Abstract
Large numbers of neuritic plaques (NP), largely composed of a fibrillar insoluble form of the β-amyloid peptide (Aβ), are found in the hippocampus and neocortex of Alzheimer's disease (AD) patients in association with damaged neuronal processes, increased numbers of activated astrocytes and microglia, and several proteins including the components of the proinflammatory complement system. These studies address the hypothesis that the activated complement system mediates the cellular changes that surround fibrillar Aβ deposits in NP. We report that Aβ peptides directly and independently activate the alternative complement pathway as well as the classical complement pathway; trigger the formation of covalent, ester-linked complexes of Aβ with activation products of the third complement component (C3); generate the cytokine-like C5a complement-activation fragment; and mediate formation of the proinflammatory C5b-9 membrane attack complex, in functionally active form able to insert into and permeabilize the membrane of neuronal precursor cells. These findings provide inflammation-based mechanisms to account for the presence of complement components in NP in association with damaged neurons and increased numbers of activated glial cells, and they have potential implications for the therapy of AD.
Keywords: Alzheimer's disease, amyloid, C3, complement, inflammation
We and others (1–3) have noted that the pathological changes which characterize Alzheimer's disease (AD)1 could all result from complement activation in neuritic plaques (NP), since this effector system has the ability to activate various cell types with release of cytokines and secondary mediators; to induce directed migration of these cells toward the complement activator; to alter cellular functions; and to damage cells (4, 5). Potential complement involvement in the brain is not dependent on disruption of the blood–brain barrier, since neurons, astrocytes, microglia, and oligodendrocytes synthesize most, and likely all, of the proteins of the complement system (6).
Since activation is a prerequisite for manifestation of all of the biological activities of the complement system, the β-amyloid peptide (Aβ) or another component of NP must possess the ability to activate complement in order for complement to be involved in mediating the pathologic cellular characteristics of AD. In this regard, we and others previously showed that fibrillar forms of Aβ bound the first reacting factor of the classical complement pathway (CCP), C1q (3, 7), and depleted the activity of the fourth complement component (C4) as well as whole complement activity (CH50), when incubated with human serum as a complement source in vitro (3, 7). Residues 14–26 of the collagen-like portion of the A polypeptide chain of the C1q molecule were implicated in binding fibrillar Aβ (7). Recent studies have confirmed this suggestive evidence of CCP activation by aggregated Aβ (8–12), and have also emphasized the critical role of the β-pleated structure of Aβ in mediating these effects (9, 11). Inhibition studies have implicated Aβ residues 1–11 in C1q binding (11, 12). The complement depletion, inhibition, and cleavage assays used in these various studies have provided suggestive evidence for CCP activation by fibrillar Aβ; however, as indirect assays, they are subject to other interpretations. In this context, we recently presented preliminary evidence suggesting that Aβ forms complexes with C3 after incubation of fibrillar Aβ with a complement source (9).
In the course of these studies, we found that the addition of fibrillar Aβ to a complement source led to the generation of covalent ester-linked complexes of Aβ with C3 activation fragments, providing unequivocal evidence for complement activation by Aβ, since covalent attachment of C3 activation fragments to complement activators represents a fundamental tenet of complement action. We also found that fibrillar Aβ possesses the ability to activate the alternative complement pathway (ACP) in serum, as well as in mixtures of the six purified proteins of the alternative pathway in physiologic concentrations, providing the first indication that Aβ independently activates both complement pathways. Additionally, we observed that such activation is highly specific for Aβ and completely independent of oxidative processes. These studies are described here. Finally, we also report for the first time that Aβ-mediated complement activation is biologically significant, as it leads to generation of the cytokine-like C5a complement-activation fragment, and mediates formation of the proinflammatory C5b-9 membrane attack complex (MAC), in functionally active form able to insert into and permeabilize the membranes of neuronal precursor cells.
Materials and Methods
Aβ-Mediated Complement Activation and Complement Activation ELISA Assays.
Aβ 1–40 and 1–42 (Bachem California, Torrance, CA; Bachem Bioscience, Inc., King of Prussia, PA; Quality Controlled Biochemicals, Inc., Hopkinton, MA; Anaspec, Inc., San Jose, CA; and California Peptide Research, Inc., Napa, CA) were dissolved in either 100% DMSO at 10 mg/ml, or double distilled (dd)H2O, gradually diluted in ddH2O to 2 mg/ml and then brought to 1 mg/ml in 0.1 M Tris buffer, pH 7.4. Aβ was used immediately (nonaggregated) or permitted to aggregate by incubation at room temperature for 48 h (Aβ 1–42) to 72 h (Aβ 1–40); further incubation for 2 wk did not decrease complement-activating potential. Preaggregated Aβ preparations were incubated with an equal volume of 1:5 normal human serum (NHS), complement-depleted sera (Advanced Research Technologies, Inc.), or the six purified proteins of the ACP (Advanced Research Technologies, Inc.) in physiologic ratios (13). Dilutions were in veronal buffered saline, pH 7.4, containing calcium and magnesium. Inhibition studies were carried out with preaggregated Aβ 1–42 in the presence of deferoxamine, glutathione, dimethylthiourea, catalase, or superoxide dismutase (SOD), all purchased from Sigma Chemical Co. (St. Louis, MO). After 1 h at 37°C, EDTA was added to stop further complement activation, and the samples were diluted (1:20–1:300) and added, in replicate, to microtitration wells precoated overnight (4°C) with 1 μg (100 μl) of mAb to Aβ (10D5), mAb to a C3b neoantigen (clone 129), or mAb to an iC3b neoantigen (Quidel, San Diego, CA) at pH 7.4, and then blocked (BLOTTO; Pierce Chemical Co., Rockford, IL). After 1 h at room temperature, bound Aβ–C3b/iC3b complexes were detected with rabbit Ab to C3 or to Aβ, horseradish peroxidase– conjugated anti–rabbit IgG (Kirkegaard & Perry Laboratories, Gaithersburg, MD), and ABTS (Kirkegaard & Perry Laboratories). C3 standard curves were generated with dilutions of purified iC3b (Advanced Research Technologies, Inc.) captured on wells precoated with anti-iC3b (Quidel). C5b-9 was captured on wells precoated with mAb to a C5b-9 neoantigen located in the poly C9 portion of the complex (Quidel) and detected with polyclonal goat Ab to C6 followed by horseradish peroxidase–conjugated mouse anti–goat IgG (Accurate Chemical and Science Corp., Westbury, NY) and ABTS. Values for the C5b-9 assay were back-calculated to the values in undiluted NHS. In some experiments, neuropeptide Y-porcine, urotensin I, or exendin 3 (all from California Peptide Research, Inc.); insulin B chain (Sigma Chemical Co.); amyloid precursor protein peptide (657– 676 (Bachem California); and adenovirus penton base 50-residue fragment (residues 317–366; reference 14), as well as preaggregated Aβ 1–42 preparations and monomeric Aβ 1–42, were dissolved in DMSO or ddH2O and aged at room temperature, exactly as described above for Aβ. In some studies, peptides, after dissolution as just described but diluted in Hepes-buffered NaCl at pH 7.4, were covalently cross-linked at a concentration of 200 μM with the primary amine reactive agent, bis(sulfosuccinimidyl)suberate (BS3; Pierce Chemical Co.), at a concentration of 5 mM. After 30 min, the reaction was terminated by quenching. All peptides were incubated with NHS for 1 h at 37°C. After dilution, complement activation was assessed by the conventional CH50 assay (15) by quantitating residual functional C3 using C3-depleted serum according to product instructions (Advanced Research Technologies, Inc.), or by evaluating C5b-9 formation.
Studies with Hydroxylamine.
After capture of complexes onto 10D5-coated wells, replicate wells were treated with 0.1 M Tris, pH 9.5, or 1 M hydroxylamine in 0.1 M Tris, pH 9.5, for 2 h at 37°C. After washing, remaining bound C3 was detected as described above. Residual Aβ was detected with rabbit Ab to Aβ, as described above. The formation of covalent complexes of Aβ with C3 activation products was also evaluated using the Western blotting procedure on SDS-PAGE gels. Replicate samples of aggregated Aβ 1–42 or Aβ 1–40 were incubated with human serum for 1 h at 37°C in the presence or absence of EDTA, and the reaction mixtures were then microfuged, washed in Tris buffer, and incubated for 3 h at 37°C with 0.1 M Tris at pH 7.4, 0.1 M Tris at pH 9.5, or 1 M hydroxylamine in 0.1 M Tris at pH 9.5. The samples were again microfuged, and washed with Tris at pH 7.4 followed by the same buffer containing 0.1% SDS. The samples were then subjected to SDS-PAGE under nonreducing conditions and electroblotted, and bands were detected with rabbit Ab to C3 followed by goat anti–rabbit IgG (Kirkegaard & Perry Laboratories) and the SuperSignal system (Pierce Chemical Co.). After stripping, Aβ was detected with 6E10 mouse Ab to Aβ (Senetek PLC, St. Louis, MO) followed by goat anti–mouse IgG (Kirkegaard & Perry Laboratories) and the SuperSignal system. In some experiments, electroblotted gels were reacted first with rabbit Ab to Aβ (generated in this laboratory) or with 6E10 mAb to Aβ (Senetek PLC) followed by goat anti–rabbit IgG (Kirkegaard & Perry Laboratories) or goat anti–mouse IgG (Kirkegaard & Perry Laboratories), stripped, and reacted with mAb anti-iC3b (Quidel) or rabbit Ab to C3 (generated in this laboratory), followed by goat anti–mouse IgG or goat anti–rabbit IgG. Quantitation was with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Mass Spectroscopy.
After solubilization in 70% formic acid, samples were analyzed by MALDI spectroscopy (Perseptive Voyager ELITE; Perseptive Biosystems, Inc., Framingham, MA).
C5a.
C5a (and C5a des-Arg) was detected in diluted samples with the Biotrak radioimmunoassay kit (Amersham Corp., Arlington Heights, IL); the samples were subjected to acid precipitation before analysis (16). Values were back-calculated to the concentrations in undiluted NHS.
C5b-9 Membrane Insertion.
Ntera2/D1 (NT2) cells (Stratagene Inc., La Jolla, CA) were grown to subconfluence, released with nonenzymatic cell dissociation solution (GIBCO BRL, Gaithersburg, MD), washed, and resuspended (2 × 107 cells/ml). NT2 cells (100 μl) were incubated with 50 μl NHS in the presence or absence of EDTA and 50 μl preaggregated Aβ. After 15 min at 37°C, the MAC was detected with rabbit Ab (Advanced Research Technologies, Inc.) or mAb (Quidel) to C5b-9 neoantigens, followed by FITC anti–rabbit or –mouse Ig and propidium iodide. Readings were performed on a FACScan® and analyzed with CellQuest software (Becton Dickinson, San Jose, CA).
Results and Discussion
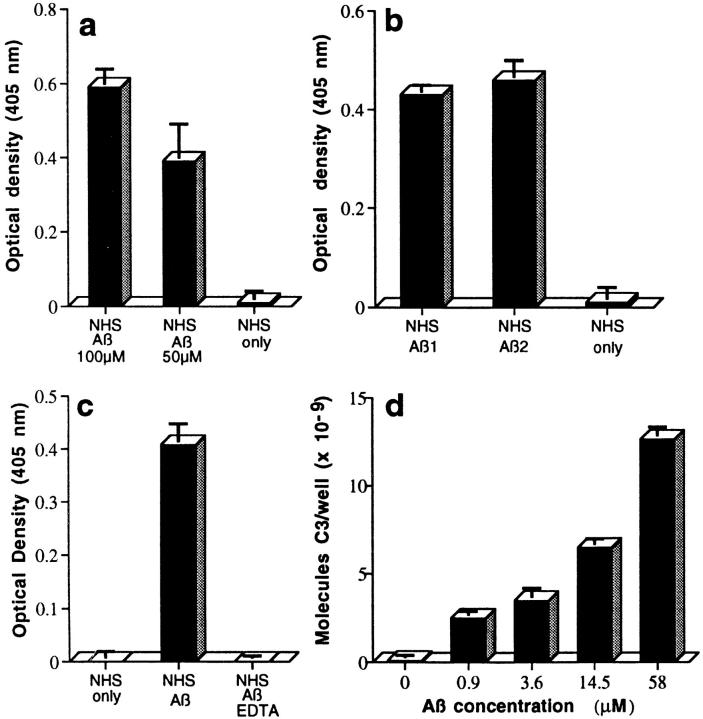
Sandwich-type ELISAs showed that complexes containing Aβ and C3b/iC3b were generated in NHS, as a complement source, after incubation with aggregated Aβ 1–42. Complexes were demonstrable after capture with mAbs to activation-dependent neoantigens in the first (C3b) or second (iC3b) C3 cleavage products and detection with rabbit Ab to Aβ (Fig. 1, a and b), as well as after capture with mAb to Aβ and detection with rabbit Ab to C3 (Fig. 1 c) or C3d (not shown). EDTA, which blocks complement activation by chelating calcium and magnesium, prevented complex formation (Fig. 1 c). ELISAs in which complexes were captured with mAb to Aβ and detected with Ab to C3 were used for most of the studies, since such ELISAs permitted quantitation by reference to included standard curves generated with purified C3 captured on wells coated with mAb to C3 and detected with rabbit Ab to C3 (Fig. 1 d). Complement activation was detectable to ∼1 μM Aβ 1–42 (Fig. 1 d). 10 different preaggregated Aβ 1–40 and 20 different preaggregated Aβ 1–42 preparations from 5 manufacturers generated such complexes. Aβ 1–42 was generally 5–10-fold more active than Aβ 1–40 in this regard. The cation-dependent formation of Aβ–C3b/iC3b complexes after incubation of aggregated Aβ with NHS provides unequivocal evidence for complement activation by aggregated Aβ.
Figure 1.
ELISA demonstration of complement-mediated formation of complexes of Aβ with C3 activation fragments (a– d ). Complexes were captured, detected, and quantitated as described in Materials and Methods. NHS or purified ACP proteins only do not contain Aβ. (a) Preaggregated Aβ 1–42 (100 μM and 50 μM) was incubated in NHS, captured with mAb to C3b, and detected with rabbit Ab to Aβ. (b) Two preaggregated Aβ 1–42 preparations (Aβ 1 and Aβ 2, at 20 μM) were incubated in NHS, captured with mAb to iC3b, and detected with rabbit Ab to Aβ. (c) Preaggregated Aβ 1–42 (58 μM) was incubated in NHS, or in NHS containing 10 mM EDTA, captured with mAb to Aβ, and detected with rabbit Ab to C3. (d ) Preaggregated Aβ 1–42 was incubated in NHS at the indicated final concentrations, captured with mAb to Aβ, and detected with rabbit Ab to C3. (e) Preaggregated Aβ 1–42 (58 μM) was incubated in NHS, factor B–depleted NHS (B-dpl ), or C1q-depleted NHS (C1q-dpl ), captured with mAb to Aβ, and detected with rabbit Ab to C3. (f ) Preaggregated Aβ 1–42 (58 μM) was incubated with the six purified ACP proteins (PAP) or NHS, captured with mAb to Aβ, and detected with rabbit Ab to C3. Background levels obtained in EDTA controls containing Aβ and purified ACP proteins or NHS, in the various experiments described above, were subtracted. ( g) Specificity of complement activation. Preaggregated Aβ preparations (20 μM) and the same concentrations of monomeric Aβ (mono), insulin B chain (Ins.B), neuropeptide Y-porcine (NPY), urotensin I (Uro.), exendin 3 (Exen), amyloid precursor peptide 657–676 (APP-pep.), and adenovirus penton base 50-residue peptide (PB50) were incubated with NHS. Complement activation was assessed by the CH50 method. Correlation coefficients for the CH50 determinations ranged from 0.995 to 1.000.
Aβ–C3b/iC3b complex formation was evident after incubation of aggregated Aβ 1–42 with NHS lacking factor B, an essential component of the ACP (Fig. 1 e); such sera contain an intact CCP, but do not permit ACP activation. A significant reduction in complex formation was also evident in C1q-depleted serum compared with NHS (Fig. 1 e). These data show that the CCP mediates complex formation by aggregated Aβ, findings that were anticipated from the results of the complement depletion assays described earlier. Unexpectedly, however, the ACP also mediated the formation of complexes, since they were also generated after the addition of aggregated Aβ 1–42 to NHS lacking factor C1q, and complex formation was reduced in factor B–depleted serum compared with NHS (Fig. 1 e), a result replicated in four additional experiments with different Aβ 1–42 preparations. The ability of Aβ 1–42 to activate the ACP was confirmed in three studies in which preaggregated Aβ 1–42 was incubated with a mixture of the six purified proteins of the ACP (factors B, D, H, and I, properdin, and C3) in physiological ratios (reference 13; Fig. 1 f ). These data document the ability of aggregated Aβ not only to activate the CCP, but also to independently activate the ACP. This is the first indication that aggregated Aβ activates the ACP; it had been presumed that complement activation by Aβ was exclusively via the CCP because of the absence of ACP components (factor B and properdin) in NP (1, 17, 18). The failure to detect ACP components in NP may be due to the extreme lability of the ACP C3 convertase.
Multiple different aggregated Aβ 1–42 preparations activated complement, as determined by the classical CH50 complement consumption technique (Fig. 1 g). The aging procedure used to aggregate Aβ generates β-pleated fibrils (9). Nonfibrillar “amorphous” aggregates of Aβ are devoid of complement-activating ability (9). In contrast to the aggregated preparations, Aβ used immediately after dissolution had limited ability to activate complement (Fig. 1 g). These data document the important role of fibril formation for complement activation by Aβ in vitro. Amylin, another peptide which spontaneously forms β-pleated fibrils, was also tested for complement-activating ability in these studies. The 37-residue amylin polypeptide represents the principal constituent of the amyloid deposits in type 2 diabetes. On SDS-PAGE gels, aged amylin migrated primarily as large SDS-insoluble stained bands. However, this fibrillar peptide did not significantly activate complement at a concentration of 100 μM (7% CH50 consumption).
The specificity of complement activation by Aβ was also evaluated by determining whether complement was activated by other small peptides (20–50 amino acids) containing multiple residues able to mediate covalent linkage to the glutamate residue of the hydrolyzed thioester of C3 (serine, tyrosine, threonine, lysine) and expressing similar overall charge to Aβ. All of the peptides were processed and aged in the same manner as Aβ. None of the peptides, including the insulin B chain (30 residues), neuropeptide Y-porcine (36 residues), urotensin I (41 residues), exendin 3 (39 residues), amyloid precursor peptide 657–676 (20 residues), and the adenovirus penton base fragment (50 residues) significantly activated complement at a concentration of 20 μM, as assessed by the classical CH50 technique (Fig. 1 g). The peptides also showed little or no ability to activate complement in other assays, including the ability to deplete residual functional C3 and form the SC5b-9 complex (not shown). To determine whether peptide aggregation would increase complement-activating ability, urotensin I, neuropeptide Y-porcine, and exendin 3 were covalently cross-linked with the primary amine reactive reagent BS3 before evaluating their ability to activate complement by the CH50 technique. Cross-linked urotensin I and neuropeptide Y-porcine gave a ladder of Coomassie-stained bands on SDS-PAGE analyses, but exendin 3 gave no stained bands, possibly due to the formation of very large aggregates. These three cross-linked peptides did not significantly activate complement (<10% CH50 depletion) at a concentration of 20 μM. In another study, cross-linked urotensin I exhibited 7% CH50 consumption at a concentration of 100 μM, whereas aggregated Aβ 1–42 showed 45% consumption. These data cumulatively demonstrate the marked specificity of complement activation by fibrillar Aβ.
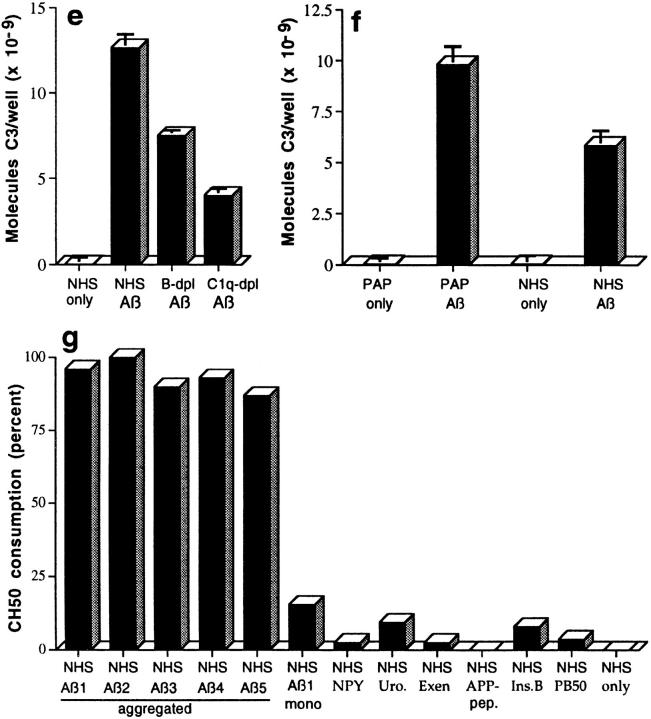
C3 preferentially binds to activators via ester bonds, although amide linkage has been described (19, 20); such bonds form between the reactive γ-carbonyl group of the glutamate residue of the activation-cleaved internal thioester bond in C3, and hydroxyl (ester) or amino (amide) groups on the activator (19). To evaluate possible ester linkage, complexes were captured with mAb to Aβ and incubated with Tris buffer containing 1 M hydroxylamine at pH 9.5 for 2 h at 37°C, a treatment which disrupts ester but not amide bonds (19, 20). Approximately 50% of the bound C3, but none of the Aβ, was removed from the captured complexes by this treatment (Fig. 2, a and b), a result replicated in two additional studies with Aβ 1–40 and 1–42.
Figure 2.
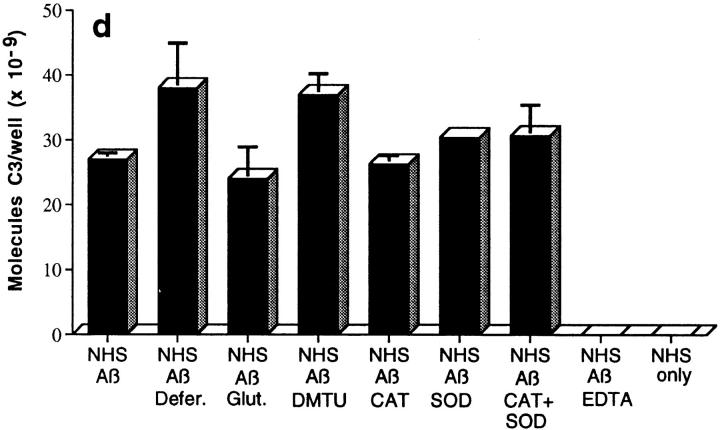
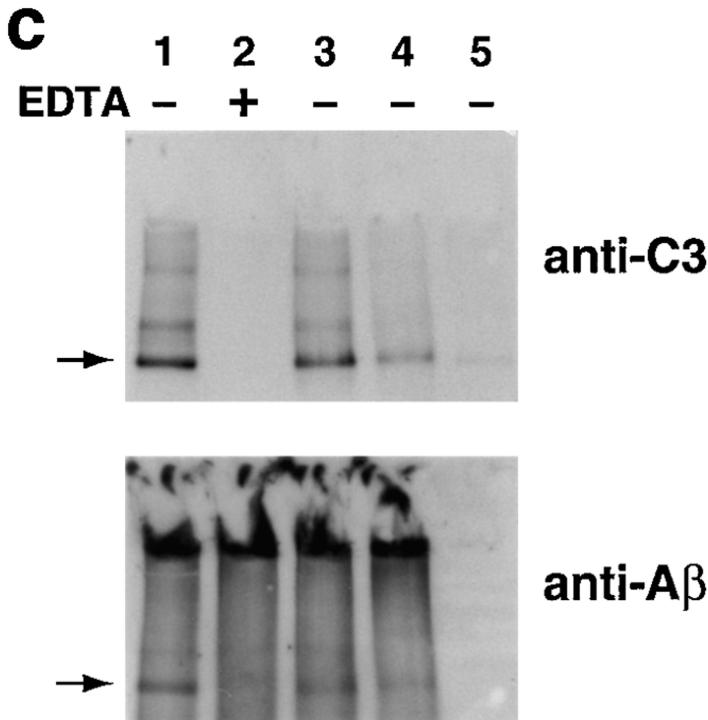
Assessment of bonds mediating binding of Aβ to C3 activation fragments. (a) Preaggregated Aβ 1–42 (25 μM) was incubated in NHS, and complexes were captured on 10D5-coated wells; NHS only does not contain Aβ. Replicate samples were treated with pH 9.5 buffer, or with the same buffer containing 1 M hydroxylamine (NH2OH ), and remaining bound C3 was then detected and quantitated as described in Materials and Methods. The control containing Aβ, NHS, and EDTA has been subtracted. (b) Replicate wells subjected to treatment with the pH 9.5 buffer or hydroxylamine were evaluated for residual bound Aβ as described in Materials and Methods. (c) Preaggregated Aβ 1–40 was incubated in NHS in the presence or absence of EDTA; lane 5 contains NHS but no Aβ. After centrifugation and washing, fibrillar Aβ pellets were incubated with pH 7.4 buffer (lanes 1 and 2), pH 9.5 buffer (lane 3), or 1 M hydroxylamine in pH 9.5 buffer (lane 4). After further washing, samples were subjected to SDS-PAGE under nonreducing conditions followed by blotting for the presence of C3 and, after stripping, for Aβ. Arrow, The C3 band at ∼180 kD. (d) Preaggregated Aβ 1–42 (50 μM) was incubated in NHS alone, and in the presence of deferoxamine (Defer.; 1 mM), glutathione (Glut.; 1 mM), dimethylthiourea (DMTU; 30 mM), catalase (CAT; 2 × 104 U/ml), SOD (10 μM), or catalase plus SOD, and the Aβ complexes with C3 activation fragments were then detected as described in Materials and Methods.
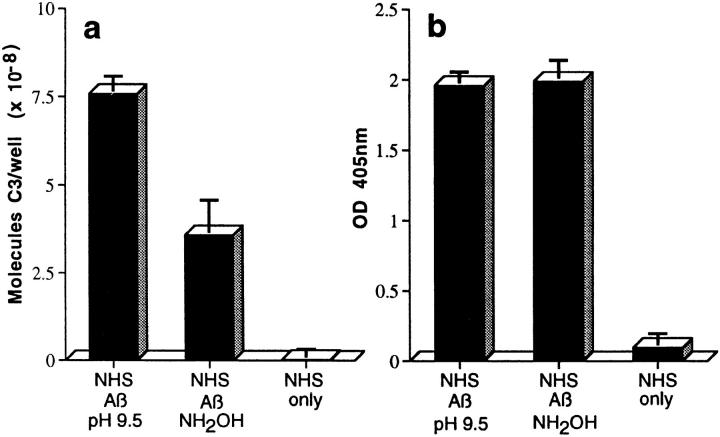
The formation of covalent complexes of Aβ with C3 activation products was also independently demonstrated using a Western blotting approach. In these studies, aggregated Aβ 1–40 was incubated with serum, and the complexes of insoluble fibrillar Aβ with C3 activation fragments were then sedimented, washed, and incubated with either 1 M hydroxylamine at pH 9.5 or control buffers. After washing, a prominent band with a molecular mass of ∼180 kD, the molecular mass of C3, as well as several higher molecular mass bands, were detected with Ab to C3 (Fig. 2 c). After stripping, the same bands were also found to react with Ab to Aβ, although the gels were darker due to the presence of large amounts of aggregated Aβ (Fig. 2 c). Bands of the same molecular masses reactive with Abs to both Aβ and C3 were also observed when the blotting studies were performed in the reverse direction, i.e., blotting first with either mAb or polyclonal Ab to Aβ followed, after stripping, by blotting with rabbit Ab or mAb to C3 (not shown). The 180-kD band and the larger bands, which contain both C3 and Aβ, undoubtedly represent complexes of Aβ monomers with C3b monomers and oligomers, since they were not evident in the reactions carried out in the presence of EDTA or in the absence of fibrillar Aβ (Fig. 2 c).
C3 was also detected in the large Aβ aggregates on the top of the gels (except for the EDTA lane) on longer exposure (not shown). The lesser reactivity of C3 in the larger Aβ aggregates at the top of the gels, compared with the C3 monomers and oligomers within the gels, indicates that not all Aβ monomers bear a molecule of C3b; this is not surprising, since Aβ is in large aggregates and, in addition, in molar excess over C3. It may also be that Aβ molecules bearing covalently bound C3b dissociate from the aggregates, in analogy to the dissociation of immune complexes by the covalent binding of C3b (21, 22).
Hydroxylamine treatment disrupted approximately half of the complexes (Fig. 2 c). Quantitative scanning of the C3 Western blot showed that the treatment with 1 M hydroxylamine at pH 9.5 removed 42% of the bound C3 compared with the pH 9.5 control; the pH 9.5 buffer treatment removed only trivial amounts (5.1%) of the bound C3 compared with the pH 7.4 treatment. The Aβ Western blot could not be satisfactorily scanned due to the large background, but visual inspection reveals the same pattern (Fig. 2 c). Identical results were obtained with Aβ 1–42 (not shown). These two independent assay systems both show that ester bonds, in part, mediate covalent attachment of C3 activation fragments to Aβ. Aβ 1–42 contains two serines, at positions 8 and 26, and a tyrosine, at position 10, which could mediate ester linkages with C3 activation fragments.
With regard to the bond(s) responsible for the nonester-linked Aβ–C3b/iC3b complexes, Aβ alone has been reported to generate free radicals (23) upon incubation in aqueous solution, and oxidative processes have been associated with Aβ denaturation, fragmentation, and oxidation (24, 25). Because of the potential relevance of these processes to the formation of complexes of Aβ with C3 activation fragments, Aβ 1–42 was assessed by MALDI mass spectroscopy after aging from 0 to 10 d. Aggregates are not detected in these assays, since the samples are dissolved in 70% formic acid for mass spectroscopic analysis. The molecular mass of the major peak in the various samples ranged from 4510 to 4514, and no other peaks were present, ruling out significant oxidation, fragmentation, and covalent cross-linking of Aβ. To determine whether oxidative processes mediated the formation of complexes of Aβ with C3 activation fragments, complement activation was carried out in the presence of deferoxamine, glutathione, dimethylthiourea, catalase, SOD, and catalase plus SOD. Since none of these antioxidants or free radical scavengers inhibited the formation of or interfered with the detection of complexes (Fig. 2 d), it is unlikely that free radical–mediated or oxidative processes are involved in the formation of complexes of Aβ with C3 activation fragments. In all likelihood, amide bonds are responsible for the remaining Aβ–C3b/iC3b complexes. Aβ 1–42 contains two lysine residues, at positions 16 and 28, which could mediate such linkages.
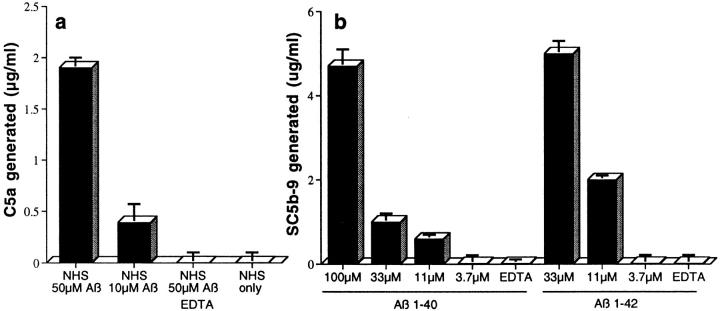
Additional studies showed that Aβ triggered activation of the terminal, proinflammatory portion of the complement-reaction sequence in NHS. C5a, a cytokine-like activation cleavage product of C5 with numerous biological properties, was efficiently generated by aggregated Aβ 1–42 in NHS, as determined by a specific radioimmunoassay which detects C5a and C5a des-Arg (lacking the COOH-terminal arginine residue) (reference 26; Fig. 3 a). A sandwich ELISA in which an mAb to a C5b-9 neoantigen located in poly C9 served as the capture Ab, and polyclonal Ab to C6 served as the detection Ab, showed that Aβ-mediated complement activation led to formation of the C5b-9 complex (Fig. 3 b). This ELISA detects C5b-9 as well as SC5b-9 complexes; the latter are formed in NHS in the absence of cells, as a consequence of the binding of S protein, a complement control protein, to the complex. Aβ 1–42 was generally more efficient in generating C5b-9 than Aβ 1–40 (Fig. 3 b). In contrast, another group recently reported that Aβ-mediated complement activation does not lead to generation of the C5b-9 complex (10). The reason(s) for their failure to demonstrate C5b-9 formation is not known. One possibility is the well-known variability in the properties of different Aβ preparations. In this regard, we have observed more variability in the ability of various Aβ 1–40 and Aβ 1–42 preparations to trigger C5b-9 complex formation, than in their ability to generate Aβ–C3b and Aβ–iC3b complexes. Other explanations could lie in slight differences in experimental conditions. For example, their C5b-9 formation experiments were carried out in NHS diluted 1:10 in phosphate-buffered NaCl; this combination provides suboptimal concentrations of calcium and magnesium, which are required for CCP and ACP activation. In this regard, we obtained 10-fold higher levels of SC5b-9 formation than they obtained with 1 μM aggregated IgG in their control studies (not shown).
Figure 3.
Aβ-mediated complement activation generates C5a and the MAC. (a) Preaggregated Aβ 1–42 was incubated in NHS, and C5a generation was then quantitated as described in Materials and Methods. (b) C5b-9 was quantitated after NHS was incubated with varying concentrations of preaggregated Aβ 1–42 or 1–40. SC5b-9 formation was quantitated as described in Materials and Methods.
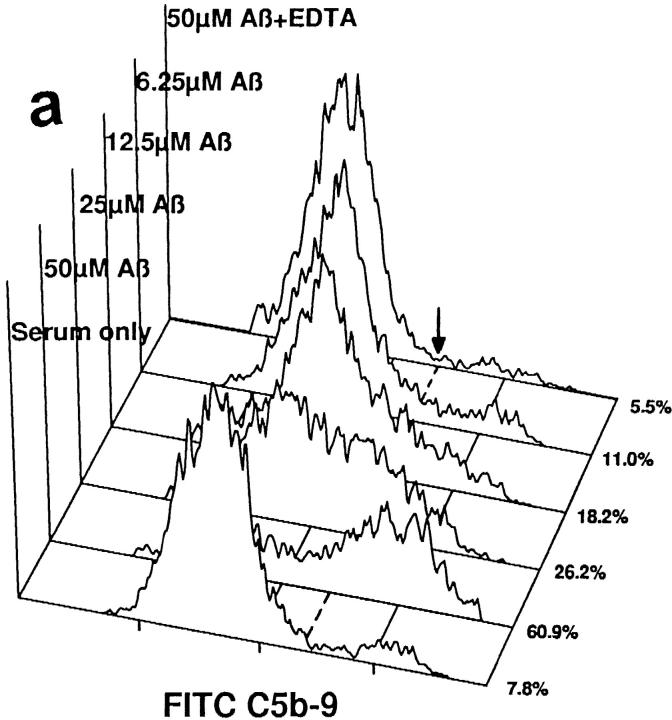
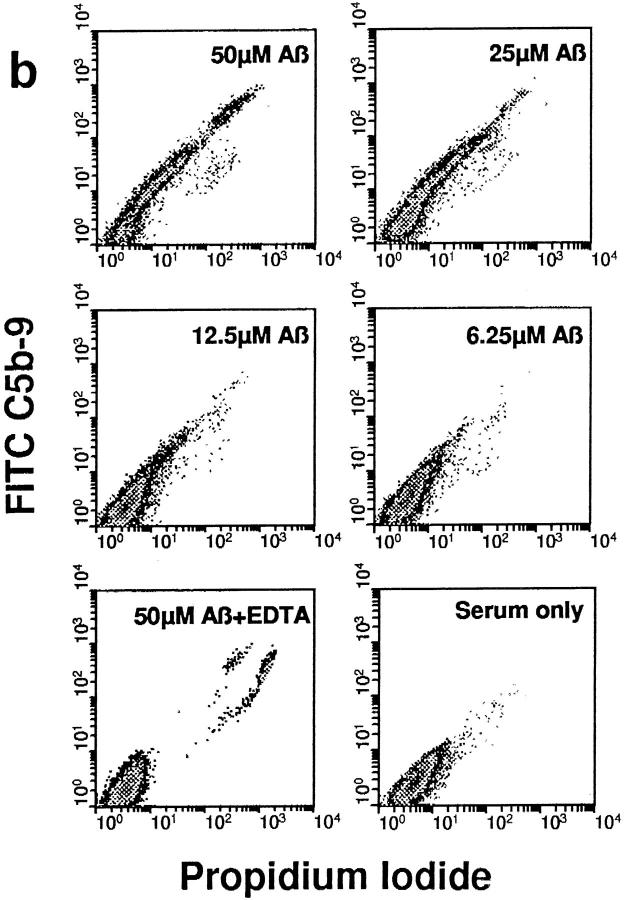
The C5b-9 complex generated by Aβ 1–42-mediated complement activation was able to insert into the membranes of NT2 cells, a committed neuronal precursor cell line, when such cells were included in reaction mixtures with aggregated Aβ and NHS (Fig. 4 a). Depicted are flow cytometric analyses with rabbit Ab to activation-specific neoantigens in the C5b-9 MAC. C5b-9 membrane insertion was likely proportional to the extent of complement activation, since it was dependent on the concentration of Aβ 1–42. mAb to C5b-9 neoantigens gave the same result (not shown). Identical Aβ 1–40 concentrations mediated lower levels of C5b-9 membrane insertion (not shown), probably because of the significantly lower levels of C5b-9 formation with Aβ 1–40. NT2 cells and other neuronal cell lines are resistant to complement-dependent cytolysis, likely because of the presence of CD59 (27), a complement regulatory protein, a finding confirmed here. Nevertheless, C5b-9 insertion into NT2 cell membranes mediated an increase in the permeability of the cells to propidium iodide that was dependent on the concentration of Aβ 1–42 (Fig. 4 b). These data indicate that C5b-9 generated by Aβ-mediated complement activation is functionally competent, since it inserts into the membranes of neuronal precursor cells and renders them permeable to small molecules.
Figure 4.
C5b-9 generated by Aβ 1–42-mediated complement activation is functionally active. (a) NT2 cells were incubated with preaggregated Aβ 1–42 at the designated concentrations in NHS, or in NHS containing 10 mM EDTA; serum only lacks Aβ. Flow cytometric analyses with a rabbit Ab to C5b-9 neoantigens are shown. Numbers (right), Percentage of C5b-9+ cells, as determined by their relationship to the marker (arrow, dashed line). (b) Density plot analyses of propidium iodide and C5b-9 (FITC C5b-9) reactivities are shown. For clarity, only live cells are depicted.
Thus, Aβ directly and independently activates the ACP as well as the CCP, leading to the formation of covalent Aβ–C3b and Aβ–iC3b complexes; generates C5a; and mediates assembly of functionally active C5b-9 complexes in vitro. These findings have potential implications for understanding the mechanisms which lead to continuing neuronal damage and altered glial functions in the vicinity of NP, and thus to the progression of AD. First, they provide an explanation for the association of bound C3 with Aβ in NP (1–3), since covalently bound C3b molecules in NP would remain bound and provide a nidus for chronic complement activation. Second, C5a generated by Aβ-mediated complement activation could be responsible for the increased numbers of activated astrocytes and microglia around NP compared with diffuse Aβ plaques (28), since these cells possess C5a receptors and are activated and migrate in response to C5a (6, 29–31). C5a could also trigger the release of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α) from glial cells, as it does from other cell types (26, 32); proinflammatory cytokines are increased in the AD brain (2, 28, 33). These cytokines could further activate glial cells and alter neuronal and glial functions (28, 32). Third, incoming activated glial cells could bind and remain adherent, via their complement receptors, to C3 activation fragments attached to Aβ (6). Fourth, C5b-9 insertion into cell membranes provides an explanation for the association of this complex with dystrophic neurites in NP (2, 3). Although not likely to be directly cytotoxic for neurons, since they bear CD59 (6, 34), C5b-9 as well as C5b-7 and C5b-8 complexes could alter neuronal functional properties over time by chronic low-level triggering of various cellular signaling pathways (35). If this inflammation-based scenario is verified, complement inhibitors should be evaluated for use in AD. Such inhibitors would need to pass the blood–brain barrier, target both complement activation pathways, and prevent C5b-9 activation.
Acknowledgments
We thank T. Hugli, D. Isenman, J. Rogers, and S. Webster for helpful discussions, G. Nemerow for PB50, and Athena Neurosciences, Inc. (South San Francisco, CA), for 10D5. We also thank Todd S. Bixby for his expert technical assistance.
This work was supported by National Institutes of Health grant NS-34682, and grant SFP-1141 from Novartis.
Abbreviations used in this paper
- ACP
alternative complement pathway
- AD
Alzheimer's disease
- Aβ
β-amyloid peptide
- CCP
classical complement pathway
- dd
double distilled
- MAC
membrane attack complex
- NP
neuritic plaques
- NHS
normal human serum
- SOD
superoxide dismutase
References
- 1.Eikelenboom P, Stam FC. Immunoglobulins and complement factors in senile plaques. Acta Neuropathol. 1982;57:239–242. doi: 10.1007/BF00685397. [DOI] [PubMed] [Google Scholar]
- 2.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 3.Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, Civin WH, Brachova L, Bradt B, Ward P, Lieberburg I. Complement activation by β-amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, N.R. 1998. Biology of the complement system. In Inflammation: Basic Principles and Clinical Correlates. J. Gallin and R. Snyderman, editors. Lippincott-Raven Publishers, Philadelphia. In press.
- 6.Morgan BP, Gasque P. Expression of complement in the brain: role in health and disease. Immunol Today. 1996;17:461–466. doi: 10.1016/0167-5699(96)20028-f. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Burdick D, Glabe CG, Cotman CW, Tenner AJ. β-Amyloid activates complement by binding to a specific region of the collagen-like domain of the C1q A chain. J Immunol. 1994;152:5050–5059. [PubMed] [Google Scholar]
- 8.Chen S, Frederickson RCA, Brunden KR. Neuroglial-mediated immunoinflammatory responses in Alzheimer's disease: complement activation and therapeutic approaches. Neurobiol Aging. 1996;17:781–787. doi: 10.1016/0197-4580(96)00103-0. [DOI] [PubMed] [Google Scholar]
- 9.Webster S, Bradt B, Rogers J, Cooper NR. Aggregation state-dependent activation of the classical complement pathway by the amyloid β peptide (Aβ) J Neurochem. 1997;69:388–398. doi: 10.1046/j.1471-4159.1997.69010388.x. [DOI] [PubMed] [Google Scholar]
- 10.Cadman ED, Puttfarcken PS. β-Amyloid peptides initiate the complement cascade without producing a comparable effect on the terminal pathway in vitro. . Exp Neurol. 1997;146:388–394. doi: 10.1006/exnr.1997.6540. [DOI] [PubMed] [Google Scholar]
- 11.Webster S, Bonnell B, Rogers J. Charge-based binding of complement component C1q to the Alzheimer amyloid β-peptide. Am J Pathol. 1997;150:1531–1536. [PMC free article] [PubMed] [Google Scholar]
- 12.Velazquez P, Cribbs DH, Poulos TL, Tenner AJ. Aspartate residue 7 in amyloid β-protein is critical for classical complement pathway activation: implications for Alzheimer's disease pathogenesis. Nat Med. 1997;3:77–79. doi: 10.1038/nm0197-77. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber RD, Pangburn MK, Lesavre PH, Müller-Eberhard HJ. Initiation of the alternative pathway of complement: recognition of activators by bound C3b and assembly of the entire pathway from six isolated proteins. Proc Natl Acad Sci USA. 1978;75:3948–3952. doi: 10.1073/pnas.75.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins αvβ3 and αvβ5promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 15.Mayer, M.M. 1961. Complement and complement fixation. In Experimental Immunochemistry. E.A. Kabat and M.M. Mayer, editors. Charles C. Thomas Publisher, Springfield, IL. 133–241.
- 16.Wagner JL, Hugli TE. Radioimmunoassay for anaphylatoxins: a sensitive method for determining complement activation products in biological fluids. Anal Biochem. 1984;136:75–88. doi: 10.1016/0003-2697(84)90308-7. [DOI] [PubMed] [Google Scholar]
- 17.McGeer PL, Akiyama H, Itagaki S, McGeer EG. Immune system response in Alzheimer's disease. Can J Neurol Sci. 1989;16:516–527. doi: 10.1017/s0317167100029863. [DOI] [PubMed] [Google Scholar]
- 18.McGeer PL, Akiyama H, Itagaki S, McGeer EG. Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci Lett. 1989;107:341–346. doi: 10.1016/0304-3940(89)90843-4. [DOI] [PubMed] [Google Scholar]
- 19.Law SKA, Lichtenberg NA, Levine RP. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979;123:1388–1394. [PubMed] [Google Scholar]
- 20.Gadd KJ, Reid KBM. The binding of complement component C3 to antibody-antigen aggregates after activation of the alternative pathway in human serum. Biochem J. 1981;195:471–480. doi: 10.1042/bj1950471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller GW, Nussenzweig V. A new complement function: solubilization of antigen-antibody aggregates. Proc Natl Acad Sci USA. 1975;72:418–422. doi: 10.1073/pnas.72.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita T, Takata Y, Tamura N. Solubilization of immune precipitates by six isolated alternative pathway proteins. J Exp Med. 1981;154:1743–1751. doi: 10.1084/jem.154.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris ME, Hensley K, Butterfield DA, Leedle RA, Carney JM. Direct evidence of oxidative injury produced by the Alzheimer's β-amyloid peptide (1-40) in cultured hippocampal neurons. Exp Neurol. 1995;131:193–202. doi: 10.1016/0014-4886(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 24.Dyrks T, Dyrks E, Hartmann T, Masters C, Beyreuther K. Amyloidogenicity of βA4 and βA4-bearing amyloid protein precursor fragments by metal-catalyzed oxidation. J Biol Chem. 1992;267:18210–18217. [PubMed] [Google Scholar]
- 25.Hensley K, Butterfield DA, Hall N, Cole P, Subramanian R, Mark R, Mattson MP, Markesbery WR, Harris ME, Aksenov M, et al. Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer's disease-associated amyloid beta peptide. Ann NY Acad Sci. 1996;786:120–134. doi: 10.1111/j.1749-6632.1996.tb39057.x. [DOI] [PubMed] [Google Scholar]
- 26.Hugli TE. Structure and function of the anaphylatoxins. Springer Semin Immunopathol. 1984;7:193–219. doi: 10.1007/BF01893020. [DOI] [PubMed] [Google Scholar]
- 27.Shen Y, Halperin JA, Lee C-M. Complement-mediated neurotoxicity is regulated by homologous restriction. Brain Res. 1995;671:282–292. doi: 10.1016/0006-8993(94)01264-i. [DOI] [PubMed] [Google Scholar]
- 28.Griffin WST, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer's disease: significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Yao J, Harvath L, Gilbert DL, Colton CA. Chemotaxis by CNS macrophage, the microglia. J Neurosci Res. 1990;27:36–42. doi: 10.1002/jnr.490270106. [DOI] [PubMed] [Google Scholar]
- 30.Lacy M, Jones J, Whittemore SR, Haviland DL, Wetsel RA, Barnum SR. Expression of the receptors for the C5a anaphylatoxin, interleukin-8 and FMLP by human astrocytes and microglia. J Neuroimmunol. 1995;61:71–78. doi: 10.1016/0165-5728(95)00075-d. [DOI] [PubMed] [Google Scholar]
- 31.Ilschner S, Nolte C, Kettenmann H. Complement factor C5a and epidermal growth factor trigger the activation of outward potassium currents in cultured murine microglia. Neuroscience. 1996;73:1109–1120. doi: 10.1016/0306-4522(96)00107-8. [DOI] [PubMed] [Google Scholar]
- 32.Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992;263:C1–C16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- 33.Selkoe DJ. Alzheimer's disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994;53:438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Vedeler C, Ulvestad E, Bjorge L, Conti G, Williams K, Mork S, Matre R. The expression of CD59 in normal human nervous tissue. Immunology. 1994;82:542–547. [PMC free article] [PubMed] [Google Scholar]
- 35.Rus HG, Niculescu F, Shin ML. Sublytic complement attack induces cell cycle in oligodendrocytes. J Immunol. 1996;156:4892–4900. [PubMed] [Google Scholar]