Abstract
We have previously shown that uncharacterized glycoprotein VI (GPVI), which is constitutively associated and coexpressed with Fc receptor γ chain (FcRγ) in human platelets, is essential for collagen-stimulated tyrosine phosphorylation of FcRγ, Syk, and phospholipase Cγ2 (PLCγ2), leading to platelet activation. Here we investigated involvement of the Src family in the proximal signals through the GPVI–FcRγ complex, using the snake venom convulxin from Crotalus durissus terrificus, which specifically recognizes GPVI and activates platelets through cross-linking GPVI. Convulxin-coupled beads precipitated the GPVI–FcRγ complex from platelet lysates. Collagen and convulxin induced tyrosine phosphorylation of FcRγ, Syk, and PLCγ2 and recruited tyrosine-phosphorylated Syk to the GPVI–FcRγ complex. Using coprecipitation methods with convulxin-coupled beads and antibodies against FcRγ and the Src family, we showed that Fyn and Lyn, but not Yes, Src, Fgr, Hck, and Lck, were physically associated with the GPVI–FcRγ complex irrespective of stimulation. Furthermore, Fyn was rapidly activated by collagen or cross-linking GPVI. The Src family–specific inhibitor PP1 dose-dependently inhibited collagen- or convulxin-induced tyrosine phosphorylation of proteins including FcRγ, Syk, and PLCγ2, accompanied by a loss of aggregation and ATP release reaction. These results indicate that the Src family plays a critical role in platelet activation via the collagen receptor GPVI–FcRγ complex.
Keywords: glycoprotein VI, Fc receptor γ chain, Src family, collagen, platelets
Platelets adhere to collagen fibers of extracellular matrix and become activated through specific membrane receptors, resulting in shape change, granule release, and aggregation. These processes are vital for the formation of a hemostatic plug under a physiologic or pathologic condition. Many platelet surface glycoproteins (GPs)1 have been proposed as receptors for collagen, including the integrin α2β1 (GPIaIIa; reference 1), GPIV (GPIIIb, CD36; reference 2), an uncharacterized protein GPVI (p62; references 3, 4), the type I collagen receptor (p65; reference 5), and 85–90-kD GPs (6). It has been well established that α2β1 plays a major role in platelet adhesion to collagen (1), although it remains unclear whether α2β1 directly transduces signals leading to platelet activation. Our laboratory and others have reported patients with mild bleeding disorders whose platelets lack GPVI but not α2β1 and show selective deficiency in collagen-induced platelet aggregation and release reaction (3, 4, 7). Several lines of evidence have demonstrated that GPVI is implicated in collagen-induced tyrosine phosphorylation of proteins (8–11), including Fc receptor γ chain (FcRγ), Syk tyrosine kinase, and phospholipase Cγ2 (PLCγ2), which are essential for platelet activation upon collagen stimulation (12–14). GPVI is constitutively associated with FcRγ (10, 11), while both of them are proportionally absent in GPVI-deficient platelets (10), suggesting their coexpression. Cross-linking GPVI by the F(ab′)2 of anti-GPVI IgG (anti-GPVI F(ab′)2) stimulates tyrosine phosphorylation of FcRγ, Syk, and PLCγ2, and recruits Syk to FcRγ, leading to Syk activation (8, 10, 11), whereas GPVI-deficient platelets specifically lack activation of Syk in response to collagen (9). These findings have revealed the GPVI–FcRγ complex as a collagen receptor responsible for platelet activation.
To further investigate the mechanisms involved in the proximal signals through the GPVI–FcRγ complex, we used the snake venom convulxin from Crotalus durissus terrificus, which has been known to be a powerful platelet activator (15, 16), and the Src family-specific inhibitor PP1 (4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; reference 17). Recent reports have shown that convulxin is a heterodimeric C-type lectin and binds to platelet surface with a high affinity on a small number of binding sites (18, 19), that 125I-convulxin specifically recognizes a glycosylated 62-kD membrane protein immunoprecipitated by the anti-GPVI IgG (18, 20), and that cross-linking GPVI with convulxin induces tyrosine phosphorylation of proteins including FcRγ, Syk, and PLCγ2 (18). In this report, we demonstrated that the Src family tyrosine kinases Fyn and Lyn were physically associated with the GPVI–FcRγ complex and that Fyn was activated by collagen stimulation or cross-linking GPVI. PP1 dose-dependently inhibited collagen- or convulxin-induced tyrosine phosphorylation of proteins including FcRγ, Syk, and PLCγ2, accompanied by a loss of aggregation and ATP release reaction. Analogy to the antigen and Fc receptor signals, the Src family kinases were involved in platelet activation through the collagen receptor GPVI coupled with FcRγ as a signal transducing subunit containing an immunoreceptor tyrosine-based activation motif (ITAM; references 21, 22).
Materials and Methods
Reagents.
Convulxin was purified with gel filtration chromatography as described by Polgár et al. (18) from lyophilized Crotalus durissus terrificus venom obtained from Sigma Chemical Co. (St. Louis, MO). CNBr-activated Sepharose 4B beads, Sepharose 4B beads, and protein A–Sepharose beads were purchased from Pharmacia Biotechnology Inc. (Uppsala, Sweden). As previously described, human anti-GPVI IgG and its F(ab′)2 fragments were prepared from serum of a patient with GPVI deficiency (3, 8), who has been followed up as an outpatient in our department. Anti-FcRγ IgG was produced by immunizing rabbits with a synthetic peptide (residues 80–86) from FcRγ as described previously (23). mAbs against Syk (clone Syk101), Lyn (clone Lyn9), and Yes (clone 3H9) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Anti-phosphotyrosine (clone 4G10) and anti-Src (clone 327) mAbs were obtained from Upstate Biotechnology, Inc. (Lake Placid, NY) and Oncogene Science, Inc. (Uniondale, NY), respectively. Anti–low affinity Fc receptor for IgG (FcγRII) mAb (clone IV.3) was from Medarex Inc. (Annandale, NJ). Affinity-purified rabbit IgG specific to Fyn (FYN3), Hck (N-30), and PLCγ2 (C19) and affinity-purified goat IgG to Lck (2102) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Affinity-purified rabbit anti-Fgr Ab was obtained from Transduction Laboratories (Lexington, KY). Mouse mAb (clone 19-1; reference 24) against the α chain of human high affinity Fc receptor for IgE (FcεRI) was a kind gift from Dr. M.-H. Jouvin and Dr. J.-P. Kinet (Beth Israel Hospital, Boston, MA). Acid soluble fibrillar collagen prepared from equine tendon was purchased from Horm-Chemie (Munich, Germany). PP1 (17) and PGE1 were kindly provided by Pfizer Central Research (Groton, CT) and Ono Pharmaceutical Co. (Osaka, Japan), respectively. Arg-Gly-Asp-Ser (RGDS) and enolase were obtained from Sigma Chemical Co. γ-[32P]ATP (3,000 Ci/mmol, 10 mCi/ml) was supplied by DuPont NEN (Boston, MA). All other reagents were obtained as previously reported (25).
Preparation and Stimulation of Platelets.
After informed consent was obtained, venous blood was collected from healthy adult donors. All experiments involving human subjects were conducted according to the principles expressed in the Declaration of Helsinki. Anticoagulation of blood and preparation of platelet-rich plasma were performed as described previously (26). Platelet-rich plasma was incubated with 1 mM aspirin for 30 min at 37°C. Gel-filtered platelets were then prepared as described previously (27) at a final concentration of 1 × 109 cells/ml in Hepes buffer (137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5.6 mM glucose, 1 mg/ml BSA, 3.3 mM NaH2PO4, and 20 mM Hepes, pH 7.4), containing 1 U/ml apyrase and 500 μM RGDS to avoid secondary effects via released ADP and aggregation. Platelets were stimulated by 50 μg/ml of collagen, 50 ng/ml of convulxin, or 150 μg/ml of anti-GPVI F(ab′)2 with gentle agitation for appropriate periods at 37°C. In some experiments, gel-filtered platelets were incubated for 3 min at 37°C with indicated concentrations of PP1 or DMSO (0.25%) as a control before stimulation. Resting or stimulated platelets were lysed with an equal volume of ice-cold 2× Triton X-100 lysis buffer (150 mM NaCl, 10 mM EGTA, 2% Triton X-100, 2 mM PMSF, 2 mM Na3VO4, 40 μg/ml leupeptin, 40 μg/ml aprotinin, and 20 mM Hepes, pH 7.4) or 2× radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 10 mM EGTA, 2% Triton X-100, 2% sodium deoxycholate, 0.2% SDS, 2 mM PMSF, 2 mM Na3VO4, 40 μg/ml leupeptin, 40 μg/ml aprotinin, and 20 mM Hepes, pH 7.4). For preparation of whole platelet lysates, gel-filtered platelets were suspended in Hepes buffer without BSA and directly lysed in an equal volume of 2× SDS sample buffer (×1; 2% SDS, 5% glycerol, 5% β-ME, and 62.5 mM Tris-HCl, pH 6.8), and boiled for 5 min.
Affinity Precipitation of Convulxin-binding Proteins.
100 μg of purified convulxin was covalently coupled to 1 ml of CNBr-activated Sepharose 4B beads according to the manufacturer's instructions. 1.2 ml of platelet lysates (from 6 × 108 cells/sample) was clarified by centrifugation at 16,000 g for 30 min, incubated for 1 h with 40 μl of Sepharose 4B (50% slurry), and then precleared by sedimentation at 16,000 g for 30 min. These steps and all subsequent steps were carried out at 4°C. The supernatants were incubated for 1 h with 40 μl of convulxin-coupled Sepharose 4B (50% slurry) or Sepharose 4B as a control. The beads were sedimented by brief centrifugation and washed four times in lysis buffer containing 0.5 M NaCl. Proteins were eluted from the beads in 90 μl of SDS sample buffer and boiled for 5 min.
Immunoprecipitation.
Immunoprecipitation of each protein and its associated proteins was performed as described previously (25), followed by two-step elution with different buffers. 1.2 ml of lysates prepared from 6 × 108 platelets in Triton X-100 lysis buffer was clarified by centrifugation at 16,000 g for 30 min, precleared with protein A–Sepharose beads, and incubated for 2 h with 5 μg of anti-FcRγ IgG, preimmune rabbit IgG, anti-FcγRII mAb, anti-Fyn Ab, anti-Lyn mAb, anti-Yes mAb, anti-Src mAb, and anti-PLCγ2 Ab. This step and all subsequent steps were performed at 4°C. For immunoprecipitation of Yes and Src, lysates were further incubated for 1 h with 25 μg of rabbit anti–mouse IgG. Immune complexes were then precipitated with protein A–Sepharose beads for 1 h, sedimented by brief centrifugation, and washed four times in Triton X-100 lysis buffer. Coprecipitated proteins were first eluted in 60 μl of RIPA buffer. Eluates were diluted with 30 μl of 3× SDS sample buffer and boiled for 5 min. The left beads were further washed four times in RIPA buffer. Immunoprecipitates were then eluted in 60 μl of SDS sample buffer and boiled for 5 min. These samples eluted by SDS sample buffer were checked with immunoblotting whether each Ab precipitated its recognizing protein.
Immunoblotting.
Immunoblot analysis for each protein was done as described previously (25). In brief, samples (the whole platelet lysates from 5 × 106 cells/lane or affinity precipitates and immunoprecipitates from 1 × 108 cells/lane) were subjected to 10% SDS-PAGE and transferred electrophoretically to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA) with a semidry blotter. For detection of FcRγ, samples were resolved on 12.5% SDS-PAGE with Tris-Tricine buffer system. The membranes were treated with Ab against phosphotyrosine (1 μg/ ml), GPVI (2.5 μg/ml), FcRγ (1 μg/ml), α chain of FcεRI (0.5 μg/ ml), Syk (2 μg/ml), Fyn (1 μg/ml), Lyn (5 μg/ml), Yes (1 μg/ml), Src (0.2 μg/ml), Fgr (1 μg/ml), Hck (2 μg/ml), Lck (1 μg/ml), or PLCγ2 (1 μg/ml), followed by the ECL chemiluminescence reaction (Amersham International plc., Little Chalfont, UK). For detection of GPVI, anti-GPVI IgG was biotinylated to enhance sensitivity (10). The densities of some protein bands on scanned images were analyzed using the public domain NIH Image program (written by Wayne Rasband at the National Institutes of Health).
Immune Complex Kinase Assay.
To examine in vitro kinase activity of Fyn and Lyn, the kinases were isolated by immunoprecipitation with 1 μg of specific Abs as described above from platelet lysates (from 3 × 108 cells/sample) prepared in RIPA buffer. Immunoprecipitates were washed four times with RIPA buffer and twice with kinase buffer (20 mM Hepes, pH 7.5, and 10 mM MnCl2), and then incubated for 5 min at room temperature with 10 μg of acid-treated enolase in 30 μl of kinase buffer including 5 μCi of γ-[32P]ATP and 1 μM of ATP. Reactions were stopped by addition of 30 μl of 2× SDS sample buffer. The samples were boiled for 3 min and subjected to 10% SDS-PAGE. Radioactivity of each protein was visualized and quantified with the Bio-Imaging Analyzer BAS-2000II (Fuji Photo Film Co., Tokyo, Japan).
Platelet Aggregation and ATP Release Reaction.
Gel-filtered platelets were prepared from platelet-rich plasma as described above but without aspirin pretreatment, suspended at a final concentration of 3 × 108 cells/ml in Hepes buffer without RGDS, and incubated with PP1 or DMSO for 3 min at 37°C before stimulation by collagen, convulxin, or thrombin. Standard aggregometry of platelets was performed according to the Born method (28) in an aggregometer (HEMA TRACER 1; Nikko Bioscience, Tokyo, Japan). Release reaction from dense granules of platelets was monitored as ATP release using luciferin-luciferase reaction in a lumi-aggregometer (Chrono-Log, Harverton, PA) as described previously (8).
Results
Precipitation of the GPVI–FcRγ Complex with Convulxin-coupled Beads from Platelet Lysates.
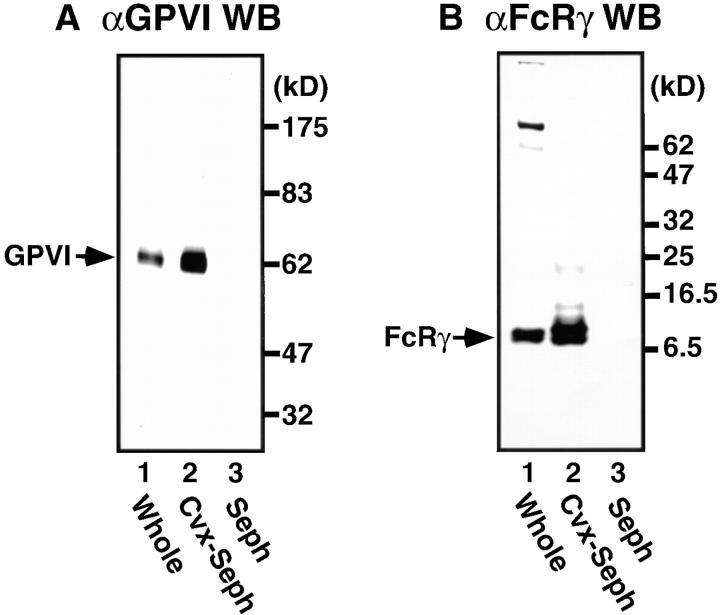
Convulxin specifically and strongly binds GPVI expressed on the platelet surface (19). 125I-convulxin also specifically recognizes GPVI blotted on polyvinylidene difluoride or nitrocellulose membranes (18, 20). Here we used Sepharose 4B beads that were covalently coupled with convulxin to isolate GPVI and associated proteins from platelet lysates. Immunoblot analysis showed that convulxin-coupled Sepharose 4B but not Sepharose 4B alone effectively precipitated both GPVI and FcRγ, which are constitutively associated with each other (10, 11), when we used Triton X-100 lysis buffer (Fig. 1, A and B). If we used more stringent RIPA buffer to solubilize platelets, only GPVI but not FcRγ was precipitated with convulxin-coupled Sepharose 4B from platelet lysates (data not shown). These findings indicate that GPVI and FcRγ were dissociated in RIPA buffer and that convulxin directly and strongly binds to GPVI. Thus, we used Triton X-100 lysis buffer to analyze molecules associated with the GPVI–FcRγ complex in the rest of studies.
Figure 1.
Affinity precipitation of the GPVI–FcRγ complex with convulxin-coupled Sepharose 4B from platelet lysates. Unstimulated gel-filtered platelets were lysed directly in SDS sample buffer or in Triton X-100 lysis buffer. Whole lysates (lane 1) and proteins precipitated by affinity with convulxin-coupled Sepharose 4B (lane 2) or Sepharose 4B alone (lane 3) were resolved by 10% (A) or 12.5% SDS-PAGE (B), transferred to nitrocellulose membranes, and immunoblotted with anti-GPVI IgG (A) or anti-FcRγ IgG (B). Molecular mass markers are indicated in kD on the right of panels.
Collagen- or Convulxin-induced Tyrosine Phosphorylation of Proteins Associated with the GPVI–FcRγ Complex.
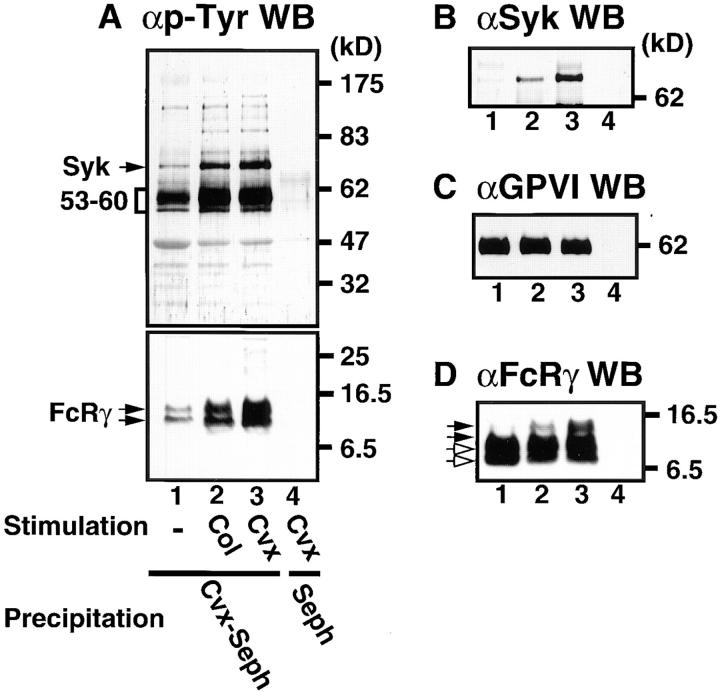
Collagen, con-vulxin, and anti-GPVI F(ab′)2 stimulate tyrosine phosphorylation of signaling molecules including FcRγ, Syk, and PLCγ2, which are essential for platelet activation by these agonists (8–14, 18). Recent studies have shown that convulxin activates platelets via cross-linking GPVI (18, 20). We examined tyrosine phosphorylation of proteins coprecipitated with the GPVI–FcRγ complex purified by convulxin-coupled Sepharose 4B from intact platelets and platelets stimulated by collagen or convulxin. Upon collagen and convulxin stimulation, we observed an increase in tyrosine phosphorylation of proteins with molecular masses of 140, 95, 85, 72, 53/60, and 10/12 kD (Fig. 2 A). We confirmed that the 72-kD band corresponded to accumulation of tyrosine-phosphorylated Syk (Fig. 2 B) and that the 10/12-kD doublets were FcRγ, which showed mobility retardation on SDS-PAGE due to phosphorylation (Fig. 2 D). GPVI, whose molecular mass is 62 kD under the reduced condition (3, 4, 8), was equally precipitated (Fig. 2 C) but not tyrosine phosphorylated irrespective of stimulation as previously reported (8, 10). Whereas collagen and convulxin stimulated tyrosine phosphorylation of PLCγ2 as previously reported (8, 14, 18, 29, 30), we could not detect PLCγ2 in the precipitated proteins with convulxin-coupled Sepharose 4B regardless of stimulation (data not shown).
Figure 2.
Tyrosine phosphorylation of proteins coprecipitated with the GPVI–FcRγ complex in collagen- and convulxin-stimulated platelets. Gel-filtered platelets were unstimulated (lane 1) or stimulated for 1 min at 37°C with 50 μg/ml of collagen (lane 2) or 50 ng/ml of convulxin (lanes 3 and 4) and lysed in Triton X-100 lysis buffer. Precipitated proteins with convulxin-coupled Sepharose 4B (lane 1–3) or Sepharose 4B (lane 4) were resolved by 10% (A, top, B, and C) or 12.5% SDS-PAGE (A, bottom and D), transferred to nitrocellulose membranes, and immunoblotted with Abs against phosphotyrosine (A), Syk (B), GPVI (C), and FcRγ (D). Molecular mass markers are indicated in kD on the right of panels. In A (bottom) and D open and closed arrows mark unphosphorylated and phosphorylated FcRγ, respectively.
Physical Association of Fyn and Lyn with the GPVI–FcRγ Complex.
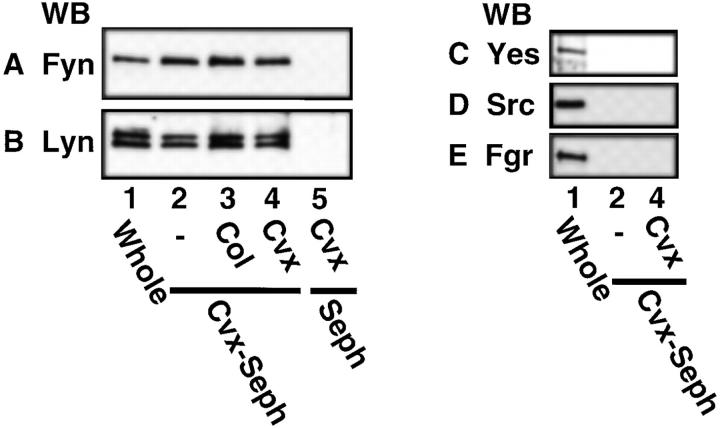
We next focused on the heavily tyrosine-phosphorylated proteins with molecular masses of 53–60 kD. The Src family kinases are cytosolic protein tyrosine kinases with molecular masses ∼60 kD and usually tyrosine phosphorylated themselves. Previous reports have shown that platelets abundantly express the Src family kinases including Src, Fyn, Yes, Lyn, Hck, Fgr, and Lck (31–36). We then examined whether the Src family kinases existed in the precipitated proteins with convulxin-coupled Sepharose 4B by immunoblotting with Ab specific to each kinase. Fig. 3 shows that convulxin-coupled Sepharose 4B precipitated Fyn (59 kD) and Lyn (53–56 kD), but not Yes (62 kD), Src (60 kD), Fgr (59 kD), and Lck (56 kD, data not shown), from lysates of resting platelets and collagen- or convulxin-stimulated platelets. Upon stimulation, protein levels of precipitated Fyn and Lyn did not change (Fig. 3, A and B). Sepharose 4B alone as a control precipitated none of these kinases (Fig. 3). We could not detect Hck with immunoblotting in whole platelet lysates or in the precipitated proteins with convulxin-coupled Sepharose 4B (data not shown). To confirm whether Fyn and Lyn precipitated with convulxin-coupled Sepharose 4B were associated with the GPVI–FcRγ complex, we next analyzed proteins coimmunoprecipitated with anti-FcRγ IgG and Abs against the Src family kinases. There was a problem that H chain of IgG used for immunoprecipitation overlapped the Src family kinases or GPVI on immunoblots due to their similar molecular masses. Hence, RIPA buffer was used to elute only coprecipitated proteins from immunoprecipitates as described in Materials and Methods, since RIPA buffer could not elute IgG and its recognizing proteins from immune complexes bound to protein A–Sepharose beads (data not shown). Fyn and Lyn but not Src were coimmunoprecipitated with anti-FcRγ IgG from platelet lysates (Fig. 4, A–C). Yes, Fgr, Hck, and Lck were not detected in coimmunoprecipitates with anti-FcRγ IgG (data not shown). Conversely, anti-Fyn or anti-Lyn Ab, but not anti-Yes or anti-Src mAb coimmunoprecipitated both GPVI and FcRγ (Fig. 4, D and E). These data clearly demonstrate that Fyn and Lyn were constitutively and specifically associated with the GPVI–FcRγ complex. Because very recent studies indicated that platelets heterogeneously express FcεRI (37, 38), which is associated with FcRγ and Lyn in other cells (39, 40), we examined whether FcεRI existed in the precipitated proteins with convulxin-coupled Sepharose 4B. We did not detect the α chain of FcεRI among them with immunoblotting (data not shown). Although collagen stimulates tyrosine phosphorylation of FcγRIIA in platelets (14), convulxin did not induce it (data not shown). Convulxin-coupled Sepharose 4B did not coprecipitate FcγRIIA (data not shown), confirming our previous findings that it is not associated with the GPVI–FcRγ complex (10).
Figure 3.
Coprecipitation of Fyn and Lyn, but not Yes, Src, and Fgr, with the GPVI–FcRγ complex from platelet lysates. Gel-filtered platelets were unstimulated (lanes 1 and 2) or stimulated for 1 min at 37°C with 50 μg/ml of collagen (lane 3) or 50 ng/ml of convulxin (lanes 4 and 5) and lysed in Triton X-100 lysis buffer (lanes 2–5) or directly in SDS sample buffer (lane 1). Whole lysates (lane 1) and precipitated proteins with convulxin-coupled Sepharose 4B (lanes 2–4) or Sepharose 4B (lane 5) were resolved by 10% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with Abs against Fyn (A), Lyn (B), Yes (C), Src (D), and Fgr (E).
Figure 4.
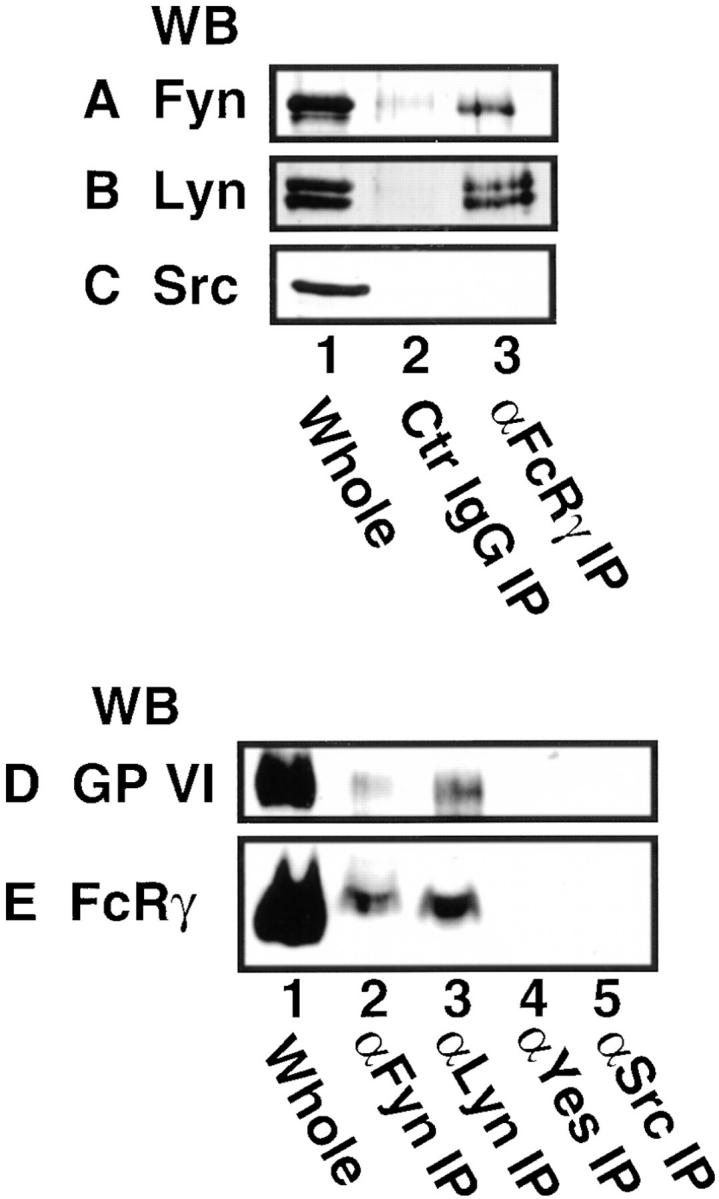
Coimmunoprecipitation of Fyn and Lyn with anti-FcRγ IgG and coimmunoprecipitation of GPVI and FcRγ with anti-Fyn and anti-Lyn Abs. Unstimulated gel-filtered platelets were lysed directly in SDS sample buffer or in Triton X-100 lysis buffer. (A–C) Whole lysates (lane 1) and immunoprecipitates with preimmune rabbit IgG (lane 2) and anti-FcRγ IgG (lane 3) were resolved by 10% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with Abs against Fyn (A), Lyn (B), and Src (C). (D and E) Whole lysates (lane 1) and immuno-precipitates with Abs against Fyn (lane 2), Lyn (lane 3), Yes (lane 4), and Src (lane 5) were resolved by 10% (D) or 12.5% (E) SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti-GPVI IgG (D) and anti-FcRγ IgG (E).
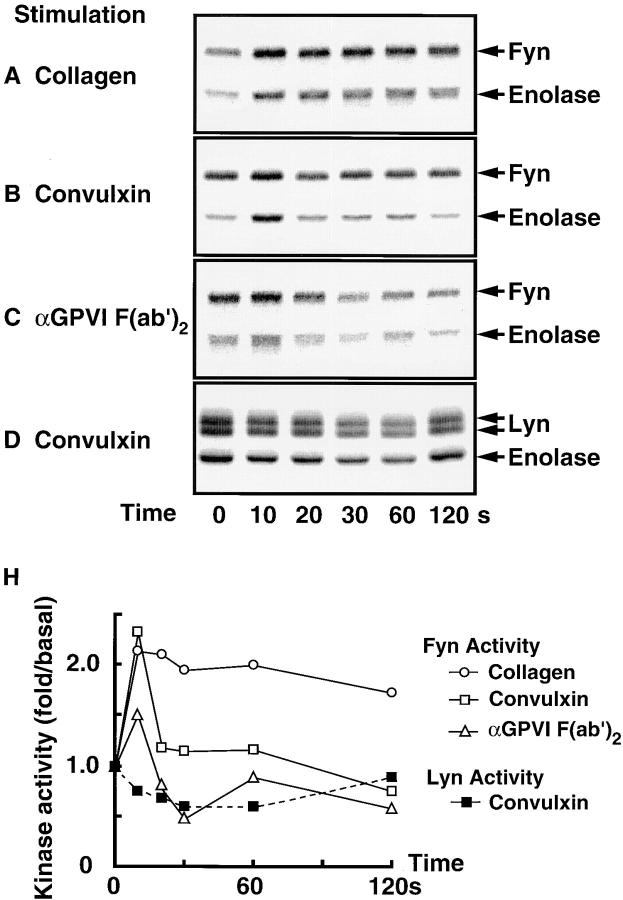
Rapid Activation of Fyn upon Collagen Stimulation or GPVI Cross-linking.
Next we examined whether collagen stimulation or cross-linking GPVI activated Fyn and Lyn. Fyn and Lyn were immunoprecipitated and subjected to an in vitro kinase assay with enolase as an exogenous substrate. We confirmed with immunoblotting that an equal amount of each kinase was immunoprecipitated (data not shown). Judging from both autophosphorylating activity and kinase activity toward enolase, Fyn was very rapidly activated by collagen stimulation or by cross-linking GPVI with convulxin or anti-GPVI F(ab′)2 (Fig. 5, A–C). Activation of Fyn reached the maximum level at 10 s and returned to the basal level within 60 s upon stimulation of convulxin or anti-GPVI F(ab′)2, whereas collagen-stimulated activation of Fyn was observed through 2 min of stimulation. We did not detect an increase in kinase activity of Lyn by collagen or cross-linking GPVI (data not shown). In some but not all experiments, we observed a slight decrease in kinase activity of Lyn upon convulxin (Fig. 5 D) or collagen stimulation (data not shown).
Figure 5.
Kinase activity of Fyn and Lyn in platelets stimulated by collagen, convulxin, or anti-GPVI F(ab′)2. Gel-filtered platelets were stimulated for the indicated periods at 37°C with collagen (A, 50 μg/ml), convulxin (B and D, 50 ng/ml), or anti-GPVI F(ab′)2 (C, 150 μg/ml) and lysed in RIPA buffer. Fyn (A–C) and Lyn (D) were immunoprecipitated from lysates and subjected to in vitro kinase assay with enolase as an exogenous substrate, followed by 10% SDS-PAGE. Radioactivity of each protein was visualized (A–D) and quantified with the Bio-Imaging Analyzer BAS-2000II. Kinase activity toward enolase was calculated (H). Positions of Fyn, Lyn, and enolase are indicated on the right.
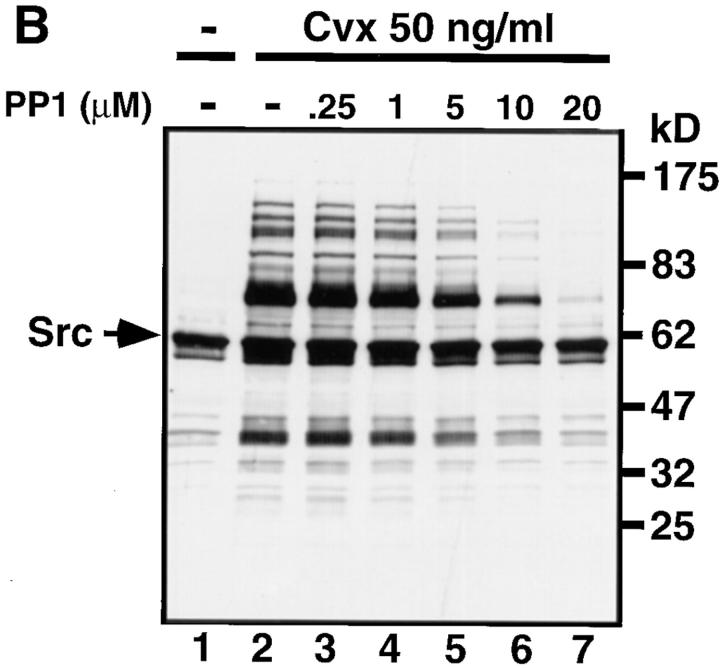
Inhibition of the Collagen- or Convulxin-induced Protein Tyro-sine Phosphorylation by the Src Family-specific Inhibitor PP1.
We used the Src family-specific inhibitor PP1 (17) to examine whether activation of Src family kinases was involved in the collagen- or convulxin-induced tyrosine phosphorylation of molecules including FcRγ, Syk, and PLCγ2. Gel-filtered platelets were preincubated with or without various concentrations of PP1 for 3 min and then stimulated with 50 μg/ml of collagen or 50 ng/ml of convulxin for appropriate times. Whole lysates were prepared from those platelets and subjected to the analysis of immunoblotting with anti-phosphotyrosine mAb (Fig. 6). Collagen stimulation induced tyrosine phosphorylation of proteins with molecular masses of 140, 120, 100, 85, 72/75, 65, 38/40, and 28/30 kD. Preincubation of platelets with PP1 inhibited the collagen-induced tyrosine phosphorylation of these proteins in a dose-dependent manner (Fig. 6 A). Convulxin stimulation induced tyrosine phosphorylation of proteins with the same molecular masses as described above, but the intensity of those protein bands was much stronger than upon collagen stimulation (Fig. 6 B). Preincubation of platelets with PP1 also inhibited the convulxin-induced tyrosine phosphorylation in a dose-dependent manner as was observed with collagen stimulation (Fig. 6 B). The heavily tyrosine-phosphorylated 60-kD protein band in both resting and activated platelets corresponded to Src with immunoblotting analysis (data not shown). Next we examined the effects of PP1 on the collagen- or convulxin-induced tyrosine phosphorylation of FcRγ, Syk, and PLCγ2 in platelets. PP1 (1–20 μM) dose-dependently inhibited the collagen-stimulated tyrosine phosphorylation of GPVI-associated FcRγ (Fig. 7 C) as well as the recruitment of tyrosine-phosphorylated Syk to the GPVI–FcRγ complex (Fig. 7, A and B). We confirmed that convulxin-coupled Sepharose 4B equally precipitated both GPVI and FcRγ, irrespective of PP1 pretreatment (Fig. 7, D and E). PP1 also reduced collagen-stimulated tyrosine phosphorylation of PLCγ2 in a dose-dependent fashion (Fig. 7 F). Essentially similar results on the PP1 effects were obtained with convulxin-stimulated platelets (data not shown).
Figure 6.
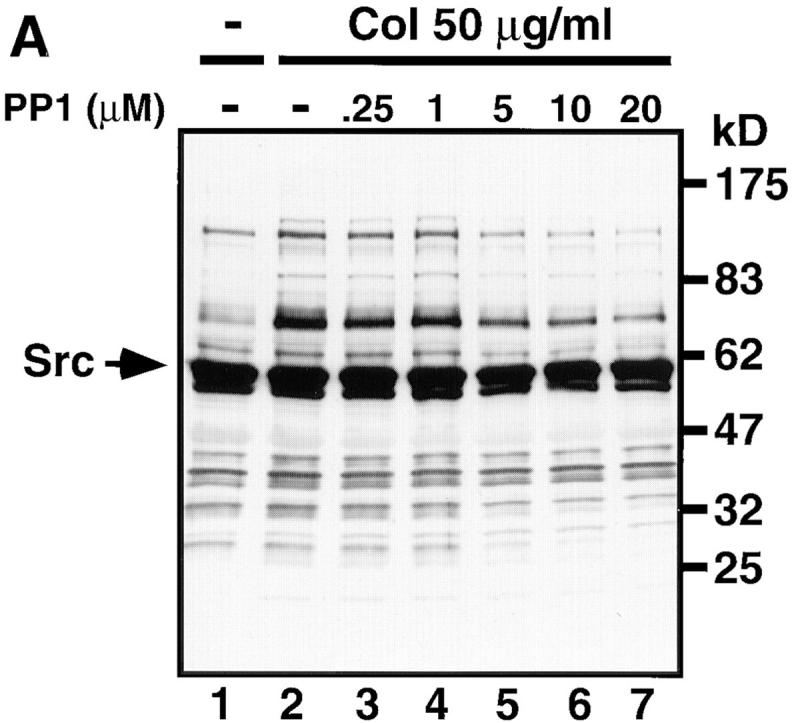
The Src family-specific inhibitor PP1 dose-dependently inhibits collagen- or convulxin-induced tyrosine phosphorylation of whole lysates. Gel-filtered platelets were preincubated for 3 min at 37°C with 0.25% DMSO (lanes 1 and 2) or the indicated concentrations of PP1 (lanes 3–7). Platelets were unstimulated (lane 1) or stimulated with 50 μg/ml of collagen for 30 s (A, lanes 2–7) or 50 ng/ml of convulxin for 15 s (B, lanes 2–7), directly lysed in SDS sample buffer, resolved by 10% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti-phosphotyrosine mAb. Molecular mass markers are indicated in kD on the right of panels. Arrows mark the position of Src.
Figure 7.
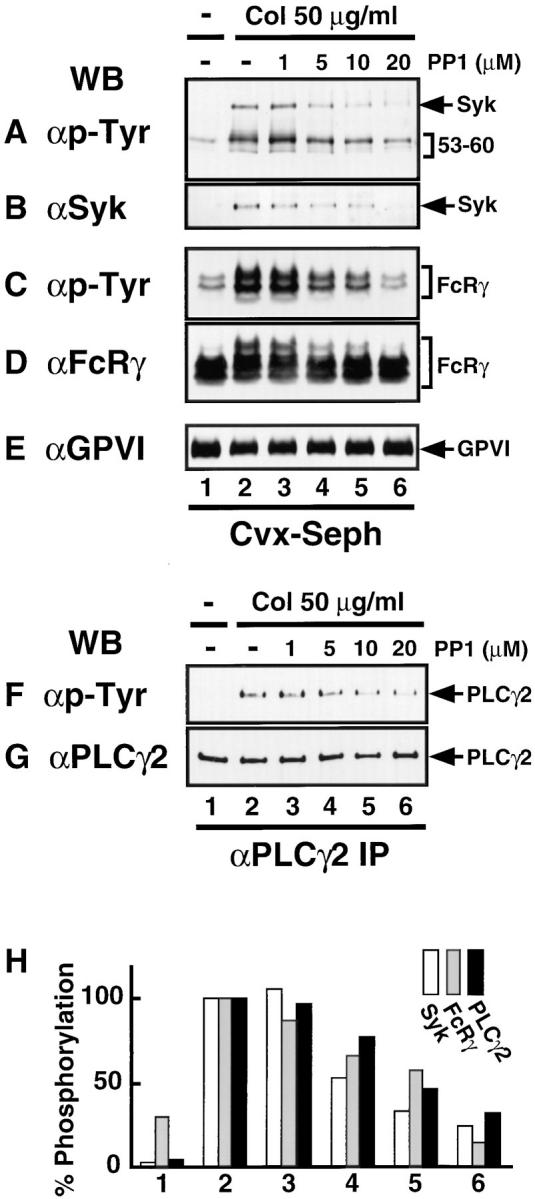
PP1 dose-dependently inhibits collagen-induced Syk recruitment and tyrosine phosphorylation of FcRγ and PLCγ2. Gel-filtered platelets were preincubated for 3 min at 37°C with 0.25% DMSO (lanes 1 and 2) or the indicated concentrations of PP1 (lanes 3–6). Platelets were unstimulated (lane 1) or stimulated with 50 μg/ml of collagen for 30 s (lanes 2–6) and lysed in Triton X-100 lysis buffer. Precipitated proteins with convulxin-coupled Sepharose 4B (A–E) or immunoprecipitates with anti-PLCγ2 Ab (F and G) were resolved by 10% (A, B, and E–G) or 12.5% (C and D) SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with Abs against phosphotyrosine (A, C, and F), Syk (B), FcRγ (D), GPVI (E), and PLCγ2 (G). In H the band densities from tyrosine-phosphorylated Syk (A), FcRγ (C), and PLCγ2 (F) were quantified and graphed as a percentage of the band density in lane 2 of each panel. The position of each protein is indicated on the right of panels.
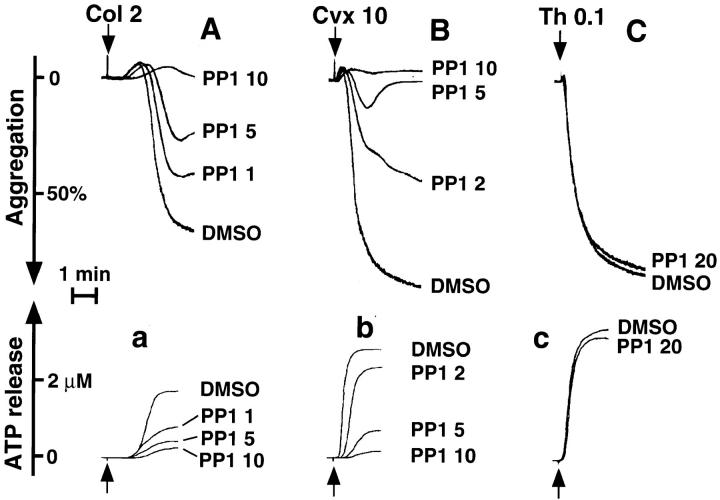
Inhibition of the Collagen- or Convulxin-induced Aggregation and ATP Release Reaction by PP1.
To investigate functional involvement of the Src family kinases in platelet activation through the GPVI–FcRγ complex, we examined the effects of PP1 on aggregation and ATP release reaction upon collagen or convulxin stimulation. PP1 alone did not cause any shape change, aggregation, or ATP release up to 20 μM of concentrations (data not shown). PP1 ranging in concentrations from 1 to 10 μM dose-dependently inhibited aggregation and ATP release caused by 2 μg/ml of collagen (Fig. 8, A and a), whereas PP1 little affected the extent of shape change but markedly delayed it (Fig. 8 A). Similar findings were also obtained when platelets were pretreated with 1–10 μM of PP1 and stimulated with 10 ng/ml of convulxin (Fig. 8, B and b). In contrast, 20 μM of PP1 did not affect aggregation and ATP release induced by 0.1 U/ml of thrombin (Fig. 8, C and c).
Figure 8.
PP1 dose-dependently inhibits collagen- or convulxin- induced aggregation and ATP release reaction. Gel-filtered platelets were preincubated for 3 min at 37°C with 0.25% DMSO or various concentrations of PP1 (μM) as indicated. Platelets were stimulated with 2 μg/ml of collagen (A and a), 10 ng/ml of convulxin (B and b), or 0.1 U/ml of thrombin (C and c) under constant stirring conditions. Aggregation curves (A–C) and ATP release (a–c) were monitored. This experiment is representative of four similar experiments from different donors.
Discussion
Because the snake venom convulxin specifically binds to GPVI (18, 20), we used convulxin-coupled Sepharose 4B beads for affinity precipitation of the GPVI–FcRγ complex and associated proteins from platelet lysates. Convulxin-coupled Sepharose 4B efficiently precipitated GPVI, FcRγ, and their associated proteins such as Syk. We also found that collagen or convulxin stimulation induced tyrosine phosphorylation of FcRγ associated with GPVI and recruitment of Syk to the GPVI–FcRγ complex, confirming the complex as a collagen receptor. These experiments showed that convulxin-coupled Sepharose 4B was a powerful tool to isolate and study the GPVI–FcRγ complex. We noticed that convulxin-coupled Sepharose 4B coprecipitated tyrosine-phosphorylated proteins with molecular masses of 53–60 kD with the GPVI–FcRγ complex. It has been established that the Src family tyrosine kinases are essential for generating initial signals through the immune receptors, which consist of ITAM-containing subunits, leading to tyrosine phosphorylation of ITAMs and Syk activation (22, 39, 40). Platelets abundantly express the Src family kinases, whose molecular masses are 53–62 kD and usually tyrosine phosphorylated (31–36). Using coprecipitation methods with convulxin-coupled Sepharose 4B, anti-FcRγ IgG, and Abs to the Src family kinases, we clearly showed that Fyn and Lyn, but not Yes, Src, Fgr, Hck, and Lck, were constitutively associated with the GPVI–FcRγ complex, irrespective of stimulation. Although GPIV (CD36) is also stably associated with Fyn, Lyn, and Yes in platelets (36), GPIV is not associated with either GPVI or FcRγ (10). Recent reports showed that platelets heterogeneously express FcεRI (37, 38), which consists of the α, β, and γ chains, and is associated with Lyn in other cells such as mast cells (39, 40). However, we denied association of the α chain of FcεRI with the GPVI–FcRγ complex. FcγRIIA, which is another ITAM-containing receptor in platelets, is not associated with the GPVI–FcRγ complex (10). These data indicate that the GPVI–FcRγ complex is a newly identified receptor that is physically associated with the Src family kinases in platelets.
Rapid activation of Src (41) and Lyn (42) but not of Fyn has been previously reported in thrombin-stimulated platelets under specific experimental conditions, such as weak stimulation or specified lysis buffers. We have reported that Src is activated upon collagen stimulation or cross-linking GPVI (8, 9). In addition, here we showed for the first time that Fyn became rapidly activated by collagen or cross-linking GPVI. We could detect the Src activation only if we used 1% Triton X-100 containing lysis buffer to lyse platelets as previously reported (8, 9). In contrast to Src, we could observe the Fyn activation only if we used RIPA buffer as described in Materials and Methods. However, we could not detect Lyn activation by collagen stimulation or cross-linking GPVI with either RIPA buffer or Triton X-100 lysis buffer. It has been well documented that members of the Src family kinases are differently distributed in resting platelets (35) and relocate to the membrane skeleton or the cytoskeleton (41–44), which is insoluble in 1% Triton X-100 containing lysis buffer, upon stimulation. These different natures of the kinases may cause the different requirements for lysis buffers to detect their activation. We observed a slight decrease in kinase activity of Lyn upon convulxin or collagen stimulation in some but not all experiments. Such a decrease in Lyn activity seems to be similar to Src activity as reported previously (9, 41). Although Src is activated by either collagen or cross-linking GPVI in normal platelets, Src is still activated by collagen in GPVI-deficient platelets lacking collagen-induced Syk activation and PLCγ2 phosphorylation (9). Taken together with our new findings that Src was not physically associated with the GPVI–FcRγ complex, Src does not seem to be directly involved at least in Syk activation and subsequent tyrosine phosphorylation of PLCγ2 upon collagen stimulation.
It has been demonstrated that PP1 selectively inhibits the Src family kinases, including Lck, Fyn, Src, Hck, and Lyn, but not the Syk family kinases, including ZAP-70 and Syk, even at concentrations over 100 μM (17, 45). We observed that PP1 (1–20 μM) dose-dependently inhibited collagen- or convulxin-induced tyrosine phosphorylation of whole lysates. PP1 also abolished tyrosine phosphorylation of GPVI-associated FcRγ, Syk, and PLCγ2 as well as recruitment of Syk to the GPVI–FcRγ complex. The concentrations of PP1 used here were similar ones that have been reported to inhibit induction of tyrosine phosphorylation or cell functions upon stimulation in T cells, RBL-2H3 cells, fibroblasts, or Mo7e cells (17, 45–47). As for immune receptors, it has been reported that FcRγ is phosphorylated on tyrosine by the Src family kinases that are associated with the receptors, such as Lyn for FcεRI (22, 39, 40). Therefore, Fyn and Lyn that were associated with the GPVI–FcRγ complex would be most probable candidate kinases responsible for phosphorylating FcRγ and subsequent signals in GPVI-mediated platelet activation by collagen. Finally, we showed that PP1 inhibited collagen- or convulxin-stimulated aggregation and ATP release reaction but not extent of shape change in a dose-dependent manner. The inhibition of aggregation and release reaction by PP1 was observed parallel to that of the tyrosine phosphorylation discussed above, suggesting that PP1 blocks platelet functions through inhibiting activation of the Src family and its downstream signals. However, when we used higher concentrations of collagen (20–50 μg/ml), aggregation and ATP release reactions became somewhat insensitive to even 20 μM of PP1, although PP1 still notably delayed beginning of aggregation and ATP release by these concentrations of the agonists (data not shown). It is probably because PP1 could not completely inhibit activation of the Src family upon strong stimulation of collagen. If it is not, there may be another possibility that PP1-insensitive bypass signals might function in platelets stimulated with higher concentrations of collagen. Although previous studies have shown that platelets are enriched in the Src family kinases (31–36), this is the first report demonstrating that the Src family is functionally related to platelet activation.
The collagen-stimulated tyrosine phosphorylation including Syk and PLCγ2 is compromised by blocking of α2β1 with anti-α2β1 mAbs (14, 48) or proteolytic cleavage of the β1 subunit with the snake venom metalloproteinase Jararhagin (49), suggesting that α2β1 is also essential for the collagen signals. However, direct cross-linking of α2β1 alone with mAbs does not cause platelet activation and protein tyrosine phosphorylation (14). In contrast to our present findings on the GPVI–FcRγ complex, α2β1 has not been shown to be physically associated with protein tyrosine kinases in platelets (48, 49). On the other hand, cross-linking the GPVI–FcRγ complex with anti-GPVI F(ab′)2, convulxin, or collagen-related peptides, which also stimulate platelets via GPVI (50), causes platelet activation and tyrosine phosphorylation of proteins including FcRγ, Syk, and PLCγ2 independently of α2β1 (8, 10–12, 18, 51, 52). In GPVI-deficient platelets, collagen stimulates α2β1-dependent activation of Src and tyrosine phosphorylation of multiple proteins but not that of Syk, PLCγ2, and Vav (9). These data seem to support the two-site, two-step model of platelet activation by collagen (53, 54). At the first step of the platelet–collagen interaction, α2β1 plays a critical role in adhesion and might modulate the GPVI–FcRγ complex-mediated responses including protein tyrosine phosphorylation as a coreceptor, leading to aggregation and release reaction. Roles of the other receptors (2, 5, 6) remain to be less clear than α2β1 and the GPVI–FcRγ complex in the collagen signals. Platelets from patients lacking GPIV (the Naka-negative phenotype) aggregate (55) and show tyrosine phosphorylation (56) normally to collagen. However, GPIV may play some roles in the collagen-induced tyrosine phosphorylation, since GPIV is physically associated with Fyn, Yes, and Lyn (36). Further studies are necessary to elucidate the complete nature of collagen signals through the GPVI– FcRγ complex, α2β1, and possibly others.
Acknowledgments
We thank Dr. M.-H. Jouvin and Dr. J.-P. Kinet for mAb to the α chain of FcεRI, Pfizer Central Research for PP1, and Ono Pharmaceutical Co. for PGE1. We also thank Dr. K. Hirai for critical reading of the manuscript and I. Nakamura for secretarial assistance.
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science and Culture of Japan, funds for comprehensive research on aging and health from the Ministry of Health and Welfare of Japan, and the Ryoichi Naito Foundation Grant for Medical Research. Y. Ezumi is a Research Fellow of the Japan Society for the Promotion of Science.
Abbreviations used in this paper
- anti-GPVI F(ab′)2
the F(ab′)2 of anti-GPVI IgG
- FcRγ
Fc receptor γ chain
- FcγRII
low affinity Fc receptor for IgG
- FcεRI
high affinity Fc receptor for IgE
- GP
glycoprotein
- GPVI
glycoprotein VI
- ITAM
immunoreceptor tyrosine-based activation motif
- PLCγ2
phospholipase Cγ2
- PP1
4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- RGDS
Arg-Gly-Asp-Ser
- RIPA
radioimmunoprecipitation assay
References
- 1.Santoro SA, Zutter MM. The α2β1integrin: a collagen receptor on platelets and other cells. Thromb Haemostasis. 1995;74:813–821. [PubMed] [Google Scholar]
- 2.Tandon NN, Kralisz U, Jamieson GA. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989;264:7576–7583. [PubMed] [Google Scholar]
- 3.Sugiyama T, Okuma M, Ushikubi F, Sensaki S, Kanaji K, Uchino H. A novel platelet aggregating factor found in a patient with defective collagen-induced platelet aggregation and autoimmune thrombocytopenia. Blood. 1987;69:1712–1720. [PubMed] [Google Scholar]
- 4.Moroi M, Jung SM, Okuma M, Shinmyozu K. A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J Clin Invest. 1989;84:1440–1445. doi: 10.1172/JCI114318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang TM, Rinaldy A, Kang AH. Cloning, characterization, and functional studies of a nonintegrin platelet receptor for type I collagen. J Clin Invest. 1997;100:514–521. doi: 10.1172/JCI119560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deckmyn H, Van Houtte E, Vermylen J. Disturbed platelet aggregation to collagen associated with an antibody against an 85- to 90-Kd platelet glycoprotein in a patient with prolonged bleeding time. Blood. 1992;79:1466–1471. [PubMed] [Google Scholar]
- 7.Arai, M., N. Yamamoto, M. Moroi, N. Akamatsu, K. Fukutake, and K. Tanoue. 1995. Platelets with 10% of the normal amount of glycoprotein VI have an impaired response to collagen that results in a mild bleeding tendency. Br. J. Haematol. 89:124–130 (erratum published 89:952). [DOI] [PubMed]
- 8.Ichinohe T, Takayama H, Ezumi Y, Yanagi S, Yamamura H, Okuma M. Cyclic AMP-insensitive activation of c-Src and Syk protein-tyrosine kinases through platelet membrane glycoprotein VI. J Biol Chem. 1995;270:28029–28036. doi: 10.1074/jbc.270.47.28029. [DOI] [PubMed] [Google Scholar]
- 9.Ichinohe T, Takayama H, Ezumi Y, Arai M, Yamamoto N, Takahashi H, Okuma M. Collagen-stimulated activation of Syk but not c-Src is severely compromised in human platelets lacking membrane glycoprotein VI. J Biol Chem. 1997;272:63–68. doi: 10.1074/jbc.272.1.63. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji M, Ezumi Y, Arai M, Takayama H. A novel association of Fc receptor γ-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J Biol Chem. 1997;272:23528–23531. doi: 10.1074/jbc.272.38.23528. [DOI] [PubMed] [Google Scholar]
- 11.Gibbins JM, Okuma M, Farndale R, Barnes M, Watson SP. Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor γ-chain. FEBS Lett. 1997;413:255–259. doi: 10.1016/s0014-5793(97)00926-5. [DOI] [PubMed] [Google Scholar]
- 12.Gibbins J, Asselin J, Farndale R, Barnes M, Law C-L, Watson SP. Tyrosine phosphorylation of the Fc receptor γ-chain in collagen-stimulated platelets. J Biol Chem. 1996;271:18095–18099. doi: 10.1074/jbc.271.30.18095. [DOI] [PubMed] [Google Scholar]
- 13.Poole A, Gibbins JM, Turner M, van Vugt MJ, van de Winkel JGJ, Saito T, Tybulewicz VLJ, Watson SP. The Fc receptor γ-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO (Eur Mol Biol Organ) J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keely PJ, Parise LV. The α2β1integrin is a necessary co-receptor for collagen-induced activation of Syk and the subsequent phosphorylation of phospholipase Cγ2 in platelets. J Biol Chem. 1996;271:26668–26676. [PubMed] [Google Scholar]
- 15.Prado-Franceschi J, Brazil OV. Convulxin, a new toxin from the venom of the South American rattlesnake Crotalus durissus terrificus. . Toxicon. 1981;19:875–887. doi: 10.1016/0041-0101(81)90085-4. [DOI] [PubMed] [Google Scholar]
- 16.Vargaftig BB, Prado-Franceschi J, Chignard M, Lefort J, Marlas G. Activation of guinea-pig platelets induced by convulxin, a substance extracted from the venom of Crotalus durissus cascavella. . Eur J Pharmacol. 1980;68:451–464. doi: 10.1016/0014-2999(80)90420-3. [DOI] [PubMed] [Google Scholar]
- 17.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 18.Polgár J, Clemetson JM, Kehrel BE, Wiedemann M, Magnenat EM, Wells TNC, Clemetson KJ. Platelet activation and signal transduction by convulxin, a C-type lectin from Crotalus durissus terrificus(tropical rattlesnake) venom via the p62/GPVI collagen receptor. J Biol Chem. 1997;272:13576–13583. doi: 10.1074/jbc.272.21.13576. [DOI] [PubMed] [Google Scholar]
- 19.Francischetti IMB, Saliou B, Leduc M, Carlini CR, Hatmi M, Randon J, Faili A, Bon C. Convulxin, a potent platelet-aggregating protein from Crotalus durissus terrificusvenom, specifically binds to platelets. Toxicon. 1997;35:1217–1228. doi: 10.1016/s0041-0101(97)00021-4. [DOI] [PubMed] [Google Scholar]
- 20.Jandrot-Perrus M, Lagrue A-H, Okuma M, Bon C. Adhesion and activation of human platelets induced by convulxin involve glycoprotein VI and integrin α2β1 . J Biol Chem. 1997;272:27035–27041. doi: 10.1074/jbc.272.43.27035. [DOI] [PubMed] [Google Scholar]
- 21.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 22.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 23.Letourneur O, Kennedy ICS, Brini AT, Ortaldo JR, O'Shea JJ, Kinet J-P. Characterization of the family of dimers associated with Fc receptors (FcεRI and FcγRIII) J Immunol. 1991;147:2652–2656. [PubMed] [Google Scholar]
- 24.Maurer D, Fiebiger E, Reininger B, Wolff-Winiski B, Jouvin M-H, Kilgus O, Kinet J-P, Stingl G. Expression of functional high affinity immunoglobulin E receptors (FcεRI) on monocytes of atopic individuals. J Exp Med. 1994;179:745–750. doi: 10.1084/jem.179.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ezumi Y, Takayama H, Okuma M. Differential regulation of protein-tyrosine phosphatases by integrin αIIbβ3through cytoskeletal reorganization and tyrosine phosphorylation in human platelets. J Biol Chem. 1995;270:11927–11934. doi: 10.1074/jbc.270.20.11927. [DOI] [PubMed] [Google Scholar]
- 26.Takayama H, Ezumi Y, Ichinohe T, Okuma M. Involvement of GPIIb-IIIa on human platelets in phosphotyrosine-specific dephosphorylation. Biochem Biophys Res Commun. 1993;194:472–477. doi: 10.1006/bbrc.1993.1843. [DOI] [PubMed] [Google Scholar]
- 27.Ezumi Y, Takayama H, Okuma M. Thrombopoietin, c-Mpl ligand, induces tyrosine phosphorylation of Tyk2, JAK2, and STAT3, and enhances agonists-induced aggregation in platelets in vitro. FEBS Lett. 1995;374:48–52. doi: 10.1016/0014-5793(95)01072-m. [DOI] [PubMed] [Google Scholar]
- 28.Born GVR. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 29.Daniel JL, Dangelmaier C, Smith JB. Evidence for a role for tyrosine phosphorylation of phospholipase Cγ2 in collagen-induced platelet cytosolic calcium mobilization. Biochem J. 1994;302:617–622. doi: 10.1042/bj3020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blake RA, Schieven GL, Watson SP. Collagen stimulates tyrosine phosphorylation of phospholipase C-γ2 but not phospholipase C-γ1 in human platelets. FEBS Lett. 1994;353:212–216. doi: 10.1016/0014-5793(94)01037-4. [DOI] [PubMed] [Google Scholar]
- 31.Golden A, Nemeth SP, Brugge JS. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci USA. 1986;83:852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varshney GC, Henry J, Kahn A, Phan-Dinh-Tuy F. Tyrosine kinases in normal human blood cells. Platelet but not erythrocyte band 3 tyrosine kinase is p60c-src . FEBS Lett. 1986;205:97–103. doi: 10.1016/0014-5793(86)80873-0. [DOI] [PubMed] [Google Scholar]
- 33.Horak ID, Corcoran ML, Thompson PA, Wahl LM, Bolen JB. Expression of p60fynin human platelets. Oncogene. 1990;5:597–602. [PubMed] [Google Scholar]
- 34.Zhao Y-H, Krueger JG, Sudol M. Expression of cellular-yesprotein in mammalian tissues. Oncogene. 1990;5:1629–1635. [PubMed] [Google Scholar]
- 35.Stenberg PE, Pestina TI, Barrie RJ, Jackson CW. The Src family kinases, Fgr, Fyn, Lck, and Lyn, colocalize with coated membranes in platelets. Blood. 1997;89:2384–2393. [PubMed] [Google Scholar]
- 36.Soslau G, Morgan DA, Jaffe JS, Brodsky I, Wang Y. Cytokine mRNA expression in human platelets and a megakaryocytic cell line and cytokine modulation of platelet function. Cytokine. 1997;9:405–411. doi: 10.1006/cyto.1996.0182. [DOI] [PubMed] [Google Scholar]
- 37.Joseph M, Gounni AS, Kusnierz J-P, Vorng H, Sarfati M, Kinet J-P, Tonnel A-B, Capron A, Capron M. Expression and functions of the high-affinity IgE receptor on human platelets and megakaryocyte precursors. Eur J Immunol. 1997;27:2212–2218. doi: 10.1002/eji.1830270914. [DOI] [PubMed] [Google Scholar]
- 38.Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 39.Daëron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 40.Huang M-M, Bolen JB, Barnwell JW, Shattil SJ, Brugge JS. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci USA. 1991;88:7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark EA, Brugge JS. Redistribution of activated pp60c-srcto integrin-dependent cytoskeletal complexes in thrombin-stimulated platelets. Mol Cell Biol. 1993;13:1863–1871. doi: 10.1128/mcb.13.3.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirao A, Hamaguchi I, Suda T, Yamaguchi N. Translocation of the Csk homologous kinase (Chk/Hyl) controls activity of CD36-anchored Lyn tyrosine kinase in thrombin-stimulated platelets. EMBO (Eur Mol Biol Organ) J. 1997;16:2342–2351. doi: 10.1093/emboj/16.9.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhar A, Shukla SD. Involvement of pp60c-srcin platelet-activating factor-stimulated platelets. Evidence for translocation from cytosol to membrane. J Biol Chem. 1991;266:18797–18801. [PubMed] [Google Scholar]
- 44.Fox JEB, Lipfert L, Clark EA, Reynolds CC, Austin CD, Brugge JS. On the role of the platelet membrane skeleton in mediating signal transduction. Association of GP IIb-IIIa, pp60c-src, pp62c-yes, and the p21rasGTPase-activating protein with the membrane skeleton. J Biol Chem. 1993;268:25973–25984. [PubMed] [Google Scholar]
- 45.Amoui M, Dráber P, Dráberová L. Src family-selective tyrosine kinase inhibitor, PP1, inhibits both FcεRI- and Thy-1-mediated activation of rat basophilic leukemia cells. Eur J Immunol. 1997;27:1881–1886. doi: 10.1002/eji.1830270810. [DOI] [PubMed] [Google Scholar]
- 46.Abe J, Takahashi M, Ishida M, Lee J-D, Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J Biol Chem. 1997;272:20389–20394. doi: 10.1074/jbc.272.33.20389. [DOI] [PubMed] [Google Scholar]
- 47.Linnekin D, DeBerry CS, Mou S. Lyn associates with the juxtamembrane region of c-Kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells. J Biol Chem. 1997;272:27450–27455. doi: 10.1074/jbc.272.43.27450. [DOI] [PubMed] [Google Scholar]
- 48.Asazuma N, Yatomi Y, Ozaki Y, Qi R, Kuroda K, Satoh K, Kume S. Protein-tyrosine phosphorylation and p72sykactivation in human platelets stimulated with collagen is dependent upon glycoprotein Ia/IIa and actin polymerization. Thromb Haemostasis. 1996;75:648–654. [PubMed] [Google Scholar]
- 49.Kamiguti AS, Markland FS, Zhou Q, Laing GD, Theakston RDG, Zuzel M. Proteolytic cleavage of the β1 subunit of platelet α2β1integrin by the metalloproteinase jararhagin compromises collagen-stimulated phosphorylation of pp72. J Biol Chem. 1997;272:32599–32605. doi: 10.1074/jbc.272.51.32599. [DOI] [PubMed] [Google Scholar]
- 50.Kehrel B, Wierwille S, Clemetson KJ, Anders O, Steiner M, Knight CG, Farndale RW, Okuma M, Barnes MJ. Glycoprotein VI is a major collagen receptor for platelet activation: it recognizes the platelet-activating quaternary structure of collagen, whereas CD36, glycoprotein IIb/IIIa, and von Willebrand factor do not. Blood. 1998;91:491–499. [PubMed] [Google Scholar]
- 51.Morton LF, Hargreaves PG, Farndale RW, Young RD, Barnes MJ. Integrin α2β1-independent activation of platelets by simple collagen-like peptides: collagen tertiary (triple-helical) and quaternary (polymeric) structures are sufficient alone for α2β1-independent platelet reactivity. Biochem J. 1995;306:337–344. doi: 10.1042/bj3060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asselin J, Gibbins JM, Achison M, Lee YH, Morton LF, Farndale RW, Barnes MJ, Watson SP. A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase Cγ2 in platelets independent of the integrin α2β1 . Blood. 1997;89:1235–1242. [PubMed] [Google Scholar]
- 53.Morton LF, Peachey AR, Barnes MJ. Platelet-reactive sites in collagens type I and type III. Evidence for separate adhesion and aggregatory sites. Biochem J. 1989;258:157–163. doi: 10.1042/bj2580157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santoro SA, Walsh JJ, Staatz WD, Baranski KJ. Distinct determinants on collagen support α2β1integrin-mediated platelet adhesion and platelet activation. Cell Regul. 1991;2:905–913. doi: 10.1091/mbc.2.11.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto N, Akamatsu N, Yamazaki H, Tanoue K. Normal aggregations of glycoprotein IV (CD36)-deficient platelets from seven healthy Japanese donors. Br J Haematol. 1992;81:86–92. doi: 10.1111/j.1365-2141.1992.tb08177.x. [DOI] [PubMed] [Google Scholar]
- 56.Daniel JL, Dangelmaier C, Strouse R, Smith JB. Collagen induces normal signal transduction in platelets deficient in CD36 (platelet glycoprotein IV) Thromb Haemostasis. 1994;71:353–356. [PubMed] [Google Scholar]