Abstract
A G protein–coupled receptor (GPCR) is encoded within the genome of Kaposi's sarcoma– associated herpesvirus (KSHV)/human herpesvirus 8, a virus that may be involved in the pathogenesis of Kaposi's sarcoma and primary effusion lymphomas. KSHV-GPCR exhibits constitutive signaling activity that causes oncogenic transformation. We report that human interferon (IFN)-γ–inducible protein 10 (HuIP-10), a C-X-C chemokine, specifically inhibits signaling of KSHV-GPCR. In contrast, monokine induced by IFN-γ (HuMig), which like HuIP-10 is an agonist of C-X-C chemokine receptor 3, does not inhibit KSHV-GPCR signaling. Moreover, HuIP-10, but not HuMig, inhibits KSHV-GPCR–induced proliferation of NIH 3T3 cells. These results show that HuIP-10 is an inverse agonist that converts KSHV-GPCR from an active to an inactive state. Thus, a human chemokine inhibits constitutive signaling and cellular proliferation that is mediated by a receptor encoded by a human disease-associated herpesvirus.
Keywords: human monokine induced by interferon γ, C-X-C chemokine receptor 3, inverse agonist, human herpesvirus 8, tumorigenesis
Kaposi's sarcoma–associated herpesvirus (KSHV)/human herpesvirus 8 is a gammaherpesvirus that may be involved in the pathogenesis of Kaposi's sarcoma (KS; reference 1) and primary effusion (or body cavity–based) lymphomas (2). KSHV contains a gene encoding a G protein– coupled receptor (KSHV-GPCR; references 3 and 4), and mRNA transcripts for KSHV-GPCR have been found in tissues from patients with KS (5) and in cell lines derived from patients with primary effusion lymphomas (2, 3). We showed that KSHV-GPCR exhibits constitutive signaling, that is, signaling in the absence of agonist, via activation of phosphoinositide-specific phospholipase C (6), and that expression of KSHV-GPCR stimulates proliferation of rat fibroblasts (6) and causes transformation of mouse NIH 3T3 fibroblasts (7) in cell culture. Moreover, NIH 3T3 cells transformed by KSHV-GPCR form tumors in mice (7). Thus, KSHV-GPCR may be a mediator of KS-induced tumorigenesis.
KSHV-GPCR shows homology to human receptors, including C-X-C chemokine receptor (CXCR)1, which binds IL-8, CXCR2, which binds IL-8 as well as several other C-X-C chemokines (8), and CXCR3, which binds human IFN-γ–inducible protein 10 (HuIP-10) and human monokine induced by IFN-γ (HuMig; references 9 and 10). However, CXCR1, CXCR2, and CXCR3 do not exhibit constitutive signaling, although like KSHV-GPCR they appear to signal via phospholipase C. For example, IL-8 activation of CXCR1 and CXCR2 stimulates formation of inositol phosphate second messenger molecules in a monkey kidney (COS) cell line (11), and HuIP-10 and HuMig activation of CXCR3 stimulates elevation of intracellular calcium, which is likely caused by phospholipase C–mediated generation of inositol 1,4,5-trisphosphate, in several cell types (9). In contrast, although several chemokines of the C-X-C and C-C families were shown to bind to KSHV-GPCR, none was found that would affect KSHV-GPCR signaling (6). In this report, we show that HuIP-10 (and two HuIP-10 analogues), but not HuMig, inhibits constitutive signaling of KSHV-GPCR. Thus, HuIP-10 is an inverse agonist of KSHV-GPCR signaling that converts the receptor from an active to an inactive state.
Materials and Methods
Inositol Phosphate Accumulation.
KSHV-GPCRs were expressed in COS-1 and NIH 3T3 cells by transfection with pcKSHV-GPCR (6) or pCEFL-KSHV-GPCR (7) using the protocols described previously. The C-X-C chemokines HuIP-10 and HuMig, the mouse homologues MuIP-10 and MuMig, and the HuIP-10 analogues were chemically synthesized using established procedures (12).
COS-1 cells transiently transfected with plasmid encoding KSHV-GPCR (5 μg/ml) or NIH 3T3 cells stably expressing KSHV-GPCRs were labeled with myo-[3H]inositol, and the formation of [3H]inositol phosphates during a 90-min incubation was measured as described (6, 7).
Mutagenesis.
The full-length KSHV-GPCR cDNA in pcDNA3.1(+) (pcKSHV-GPCR; reference 6) was used for mutation. Mutants were prepared by PCR and were subcloned directly into pcKSHV-GPCR after digesting with EcoRI and EcoRV. Mutant KSHV-GPCR sequences were confirmed by the dideoxy chain termination method.
Cell Proliferation Assays.
KSHV-GPCR–expressing NIH 3T3 cells were grown in DME supplemented with 10% calf serum. DNA synthesis was measured by incubating KSHV-GPCR–expressing NIH 3T3 cells in medium supplemented with 1% calf serum and the indicated concentration of HuIP-10 for 2 d before incubation with [3H]thymidine (2 μCi/ml) for 4–6 h.
Statistical Analysis.
Statistical significance was determined in each experiment by Student's t test.
Results and Discussion
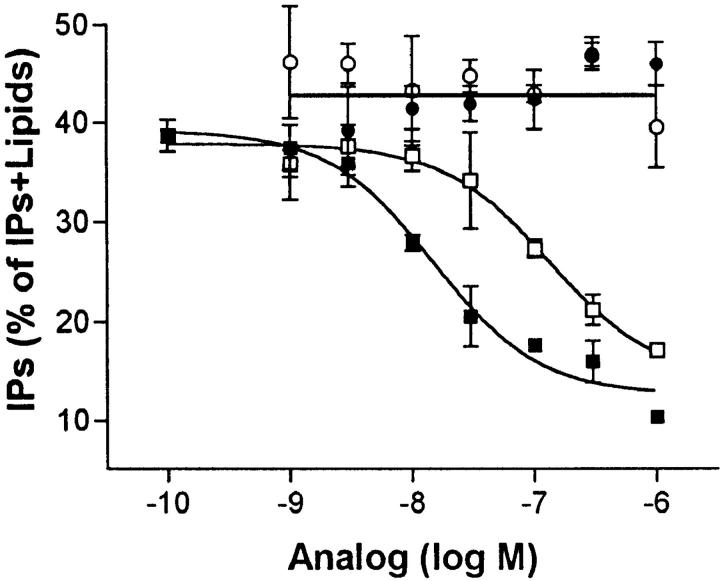
Fig. 1 illustrates that HuIP-10 specifically inhibits inositol phosphate second messenger formation in COS-1 cells expressing KSHV-GPCRs. The dose of HuIP-10 that causes 50% inhibition of inositol phosphate formation is 15 nM. HuIP-10 had no effect on inositol phosphate production by COS-1 cells that did not express KSHV-GPCRs (data not shown). These data are consistent with the idea that HuIP-10 is a specific inverse agonist (or negative antagonist; reference 13) of KSHV-GPCR.
Figure 1.
Effects of HuIP-10 (filled squares), HuMig (filled circles), MuIP-10 (open squares), and MuMig (open circles) on constitutive KSHV-GPCR signaling. Inositol phosphate accumulation in COS-1 cells transiently transfected with plasmid encoding KSHV-GPCR was measured as described in Materials and Methods. Chemokine analogues were added at the concentrations indicated 15 min before LiCl (10 mM). Untransfected cells and cells transfected with plasmid without DNA encoding KSHV-GPCR (“mock”) or with plasmid encoding the receptor for thyrotropin-releasing hormone were studied in parallel. There was no effect of any of these chemokines on inositol phosphate production in untransfected cells, in mock-transfected cells, or in cells expressing thyrotropin-releasing hormone receptors (not shown). The dose of HuIP-10 that caused 50% inhibition of inositol phosphate formation was 15 nM (7.2–32 nM, 95% confidence interval). The data represent the mean ± SD of triplicate determinations in a representative of three experiments.
As noted above, HuIP-10 is a ligand for CXCR3, which also binds HuMig (10). HuIP-10 and HuMig are CXCR3 agonists (9, 14). To further characterize the effects of these chemokines on KSHV-GPCR signaling, the effects of HuMig and of the mouse homologues MuIP-10 and MuMig were determined. MuIP-10, like HuIP-10, inhibits signaling by KSHV-GPCR, whereas HuMig and MuMig have no effect on KSHV-GPCR signaling (Fig. 1). Thus, the IP-10 homologues are inverse agonists, whereas the Mig homologues exhibit no detectable activity at KSHV-GPCR.
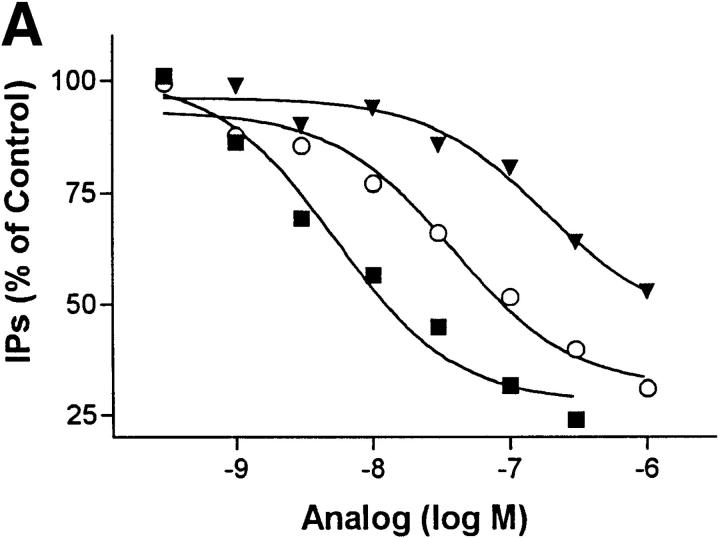
HuIP-10 is a 77–amino acid polypeptide. To gain insight into the structural domain of HuIP-10 involved in inhibition of KSHV-GPCR signaling, two analogues truncated at the NH2 terminus were studied. HuIP-10(4–77) and HuIP-10(9–77) are lacking the first three or eight amino acid residues, respectively, of HuIP-10. Both HuIP-10(4–77) and HuIP-10(9–77) inhibited KSHV-GPCR signaling although with lower potencies than HuIP-10 (3- and 10-fold lower, respectively; Fig. 2 A). Thus, the NH2 terminus of HuIP-10 is not needed for inhibition of KSHV-GPCR signaling. This is an interesting finding because the agonist activity of several members of the C-X-C chemokine family, including HuIP-10, has been shown to be dependent on their NH2-terminal domains (8). For example, deletion of the NH2 terminus of IL-8 causes loss of agonist activity and forms peptides that act as competitive antagonists (15). The active domain (or pharmacophore) for inhibition of KSHV-GPCR signaling is not part of the NH2 terminus of HuIP-10.
Figure 2.
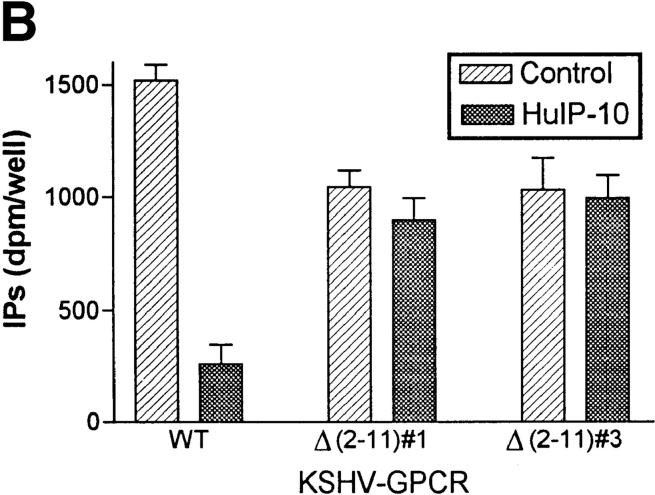
Effects of truncations of the NH2 termini of HuIP-10 and KSHV-GPCR on inhibition of KSHV-GPCR signaling. Inositol phosphate accumulation in COS-1 cells transiently transfected with plasmid encoding KSHV-GPCR or KSHV-GPCR(Δ2–11) was measured as described in Materials and Methods. HuIP-10, HuIP-10(4–77), or HuIP-10(9–77) was added at the concentrations indicated 15 min before LiCl (10 mM). (A) Effect of truncating the NH2 terminus of the ligand HuIP-10. The data represent the mean ± SD of triplicate determinations in a representative of three experiments. Filled squares, HuIP-10; open circles, HuIP-10(4–77); filled triangles, HuIP-10(9–77). (B) Effect of truncating the NH2 terminus of KSHV-GPCR. The data represent the mean ± SD of triplicate determinations in a representative experiment in which two plasmid clones encoding KSHV-GPCR(Δ2–11) were tested. WT, wild-type.
It has been found that the putative extracellular NH2 termini of CXCR1 and CXCR2 are important for interacting with IL-8 (16). Therefore, we constructed a mutant KSHV-GPCR in which amino acid residues from positions 2–11 were deleted [KSHV-GPCR(Δ2–11)]. This mutant receptor exhibited constitutive signaling activity that was 70% (P >0.005) of that of KSHV-GPCR (Fig. 2 B). Therefore, residues within the NH2 terminus may affect constitutive signaling by KSHV-GPCR. However, since signaling activity is directly related to receptor number (6), it is possible that the modest decrease in signaling is due to decreased expression. More importantly, the constitutive signaling activity exhibited by KSHV-GPCR(Δ2–11) was not inhibited by HuIP-10. As we have not been able to measure specific HuIP-10 binding, perhaps because of binding to cell surface heparin sulfates (17), we do not know whether this lack of effect of HuIP-10 is because it does not bind to KSHV-GPCR(Δ2–11) or whether it binds but does not inactivate KSHV-GPCR(Δ2–11). However, it is clear that the NH2 terminus of KSHV-GPCR is important for the inverse agonist action of HuIP-10.
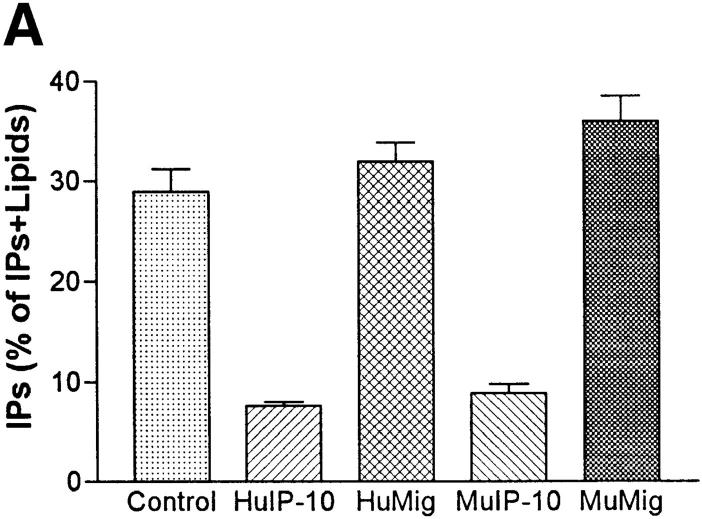
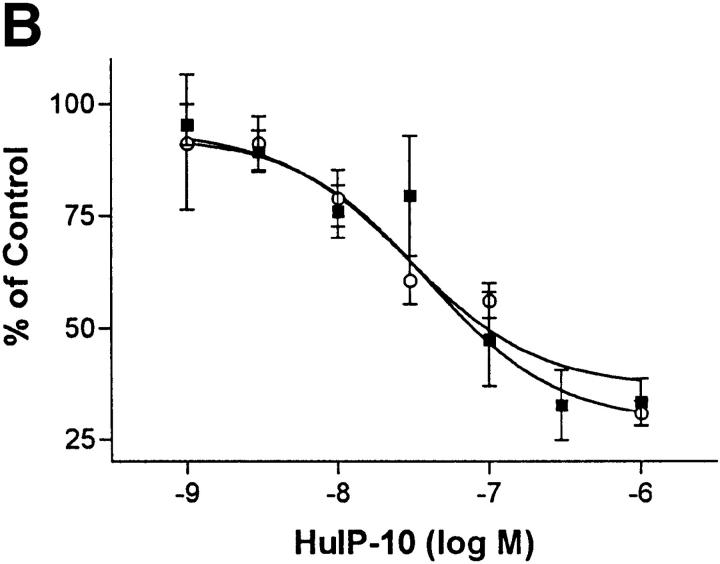
Fibroblasts may be a good model for the study of KSHV-GPCR function because fibroblasts are typically present in KS lesions (1) and KSHV-like viruses are found in retroperitoneal fibromatosis tissues in monkeys (18). Expression of KSHV-GPCR results in stimulation of proliferation of NIH 3T3 fibroblasts (7). Stimulation of the proliferation of target cells by KSHV-GPCR could be a component of the pathogenesis of KS or primary effusion lymphomas (1, 2). To determine whether inhibition of KSHV-GPCR signaling would inhibit the proliferative response, the effects of the IP-10 homologues were measured in transfected NIH 3T3 cells stably expressing KSHV-GPCRs. Fig. 3 (top) illustrates that HuIP-10 and MuIP-10 inhibit KSHV-GPCR signaling in NIH 3T3 cells, whereas HuMig and MuMig have no effect. These findings are identical to those in COS-1 cells. Moreover, HuIP-10 causes dose-dependent inhibitions of KSHV-GPCR signaling and stimulated DNA synthesis in these cells (Fig. 3, bottom). The concentrations required for 50% inhibition (IC50s) of these effects are similar: 39 nM for inhibition of inositol phosphate second messenger formation, and 29 nM for inhibition of DNA synthesis. These results indicate that HuIP-10 is an inverse agonist of constitutive signaling by KSHV-GPCR in NIH 3T3 cells that inhibits KSHV-GPCR–stimulated cell proliferation.
Figure 3.
Inhibition of constitutive KSHV-GPCR signaling and DNA synthesis in NIH 3T3 mouse fibroblasts. (A) Inositol phosphate accumulation in NIH 3T3 cells stably expressing KSHV-GPCRs (reference 7). HuIP-10, HuMig, MuIP-10, or MuMig (300 μM) was added 15 min before the addition of LiCl (10 mM). The data represent the mean ± SD of triplicate determinations in one of two experiments. (B) Dose-dependent effects of HuIP-10 on inositol phosphate formation (open circles) and DNA synthesis (filled squares). The concentration of HuIP-10 required for 50% inhibition (IC50) of inositol phosphate second messenger formation is 39 nM (9–160 nM; 95% confidence interval), and for 50% inhibition of DNA synthesis is 29 nM (8–110 nM, 95% confidence interval). The data represent the mean ± SE of triplicate determinations in three experiments.
IP-10 homologues are CXCR3 agonists (9), but we show herein that they are inverse agonists at KSHV-GPCR. In the majority of instances where two GPCRs interact with the same ligand, the receptors respond similarly to that ligand (19). However, there are examples of mutations of GPCRs engineered in the laboratory that have changed an antagonist or inverse agonist into a partial agonist (20, 21). The different effects of the IP-10 homologues at KSHV-GPCR and CXCR3 may represent a related circumstance in which a pirated mammalian receptor is mutated within a virus and is then antagonized by a naturally occurring agonist.
KSHV-GPCR interacts with a much broader array of chemokines (6) than most mammalian receptors (8). Indeed, KSHV-GPCR interacts with C-X-C chemokines including IL-8, neutrophil activating protein 2, platelet factor 4, melanoma growth stimulatory activity, IP-10 analogues, and C-C chemokines including regulated on activation, normal T expressed and secreted (RANTES) and I-309. However, of these chemokines only the IP-10 analogues inhibit KSHV-GPCR signaling. It is noteworthy that in addition to encoding KSHV-GPCR, there are three chemokine analogues encoded within the KSHV genome. One of these, vMIP-II, is a broad-spectrum antagonist of chemokine receptors (22), but stimulates chemotaxis of eosinophils via chemokine receptor CCR3 (23). vMIP-I and vMIP-II block infection by HIV-1 by binding to chemokine receptors CCR3 and CCR5, and stimulate angiogenesis (23). It is not known whether any of these chemokines affects KSHV-GPCR signaling.
Evidence continues to accumulate supporting the idea that KSHV is involved in the pathogenesis of KS (1) and primary effusion lymphomas (2). More recently, it has been proposed that KSHV could also be involved in the development of multiple myeloma (24). From the perspective of demonstrating a pathogenic role for KSHV-GPCR in these diseases, it will be important to develop an animal model and to show that inhibition of KSHV-GPCR signaling inhibits tumorigenesis in KSHV-infected animals. The discovery of an inhibitor of the constitutive signaling activity of KSHV-GPCR will allow direct testing in an animal model of the hypothesis that KSHV infection leads to expression of KSHV-GPCR and KSHV-GPCR signaling leads to tumorigenesis.
Acknowledgments
This work was supported by National Institutes of Health grants DK-43036 and CA-75918.
References
- 1.Offermann MK. Kaposi's sarcoma and HHV-8. Trends Microbiol. 1996;4:419. doi: 10.1016/0966-842x(96)84953-5. [DOI] [PubMed] [Google Scholar]
- 2.Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Said J, Knowles DM. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 3.Cesarman E, Nador RG, Bai F, Bohenzky RA, Russo JJ, Moore PS, Chang Y, Knowles DM. Kaposi's sarcoma associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang YA, Moore PS. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvanitakis L, Mesri EA, Nador RG, Said JW, Asch AS, Knowles DM, Cesarman E. Establishment and characterization of a body cavity-based lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;86:2708–2714. [PubMed] [Google Scholar]
- 6.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 7.Bais C, Santomasso B, Coso O, Arvanitakis L, Geras-Raaka E, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 8.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 9.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukocyte Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 11.Wu D, LaRosa GJ, Simon MI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 12.Clark-Lewis I, Moser B, Walz A, Baggiolini M, Scott GJ, Aebersold R. Chemical synthesis, purification, and characterization of two inflammatory proteins, neutrophil activating peptide 1 (interleukin-8) and neutrophil activating peptide 2. Biochemistry. 1991;30:3128–3135. doi: 10.1021/bi00226a021. [DOI] [PubMed] [Google Scholar]
- 13.Schutz W, Freissmuth M. Reverse intrinsic activity of antagonists on G protein-coupled receptors. Trends Pharmacol Sci. 1992;13:376–380. doi: 10.1016/0165-6147(92)90116-n. [DOI] [PubMed] [Google Scholar]
- 14.Maghazachi AA, Skalhegg BS, Rolstad B, Al-Aoukaty A. Interferon-inducible protein-10 and lymphotactin induce chemotaxis and mobilization of intracellular calcium in natural killer cells through pertussis toxin-sensitive and -insensitive heterotrimeric G-proteins. FASEB (Fed Am Soc Exp Biol) J. 1997;11:765–774. doi: 10.1096/fasebj.11.10.9271361. [DOI] [PubMed] [Google Scholar]
- 15.Moser B, Dewald B, Barella L, Schumacher C, Baggiolini M, Clark-Lewis I. Interleukin-8 antagonists generated by N-terminal modification. J Biol Chem. 1993;268:7125–7128. [PubMed] [Google Scholar]
- 16.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 17.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182:219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose TM, Strand KB, Schultz ER, Schaefer G, Rankin GW, Jr, Thouless ME, Tsai C-C, Bosch ML. Identification of two homologs of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaquespecies. J Virol. 1997;71:4138–4144. doi: 10.1128/jvi.71.5.4138-4144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strader CD, Fong TM, Graziano MP, Tota MR. The family of G-protein-coupled receptors. FASEB (Fed Am Soc Exp Biol) J. 1995;9:745–754. [PubMed] [Google Scholar]
- 20.Strader CD, Candelore MR, Hill WS, Dixon RAF, Sigal IS. A single amino acid substitution in the β-adrenergic receptor promotes partial agonist activity from antagonists. J Biol Chem. 1989;264:16470–16477. [PubMed] [Google Scholar]
- 21.Groblewski T, Maigret B, Larguier R, Lombard C, Bonnafous JC, Marie J. Mutation of Asn111 in the third transmembrane domain of the AT1Aangiotensin II receptor induces its constitutive activation. J Biol Chem. 1997;272:1822–1826. doi: 10.1074/jbc.272.3.1822. [DOI] [PubMed] [Google Scholar]
- 22.Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, Alouani S, Power CA, Lüttichau HR, Gerstoft J, Clapham PR, et al. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science. 1997;277:1656–1659. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 23.Boshoff C, Endo Y, Collins PD, Takeuchi Y, Reeves JD, Schweikart VL, Siani MA, Sasaki T, Williams TJ, Gray PW, et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 24.Rettig MB, Ma HJ, Vescio RA, Pold M, Schiller G, Belson D, Savage A, Nishikubo C, Wu C, Fraser J, et al. Kaposi's sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science. 1997;276:1851–1854. doi: 10.1126/science.276.5320.1851. [DOI] [PubMed] [Google Scholar]