Abstract
Tumor necrosis factor (TNF) signaling leads to pleiotropic responses in a wide range of cell types, in part by activating antiapoptotic and proapoptotic signaling pathways. Thus, although TNF can cause apoptosis and may prove useful in the treatment of malignancies, most cells are resistant to TNF-induced cell death unless de novo protein synthesis is inhibited. Previous studies suggested that TNF activation of the nuclear factor (NF)-κB transcription factor family antagonizes the proapoptotic signals initiated by TNF-α. TNF receptor–associated factor (TRAF)2 has also been shown to mediate crucial antiapoptotic signals during TNF stimulation, yet is not essential in activation of NF-κB under physiologic conditions, thus raising questions about the relationship between these antiapoptotic pathways. We report here that inhibition of TRAF2 and NF-κB function in primary cells, by coexpression of a constitutive repressor of multiple NF-κB/Rel proteins (IκBα.DN) and a dominant negative form of TRAF2 (TRAF2.DN), synergistically enhanced TNF-induced apoptosis. The effects were stimulus dependent, such that neither inhibitory molecule affected Fas- and daunorubicin-induced apoptosis to the same degree as TNF-induced death. These findings indicate that the NF-κB and TRAF2 pathways activate independent antiapoptotic mechanisms which act in concert to suppress the proapoptotic signals induced by TNF-α.
Keywords: apoptosis, tumor necrosis factor receptor–associated factor 2, nuclear factor κB, tumor necrosis factor, signal transduction
Oligomerization of TNFR1 by TNF-α results in multiple intracellular changes, including activation of the transcription factor nuclear factor (NF)-κB, the c-Jun NH2-terminal kinase (JNK), and apoptosis (1). These events are initiated by the TNF-dependent recruitment of the adapter molecule TNFR death domain–associated protein (TRADD) to the TNFR1 complex (2, 3). Previous studies suggested that TNFR1–induced apoptosis and proinflammatory responses (such as those mediated by NF-κB and JNK activation) are mutually exclusive and are initiated by separate downstream components of the TNFR signal transduction machinery (3, 4). Recent experiments have provided support for this paradigm by demonstrating that the proinflammatory signaling initiated by TNFR1 is closely linked to protection against apoptosis. Specifically, JNK activation by TNF requires the signal adapter TNFR-associated factor (TRAF)2 but not the adapter receptor- interacting protein (RIP), whereas TNF-induced NF-κB activation requires the adapter RIP but not TRAF2 (5–8). Each of these signal transducers also appears to initiate antiapoptotic signals that inhibit cell death during TNF stimulation. For example, it was recently shown that inhibition of the TRAF2 signaling pathway, either by overexpression of a dominant negative TRAF2 (TRAF2.DN) or by gene targeting and inactivation, enhanced TNF-induced apoptosis of normal murine lymphocytes or fibroblasts (5, 6). In other studies, NF-κB inactivation enhanced the sensitivity of cultured cell lines and embryonic fibroblasts to TNF- induced apoptosis (4, 9–11). However, the relationship between these pathways and whether they converge on the same antiapoptotic effector molecules are not known.
Since TNF-α triggers TRAF2/JNK and NF-κB activation by independent signaling pathways, it was thought that TRAF2- and NF-κB–mediated pathways provide independent, nonoverlapping antiapoptotic signals that were not expected to interact with each other during TNF- induced apoptosis. In contrast to this current paradigm, we show in this study that TRAF2- and NF-κB–mediated signals play synergistic roles in preventing TNF-induced apoptosis.
Materials and Methods
Transgenic Mice.
Transgenic mice expressing IκBα.DN or TRAF2.DN were described previously (5, 12).
Kinase and NF-κB Assays.
In vitro JNK assays were done as described previously (5). In brief, 2–5 × 106 cells were treated with medium alone or rMu–TNF-α (R&D Systems, Inc., Minneapolis, MN) and lysed. For the kinase reaction, 1.5–3.0 μg of purified glutathione S-transferase (GST)–c-Jun(1-79) (a gift from Dr. H. Hanafusa, The Rockefeller University), 0.5 μCi of [γ-32P] ATP and ATP (20 μM) was incubated with the immunoprecipitated JNK in a total volume of 30 μl of JNK reaction buffer for 20 min at 30°C. The reactions were stopped with 2× loading buffer, boiled for 5 min, and run on a 12% SDS-PAGE gel. To measure NF-κB activation, nuclear extracts prepared from thymocyte lysates stimulated with medium alone or TNF were used for gel-shift assays as outlined previously (5).
Cell Killing Assay.
For thymocyte apoptosis assays, freshly isolated thymocytes (2 × 105 cells/well) were treated with the indicated amounts of rMu–TNF-α (R&D Systems, Inc.), or anti– Mu-Fas mAb (Jo2; PharMingen, San Diego, CA) for 22 h in the presence or absence of 30 μg/ml cycloheximide (CHX; Sigma Chemical Co., St. Louis, MO), with anti-CD3 (10 μg/ml) and anti-CD28 antibody (10 μg/ml), or with the indicated amounts of daunorubicin. Viable cells were quantified for 30 s at a constant rate by flow cytometry after staining with 5 μg/ml propidium iodide (PI) as described previously (5, 13). Specific cell death (%) was determined from the percentages of viable (PI-negative) cells and was calculated as 100 × (1 − no. of viable cells in a treated sample/no. of viable cells in the control).
Results and Discussion
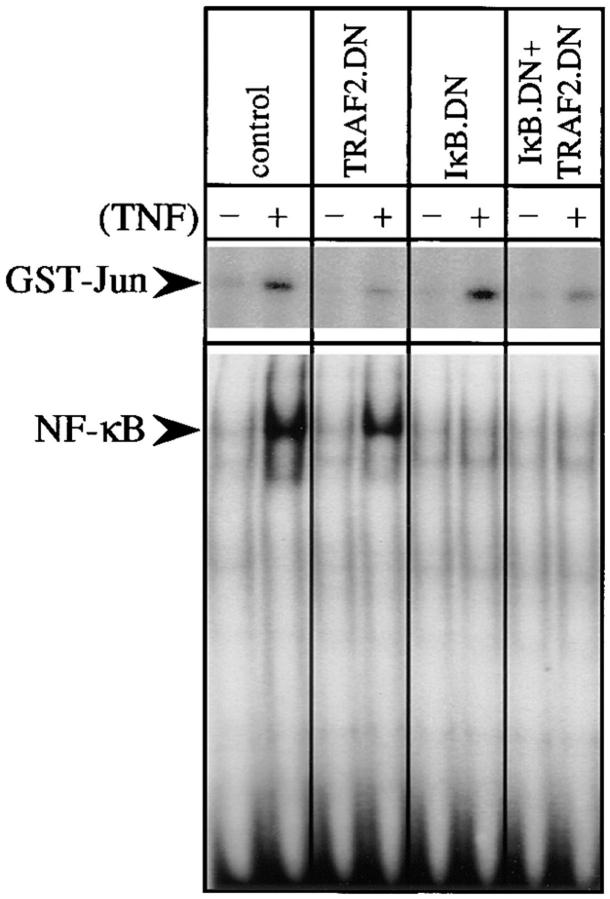
To determine the relationship between TRAF2- and NF-κB–dependent antiapoptotic signaling pathways during TNF stimulation of normal cells, we have tested the role of these proteins in the TNF-induced apoptosis of thymocytes from transgenic mice expressing trans-dominant repressors of IκBα (IκBα.DN [12]) and/or TRAF2 (TRAF2.DN [5]). Expression of IκBα.DN, which lacks sequences required for signal-dependent degradation and functions as a constitutive repressor of multiple NF-κB/Rel proteins (14), abolished the activation of NF-κB, but not JNK, in thymocytes upon TNF stimulation (Fig. 1). As reported previously (5), expression of TRAF2.DN, which lacks the NH2-terminal RING and zinc finger domains, impaired the activation of JNK by TNF, but not that of NF-κB. These results show that the activation of JNK and NF-κB by TNF in thymocytes is mediated by two discrete signaling pathways (Fig. 1). When both IκBα.DN and TRAF2.DN were expressed, the activation of NF-κB and JNK by TNF were both severely impaired (Fig. 1).
Figure 1.
TNF-induced JNK and NF-κB activation are mediated by distinct signal transduction pathways. (Top) Cell lysates prepared from thymocytes unstimulated (−) or stimulated (+) with TNF (10 ng/ml) for 2.5 min were used to measure JNK activity by immunocomplex kinase assay with GST–c-Jun(1-79) as a substrate. (Bottom) Nuclear extracts (4 μg) prepared from thymocytes unstimulated (−) or stimulated (+) with TNF for 15 min were used in gel-shift assays of NF-κB/Rel binding activity.
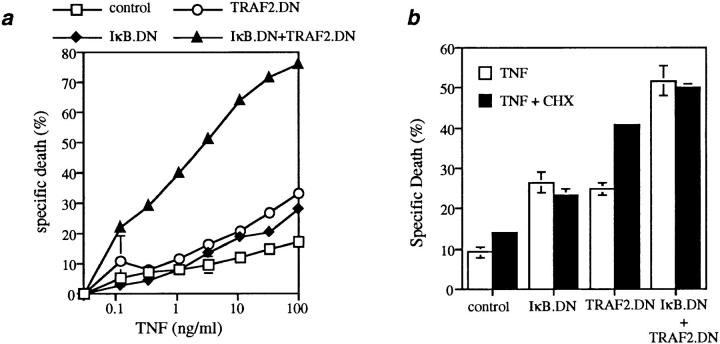
To determine the relationship between the NF-κB and TRAF2 pathways in normal cells, we measured TNF-α–induced death using thymocytes. Cells from control mice were relatively resistant to TNF-induced apoptosis, which could be enhanced to a modest degree by treatment with CHX to prevent new protein synthesis (Fig. 2, a and b). Inhibition of TRAF2 by TRAF2.DN expression potentiated the TNF-induced apoptosis of thymocytes, and this level of killing could be further increased by CHX treatment, suggesting that TNF retained the ability to induce synthesis of antiapoptotic proteins despite the observed impairment of JNK activation (Fig. 2, a and b). To determine whether NF-κB activation suppresses the TNF-induced apoptosis of normal T lymphoid cells, thymocytes from IκBα.DN mice were treated with increasing concentrations of TNF. Inhibition of NF-κB activation by overexpression of IκBα.DN significantly enhanced TNF-induced apoptosis of thymocytes (Fig. 2 a). Of note, CHX treatment of thymocytes from IκBα.DN mice led to no further enhancement in their TNF-induced apoptosis, suggesting that the effect of CHX on TNF-induced apoptosis is mediated mostly through inhibition of the synthesis of NF-κB– dependent antiapoptotic proteins (Fig. 2, a and b).
Figure 2.
Inhibition of TRAF2 and NF-κB activity synergistically sensitizes thymocytes to TNF-induced apoptosis. (a) Thymocytes (2 × 105/ well) from the indicated mice (6–8 wk old) were treated for 22 h with increasing amounts of murine TNF-α as indicated, and the percentages of cell death are shown as mean ± SD of triplicate samples from each group. Similar results were also obtained with human TNF-α (data not shown), which is specific for murine TNFR1. Representative data from one of five experiments are shown. Of note, the number and distribution of the major thymic subsets were normal, with the exception of a previously reported decrease in single positive cells in IκBα.DN-expressing mice and in double-TG mice expressing both TRAF2.DN and IκBα.DN (data not shown); however, this subset represented <1% of thymocytes in wild-type littermates. (b) Thymocytes from the indicated mice were treated overnight with TNF (33 ng/ml), with or without CHX (30 μg/ml), as indicated. Background cell death of thymocytes in medium ± CHX alone was ∼20–30%. Mean (± SD) data from one of three experiments, all of which yielded similar results, are shown.
Strikingly, when thymocytes from IκBα.DN × TRAF2. DN double-transgenic (TG) mice were treated with increasing concentrations of TNF-α, they were at least 1,000 times more sensitive to TNF-induced apoptosis than cells from normal mice, and at least 100 times more sensitive than those from either IκBα.DN or TRAF2.DN TG mice (Fig. 2 a). These results indicate that NF-κB– and TRAF2-dependent antiapoptotic signals synergistically protect cells from TNF-induced apoptosis. Moreover, treatment of thymocytes from IκBα.DN × TRAF2.DN double-TG mice with TNF-α induced a level of apoptosis similar to that induced by TNF in CHX-treated thymocytes from TRAF2.DN single-TG mice (Fig. 2 b). This finding further supports the conclusion that the principal effect of CHX is to inhibit de novo synthesis of antiapoptotic proteins that are regulated by the state of NF-κB activation.
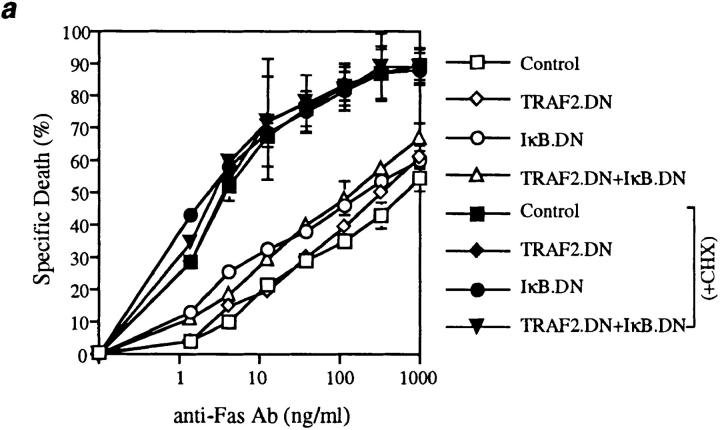
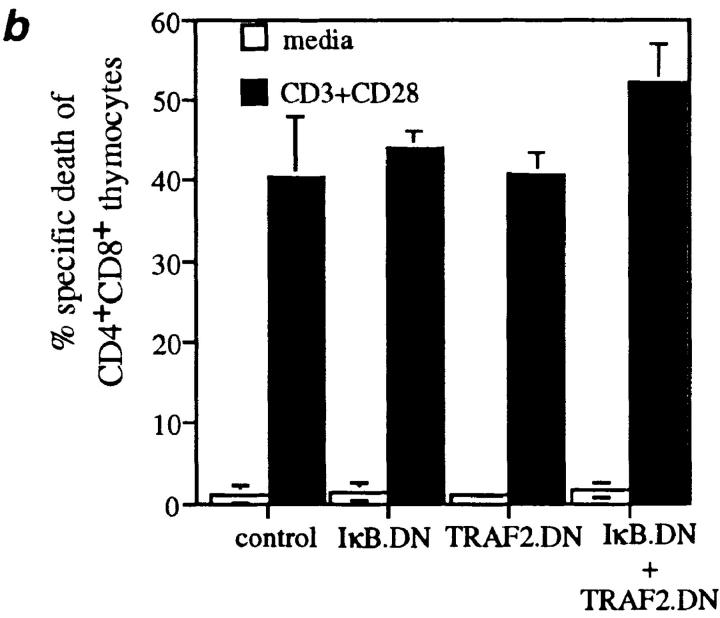
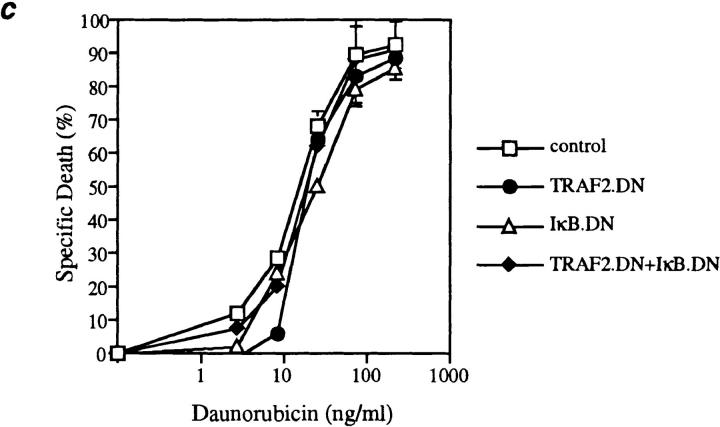
Fas death domain–associated protein (FADD), an effector of TNF-induced apoptosis recruited by heterotypic death domain interactions with TRADD, is also an effector of apoptosis induced by the Fas receptor (3, 15, 16). Moreover, NF-κB signaling occurs in thymocytes in situ (17, 18), suggesting that the activation state of NF-κB could influence Fas-induced apoptosis. To investigate this possibility, thymocytes from the four sets of mice were cultured in the presence of increasing concentrations of a cross-linking anti-Fas antibody (Fig. 3 a). Compared with the dramatic increase in TNF-induced apoptosis caused by simultaneous inhibition of TRAF2 and NF-κB activation, increased sensitivity to Fas-induced apoptosis was minimal. In addition, when CD4+CD8+ thymocytes were activated by anti-CD3 and anti-CD28 antibodies, physiological stimuli involved in thymocyte selection, the induction of apoptosis in these cells was not affected by IκBα.DN and TRAF2.DN expression (Fig. 3 b). TRADD and the NF-κB signaling pathway have also been implicated in the apoptosis induced by interactions between TNF-related apoptosis–inducing ligand (TRAIL) and death receptors (DR4, DR5 [19, 20]). However, TRAIL did not induce any significant cell death in wild-type thymocytes or in thymocytes from transgenic mice expressing TRAF2.DN and IκBα.DN (data not shown). Finally, NF-κB activation has been suggested to play a role in the induction of apoptosis of some tumor cells by cancer chemotherapeutic compounds such as daunorubicin (11), a finding offering the hope of potential therapeutic benefits. The therapeutic window for such effects would be more favorable if they were restricted to cancer cells and did not affect primary cells. Importantly, then, neither TRAF2 nor NF-κB activation played any role during daunorubicin-induced apoptosis of thymocytes (Fig. 3 c). Therefore, these results suggest that in nonneoplastic cells, the antiapoptotic role of TRAF2 and NF-κB may be restricted to the context of TNF signaling.
Figure 3.
Inhibition of TRAF2 and NF-κB activity does not affect the sensitivity of thymocytes to apoptosis induced by other stimuli. Thymocytes (2 × 105/well) from the indicated mice were treated for 22 h with (a) anti-Fas antibody (Jo2); (b) anti-CD3 and anti-CD28 antibodies; or (c) daunorubicin. Mean (± SD) data from one of three experiments with similar results are shown.
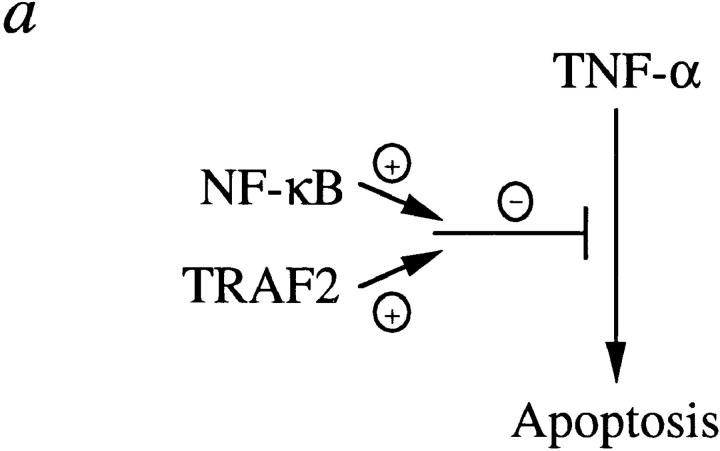
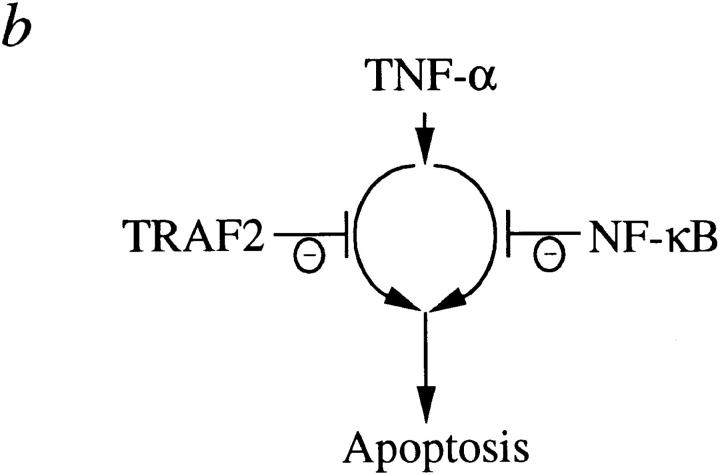
As shown in this study and in previous reports (5, 6, 8), TNF-induced JNK and NF-κB activation appear to be triggered by independent signaling pathways, with the former but not the latter mediated by TRAF2. In addition, NF-κB–mediated antiapoptosis required de novo protein synthesis, whereas TRAF2-mediated antiapoptosis did not. Therefore, it was surprising to see that TRAF2 and NF-κB played synergistic roles in preventing TNF-induced apoptosis. How might this happen? One possibility is that TRAF2- and NF-κB–mediated pathways reconverge at some point to effect antiapoptosis during TNF stimulation (Fig. 4 a); for instance, TRAF2 may be responsible for recruitment of an antiapoptotic protein whose basal level of expression is induced by TNF-α in an NF-κB–dependent, JNK-independent manner. Alternatively, TRAF2/JNK and NF-κB activation could lead to the independent inhibition of discrete apoptotic effectors whose synergistic action is required for efficient induction of apoptosis (Fig. 4 b).
Figure 4.
Models for antiapoptotic synergism induced by TNF-α.
Although TNF was identified by its ability to kill various tumor cells, most cells do not undergo apoptosis in response to TNF. Our results indicate that two distinct antiapoptotic signals are generated by TNF stimulation, and that removal of either one is insufficient to allow efficient apoptosis induction by TNF. Thus, the combined inhibition of both antiapoptotic pathways during TNF stimulation may enhance the efficacy of TNF-based antineoplastic therapies.
Acknowledgments
We thank Brian Wong for his critical comments, J. Tschopp for soluble TRAIL, and Yaneth Castellanos for technical help.
Footnotes
M. Boothby is supported by a grant from the National Institutes of Health (AI-36997), and by a Leukemia Society of America Scholar Award. Y. Choi is an investigator of the Howard Hughes Medical Institute.
References
- 1.Baker SJ, Reddy EP. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene. 1996;12:1–9. [PubMed] [Google Scholar]
- 2.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 3.Hsu H, Shu HB, Pan M-G, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z-G, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 5.Lee SY, Reichlin A, Santana A, Sokol K, Nussenzweig M, Choi Y. TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 6.Yeh W-C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham F, de la Pompa JL, Ferrick D, Hum B, Iscove N, et al. Early lethality, functional NF-κB activation and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 7.Hsu H, Huang J, Shu H-B, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 8.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 9.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 10.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 11.Wang C-Y, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 12.Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-κB. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumor necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 14.Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 15.Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 16.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 17.Moore NC, Girdlestone J, Anderson G, Owen JJT, Jenkinson EJ. Stimulation of thymocytes before and after positive selection results in the induction of different NF-κB/Rel protein complexes. J Immunol. 1995;155:4653–4660. [PubMed] [Google Scholar]
- 18.Sen J, Venkataraman L, Shinkai Y, Pierce JW, Alt FW, Burakoff SJ, Sen R. Expression and induction of nuclear factor-κB-related proteins in thymocytes. J Immunol. 1995;154:3213–3221. [PubMed] [Google Scholar]
- 19.Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-κB pathway. Immunity. 1997;7:821–830. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 20.Schneider P, Thome M, Burns K, Bodmer J-L, Hofmann K, Kataoka T, Holler N, Tschopp J. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-κB. Immunity. 1997;7:831–836. doi: 10.1016/s1074-7613(00)80401-x. [DOI] [PubMed] [Google Scholar]