Abstract
The Legionnaires' disease bacterium, Legionella pneumophila, is a facultative intracellular pathogen that invades and replicates within two evolutionarily distant hosts, free living protozoa and mammalian cells. Invasion and intracellular replication within protozoa are thought to be major factors in the transmission of Legionnaires' disease. We have recently reported the identification of a galactose/N-acetyl-d-galactosamine (Gal/GalNAc) lectin in the protozoan host Hartmannella vermiformis as a receptor for attachment and invasion by L. pneumophila (Venkataraman, C., B.J. Haack, S. Bondada, and Y.A. Kwaik. 1997. J. Exp. Med. 186:537–547). In this report, we extended our studies to the effects of bacterial attachment and invasion on the cytoskeletal proteins of H. vermiformis. We first identified the presence of many protozoan cytoskeletal proteins that were putative homologues to their mammalian counterparts, including actin, pp125FAK, paxillin, and vinculin, all of which were basally tyrosine phosphorylated in resting H. vermiformis. In addition to L. pneumophila–induced tyrosine dephosphorylation of the lectin, bacterial attachment and invasion was associated with tyrosine dephosphorylation of paxillin, pp125FAK, and vinculin, whereas actin was minimally affected. Inhibition of bacterial attachment to H. vermiformis by Gal or GalNAc monomers blocked bacteria-induced tyrosine dephosphorylation of detergent-insoluble proteins. In contrast, inhibition of bacterial invasion but not attachment failed to block bacteria-induced tyrosine dephosphorylation of H. vermiformis proteins. This was further supported by the observation that 10 mutants of L. pneumophila that were defective in invasion of H. vermiformis were capable of inducing tyrosine dephosphorylation of H. vermiformis proteins. Entry of L. pneumophila into H. vermiformis was predominantly mediated by noncoated receptor-mediated endocytosis (93%) but coiling phagocytosis was infrequently observed (7%). We conclude that attachment but not invasion by L. pneumophila into H. vermiformis was sufficient and essential to induce protein tyrosine dephosphorylation in H. vermiformis. These manipulations of host cell processes were associated with, or followed by, entry of the bacteria by a noncoated receptor-mediated endocytosis. A model for attachment and entry of L. pneumophila into H. vermiformis is proposed.
Keywords: intracellular, bacteria, protozoa, cytoskeleton, lectin
Entry of intracellular bacteria into host cells involves interaction with the host cell receptor and manipulation of host signaling and cytoskeletal processes to induce bacterial uptake (1, 2). Invasion of epithelial cells by Shigella flexneri and Yersinia enterocolitica requires contact with the β1 integrins present on host cell surfaces (1). Integrins are heterodimeric transmembrane receptors comprising of α and β subunits that serve as a link between the extracellular matrix and the actin cytoskeleton at sites of close cell–substratum contact (3). Clustering of integrins upon binding to their ligands induces tyrosine phosphorylation of integrins and recruitment of several cytoskeletal and regulatory proteins forming focal adhesions at contact sites (3–5). Protein tyrosine phosphatases are involved in the regulation of focal contacts and their stability (6–9).
The Legionnaires' disease bacterium, Legionella pneumophila, is a facultative intracellular pathogen that invades and replicates within a rough endoplasmic reticulum (RER)1-surrounded phagosome in human macrophages and epithelial cells (10). In the aquatic environment, L. pneumophila is a parasite of protozoa. The bacteria are transmitted by aerosols generated in the environment. Intracellular replication within protozoa probably play major factors in bacterial amplification and transmission to humans (10–13). Interestingly, similar to the intracellular infection of macrophages, the bacteria are also targeted within protozoa into a replicative phagosome surrounded by the RER (14). Although L. pneumophila has loci that are required for survival within both macrophages and protozoa, the bacterium has mammalian-specific infectivity loci (mil) that are not required for infectivity of protozoa (15, 16).
Initial attachment of L. pneumophila to protozoa is mediated by the bacterial type IV pili, but the receptor for these pili is not known (17). Recently, we have demonstrated that attachment and invasion of L. pneumophila into its protozoan host, H. vermiformis, is mediated by bacterial attachment to a 170-kD galactose/N-acetyl-d-galactosamine (Gal/ GalNAc) inhibitable lectin, a homologue of a β2 integrin– like Gal/GalNAc lectin of the human pathogen, Entamoeba histolytica (18–20). Bacterial uptake by H. vermiformis is insensitive to the inhibitory effects of cytochalasin D (21). In addition, many genes are induced upon bacterial attachment and invasion, and inhibition of protein synthesis of H. vermiformis blocks bacterial entry (22).
Attachment of L. pneumophila to the Gal/GalNAc lectin of H. vermiformis is associated with a time-dependent and reversible tyrosine dephosphorylation of several host cell proteins, including the 170-kD lectin (18). This bacteria- induced host tyrosine dephosphorylation is blocked by prior treatment of H. vermiformis with tyrosine phosphatase inhibitors, indicating a bacterial attachment-mediated induction of a tyrosine phosphatase activity (18). It is not known whether protein tyrosine dephosphorylation in H. vermiformis plays any role in modulating host cytoskeletal events upon attachment and entry of L. pneumophila into the protozoan host.
Focal adhesions have been recognized in Entamoeba histolytica and Acanthamoebae, and have been shown to be involved in attachment to surfaces, interaction with the receptor upon engagement to ligands, and locomotion of the parasite (23–28). The protozoan focal adhesions are also disrupted by cytochalasin D, similar to the mammalian focal adhesions (26). Protozoan focal adhesions are composed of cytoskeletal and regulatory proteins that are antigenically and functionally homologous to their mammalian counterparts, including actin, α-spectrin, myosin II, pp125FAK, vinculin, α-actinin, protein kinase C, and MAP kinase (25, 27, 28). Many of these protozoan proteins, similar to their mammalian homologues, have been also shown to undergo tyrosine phosphorylation upon receptor engagement (25, 27, 28). These observations indicate that many aspects of receptor-mediated signal transduction are highly conserved through evolution.
In this report, we examined the events leading to cytoskeletal changes during the uptake of L. pneumophila by H. vermiformis. Attachment of L. pneumophila to H. vermiformis is associated with a time-dependent and reversible tyrosine dephosphorylation of many putative homologues of mammalian cytoskeletal proteins, including, paxillin, vinculin, and pp125FAK whereas actin was minimally affected. These alterations in host cell processes were associated with or followed by a noncoated receptor-mediated endocytic uptake of the bacteria.
Materials and Methods
Bacterial and Protozoan Strains and Culture.
Legionella pneumophila AA100 is a virulent clinical isolate that has been described previously (29). The construction of a mini Tn10::kan transposon insertion library in L. pneumophila AA100 has been described previously (15). The mini Tn10::kan insertion mutants of L. pneumophila (GF162, GG104, GB112, GM128, GO128, GP65, GQ262, and GT251) were selected according to their defects in invasion of H. vermiformis, using gentamicin protection assays, as we described previously (18). In brief, after 1 h of infection, extracellular bacteria were killed with gentamicin, and intracellular bacteria were plated for colony enumeration. The percentage of invasion by the mutants was obtained relative to the wild-type strain. Through Southern hybridizations, these mutants have been confirmed to contain distinct insertions in their chromosomes (15). L. pneumophila was grown on buffered charcoal yeast extract agar plates at 37°C, or in the presence of 50 μg/ml of kanamycin for the mutants. For infections, bacteria grown for 48 h on agar plates were resuspended in serum-free axenic medium to a desired concentration.
H. vermiformis strain CDC-19 (American Type Culture Collection [ATCC] 50237) is an established model for the study of pathogenesis of L. pneumophila, and was isolated from a water source of an outbreak of nosocomial Legionnaires' disease (14, 30). The amebae were maintained in ATCC culture medium 1034 (30).
Reagents and Antibodies.
Biotinylated and horseradish peroxidase (HRP)-conjugated recombinant antiphosphotyrosine antibodies were purchased from Transduction Laboratories (Lexington, KY). D(+)-Galactose, N-acetyl-d-galactosamine (GalNAc), D(+)-mannose, and antibodies to vinculin (monoclonal mouse anti–human vinculin, IgG1, clone hVIN-1), and actin (monoclonal mouse anti–amebae actin, IgG1, clone KJ43A) were obtained from Sigma Chemical Co. (St. Louis, MO). Antipaxillin (monoclonal mouse anti–chicken paxillin antibody, IgG1, clone Z035) and anti-pp125FAK (polyclonal rabbit anti–human pp125FAK antibody) antibodies were from Zymed Laboratories (San Francisco, CA) and Santa Cruz Biotechnology Inc. (Santa Cruz, CA), respectively. Methylamine was purchased from Sigma Chemical Co.
Detection of Tyrosine-phosphorylated Proteins in H. vermiformis upon Contact with L. pneumophila.
H. vermiformis were harvested and infected with L. pneumophila as we described previously (18). At several time intervals of coincubation at 37°C, amebal cell lysates were prepared for immunoblot and immunoprecipitation analysis as described below.
To examine the ability of some sugars to block tyrosine dephosphorylation of amebal proteins upon contact with L. pneumophila, H. vermiformis were preincubated before the infection in the presence of different sugars. Preincubation of amebae was performed for 15 min on ice followed by coincubation with the bacteria at 37°C. At the end of the coincubation period, amebal cell lysates were prepared as described below.
Preparation of Cell Lysates.
After incubation of H. vermiformis with L. pneumophila, infections were stopped using cold stop buffer containing protease and phosphatase inhibitors (18). Cells were washed three times with stop buffer and pelleted by low speed centrifugation at 735 g for 2 min. The amebae were lysed using cold 0.1% Triton X-100 lysis buffer, and the detergent-soluble and -insoluble fractions were separated by centrifugation, as described previously (18). Proteins from detergent-insoluble fraction (pellet) were resuspended in 50 μl of lysis buffer and sonicated for 5 s.
Western Blotting and Immunoprecipitation Studies.
Proteins from detergent-soluble and -insoluble fractions were resolved on 10% SDS-PAGE under reducing conditions. After transfer onto Immobilon-P (Millipore, Bedford, MA), membranes were incubated in a blocking buffer containing 1.5% BSA for 30 min. Membranes were probed with antiphosphotyrosine antibody (RC-20; Transduction Laboratories). After extensive washing, blots were developed using the enhanced chemiluminescence kit (DuPont NEN, Boston, MA) according to the manufacturer's instructions.
For immunoprecipitations, cell extracts were incubated with biotinylated anti-phosphotyrosine antibodies and immune complexes were collected using avidin-agarose beads (Pierce Chemical Co., Rockford, IL). The beads were washed extensively with lysis buffer and eluted proteins were resolved under SDS-PAGE conditions. After transfer onto Immobilon-P membranes, the blots were probed with antibodies to pp125FAK, paxillin, or vinculin. Anti-phosphotyrosine immunoprecipitates were also analyzed by immunoblotting with antiactin antibody in the same experiment. This was followed by incubation with either HRP-conjugated goat anti– mouse or goat anti–rabbit antibody (Santa Cruz Biotechnology Inc.) and proteins were visualized as described above. The relative intensities of the protein bands were quantitated using the NIH Image program (version 1.6). The results were expressed as percentages of uninfected controls. The percent tyrosine phosphorylation of each of the cytoskeletal proteins compared with uninfected controls (PY%) was calculated as a ratio of tyrosine-phosphorylated paxillin, pp125FAK or vinculin to the tyrosine-phosphorylated actin for that time point during infection in the same experiment.
Transmission Electron Microscopy.
H. vermiformis were infected with L. pneumophila using a multiplicity of infection (moi) of 100 at 4°C to allow attachment, followed by a temperature shift to 37°C for 8 min to allow uptake. Preparation of ultrathin sections was performed as described previously (15). In brief, infected amebae were extensively washed with tissue culture medium to remove extracellular bacteria, fixed with 3.5% glutaraldehyde followed by 1% OsO4, dehydrated by ethanol, and embedded in Eponate 12 resin (Ted Pella, Redding, CA). Ultrathin sections were stained with uranyl acetate followed by lead citrate and examined by a Hitachi H-7000/STEM electron microscope (Hitachi Inc., Tokyo, Japan) at 75 KV. Multiple sections of different samples were examined.
Results
Attachment and Invasion of H. vermiformis by L. pneumophila Is Associated with Tyrosine Dephosphorylation of Several Detergent-insoluble Proteins.
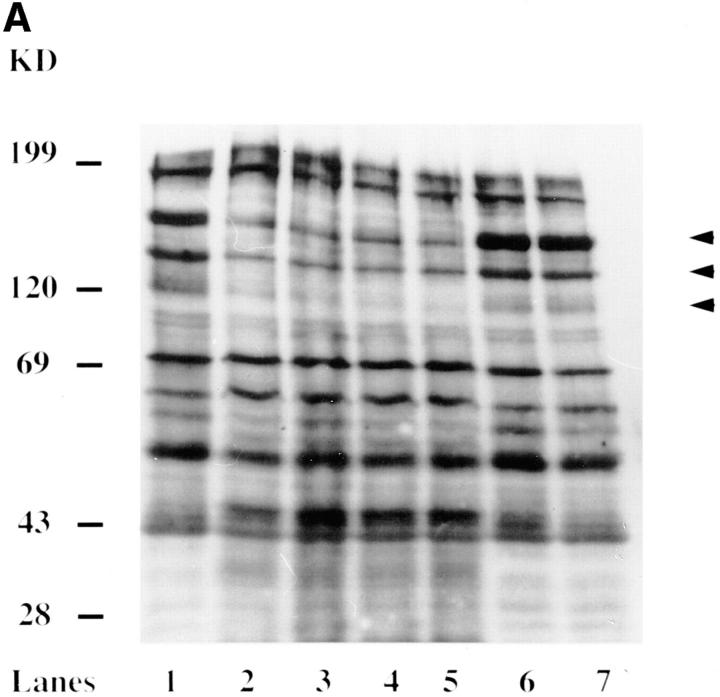
Previously, we have shown that attachment and entry of L. pneumophila into its protozoan host, H. vermiformis, was associated with a time-dependent and reversible tyrosine dephosphorylation of multiple host proteins in the detergent-soluble fraction (18). To further investigate this, we examined the changes in the tyrosine phosphorylation status of proteins in the detergent-insoluble fractions, which constitute most of the cytoskeletal proteins. H. vermiformis was infected with L. pneumophila for several time intervals and detergent-insoluble fractions of H. vermiformis were subjected to antiphosphotyrosine immunoblot analysis. In contrast to uninfected H. vermiformis at time zero (Fig. 1 A, lane 1) or 30 min incubated under identical conditions of infection (Fig. 1 A, lane 6), tyrosine dephosphorylation of many proteins was seen in infected H. vermiformis (Fig. 1). Prominent tyrosine dephosphorylation of many proteins with apparent molecular mass of 170, 150, and 120–125 kD indicated by arrowheads was detectable as early as 1 min, and remained dephosphorylated during the 30-min infection period (Fig. 1 A, lanes 2–5). Although the degree of protein tyrosine dephosphorylation varied slightly between experiments, similar to our observations with the detergent-soluble proteins (18), the observations of tyrosine dephosphorylation of the 170-, 150-, and 120–125-kD was always consistent in several experiments (data not shown). Electrotransfer of the proteins >200-kD species was inconsistent but when it occurred the 200-kD species was dephosphorylated upon infection (data not shown). Tyrosine phosphorylation of the proteins of molecular masses <69 kD was variable, and their dephosphorylation upon infection was inconsistent in multiple experiments (data not shown). The molecular masses of the major tyrosine-dephosphorylated proteins corresponded to some known cytoskeletal proteins such as vinculin (120 kD) and myosin II (200 kD). This process of protein tyrosine dephosphorylation was highly reversible. When H. vermiformis was infected for 30 min with L. pneumophila and the cells were washed to remove extracellular bacteria and further incubated in medium for 15 min, the tyrosine phosphorylation pattern of proteins returned to normal levels similar to uninfected cells (Fig. 1 A, compare lane 1 with 7). Protein tyrosine dephosphorylation in H. vermiformis in response to invasion by L. pneumophila was not induced by supernatant from Legionella culture (Fig. 1 B, lane 3) or equivalent numbers of the Escherichia coli strain HB101 (lane 4).
Figure 1.

Attachment to and invasion of H. vermiformis by L. pneumophila is associated with a time-dependent and reversible tyrosine dephosphorylation of detergent-insoluble proteins. (A) Detergent-insoluble protein extracts were prepared from resting H. vermiformis (lane 1) or after infection by L. pneumophila for 1, 5, 15, and 30 min (lanes 2–5, respectively) and subjected to immunoblot analysis with antiphosphotyrosine antibody. Lane 6 represents amebal proteins from uninfected cells incubated for 30 min under identical conditions. Lane 7 contains proteins from H. vermiformis infected for 30 min, after which extracellular bacteria were washed away and the cells were further incubated at 37°C for 15 min. (B) Detergent-insoluble extracts were prepared from resting H. vermiformis (lane 1), or after infection with L. pneumophila for 20 min (lane 2) and subjected to antiphosphotyrosine analysis. Lanes 3 and 4 represent cell extracts prepared from H. vermiformis incubated with supernatant from Legionella culture and equivalent numbers of E. coli strain, HB101 for 20 min, respectively. Arrowheads represent the prominent proteins that underwent tyrosine dephosphorylation upon infection. Results are representative of three independent experiments.
Cytoskeletal Proteins Are Basally Tyrosine Phosphorylated in H. vermiformis.
Since many detergent-insoluble proteins underwent tyrosine dephosphorylation, we asked whether the increase in tyrosine phosphatase activity upon infection by L. pneumophila (18) altered the phosphorylation status of host cytoskeletal proteins. Many protozoan cytoskeletal and regulatory proteins involved in formation of focal adhesions have been shown to be homologous to their mammalian counterparts, including actin, myosin II, pp125FAK, vinculin, α-actinin, protein kinase C, and MAP kinase (25, 27, 28). In addition, some of these protozoan proteins have been also shown to be tyrosine phosphorylated, such as pp125FAK (28). Many of the protozoan cytoskeletal proteins are antigenically similar to higher eukaryotic homologues, since they are recognized by antibodies raised against the mammalian or avian homologous proteins, including pp125FAK, actin, vinculin, and α-actinin (25, 27, 28, 28).
As an initial step, we sought to identify some of the cytoskeletal proteins that may be tyrosine phosphorylated in uninfected cells to monitor changes after infection. As a screening procedure, phosphotyrosine immunoprecipitates of amebal cell lysates (PY/IP) were immunoblotted with appropriate antibodies that recognized cytoskeletal proteins.
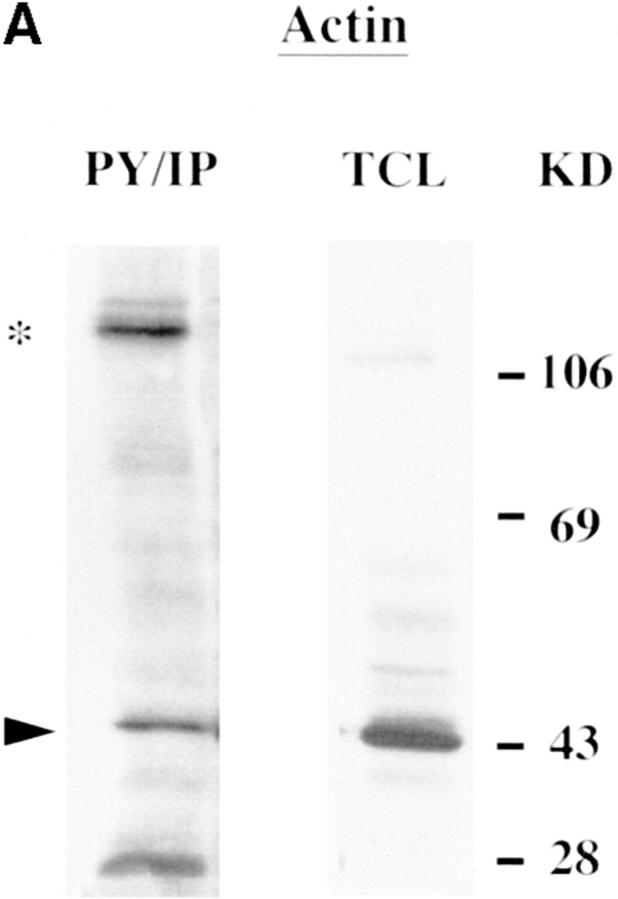
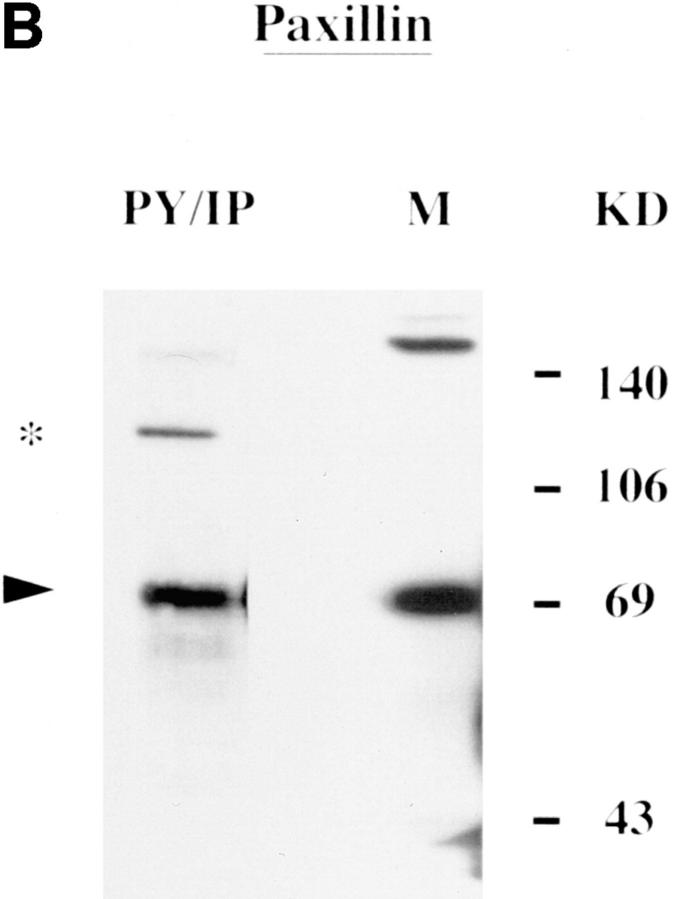
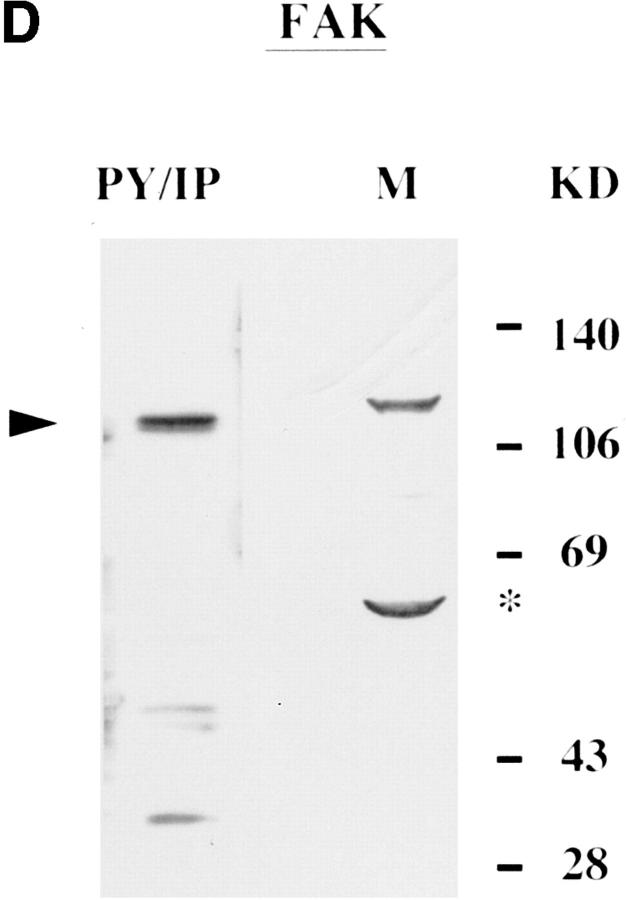
Actin, paxillin, vinculin, and pp125FAK appeared as 43-, 69-, 116–120-, and 100–105-kD proteins, respectively (Fig. 2, A–D). All four proteins were basally tyrosine phosphorylated in uninfected H. vermiformis (Fig. 2, A–D, PY/IP). The anti pp125FAK, and antivinculin antibodies were generated against the respective mammalian proteins, while the antiactin was raised against protozoan actin (see Materials and Methods). Antibodies to paxillin, vinculin, and pp125FAK identified more than one protein band in Western blot analysis but the most prominent band comigrated with their mammalian homologues from murine B (Fig. 2, B–D, lane M) and T lymphocytes (data not shown). Tyrosine-phosphorylated FAK migrated with slightly higher mobility when compared with its mammalian counterpart (Fig. 2 D). Antibodies to several other cytoskeletal proteins failed to recognize H. vermiformis proteins in Western blot analysis (data not shown). These experiments identified four of the cytoskeletal proteins, actin, paxillin, vinculin, and pp125FAK as basally tyrosine-phosphorylated proteins in H. vermiformis. These data are the first demonstration of the presence of cytoskeletal protein homologues in the protozoan H. vermiformis that were immunologically cross-reactive with their mammalian counterparts.
Figure 2.
Presence of tyrosine-phosphorylated cytoskeletal proteins in H. vermiformis. Phosphotyrosine immunoprecipitates (PY/IP) of resting H. vermiformis were resolved under SDS-PAGE conditions and immunoblotted with antibodies to actin, paxillin, vinculin, or pp125FAK, followed by appropriate HRP-conjugated secondary antibodies (A–D). Total cell lysates from 107 murine splenic B lymphocytes (M) was used as a positive control for comparison, except in A where total cell lysate from H. vermiformis was used (TCL), since antiactin was amebae specific. Arrowheads indicate the relative positions of the cytoskeletal proteins and asterisks represent other cross-reactive protein bands under these immunoblotting conditions. Results are representative of two independent experiments.
Attachment and Invasion of H. vermiformis by L. pneumophila Is Associated with Tyrosine Dephosphorylation of Paxillin, Vinculin, and pp125FAK.
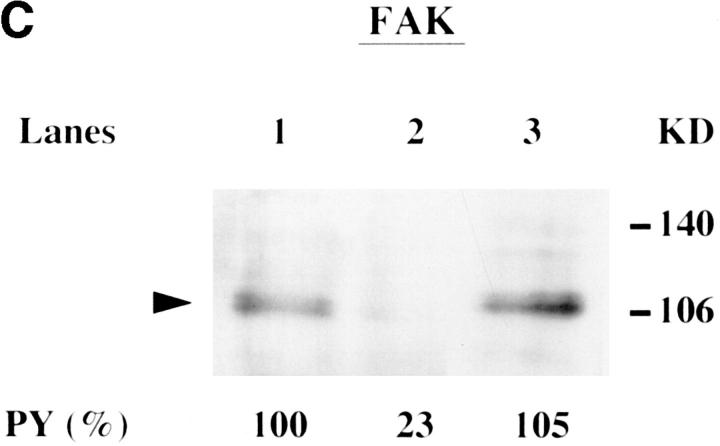
Changes in tyrosine phosphorylation status of cytoskeletal proteins are important in the formation of focal contacts, which are regulated in part by tyrosine phosphatases (3, 6–9). Since attachment and invasion of H. vermiformis by L. pneumophila resulted in the tyrosine dephosphorylation of multiple detergent-insoluble host proteins, we decided to determine whether any of the cytoskeletal protein homologues were substrates for this increased tyrosine phosphatase activity mediated by attachment and invasion by L. pneumophila (18). H. vermiformis was left uninfected or infected with L. pneumophila for 5 or 20 min, and tyrosine-phosphorylated proteins were immunoprecipitated with antiphosphotyrosine antibody, and subsequently subjected to immunoblotting with antibodies to actin, paxillin, vinculin, or pp125FAK. A time-dependent tyrosine dephosphorylation of paxillin, vinculin, and pp125FAK was observed upon attachment and invasion by L. pneumophila (Fig. 3, A–C). Tyrosine phosphorylation of actin was minimally affected in these experiments (data not shown), and was used as an internal control in these experiments. The percent tyrosine phosphorylation of each of the cytoskeletal protein compared with uninfected controls (PY%) was calculated as a ratio of tyrosine-phosphorylated paxillin, pp125FAK, or vinculin to the tyrosine-phosphorylated actin for that time point in the same experiment. Thus, it is possible that the degree of phosphorylation of vinculin, paxillin, or pp125FAK may be slightly underestimated due to a minimal decrease in actin tyrosine phosphorylation upon infection. Despite this potential underestimate, we consistently observed tyrosine dephosphorylation of paxillin, pp125FAK, and vinculin upon infection when compared with actin in the same experiment for the same time point of infection (data not shown). After 20 min of infection, the relative tyrosine phosphorylation of paxillin, vinculin, and pp125FAK was 60, 50, and 23%, respectively, compared with their levels in uninfected cells. The relative tyrosine phosphorylation of pp125FAK was ∼20% within 5 min of the infection (data not shown). Specificity of these L. pneumophila–induced host cell processes was confirmed by the observation that no loss of tyrosine phosphorylation of the cytoskeletal proteins was detected when H. vermiformis was incubated for 20 min with formalin-killed L. pneumophila (Fig. 3, A and B, lane 4 and C, lane 3). Note that vinculin was the only protein (among the proteins studied) with a molecular mass that corresponded to a protein that was dramatically tyrosine dephosphorylated upon infection (Fig. 1 A). It is possible that the other two proteins comigrated with other bands that masked the detection of their tyrosine dephosphorylation, which was detectable after immunoprecipitation (Fig. 3). Alternatively, the proteins underwent minor tyrosine dephosphorylation that was undetectable in simple immunoblots that detected the major dephosphorylated proteins.
Figure 3.
Paxillin, vinculin and pp125FAK are tyrosine dephosphorylated upon attachment and invasion of H. vermiformis by L. pneumophila. (A and B) H. vermiformis were left uninfected (lane 1), infected with L. pneumophila for 5 min (lane 2) or 20 min (lane 3). Lane 4 represents H. vermiformis infected with formalin-killed L. pneumophila for 20 min. Amebal cell extracts were immunoprecipitated with antiphosphotyrosine antibody followed by immunoblotting with antibodies to paxillin (A) or vinculin (B). In C, cell extracts from H. vermiformis left uninfected (lane 1) or infected with live (lane 2) or with formaline-killed bacteria (lane 3) for 20 min were immunoprecipitated with antiphosphotyrosine antibody and probed with antipp125FAK antibody. Tyrosine dephosphorylation of pp125FAK was maximal after 5 min of infection with live L. pneumophila (data not shown). The relative intensities of the protein bands were quantitated using the NIH Image program (version 1.6). The results were expressed as percentages of uninfected controls as described in Materials and Methods. Results are representative of two to three independent experiments for each of these proteins. Arrowhead indicates the relative positions of the cytoskeletal proteins.
Attachment of L. pneumophila to H. vermiformis is Essential to Induce Tyrosine Dephosphorylation of Detergent-insoluble Proteins.
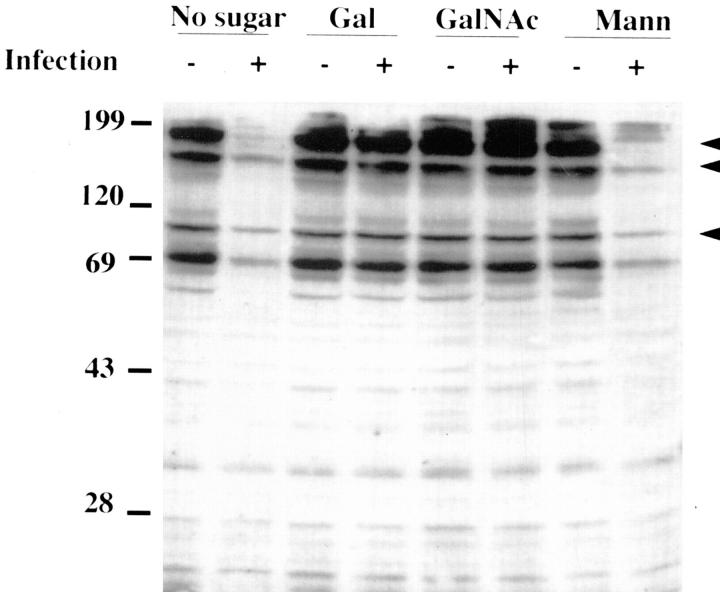
We have previously shown that L. pneumophila– induced tyrosine dephosphorylation of H. vermiformis detergent-soluble proteins is inhibited by the monovalent sugars Gal and GalNAc, by inhibiting bacterial attachment to the lectin (18). Hence, we tested whether tyrosine dephosphorylation of detergent-insoluble proteins was altered in the presence of Gal or GalNAc during infection of H. vermiformis by L. pneumophila. Another monovalent sugar, mannose, was used as a negative control since it does not block L. pneumophila–induced tyrosine dephosphorylation of H. vermiformis proteins (18). H. vermiformis were preincubated in the presence of Gal, GalNAc, or mannose and later infected for 40 min by L. pneumophila. Cell extracts were prepared and immunoblotted with antiphosphotyrosine antibody. Tyrosine dephosphorylation of detergent-insoluble proteins was completely blocked throughout the 40-min infection period in the presence of Gal and GalNAc but not mannose (Fig. 4). These results indicated that bacterial attachment to the lectin was essential to mediate induction of tyrosine dephosphorylation of the host detergent-insoluble proteins.
Figure 4.
Attachment of L. pneumophila to H. vermiformis is required to induce tyrosine dephosphorylation of detergent-insoluble proteins. H. vermiformis were preincubated for 15 min in the presence of 100 mM of either Gal, GalNAc, or mannose (mann) for 15 min and later coincubated with (+) or without (−) L. pneumophila for 30 min. Cell extracts were prepared and probed with antiphosphotyrosine antibody. Arrowheads indicate prominent tyrosine-dephosphorylated proteins. Results are representative of three independent experiments.
Attachment but Not Entry of L. pneumophila Is Sufficient to Induce Tyrosine Dephosphorylation of H. vermiformis Proteins.
Next, we asked whether entry of L. pneumophila was essential to induce tyrosine dephosphorylation of host cell proteins or that bacterial attachment was sufficient to induce these host cell processes. We used two strategies to answer these questions.
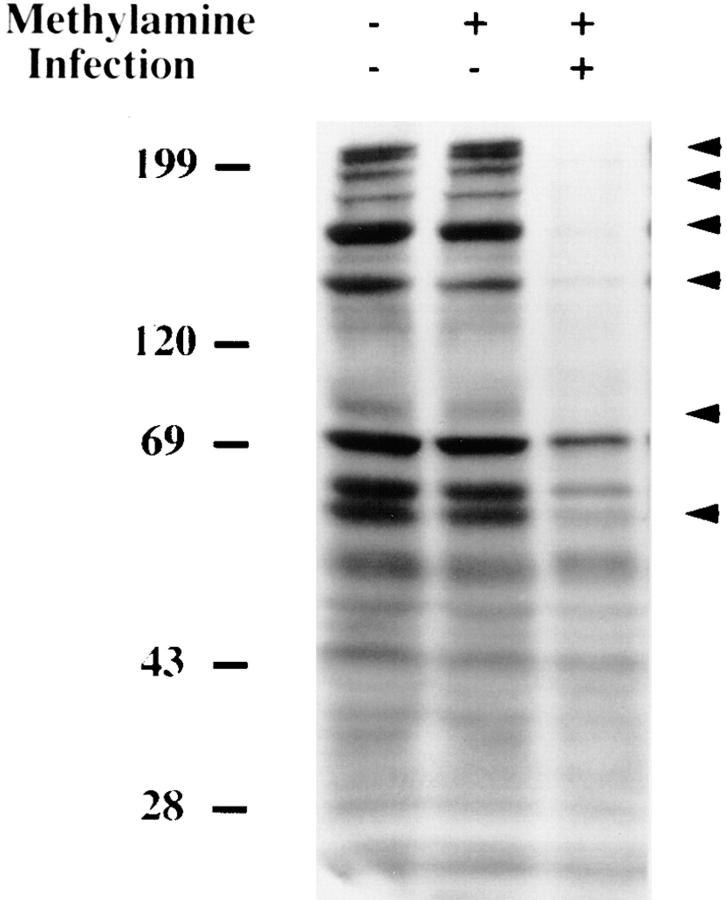
First, we used methylamine, an inhibitor of receptor-mediated endocytosis (31) that has been shown to block entry of L. pneumophila into protozoa, including H. vermiformis (15, 21, 32). H. vermiformis was pretreated with 50 mM of methylamine for 30 min and later infected with L. pneumophila. Under these conditions, methylamine caused complete inhibition of bacterial entry, as confirmed by gentamicin protection assays (18), but had no effect on the number of attached bacteria compared with untreated cells (data not shown). Phosphotyrosine analysis of H. vermiformis total proteins before and after infection showed that inhibition of bacterial entry did not block bacteria-induced tyrosine dephosphorylation of host cell proteins (Fig. 5). Incubation with methylamine did not alter the pattern of protein tyrosine phosphorylation in uninfected cells (Fig. 5, lane 2). These results indicated that entry of L. pneumophila into H. vermiformis was not required to induce tyrosine dephosphorylation of host proteins, and that bacterial attachment was sufficient to induce tyrosine dephosphorylation of H. vermiformis proteins. The increased level of bacteria-induced tyrosine dephosphorylation under these conditions (compared with Fig. 1 A) may be due to continuous attachment of the bacteria in the absence of uptake throughout the infection period.
Figure 5.
L. pneumophila–induced tyrosine dephosphorylation of H. vermiformis proteins, is not inhibited by blocking bacterial entry. Tyrosine dephosphorylation of H. vermiformis by L. pneumophila in the presence of methylamine. Amebae were preincubated for 30 min with 50 mM methylamine and infected with L. pneumophila for 20 min. Cell extracts were immunoblotted with antiphosphotyrosine antibody. Results are representative of two independent experiments.
Our second strategy was to examine whether mutants of L. pneumophila that were defective in entry into H. vermiformis were capable of inducing tyrosine dephosphorylation of H. vermiformis. Among a bank of 5,200 mini Tn10::kan insertion mutants of L. pneumophila (15), we isolated 10 mutants that were defective in invasion of H. vermiformis using gentamicin protection assays, as we described previously (18). Their invasion level was 3–79% of the parent strain AA100 (Fig. 6 A). Interestingly, similar to the parent strain, the mutants were capable of inducing tyrosine dephosphorylation of H. vermiformis proteins (Fig. 6 B). These data supported our observations that entry of L. pneumophila into H. vermiformis was not required to induce tyrosine dephosphorylation of host cell proteins. It is important to note that most of the tested mutants are defective in attachment to H. vermiformis, indicating that the bacterial ligand that recognizes the lectin is intact in these mutants (32).
Figure 6.

Invasion-defective mutants of L. pneumophila are capable of inducing tyrosine dephosphorylation of H. vermiformis proteins. (A) H. vermiformis was infected for 1 h with an equivalent number of bacteria of the parent strain AA100 or each of the 10 mutants. The percentage of invasion was derived by the number of internalized bacteria of each mutant compared with the parent strain. Extracellular bacteria were killed by gentamicin treatment, and the number of intracellular bacteria was determined (18). This is representative of three independent experiments. (B) Phosphotyrosine immunoblot of detergent-soluble extracts of uninfected H. vermiformis or infected by the parent strain AA100 or the mutants for 20 min. The middle arrowhead indicates the 170-kD Gal/GalNAc lectin, whereas the other arrowheads represent other proteins that underwent tyrosine dephosphorylation upon infection. Results are representative of two independent experiments.
Ultrastructural Characteristics of Entry of L. pneumophila into H. vermiformis.
Tyrosine dephosphorylation of many cytoskeletal proteins in H. vermiformis upon attachment and invasion by L. pneumophila suggested disassembly of the cytoskeleton (6–9). In addition, cytoskeletal disruption of H. vermiformis by cytochalasin D does not interfere with bacterial entry (21). In contrast, uptake of L. pneumophila by human macrophages occurred through a coiling phagocytic mechanism, and the uptake is inhibited by cytochalasin D (15, 21, 33). These observations indicate that uptake of L. pneumophila by H. vermiformis is different from that of mammalian macrophages. Therefore, we examined the mode of bacterial entry into H. vermiformis by transmission electron microscopy. Among 73 internalization events examined, 5 (7%) were manifested as coiling phagocytosis (Fig. 7, A and B; reference 33). It will be interesting to test whether the coiling phagocytosis seen during bacterial uptake by H. vermiformis is affected by cytochalasin D. 68 (93%) uptake events were manifested as a cup-shaped invagination of the plasma membrane (Fig. 7, C and D). These internalization events were noncoated endocytic processes, differing from the classical receptor-mediated endocytosis of the majority of ligands through clathrin-coated pits (34). It is important to note that no direct contact between the bacteria and plasma membrane of the amebae was detected.
Figure 7.
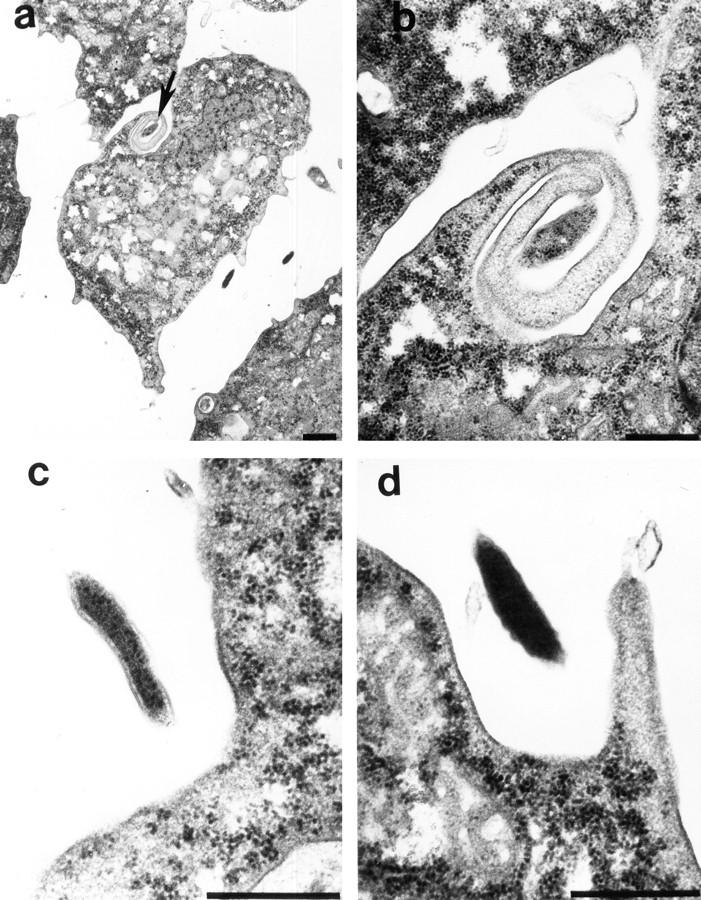
Transmission electron micrographs of uptake of L. pneumophila by H. vermiformis. (a) Coiling phagocytosis is indicated by the arrow, and the pseudopodia-like coil surrounding L. pneumophila is enlarged in b. c and d are different stages of invagination of the plasma membrane of H. vermiformis to internalize L. pneumophila. Bars: (a) 1 μm; (b–d) 0.5 μm.
Discussion
Our data showed that attachment of L. pneumophila to the Gal/GalNAc lectin receptor of H. vermiformis induces a prominent tyrosine dephosphorylation of many host cell proteins, including the Gal/GalNAc lectin receptor (18) and several putative cytoskeletal protein homologues. Bacterial attachment but not invasion is essential and sufficient to induce protein tyrosine dephosphorylation in H. vermiformis. Inhibition of bacterial attachment by Gal or GalNAc effectively blocked bacterial-induced tyrosine dephosphorylation of H. vermiformis proteins. Inhibition of bacterial invasion of H. vermiformis had no detectable effect on the ability of the bacteria to induce tyrosine dephosphorylation of host cell proteins. Moreover, L. pneumophila mutants defective in invasion of H. vermiformis were as efficient as the wild-type strain in their ability to induce tyrosine dephosphorylation of host proteins. Tyrosine dephosphorylation of the cytoskeletal protein homologues suggested disruption of the cytoskeleton upon bacterial attachment.
The Gal/GalNAc lectin homologue of E. histolytica is a β2-like integrin (19, 20). In general, binding of a ligand to the extracellular domain of the integrin results in tyrosine phosphorylation of the cytoplasmic domain of the β chain of the integrin, clustering of the integrin receptor, and recruitment of many regulatory and cytoskeletal proteins (3, 35). Tyrosine phosphorylation of the cytoplasmic domain of integrins is important for internalization and targeting of the ligands to the appropriate endosomal compartment (36, 37). Disruption of tyrosine phosphorylation of the cytoplasmic domain of integrins blocks ligand internalization (35, 36, 38), disrupts cytoskeletal rearrangements (3, 35), or alters targeting of the internalized ligand through the endosomal pathway (37). Interestingly, invasion of mammalian cells by the intracellular bacterium, Yersinia pseudotuberculosis is mediated by binding to β1 integrin and is enhanced by mutations in the cytoplasmic domain of the integrin that disrupts its association with the cytoskeleton (39). Similar substitutions of residues in the cytoplasmic tail of the low density lipoprotein receptor results in the rapid endocytosis of the receptor (40). These studies suggest that mutations that disrupt association between the receptor and cytoskeletal components favor efficient internalization of certain ligands (39).
Disassembly of focal contacts and disruption of cytoskeletal rearrangements involves activation of protein tyrosine phosphatases (6, 7). In addition, inhibitors of tyrosine kinases inhibit the formation of focal contacts (3). It is tempting to speculate that tyrosine dephosphorylation of the lectin and the putative cytoskeletal protein homologues are collectively indicative of bacterial attachment-mediated cytoskeletal disruption of the protozoan host. These predictions are supported by the observations that uptake of L. pneumophila by H. vermiformis is not blocked by cytoskeletal disruption of the protozoan host (21, 32). Similarly, uptake of the intracellular bacterium Chlamydia by epithelial cells is also not affected by disruption of the host cytoskeleton (41). In addition, invasion of mammalian cells by many viruses is associated with disruption of the cytoskeleton (42).
Transmission electron microscopy in this study showed that a small proportion of bacterial uptake occurs by coiling phagocytosis. No coated pits are observed in the coil structure. This mode of uptake is morphologically similar to the coiling phagocytosis of complement-opsonized L. pneumophila by human monocytes (33). However, most of the uptake events of L. pneumophila by H. vermiformis occur by a cup-shaped invagination of the plasma membrane through which the bacterium sinks into the host cell. In addition, there is no direct contact between the bacterium and the plasma membrane in this invagination. We think that initial contacts are made between L. pneumophila and H. vermiformis followed by signaling of the protozoan host to take up the bacteria by a trigger and not a zipper mode of uptake (43).
Based on our current data and previous studies (18, 21, 22), we propose the following model of attachment and invasion of the protozoan host H. vermiformis by the Legionnaires' disease bacterium, L. pneumophila. Attachment of L. pneumophila to the Gal/GalNAc lectin receptor of H. vermiformis is associated with induction of a tyrosine phosphatase activity that results in dephosphorylation of the lectin receptor (18) and several cytoskeletal proteins. We propose that these alterations in the protozoan host are manifested in disruption of the cytoskeleton to avoid shedding of the bacteria. These events are associated with, or followed by, a triggering mechanism of receptor-mediated endocytosis to internalize L. pneumophila. In addition, bacterial attachment and invasion is also associated with induced expression of specific protozoan genes and these induced genes may be important for entry of L. pneumophila (22). Some of the induced gene products may be the lectin receptor or its associated proteins. The internalized bacteria are targeted into a RER-surrounded phagosome that is excluded from the classical endosomal–lysosomal degradation pathway. Future studies will shed light on the intriguing ability of this intracellular bacterial pathogen to manipulate cell biological processes within two evolutionarily distant hosts, humans and protozoa.
Acknowledgments
This work is supported by grants AI21490 and AG05731 awarded to S. Bondada and grant R29AI38410 awarded to Y. Abu Kwaik.
Abbreviations used in this paper
- GalNAc
D(+)-Galactose, N-acetyl-d-galactosamine
- HRP
horseradish peroxidase
- mil
mammalian-specific infectivity
- moi
multiplicity of infection
- RER
rough endoplasmic reticulum
Footnotes
The authors gratefully thank Dr. E. Tannich for the generous gift of the anti Gal/GalNAc lectin antiserum. We thank Drs. Charles E. Snow and Thomas L. Roszman for their critical and helpful discussion and comments on the manuscript and Mr. Michael Rock for technical help in screening for various cytoskeletal proteins in human T lymphocytes and H. vermiformis.
References
- 1.Finlay BB, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 2.Menard R, Dehio C, Sansonetti PJ. Bacterial entry into epithelial cells: the paradigm of Shigella. . Trends Microbiol. 1996;4:220–226. doi: 10.1016/0966-842X(96)10039-1. [DOI] [PubMed] [Google Scholar]
- 3.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 4.Heldin C-H. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto S, Teramoto H, Coso OA, Sutkind S, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duntlevy JR, Couchman JR. Controlled induction of focal adhesion disassembly and migration in primary fibroblasts. J Cell Sci. 1993;105:489–500. doi: 10.1242/jcs.105.2.489. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura TY, Yamamoto I, Nishitani H, Matozaki T, Suzuki T, Wakabayashi S, Shigekawa M, Goshima K. Detachment of cultured cells from the substratum induced by the neutrophil-derived oxidant NH2Cl: synergistic role of phosphotyrosine and intracellular Ca2+concentration. J Cell Biol. 1995;131:509–524. doi: 10.1083/jcb.131.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defilippi P, Retta SF, Olivo C, Palmieri M, Venturino M, Silengo L, Tarone G. p125FAKtyrosine phosphorylation and focal adhesion assembly: studies with phosphotyrosine phosphatase inhibitors. Exp Cell Res. 1995;221:141–152. doi: 10.1006/excr.1995.1361. [DOI] [PubMed] [Google Scholar]
- 9.Serra-Pages C, Kedershal NL, Fazikas L, Medley Q, Debant A, Streuli M. The LAR transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting protein co-localize at focal adhesions. EMBO (Eur Mol Biol Organ) J. 1995;14:2827–2838. doi: 10.1002/j.1460-2075.1995.tb07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields BS. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 11.Abu Kwaik, Y., L.-Y. Gao, O.S. Harb, and B.J. Stone. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophilaand phenotypic characterization of a null mutant. Mol Microbiol. 1997;24:629–642. doi: 10.1046/j.1365-2958.1997.3661739.x. [DOI] [PubMed] [Google Scholar]
- 12.Cirillo JD, Tompkins LS, Falkow S. Growth of Legionella pneumophila in Acanthamoeba castellaniienhances invasion. Infect Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brieland JK, Fantone JC, Remick DG, LeGendre M, McClain M, Engleberg NC. The role of Legionella pneumophila-infected Hartmannella vermiformisas an infectious particle in a murine model of Legionnaires' disease. Infect Immun. 1997;65:4892–4896. doi: 10.1128/iai.65.12.5330-5333.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu Kwaik, Y. The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformisis surrounded by the rough endoplasmic reticulum. Appl Environ Microbiol. 1996;62:2022–2028. doi: 10.1128/aem.62.6.2022-2028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao L-Y, Harb OS, Abu Y, Kwaik Utilization of similar mechanisms by Legionella pneumophilato parasitize two evolutionarily distant hosts, mammalian and protozoan cells. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao L-Y, Harb OS, Abu Y, Kwaik Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophilathat are not required for infectivity of protozoa. Infect Immun. 1998;66:883–892. doi: 10.1128/iai.66.3.883-892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone BJ, Abu Y, Kwaik Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect Immun. 1998;66:1768–1775. doi: 10.1128/iai.66.4.1768-1775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkataraman C, Haack BJ, Bondada S, Abu Y, Kwaik Identification of a Gal/GalNAc lectin in the protozoan Hartmannella vermiformis as a potential receptor for attachment and invasion by the Legionnaires' disease bacterium, Legionella pneumophila. . J Exp Med. 1997;186:537–547. doi: 10.1084/jem.186.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tannich E, Ebert F, Horstmann RD. Primary structure of the 170-kD surface lectin of pathogenic Entamoeba histolytica. . Proc Natl Acad Sci USA. 1991;88:1849–1853. doi: 10.1073/pnas.88.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams SA, Robson SC, Gathiram V, Jackson TFHG, Pillay TS, Kirsch RE, Makgoba MW. Immunological similarity between the 170 kD amoebic adherence glycoprotein and human β2 integrins. Lancet. 1993;341:17–19. doi: 10.1016/0140-6736(93)92483-a. [DOI] [PubMed] [Google Scholar]
- 21.King CH, Fields BS, Shotts EB, Jr, White EH. Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophilain amoebae and human monocyte-like cells. Infect Immun. 1991;59:758–763. doi: 10.1128/iai.59.3.758-763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu Kwaik, Y., B.S. Fields, and N.C. Engleberg. Protein expression by the protozoan Hartmannella vermiformis upon contact with its bacterial parasite Legionella pneumophila. . Infect Immun. 1994;62:1860–1866. doi: 10.1128/iai.62.5.1860-1866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwiatkowska K, Sobota A. Local accumulation of α-spectrin-related protein under plasma membrane during capping and phagocytosis in Acanthamoeba. . Cell Motil Cytoskeleton. 1997;36:253–265. doi: 10.1002/(SICI)1097-0169(1997)36:3<253::AID-CM6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Santiago A, Carbajal ME, Benitez-King G, Meza I. Entamoeba histolytica: PKC transduction pathway activation in the trophozoite-fibronectin interaction. Exp Parasitol. 1994;79:436–444. doi: 10.1006/expr.1994.1105. [DOI] [PubMed] [Google Scholar]
- 25.Bailey GB, Shen PS, Beanan MJ, McCoomer NE. Actin associated proteins of Entamoeba histolytica. . Arch Med Res. 1992;23:129–132. [PubMed] [Google Scholar]
- 26.Vazquez-Prado J, Meza I. Fibronectin “receptor” in Entamoeba histolytica: purification and association with the cytoskeleton. Arch Med Res. 1992;23:125–128. [PubMed] [Google Scholar]
- 27.Vazquez J, Franco E, Reyes G, Meza I. Characterization of adhesion plates induced by the interaction of Entamoeba histolyticatrophozoites with fibronectin. Cell Motil Cytoskeleton. 1995;32:37–45. doi: 10.1002/cm.970320105. [DOI] [PubMed] [Google Scholar]
- 28.Pe'rez E, Munoz MDL, Ortega A. Entamoeba histolytica: Involvement of pp125FAKin collagen-induced signal transduction. Exp Parasitol. 1996;82:164–170. doi: 10.1006/expr.1996.0021. [DOI] [PubMed] [Google Scholar]
- 29.Abu Kwaik, Y., B.I. Eisenstein, and N.C. Engleberg. Phenotypic modulation by Legionella pneumophilaupon infection of macrophages. Infect Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fields BS, Nerad TA, Sawyer TK, King CH, Barbaree JM, Martin WT, Morrill WE, Sanden GN. Characterization of an axenic strain of Hartmannella vermiformisobtained from an investigation of nosocomial legionellosis. J Protozool. 1990;37:581–583. doi: 10.1111/j.1550-7408.1990.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 31.Davies PJA, Davies DR, Levitzki A, Maxfield FR, Milhaud P, Willingham MC, Pastan IH. Transglutaminase is essential in receptor-mediated endocytosis of α2-macroglobulin and polypeptide hormones. Nature. 1980;283:162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- 32.Harb OS, Venkataraman C, Haack BJ, Gao L-Y, Abu Y, Kwaik Heterogeneity in the attachment and uptake mechanisms of the Legionnaires' disease bacterium, Legionella pneumophila, by protozoan hosts. Appl Environ Microbiol. 1998;64:126–132. doi: 10.1128/aem.64.1.126-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horwitz MA. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila)occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz AL. Receptor cell biology: receptor-mediated endocytosis. Pediatr Res. 1995;38:835–843. doi: 10.1203/00006450-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg S, Chang P, Silverstein SC. Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1993;177:529–534. doi: 10.1084/jem.177.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fallon RJ, Danaher M, Slayors RL, Saxena A. Defective asialoglycoprotein receptor endocytosis mediated by tyrosine kinase inhibitors. Requirement for a tyrosine in the receptor internalization signal. J Biol Chem. 1994;269:11011–11017. [PubMed] [Google Scholar]
- 37.Weiser P, Muller R, Braun U, Reth M. Endosomal targeting by the cytoplamic tail of membrane immunoglobulin. Science. 1997;276:407–411. doi: 10.1126/science.276.5311.407. [DOI] [PubMed] [Google Scholar]
- 38.Yokota A, Yukawa K, Yamamoto A, Sugiyama K, Suemura M, Tashiro Y, Kishimoto T, Kikutani H. Two forms of low-affinity Fc receptor for IgE differentially mediate endocytosis and phagocytosis: identification of the critical cytoplasmic domains. Proc Natl Acad Sci USA. 1992;89:5030–5034. doi: 10.1073/pnas.89.11.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nhieu GTV, Krukonis ES, Reszka AA, Horwitz AF, Isberg RR. Mutations in the cytoplasmic domain of the integrin β1 chain indicate a role for endocytosis factors in bacterial internalization. J Biol Chem. 1996;271:7665–7672. doi: 10.1074/jbc.271.13.7665. [DOI] [PubMed] [Google Scholar]
- 40.Davis CG, van Driel IR, Russel DW, Brown MS, Goldstein JL. The low density lipoprotein receptor. Identification of amino acids in cytoplasmic domain required for rapid endocytosis. J Biol Chem. 1987;262:4075–4082. [PubMed] [Google Scholar]
- 41.Reynolds DJ, Pearce JH. Characterization of cytochalasin D-resistant (pinocytic) mechanisms of endocytosis utilized by chlamydiae. Infect Immun. 1990;58:3208–3216. doi: 10.1128/iai.58.10.3208-3216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cudmore S, Reckmann I, Way M. Viral manipulations of the actin cytoskeleton. Trends Microbiol. 1997;5:142–148. doi: 10.1016/S0966-842X(97)01011-1. [DOI] [PubMed] [Google Scholar]
- 43.Swanson JA, Baer SC. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5:89–93. doi: 10.1016/s0962-8924(00)88956-4. [DOI] [PubMed] [Google Scholar]