus PepG polymer synergizes with LTA from the cell wall of S. aureus to induce iNOS in macrophages and in the anesthetized rat (18). In agreement with the present observations, the PepG fragment NAG-NAM-l-ala-d-isoglutamine or NAM-l-ala-d-isoglutamine synergized with LTA (0.1 μg/ ml) to induce an enhanced formation of nitrite in macrophages (Fig. 4 B). Again, this synergy was stereoselective, as the induction of nitrite formation was decreased for the stereoisomers of NAM-l-ala-d-isoglutamine (Fig. 4 B). None of the other fragments of PepG tested synergized with LTA to cause the formation of nitrite (results not shown).
Polymyxin B (an agent that binds and inactivates endotoxin) at a concentration sufficient to abolish the increase in nitrite caused by LPS in macrophages (17) did not affect the nitrite production caused by either PepG or its fragment NAG-NAM-l-ala-d-isoglutamine in the presence of IFN-γ in the same cells (results not shown).
iNOS Induction after Separation of Commercially Available S. aureus LTA Extract.
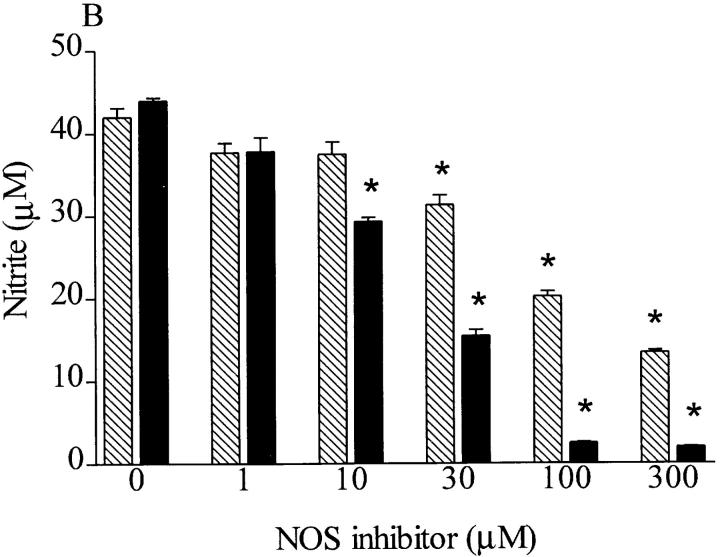
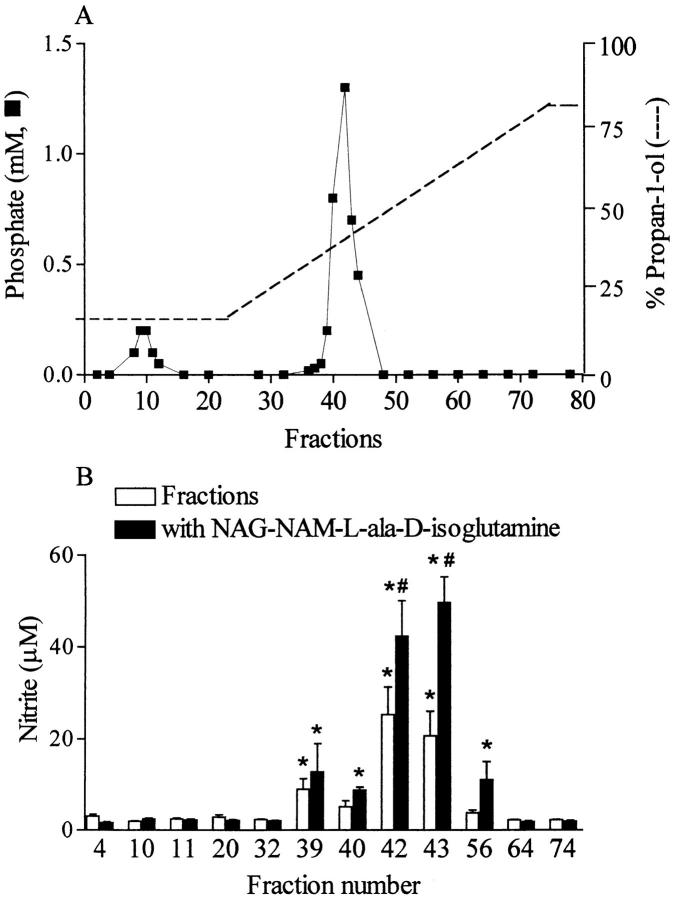
There are several studies reporting that LTA (Sigma Chemical Co.) can induce iNOS activity in macrophages or RASMC in culture and in vivo in anesthetized rats (15, 16, 28). This commercially available LTA is extracted by the hot phenol–water procedure and is still contaminated with various bacterial components, including polysaccharides, proteins, and nucleic acids. Two recent reports indicate that components other than purified LTA contribute to (a) the release of IL-6 in a human astrocytoma cell line; (b) the release of IL-1, IL-6, and TNF-α from human monocytes; and (c) the induction of iNOS in mononuclear cells (8, 29). Therefore, S. aureus LTA obtained from Sigma Chemical Co. was subjected to separation by hydrophobic interaction column chromatography, and relevant fractions were obtained. As LTA contains phosphates, detectable amounts of phosphate were observed in fractions 8–12 (corresponding to material not bound by the column matrix) and in fractions 36–44 (Fig. 5). Only the latter fractions induced the accumulation of nitrite in macrophages when given to these cells either alone or in combination with the PepG fragment NAG-NAM-l-ala-d-isoglutamine. Most notably, the increase in nitrite afforded by these fractions was not affected by polymyxin B (0.5 μg/ml; results not shown), demonstrating that it was not due to the contamination of these fractions with LPS.
Figure 5.
Purification of LTA from S. aureus with an octyl–sepharose column. The LTA extract was fractionated on an octyl–sepharose CL-4B column equilibrated with 0.1 M sodium acetate buffer (pH 4.7) containing 15% propan-1-ol. The column was successfully eluted with the buffer described above and a linear gradient of 15–80% propan-1-ol in the same buffer. Fractions (2 ml each) were collected and tested for (A) phosphate content as well as (B) ability to induce nitrite formation in macrophages with or without NAG-NAM-l-ala-d-isoglutamine (1 μg/ml). Data are expressed as means ± SE of four wells from six independent experiments. *P <0.05 versus baseline, # P <0.05 versus fractions alone. The chromatogram is representative of two independent separations.
Lack of Synergy between PepG and IFN-γ or LTA in RASMC.
Treatment of RASMC for 48 h with S. aureus LTA resulted in only a small increase in nitrite accumulation, whereas S. aureus PepG, IFN-γ, or NAG-NAM- l-ala-d-isoglutamine did not induce a significant increase (Fig. 6). LTA synergized with IFN-γ in RASMC to induce a marked accumulation of nitrite. However, a combination of NAG-NAM-l-ala-d-isoglutamine or PepG with LTA or IFN-γ did not significantly enhance the release of nitrite from these cells compared with treatment with LTA or IFN-γ alone (Fig. 6).
Figure 6.
Neither S. aureus PepG (30 μg/ml) nor NAG-NAM-l-ala- d-isoglutamine (10 μg/ml) potentiated the increases in nitrite caused by S. aureus LTA alone (100 μg/ml) in the supernatant of RASMC. However, IFN-γ (10 IU/ml) synergized with LTA but not with either NAG-NAM-l-ala-d-isoglutamine or PepG to cause enhanced formation of nitrite. Data are expressed as means ± SE of four wells from three to four independent experiments.
Comparison of Nitrite Formation Induced by S. aureus and B. subtilis in Macrophages.
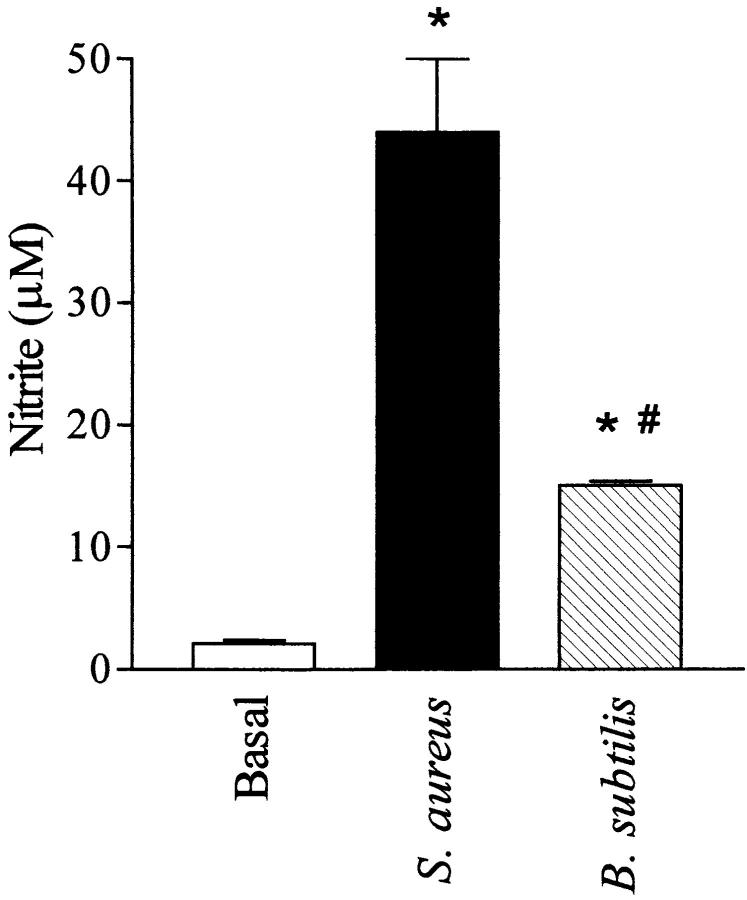
Incubation of macrophages with whole heat-killed S. aureus (108 cells/ml) for 24 h resulted in a marked elevation of nitrite formation (Fig. 7). However, the induction of nitrite formation by the nonpathogenic bacterium B. subtilis (108 cells/ml) was only ∼30% of that elicited by S. aureus (Fig. 7).
Figure 7.
Effects of heat-killed whole bacteria S. aureus (108 cells/ml) or B. subtilis (108 cells/ ml) on the nitrite formation of macrophages. Data are expressed as means ± SE of six wells from three independent experiments. *P <0.05 versus untreated cells, # P <0.05 versus S. aureus.
We confirm that LTA from S. aureus elicits a concentration-dependent increase in nitrite formation from macrophages (Table 1). In contrast, LTA from B. subtilis (0.01–30 μg/ml) did not cause a significant increase in nitrite production from baseline within 24 h (Table 1).
Table 1.
Effect of LTA and PepG from S. aureus or B. subtilis on Nitrite Formation in Macrophages
| Component | Nitrite formation at concentration (μg/ml) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.1 | 1 | 10 | 30 | ||||||
| μM | μM | μM | μM | μM | ||||||
| S. aureus LTA | 1.8 ± 0.2 | 14.0 ± 2.3* | 39.9 ± 1.2* | 51.6 ± 1.2* | 56.8 ± 1.7* | |||||
| S. aureus PepG | 1.4 ± 0.2 | 2.0 ± 0.1 | 2.1 ± 0.3 | 2.9 ± 0.4 | 3.3 ± 0.8 | |||||
| B. subtilis LTA | 1.9 ± 0.1 | 1.7 ± 0.1 | 2.1 ± 0.2 | 2.4 ± 0.2 | 5.7 ± 1.1 | |||||
| B. subtilis PepG | 2.0 ± 0.1 | 1.5 ± 0.1 | 2.1 ± 0.2 | 6.6 ± 0.2 | 17.0 ± 0.9* | |||||
Data are given as mean ± SE of 12 wells from four independent experiments.
P <0.05 compared with untreated cells (Dunnett's test).
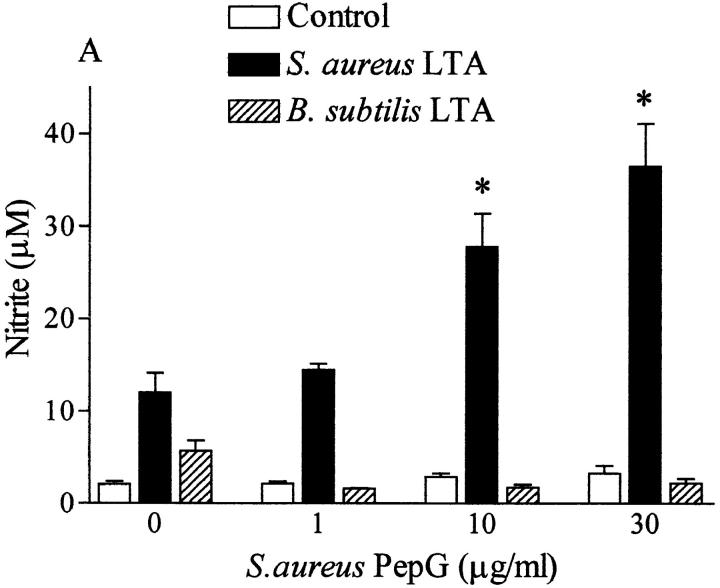
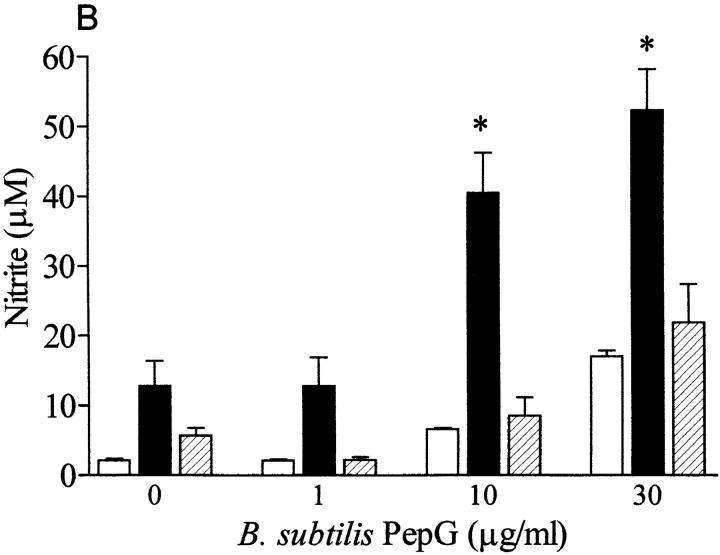
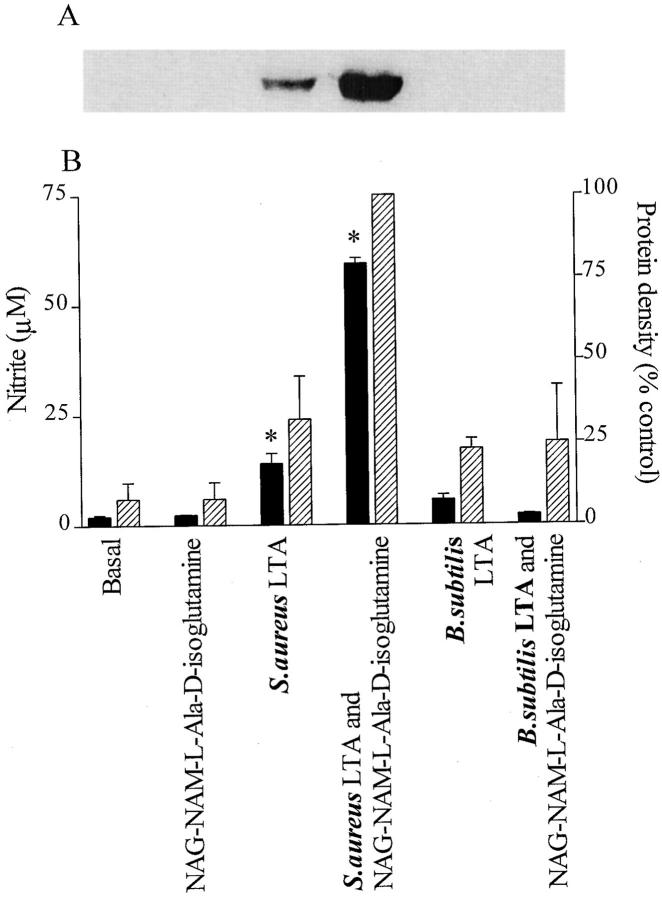
Although incubation of macrophages with PepG from S. aureus (up to 30 μg/ml, in the absence of IFN-γ) did not result in an enhanced production of nitrite, PepG from B. subtilis elicited a small but significant increase in nitrite (at the highest concentration tested; Table 1). The PepG from S. aureus synergized only with LTA from S. aureus, not with LTA from B. subtilis, to induce nitrite formation (Fig. 8 A). Similarly, coincubation of macrophages for 24 h with PepG from B. subtilis and LTA from S. aureus markedly enhanced the nitrite formation compared with that elicited by either component alone, but no synergy was observed between PepG and LTA from B. subtilis (Fig. 8 B). Furthermore, NAG–NAM–l–ala–d–isoglutamine present in the PepG monomer of both S. aureus and B. subtilis synergized only with S. aureus LTA, not with (at 100-fold concentration) B. subtilis LTA, for the induction of iNOS protein and nitrite formation in macrophages (Fig. 9).
Figure 8.
LTA (0.1 μg/ml) from S. aureus synergized with PepG from either (A) S. aureus or (B) B. subtilis to cause nitrite formation in macrophages. In contrast, a 100-fold concentration of LTA from B. subtilis (10 μg/ml) did not synergize with PepG from either (A) S. aureus or (B) B. subtilis to cause nitrite accumulation. The nitrite formation induced by LTA plus PepG from B. subtilis is due to PepG alone (see Table 1). Data are expressed as means ± SE of nine wells from three independent experiments. *P <0.05 versus respective basal levels.
Figure 9.
LTA from S. aureus (0.1 μg/ml) but not from B. subtilis (10 μg/ml) synergizes with the PepG fragment NAG-NAM-l-ala-d-isoglutamine (1 μg/ml) to cause (A) the expression of iNOS protein and (B) nitrite formation in macrophages. LTA from S. aureus at 0.1 μg/ml alone produced significant nitrite levels and the expression of iNOS protein, whereas NAG-NAM-l-ala-d-isoglutamine (10 μg/ml) alone did not produce any of these responses. Data are expressed as means ± SE of nine wells from three independent experiments. The Western blot is representative of four separate experiments.
Synergy between B. subtilis PepG and S. aureus LTA Causes Vascular Hyporeactivity and Multiple Organ Failure.
Treatment of rats with S. aureus LTA (3 mg/kg) and B. subtilis PepG (1 mg/kg) resulted in a significant decrease in blood pressure, from 125 ± 3 mmHg at time 0 to 84 ± 6 mmHg at 6 h (P <0.05 compared with sham-operated rats at the same time point [117 ± 3 mmHg]). Similarly, administration of other cell wall components also produced a delayed hypotension, but these were not significantly different from sham-operated animals (S. aureus LTA [3 mg/kg], 97 ± 6 mmHg; B. subtilis PepG [1 mg/kg], 82 ± 12 mmHg; B. subtilis LTA [3 mg/kg], 94 ± 7 mmHg; B. subtilis LTA [3 mg/kg] and B. subtilis PepG [1 mg/kg], 89 ± 7 mmHg; n = 5–7). Only in rats cotreated with S. aureus LTA and B. subtilis PepG was the delayed hypotension accompanied by a severe loss in the pressor response elicited by norepinephrine compared with sham-operated rats (Fig. 10 A). No significant change in the pressor response to norepinephrine was observed in rats receiving LTA and PepG from B. subtilis. There were no significant changes in the heart rate during the course of the experiment in any of the treatment groups (results not shown).
Figure 10.


Effect of administration of various cell wall components from S. aureus and B. subtilis on the (A) pressor response to norepinephrine, (B) serum ALT, (C) serum creatinine, and (D) iNOS activity in lung homogenates. Data are expressed as means ± SE (n = 5–7). *P <0.05 versus sham, # P <0.05 versus S. aureus LTA alone.
Administration of S. aureus LTA (3 mg/kg) and B. subtilis PepG (1 mg/kg) resulted, within 6 h, in a significant elevation of serum levels of ALT (an indicator of hepatocellular injury), urea (results not shown) and creatinine (indicators of renal failure), and enhanced activity of iNOS in lung homogenates compared with sham-operated rats or rats receiving either component alone (Fig. 10, B–D). Rats receiving B. subtilis LTA (3 mg/kg) in combination with B. subtilis PepG did not show a significant rise in the serum levels of these biochemical markers of organ failure or in lung iNOS activity.
Discussion
The cell wall component PepG derived from S. aureus synergizes with IFN-γ to cause the induction of iNOS protein and activity in J774.2 macrophages, as demonstrated by protein Western blot analysis and the accumulation of nitrite in the supernatant, which was attenuated by the NOS inhibitors L-NMMA or AE-ITU. In the presence of IFN-γ or LTA, NAG-NAM-l-ala-d-isoglutamine, a small moiety within the PepG monomer, also caused an increase in NO formation by these cells. In contrast, neither of the sugars NAG or NAM nor the stem peptide (l-ala-d-isoglutaminyl-l-lys-d-ala-d-ala) or the pentaglycine bridge caused an increase in NO formation in J774.2 macrophages. Taken together, these results strongly suggest that the NAG-NAM-l-ala-d-isoglutamine moiety accounts for the induction of iNOS attributed to the whole PepG polymer. This conclusion is further supported by the following findings. (a) Hydrolysis of the glycosidic bond between NAG and NAM (sugar backbone of PepG) with two different muramidases (mutanolysin or M1) also reduced the NO formation elicited by the whole PepG polymer (in the presence of IFN-γ) by approximately one third. We also found that the NAM-l-ala-d-isoglutamine moiety was less potent than NAG-NAM-l-ala-d-isoglutamine to induce this response. (b) Hydrolysis of the amide bond between NAM and l-ala in PepG with SALE (30) completely abolished the NO formation caused by the whole PepG polymer (in the presence of IFN-γ). Similarly, mutanolysin, M1, or SALE have also been reported to attenuate the formation of TNF-α elicited by PepG from Staphylococcus epidermidis in human monocytes (21). It could be argued that the observed effects of SALE are due to its ability to cleave the pentaglycine bridge between PepG monomers rather than its amidase activity. However, this is not the case, as the hydrolysis of this glycine bridge by lysostaphin (31) enhanced (rather than decreased) the formation of NO. This finding is not entirely surprising, as the cleavage of the glycine bridge by lysostaphin is likely to facilitate the interaction of the active moeity, NAG-NAM-l-ala-d-isoglutamine, with the macrophages. These results support our view that NAM-l-ala-d-isoglutamine is the smallest possible fragment of PepG that is able to synergize with IFN-γ to induce iNOS in macrophages, while an extra NAG residue results in a more potent induction.
We have reported previously that the PepG polymer synergizes with another gram-positive cell wall component, LTA (18). Indeed, the identified active moieties (NAG-)NAM-l-ala-d-isoglutamine also synergize with LTA to cause induction of iNOS in macrophages. This synergy with IFN-γ or LTA is stereoselective, as a decrease in activity is observed with the stereoenantiomers NAM-l-ala- L-isoglutamine and NAM-D-ala-d-isoglutamine. This conformational requirement suggests a distinct receptor–ligand interaction. These results are in agreement with reports demonstrating that NAM-l-ala-d-isoglutamine, also known as adjuvant or muramyl peptide, is the smallest biologically active structure of bacterial PepGs (32, 33). Additionally, computer modeling studies have shown that a specific conformation of NAM-l-ala-d-isoglutamine is required to exert maximal biological activity (34).
Commercially available LTA is prepared by hot phenol– water extraction of whole bacteria (35) and may still contain a variety of bacterial components coextracted during the procedure. Further purification of this “crude” LTA from S. aureus by hydrophobic interaction column chromatography shows that the increase in NO formation is caused by those fractions containing phosphate (a major component of the poly(glycerophosphate) backbone of LTA) and eluted at 35–55% vol/vol propanol, the expected hydrophobicity of LTA (13, 35). Similar results are found for the synergy of these fractions with NAG-NAM-l-ala- d-isoglutamine. Thus, the results strongly suggest that purified LTA induces the formation of NO by macrophages. The LTA fractions 40 and 43 contain similar phosphate concentrations; however, the more lipophilic fraction, 43, elicited a fivefold higher increase in NO formation than fraction 40. This suggests that the activity of LTA to induce iNOS increases with lipophilicity of the LTA fractions. Similar results have been reported for two LTA fractions isolated from Streptococcus faecalis (36). The more lipophilic LTA fraction causes the release of TNF-α from macrophages and regression of tumors in mice, whereas the more hydrophilic is inactive. After column chromatography, fractions with significant concentrations of phosphate are also eluted at 15% vol/vol propanol, representing hydrophilic fractions that do not contain LTA. Kusunoki et al. (22) have shown that compound(s) in these fractions (and not in lipophilic fractions containing purified LTA) elicit the release of IL-6 in human astrocytoma cells. However, in the present study, the equivalent fractions do not induce the formation of NO in murine macrophages. Therefore, it is unlikely that components other than the purified LTA contribute to the induction of iNOS in macrophages elicited by commercially available LTA extract.
S. aureus is a major cause of nosocomial infections and gram-positive septic shock (1), whereas B. subtilis is a nonpathogenic gram-positive bacterium (37, 38). We report here that macrophages challenged with whole heat-killed B. subtilis bacteria produce far less NO than macrophages activated with heat-killed S. aureus. Similarly, LTA from S. aureus is more potent than LTA from B. subtilis in enhancing the formation of NO in macrophages. It has been reported that LTA from S. aureus is also more potent in the activation of arachidonic acid metabolism in mouse macrophages than LTA from B. subtilis (39). The structure of LTA from S. aureus and B. subtilis is very similar, except that the glycosylation and alanine substitution of the polyglycerophosphate backbone is higher in S. aureus (13, 40, 41). This suggests that the lipophilic portion of LTA may be important for the biological activity. This notion is supported by findings that the immunodeterminants of LTA of various gram-positive bacteria are located mainly in their polyglycerophosphate moieties, including its glycosyl and alanyl substituents (42). Also, in the study by Takada et al. (36), the more lipophilic LTA fraction of S. faecalis with a high alanine content was more active than the hydrophilic LTA fraction with a low alanine content. In the present study, the more lipophilic fraction from LTA of S. aureus was also associated with higher activity. Thus, the degree of alanine substitution in LTA may be important for the induction of iNOS in macrophages, and may explain the lower activity of LTA from B. subtilis, but the exact role of alanine substitution remains to be further elucidated.
LTA from S. aureus synergizes with NAG-NAM-l-ala- d-isoglutamine, the active moiety of PepG present in the PepG of both S. aureus and B. subtilis (33, 43), to induce iNOS activity in macrophages. In contrast, LTA from B. subtilis does not synergize with this moiety to enhance the formation of NO. In support of this, PepG from either S. aureus or B. subtilis synergizes with LTA from S. aureus but not from B. subtilis to cause NO formation in these cells. These results further indicate that the structure of LTA determines whether or not a particular LTA is able to synergize with PepG to induce iNOS activity. The activity of PepG seems largely dependent on the NAG-NAM-l-ala- d-isoglutamine moiety, which is one of the most conserved parts of PepG from (pathogenic and nonpathogenic) gram-positive organisms (43). The diversity of PepG among gram-positive organisms is limited to the free combination of 11 different types of peptide side chains with two types of cross-linkage (44). Thus, our results suggest that the ability of gram-positive organisms to induce iNOS activity in macrophages, and possibly its pathogenicity, reside within the LTA component, while PepG serves to amplify a given biological response.
Surprisingly, in this study we found that neither NAG-NAM-l-ala-d-isoglutamine nor PepG from S. aureus synergizes with IFN-γ or LTA to induce NO formation in RASMC. The reason for this is not clear, but may be that these cells do not possess the “receptor” to which PepG binds to activate these cells (45, 46). On the other hand, it is possible that the PepG polymer has to be digested by macrophages to release biologically active fragments, such as NAG-NAM-l-ala-d-isoglutamine, whereas smooth muscle cells are unable to digest PepG (47). Indeed, B. subtilis PepG given to alveolar macrophages releases predominantly NAG-NAM-l-ala-d-isoglutamine (48). But our finding that direct exposure of smooth muscle cells to this fragment (in the presence of IFN-γ or LTA) does not result in an enhanced formation of NO would argue against this possibility. Thus, the reason underlying this lack of synergy in smooth muscle cells remains to be established.
Previously, we have shown that the cell wall components LTA and PepG from S. aureus synergize in vivo to cause circulatory and multiple organ failure in anesthetized rats (18). We find here that LTA or PepG from B. subtilis when given either alone or in combination to anesthetized rats does not induce vascular hyporeactivity to norepinephrine, hepatic injury, or renal failure. The inability of the LTA and PepG–teichoic acid complex from B. subtilis to cause death in mice has been documented previously (37, 38). As in macrophages, PepG from B. subtilis and LTA from S. aureus synergized to cause vascular hyporeactivity to norepinephrine, liver injury, renal failure, and an enhanced iNOS activity in the lungs of rats (although either component alone was inactive). Thus, the importance of the LTA structure for synergy among the cell wall components extends from the in vitro (macrophages) to the in vivo (anesthetized rats) situation. In these experiments, the circulatory and multiple organ failure is associated with the induction of iNOS activity, suggesting a causative role for NO. Previous studies indicate that inhibition of NO synthesis prevents the circulatory failure and liver injury but not the renal failure elicited by the combined cell wall components from S. aureus (19). Thus, an enhanced formation of NO by iNOS accounts for the circulatory failure and contributes to the organ injury associated with gram-positive shock.
In summary, NAG-NAM-l-ala-d-isoglutamine is the bioactive moiety in PepG responsible for the synergy of PepG with IFN-γ or LTA to induce iNOS in macrophages. The finding that LTA from S. aureus but not B. subtilis synergizes with PepG from either bacterium to induce (a) NO formation in macrophages and (b) iNOS activity, shock, and multiple organ failure in anesthetized rats suggests that the structure of LTA determines the pathogenicity of a particular bacterium, whereas PepG acts to amplify any response induced by LTA.
Abbreviations used in this paper
- AE-ITU
aminoethyl-isothiourea
- ALT
alanine aminotransferase
- d- or l-ala
d- or l-alanine
- iNOS
inducible NOS
- l-lys
l-lysine
- L-NMMA
N G-methyl-l-arginine
- LTA
lipoteichoic acid
- MTT
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAG
N-acetylglucosamine
- NAM
N-acetylmuramic acid
- NAG-NAM-l-ala- d-isoglutamine
N-acetylglucosamine-β-[1→ 4]-N-acetylmuramyl-l-alanine- d-isoglutamine
- NO
nitric oxide
- NOS
NO synthase
- PepG
peptidoglycan
- RASMC
rat aortic smooth muscle cell(s)
- SALE
Staphylococcus aureus lytic enzyme
Footnotes
C. Thiemermann is a Senior Fellow of the British Heart Foundation (FS 016/96). S.J. Foster is supported by the Royal Society. This work was supported by a Biomed II grant of the European Commission.
References
- 1.Bone RC. Gram-positive organisms and sepsis. Arch Intern Med. 1994;154:26–34. [PubMed] [Google Scholar]
- 2.Nogare AR. Southwestern Internal Medicine Conference: septic shock. Am J Med Sci. 1991;302:50–65. [Google Scholar]
- 3.Natanson C, Danner RL, Elin RJ, Hosseini JM, Peart KW, Banks SM, Macvittie TJ, Walker RI, Parrillo JE. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureuschallenges in a canine model of human septic shock. J Clin Invest. 1989;83:243–251. doi: 10.1172/JCI113866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakabayashi G, Gelfand JA, Jung WK, Connolly RJ, Burke JF, Dinarello CA. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. . J Clin Invest. 1991;87:1925–1935. doi: 10.1172/JCI115218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattsson E, Verhage L, Rollof J, Fleer A, Verhoef J, Vandijk H. Peptidoglycan and teichoic acid from Staphylococcus epidermidisstimulate human monocytes to release tumor necrosis factor-α, interleukin-1β and interleukin-6. FEMS Immunol Med Microbiol. 1993;7:281–287. doi: 10.1111/j.1574-695X.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 6.Heumann D, Barras C, Severin A, Glauser MP, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor-α and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhakdi S, Muhly M, Korom S, Hugo F. Release of interleukin-1β associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989;57:3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhakdi S, Klonisch T, Nuber P, Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 10.Moncada S, Higgs A. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB (Fed Am Soc Exp Biol) J. 1995;9:1319–1330. [PubMed] [Google Scholar]
- 11.Thiemermann C. The role of the l-arginine: nitric oxide pathway in circulatory shock. Adv Pharmacol. 1994;28:45–79. doi: 10.1016/s1054-3589(08)60493-7. [DOI] [PubMed] [Google Scholar]
- 12.Thiemermann C. The use of selective inhibitors of inducible nitric oxide synthase. Sepsis. 1998;1:123–129. [Google Scholar]
- 13.Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- 14.Shaw N. Lipid composition as a guide to the classification of bacteria. Adv Appl Microbiol. 1974;17:63–108. doi: 10.1016/s0065-2164(08)70555-0. [DOI] [PubMed] [Google Scholar]
- 15.Auguet M, Lonchampt M-O, Delaflotte S, Goulin-Schulz J, Chabrier PE, Braquet P. Induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureusin vascular smooth muscle cells. FEBS (Fed Eur Biol Soc) Lett. 1992;297:183–185. doi: 10.1016/0014-5793(92)80356-l. [DOI] [PubMed] [Google Scholar]
- 16.De Kimpe SJ, Hunter ML, Bryant CE, Thiemermann C, Vane JR. Delayed circulatory failure due to the induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureusin anaesthetised rats. Br J Pharmacol. 1995;114:1317–1323. doi: 10.1111/j.1476-5381.1995.tb13349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kengatharan M, De Kimpe SJ, Thiemermann C. Analysis of the signal transduction in the induction of nitric oxide synthase by lipoteichoic acid in macrophages. Br J Pharmacol. 1996;117:1163–1170. doi: 10.1111/j.1476-5381.1996.tb16711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Kimpe SJ, Kengatharan M, Thiemermann C, Vane JR. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureusact in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kengatharan MK, De Kimpe SJ, Thiemermann C. Role of nitric oxide synthase in the circulatory failure and organ injury in a rodent model of gram-positive shock. Br J Pharmacol. 1996;119:1411–1421. doi: 10.1111/j.1476-5381.1996.tb16053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster SJ. Analysis of the autolysins of Bacillus subtilis 168during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J Bacteriol. 1992;174:464–470. doi: 10.1128/jb.174.2.464-470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timmerman CP, Mattsson E, Martinez-Martinez L, De Graaf L, Vanstrijp JAG, Verbrugh HA, Verhoef J, Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993;61:4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusunoki T, Hailman E, Juan TSC, Lichenstein HS, Wright SD. Molecules from Staphylococcus aureusthat bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross SS, Stuehr DJ, Aisaka K, Jaffe EA, Levi R, Griffith OW. Macrophages and endothelial cell nitric oxide synthesis: cell-type selective inhibition by N G-amino-arginine, N G-nitro-l-arginine and N G-methyl-arginine. Biochem Biophys Res Commun. 1990;170:96–103. doi: 10.1016/0006-291x(90)91245-n. [DOI] [PubMed] [Google Scholar]
- 24.Bryant CE, Tomlinson A, Mitchell JA, Thiemermann C, Willoughby DA. Nitric oxide synthase in the rat fallopian tube is regulated during the estrous cycle. J Endocrinol. 1995;146:149–157. doi: 10.1677/joe.0.1460149. [DOI] [PubMed] [Google Scholar]
- 25.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baue, A.E. 1993. The multiple organ or system failure syndrome. In Pathophysiology of Shock, Sepsis and Organ Failure. G. Schlag and H. Redl, editors. Springer-Verlag, Berlin. 1004–1018.
- 27.Wallenstein S, Zucker LC, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Cunha FQ, Moss DW, Leal LMCC, Moncada S, Liew FY. Induction of macrophage parasiticidal activity by Staphylococcus aureusand exotoxins through the nitric oxide synthesis pathway. Immunology. 1993;78:563–567. [PMC free article] [PubMed] [Google Scholar]
- 29.Keller R, Fischer W, Keist R, Bassetti S. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect Immun. 1992;60:3664–3672. doi: 10.1128/iai.60.9.3664-3672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawata S, Takemura T, Yokogawa K, Kotani S. Studies on lytic enzymes toward cariogenic streptococci .9. isolation of bacteriolytic endopeptidase from a strain of cytophaga and its application to preparation of hydrosoluble polysaccharide peptide from Staphylococcus epidermidispeptidoglycan. Agric Biol Chem. 1984;48:2253–2263. [Google Scholar]
- 31.Wadstrom T, Vesterberg O. Studies on endo-β-acetylglucosaminidase, staphylolytic peptidase, and N-acetylmuramyl-l-alanine amidase in lysostaphin and from Staphylococcus aureus. . Acta Pathol Microbiol Scand [B] Microbiol Immunol. 1971;79:248–264. doi: 10.1111/j.1699-0463.1971.tb02152.x. [DOI] [PubMed] [Google Scholar]
- 32.Chedid L, Lederer E. Past, present and future of the synthetic immunoadjuvant MDP and its analogs. Biochem Pharmacol. 1978;27:2183–2186. doi: 10.1016/0006-2952(78)90074-6. [DOI] [PubMed] [Google Scholar]
- 33.Seidl, P.H., and K.H. Schleifer. 1986. Structure and immunochemistry of peptidoglycan. In Biological Properties of Peptidoglycans. P.H. Seidl and K.H. Schleifer, editors. Walter de Gruyter & Co., New York. 1–20.
- 34.Pristovsek P, Kidric J, Hadzi D. Bioactive conformations of small peptides: a method for selection of candidates based on conformations of active and inactive analogues and its application to muramyl dipeptide. J Chem Inf Comput Sci. 1995;35:633–639. doi: 10.1021/ci00025a034. [DOI] [PubMed] [Google Scholar]
- 35.Fischer W. Molecular analysis of lipid macroamphiphiles by hydrophobic interaction chromatography. J Microbiol Methods. 1996;25:129–144. [Google Scholar]
- 36.Takada H, Kawabata Y, Arakaki R, Kusumoto S, Fukase K, Suda Y, Yoshimura T, Kokeguchi S, Kato K, Komuro T, et al. Molecular and structural requirements of a lipoteichoic acid from Enterococcus hiraeATCC 9790 for cytokine-inducing, antitumor, and antigenic activities. Infect Immun. 1995;63:57–65. doi: 10.1128/iai.63.1.57-65.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Himanen JP, Pyhala L, Olander RM, Merimskaya O, Kuzina T, Lysyuk O, Pronin A, Sanin A, Helander IM, Sarvas M. Biological activities of lipoteichoic acid and peptidoglycan-teichoic acid of Bacillus subtilis 168 (Marburg). . J Gen Microbiol. 1993;139:2659–2665. doi: 10.1099/00221287-139-11-2659. [DOI] [PubMed] [Google Scholar]
- 38.Himanen JP, Sarvas M, Helander IM. Assessment of nonprotein impurities in potential vaccine proteins produced by Bacillus subtilis. . Vaccine. 1993;11:970–973. doi: 10.1016/0264-410x(93)90388-e. [DOI] [PubMed] [Google Scholar]
- 39.Card GL, Jasuja RR, Gustafson GL. Activation of arachidonic acid metabolism in mouse macrophages by bacterial amphiphiles. J Leukocyte Biol. 1994;56:723–728. doi: 10.1002/jlb.56.6.723. [DOI] [PubMed] [Google Scholar]
- 40.Fischer W, Rosel P. The alanine ester substitution of lipoteichoic acid (LTA) in Staphylococcus aureus. . FEBS (Fed Eur Biol Soc) Lett. 1980;119:224–226. doi: 10.1016/0014-5793(80)80257-2. [DOI] [PubMed] [Google Scholar]
- 41.Fischer W, Mannsfeld T, Hagen G, Fischer G. On the basic structure of poly(glycerophosphate) lipoteichoic acids. Biochem Cell Biol. 1990;68:33–43. doi: 10.1139/o90-005. [DOI] [PubMed] [Google Scholar]
- 42.Wicken AJ, Knox KW. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975;187:1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- 43.Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glauner B. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem. 1988;172:451–464. doi: 10.1016/0003-2697(88)90468-x. [DOI] [PubMed] [Google Scholar]
- 45.Dziarski R. Demonstration of peptidoglycan binding sites on lymphocytes and macrophages by photoaffinity cross-linking. J Biol Chem. 1991;266:4713–4718. [PubMed] [Google Scholar]
- 46.Rabin RL, Bieber MM, Teng NNH. Lipopolysaccharide and peptidoglycan share binding sites on human peripheral blood monocytes. J Infect Dis. 1993;168:135–142. doi: 10.1093/infdis/168.1.135. [DOI] [PubMed] [Google Scholar]
- 47.Ginsburg I. The biochemistry of bacteriolysis: paradoxes, facts and myths. Microbiol Sci. 1988;5:137–142. [PubMed] [Google Scholar]
- 48.Vermeulen MW, Gray GR. Processing of Bacillus subtilispeptidoglycan by a mouse macrophage cell line. Infect Immun. 1984;46:476–483. doi: 10.1128/iai.46.2.476-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]