Abstract
This report investigates the role of OX40 ligand (OX40L) and its receptor, OX40, expressed on activated B and T cells, respectively, in promoting the differentiation of T helper type 2 (Th2) CD4 T cells. These molecules are expressed in vivo by day 2 after priming with T cell– dependent antigens. Their expression coincides with the appearance of immunoglobulin (Ig)G switch transcripts and mRNA for interleukin (IL)-4 and interferon (IFN)-γ, suggesting that this molecular interaction plays a role in early cognate interactions between B and T cells. In vitro, we report that costimulation of naive, CD62Lhigh CD4 T cells through OX40 promotes IL-4 expression and upregulates mRNA for the chemokine receptor, blr-1, whose ligand is expressed in B follicles and attracts lymphocytes to this location. Furthermore, T cell stimulation through OX40 inhibits IFN-γ expression in both CD8 T cells and IL-12–stimulated CD4 T cells. Although this signal initiates IL-4 expression, IL-4 itself is strongly synergistic. Our data suggest that OX40L on antigen-activated B cells instructs naive T cells to differentiate into Th2 cells and migrate into B follicles, where T cell–dependent germinal centers develop.
Keywords: Th1–Th2 differentiation, OX40/OX40 ligand, cytokine, T cell priming, cognate B cell–T cell interaction
The mammalian immune system has evolved to deal with two principal groups of pathogens: extracellular organisms, which are opsonized by antibody and complement and are killed when ingested by phagocytes; and intracellular bacterial organisms like mycobacteria, which escape the normal intracellular killing mechanisms and replicate inside host cells. T cells play a crucial role in orchestrating host defences against both types of pathogen. In mice and humans, two distinct patterns of Th cell have been described. Th1 cells, by virtue of their capacity to secrete IFN-γ, play a crucial role in eliminating intracellular bacteria in mice and humans (1); Th2 CD4 T cells are more effective helpers for antibody responses.
Recent experiments suggest that commitment of CD4 T cells to Th1 or Th2 subsets occurs at or shortly after T cell priming on dendritic cells (DC)1 (2). It is well established that IL-12 plays the key role in directing Th1 cell CD4 differentiation (3), and this cytokine can be secreted by activated DC (4, 5). The factors that initiate Th2 CD4 differentiation are less well understood. IL-4 promotes Th2 CD4 differentiation and inhibits Th1 cells by downregulating the β chain of the IL-12 receptor (6). It has been suggested that NK1.1 cells (7) are the initial sources of IL-4, but NK1.1-deficient mice make normal Th2 responses (8).
Alternatively, there is evidence that B cells evoke Th2 differentiation (9, 10). The appearance of IgG switch transcripts coincident with the appearance of mRNA for cytokines during immune responses to T cell–dependent antigens supports the notion that B cells interacting with T cells might play a role in initiating IL-4 secretion (2).
It has been reported previously that the expression of OX40 on T cells in the T zone is maximal 3 d after priming (11). In this paper, we confirm that the B cell activation antigen, OX40 ligand (OX40L), and the T cell activation antigen, OX40, are expressed by day 2 at the time of T cell priming in vivo. We report that costimulation of naive CD62Lhigh T cells through OX40 promotes IL-4 expression and upregulates the chemokine receptor, CXCR5 (blr-1), whose ligand is expressed in B follicles (12). Although OX40L initiates IL-4 responses, subsequent differentiation is IL-4 dependent. In addition, IL-4 inhibits IFN-γ expression in both CD8- and IL-12–stimulated CD4 T cells. Our data suggests a mechanism whereby antigen-activated B cells promote the differentiation of Th2 cells and their recruitment to B follicles, where T cell–dependent germinal centers develop.
Materials and Methods
Construction of Murine OX40L Transfectant.
Construction and expression of stable transfectants expressing murine (m)OX40L protein were carried out as follows.
The primers used to amplify mOX40L were 5′ (TATATAGAGCTCACCATGGAAGGGGAAGGGGTTCAACCCC) and 3′ (ATATATAAGCTTACTTACCTCACAGTGGTACTTGGTTCACAGT).
Reverse transcription (RT)-PCR using immunized mouse spleen cDNA as a template was used to amplify cDNA encoding mOX40L. The PCR product was subcloned into an expression plasmid containing prokaryotic (Ampicillin) and eukaryotic (histidinol) selection markers, and promoters and enhancers for murine B cells and plasma cells. The plasmid has been described elsewhere (13).
Construction of mOX40–Human γ1.
The primers used to amplify mOX40 were 5′ (TATATAGAGCTCACCATGGCATGTATGTGTGGGTTCAGCAGCC) and 3′ (ATATATAAGCTTACTTACCCTCAGGAGTCACCAAGGTGGG).
Chimeric Ig molecules expressing the extracellular portions of the mouse OX40 gene (14) and the human IgG1 constant domains were created as follows. External primers encoding the 5′ portion and the 3′ portion of mOX40 were used to amplify the extracellular portion of mOX40 (from mouse spleen cDNA). Each primer contained appropriate restriction sites for subcloning into the human IgG1 expression vector (13), together with a 3′ splice donor site within the 3′ primer to correctly splice to the human (h)γ1 exons.
Transfection of Plasmids into J558L.
Plasmids containing the correct insert were linearized and electroporated into the mammalian plasmacytoma cell line, J558L, and selected in 10 mM Histidinol (Sigma Chemical Co., St. Louis, MO) for the OX40L transfectant, or mycophenolic acid and Xanthine (Sigma Chemical Co.) for mOX40-hγ1 (13). The fusion proteins were detected by hemagglutination of SRBC coated with an antibody to human IgG. Strongly secreting clones were grown, and supernatants were purified over protein A as described previously (15).
mOX40L transfectants that stained appropriately with mOX40-hγ1 but not an irrelevant fusion protein were selected and used for T cell stimulation studies (data not shown).
Preparation of CD4CD62Lhigh and CD8CD62Lhigh T Cells.
6–12-wk-old female BALB/c or C57BL/6 mice were killed, and their spleen and LNs were removed. Cell suspensions were made by crushing the tissues between gauze. After separation with Ficoll-paque (Amersham Pharmacia Biotech, Herts, UK), the cell suspensions were pooled and incubated with FITC-conjugated anti-CD4 or anti-CD8 antibody (Southern Biotechnology Associates, Inc., Birmingham, AL) at 20 μl/108 cells, for 15 min at 4°C. FITC-stained single cells were positively selected using MACS® anti-FITC (isomer 1) Multisort kit (Miltenyi Biotec Ltd., Surrey, UK) and a miniMACS® separation unit according to the manufacturer's protocol. The resulting positive fraction was further separated according to expression of CD62L using MACs® anti– mouse CD62L microbeads (Miltenyi Biotec Ltd.), again according to the manufacturer's protocol. The resulting population was typically >95% positive for CD4 or CD8 and CD62L as determined by flow cytometry using a FACScan® (Becton Dickinson, Mountain View, CA).
T Cell Stimulation, Cytokines, and Antibodies.
For cell culture, J558L and the mOX40L and hCD27L transfectants were fixed in 1% formaldehyde for 45 min at 4°C and washed thoroughly. Viability after fixation was undetectable, but OX40L transfectants could still be stained with the mOX40-hγ1 fusion protein. RT-PCR did not give a β-actin signal from fixed cells, and only cell debris remained after 3 d in culture (data not shown).
24-well plates (GIBCO BRL, Paisley, UK) were coated overnight with anti-CD3 antibody (10 μg/ml; PharMingen, San Diego, CA) at 4°C. CD4+CD62L+, CD4+CD62L− enriched, or CD8+ CD62L+ T cells (1–2 × 106 cells/well) were cultured either alone or with the addition of 5 × 105 cells per well of fixed OX40L transfectants or the fixed parental cell line (J558L). The cultures were incubated with soluble anti-CD28 antibody (10% final concentration from a supernatant of a hamster anti–mouse CD28 (clone 37.51; a gift from Dr. Jim Allison, University of California, Berkeley, CA) in RPMI 1640 medium containing l-glutamine (GIBCO BRL) supplemented with 10 U/ml penicillin/100 μg/ml streptomycin (GIBCO BRL), ciprofloxacin (10 μg/ml; Sigma Chemical Co.), and 10% FCS (GIBCO BRL) at 37°C with 5% CO2.
For blocking experiments, purified rat anti–mouse IL-4 antibody (11B11) or anti–mouse IFN-γ antibody (XMG1.2) was added to the cultures at a concentration of 50 μg/ml. Recombinant mouse IL-12 (R&D Systems, Abingdon, UK) was used at 50 ng/ml, which in our hands gave optimal induction of IFN-γ.
Restimulation of T Cells for FACS® Analysis for CD40L, IL-4, and IFN-γ.
At the required time points, the cultures were harvested and washed thoroughly to remove antibodies. The cultures were restimulated with anti-CD3 mAb for 4 h at 37°C as described above in the presence of GolgiStop™ according to the manufacturer's protocol (Cytofix/Cytoperm Plus™ cytostain kit; PharMingen). Without GolgiStop™, intracellular cytokine staining was not detectable. Cells incubated with GolgiStop™ but not restimulated gave a similar pattern of staining to restimulated cells, but the intensity was less (data not shown).
After restimulation, the cells were fixed and permeabilized according to the manufacturer's protocol for the cytostain kit. The cells were stained optimally with anti-CD4 FITC or anti-CD8 FITC mAb (Southern Biotechnology Associates, Inc.), biotinylated anti–IL-4, biotinylated anti–IFN-γ (PharMingen), and biotinylated MR1 anti-CD40L antibody (a gift of Dr. Randy Noelle, Dartmouth Medical School, Lebanon, NH). After washing, the cells were stained in a second step with neutralite avidin PE (Southern Biotechnology Associates, Inc.) at 1:200 final concentration. For each sample, 104 live gated cells were analyzed using a FACScan® (Becton Dickinson).
Semiquantitative RT-PCR.
To obtain cell samples for cDNA preparation, the above culture conditions were duplicated on rat anti–mouse CD3 (10 μg/ml)–coated 96-well plates. CD4+ CD62L+ cells were plated in triplicate at 105 cells/well with the addition of 3 × 104 cells/well of the fixed transfected cell line. Cell cultures were harvested without restimulation at time 0, and after 24, 48, and 72 h. The cells were washed in PBS, and the dry pellet was frozen at −70°C before cDNA preparation. cDNA was also made from the J558L cell line and transfectants. Living cells gave a strong β-actin signal, but no signal was detected for IL-4, IFN-γ, or blr-1. Fixed cell lines did not give a β-actin signal.
For in vivo studies, mice were killed by CO2 asphyxiation at different time points after immunization. Draining popliteal LNs were removed and put on aluminium foil in a defined orientation, embedded in OCT compound (Miles Inc., Elkhart, IN), and frozen by sequential dipping in liquid N2. Tissues were stored in sealed polythene bags at –70°C until use. 5-μm cryostat sections of the tissue were mounted on four-spot glass slides for immunohistology. After cutting the first eight sections, which were used for immunohistology, three 24-μm sections of LN were cut, placed in a polypropylene microfuge tube, and stored at –70°C for mRNA extraction. cDNA samples were prepared from tissue sections from popliteal LNs taken from mice at different time points during immune responses to Swiss-type mouse mammary tumor virus (MMTV) or (4-hydroxy-3-nitrophenyl) acetyl– chicken γ-globulin (NP-CγG) as described (2).
cDNA prepared from the cells or tissue sections was diluted to a final volume of 100 μl. The PCR β-actin signal was used to correct for differences in the amount of starting cDNA from each sample. The specific primers were as follows: β-actin (Stratagene, Cambridge, UK), 5′ (AGCGGGAAATCGTGCGTG) and 5′ (CAGGGTACATGGTGGTGCC); IL-4 (16), 5′ (GAATGTACCAGGAGCCATATC) and 5′ (CTCAGTACTACGAGTAATCCA); IFN-γ (16), 5′ (AACGCTACACACTGCATCTTGG) and 5′ (GACTTCAAAGAGTCTGAGG); blr-1 (17), 5′ (TTCCTGTTCCACCTCGCAGTAGC) and 3′ (CCATCCCATCACAAGCATCGG); mOX40, 5′ (TGTATGTGTGGGTTCAGCAGCC) and 5′ (CCCTCAGGAGTCACCAAGGTGGG); mOX40L, 5′ (ATGGAAGGGGAAGGGGTTCAACC) and 5′ (TCACAGTGGTACTTGGTTCACAG).
Buffers were used as supplied with the enzymes, plus 1 μM of each primer, 200 μM of each dNTP, and 1.5 mM MgCl2 for β-actin, IL-4, and IFN-γ cDNAs, or 2.0 mM MgCl2 for blr-1 cDNAs. cDNAs were amplified using 0.1 μl AmpliTaq Gold (Perkin-Elmer, Langen, Germany). PCR was performed with 2 μl cDNA template in a 20 μl vol, and the reaction mix was overlaid with one drop of mineral oil (Sigma Chemical Co.). The PCRs were performed in 0.2 ml polypropylene plates in a Touch Down thermal cycler (Hybaid Ltd., Ashford, Middlesex, UK). Cycling was done with an initial denaturation step of 9 min, followed by 30 s at 94°C, 30 s at annealing temperature (60°C for β-actin, 55°C for blr-1, and 50°C for cytokine mRNA), and 2 min plus 2 s for every cycle at 72°C. Cycle numbers were 22 for β-actin, 30 for IL-4 and IFN-γ mRNA, and 35 for blr-1 mRNA. After a final elongation step of 15 min at 72°C, the PCR product was separated on a 1.5% agarose gel containing ethidium bromide, and photographed.
For semiquantitative PCR of OX40 and OX40L mRNA, ∼10 fewer cycles than were required to detect PCR product using ethidium bromide gels were done to quantitate OX40 and OX40L cDNAs by PCR Southern blot or dot blot analysis. For each set of primers, three different numbers of PCR cycles were performed to ensure that amplification was logarithmic and that the conditions were not saturating. The PCR product was separated on a 1.5% agarose gel and transferred onto prewetted Hybond-N+ membrane (Amersham International, Little Chalfont, UK) by capillary transfer under alkaline conditions. The membrane was hybridized with a 32P-labeled purified PCR product used as a probe and imaged using a PhosphorImager (Molecular Dynamics Ltd., Buckinghamshire, UK).
Using ImageQuant software (Molecular Dynamics Ltd.), a grid was laid over PCR bands, with individual fields covering the central 50% of a band. The signal in each field was calculated, and these figures were transferred to spreadsheet software to sort the randomized files to the correct order. The average of the three PCRs with different cycle number for each gene was taken and divided by the average of the three corresponding β-actin PCRs. These values represent the relative amount of mRNA for each gene per cell. This value was multiplied by the size of the section area (determined by microscopy on adjacent sections to those taken for cDNA using the point counting technique [18]) to give mRNA amount per section.
Results
In Vivo, The Timing of Expression of OX40 and OX40L Correlates with Priming of B and T Cells.
During immune responses to the nominal haptenated protein antigen NP-CγG and the infectious viral superantigen MMTV (2), T cell priming occurs around day 3. The predominant cytokine associated with MMTV is IFN-γ, whereas IL-4 is secreted in response to NP-CγG. Antigen presentation of the superantigen by MMTV on B cells is associated with T cell–driven proliferation of large numbers of B cells. The response to NP-CγG is of lower magnitude, with activation of antigen-specific B cells. We examined mRNA expression of OX40L and OX40 during these two immune responses (see Fig. 1, A–D).
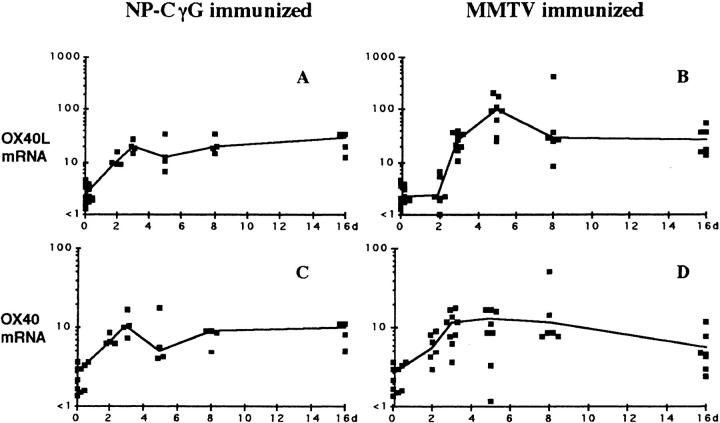
Figure 1.
Induction of OX40 and OX40L after immunization. Time course of induction of mRNA for OX40 and OX40L in popliteal LNs in mice after immunization with NP-CγG (plus killed Bordetella pertussis 5 × 108) or MMTV. Symbols show the amount of mRNA for OX40L (A and B) and OX40 mRNA (C and D) per LN section by semiquantitative PCR. Symbols, Results for individual mice; solid lines, median values.
During immune responses to MMTV, which potently activates B cells (19), OX40L is strongly induced. There is a 10-fold increase in mRNA expression between days 2 and 3, and by day 5, the peak of the B cell response, there is a further 5-fold increase in mRNA (Fig. 1 B). OX40 mRNA is also upregulated about fivefold by day 2 of the immune response (Fig. 1 D). The response to the nominal protein antigen NP-CγG is less marked but exhibits similar kinetics. Increased OX40 and OX40L mRNA is in evidence by day 2 and peaks on day 3 of the immune response, coinciding with the early cognate interaction among B cells, T cells, and DC. mRNA levels for OX40 and OX40L remain elevated above baseline levels for several weeks in both immune responses. The predominant site of B–T interaction is within the light zone of germinal centers at these late time points. In summary, these results indicate that OX40 and its ligand are expressed from the time of B and T cell priming in vivo, and could therefore play a role in directing subsequent T and B cell differentiation.
Stimulation of Naive CD4 T Cells (CD62high) through CD3 and CD28 Is Insufficient to Induce Expression of Intracytoplasmic IL-4 or IFN-γ.
Previous evidence has suggested that cross-linking of OX40L on B cells by T cell OX40 delivers a B cell differentiation signal (20). To dissect the effects of OX40L on signaling through T cell OX40, we developed an in vitro model to study naive CD4 T cell activation and differentiation.
CD4+CD62Lhigh T cells that were >95% pure were compared with total or CD4+CD62Llow T cells for their capacity to upregulate the expression of intracytoplasmic IL-4 and IFN-γ after activation through CD3 and CD28. In our system, CD4 T cells are activated for short periods (up to 3 d). This time frame was chosen to mimic as closely as possible the in vivo situation (2).
Before staining for intracytoplasmic IL-4 and IFN-γ, T cells were restimulated by immobilized mAb to CD3 for 4 h in the presence of GolgiStop™, which causes newly synthesized proteins to accumulate within the Golgi compartment. Without GolgiStop™, cytokine expression could not be detected. If GolgiStop™ was added to cultures without restimulating with anti-CD3 mAb, a similar pattern of cytokine expression was observed, but levels were much lower (data not shown).
Results of a typical experiment are shown in Fig. 2. In the absence of IL-12 or other costimuli besides CD3 and CD28, a substantial fraction of CD62Llow-enriched CD4 T cells are induced to express IL-4, but few express IFN-γ (Fig. 2, A and B). In contrast, IFN-γ was strongly expressed by ∼30% of naive CD8+CD62Lhigh cells 72 h after activation with the same CD3 and CD28 stimulus (Fig. 2 D). Almost no CD8 T cells expressed detectable levels of IL-4 (Fig. 2 C).
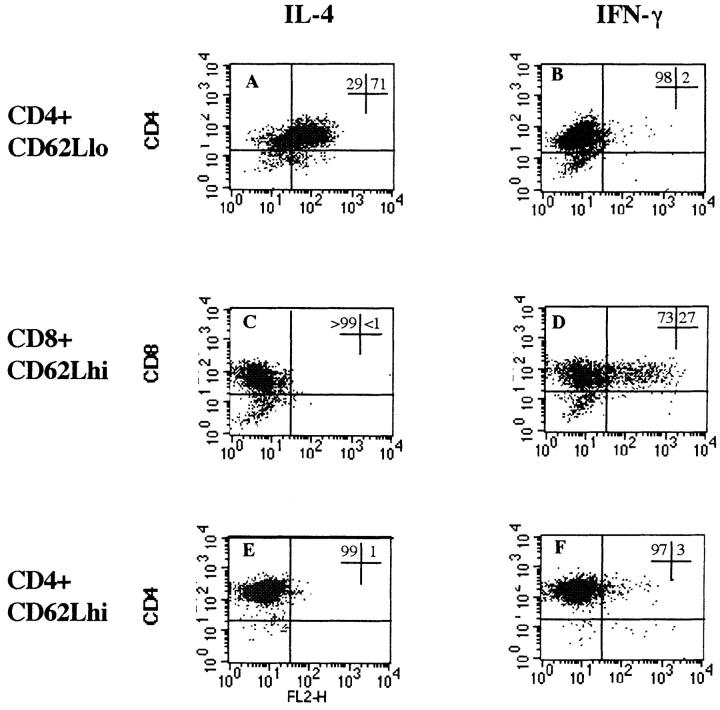
Figure 2.
Intracellular staining for IL-4 and IFN-γ on activated subpopulations of T cells. Purified subpopulations of CD4 and CD8 T cells, which had been activated for 3 d with immobilized mAb to CD3 and soluble mAb to CD28, were restimulated through CD3 for 4 h in the presence of GolgiStop™ to cause accumulation of intracellular cytokines before staining on the FACs®. Histograms show the proportion of cells positive compared with a negative control mAb: CD4+CD62Llow T cells enriched for activated and memory T cells (A) IL-4 and (B) IFN-γ; CD8+ CD62Lhigh naive T cells (C) IL-4 and (D) IFN-γ; and CD4+CD62Lhi naive T cells (E) IL-4 and (F) IFN-γ. lo, Low; hi, high.
Naive CD4+CD62Lhigh T cells activated in parallel failed to express either IL-4 or IFN-γ (Fig. 2, E and F). This was not because of lack of activation of these T cells, as they were stimulated to divide and upregulate expression of CD40L (see Fig. 3 A).
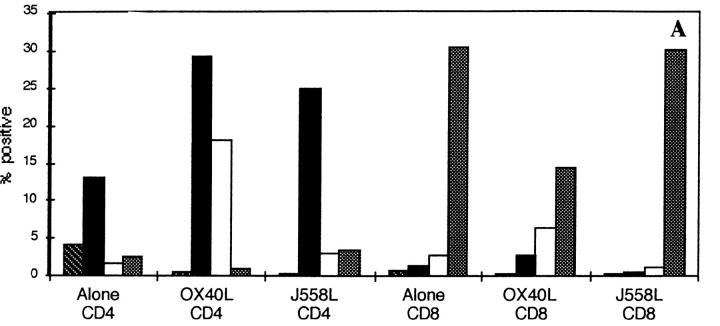
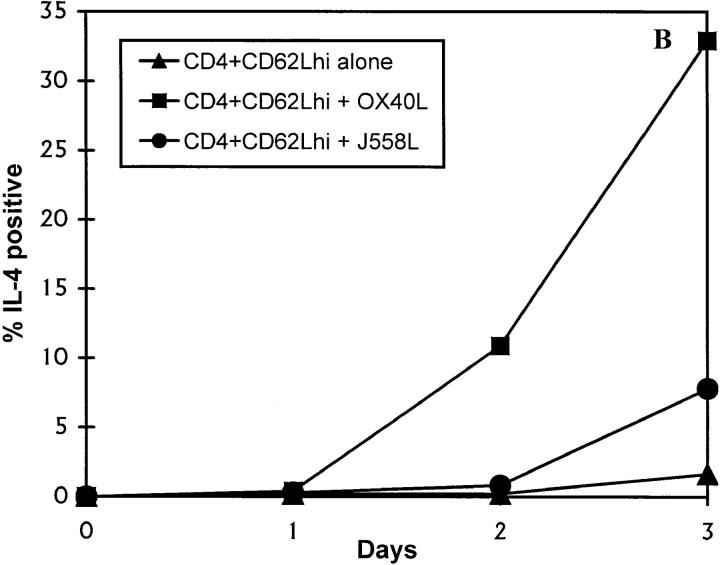
Figure 3.
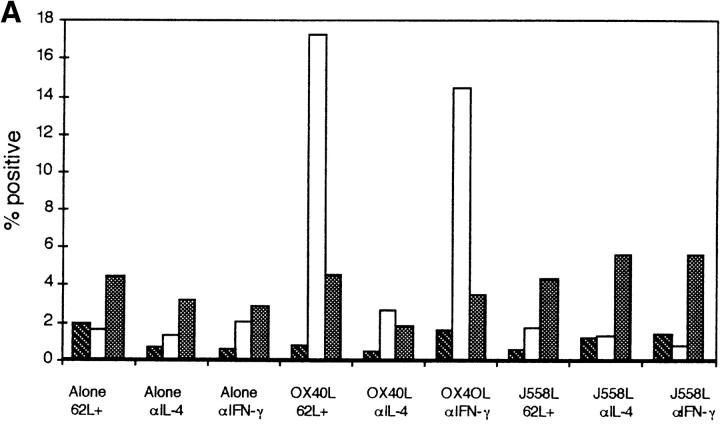
Induction of IL-4 expression by OX40L. CD62Lhigh CD8 and CD4 T cells were activated through CD3 and CD28 for 3 d in the presence of a fixed OX40L transfectant or the control parental cell line. Cells were restimulated through CD3 for 4 h in the presence of GolgiStop™ and stained for intracellular expression. (A) Staining with a control mAb (striped bars), anti-CD40L (black bars), anti–IL-4 (white bars), and anti–IFN-γ (stippled bars), on purified CD4 or CD8 CD62Lhigh T cells. The above data are representative of at least 10 different experiments. (B) Timing of induction of IL-4 expression in naive T cells by OX40L. Cells were stimulated as above. Data are representative of two experiments. hi, High.
OX40L Induces IL-4 but not IFN-γ Expression in Naive CD4 CD62Lhigh T Cells.
The above data indicated that naive CD4 T cells need signals other than those through their TCR and CD28 to upregulate expression of IL-4 or IFN-γ. IL-12, which can be produced by activated DC (4, 5), has been shown to be the principal cytokine involved in differentiation of Th1 CD4 T cells. Because we had observed that OX40L was upregulated at or about the time of T cell priming, we investigated whether this molecule could increase expression of IL-4 or IFN-γ within the 3-d time frame of priming of naive CD4 T cells in vivo. As can be seen from Fig. 3 A, OX40L transfectants but not the parental cell line, J558L, induced substantial expression of IL-4 but not IFN-γ by day 3. The effect of the OX40L transfectant could consistently be partially abrogated (∼30%) by preincubating the transfectant cell line with OX40-hγ1 (10 μg/ml) before the addition of T cells. Because the T cells are in contact with the OX40L transfectant for several days, we found it impossible to obtain complete inhibition of the OX40L effect with the Ig fusion protein (data not shown). The effects of OX40L cannot simply be attributable to better activation, as levels of CD40L induced on CD4 T cells were comparable. CD4 T cells enriched for activated CD62Llow cells expressed high levels of IL-4, irrespective of the costimulus (data not shown). A time course of induction of expression of IL-4 by OX40L showed no expression until day 2 (Fig. 3 B), a result consistent with the induction of IL-4 in vivo (2). These experiments were performed in both Balb/c and C57Bl/6 inbred strains of mice. The yield of CD4+CD62Lhigh cells was consistently greater from Balb/c mice (∼30%), and these cells survived much better in culture. In both strains of mice, OX40L induced expression of intracellular IL-4, although the proportion of CD4 T cells expressing IL-4 was less in the C57Bl/6 strain (∼50% of that seen in Balb/c mice; three experiments). Data is shown for Balb/c mice, which were used in most experiments because of the increased yield and better survival in culture of purified T cells.
Induction of IL-4 in CD4 T Cells by OX40L Is Dependent on IL-4 and Blocked by Neutralizing Anti–IL-4 Antibodies.
The above experiments indicated that OX40L upregulated the expression of IL-4 in naive CD4 T cells. It is well established that IL-4 itself promotes CD4 Th2 differentiation. To test whether OX40L was dependent on IL-4 for its effect, T cells were stimulated for 3 d in the presence of neutralizing IL-4 and IFN-γ mAb. Cells were washed extensively before restimulation to remove excess mAb that could interfere with the intracellular staining process. As can be seen from Fig. 4 A, anti–IL-4 mAb blocked the induction of intracytoplasmic IL-4 in CD4 T cells. mAb to IFN-γ partially blocked IL-4 expression, but this effect was much less marked.
Figure 4.
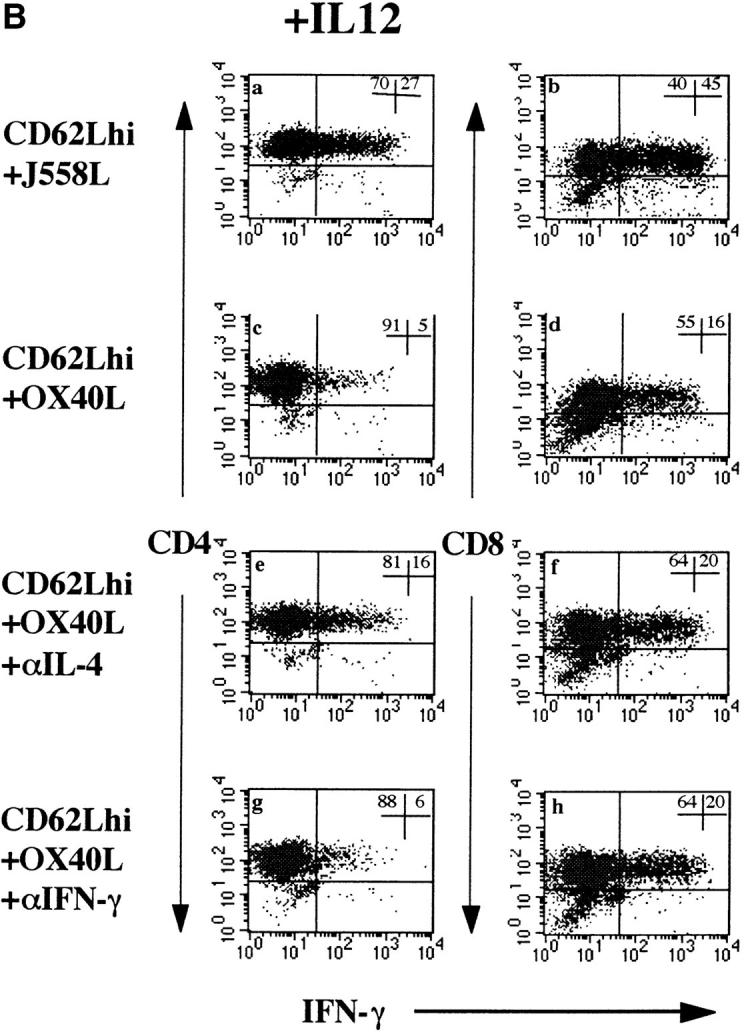
(A) Dependence of OX40L effect on IL-4 and IFN-γ secretion. Purified CD4+CD62Lhigh T cells were stimulated as described in the legend to Fig. 3 in the presence or absence of neutralizing mAbs to IL-4 and IFN-γ at a final concentration of 50 μg/ml. Before restimulation, cells were washed three times in medium to remove excess cytokine antibody. This was done to prevent interference with subsequent staining for intracellular cytokines. A shows the percentage of IL-4– or IFN-γ–positive CD4+ T cells when T cells were stimulated alone, with J558L, or with OX40L-fixed transfectants. Data shown are representative of six experiments in Balb/c mice. C57Bl/6 mice gave qualitatively similar results, but the degree of IL-4 expression was less (three experiments). Striped bars, Control; white bars, IL-4; stippled bars, IFN-γ. (B) Effect of OX40L on intracellular expression of IFN-γ in CD4 and CD8 T cells. CD4 and CD8 T cells were activated for 3 d as described in the legend to Fig. 2. To induce IFN-γ expression in CD4 T cells, IL-12 was added to cultures at a final concentration of 50 ng/ml. a, c, e, and g, Effects of OX40L and blocking cytokine mAbs on IFN-γ expression in CD4 T cells. b, d, f, and h, Effects on CD8 T cells. Results are representative of five separate experiments. hi, High.
IL-12–induced Th1 Differentiation of CD4 T Cells Is Substantially Blocked by OX40L.
A substantial proportion of naive CD8 (Fig. 2 D) but not CD4 (Fig. 2 F) T cells expresses IFN-γ when costimulated with CD3 and CD28 alone. However, if IL-12 is added to cultures, IFN-γ is strongly upregulated within activated CD62Lhigh CD4 T cells (Fig. 4 B, a). In the presence of IL-12, substantial numbers (∼25%) of activated CD4 cells express IFN-γ by day 3, and this is substantially inhibited in the presence of OX40L (5%). OX40-hγ1 abrogated the effect of OX40L on IFN-γ expression, confirming that the effects were attributable to this molecule (data not shown). The effect of OX40L on IFN-γ expression is inhibited when blocking IL-4 mAbs are added (Fig. 4 B, e), suggesting that its effects on CD4 Th1 differentiation are mediated by IL-4. IFN-γ blocking mAbs also inhibit CD4 Th1 differentiation (Fig. 4 B, g), confirming a previous report (6). Qualitatively similar experimental results were obtained using C57Bl/6 mice, but the inhibition of IFN-γ expression was less marked, correlating with the lower induction of IL-4.
OX40L also inhibits induction of IFN-γ from 45 to 16% in naive CD8 T cells (Fig. 4 B, g). This effect is resistant to blocking mAbs to either IL-4 or IFN-γ (Fig. 4 B, f and h).
Expression of mRNA for the Chemokine Receptor, Blr-1, Is Upregulated by OX40L.
Differentiation of CD4 T cells is associated not only with distinct cytokines but also with the propensity to migrate to different sites. Th1 cells express adhesion molecules which allow them to migrate to where endothelium is inflamed (21). In contrast, Th2 cells express the eotaxin receptor (CCR3), which perhaps allows them to migrate to sites of allergic responses (22). Another chemokine receptor, blr-1 (CXCR5), is implicated in the migration of B and T cells into follicles (23) where the ligand is expressed (12). We examined the expression of mRNA for this chemokine receptor in parallel with cytokine expression on CD4 T cells activated with and without OX40L.
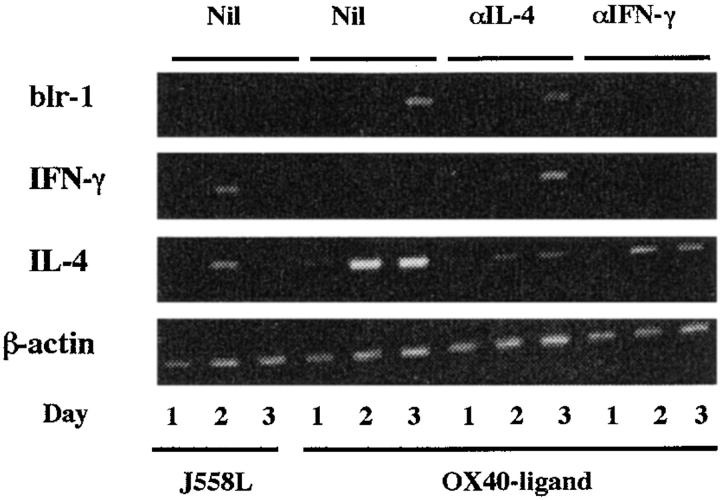
Blr-1 was not expressed on naive CD4 T cells, but after 3 d costimulation with OX40L but not the parental line, J558L, blr-1 mRNA was strongly upregulated (Fig. 5). Expression of this mRNA was blocked only partially by neutralizing mAb to IL-4.
Figure 5.
OX40L induces IL-4 and blr-1 mRNA in naive CD4 T cells. The ethidium bromide gel shows semiquantitative RT-PCR for IL-4, IFN-γ, and blr-1 in cell samples of activated CD4 CD62Lhigh T cells at the times indicated after culture. Amounts of mRNA were adjusted to give comparable β-actin signals. T cells had been stimulated in the presence of fixed parental cell line J558L or OX40L transfectant with and without mAb to IL-4 and IFN-γ. RT-PCR of the fixed cell lines did not yield cDNA that could be amplified. Results are representative of three experiments. Nil, no blocking mAb added.
The expression of mRNA for IFN-γ and IL-4 mRNA paralleled that seen by staining for intracellular cytokines, even though cells had not been restimulated. This confirms that restimulation does not alter the pattern of cytokines expressed. In these experiments, blocking mAb to IL-4 inhibits mRNA expression, showing that the effects of OX40L on IL-4 expression are dependent on IL-4.
Discussion
T and B cell immune responses to protein antigens are initiated in the T zone (24, 25). Some B cells differentiate locally to plasma cells, while others are induced to migrate into B follicles and form germinal centers with the help of antigen-specific T cells. Recent data have shown that T cell cytokine commitment starts ∼2 d after immunization: soluble protein antigens evoke predominantly Th2 CD4 T cell responses, whereas responses to viral antigens are more complex and show a mixture of Th1 and Th2 cytokines (2). It is crucial that CD4 T cells make the appropriate cytokine response, as resistance to intracellular bacterial infections like mycobacteria is dependent on IFN-γ production. How do CD4 T cells decide? We present evidence in this paper that effector cells play an important role in the decision-making process by interacting with CD4 T cells at the time of T cell priming.
It has been reported previously that cross-linking of Ig receptors or CD40 ligation of B cells upregulates the expression of OX40L (20), and there is experimental evidence that blocking this interaction abrogates B cell differentiation in the T zone in vivo (11). We have found that OX40L and its receptor, OX40, are upregulated between days 2 and 3, when T and B cells are primed in vivo. To study whether OX40L induces differentiation of naive T cells, an in vitro system was developed. In this model, signals through CD28 and the TCR were insufficient to induce either IL-4 or IFN-γ expression during the first 3 d of priming of naive CD4 cells, which nevertheless proliferated (data not shown) and expressed CD40L. This time frame was chosen because it reflected the commitment of CD4 T cells to IL-4 or IFN-γ production in vivo. The addition of fixed OX40L but not control transfectants induced IL-4 expression that was detectable by day 2 and strongly expressed by day 3. This temporal expression coincided with that seen in vivo. This effect was inhibited if IL-4 was neutralized during the priming process, suggesting that OX40L initiates Th2 differentiation but IL-4 plays the dominant role thereafter. In addition, OX40L upregulated the expression of blr-1, a chemokine receptor implicated in the migration of T and B cells into follicles (12, 23).
In contrast to naive CD4 T cells, naive CD8 T cells produced IFN-γ when costimulated through CD28 and TCR alone. IFN-γ expression in naive CD4 T cells could be readily induced by IL-12, but expression was substantially inhibited by OX40L. This effect was mediated in part by IL-4, as neutralizing IL-4 antibodies mitigated this effect.
A Model for Th2 Decision-making in Naive CD4 T Cells.
There is increasing evidence that the type of antigen or the way in which it is presented to the immune system can regulate subsequent cytokine responses by CD4 T cells (2, 5). It has been shown previously that antigen dosage can regulate commitment to Th1 or Th2 CD4 pathways in vitro (26). However, the cytokine IL-12 must play the crucial role in Th1 differentiation, as IL-12–deficient mice and humans cannot mount effective IFN-γ responses to intracellular pathogens. IL-6 has been proposed be the Th2 cytokine equivalent of IL-12, as it is able to initiate IL-4 secretion in CD4 T cells (27). However, the phenotype of IL-6–deficient mice, which make Th2 responses, suggests that this cannot be an exclusive mechanism (28).
Our data are consistent with a model where CD4 T cells are not committed initially to make either IL-4 or IFN-γ when they are activated by signals through CD28 and the TCR on DC. Instead, their differentiation is guided by secondary signals from the effector cell with which they interact. We show here that OX40L is a sufficient accessory signal to initiate early Th2 differentiation in naive CD4 T cells. We propose that antigen-activated B cells, which are programmed to migrate to T cell areas (29), engage CD4 T cells around the time of their priming. Signaling through CD40 or surface Ig on B cells upregulates the expression of OX40L, which engages OX40 on the activated cognate CD4 T cell. This signal sets in motion a self-reinforcing IL-4 loop in primed CD4 T cells that further promotes Th2 differentiation. IL-12 can be produced by DC (4, 5), and a recent report suggests that human DC can express OX40L (30). By inducing IL-12 or OX40L, respectively, antigens might effectively evoke Th1- and Th2-promoting DC.
OX40L Induces Naive T Cells to Express Blr-1, A Chemokine Receptor Whose Ligand Is Expressed in B Follicles.
Th1 differentiation is associated with the upregulation of chemokine receptors CXCR3 and CCR5 (31) and the expression of adhesion molecules that allow them to migrate into inflamed tissue (21). In contrast, Th2 cells express the eotaxin receptor, CCR3, which allows them to migrate to allergic sites (22). Some T cells express CXCR5 (blr-1), a chemokine receptor whose ligand is expressed in follicles. Our data show that OX40L upregulates blr-1, and therefore directs not only Th2 differentiation but also migration of T cells into follicles to help B cells. Although blocking OX40 interactions in vivo does not abrogate germinal center development (11), this probably reflects redundancy within the CD40 family of receptor ligands. We have found that CD27L, which can be expressed on activated B cells (32) and whose receptor is expressed on T cells, induces similar effects on naive CD4 T cells (our unpublished observations).
Production of IFN-γ by Activated Naive CD8 T Cells Helps CD4 T Cells Make Th1 Responses.
IL-12 and IFN-γ play a crucial role in responses to intracellular bacterial antigens. Although the immune system may have evolved to recognize specific intracellular pathogens (5), it seems likely that there is some default mechanism for recognizing such infections. Differential processing of antigen by DC might determine whether they produced IL-12.
Alternatively, the experiments described here raise a second possibility. During responses to extracellular antigens, CD8 T cells are not generally primed as antigen is presented in association with MHC class II molecules. This is not the case with intracellular infections where both CD8 and CD4 T cells are activated by antigens presented on MHC class I and II molecules, respectively. Most CD8 responses require Th1 CD4 help (IL-2), but unlike B cells, CD8 lymphocytes do not engage cognately with CD4 T cells. The default expression of IFN-γ (which promotes Th1 differentiation [6]) by naive CD8 T cells during T cell priming perhaps offers a mechanism for instructing naive CD4 T cells to remain responsive to IL-12 (6), and DC to produce IL-12 (33). This idea is supported by recent data suggesting that CD8 T cells play an important role in the generation of protective Th1 CD4 responses (34), and in particular IFN-γ produced by these CD8 T cells (35).
Most Responses Require a Mixture of Th1 and Th2 CD T Cells.
Most intracellular infections, particularly viruses, require a combination of high affinity antibody and cytotoxic CD8 T cells for the best protective immunity. We show here that OX40L will inhibit Th1 differentiation promoted by IL-12. This suggests a mechanism whereby B cells can efficiently evoke Th2 cytokines from CD4 T cells in an environment where IL-12 is inducing IFN-γ. This is demonstrated in the response to MMTV, where IL-4 production after IFN-γ is associated with the development of germinal centers (2) and the production of neutralizing antiviral envelope antibodies (19).
Acknowledgments
The authors would like to thank Ian MacLennan, Matt Cook, Lucy Walker, and Carola Garcia for their comments on the manuscript.
This work was supported by grants from The Royal Society and from the Wellcome Trust to P. Lane. S. Flynn is supported by a Medical Research Council Ph.D. studentship.
Abbreviations used in this paper
- DC
dendritic cell(s)
- h
human
- L
ligand
- m
murine
- MMTV
Swiss-type mouse mammary tumor virus
- NP-CγG
(4-hydroxy-3-nitrophenyl) acetyl–chicken γ globulin
- RT
reverse transcription
References
- 1.Kumararatne DS. Tuberculosis and immunodeficiency— of mice and men. Clin Exp Immunol. 1997;107:11–14. doi: 10.1046/j.1365-2249.1997.d01-910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toellner K, Luther S, Sze DM-Y, Choy RK-W, Taylor DR, MacLennan ICM, Acha-Orbea H. Th1 and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–1201. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 4.Cella M, Scheidegger D, Palmer-Lehman K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell costimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sousa CR, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand–independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendelac A. Mouse NK1+ T cells. Curr Opin Immunol. 1995;7:367–74. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 8.von der Weid T, Beebe AM, Roopenian DC, Coffman RL. Early production of IL-4 and induction of Th2 responses in the lymph node originate from an MHC class I-independent CD4+NK1.1−T cell population. J Immunol. 1996;157:4421–4427. [PubMed] [Google Scholar]
- 9.Stockinger B, Zal T, Zal A, Gray D. B cells solicit their own help from T cells. J Exp Med. 1995;183:891–899. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason D. The role of B cells in the programming of T cells for IL-4 synthesis. J Exp Med. 1996;183:717–719. doi: 10.1084/jem.183.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stüber E, Strober W. The T cell–B cell interaction via OX40-OX40L is necessary for the cell-dependent humoral immune response. J Exp Med. 1996;183:979–989. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma type receptor-1. Nature. 1998;391:799–802. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 13.Traunecker A, Oliveri F, Karjalainen K. Myeloma based expression system for production of large mammalian proteins. Trends Biotechnol. 1991;9:109–113. doi: 10.1016/0167-7799(91)90038-j. [DOI] [PubMed] [Google Scholar]
- 14.Calderhead DM, Buhlmann JE, Vandeneertwegh AJM, Claassen E, Noelle RJ, Fell HP. Cloning of mouse Ox40: a T cell activation marker that may mediate T-B cell interactions. J Immunol. 1993;151:5261–5271. [PubMed] [Google Scholar]
- 15.Lane P, Gerhard W, Hubele S, Lanzavecchia A, McConnell F. Expression and functional properties of mouse B7/BB1 using a fusion protein between mouse CTLA4 and human γ 1. Immunology. 1993;80:56–61. [PMC free article] [PubMed] [Google Scholar]
- 16.Svetic A, Finkelman FD, Jian YC, Dieffenbach CW, Scott DE, McCarthy KF, Steinberg AD, Gause WC. Cytokine gene expression after in vivoprimary immunization with goat antibody to mouse IgD antibody. J Immunol. 1991;147:2391–2397. [PubMed] [Google Scholar]
- 17.Forster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein–coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- 18.Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Lab Invest. 1963;12:131–155. [PubMed] [Google Scholar]
- 19.Luther SA, Maillard I, Luthi F, Scarpellino L, Diggelmann H, Acha-Orbea H. Early neutralizing antibody response against mouse mammary tumor virus: critical role of viral infection and superantigen-reactive T cells. J Immunol. 1997;159:2807–2814. [PubMed] [Google Scholar]
- 20.Stüber E, Neurath M, Calderhead D, Fell HP, Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 21.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 23.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 24.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 26.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4–producing CD4+T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopf M, Legros G, Coyle AJ, Koscovilbois M, Brombacher F. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol Rev. 1995;148:45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 29.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 30.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- 31.Bonecchi R, Bianchi G, Bordignon PP, Dambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lens SMA, Dejong RL, Hooibrink B, Koopman B, Pals ST, Vanoers MHJ, Vanlier RAW. Phenotype and function of human B-cells expressing CD70 (CD27 ligand) Eur J Immunol. 1996;26:2964–2971. doi: 10.1002/eji.1830261223. [DOI] [PubMed] [Google Scholar]
- 33.Hilkens CMU, Kalinski P, deBoer M, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90:1920–1926. [PubMed] [Google Scholar]
- 34.Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ. Protection against Mycobacterium tuberculosis infection by CD8+T cells requires the production of gamma interferon. Infect Immun. 1998;66:830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]