Abstract
CC chemokines produced by CD8+ T cells are known to act as HIV-suppressive factors. We studied the possible role of these chemokines in HIV-1–specific killing of target cells. We found that the activity of cytotoxic T lymphocytes (CTLs) in CTL lines or freshly isolated peripheral blood mononuclear cells from HIV-1–infected individuals is markedly enhanced by RANTES (regulated on activation, normal T cell expressed and secreted) and virtually abolished by an antibody neutralizing RANTES or the RANTES receptor antagonist RANTES(9-68). Lysis was mediated by CD8+ major histocompatibility complex class I–restricted T cells and was obtained with target cells expressing epitopes of the HIV-1LAI proteins Gag, Pol, Env, and Nef. The cytolytic activity observed in the presence or absence of added RANTES could be abolished by pretreatment of the CTLs with pertussis toxin, indicating that the effect is mediated by a G protein–coupled receptor. The chemokines monocyte chemotactic protein (MCP)-3, MCP-4, and eotaxin acted like RANTES, whereas macrophage inflammatory protein (MIP)-1α, MIP-1β, MCP-1, and stromal cell–derived factor 1 were inactive, suggesting a role for the eotaxin receptor, CCR3, and ruling out the involvement of CCR1, CCR2, CCR5, and CXCR4. CTL activity was abrogated by an antibody that blocks CCR3, further indicating that specific lysis is triggered via this chemokine receptor. These observations reveal a novel mechanism for the induction of HIV-1–specific cytotoxicity that depends on RANTES acting via CCR3.
Keywords: CD8+ T lymphocytes, monocyte chemotactic protein 3, monocyte chemotactic protein 4, eotaxin, RANTES antagonist
irus-specific cytolysis is considered a major defense function of CD8+ T lymphocytes that eliminate productively infected cells and control the viral load during the asymptomatic stages after HIV infection (1). HIV-1–specific CTLs have been studied extensively, and it has been shown that they are present in high numbers in the blood and and tissues (2–4). However, specific CTL activity declines with the development of AIDS (5, 6). CD8+ T cells are an important source of chemokines that were shown to inhibit HIV entry into CD4-bearing cells and to limit infection in vitro (7) and in vivo (8). On the other hand, chemokines are potent attractants of T lymphocytes and NK cells (9) and could, therefore, enhance antiviral effector functions by stimulating cytotoxicity. This action would differ from the well-established role of some chemokines as competitors for HIV coreceptor usage (10).
Monitoring of specific cytolytic activity is a way to assess the antiviral defense of HIV-1–infected individuals (11, 12). Activated, CD8+ HIV-1–specific CTLs are known to produce interferons and chemokines (13), but other factors or mechanisms may contribute to their overall HIV-1–suppressive activity (7, 14). We have now studied the effects of chemokines on PBMCs and PBMC-derived, HIV-1–specific CTL lines generated from HIV-1–infected individuals who were monitored for several years. We present data indicating that the HIV-1–specific lytic activity of patient-derived CTLs is enhanced by RANTES (regulated on activation, normal T cell expressed and secreted), which acts on the CTLs through the chemokine receptor CCR3.
Materials and Methods
HIV-1–infected Donors.
The donors of PBMCs used to establish CTL lines and target cells were eight HLA-typed, HIV-1–infected patients from the French cohort IMMUNOCO with CD4+ T lymphocyte counts between 150 and 1,050/μl. They were recruited in 1991 and were followed for 5 yr on the basis of clinical and functional tests.
Generation of HIV-1–specific CTL Lines.
CTL lines were prepared according to established methods (11, 12). PBMCs were isolated from the venous blood of HIV-1–infected individuals by centrifugation in Ficoll-Hypaque density gradients (Eurobio, Les Ulis, France) and were activated overnight with 1 μg/ml PHA (HA-16; Murex Diagnostic Ltd., Dartford, England). Polyspecific CTL lines were generated by coculture with irradiated autologous PHA blasts, and CTL lines specific for the Pol epitope of HIV-1LAI (amino acid 476–484) by coculture with irradiated autologous PHA blasts that were pre-incubated for 2 h at 37°C with 10 μM of the synthetic peptide 476–484 (15). The appropriate irradiated PHA blasts were added every tenth day, and the cocultures were performed in the presence of recombinant human IL-2 (20 U/ml; Boehringer Mannheim, Germany) supplied every third day. The CTLs were used for the cytotoxicity assays after 20 d of coculture.
Target Cells.
Three types of target cells were used: autologous EBV-transformed lymphoblastoid B cell lines infected with vaccinia virus expressing the HIV-1LAI proteins Gag, Pol, or Env; autologous EBV-transformed lymphoblastoid B cell lines pulsed with the synthetic peptide 476–484 corresponding to the Pol epitope of HIV-1LAI; and the human plasmocytoid cell line Hmy cotransfected with the HLA-A2 and the HIV-1LAI nef genes. The EBV-transformed lymphoblastoid B cell lines were established for each CTL donor. Infection with wild-type vaccinia virus or with vaccinia virus expressing the HIV-1LAI proteins Gag, Pol, or Env (Transgene, Strasbourg, France) was performed for 18 h at 37°C at a multiplicity of 5 PFU/cell. Pulsing was performed with 10 μM of the synthetic peptide 476–484 for 2 h at 37°C using EBV-transformed cells already labeled with 51Cr (12). EBV-transformed cells infected with wild-type vaccinia virus or not pulsed with the synthetic Pol peptide and Hmy cells transfected with the HLA-A2 gene alone were used as the corresponding controls. The Hmy cell lines transfected with the HLA-A2 or HLA-A2 and the HIV-1LAI nef genes (16, 17) were provided by Dr. W. Biddison (National Institutes of Health, Bethesda, MD).
HIV-1–specific CTL Activity.
The target cells were labeled for 2 h at 37°C with 100 μCi per 106 cells Na51Cr (Amersham, Les Ulis, France), and washed twice with culture medium. The target cells were distributed in round-bottomed 96-well microtiter plates (4 × 103 cells per well), and the effector cells were added at E/T ratios ranging between 120:1 and 3:1. The plates were centrifuged at 300 rpm for 2 min and incubated for 4 h at 37°C. The supernatants were then collected and 51Cr-release was measured in a gamma counter. The relative specific 51Cr-release was calculated as previously described (12). Values for spontaneous 51Cr-release, which are deducted in the calculation, were between 10 and 20% of the total incorporated radioactivity. The results are presented after subtraction of the nonspecific lysis obtained with control targets. All experiments were performed in triplicate. SE of triplicates was always <5% of the mean value and was omitted for clarity.
NK and Lymphokine-activated Killer Cell Generation and Activity.
PBMCs from healthy donors were isolated by centrifugation in a Ficoll-Hypaque density gradient and were immediately tested for NK activity on K562 target cells. Alternatively, the PBMCs were cultured for 48 h with 100 U/ml of recombinant IL-2 and tested for lymphokine-activated killer (LAK) activity on Daudi target cells. The experiments were performed at E/T ratios ranging between 70:1 and 7:1. The conditions for the Cr release assays were the same as for the experiments with CTLs.
Flow Cytometry.
CCR3 expression was assessed on effector and target cells using anti-CCR3 (see Reagents). For double fluorescence analysis of PBMCs, 5 × 105 cells were incubated for 30 min at 4°C with 10 μg of anti-CCR3, washed with PBS, fixed with 1% paraformaldehyde in PBS, and analyzed on a FACScan® flow cytometer (Becton Dickinson, San Jose, CA). 104 events per sample were collected and analyzed using the Cellquest software (Becton Dickinson).
Reagents.
RPMI 1640 medium (Gibco Life Technologies, Cergy Pontoise, France) supplemented with 10% heat-inactivated FCS, and standard concentrations of l-glutamine, sodium pyruvate, penicillin, and streptomycin was used as the medium in all cultures and for dissolving additions. The chemokines and the chemokine antagonist RANTES(9-68) were prepared by chemical synthesis (18). If not stated otherwise they were added to the assays at the time of mixing effector and target cells. RANTES determination in cell supernatants was performed by ELISA (R&D Systems, Minneapolis, MN) and the data were analyzed using the Softmax program (Molecular Devices, Sunnyvale, CA). A standard concentration curve was assayed with each analysis. Four neutralizing monoclonal antibodies were used: anti-RANTES (R&D Systems, Minneapolis, MN); anti-CCR3 (7B11; LeukoSite, Cambridge, MA); anti-CD4 (IOT4; Immunotech, Marseille, France); and anti-CD8 (B9-11; Immunotech). An anti-VLA6 monoclonal of the same isotype (Immunotech) was used as irrelevant antibody control. The antibodies were added to the suspension containing the CTLs shortly before mixing with the targets at the concentration of 1 μg/ml, which was found, in concentration-dependence assays, to be sufficient for neutralization. Pretreatment of CTLs with Bordetella pertussis toxin (Sigma-Aldrich, St. Quentin Fallavier, France) was performed for 1.5 h at 37°C in the standard medium, followed by washing.
Results and Discussion
RANTES Enhances CTL Activity.
The effect of RANTES on HIV-1–specific cytotoxicity was examined using polyspecific MHC class I–restricted CTL lines established from HIV-1–infected donors that recognize the HIV-1LAI Env, Pol, Gag, or Nef gene products. As shown in Fig. 1 A, the specific lysis of autologous B cell lines expressing Gag, was markedly enhanced by RANTES and was suppressed by a RANTES-neutralizing antibody. Similar effects were obtained when the lytic activity of patient-derived HIV-1– specific CTLs was tested with two other targets, autologous B cells pulsed with a HIV-1 Pol peptide (Fig. 1 B) and a human plasmocytoid cell line, Hmy, cotransfected with the HLA-A2 and the HIV-1LAI nef genes (Fig. 1 C). By contrast, neither RANTES nor the RANTES-neutralizing antibody influenced the lysis mediated by blood-derived NK or LAK cells, as illustrated for NK cells in Fig. 1 D. As shown in Fig. 1 E, the enhancing effect of RANTES and the inhibition of CTL activity by either neutralizing RANTES or blocking its receptor with the antagonist RANTES(9-68) were regularly observed with several CTL lines specific for Gag, Pol, or Env derived from HIV-1– infected individuals. Together these results indicate that RANTES was required for the lytic activity of HIV-1–specific CTLs of the cohort patients. Comparison of CTLs from different individuals (Fig. 1 E) shows that the effects were similar for different HIV-1 epitopes presented by the target cells, and were highly reproducible. The use of different types of target cells (Fig. 1, A–C) indicates that the procedure used to induce expression of HIV-1–specific epitopes had no influence on the outcome of the experiments.
Figure 1.
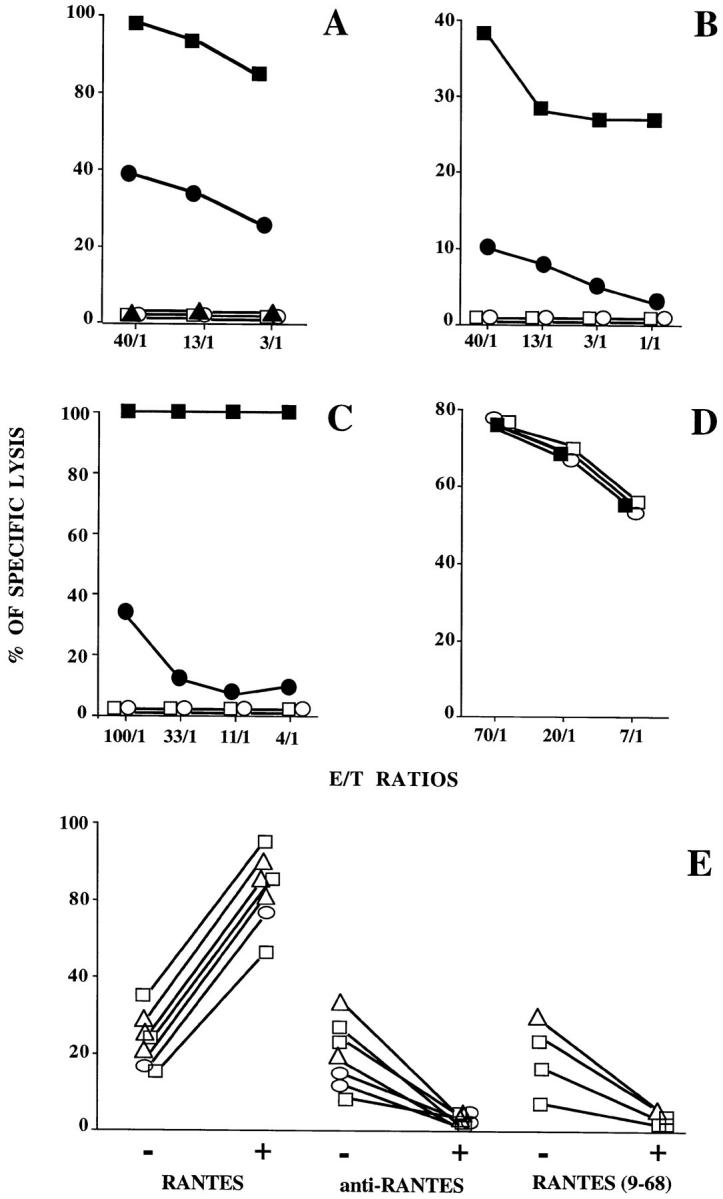
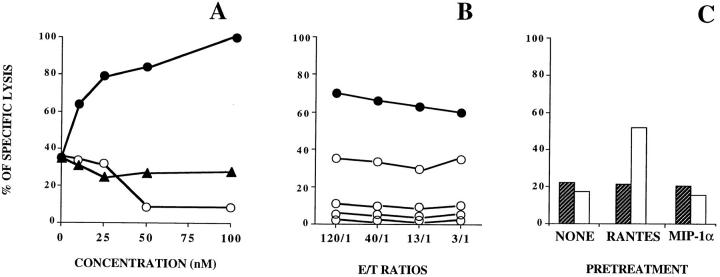
RANTES enhances HIV-1–specific CTL activity. (A) Transformed autologous B cells infected with recombinant vaccinia virus expressing Gag HIV-1 antigens were used as targets. Lysis by HIV-1–specific CTLs was determined in the presence of 25 nM RANTES (closed squares), 1 μg/ml anti-RANTES (open circles), or without additions (closed circles). No lysis was obtained in the presence or absence of 25 nM RANTES (open squares and closed triangles, respectively) when transformed autologous B cells infected with wild-type recombinant vaccinia virus were used as targets. Similar effects were obtained in eight experiments with CTLs from different HIV-1–infected donors. (B) Transformed autologous B cells preincubated with the synthetic peptide 476–484 corresponding to the Pol epitope of HIV-1LAI were used as targets. Lysis by HIV-1–specific CTLs was determined in the presence of 25 nM RANTES (closed squares), 25 nM RANTES(9-68) (open squares), 1 μg/ml anti-RANTES (open circles), or without additions (closed circles). Data from one experiment. (C) Human plasmocytoid cells Hmy cotransformed with the HLA-A2 and the HIV-1LAI nef genes were used as targets. Lysis by HIV-1–specific CTLs was determined in the presence of 25 nM RANTES (closed squares), 25 nM RANTES(9-68) (open squares), 1 μg/ml anti-RANTES (open circles), or without additions (closed circles). The data are representative for two experiments. (D) RANTES does not affect the lytic activity of NK cells. Lysis of K562 target cells by NK cells obtained from venous blood of healthy donors was determined in the presence of 25 nM RANTES (closed squares), 1 μg/ml anti-RANTES (open circles), or without additions (open squares). The data are representative for four experiments. (E) Effects of 25 nM RANTES, 1 μg/ml anti-RANTES and 25 nM RANTES(9−68) on the activity of HIV-1–specific CTLs from eight different donors. Transformed autologous B cells infected with recombinant vaccinia virus expressing Gag (squares), Pol (circles), or Env (triangles) were used as targets. Data are shown for an E/T ratio of 40:1. The straight lines connect the lytic activity values obtained in the absence (−) or presence (+) of the added reagent as indicated.
The effects of RANTES and RANTES(9-68) were concentration dependent (Fig. 2 A). Specific lysis was nearly doubled at 10 nM RANTES and approached a maximum between 50 and 100 nM, whereas inhibition by RANTES (9-68) was observed above 25 nM and was nearly complete at 50 and 100 nM. On the other hand, no effect was obtained with increasing concentrations of macrophage inflammatory protein (MIP)-1α. The concentrations shown are similar to those required for leukocyte activation by chemokines and the inhibition of functional responses by chemokine antagonists (19–21). Since chemokine antagonists have lower receptor affinity than the chemokines themselves (20), it is not surprising that higher concentrations of RANTES(9-68) were required to achieve a biological effect. As shown in Fig. 2 B, pretreatment of the CTLs with increasing concentrations of pertussis toxin progressively decreased the cytolytic activity observed in the presence of RANTES, indicating that the chemokine effect is mediated by a G protein–coupled receptor (9). Pertussis toxin also abrogated CTL activity in the absence of added RANTES (data not shown). To identify the cells responding to RANTES, effector and target cells were pretreated separately before testing cytotoxicity (Fig. 2 C). Pretreatment of the effector cells with RANTES yielded similar results as shown in Fig. 1 when the chemokine was added at the time of mixing effectors and targets. By contrast, no changes were observed when the same pretreatment was applied to the target cells. MIP-1α and MIP-1β, which are produced by CD8+ T cells together with RANTES (22, 23), were ineffective, as shown in Fig. 2 C for MIP-1α, suggesting that MIP-1α and MIP-1β do not share the relevant receptor with RANTES. These results indicate that RANTES acts on the CTLs and has no toxic or other apparent effect on the target cells. Additional support for this conclusion comes from the observed inhibition of the effect of RANTES after pretreatment of the CTLs with pertussis toxin (Fig. 2 B).
Figure 2.
(A) Concentration dependence of the effect of RANTES on HIV-1–specific cytolysis. Transformed autologous B cells infected with recombinant vaccinia virus expressing Gag were used as targets. Lysis by HIV-1–specific CTLs was assessed at an E/T ratio of 120:1 in the presence of increasing concentrations of RANTES (closed circles), the antagonist RANTES(9-68) (open circles), or MIP-1α (closed triangles). The data are representative for two experiments. (B) Effect of B. pertussis toxin. The effector cells were pretreated with RANTES in the absence (closed circles) or the presence (open circles) of pertussis toxin, washed, and then mixed with target cells expressing HIV-1 Pol protein. The toxin concentration was 10, 100, 1,000, or 5,000 ng/ml (open circles, top to bottom). The data are representative for four experiments. (C) RANTES acts on the HIV-1–specific CTLs but not on the target cells. Lysis was assessed at a similar E/T ratio after pretreatment of the effector (white bars) or the target cells (hatched bars) with RANTES, MIP-1α (all 25 nM) or without additions. Pretreatment was performed for 1 h at 37°C followed by washing, immediately before the Cr-release assay. The data are representative for two experiments.
CTL Activity Mediated by CCR3.
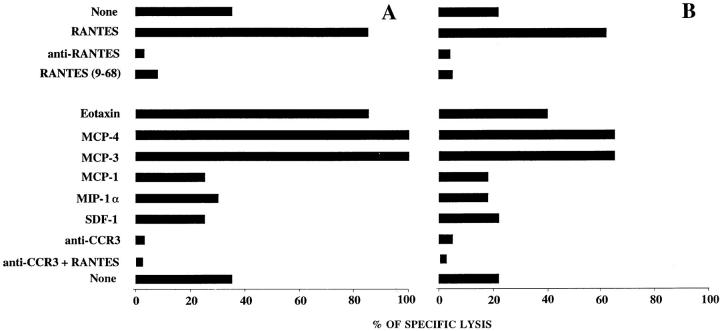
RANTES binds to several receptors. It attracts monocytes (19, 24) and T lymphocytes (25) via CCR1, and eosinophils and basophils via CCR3 (26, 27) and acts on T lymphocytes via CCR5 (28). CCR4 was also reported as a candidate receptor (29), but was later shown to bind a novel CC chemokine called TARC (30). To identify the receptor involved in HIV-1– specific CTL activity, we tested the effect of several well-characterized chemokines. As shown in Fig. 3, RANTES could be substituted by eotaxin, monocyte chemotactic protein (MCP)-3 and MCP-4, but not by MIP-1α, MCP-1, or stromal cell–derived factor (SDF)-1. This suggests that the observed HIV-1–specific CTL activity depends on CCR3 and rules out effects via CCR1, CCR2, CCR5, and CXCR4. To definitively prove the involvement of CCR3, we used a monoclonal antibody that selectively blocks the function of this receptor (26). Fig. 3 shows that target cell lysis was abrogated in the presence of the antibody confirming that HIV-1–specific cytotoxicity is mediated by chemokines that act via CCR3. Anti-CCR3 also prevented the lysis of autologous B cells pulsed with a HIV-1 Pol peptide 476–484 and of the Hmy plasmocytoid cells expressing HLA-A2 and HIV-1LAI Nef, but had no effect on the lysis by NK cells (data not shown). We tested the expression of CCR3 in several HIV-1–specific CTL lines established from HIV-1– infected individuals. The receptor was detectable in all lines, and the percentage of positive cells varied between 3 and 10 depending on the donor. In no instance CCR3 was detected on the target cells (data not shown). Although several chemokines that bind to CCR3 enhanced CTL activity, RANTES appears to be the biologically relevant stimulus, since lysis was virtually abolished when this chemokine was selectively neutralized with the anti-RANTES antibody. It has been previously reported that CD8+ T cells produce RANTES and other CC chemokines (31–34). We have determined the production of RANTES by two CTL lines for the duration of the cytotoxicity assay at an E/T ratio of 120:1, and found concentrations of 52 ± 5 and 138 ± 22 pg/ml ± SD in the culture medium under conditions that yielded 20 and 40% specific lysis, respectively. These concentrations are considerably lower than those added in this study to enhance lysis. The low level of RANTES detected in these assays may be related to the weak lytic activity observed even at high E/T ratios. On the other hand, RANTES is likely to bind to CCR3 as it is released by the producing cells, and the assay in the supernatant may underestimate the effective concentrations.
Figure 3.
Lysis by HIV-1–specific CTLs is mediated by CCR3. Transformed autologous B cells infected with recombinant vaccinia virus expressing Gag were used as target cells. Lysis by HIV-1–specific CTL lines obtained from two individuals (A and B) was determined at an E/T ratio of 120:1 in the presence of different chemokines (all 25 nM), 25 nM RANTES(9-68), 1 μg/ml anti-RANTES, 1 μg/ml anti-CCR3, 1 μg/ml anti-CCR3 plus 25 nM RANTES, or without additions. Similar effects were obtained in eight experiments with CTLs from different HIV-1–infected donors.
After having shown that RANTES is a major mediator of lysis by CTL lines derived from HIV-1–infected patients, it was important to assess whether the activity of freshly isolated PBMCs was also RANTES-dependent. As shown in Fig. 4, HIV-1–specific lysis by PBMCs that were freshly isolated from infected individuals was enhanced by RANTES and virtually abolished by antibodies that neutralize RANTES or block CCR3. In addition, Fig. 4 shows that CTL lines established from the PBMCs of both donors lysed autologous but not mismatched targets. The lytic activity was abrogated by anti-CD8 antibodies, both in the presence and absence of RANTES indicating that it was mediated by CD8+ T lymphocytes. Anti-CD4 antibodies, by contrast, were without effect (data not shown).
Figure 4.
(Top) RANTES enhances the CTL activity of freshly isolated PBMCs. The lytic activity of PBMCs obtained from two HIV-1– infected individuals (A and B) was assayed at an E/T ratio of 120:1 against transformed autologous B cells infected with recombinant vaccinia virus expressing Pol (A) and Gag (B) in the presence of 25 nM RANTES, 1 μg/ml anti-RANTES, 1 μg/ml anti-CCR3, 1 μg/ml anti-CCR3 plus 25 nM RANTES, or without additions. (Middle and bottom) RANTES activity is mediated by MHC-restricted CD8+ T cells. HIV-1–specific lysis by CTL lines derived from the PBMCs of the same patients (A and B) was determined against autologous and mismatched transformed B cells infected with recombinant vaccinia virus expressing Pol (A) and Gag (B) in the presence of 25 nM RANTES, 1 μg/ml anti-RANTES, 1 μg/ml anti-CD8, 1 μg/ml anti-CD8 plus 25 nM RANTES, or without additions.
This study shows that RANTES mediates the HIV-1– specific killing of target cells by MHC-restricted CD8+ T lymphocytes through a mechanism that depends on the chemokine receptor CCR3. It is of interest that RANTES is produced by CD8+ T cells after antigen-specific stimulation (35) and that CCR3 is expressed in CD4+ and CD8+ T lymphocytes (36). MIP-1α, MIP-1β, RANTES, and MCP-1 were reported to enhance the proliferation of human T cells in response to anti-CD3 and of T cell clones challenged with the relevant antigen (37), suggesting a role of CC chemokines in the activation of polyclonal and antigen-specific cytotoxicity (38). A costimulatory function of RANTES cannot be ruled out although the selectivity observed in this study suggests a different mechanism. Our observations reveal a new facet of the antiviral properties of RANTES. The involvement of CCR3 suggests the possibility to enhance its expression by CD8+ effector T cells in order to prevent the decline of CTL activity in AIDS and to improve cellular defense in other immunodeficiencies. In addition, retroviral gene transduction approaches could be used to regulate the endogenous expression of RANTES or RANTES-inducing cytokines.
Acknowledgments
This study was supported by the Agence Nationale pour la Recherche sur le Sida (ANRS), the Swiss National Science Foundation (Grant 31-39744.93), and the Protein Engineering Network of Centers of Excellence (PENCE), Canada.
Footnotes
We are particularly indebted to Dr. Fernando Arenzana (Institut Pasteur, Paris) and Beatrice Dewald (Theodor Kocher Institute, Bern) for discussions and important suggestions.
References
- 1.Autran B, Hadida F, Haas G. Evolution and plasticity of CTL responses against HIV. Curr Opin Immunol. 1996;8:546–553. doi: 10.1016/s0952-7915(96)80045-8. [DOI] [PubMed] [Google Scholar]
- 2.Gotch FM, Nixon DF, Alp N, McMichael AJ, Borysievicz LK. High frequency of memory and effector Gag-specific cytotoxic T lymphocytes in HIV seropositive individuals. Int Immunol. 1990;2:707–712. doi: 10.1093/intimm/2.8.707. [DOI] [PubMed] [Google Scholar]
- 3.Hoffenbach A, Langlade-Demoyen P, Vilmer E, Dadaglio G, Michel F, Mayaud C, Autran B, Plata F. Very high frequencies of HIV specific cytotoxic T lymphocytes in humans. J Immunol. 1989;142:452–456. [PubMed] [Google Scholar]
- 4.Hadida F, Parrot A, Kieny MP, Sadat-Sowti B, Mayaud C, Debré P, Autran B. Carboxyl-terminal and central regions of human immunodeficiency virus-1 Nef recognized by cytotoxic T lymphocytes from lymphoid organs. J Clin Invest. 1992;89:53–60. doi: 10.1172/JCI115585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joly P, Guillon JM, Mayaud C, Plata F, Theodorou I, Denis M, Debré P, Autran B. Cell mediated suppression of HIV-specific cytotoxic T lymphocytes. J Immunol. 1989;143:2193–2201. [PubMed] [Google Scholar]
- 6.Rivière Y, McChesney MB, Porrot F, Tanneau-Salvadori F, Sansonetti P, Lopez O, Pialoux G, Feuillie V, Mollereau M, Chamaret S, et al. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res Hum Retroviruses. 1995;11:903–907. doi: 10.1089/aid.1995.11.903. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 8.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapouméroulie C, Cognaux J, Forceille C, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 9.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 10.D'Souza MP, Harden VA. Chemokines and HIV-1 second receptors. Confluence of two fields generates optimism in AIDS research. Nat Med. 1996;2:1293–1300. doi: 10.1038/nm1296-1293. [DOI] [PubMed] [Google Scholar]
- 11.Nixon DF, Townsend ARM, Elvin JG, Rizza CR, Gallwey J, McMichael AJ. HIV-1 Gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988;336:484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- 12.Hadida F, Haas G, Zimmermann N, Hosmalin A, Spohn R, Jung R, Debré P, Autran B. Cytotoxic T lymphocytes from lymphoid organs recognize an optimal HLA-A2 and -B52 restricted nonapeptide and several epitopes in the C- terminal region of HIV-1 Nef. J Immunol. 1995;154:4174–4186. [PubMed] [Google Scholar]
- 13.Devergne O, Raphael M, Autran B, Leger-Ravet MB, Coumbaras J, Crevon MC, Galanaud P, Emilie D. Intratumoral activation of DC8+positive cytotoxic lymphocytes in acquired immunodeficiency syndrome lymphomas. Hum Pathol. 1995;26:284–290. doi: 10.1016/0046-8177(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 14.Walker CM, Moody DJ, Stites DP, Levy JA. CD8+lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–1565. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 15.Tsomides TJ, Walker BD, Eisen HN. An optimal viral peptide recognized by CD8+T cells binds very tightly to the restricting class I major histocompatibility complex protein on intact cells but not to the purified class I protein. Proc Natl Acad Sci USA. 1991;88:11276–11280. doi: 10.1073/pnas.88.24.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salter RD, Clayberger C, Lomen CE, Krensky AM, Parham P. In vitro mutagenesis at a single residue introduces B and T cell epitopes into a class I HLA molecule. Eur J Immunol. 1982;166:283–290. doi: 10.1084/jem.166.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucchiari M, Niedermann G, Leipner C, Meyerhans A, Eichmann K, Maier B. Human immune response to HIV-1-Nef. I. CD4SRO−T lymphocytes of non-infected donors contain cytotoxic T lymphocyte precursors at high frequency. Int Immunol. 1994;6:1739–1749. doi: 10.1093/intimm/6.11.1739. [DOI] [PubMed] [Google Scholar]
- 18.Clark-Lewis I, Moser B, Walz A, Baggiolini M, Scott GJ, Aebersold R. Chemical synthesis, purification, and characterization of two inflammatory proteins, neutrophil activating peptide 1 (interleukin-8) and neutrophil activating peptide 2. Biochemistry. 1991;30:3128–3135. doi: 10.1021/bi00226a021. [DOI] [PubMed] [Google Scholar]
- 19.Uguccioni M, D'Apuzzo M, Loetscher M, Dewald B, Baggiolini M. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1α and MIP-1β on human monocytes. Eur J Immunol. 1995;25:64–68. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- 20.Gong J-H, Uguccioni M, Dewald B, Baggiolini M, Clark-Lewis I. RANTES and MCP-3 antagonists bind multiple chemokine receptors. J Biol Chem. 1996;271:10521–10527. doi: 10.1074/jbc.271.18.10521. [DOI] [PubMed] [Google Scholar]
- 21.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1α induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conlon K, Lloyd A, Chattopadhyay U, Lukacs N, Kunkel S, Schall T, Taub D, Morimoto C, Osborne J, Oppenheim J, et al. CD8+ and CD45RA+human peripheral blood lymphocytes are potent sources of macrophage inflammatory protein 1α, interleukin-8 and RANTES. Eur J Immunol. 1995;25:751–756. doi: 10.1002/eji.1830250319. [DOI] [PubMed] [Google Scholar]
- 23.Bazan JF, Schall TJ. Interleukin-16 or not. Nature. 1996;381:29–30. doi: 10.1038/381029a0. [DOI] [PubMed] [Google Scholar]
- 24.Proudfoot AEI, Power CA, Hoogewerf A, Montjovent MO, Borlat F, Wells TNC. Characterization of the RANTES/MIP-1α receptor (CC CKR-1) stably transfected in HEK 293 cells and the recombinant ligands. FEBS Lett. 1995;376:19–23. doi: 10.1016/0014-5793(95)01235-x. [DOI] [PubMed] [Google Scholar]
- 25.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heath H, Qin SX, Rao P, Wu LJ, LaRosa G, Kassam N, Ponath PD, Mackay CR. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest. 1997;99:178–184. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada H, Hirai K, Miyamasu M, Iikura M, Misaki Y, Shoji S, Takaishi T, Kasahara T, Morita Y, Ito K. Eotaxin is a potent chemotaxin for human basophils. Biochem Biophys Res Commun. 1997;231:365–368. doi: 10.1006/bbrc.1997.6100. [DOI] [PubMed] [Google Scholar]
- 28.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 29.Power CA, Meyer A, Nemeth K, Bacon KB, Hoogewerf AJ, Proudfoot AEI, Wells TNC. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J Biol Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- 30.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell–directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 31.Cao JX, Gershon PD, Black DN. Sequence analysis of HindIII Q2 fragment of capripoxvirus reveals a putative gene encoding a G-protein–coupled chemokine receptor homologue. Virology. 1995;209:207–212. doi: 10.1006/viro.1995.1244. [DOI] [PubMed] [Google Scholar]
- 32.Zanussi S, D'Andrea M, Simonelli C, Battiston V, Tirelli U, De Paoli P. CD8+cells in HIV infection produce macrophage inflammatory protein-1α and RANTES: a comparative study in long term survivors and progressor patients. Immunol Lett. 1996;53:105–108. doi: 10.1016/s0165-2478(96)02617-x. [DOI] [PubMed] [Google Scholar]
- 33.Yang OO, Kalams SA, Trocha A, Cao HY, Luster A, Johnson RP, Walker BD. Suppression of human immunodeficiency virus type 1 replication by CD8+cells: evidence for HLA class I–restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scala E, D'Offizi G, Rosso R, Turriziani O, Ferrara R, Mazzone AM, Antonelli G, Aiuti F, Paganelli R. C-C chemokines, IL-16, and soluble antiviral factor activity are increased in cloned T cells from subjects with long-term nonprogressive HIV infection. J Immunol. 1997;158:4485–4492. [PubMed] [Google Scholar]
- 35.Price DA, Sewell AK, Dong T, Tan R, Goulder JR, Rowland-Jones SL, Phillips RE. Antigen-specific release of β-chemokines by anti–HIV-1 cytotoxic T lymphocytes. Curr Biol. 1998;8:355–358. doi: 10.1016/s0960-9822(98)70138-1. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 37.Taub DD, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Chemokines and T lymphocyte activation: I. β chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- 38.Taub DD, Ortaldo JR, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. β chemokines costimulate lymphocyte cytolysis, proliferation, and lymphokine production. J Leukocyte Biol. 1996;59:81–89. doi: 10.1002/jlb.59.1.81. [DOI] [PubMed] [Google Scholar]