Abstract
Interleukin (IL)-5 has been shown to activate many signaling molecules in eosinophils, but their functional relevance remains unknown. We have examined the functional relevance of Lyn, Jak2, and Raf-1 kinases in eosinophil survival, upregulation of adhesion molecules and degranulation. To this goal we used Lyn and Raf-1 antisense (AS) oligodeoxynucleotides (ODN) to inhibit the expression of these proteins and tyrphostin AG490 to specifically block the activation of Jak2. We have demonstrated that all three kinases are important for IL-5– induced suppression of eosinophil apoptosis. However, Lyn and Jak2 tyrosine kinases are not important for the upregulation of CD11b and the secretion of eosinophil cationic protein. In contrast, Raf-1 kinase is critical for both these functions. This is the first identification of specific signaling molecules responsible for three important functions of eosinophils. We have established a central role for Raf-1 kinase in regulating eosinophil survival, expression of β2 integrins and degranulation. Further, there appears to be a dissociation between two receptor-associated tyrosine kinases, i.e., Lyn and Jak2, and the activation of Raf-1 kinase. The delineation of the functional relevance of signaling molecules will help design therapeutic approaches targeting specific eosinophil function.
Keywords: eosinophil, signal transduction, interleukin 5, Raf-1 kinase, degranulation
Eosinophils are major inflammatory effector cells, with blood and tissue eosinophilia being a feature of many allergic disorders (1). Activated eosinophils can perform a variety of specialized cellular functions, including cellular adhesion, chemotaxis, generation of reactive oxygen metabolites, and secretion of cytotoxic enzymes (2). During an allergic response, after exposure to a wide range of activators and chemotactic factors, eosinophils attach to the endothelial wall of the blood vessel at the site of the allergic reaction. The process of cell attachment and tissue localization is mediated by families of cell adhesion molecules that are expressed on both eosinophils and vascular endothelium (3). Eosinophils then migrate out of the bloodstream into tissue and degranulate readily, releasing cytotoxic products such as eosinophil cationic protein (ECP)1 and major basic protein. Interleukin-5 is the principal regulatory cytokine that modulates all described functions of eosinophils. First, it stimulates differentiation of eosinophils from bone marrow cells leading to blood eosinophilia (4). Second, it upregulates the expression of β2 integrins, leading to increased recruitment of eosinophils from circulation (5). By inhibiting apoptosis, IL-5 prolongs survival of eosinophils resulting both in blood and tissue eosinophilia (6). Finally, IL-5 primes eosinophils for degranulation and release of reactive oxygen metabolites (7).
The exact mechanism of how IL-5 stimulates functions of eosinophils is poorly understood. The earliest signaling events to occur after stimulation of the IL-5 receptor by the ligand involve the phosphorylation and activation of series of protein tyrosine kinases, including Lyn, Syk, Jak2, and Fyn (8–11). The consequence of activation of tyrosine kinases is the propagation of signal through the Ras-Raf-1–mitogen-activated protein (MAP) kinase pathway and the Jak-STAT pathway. Recently, Yosefi et al. (11) have shown that Lyn and Syk tyrosine kinases are necessary for IL-5–induced inhibition of apoptosis in eosinophils. We have reported the requirement for SHP-2 tyrosine phosphatase in IL-5–stimulated prolongation of eosinophil survival (12). To date, these are the only reports showing requirement of signaling molecules for IL-5–induced function of eosinophils.
The role of Jak2, Raf-1, MEK, and MAP kinases in mediating diverse cellular functions such as prolongation of eosinophil survival and activation is not known. An approach using a dominant negative mutant indicated a role for Jak2 in GM-CSF–induced cell proliferation (13). Further, depletion of Raf-1 kinase by antisense oligodeoxynucleotides resulted in inhibition of GM-CSF and IL-3–induced proliferation and colony formation in leukemic cell lines (14). The latter findings indicate that Raf-1 kinase is necessary for activation of different cellular functions. In this study we took advantage of newly synthesized specific inhibitors and/or antisense oligodeoxynucleotides to alter activation or expression of the tyrosine kinases Jak2 and Lyn and the serine/threonine kinase Raf-1 in human eosinophils. Having such altered cells, we asked about their ability to respond to IL-5 with prolongation of survival, upregulation of CD11b adhesion molecule and priming for ECP release. Our findings revealed different requirements of the studied kinases for mediating IL-5–induced survival and activation of eosinophils.
Materials and Methods
Reagents.
Percoll was purchased from Pharmacia Biotech (Piscataway, NJ). The mAb against anti-phosphotyrosine (clone 4G10) was obtained from the Upstate Biotechnology Inc. (Lake Placid, NY). Rabbit polyclonal anti-MAP/extracellular signal-regulated kinase (ERK)2, anti-Jak2, anti-Lyn, and anti–Raf-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal FITC-conjugated anti-CD11b and its isotype control antibody were obtained from Sigma Chemical Co. (St. Louis, MO). The Jak2 inhibitor tyrphostin AG490 was purchased from Calbiochem-Novabiochem Corp. (La Jolla, CA), and resuspended in DMSO. Enhanced chemiluminescence detection system was purchased from Amersham Corp. (Arlington Heights, IL).
Eosinophil Purification.
Peripheral blood for eosinophil purification was obtained from subjects with mild to moderate eosinophilia (6–12%). Eosinophils were isolated by sedimentation with 3% hydroxyethyl starch followed by centrifugation on discontinuous Percoll gradients according to the method of Gartner (15) as described previously. The cells were further purified by negative selection using anti-CD16 immunomagnetic beads (Miltenyi Biotec, Sunnyvale, CA). Eosinophils (>99% purity) were then suspended in RPMI 1640 in tubes coated with 3% human serum albumin.
Preparation of Cytosolic Cell Extracts and Immunoprecipitation.
Eosinophils (1–4 × 106) were incubated with IL-5 at a concentration of 10−10 M or medium at 37°C for 5 min or as indicated in the text. The stimulation was terminated by addition of 1 vol of ice-cold PBS containing 1 mM Na3VO4. The cells were pelleted by centrifugation, washed rapidly with PBS and lysed in a buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EGTA, 0.25% sodium deoxycholate, 1 μM PMSF, 1 μM Na3VO4, 1 mM NaF, 0.7% Triton X-100, and 1 μg/ml of aprotinin, leupeptin, and pepstatin. After an incubation on ice for 10 min, the lysates were passed several times through a 26-gauge needle and detergent-insoluble materials were removed by centrifugation at 4°C at 12,000 g. The protein concentration was determined using bicinchoninic acid assay (Pierce Chemical Co., Rockford, IL). Cell lysates were then resolved on SDS-PAGE and subjected to Western blotting for detection of Lyn, Raf-1, and ERK-2 kinases. In other experiments cell lysates were immunoprecipitated with anti-Jak2, anti-Lyn, or anti–ERK-2 antibodies to study kinase phosphorylation. For this purpose, the cell lysates were precleared by incubation with 20 μl of the Protein A/G Plus Agarose (Santa Cruz Biotechnology) for 2 h. After removal of the beads, the lysates were incubated with an appropriate antibody and Protein A/G Plus Agarose for 4 h at 4°C. The immunoprecipitates were washed three times with the cold lysis buffer and boiled in the Laemmli sample buffer.
For the autophosphorylation assay anti-Lyn immunoprecipitates were suspended in 20 μl kinase buffer (10 mM Hepes, pH 7.4, 50 mM NaCl, 5 mM MgCl2, 5 mM MnCl2, and 0.1 mM Na3VO4) with 0.25 mCi/ml γ-[32P]ATP for 60 min at room temperature. The reaction was stopped by washing immunoprecipitates three times with the lysis buffer and by adding the Laemmli's sample buffer. The kinase reaction products were then applied to SDS-PAGE and autoradiography.
Gel Electrophoresis and Immunoblotting.
SDS–polyacrylamide gels were prepared according to the Laemmli protocol and used for immunoblotting. The concentration of polyacrylamide was 7 or 12% depending on the molecular mass range of the proteins studied. Gels were blotted onto Hybond membranes for Western blotting using the enhanced chemiluminescence system. Blots were incubated in a blocking buffer containing 5% BSA in TBST buffer (20 mM Tris-base, 137 mM NaCl, pH 7.6, and 0.05% Tween 20) for 1 h followed by incubation in the primary Ab (0.1 μg/ml) for 1 h. After washing five times in TBST buffer, blots were incubated for 30 min with a HRP-conjugated secondary antibody (0.1 μg/ml) directed against primary Ab. The blots were developed with the enhanced chemiluminescence substrate according to manufacturer's protocol. In some experiments blots were reprobed with another Ab after stripping in a buffer of 62.5 mM Tris-HCl, pH 6.7, 100 μM 2-mercaptoethanol, and 2% SDS at 50°C for 30 min.
Antisense Oligodeoxynucleotides.
Two 15-mer Lyn sense (SS) and antisense (AS) oligodeoxynucleotides (ODN) were synthesized by Operon Technologies (Alameda, CA) based on previously published sequence information (11). These oligodeoxynucleotides have been reported to decrease the expression of Lyn in human eosinophils (11). Sequences used were as follows: AS Lyn (CATATTTCCCGCTCG) and SS Lyn (CGAGCGGGAAATATG). Two 18-mer Raf-1 ODN, AS (TCCCTGTATGTGCTCCAT) and SS (ATGGAGCACATACAGGGA), were synthesized according to a published report that has proven to block the expression of Raf-1, but not A-Raf, and inhibit GM-CSF–dependent cell proliferation (14). The ODNs were phosphorothioate modified and resuspended in sterile H2O at 100 μM concentration.
Stimulation of Eosinophils.
Purified eosinophils were suspended at 106/ml in RPMI 1640 and cultured in duplicate in the presence of ODNs or inhibitors at appropriate concentrations. All cultures and assays were performed in wells or tubes coated with 3% HSA (Sigma Chemical Co.). After a 6-h incubation, eosinophils were counted and cultured with IL-5 (10−10M) for 1.5 h or as indicated in the text. At this time duplicates were lysed and subjected to immunoblotting for appropriate kinase expression or phosphorylation. After treatment with IL-5, cells were washed, counted, and resuspended in duplicate in RPMI 1640 without IL-5, FCS, inhibitors and ODNs for survival assay (0.5 × 106/ ml), degranulation assay (0.2 × 106/ml), or flow cytometric analysis of CD11b expression (0.5 × 106/ml). All four assays were performed simultaneously on eosinophils from the same donor.
Survival Assay.
For survival studies, eosinophils (0.5 × 106 cells/ml) were incubated with ODNs or inhibitors for 6 h or as indicated in the text and then stimulated with IL-5 (10−10 M) for 1.5 h. Subsequently, the cells were cultured for 32 h. The relative number of dead cells was determined by uptake of 5 mM propidium iodide (Sigma Chemical Co.) as determined under a fluorescence microscope.
Flow Cytometry.
Eosinophils (0.5 × 106 cells/ml) were incubated in the presence or absence of IL-5. After 1.5 h of stimulation, eosinophils were washed in FACS buffer (HBSS containing 1% BSA and 0.1% NaN3) and stained with an anti–human CD11b FITC-conjugated mAb or with isotype control for 1 h at 4°C. The cells were finally washed in the FACS® buffer, fixed with 1% paraformaldehyde and 10,000 cells were analyzed by single-color flow cytometry on a FACScan® (Becton Dickinson, San Jose, CA).
Degranulation Assay.
Eosinophils were primed with IL-5 and then stimulated with a suboptimal dose of PMA or platelet-activating factor (PAF) that did not cause degranulation on its own. In brief, after stimulation with IL-5 or buffer for 1.5 h, eosinophils were incubated in RPMI 1640 (0.2 × 106 cells/ml) with PMA (0.1 ng/ml) or PAF (10−8 M) for 30 min. After incubation, supernatants were collected and frozen at −70°C until assayed for ECP by radioimmunoassay (Pharmacia Biotech).
Statistical Analyses.
Results are expressed as mean ± SD. All statistical analyses were performed using Student's paired t test. P <0.05 was considered significant.
Results
Effect of AS ODNs on Lyn Kinase Expression.
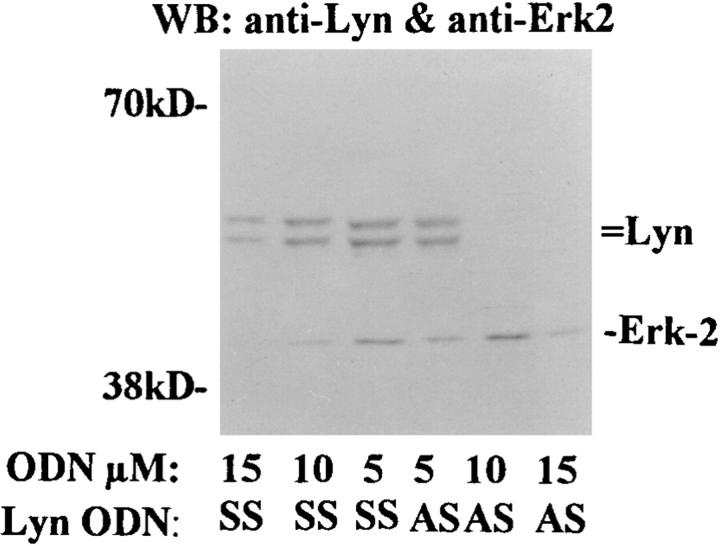
One of the earliest events that occurs after IL-5 stimulation is the activation and phosphorylation of the receptor-bound Lyn tyrosine kinase. This kinase has been already shown to be essential for mediating IL-5–dependent inhibition of apoptosis in human eosinophils. To determine the role of Lyn kinase in IL-5–induced function of eosinophils, first we evaluated the effect of Lyn AS ODN on the tyrosine kinase expression. Since eosinophils are terminally differentiated cells with a 3–4-d life span, the use of AS ODN is the most practical method to specifically alter expression of Lyn kinase. As demonstrated in Fig. 1, eosinophils exposed to 10 μM AS ODN for 6 h expressed little or no detectable p53/p56 Lyn kinase, whereas SS ODN did not alter Lyn level. The AS ODN used in our assay did not alter expression of a downstream signaling molecule MAP/ERK2 kinase (Fig. 1) and another tyrosine kinase Jak2 (data not shown). Higher concentrations (15 μM) of both AS and SS ODN nonspecifically inhibited both Lyn and ERK2 expression. For this reason all experiments were performed with 10 μM concentration of Lyn ODN. The viability of eosinophils assessed at this time (immediately before stimulation with IL-5) always exceeded 90% and was not different from control samples, indicating that at 10 μM concentration the ODNs were not toxic to the cells.
Figure 1.
The effect of Lyn AS ODN on Lyn and ERK-2 kinases expression. Purified eosinophils were incubated with Lyn antisense (AS ) or sense (SS ) ODN for 6 h. The cells were then washed and lysed. The lysates were electrophoresed, transferred to Hybond membrane, and then Western blotted with anti-Lyn and anti–ERK-2 antibodies. The blot was developed with the enhanced chemiluminescence system. Lyn AS ODN at 10 μM concentration blocked the expression of Lyn but not ERK-2 kinase (n = 3). At 15 μM concentration both AS and SS ODN inhibited the expression of the kinases.
Effect of Tyrphostin AG490 on Jak2 Inhibition.
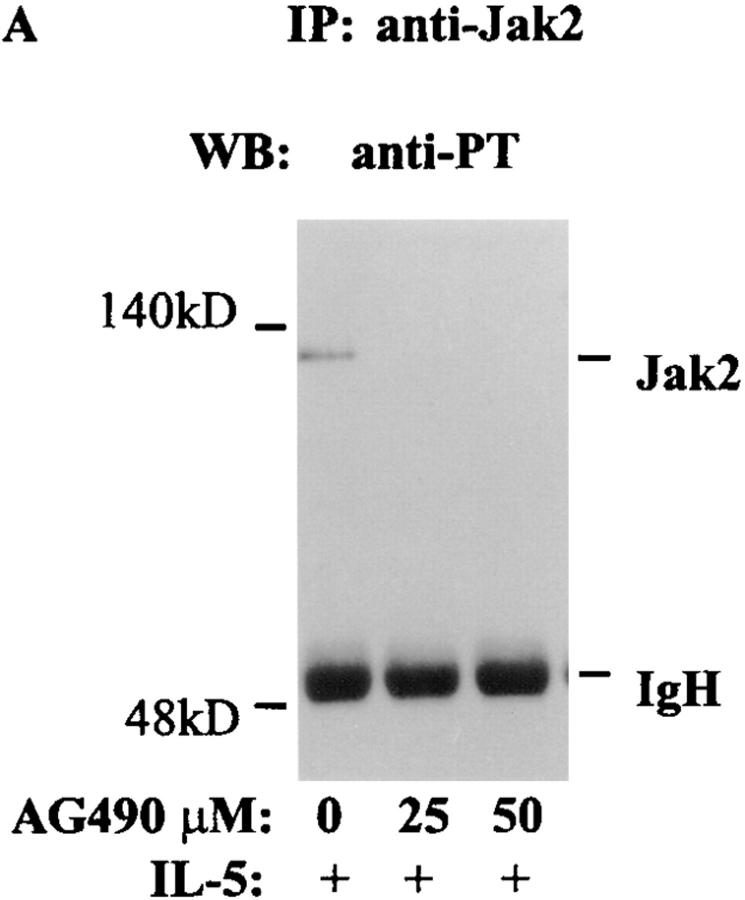
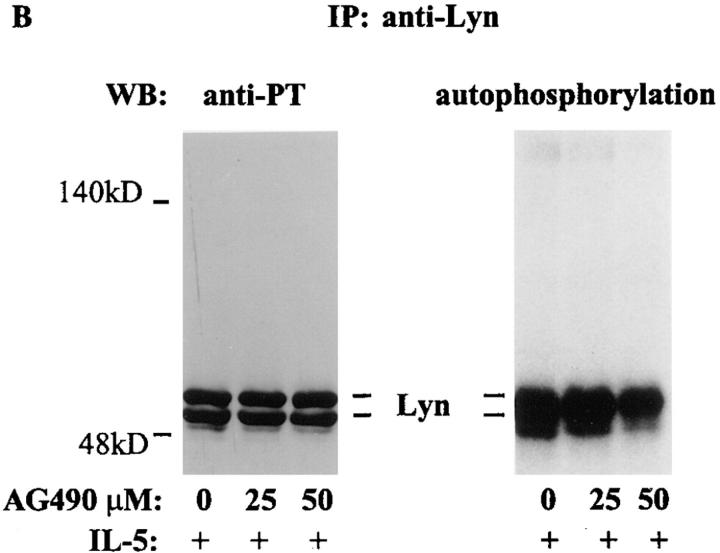
We examined the biological relevance of activation of Jak2 by IL-5. For this purpose eosinophils were treated with AG490 (25 and 50 μM) for 6 h (19). The inhibitor at a concentration <50 μM was not toxic to eosinophils. More than 90% of eosinophils were viable after 6 h of incubation with AG490. At 25 μM concentration, AG490 completely blocked IL-5–induced tyrosine phosphorylation of Jak2 (Fig. 2 A). In contrast, preincubation of eosinophils with AG490 at 25–50 μM concentrations had no effect on IL-5–induced tyrosine phosphorylation of p53 and p56 Lyn kinases (Fig. 2 B). We also studied the effect of AG490 on Lyn kinase activation. Activated Lyn kinase has previously been shown to undergo autophosphorylation. AG490 at 25 μM concentration did not affect autophosphorylation of Lyn kinase (Fig. 2 B, right), confirming its specificity for Jak2 kinase at low concentrations (19). Interestingly, at 50 μM concentration of AG490 some inhibition of Lyn autophosphorylation was noted. In subsequent experiments we used a 25 μM concentration of AG490.
Figure 2.
The effect of AG490 on IL-5–induced tyrosine phosphorylation of Jak2 and Lyn and autophosphorylation of Lyn kinase. Eosinophils were pretreated with AG490 for 6 h and then stimulated with IL-5. The cells were lysed and immunoprecipitated with antibodies against p53/56 Lyn or Jak2 kinases. The immunoprecipitates were immunoblotted with an antiphosphotyrosine antibody. Further, the anti-Lyn immunoprecipitate was used in an autophosphorylation assay. IL-5–induced tyrosine phosphorylation of Jak2 (A) and p53/56 Lyn (B, left) kinases. AG490 at 25 and 50 μM concentrations inhibited tyrosine phosphorylation of Jak2 but not Lyn kinase (n = 3). AG490 did not affect autophosphorylation of Lyn kinase at 25 μM but had some inhibitory effects at 50 μM concentration (B, right).
Effect of ODN on Raf-1 Expression.
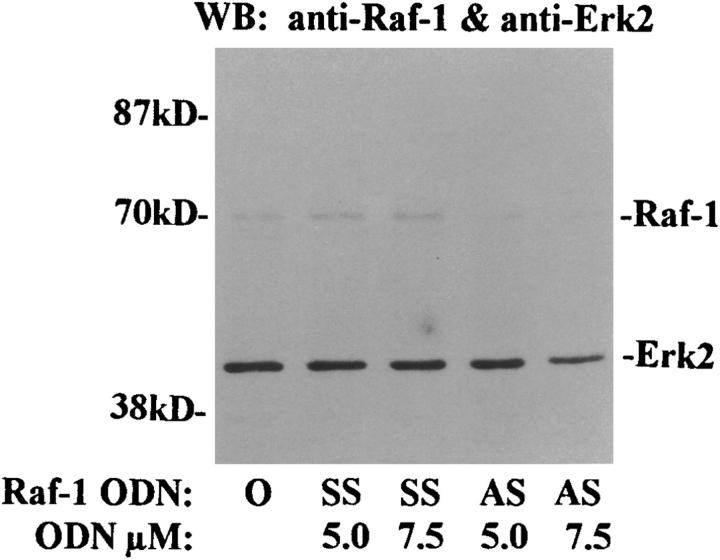
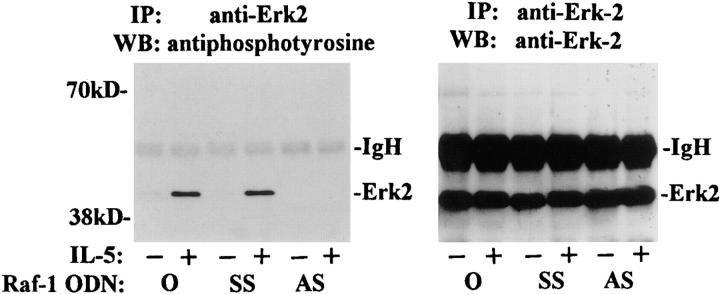
We previously reported that IL-5 stimulated the phosphorylation and activation of Raf-1 kinase in eosinophils. To assess the role of Raf-1 kinase in transducing signals for eosinophil function, we performed several sets of experiments using Raf-1 AS ODN. First, we asked, whether the treatment with AS ODN had any effect on the expression of Raf-1 and a downstream signaling molecule, ERK2. As shown on Fig. 3 a, Raf-1 AS, at 7.5 μM concentration, almost completely inhibited the expression of Raf-1 without affecting ERK2 expression. The SS ODN did not inhibit Raf-1 expression. Next, we studied the effect of Raf-1 AS ODN on ERK2 activation. Eosinophils treated with medium or SS ODN showed tyrosine phosphorylation of ERK2 upon stimulation with IL-5. However, this tyrosine phosphorylation was completely inhibited by the AS ODN (Fig. 3 b, left). When the membrane was reprobed with the anti–ERK-2 antibody, we found that there was no change in ERK-2 expression after ODN treatment (Fig. 3 b, right). The results suggest that Raf-1 is necessary for phosphorylation and perhaps activation of ERK-2 kinase. In a separate experiment, the necrotic and apoptotic effects of various treatments were studied by flow cytometric analysis of annexin V and 7-amino actinomycin D (7-AAD)-stained cells. The percentage of cells showing necrotic death was <1% in AS ODN- and AG490-treated cells and was not different from control buffer–treated cells. Both Lyn and Raf-1 AS ODN as well as AG490 reversed the antiapoptotic effect of IL-5 on eosinophils (data not shown).
Figure 3.
The effect of Raf-1 antisense ODN on the expression of Raf-1 and ERK-2 kinases (A) and phosphorylation of ERK-2 (B). (A) Purified eosinophils were incubated with medium (O), Raf-1 AS (AS), or SS ODN (SS ) for 6 h. The cells were then washed and lysed. The lysates were electrophoresed and immunoblotted with anti– Raf-1 and anti–ERK-2 antibodies. Raf-1 AS ODN at 5 and 7.5 μM concentrations blocked the expression of Raf-1 but not ERK-2 kinase (n = 3). The SS ODN did not affect the expression of the kinases. (B) Cells were pretreated with medium (O) or 7.5 μM concentration of Raf-1 AS (AS ) and SS ODN (SS ) for 6 h and then stimulated with (+) or without (−) IL-5. The cells were lysed, immunoprecipitated with an anti-ERK2 antibody, and immunoblotted with an antiphosphotyrosine antibody. IL-5–induced tyrosine phosphorylation of ERK-2 kinase. Raf-1 AS but not SS ODN inhibited the phosphorylation of ERK-2 kinase (left). The membrane was then stripped and reprobed with the anti–ERK-2 antibody (right), which showed an equal amount of ERK-2 protein in each lane.
Having established specific inhibition of Lyn, Jak2, and Raf-1 kinases, we studied the effect of the AS ODN and AG490 on eosinophil survival, upregulation of CD11b adhesion molecule and the release of eosinophil cationic protein. In all subsequent experiments, eosinophils were preincubated with the ODN or AG490 for 6 h and then stimulated with IL-5 for 1.5 h. The cells were then aliquoted for three separate and parallel experiments: (a) survival assay after 32 h; (b) measurement of CD11b upregulation by flow cytometry; and (c) ECP secretion after stimulation with a subthreshold concentration of PMA.
The Effect of AS ODN and AG490 on Eosinophil Survival.
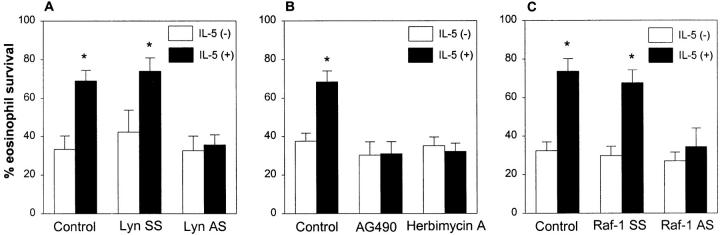
After preincubation with the inhibitors (ODN and AG490) and stimulation with IL-5, eosinophils were resuspended in RPMI 1640 without IL-5, inhibitors, and FCS. We excluded FCS from this stage of experiment because serum has been shown to alleviate the requirement for the Ras pathway for GM-CSF dependent viability and proliferation in mutant cells with βc receptor lacking amino acid residues 626–763 (17). The viability of the cells cultured without serum was 69 ± 5% when assessed 32 h after stimulation with IL-5. In contrast to IL-5–stimulated cells, there was 33 ± 7% viable cells in samples incubated with medium alone in lieu of IL-5 (Fig. 4 A). Lyn AS ODN blocked the ability of IL-5 to prevent eosinophil death (35 ± 5% vs. 74 ± 7% vs. 69 ± 5% for AS-, SS-, and medium-treated cells, respectively, P <0.01). The results indicate a critical role of Lyn kinase in IL-5–induced survival of eosinophils and confirm a previously published report (11).
Figure 4.
The effect of Lyn AS ODN (A), AG490 (B) and Raf-1 AS ODN (C ) on survival-prolonging activity of IL-5 on eosinophils. (A) Eosinophils were pretreated with 10 μM concentration of Lyn AS and SS ODN or medium (Control ) for 6 h. The cells were then stimulated with (+) or without (−) IL-5 (10−10 M) for 1.5 h. Eosinophil viability was assessed 32 h later. The control and Lyn SS-treated cells showed significant prolongation of eosinophil survival (n = 5, *P <0.01, paired t test) after IL-5 stimulation. This survival prolonging effect of IL-5 was blocked by pretreatment of cells with Lyn AS ODN. (B) Purified eosinophils were pretreated with buffer (Control ), AG490 (25 μM) or Herbimycin A (2 μM) for 6 h. The cells were then stimulated with (+) or without (−) IL-5 for 1.5 h. Eosinophil viability was assessed 32 h later. The IL-5–induced prolongation of eosinophil survival was completely abrogated by both AG490 and Herbimycin A (n = 5, *P <0.01). (C ) Eosinophils were pretreated with 7.5 μM concentration of Raf-1 AS, SS ODN, or medium (Control ) for 6 h. The cells were then stimulated with (+) or without (−) IL-5 (10−10 M) for 1.5 h. Eosinophil viability was assessed 32 h later. The control and SS ODN-treated cells showed significant prolongation of eosinophil survival (n = 5, *P <0.01, paired t test) after IL-5 stimulation. Raf-1 AS ODN completely blocked this survival prolonging effect of IL-5.
The incubation of eosinophils with AG490 blocked the IL-5–induced prolongation of eosinophil survival (Fig. 4 B). There were 31 ± 6% viable eosinophils 32 h after stimulation with IL-5 in samples pretreated with AG490. The dead cells had characteristics of apoptosis by microscopic examination. Similar results were obtained with a broad spectrum tyrosine kinase inhibitor, herbimycin A (Fig. 4 B). At 2 μM concentration, herbimycin A blocked both Lyn and Jak2 tyrosine phosphorylation in eosinophils in our control immunoblottings. As expected, this inhibitor blocked IL-5–induced prolongation of viability to a degree similar to that observed with AG490 and Lyn antisense ODN. Like Lyn AS ODN and AG490, Raf-1 AS ODN abrogated the IL-5–induced prolongation of eosinophil survival (34 ± 9% vs. 74 ± 7% in control cells; Fig. 4 C).
The Effect of AS ODN and AG490 on CD11b Expression.
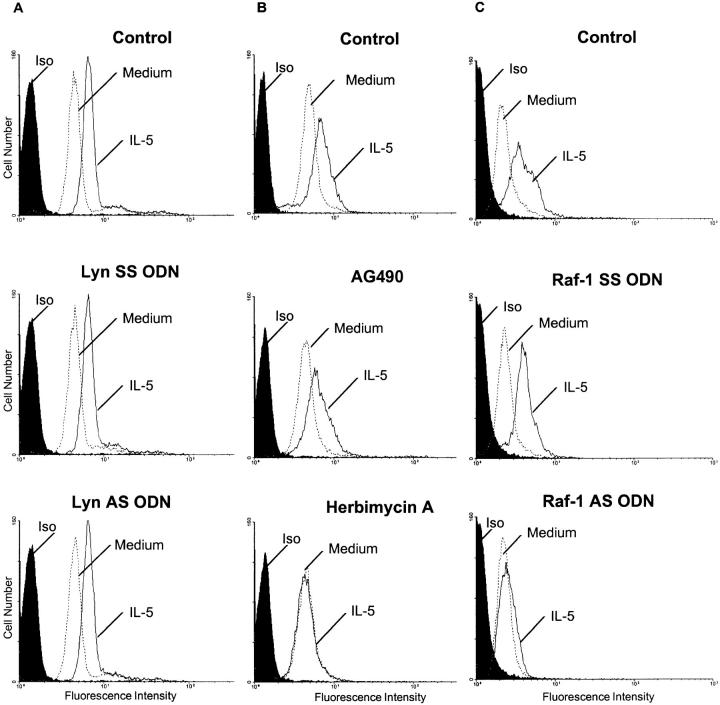
We examined the effect of the AS ODN and AG490 on IL-5–induced upregulation of CD11b adhesion molecule. CD11b/CD18 complex is constitutively expressed on eosinophils and its surface density can be upregulated by stimulation with IL-5 and GM-CSF. Indeed, a considerable upregulation of CD11b expression was found as early as 60 min after stimulation with IL-5, reaching the peak at 1.5 h. This increase in CD11b was not inhibited in cells treated with Lyn AS or SS ODN indicating that Lyn was not important in this process (Fig. 5 A). Similarly, AG490 did not affect the expression of CD11b (Fig. 5 B). Eosinophils treated with AG490 were still able to upregulate CD11b expression in response to IL-5. However, herbimycin, at 2 μM concentration, inhibited the upregulation of CD11b expression in response to IL-5. Thus, we conclude that protein tyrosine phosphorylation is important for CD11b upregulation, but Lyn and Jak2 kinases are not essential for this process. However, unlike Lyn AS ODN and AG490, Raf-1 AS ODN blocked the IL-5– stimulated upregulation of CD11b on eosinophils (Fig. 5 C). The SS ODN had no effects.
Figure 5.
The effect of Lyn AS ODN (A), AG490 (B), and Raf-1 AS ODN (C ) on CD11b expression. (A). Eosinophils were pretreated with medium (Control ), Lyn SS, or AS ODN and then stimulated with medium or IL-5. Then the cells were stained with an anti-CD11b FITC-conjugated mAb or isotype control mAb and analyzed by a FACScan®. In control experiments, IL-5 upregulated the expression of CD11b. Neither AS nor SS Lyn ODN had any effect on eosinophil CD11b expression (n = 3). (B). Jak2 kinase and CD11b expression. Eosinophils were pretreated with medium (Control ), AG490 (25 μM) or Herbimycin A (2 μM) and then stimulated with medium or IL-5. Then the cells were stained with the anti-CD11b FITC-conjugated mAb or isotype control mAb. In control experiments, IL-5 upregulated the expression of CD11b. The Jak2 inhibitor AG490 did not block the upregulation of CD11b, but a broad spectrum tyrosine kinase inhibitor Herbimycin A did (n = 3). (C ) Eosinophils were pretreated with medium (Control ), Raf-1 SS or AS ODN and then stimulated with medium or IL-5. Then the cells were stained with the anti-CD11b FITC-conjugated mAb or isotype control mAb. In control and Raf-1 SS ODN experiments, IL-5 upregulated the expression of CD11b. Raf-1 AS ODN blocked the upregulation of CD11b by IL-5 (n = 3).
The Effect of AS ODN and AG490 on ECP Release from Eosinophils.
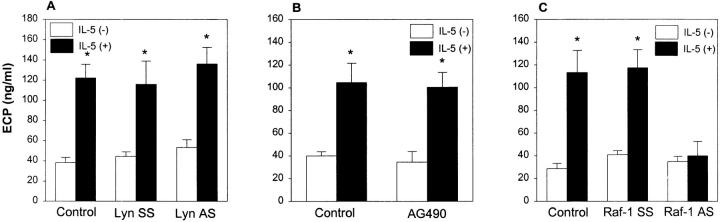
It has been previously reported that tyrosine phosphorylation is involved in cytokine priming of the respiratory burst and degranulation (18). In our study, we asked whether Lyn kinase participated in the priming of eosinophils for ECP release. As a degranulating stimulus we used phorbol 12-myristate 13-acetate (PMA) at the suboptimal concentration of 0.1 ng/ml. This dose of PMA did not induce ECP release above the background level (data not shown). However, when eosinophils were preincubated with IL-5 for 1.5 h, they became more susceptible to stimulation with PMA and released three times more ECP (122 ± 13 ng/ml vs. 38 ± 5 ng/ml of ECP for unprimed eosinophils, P <0.01). Neither Lyn AS nor SS ODN modified IL-5 priming of ECP secretion (Fig. 6 A). Similar to Lyn AS ODN, AG490 did not block ECP secretion (Fig. 6 B). The data indicate that Lyn and Jak2 are not required for IL-5 priming for ECP release.
Figure 6.
The effect of Lyn AS ODN (A), AG490 (B), and Raf-1 AS ODN (C ) on ECP release. (A) Eosinophils were pretreated with medium (Control ), Lyn SS or AS ODN and, then, primed with (+) or without (−) IL-5. The cells were then challenged with a suboptimal concentration of PMA (0.1 ng/ml) which by itself did not induce ECP release. The IL-5–priming of cells caused significant secretion of ECP from eosinophils (*P <0.01, n = 4). Neither AS nor SS Lyn ODN affected ECP release. (B). Eosinophils were pretreated with medium (Control ) or AG490 (25 μM) and then primed with (+) or without (−) IL-5 as described above. The cells were then challenged with a suboptimal concentration of PMA (0.1 ng/ml). The IL-5–priming of cells caused significant secretion of ECP from eosinophils (*P <0.05, n = 4). Pretreatment of cells with AG490 did not block ECP release. (C ) Eosinophils were pretreated with medium (Control), Raf-1 SS or AS ODN and, then, primed with (+) or without (−) IL-5. The cells were then challenged with PMA (0.1 ng/ml). The IL-5–priming of cells caused significant secretion of ECP from control and SS ODN-treated eosinophils (*P <0.05, n = 4). The Raf-1 AS ODN completely prevented ECP release.
In contrast to Lyn AS ODN and AG490, Raf-1 AS ODN almost completely blocked ECP secretion from eosinophils (Fig. 6 C). The SS ODN had no effects. There are some suggestions from the literature that PMA signaling may involve Raf-1 kinase. Thus, the PMA stimulation of cells may be more susceptible to RAF-1 AS inhibition. To address this issue, we performed experiments using a different eosinophil degranulator, PAF, at a subthreshold concentration. We observed a similar inhibition of ECP release from Raf-1 AS ODN-treated cells (132 ± 22 ng/ml for medium-pretreated cells vs. 42 ± 6 ng/ml for AS-treated cells, n = 3). Thus, although we cannot rule out that Raf-1 is involved in PAF signaling, our results suggest that this kinase is required for transducing signals for ECP release.
Discussion
In this study, we have prepared eosinophils lacking active forms of Lyn, Jak2, and Raf-1 kinases and analyzed their physiologic responses to IL-5. We have made the following observations. (a) IL-5 is unable to inhibit apoptosis of cells not expressing active Lyn, Jak2, and Raf-1 kinases. (b) The induction of CD11b expression and ECP secretion by IL-5 are intact in the absence of Lyn and Jak2 activation. However, other tyrosine kinases are important for CD11b upregulation. (c) The IL-5–mediated suppression of eosinophil apoptosis, upregulation of CD11b, and ECP secretion are impaired in eosinophils lacking Raf-1 kinase. Based on these results, we conclude that Lyn, Jak2, and Raf-1 kinases are necessary for the antiapoptotic effect of IL-5, whereas Raf-1 is additionally essential for eosinophil activation and degranulation. Previously, Lyn kinase was found to be important for eosinophil survival (11). However, the role of Lyn in β2 integrin expression and eosinophil degranulation has been unknown. This is the first report on the importance of Lyn, Jak2, and Raf-1 kinase in three important functions of eosinophils. We have established a central role for Raf-1 kinase in the multifaceted regulation of eosinophil function. Further, our work reveals a differential requirement of signaling molecules for survival, adhesion, and exocytosis of eosinophils.
Eosinophils are terminally differentiated cells that die rapidly in the tissue or culture due to apoptosis. They exhibit the classical changes in morphology including cytoplasmic and nuclear condensation resulting in cell shrinkage, DNA fragmentation, and loss of nucleoli (20). It is an important mechanism by which eosinophils are cleared from the tissue without eliciting inflammation (21). It has been known for many years that IL-5, GM-CSF, and IL-3 enhance their survival in culture for up to two weeks. The inhibition of eosinophil apoptosis by interleukins is considered an important mechanism for the development of blood and tissue eosinophilia in diseases such as asthma and other allergic disorders. However, an understanding of the induction of apoptosis and its inhibition is still fragmentary and mainly originates from cell lines that may not directly represent mature hematopoietic cells. Several genes have been identified that participate either as inducers or repressors of the programmed cell death (22). Among these, Bcl-2 and Bax are homologous proteins that have opposing effects on apoptosis. Bcl-2 serves to prolong cell survival, whereas Bax acts as an accelerator of apoptosis (23). These proteins act as ion channels and adaptor proteins regulating the release of mitochondrial proteins into the cytosol in order to activate the cysteine proteases (caspases) that are the terminal effectors of apoptosis. Overexpression of Bcl-2 inhibits mitochondrial permeability transition, whereas Bax overexpression induces it. One mechanism of inhibition of apoptosis by Bcl-2 is its ability to associate with Raf-1, translocating this kinase from the cytosol to the mitochondrial membrane (24). Once there, Raf-1 induces phosphorylation of BAD, a proapoptotic Bcl-2 family protein that abrogates the cytoprotective functions of Bcl-2 by heterodimerizing with it (25). This mechanism could explain our findings on the requirement of Raf-1 kinase for antiapoptotic effect of IL-5. In this regard, IL-3 has previously been shown to induce Bcl-2 in myeloid cell precursors (26).
Another mechanism by which Raf-1 could transduce the antiapoptotic signal is through activation of downstream signaling molecules such as MEK and MAP kinases and/or NFκB nuclear factor. As we have shown in this work, inhibition of Raf-1 expression prevents tyrosine phosphorylation of ERK-2 MAP kinase. MAP kinases are known to induce c-fos and c-jun (27). The truncation of the βc receptor at 516 or 626 amino acid residues abolishes activation of Ras-Raf-MAP, c-fos and c-jun and inhibits the antiapoptotic effect of GM-CSF (28). Moreover, the contribution of MAP kinases in regulation of STAT1/3 nuclear factors has recently been reported (29). Because Raf-1 is essential for both MAP activation and prolongation of survival, the viability signaling may be linked to molecules and genes activated by MAP kinases.
There are reports of NFκB activation by Raf-1 kinase (30). NFκB consists of two subunits (p50 and p65) and exists in a complex with IκB in resting cells. Activated Raf-1 phosphorylates IκB releasing NFκB that enters the nucleus and activates various genes carrying the NFκB response element. Cells lacking NFκB are more sensitive to TNF-α–induced cytotoxicity, confirming that one of the target genes for NFκB is a gene encoding a survival factor (31). Although Raf-1 activation by antiapoptotic cytokines such as IL-3, GM-CSF, and IL-5 is well documented, the activation of NFκB in eosinophils has not been investigated.
In this work we confirmed the essential role of Lyn kinase in the antiapoptotic effect of IL-5 as previously reported (11). Lyn kinase is one of the earliest activated kinases after IL-5 stimulation. It is constitutively associated with the βc receptor for IL-5. It may serve to activate the cytosolic tyrosine kinase Syk (8, 11). Other potential substrates for Lyn kinase are Raf-1 kinase and SHP2 tyrosine phosphatase. Our results suggest a dissociation of Lyn from Raf-1 activation since they have nonoverlapping functions.
This is the first report on the importance of Jak2 kinase in eosinophil survival. Jak2 kinase is constitutively associated with the βc subunit of the IL-5 receptor and is activated upon ligand binding. There are several possible target molecules for Jak2 kinase: IL-5 βc receptor, STAT nuclear factors, SHP2 tyrosine phosphatase, and Raf-1. Jak2 is essential for phosphorylation of the receptor as IL-5Rβc phosphorylation by GM-CSF is abolished in mutant Jak2-negative BA/F3 cells (13). Interestingly, one of the tyrosine residues phosphorylated by Jak2 is Y750, which is considered a viability domain of the IL-5βc receptor. This tyrosine residue was found to be necessary for activation of Shc, p21 ras, Raf-1, and MAP kinases. STAT nuclear factors are the first described downstream molecules for the Janus kinase family. The Jak-STAT pathway is considered critical for cytokine-stimulated proliferation of cells. Their function in nonproliferating cells such as eosinophils is unknown. Our results define a role for Jak2 in survival but not for eosinophil activation.
Human eosinophils constitutively express CD11b/CD18 adhesion molecule. In vitro CD11b/CD18 can be upregulated by a number of stimuli including IL-5, GM-CSF, and PAF (32, 33). CD11b upregulation on eosinophils is probably analogous to that on neutrophils, in which CD11b is rapidly mobilized from cytoplasmic stores and upregulation does not require de novo protein synthesis (34). Little is known on signaling events leading to increased expression of CD11b/CD18. It has been shown that antisense oligodeoxynucleotides to the p65 subunit of NFκB block CD11b expression and alter adhesion properties of differentiated HL-60 granulocytes (35). In our work, we could not upregulate CD11b expression in eosinophils lacking Raf-1 kinase. It is possible that the CD11b upregulation through Raf-1 is linked to NFκB activation.
The suppression of Lyn and Jak2 did not block CD11b upregulation by IL-5. The result does not imply that tyrosine phosphorylation is dispensable for the function, because treatment of eosinophils with herbimycin A completely inhibited IL-5–induced upregulation of CD11b. The data are in agreement with the study of Nishizumi et al. (36) who found that Lyn-deficient mast cells had impaired tyrosine phosphorylation and Ca2+ mobilization but retained capability for adhesion and degranulation. There are two possible interpretations of our results. When Lyn is inhibited, Jak2 induces Raf-1 activation. Likewise, when Jak2 is inhibited, Lyn may signal for Raf-1 activation. Alternatively, a third tyrosine kinase (or kinases) is responsible for Raf-1 signaling. Among the candidates are Fyn, Btk, Fes, and Syk, all of which have been shown to be activated by IL-5 (10, 37, 38). Additional pathways may include protein kinase C.
Recently, it has become evident that CD11b/CD18 is not simply an adhesion site on the cell surface, but may modulate a number of cellular processes such as eosinophil degranulation and superoxide production. For example, superoxide production and release of EDN induced by GM-CSF is abolished by the treatment of eosinophils with anti-CD11b and anti-CD18 mAbs (39). Our finding that similar signaling processes are involved in both CD11b upregulation and degranulation may indicate that these events are closely linked. We have used IL-5 for eosinophil priming and PMA or PAF for the induction of ECP release. Raf-1 appears to control the signaling processes originating from all these diverse stimuli, leading to ECP secretion. Based on these observations we believe that Raf-1 is a critical signal for eosinophil degranulation.
In summary, we have defined specific functions of Lyn, Jak2, and Raf-1 kinases in eosinophil activation. Eosinophils play a pivotal role in asthma and other allergic diseases. The identification of the critical signaling molecules for eosinophil survival, adhesion, and degranulation will help develop specific inhibitors for use in allergic and other eosinophilic disorders.
Acknowledgments
This work was supported by grants from the National Institutes of Health (RO1 35713 and RO1 15939) and the John Sealy Memorial Foundation. K. Pazdrak was supported by a McLaughlin Foundation Postdoctoral Fellowship, Texas Allergy and Immunology Society Memorial Foundation, and the President's Grant-In-Aid from the American Academy of Allergy, Asthma, and Immunology.
Abbreviations used in this paper
- AS
antisense
- ECP
eosinophil cationic protein
- ERK
extracellular signal-regulated kinase
- MAP kinase
mitogen-activated protein kinase
- ODN
oligodeoxynucleotide
- PAF
platelet-activating factor
- SS
sense
References
- 1.Corrigan CJ, Kay AB. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992;13:501–503. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- 2.Owen, W.F., Jr., and K.F. Austen. 1994. Cytokine regulation of eosinophil-mediated inflammatory reactions by modulation of eosinophil programmed cell death and subsequent priming for augmented function. In Eosinophils In Allergy and Inflammation. G.J. Gleich and A.B. Kay, editors. Marcel Decker, New York. 239–253.
- 3.Resnick MB, Weller PF. Mechanisms of eosinophil recruitment. Am J Res Cell Mol Biol. 1993;8:349–355. doi: 10.1165/ajrcmb/8.4.349. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi Y, Tsuda T, Suda J, Eguchi M, Miura Y, Harada N, Tominaga A, Takatsu K. Purified interleukin-5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh GM, Hartnell A, Wardlaw AJ, Kurihara K, Sanderson CJ, Kay AB. IL-5 enhances the in vitro adhesion of human eosinophils, but not neutrophils, in a leukocyte integrin (CD11/CD18)-dependent manner. Immunology. 1990;71:258–265. [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi Y, Sudo T, Ohata S, Tominaga K, Miura Y, Kasa T. Analysis of the survival of mature eosinophils: interleukin-5 prevents apoptosis in mature human eosinophils. Blood. 1991;78:2542–2547. [PubMed] [Google Scholar]
- 7.Fujisawa T, Abu-Ghazaleh R, Kita H, Sanderson CJ, Gleich GJ. Regulatory effect of cytokines on eosinophil degranulation. J Immunol. 1990;144:642–646. [PubMed] [Google Scholar]
- 8.Pazdrak K, Schreiber D, Forsythe P, Justement L, Alam R. The signal transduction mechanism of IL-5 in eosinophils: the involvement of Lyn tyrosine kinase and the Ras-Raf-1-MEK-MAP kinase pathway. J Exp Med. 1995;181:1827–1834. doi: 10.1084/jem.181.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pazdrak K, Stafford S, Alam R. The activation of the Jak-STAT1 signaling pathway in eosinophils by IL-5. J Immunol. 1995;155:397–402. [PubMed] [Google Scholar]
- 10.Appleby MW, Kerner JD, Chien S, Maliszewski CR, Bondadaa S, Perlmutter RM. Involvement of p59fynT in interleukin-5 receptor signaling. J Exp Med. 1995;182:811–820. doi: 10.1084/jem.182.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousefi S, Hoessli DC, Blaser K, Mills GB, Simon HU. Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med. 1996;183:1407–1414. doi: 10.1084/jem.183.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pazdrak K, Adachi T, Alam R. SHPTP2/SHP2 tyrosine phosphatase is a positive regulator of the interleukin-5 receptor signal transduction pathways leading to the prolongation of eosinophil survival. J Exp Med. 1997;186:561–568. doi: 10.1084/jem.186.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe S, Itoh T, Arai K. Jak2 is essential for activation of c-fos and c-mycpromoters and cell proliferation through the human granulocyte-macrophage colony-stimulating factor receptor in BA/F3 cells. J Biol Chem. 1996;271:12681–12686. doi: 10.1074/jbc.271.21.12681. [DOI] [PubMed] [Google Scholar]
- 14.Muszynski KW, Ruscetti FW, Heidecker G, Rapp U, Troppmair J, Gooya JM, Keller JR. Raf-1 protein is required for growth factor–induced proliferation of hematopoietic cells. J Exp Med. 1995;181:2189–2199. doi: 10.1084/jem.181.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gartner I. Separation of human eosinophils in density gradients of polyvinylpyrrolidone-coated silica gel (Percoll) Immunology. 1980;40:133–136. [PMC free article] [PubMed] [Google Scholar]
- 16.Pazdrak K, Justement L, Alam R. Mechanism of inhibition of eosinophil activation by transforming growth factor-β. J Immunol. 1995;155:4454–4458. [PubMed] [Google Scholar]
- 17.Inhorn RC, Carlesso N, Durstin M, Frank DA, Griffin JD. Identification of a viability domain in the granulocyte/macrophage colony-stimulating factor receptor β chain involving tyrosine-750. Proc Natl Acad Sci USA. 1995;92:8665–8669. doi: 10.1073/pnas.92.19.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van der Bruggen T, Kok PTM, Raaijmakers JAM, Verhoeven AJ, Kessels RGC, Lammers JWJ, Koenderman L. Cytokine priming of the respiratory burst in human eosinophils is Ca2+independent and accompanied by induction of tyrosine kinase activity. J Leukoc Biol. 1993;53:347–353. doi: 10.1002/jlb.53.4.347. [DOI] [PubMed] [Google Scholar]
- 19.Meydean NT, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, et al. Inhibition of acute lymphoblastic leukemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 20.Beauvais F, Michel L, Dubertret Human eosinophils in culture undergo a striking and rapid shrinkage during apoptosis. Role of K+ channels. J Leukoc Biol. 1995;57:861–865. doi: 10.1002/jlb.57.6.851. [DOI] [PubMed] [Google Scholar]
- 21.Wooley KL, Gibson PG, Carty K, Wilson AJ, Twaddell SH, Wooley MJ. Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am J Respir Crit Care Med. 1996;154:237–243. doi: 10.1164/ajrccm.154.1.8680686. [DOI] [PubMed] [Google Scholar]
- 22.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 23.Middleton G, Nunez G, Davies AM. Bax promotes neuronal survival and antagonises the survival effects of neurotrophic factors. Development (Camb) 1996;122:695–701. doi: 10.1242/dev.122.2.695. [DOI] [PubMed] [Google Scholar]
- 24.Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 25.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-XL. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 26.Baffy G, Miyashita T, Wiliamson JR, Reed JC. Apoptosis induced by withdrawal of IL-3 from IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993;268:6511–6519. [PubMed] [Google Scholar]
- 27.Pulverer BJ, Kyriakis JM, Avruch J, Nikolakaki E, Woodgett JR. Phosphorylation of c-junmediated by MAP kinase. Nature. 1991;353:670–672. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 28.Sato N, Sakamaki K, Terada N, Arai K, Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common β subunit responsible for different signaling. EMBO (Eur Mol Biol Organ) J. 1993;11:4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajote D, Sadowski HB, Haman A, Gopalbhai K, Meloche S, Liu L, Krystal G, Hoang T. Contribution of both STAT and SRF/TCF to c-fos promoter activation by GM-CSF. Blood. 1996;88:2906–2916. [PubMed] [Google Scholar]
- 30.Li S, Sedivy JM. Raf-1 protein kinase activates the NFκB transcription factor by dissociating the cytoplasmic NFκB-IκB complex. Proc Natl Acad Sci USA. 1993;90:9247–9251. doi: 10.1073/pnas.90.20.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beg AA, Baltimore D. An essential role for NFκB in preventing TNF-α–induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 32.Neeley SP, Hamman KJ, White SR, Baranowski SL, Burch RA, Leff AR. Selective regulation of expression of surface adhesion molecules Mac-1, L-selectin, and VLA-4 on human eosinophils and neutrophils. Am J Respir Cell Mol Biol. 1993;8:633–638. doi: 10.1165/ajrcmb/8.6.633. [DOI] [PubMed] [Google Scholar]
- 33.Blom M, Tool ATJ, Kok PTM, Koenderman L, Roos D, Verhoeven AJ. GM-CSF, IL-3 and IL-5 greatly enhance the interaction of human eosinophils with opsonized particles by changing the affinity of complement receptor type 3. Blood. 1994;83:2978–2984. [PubMed] [Google Scholar]
- 34.O'Shea J, Brown EJ, Seligmann BE, Metcalf JA, Frank MM, Gallin JI. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985;134:2580–2585. [PubMed] [Google Scholar]
- 35.Sokoloski J, Sartorelli AC, Rosen CA, Narayanan R. Antisense oligonucleotides to the p65 subunit of NFkB block CD11b expression and alter adhesion properties of differentiated HL-60 granulocytes. Blood. 1993;82:625–632. [PubMed] [Google Scholar]
- 36.Nishizumi H, Yamamoto T. Impaired tyrosine phosphorylation and Ca2+mobilization, but not degranulation, in Lyn-deficient bone marrow-derived mast cells. J Immunol. 1997;158:2350–2355. [PubMed] [Google Scholar]
- 37.Sato S, Katagiri T, Takaki S, Kikuchi Y, Hitoshi Y, Yonehara S, Tsukada S, Kitamura D, Watanabe T, Witte O, Takatsu K. IL-5 receptor-mediated tyrosine phosphorylation of SH2/SH3-containing proteins and activation of Bruton's tyrosine and Janus 2 kinases. J Exp Med. 1994;180:2101–2111. doi: 10.1084/jem.180.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gold MR, Duronio V, Saxena SP, Schrader JW, Aebersold R. Multiple cytokines activate PI-3 kinase in hematopoietic cells. Association of the enzyme with various tyrosine-phosphorylated proteins. J Biol Chem. 1994;269:5403–5412. [PubMed] [Google Scholar]
- 39.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony stimulating factor and platelet-activating factor. J Immunol. 1994;152:5457–5467. [PubMed] [Google Scholar]