Abstract
The expression of the murine interleukin (IL)-2 receptor α chain/CD25 is strongly induced at the transcriptional level after T cell activation. We show here that nuclear factor of activated T cell (NF-AT) factors are involved in the control of CD25 promoter induction in T cells. NF-ATp and NF-ATc bind to two sites around positions −585 and −650 located upstream of the proximal CD25 promoter. Immediately 3′ from these NF-AT motifs, nonconsensus sites are located for the binding of AP-1–like factors. Mutations of sites that suppress NF-AT binding impair the induction and strong NF-ATp–mediated transactivation of the CD25 promoter in T cells. In T lymphocytes from NF-ATp–deficient mice, the expression of CD25 is severely impaired, leading to a delayed IL-2 receptor expression after T cell receptor (TCR)/CD3 stimulation. Our data indicate an important role for NF-AT in the faithful expression of high affinity IL-2 receptors and a close link between the TCR-mediated induction of IL-2 and IL-2 receptor α chain promoters, both of which are regulated by NF-AT factors.
Keywords: interleukin 2 receptor, nuclear factor of activated T cells, transcription factors, T cells, NF-AT factors
The high affinity IL-2 receptor consists of three individual polypeptides, the α, β, and γ chains. Although the β and γ chains are shared by other lymphokine receptors, the α chain (CD25) is restricted to the IL-2 receptor, and is expressed by a variety of lymphoid cells (for review see reference 1). The induction of CD25 in T cells is controlled at the transcriptional level through two DNA sequence elements, a proximal promoter/enhancer spanning the nucleotides between positions −54 and −584 in the mouse and −64 and −276 in humans, and a distal enhancer spanning ∼80 nucleotides around position −1350 in the mouse and −3750 (or −4150, according to another nomenclature) in the human CD25 gene (2–6). The activity of the promoter is rapidly induced by TCR-mediated signals or IL-1, and is controlled by an array of transcription factors, in particular by nuclear factor (NF)-κB, Elf-1, SRF, and HMG I(Y). The induction of the distal enhancer is controlled by IL-2, which induces signal transducer and activator of transcription (Stat)5, a member of the family of Stat transcription factors. Stat5 binds in concert with Elf-1, HMG I(Y), and GATA factors to multiple sites of the distal enhancer and contributes to its IL-2–mediated full expression in activated peripheral T lymphocytes (4–6).
Nuclear factor of activated T cell (NF-AT) factors comprise a family of transcription factors that contribute to the induced expression of numerous lymphokine and receptor genes in T cells. Similar to NF-κB factors, the nuclear translocation and activity of NF-AT factors is stimulated by TCR-mediated signals (for review see reference 7). The DNA-binding domains of NF-AT and NF-κB/Rel factors share a common architecture (8) and, therefore, recognize overlapping DNA sequence motifs. These common properties between NF-AT and NF-κB (a major regulator of the CD25 promoter), and reports on the inhibition of CD25 expression by cyclosporin A (9) (an inhibitor of phosphatase calcineurin and, therefore, of nuclear translocation of NF-AT; reference 7), prompted us to investigate whether NF-AT factors participate in CD25 promoter control. We show here that NF-ATp and NF-ATc bind to two sites located immediately upstream of the proximal CD25 promoter. Mutations within the NF-AT sites that suppress NF-AT binding impair CD25 promoter induction. Accordingly, the induction of CD25 is markedly delayed in T cells from NF-ATp–deficient mice. These findings implicate an important role for NF-AT factors in the inducible expression of high affinity IL-2 receptors after T cell activation.
Materials and Methods
Cell Culture, Construction, and Transfection of CD25 Promoter Luciferase Plasmids.
Murine El4 T thymoma cells and human Jurkat T leukemia cells were grown in RPMI medium containing 5% FCS. 2 × 107 cells were transfected using the DEAE dextran protocol with 2.5 μg DNA of the CD25 promoter-luciferase reporter constructs alone or 0.5–2.5 μg DNA of reporter constructs (as indicated in the figure legends) along with 2 μg of a pLGP3-based vector expressing full-length murine NF-ATp (NF-AT1-C; reference 10) or an RSV-LTR vector expressing human NF-ATc. Human 293 embryonic kidney cells were cultured in DMEM and transfected using a calcium phosphate transfection protocol. The luciferase reporter gene construct contains the wild-type murine CD25 promoter spanning the nucleotides up to position −2556 (4). Mutations in one or both of the NF-ATp binding sites around positions −585 and −650 were introduced into the promoter fragment from +1 to −800 using the QuikChangeTM site-directed mutagenesis kit (Stratagene Corp., La Jolla, CA) according to the manufacturer's instructions.
The following oligonucleotides were used for the mutagenesis of NF-AT sites:
(i) (−667) GCTAGACTTAAAATCTATCATTGCAGCTGTAAACAC (−632)
CGATCTGAATTTTAGATAGTAACGTCGACATTTGTG; and
(ii) (−596) CCCACACCCATGATACTATGAATCGTGCATCAGAG (−562)
GGGTGAGGGTACTATGATACTTAGCACGTAGTCTC
The underlined nucleotides indicate the mutations.
Immunofluorescence and Flow Cytometry.
For Ab stainings, 2–8 × 105 cells were incubated on ice with mAbs at saturating concentrations. Fluorescein- and PE-labeled mAbs (Pharmingen, San Diego, CA) were used for two- and three-color immunofluorescence. For three-color flow cytometry, cells were stained first with biotinylated mAbs (PharMingen) for 15 min and were subsequently incubated with streptavidin-Red670 (GIBCO BRL, Eggenstein, Germany) and FITC- and PE-labeled mAbs for 15 min. Results obtained after analysis on a FACScan® flow cytometer (Becton Dickinson, Mountain View, CA) using Lysys II software (Becton Dickinson) are shown as log dot-plots or histograms.
DNase I Footprint Protection Assays and EMSAs.
In DNase I footprint protection assays, end-labeled DNA probes were prepared using [γ-32P]ATP and polynucleotide kinase. 104 cpm (∼0.2 ng) of the following DNA fragments from the murine CD25 promoter (4) were used: (a) the HindIII–SacII fragment spanning the nucleotides from position +94 to −268; and (b) the SacII–BglII fragment spanning the nucleotides from −268 to −801. Fragment (a) was recut with EspI, and fragment (b) with DraI generating DNA fragments of ∼150–300 bp. These were incubated for 60 min with a bacterially expressed glutathione S-transferase (GST)–NF-ATp protein (11) containing the DNA-binding domain of murine NF-ATp. The samples were processed and fractionated on 6% polyacrylamide, 42% urea-sequencing gels.
Electromobility shift assays (EMSAs) were performed as previously described (11), using 2 μg nuclear proteins and 0.5 μg poly [d(I-C)]. In supershift EMSAs, 0.5 μg of either an NF-ATp–specific Ab (Cat. no. 06-348; UBI) or an NF-ATc–specific mAb (7A6) (12) were added to the incubations. When the DNA binding of GST–NF-ATp was tested, 0.5–1.5 μg of bacterial proteins prepared by affinity column chromatography (11) were incubated along with 0.5 μg poly [d(I-C)]. The following oligonucleotides were used as probes:
(iii) (−596)gatcCCCACACCCATGGAACTATGAATCGTG (−571)
GGGTGTGGGTACCTTGATACTTAGCACctag;
(iv) (−663)gatcGACTTAAAATCTTCCATTGCAGCTGTA (−635)
CTGAATTTTAGAAGGTAACGTCGACATctag; and
(v) (−667)GCTAGACTTAAAATCTTCCATTGCAGCTGTAAACAC (−632)
CGATCTGAATTTTAGAAGGTAACGTCGACATTTGTG
The small letters indicate linker nucleotides.
Results and Discussion
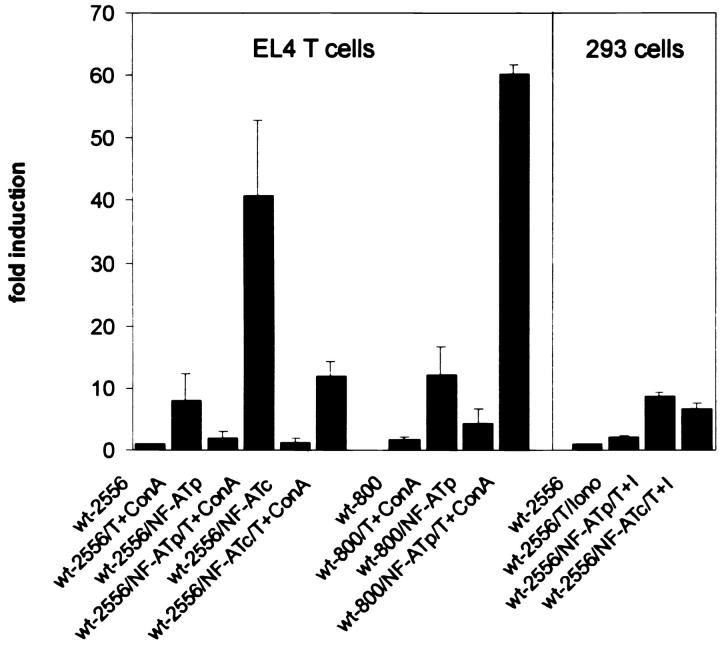
To determine whether the CD25 promoter is a target for NF-AT, we cotransfected a CD25 promoter–driven luciferase reporter gene with NF-ATp– and NF-ATc–specific expression vectors into El4 T and 293 cells. Treatment of 293 cells with TPA plus ionomycin (T+I) led to a <2-fold, and treatment of El4 cells with T+Con A led to an 8–9-fold, induction of activity of CD25 promoter spanning the nucleotides up to position −2556 (4), and to a 12-fold induction of a shorter CD25 promoter reaching up to −800. Cotransfection of an NF-ATp expression vector into El4 cells resulted in a strong, 40-fold induction of activity of the longer CD25 promoter and in an up to 60-fold induction of the shorter CD25 promoter fragment after T+Con A treatment of cells (Fig. 1). Cotransfection with the NF-ATc vector gave rise to only a slight increase in promoter activity. In 293 cells, the overexpression of both NF-AT factors resulted in a six- to ninefold increase in CD25 promoter activity (Fig. 1).
Figure 1.
NF-ATp transactivates the murine CD25 promoter in T cells. 2.5 μg DNA of luciferase reporter gene constructs controlled by murine CD25 promoters up to position −2556 (wt-2556) and −800 (wt-800) were transfected into murine El4 T thymoma cells or human embryonic 293 kidney cells, along with an empty RSV-based expression vector or vectors expressing NF-ATp (10) or NF-ATc. The cells were induced as indicated for 18 h. To calculate the extent of induction, the activity of the CD25 wild-type promoter in nonstimulated cells was used as a reference point (onefold).
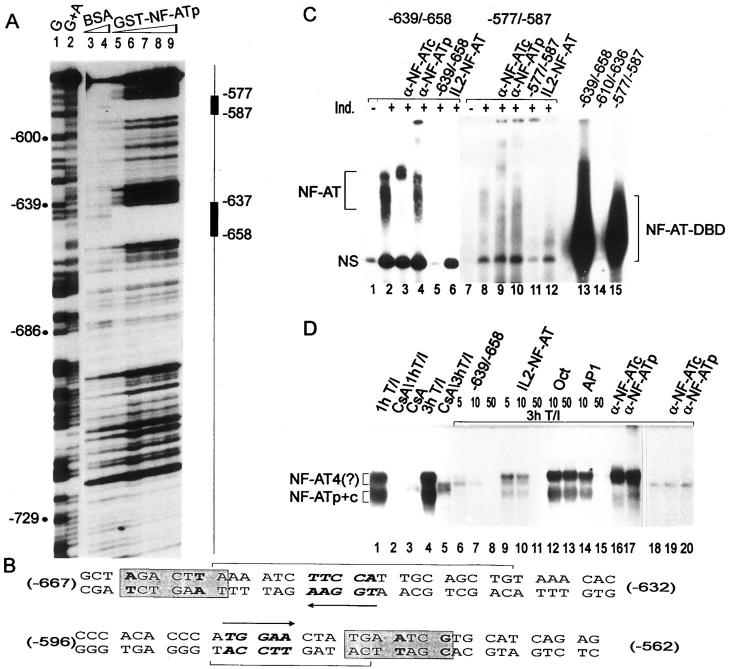
To demonstrate the binding of NF-ATp to the CD25 promoter, GST–NF-ATp encoding its DNA-binding domain was incubated with DNA fragments containing the first 800 bp of the promoter region in DNase I footprint protection assays. Two prominent footprints were detected, spanning the nucleotides from −577 to −587 and −639 to −658, respectively (Fig. 2 A). These comprise the NF-AT “core” binding sequence TGGAA (7) in opposite orientations (Fig. 2 B). When probes of these oligonucleotides were incubated with GST–NF-ATp in EMSAs, both probes were bound by NF-ATp, whereas a third probe spanning the unprotected nucleotides from −619 to −636 was unable to bind (Fig. 2 C, lanes 13–15). Using nuclear proteins from murine splenocytes, the generation of typical inducible NF-AT complexes was detected with the −639/−658 probe (Fig. 2 C, lanes 1–6) and, although far more weakly, with the −577/−587 probe (Fig. 2 C, lanes 7–12). The generation of these complexes was enhanced after induction of cells with T+I (lanes 1, 2, 7, and 8) and efficiently competed with a 100-fold molar excess of the distal IL-2 NF-AT site (Fig. 2 C, lanes 6 and 12). Moreover, the complexes were supershifted in EMSAs using NF-ATp– and NF-ATc–specific Abs (lanes 3, 4, 9, and 10).
Figure 2.
Binding of NF-AT to the CD25 promoter. (A) DNase I footprint protection assay. A CD25 promoter probe spanning the nucleotides from −548 to −804 was incubated with 3 and 5 μg BSA (lanes 3 and 4) or 1–5 μg GST–NF-ATp protein (lanes 5–9). The NF-ATp–specific footprints are indicated. G and G+A, chemical sequencing reactions. (B) Sequences of footprint regions. The footprints are indicated in brackets. The arrows indicate the direction of TGGAA NF-AT “core” motifs. The TPA responsive element–like sequence motifs 3′ from the NF-AT motifs are boxed. (C) EMSAs with the NF-AT sites. In lanes 1–12, nuclear proteins from murine splenocytes are shown, and in lanes 13–15, GST–NF-ATp was used with the −639/−658 (lanes 1–6 and 13) and −577/−587 probes (lanes 7–12 and 15). In lane 14, a probe of the −610/−636 site was used as a control. 2 μg of nuclear proteins from uninduced splenocytes (−) or splenocytes induced for 2 h with T+I (+) were incubated in the absence or presence of 0.5 μg NF-ATc– or NF-ATp–specific Abs, or in the presence of a 200-fold molar excess of homologous unlabeled probes or distal IL-2 NF-AT site as indicated. Note that the autoradiograph of lanes 1–6 was exposed for 16 h, and that of lanes 7–12 for 3 d. NS, nonspecific complex. Free probes are cut off. (D) EMSAs of the −639/−658 site using nuclear proteins from Jurkat cells. A probe of the −639/−658 site was incubated with 2 μg of nuclear proteins from Jurkat cells treated with T+I in the absence or presence of 100 ng/ml cyclosporin A (CsA). For competition the following DNAs were used: 5–50 ng of the cold −639/−658 site and the distal IL-2 NF-AT site, or 10 and 50 ng of the proximal IL-2 octamer site (11) and a consensus AP-1 site. In lanes 16 and 17, 0.5 μg of NF-AT–specific Abs were added. In lanes 18–20, a −632/−667 probe mutated in the NF-AT site (see oligonucleotide a in Materials and Methods) was incubated without or with NF-AT–specific Abs.
The −639/−658 site corresponds to a high-affinity NF-AT binding site. This could be seen best in EMSA competition assays comparing the binding of NF-AT factors in nuclear protein preparations from T+I–induced Jurkat cells to this site and the distal NF-AT site of the murine IL-2 promoter (Fig. 2 D). Although 5–10 ng of the −639/−658 site was sufficient to suppress almost all NF-AT binding, 10–50 ng of the distal IL-2 NF-AT site was necessary to see the same effect (Fig. 2 D, lanes 6–11). 50 ng of an AP-1 site was also able to suppress NF-AT complex formation, whereas the same amount of the upstream promoter site, i.e., an efficient octamer but poor AP-1 site from the IL-2 promoter (11), was without effect on factor binding (Fig. 2 D, lanes 12–15). In contrast to the −639/−658 site, the −577/−587 NF-ATp binding site is a low-affinity NF-AT site (Fig. 2 C, lanes 7–12; note that lanes 7–12 were exposed five times longer than lanes 1–6).
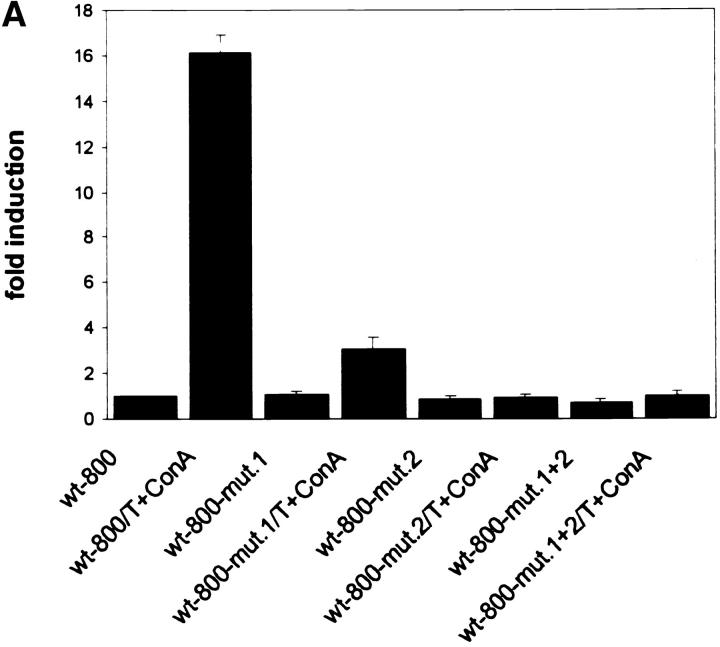
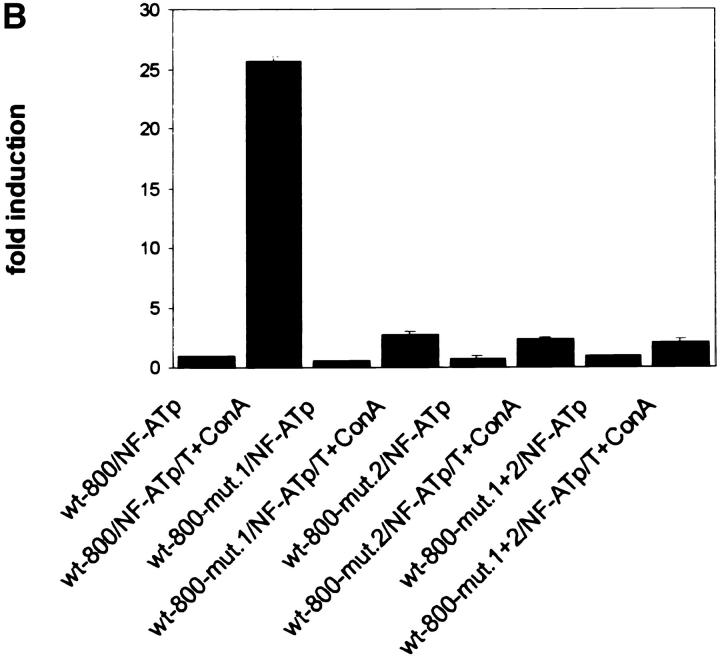
To demonstrate a functional role for the two NF-ATp sites, we introduced mutations into the NF-AT motifs of each site, or into both sites, in the context of the 800-bp wild-type CD25 promoter fragment. These mutations led to a loss of NF-AT binding in EMSAs using nuclear proteins from induced Jurkat cells (see Fig. 2 D, lanes 18–20) or GST–NF-ATp (data not shown). When the mutated CD25 promoter/luciferase constructs were transfected into El4 T cells alone or with an NF-ATp expression vector their induction was severely impaired compared with the wild-type promoter. The T+Con A–mediated 16-fold induction of the 800-bp CD25 promoter fragment was almost abolished (Fig. 3 A) and its >25-fold transactivation by NF-ATp was reduced to a 2–3-fold increase for the mutated promoter (Fig. 3 B).
Figure 3.
The NF-ATp sites contribute to the induction of CD25 promoter. (A) Mutations within the NF-ATp sites that suppress NF-AT binding interfere with the T+Con A–mediated CD25 promoter induction in El4 cells. 2.5 μg of luciferase constructs containing the wild-type CD25 promoter up to −800 bp or a promoter with mutations in the −658/−639 (mut. 1), the −587/−577 site (mut. 2), or in both sites (mut. 1+2) was transfected into El4 cells that were induced for 12 h. (B) Mutations of NF-AT sites suppress the NF-ATp–mediated transactivation of the CD25 promoter. 0.5 μg of the CD25 luciferase constructs was cotransfected with an NF-ATp expression vector into El4 cells that were stimulated for 12 h. To calculate the extent of induction, the activity of the CD25 wild-type promoter in nonstimulated cells was used as a reference point (onefold).
The importance of NF-ATp sites for the CD25 expression is underlined by defects in the CD25 surface expression on LN T cells from NF-ATp−/− mice established in our laboratory (13). When LN T cells from wild-type mice were stimulated with plate-bound α-CD3 Abs for 2–24 h in vitro, a marked increase of CD25 surface expression was detected after 6–12 h, which became even more pronounced after 24 h. Due to the strong stimulation of the CD25 promoter by secreted IL-2 (2), >50% of T cells express large amounts of CD25 48 h after stimulation (Fig. 4). On NF-ATp−/− LN T cells, CD25 expression was found to be distinctly delayed, becoming clearly detectable only 24 h after induction in spite of high, unimpaired IL-2 production of NF-ATp−/− T cells (13). In addition, fewer cells expressed high levels of CD25 after induction for 48 h (Fig. 4).
Figure 4.
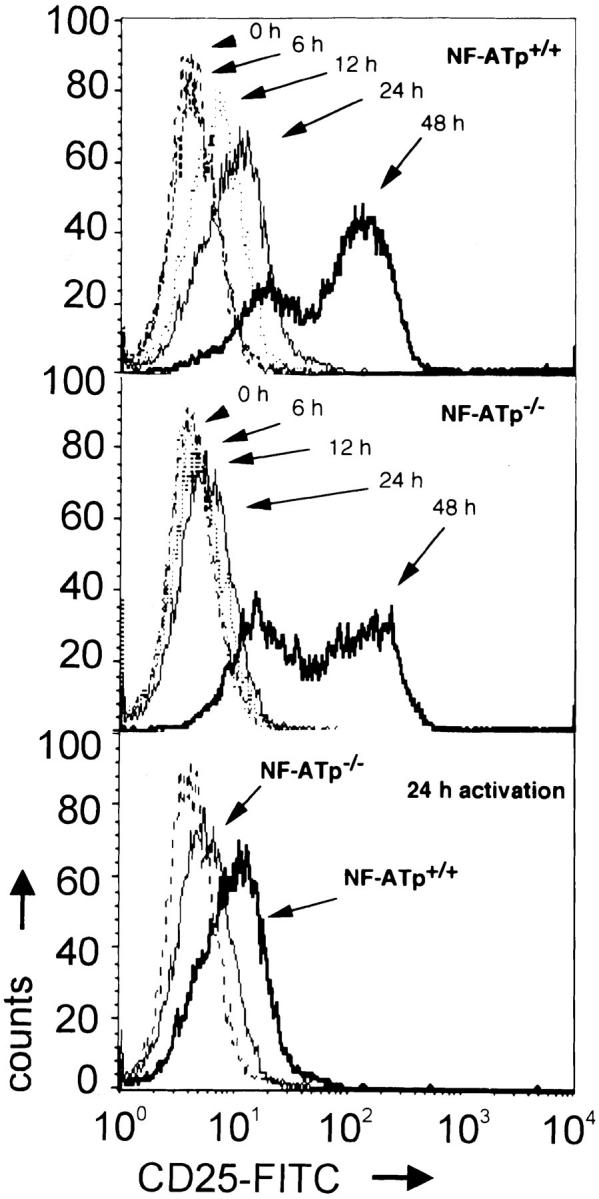
Impaired CD25 expression on NF-ATp−/− LN T cells. LN T cells from NF-ATp+/+ and NF-ATp−/− mice (13) were treated with plate-bound α-CD3 mAb in vitro. The cells were stained with mAbs directed against murine CD25 (7D4; PharMingen) and α/β-TCR (H57-597; PharMingen). The expression of CD25 on α/β-TCR+ LN cells is shown. The lowest panel shows a comparison of CD25 expression on NF-ATp+/+ and NF-ATp−/− TCR+ LN cells stimulated for 24 h. The dotted line indicates the isotype control staining of LN cells.
The two NF-ATp binding sites are located near the CD25 promoter and therefore appear to be involved in the rapid induction of the murine CD25 gene in resting T cells. The core sequences of these sites, TGGAA, differ slightly from the AGGAAAA core motifs of the IL-2 and IL-4 promoters and are the strongest NF-ATp binding sites in the human GM-CSF enhancer (14). As indicated in Fig. 2 B, 7–9 bp 3′ to the NF-AT motifs are situated TPA- responsive element–like sequences that might allow the concerted binding of AP-1 and NF-AT. In EMSAs we detected a specific binding of GST–c-Jun to both sites (data not shown), but it remains to be shown which proteins of the AP-1 family bind and regulate the CD25 promoter in vivo.
Finally, it should be pointed out that several properties of NF-ATp−/− mice, such as the impaired clonal deletion of T cells and expansion of lymphoid organs (13, 15, 16), are shared by the mice deficient for IL-2 and IL-2 receptors (17–19). We assume that the impaired CD25 expression might contribute to the development of this phenotype in NF-ATp−/− mice, which is reminiscent of other mice with defects in the IL-2 signaling system.
Acknowledgments
We are indebted to Daniela Räth and Heidi Runknagel for excellent technical assistance. We wish to thank Dr. M. Nabholz (Swiss Institute for Experimental Cancer Research, Epalinges, Switzerland) and Dr. A. Rao (Center for Blood Research and Department of Pathology, Harvard Medical School, Boston, MA) for DNA constructs.
This work was supported by grants from the Wilhelm-Sander-Stiftung (to E. Serfling) and the Deutsche Forschungsgemeinschaft, SFBs 165 and 465 (to A. Schimpl and E. Serfling).
Footnotes
The first two authors contributed equally to this paper.
References
- 1.Minami J, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 2.Sperisen P, Wang SM, Soldaini E, Pla M, Rusterholz C, Bucher P, Corthesy P, Reichenbach P, Nabholz M. Mouse interleukin-2 receptor α gene expression. Interleukin-1 and interleukin-2 control transcription via distinct cis-acting elements. J Biol Chem. 1995;277:10743–10752. doi: 10.1074/jbc.270.18.10743. [DOI] [PubMed] [Google Scholar]
- 3.John S, Reeves RB, Lin J-Y, Child R, Leiden JM, Thompson CB, Leonard WJ. Regulation of cell-type–specific interleukin-2 receptor α chain gene expression: potential role of physical interactions between Elf-1, HMG-I(Y), and NF-κB family proteins. Mol Cell Biol. 1995;15:1786–1796. doi: 10.1128/mcb.15.3.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serdobova I, Pla M, Reichenbach P, Sperisen P, Ghysdael J, Wilson A, Freeman J, Nabholz M. Elf-1 contributes to the function of the complex interleukin (IL)- 2–responsive enhancer in mouse IL-2 receptor α gene. J Exp Med. 1997;185:1211–1221. doi: 10.1084/jem.185.7.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John S, Robbins CM, Leonard WJ. An IL-2 response element in the human IL-2 receptor α chain promoter is a composite element that binds Stat5, Elf-1, HMG-I(Y) and a GATA family protein. EMBO (Eur Mol Biol Organ) J. 1996;15:5627–5635. [PMC free article] [PubMed] [Google Scholar]
- 6.Lecine P, Algarte M, Rameil P, Beadling C, Bucher P, Nabholz M, Imbert J. Elf-1 and Stat5 bind to a critical element in a new enhancer of the human interleukin-2 receptor α chain. Mol Cell Biol. 1996;16:6829–6840. doi: 10.1128/mcb.16.12.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 8.Wolf SA, Zhou P, Dötsch V, Chen L, You A, Ho SN, Crabtree GR, Wagner G, Verdine GL. Unusual Rel-like architecture in the DNA-binding domain of the transcription factor NF-ATc. Nature. 1997;385:172–176. doi: 10.1038/385172a0. [DOI] [PubMed] [Google Scholar]
- 9.Gauchat J-F, Khandjian EW, Weil R. Cyclosporin A prevents induction of the interleukin 2 receptor gene in cultured murine thymocytes. Proc Natl Acad Sci USA. 1986;83:6430–6434. doi: 10.1073/pnas.83.17.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo C, Burgeon E, Carew JA, McCaffrey PG, Badalian TM, Lane WS, Hogan PG, Rao A. Recombinant NFAT1 (NFATp) is regulated by calcineurin in T cells and mediates transcription of several cytokine genes. Mol Cell Biol. 1996;16:3955–3966. doi: 10.1128/mcb.16.7.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeuffer I, Klein-Hessling S, Heinfling A, Chuvpilo S, Escher C, Brabletz T, Hentsch B, Schwarzenbach H, Matthias P, Serfling E. Octamer factors exert a dual effect on the IL-2 and IL-4 promoters. J Immunol. 1994;153:5572–5585. [PubMed] [Google Scholar]
- 12.Northrop JP, Ho SN, Chen L, Thomas DJ, Timmerman LA, Nolan GP, Admon A, Crabtree GR. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 13.Schuh K, Kneitz B, Heyer J, Siebelt F, Fischer C, Jankevics E, Rüde E, Schmitt E, Schimpl A, Serfling E. NF-ATp plays a prominent role in the transcriptional induction of Th2-type lymphokines. Immunol Lett. 1997;57:171–175. doi: 10.1016/s0165-2478(97)00068-0. [DOI] [PubMed] [Google Scholar]
- 14.Cockerill PN, Bert AG, Jenkins F, Ryan GR, Shannon MF, Vadas M A. Human granulocyte-macrophage colony-stimulating factor enhancer function is associated with cooperative interactions between AP-1 and NFATp/c. Mol Cell Biol. 1995;15:2071–2079. doi: 10.1128/mcb.15.4.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodge MR, Ranger AM, de la Brousse FC, Hoey T, Grusby MJ, Glimcher LH. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 16.Xanthoudakis S, Viola JPB, Shaw KTY, Luo C, Wallace JD, Bozza PT, Curran T, Rao A. An enhanced immune response in mice lacking the transcription factor NFAT1. Science. 1996;272:892–895. doi: 10.1126/science.272.5263.892. [DOI] [PubMed] [Google Scholar]
- 17.Kneitz B, Herrmann T, Yonehara S, Schimpl A. Normal clonal expansion but impaired Fas-mediated cell death and anergy induction in interleukin-2–deficient mice. Eur J Immunol. 1995;25:2572–2577. doi: 10.1002/eji.1830250925. [DOI] [PubMed] [Google Scholar]
- 18.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Kündig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JL, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]