Abstract
During apoptotic cell death, cell surface ligands initiate phagocytosis of the dying cell. Clearance of these apoptotic cells is thought to occur without an immune response. Since a number of autoantigens are located at the cell surface or within apoptotic blebs, we examined whether exposure of mice to syngeneic apoptotic cells by the intravenous route could induce autoantibody production. Normal mice injected with syngeneic apoptotic thymocytes developed antinuclear autoantibodies and anticardiolipin and anti-ssDNA antibodies. The autoantibody levels were generally lower than those observed in MRL/Faslpr mice and were transient. Surprisingly, six out of six immunized mice demonstrated immunoglobulin G deposition in the glomeruli several months after immunization. These findings indicate that systemic exposure to apoptotic cells can induce an immune response in normal mice, and may help to explain antigen selection and initiation of the immune response in diseases characterized by increased rates of apoptosis such as AIDS and, possibly, systemic lupus erythematosus.
Keywords: apoptosis, autoimmunity, systemic lupus erythematosus, anti-DNA, anticardiolipin
Apoptosis is of basic biomedical interest and is of particular importance in the pathogenesis of systemic autoimmune diseases, as exemplified by Fas and Fas ligand mutations in mice and humans (for review see reference 1). It is unclear whether there are primary defects in apoptotic pathways in cells from patients with systemic lupus erythematosus (SLE), although most patients do not have defective expression or function of Fas/Fas ligand (1). It has long been appreciated that DNA and histones are major autoantigens in SLE but only more recently recognized that the DNA–histone complex, i.e., nucleosomes, are the preferred targets of autoantibodies (2). Unanswered questions are how and why nucleosomes and several other intracellular antigen targets become antigenic in SLE.
One possible explanation for antigen selection is apoptosis. During apoptosis, the cell membrane forms cytoplasmic blebs, some of which are shed as apoptotic bodies. Casciola-Rosen et al. (3) have shown that UV-induced apoptosis of keratinocytes leads to redistribution of several autoantigens to apoptotic blebs. Phosphatidylserine (PS), an acidic phospholipid autoantigen (4) that normally resides on the inside of the cell, flips to the outside of the cell membrane when the cell undergoes apoptosis (5). Together, these findings provide a hypothesis for antigen selection in SLE, viz. that SLE patients respond to PS and a subset of intracellular nucleoproteins translocated to cell surface blebs during apoptosis. To test whether apoptotic cells could, under some circumstances be immunogenic, we immunized normal mice with syngeneic apoptotic cells.
Materials and Methods
Apoptosis Induction and Analysis.
Apoptosis of thymocytes was induced by γ-irradiation (600 rads). Apoptosis was quantitated by annexin-FITC/propidium iodide (PI) staining and flow cytometry (6), and by quantitation of the subdiploid peak after PI staining (7).
Immunization Protocol.
C3H-SnJ (C3H), BALB/c, C57BL/6 (B6), and MRL/MpJ-Fasl pr mice (MRL/lpr) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Thymocytes and splenocytes were prepared from 6–8-wk-old mice as described previously (8). The thymocytes were either irradiated to induce apoptosis as above or lysed by three freeze–thaw cycles. The thymocyte cell lysates and splenocytes (107 syngeneic cells per mouse) were injected intravenously without any further manipulation. The irradiated thymocytes were incubated in medium at 37°C for 4 h to allow apoptotic changes to occur and 107 syngeneic cells were injected intravenously per recipient. The injections were performed weekly for a total of four injections.
Immune Response.
Serum samples were obtained immediately before immunization and once every 2 wk after immunization for up to 30 wk. Antinuclear antibodies (ANAs) were detected by indirect immunofluorescence on Hep-2 cells or a mouse T cell line (AE.7). Total serum IgG and IgM, anti-ssDNA, anticardiolipin (AcL), and rheumatoid factor autoantibodies were quantified by ELISAs as described previously (8, 9). Sera were diluted 1:50 for the ANA and 1:100 for the autoantibody screens. Values >3 SD above the mean derived from syngeneic normal age-matched controls was considered positive for ELISA. Inhibition studies for anti-ssDNA and AcL were performed as described previously (26). Antibodies to protein antigens were tested by Western blot analysis using cell extracts as well as human recombinant Ro/SSA, La/SSB, Sm, and ribosomal P proteins (10, 11).
Clinical and Pathological Evaluation.
Mice were examined bimonthly for clinical signs of disease and for hematuria or proteinuria using N-Multistix SG (Bayer, IN). Histological evaluation of kidneys was performed as previously described (8). Immunofluorescence was examined using a Nikon microphot-fxa immunofluorescence microscope. The time (seconds) required for the photometer to obtain sufficient signal for photography (inversely proportional to the intensity of the immunofluorescence signal) was recorded.
Results
Normal Mice Injected with Syngeneic Apoptotic Thymocytes Develop ANAs.
Irradiation of thymocytes induced apoptosis in ∼70% of cells as determined by annexin V staining. Only a small proportion of the cells were in advanced stages of cell death as determined by admission of PI (<5%) or trypan blue (<2%) (data not shown).
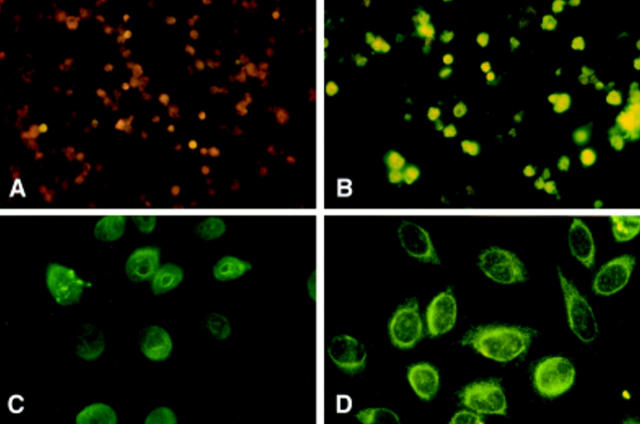
To determine whether exposure to large numbers of syngeneic apoptotic cells could evoke an immune response in normal mice, we injected 107 cells per mouse by the intravenous route. The majority (12 out of 16) of normal C3H mice injected with apoptotic cells developed positive IgM ANAs, and approximately half (8 out of 15) developed IgG ANAs by 4–6 wk after initial immunization (Table 1 and Fig. 1). Although the ANA patterns were heterogeneous, the most common patterns observed were nuclear rim with speckled intranuclear staining (Fig. 1). Similar results were obtained by immunization of BALB/c and B6 mouse strains, indicating that these results were not strain specific. Since nonirradiated thymocytes contained ≥10% annexin-binding cells after isolation (data not shown), splenocytes (<5% annexin positive) rather than thymocytes were used as a control for these experiments. Few of the nonimmunized mice or mice immunized with splenocytes developed ANAs (Table 1).
Table 1.
Number and Percentage of ANA-positive Sera
| Strain | Immunogen | IgM ANA | IgG ANA | |||
|---|---|---|---|---|---|---|
| C3H/SnJ | Irr-T | 12/16 (75) | 8/15 (53) | |||
| SPL | 1/6 (17) | 0/6 (0) | ||||
| Lys-T | 2/4 (50) | 1/4 (25) | ||||
| Nil | 0/10 (0) | 0/10 (0) | ||||
| BALB/c | Irr-T | 7/9 (78) | 5/9 (56) | |||
| SPL | 1/3 (33) | 0/3 (0) | ||||
| Lys-T | ND | ND | ||||
| Nil | 1/10 (10) | 1/10 (10) | ||||
| C57BL/6 | Irr-T | 4/6 (67) | 3/6 (50) | |||
| SPL | 0/3 (0) | 0 (0) | ||||
| Lys-T | ND | ND | ||||
| Nil | 0/5 (0) | 0/5 (0) |
Mice were immunized with irradiated apoptotic thymocytes (Irr-T), nonapoptotic splenocytes (SPL), a thymocyte lysate (Lys-T), or saline (Nil). Percentages are shown in parentheses. Representative examples are shown in Fig. 1.
Figure 1.
Injection of apoptotic cells induces ANAs. Serum obtained from normal C3H mice either uninjected (A) or injected with syngeneic apoptotic cells (B–D) were tested for ANAs by indirect immunofluorescence at a 1:50 dilution, 4–6 wk after the first injection. The cell lines used for ANA detection were the T cell lines, AE.7 (A and B) or HEP-2 (C and D). These figures are representative of the results summarized in Table 1.
Normal Mice Injected with Syngeneic Apoptotic Thymocytes Develop anti-ssDNA and AcL Autoantibodies.
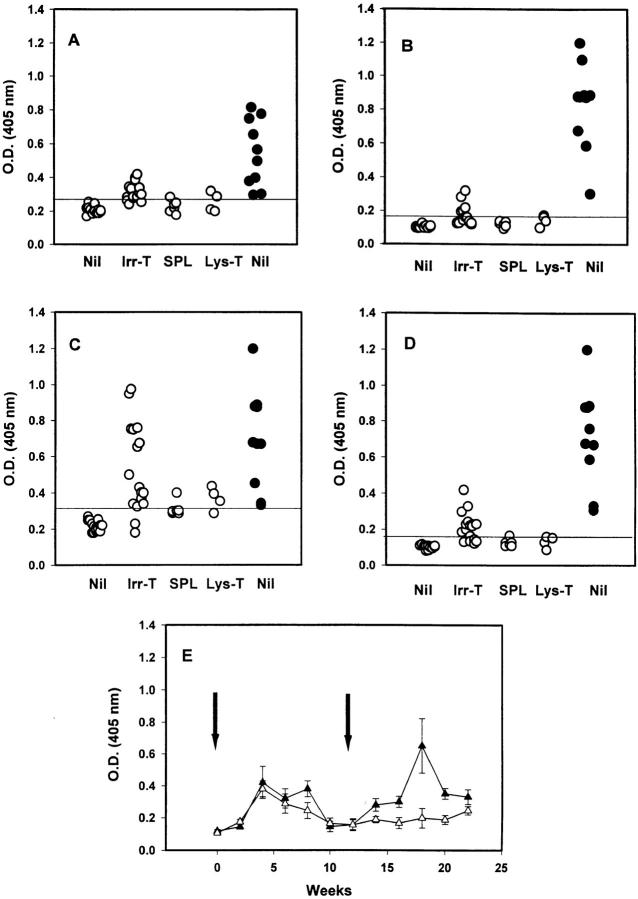
Most of the C3H mice injected with apoptotic thymocytes produced modest titers of anti-ssDNA antibodies of both the IgM and IgG isotypes (Fig. 2, A and B). Similar results were seen in BALB/c, and B6 mouse strains (data not shown). Anti-dsDNA antibodies and rheumatoid factor were not significantly elevated in comparison to age-matched control (data not shown).
Figure 2.
Mice injected with apoptotic thymocytes produce anti- ssDNA and AcL autoantibodies. C3H mice (open circles) were immunized with saline (Nil), irradiated thymocytes (Irr-T), nonirradiated splenocytes (SPL), or a lysate from irradiated thymocytes (Lys-T). Sera obtained 2–6 wk after initial injection were tested for anti-ssDNA and AcL autoantibodies by ELISA as described in Materials and Methods. The results of IgM anti-ssDNA (A), IgG anti-ssDNA (B), IgM AcL (C), and IgG AcL (D) are shown. Results greater than the mean + 3 SD (horizontal line) obtained from age-matched controls were regarded as positive. Anti-ssDNA and AcL levels of 5–6-mo-old MRL/lpr mice (closed circles) are shown for comparison. The kinetics of IgG AcL antibody production are shown in E. Six C3H mice were immunized weekly with irradiated thymocytes (time 0 [left arrow] to wk 4). Starting at wk 12 (right arrow), three of these mice (closed triangles) were reimmunized weekly and three mice (open triangles) were used as controls. Serum was collected at the times shown and tested for AcL binding as described in Materials and Methods.
Compared with the low titers of anti-ssDNA produced in response to immunization with apoptotic thymocytes, some C3H mice produced quite striking elevations of AcL antibodies similar to the autoimmune MRL/lpr-positive controls (Fig. 2, C and D). As with ANAs and anti-ssDNA, these responses were either absent or substantially lower in the control-immunized mice. Similar results were observed in the other normal strains injected with apoptotic thymocytes, although the frequency of response was slightly lower (IgM and IgG AcL were positive in 7 out of 9 and 6 out of 9 BALB/c mice and 4 out of 6 and 2 out of 6 B6 mice, respectively). As shown in Fig. 2 E, the increases in AcL autoantibodies observed 2–4 wk after the first injection declined by week 10 and, for the most part, returned to baseline (similar to aged-matched, nonimmunized mice). To verify that the increases in autoantibody production were the result of the injection of apoptotic cells, we repeated the series of four injections with syngeneic apoptotic cells 8 wk after the last injection in the same mice. Re-injection of apoptotic cells once again induced the autoantibody response that declined after week 20 (Fig. 2 E).
Negatively charged phospholipids as well as apoptotic cells bind to β-2 glycoprotein I (β-2GPI), an important cofactor for recognition of AcL antibodies. Since immunization of mice with xenogenic, but not syngeneic β-2GPI induces AcL (9), we repeated these experiments with syngeneic apoptotic thymocytes that had no contact with foreign serum. The frequency and levels of autoantibodies produced in these mice were virtually identical to those described above (data not shown), indicating that the immune response observed was not caused by immunization with bovine β-2GPI or other FCS-derived products.
The Character of AcL.
Since DNA and cardiolipin are highly charged polyanionic molecules, binding to ssDNA and AcL could be due to a polyreactive antibody population (12). Therefore, we performed cross-absorption experiments with ssDNA and cardiolipin micelles. The anti-ssDNA and AcL antibodies could be inhibited by 50% with 1–10 μg/ml of cardiolipin micelles or ssDNA, respectively, indicating that these antibodies have some features in common with the polyreactive autoantibodies described in autoimmune strains of mice.
Total Ig Levels and Autoantibodies to Protein Antigens.
No significant differences in total IgM concentrations were observed between mice injected with apoptotic thymocytes and those injected with splenocytes (data not shown). IgG concentrations were significantly higher in mice with apoptotic cells at 6 wk after initial immunization compared with splenocyte-immunized controls (5.9 ± 0.88 and 2.57 ± 0.50 mg/ml, respectively, P <0.004). These findings are consistent with a modest polyclonal activation of the immune system by the apoptotic cells. Western blot analysis for antibodies to whole cell extracts (both apoptotic and nonapoptotic), nuclear extracts, or specific recombinant autoantigens were negative (data not shown).
Clinical Evaluation.
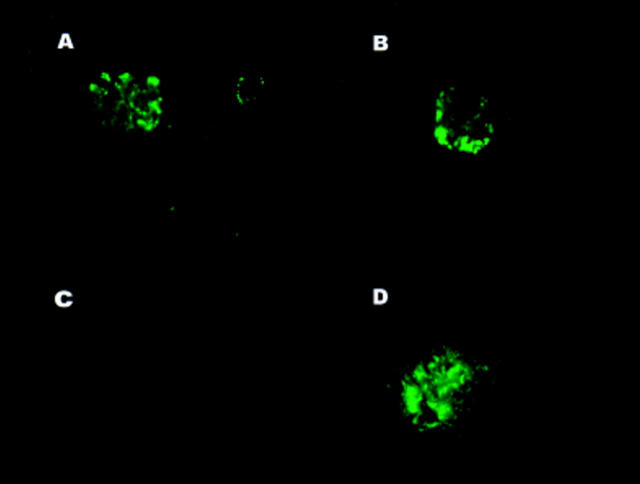
No obvious clinical changes were noted in the immunized mice. Proteinuria did not exceed 1+ (as is seen in normal age-matched controls) and hematuria was not detected. Light microscopic evaluation of the kidney was normal with the exception of occasional mild mesangial proliferation. IgG deposition was not detected in the glomeruli of any of the nonimmunized controls or splenocyte-immunized (n = 4) mice, but was positive in all six normal mice injected with apoptotic cells (Fig. 3). Of note, of three kidneys examined from mice immunized with thymocyte lysate, all had IgG deposition, although the staining was of lower intensity. The exposure time (seconds) for each group (n = 3–7 mice per group) were as follows: nonimmunized control, >20; nonimmunized MRL/ lpr, 4.1 ± 1.5; splenocyte-immunized, >20; apoptotic cell– immunized, 7.6 ± 0.5; lysate-immunized, 12.4 ± 1.0.
Figure 3.
Immunization with apoptotic thymocytes results in IgG deposition in renal glomeruli. Kidneys obtained from normal C3H mice immunized with apoptotic thymocytes (A and B), were tested for IgG deposition by immunofluorescence. Nonimmunized, age-matched C3H and MRL/lpr mice were used as a negative (C) and positive (D) controls.
Discussion
An important difference between programmed (apoptotic) versus accidental/toxic (necrotic) death is that programmed cell death results in the ordered fragmentation of the cell leading to rapid phagocytosis by neighboring cells and/or professional phagocytes without cell activation and inflammation (13). The major observation reported here is that administration of apoptotic cells by the intravenous route is able to induce autoantibodies in normal mice. Autoantibody production reflected an immune response to the immunogen since, after subsidence of autoantibody levels, repeated administration of apoptotic cells once again induced circulating autoantibodies. The ability to induce autoantibodies by the intravenous route indicates that handling of apoptotic cells is not always “silent”.
Nonapoptotic splenocytes and the thymocyte lysate (which contained ∼10% annexin V–positive cells before lysis) induced only slight increases in autoantibody levels in this model, suggesting that the production of autoantibodies in response to apoptotic thymocytes could not simply be explained by exposure to high concentrations of cellular molecules or to “danger” stimuli released by injection. Since a high titer of AcL antibodies were also produced when irradiated thymocytes were prepared in the absence of fetal bovine serum, a role for heterologous β-2GPI (9) can be excluded. These findings indicate that the induction of anti-ssDNA and AcL antibodies reflects an immune response to intact apoptotic cells or apoptotic bodies. At present, we cannot distinguish whether the lower immune response observed after injection of the thymocyte lysate (see also below) was due to contamination by blebs derived from the apoptotic population of cells or whether both sources induce an immune response.
The immunogenicity of syngeneic apoptotic cells reported here is surprising. Numerous studies over the years have shown anti-DNA and other autoantibodies are rarely generated by immunization with self-antigen unless special adjuvants and/or a foreign carrier are used concomitantly (14). Furthermore, injection of antigen by the intravenous route is thought of as a tolerogenic rather than an immunogenic pathway (15). The normal immune system is exposed to many millions of apoptotic cells per day. However, apoptosis occurs predominantly in the central lymphoid organs such as the thymus and bone marrow, where the clearance of the cells is remarkably efficient. The products of apoptotic cells may induce tolerance under these circumstances due to lack of induction of costimulatory signals.
Several possibilities may explain the induction of autoantibodies in the present study. The quantity of apoptotic material injected may have overwhelmed the normal clearance capacity of macrophages leading to abnormal presentation to B cells or dendritic cells. Phosphatidylserine is exposed on the apoptotic cell surface membrane (5) and could provide signal 1 to potentially autoreactive B cells. Since this antigen would be multivalent, it may be sufficient to act as a T cell–independent antigen. It is less clear whether nucleosomes and other lupus autoantigens are actually exposed on the outer surface of apoptotic blebs, although it is possible that even a small degree of breakdown of the apoptotic blebs would release nucleosomes resulting in anti-ssDNA production. A recent study reports that uptake of apoptotic cells by LPS-stimulated macrophages results in the enhanced secretion of IL-10 (16). Although IL-10 is immunosuppressive for T cells and enhances apoptosis of SLE T cells in vitro (17), it augments B cell activation and is produced at high concentration in patients with SLE (18).
We also observed partial cross-reactivity between anti-ssDNA and AcL autoantibodies as has been shown with certain mAbs derived from MRL/lpr mice (12), suggesting that some of the autoantibodies generated may be low affinity and polyreactive. The increased production of total IgG in recipient mice argues that some degree of polyclonal B cell stimulation was induced, similar to that observed after exposure of B cells to nucleosomes in vitro (19). The antibodies induced could also reflect expression of “natural antibodies”.
Finally, since apoptosis and irradiation are associated with numerous intracellular alterations, protein modification in the apoptotic cell or induction of costimulatory molecules in the phagocyte could contribute to antibody production. However, autoantibodies were predominantly targeted to nonprotein antigens and were transient. The absence of anti-dsDNA antibodies in this study also argues that T cell tolerance to nucleosomes is either maintained or only transiently lost in normal strains of mice.
Although none of the mice in this study developed overt clinical disease, IgG deposits were consistently found in the kidneys of mice immunized with apoptotic cells several months after immunization. Even more surprising, mice immunized with thymocyte lysates also had glomerular IgG, although of lower intensity. These findings may be explained by the deposition of nucleosomes in glomeruli (20) followed by in situ fixation of anti-ssDNA or polyreactive antibodies. Alternatively, once low titer anti-DNA antibodies were induced, antigen–antibody complexes may have formed in the circulation with subsequent renal deposition. As discussed above, the failure to induce high affinity IgG anti-dsDNA antibodies most likely accounts for the lack of lupus-like glomerulonephritis in the immunized mice.
The findings in this study suggest that, in diseases characterized by a continuous source of apoptotic cells in the peripheral immune system, autoantibodies directed against phospholipids, ssDNA, and possibly other antigens may be generated as an immune response. Patients with AIDS have extensive apoptosis of CD4+ T cells (21) and SLE patients have lymphopenia as well as evidence for an increased rate of activation-induced cell death (17, 22, 23). The production of anti-ssDNA, AcL, and antibodies to other cell surface antigens that occurs in SLE and HIV infections (24) may therefore be induced by a mechanism similar to that described herein. On the other hand, this study also suggests that systemic exposure to normal syngeneic apoptotic cells is probably not a sufficient explanation for induction of high titer, high affinity, pathogenic autoantibodies. Additional immunoregulatory defects are most likely required for the full induction of systemic autoimmune disorders. It will be of considerable interest to determine whether an increased apoptotic load accelerates autoimmunity in mice with genetic defects predisposing to systemic autoimmunity and whether some autoimmunity susceptibility genes relate to uptake and/or handling of apoptotic cells.
Acknowledgments
Supported in part by grants from the National Institutes of Health (SCOR in SLE P50AR-42588), aided by a grant from the Arthritis Foundation, NY Chapter (to D. Mevorach).
Footnotes
Support from Drs. S. Paget, A. Gibofsky, N. Brot, and M. Mevorach is acknowledged.
References
- 1.Vaishnaw AK, McNally JD, Elkon KB. Apoptosis in the rheumatic diseases. Arthritis Rheum. 1997;40:1917–1927. doi: 10.1002/art.1780401102. [DOI] [PubMed] [Google Scholar]
- 2.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauch J, Janoff AS. Antibodies against phospholipids other than cardiolipin: potential roles for both phospholipid and protein. Lupus. 1996;5:498–502. doi: 10.1177/096120339600500534. [DOI] [PubMed] [Google Scholar]
- 5.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein-labeled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 7.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocytes apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:1173–1181. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 8.Bullard DC, King PD, Hicks MJ, Dupont B, Beaudet AL, Elkon KB. ICAM-1 deficiency protects MRL/MpJ-Faslpr mice from early lethality. J Immunol. 1997;159:2058–2067. [PubMed] [Google Scholar]
- 9.Gharavi AE, Sammaritano LR, Wen J, Elkon KB. Induction of antiphospholipid autoantibodies by immunization with 2 glycoprotein I (apolipoprotein H) J Clin Invest. 1992;90:1105–1109. doi: 10.1172/JCI115927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bini P, Chu JL, Okolo C, Elkon KB. Analysis of autoantibodies to recombinant La (SS-B) peptides in systemic lupus erythematosus and primary Sjogren's syndrome. J Clin Invest. 1990;85:325–333. doi: 10.1172/JCI114441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkon KB, Hines JJ, Chu JL, Parnassa AP. Epitope mapping of recombinant HeLa SmB and B′ peptides obtained by the polymerase chain reaction. J Immunol. 1990;145:636–643. [PubMed] [Google Scholar]
- 12.Lafer E, Rauch J, Andrzejewski C, Mudd D, Furie B, Schwartz RS, Stollar BD. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981;153:897–904. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz RS, Stollar BD. Origins of anti-DNA antibodies. J Clin Invest. 1985;75:321–327. doi: 10.1172/JCI111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz, R.H. 1993. Immunological tolerance. In Fundamental Immunology, 3rd edition. W. E. Paul, editor. Raven Press, New York. 677–731.
- 16.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 17.Georgescu L, Vakkalanka RK, Elkon KB, Crow MK. Interleukin-10 promotes activation-induced cell death of SLE lymphocytes mediated by Fas ligand. J Clin Invest. 1997;100:2622–2633. doi: 10.1172/JCI119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Morel-Fourrier B, Brouet J-C, Alarcon-Segovia D, Galanaud P, Emilie D. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–844. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell DA, Morrison B. The spontaneous apoptotic cell death of normal human lymphocytes in vitro: the release of, and immunoproliferative response to, nucleosomes in vitro. Clin Immunol Immunopathol. 1991;60:13–26. doi: 10.1016/0090-1229(91)90108-m. [DOI] [PubMed] [Google Scholar]
- 20.Termaat RM, Assmann KJ, Dijkman HB, van Gompel F, Smeenk RJ, Berden JH. Anti-DNA antibodies can bind to the glomerulus via two distinct mechanisms. Kidney Int. 1992;42:1363–1371. doi: 10.1038/ki.1992.428. [DOI] [PubMed] [Google Scholar]
- 21.Meyaard L, Otto SA, Jonker RR, Mijnster MJ, Keet RPM, Miedema F. Programmed death of T cells in HIV infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 22.Emlen W, Niebur J-A, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994;152:3685–3692. [PubMed] [Google Scholar]
- 23.Lorenz HM, Grunke M, Hieronymus T, Herrmann M, Kuhnel A, Manger B, Kalden JR. In vitro apoptosis and expression of apoptosis-related molecules in lymphocytes from patients with systemic lupus erythematosus and other autoimmune diseases. Arthritis Rheum. 1997;40:306–317. doi: 10.1002/art.1780400216. [DOI] [PubMed] [Google Scholar]
- 24.Solinger AM, Hess EV. Induction of autoantibodies by human immunodeficiency virus infection and their significance. Rheum Dis Clin N Am. 1991;17:157–176. [PubMed] [Google Scholar]