Abstract
Anti-DNA antibodies are regulated in normal individuals but are found in high concentration in the serum of systemic lupus erythematosus (SLE) patients and the MRL lpr/lpr mouse model of SLE. We previously studied the regulation of anti–double-stranded (ds)DNA and anti–single-stranded (ss)DNA B cells in a nonautoimmune background by generating mice carrying immunoglobulin transgenes coding for anti-DNAs derived from MRL lpr/lpr. Anti-dsDNA B cells undergo receptor editing, but anti-ssDNA B cells seem to be functionally silenced. Here we have investigated how anti-DNA B cells are regulated in recombination- activating gene (RAG)-2−/− mice. In this setting, anti-dsDNA B cells are eliminated by apoptosis in the bone marrow and anti-ssDNA B cells are partially activated.
Keywords: anti-DNA antibody, B cell deletion, B cell anergy, recombination-activating gene deficiency, apoptosis
Astriking feature of Lupus is that individual SLE patients express unique subsets of the many anti-nuclear antibodies (ANAs)1 seen in the population of individuals with this disease (1). As pointed out by Hardin (2), these idiosyncratic spectra of ANAs would not be expected by nonspecific activation of the repertoire and suggest instead that autoantibodies arise by immunization. The oligoclonal nature of autoantibodies and the evidence for positive selection of somatic mutants of autoantibodies provides support for this notion (3, 4). Craft and Hardin (5) also noted that these subsets of ANAs include antibodies to “physically-linked epitopes”, e.g., anti-DNA with antihistone(s). They interpreted such linked sets to mean that the activating antigen must be a nucleoprotein particle. An elegant extension of this insight is the demonstration that the blebs on the surface of apoptotic cells contain the targets of Lupus autoantibodies such as DNA, Ro, La, and ribonucleoprotein and could thereby be the delivery vehicles of self-antigen (6).
The observation that self-antigens activate autoreactive B cells in disease implies that such B cells are normally under active, negative regulation. Indeed, B cells specific for both facultative and constitutive self-antigens have been shown to be deleted or inactivated (7–10). The exact site and stage of these manifestations of tolerance have been obscured by receptor editing, a process that rescues autoreactive B cells by revising their receptors (11–16). It has been possible to study deletion directly in mice that are either unable to edit (SCID or recombination-activating gene [RAG]−/−) or lack a substrate for editing (H or L chain–deficient mice) (17–20). Mature B cells can be generated by crossing H and L chain transgenes onto either RAG−/− or SCID mice. These immunodeficient mice can then delete or arrest autoreactive B cells, and thereby provide a powerful tool for studying tolerance in the absence of rearrangement.
We have generated transgenic (tg) mice expressing anti-DNA antibodies characteristic of those found in autoimmune disease (10). In this study we have crossed these anti– double-stranded (ds)DNA (3H9RVk4) and anti-ssDNA (3H9RVk8) tg mice onto RAG-2−/− mice. This model allows us to determine whether anti-DNA B cells are deleted by programmed cell death, whether apoptotic cells present the immunogens that drive DNA-specific B cells, and whether negative regulation of these autospecificities is sustained in the absence of T cells. We find that in RAG-2−/− mice, dsDNA B cells are eliminated by apoptosis but ssDNA B cells appear to be activated. These data show that deletion efficiently regulates anti-dsDNA and suggest a role for T cells in B cell anergy but not central deletion.
Materials and Methods
Mice.
The generation of 3H9 site-directed tg (3H9R), Vk4, and Vk8 tg mice has been described previously (15, 16). All three lines were crossed to RAG-2−/− mice on an inbred 129/SvEv background (Taconic Farms, Inc., Germantown, NY). 3H9R/ RAG-2−/− and 3H9R mice were crossed to Vk4/RAG-2−/− and Vk8RAG-2−/− to produce 3H9RVk4/RAG-2−/− and 3H9RVk8/RAG-2−/− or double tg RAG-2+/− mice, respectively. The genotypes of mice were determined by PCR as described previously (15, 16). All experimental mice were 10–16 wk of age.
Flow Cytometric Analysis.
Single-cell suspensions were prepared from the bone marrow and spleen as described previously (20). Cell staining and four-color FACS® analysis were conducted as described previously (20). Pro-B, pre-B, immature B, and mature B cells were visualized by incubating bone marrow and splenic cells with a combination of FITC-S7, an antibody against CD43 (21); allophycocyanin-6B2, an anti-B220 antibody (PharMingen, San Diego, CA); Texas red goat anti–mouse IgM (Southern Biotechnology Associates, Inc., Birmingham, AL); and biotin-conjugated rat anti–mouse IgD (Southern Biotechnology Associates, Inc.). PE-streptavidin (Molecular Probes, Inc., Eugene, OR) was used to reveal the biotin reagents. An anti- dsDNA antibody, 3H9Vk4, and an antiidiotypic antibody, 1.209, specific for various 3H9 heavy and κ light chain combinations (8), were conjugated with FITC following the manufacturer's instruction (Molecular Probes). Dead cells were excluded by staining with propidium iodide and cell samples were analyzed using a FACSvantage® (Becton Dickinson, San Jose, CA).
ELISAs.
Igs in serum and supernatants were measured as described previously (20). Binding to ssDNA and dsDNA was tested by a two-step solution phase ELISA as previously described (13).
TUNEL (TdT-mediated dUTP Nick-end Labeling) Assay.
Apoptosis of bone marrow cells was examined using the In Situ Cell Death Detection Kit (Boehringer Mannheim, Indianapolis, IN). The cell suspensions were prepared following the procedures provided by the company, except that the cells were stained with allophycocyanin-B220 before fixation. Apoptotic cells were analyzed by flow cytometry.
Induction of Apoptosis and Gel Electrophoresis DNA Fragmentation Assay.
Isolated splenic cells were incubated with ionomycin, a Ca2+ agonist (Sigma Chemical Co., St. Louis, MO), at 1 μg/ml at 37°C overnight. Apoptosis of splenic cells was confirmed by gel electrophoresis. DNA was prepared for gel electrophoresis as follows: cell pellets obtained by centrifugation at 13,000 rpm for 30 s were lysed with 400 μl of lysis buffer containing 0.2% Triton X-100, 10 mM Tris, and 1 mM EDTA, pH 8.0. DNA fragments were precipitated from 200 μl of cell lysate with 0.5 M NaCl and 1 vol isopropanol at −20°C overnight. DNA fragments were washed with 70% ETOH and resuspended in 100 μl of water. Electrophoresis was performed on 1% agarose gels.
T Cell Depletion.
T cells were depleted using anti-Thy 1.2 antibody (J1j10; American Type Culture Collection, Rockville, MD) and rabbit complement (Accurate Chemical & Scientific Co., Westbury, NY). Spleen cells were incubated with anti-Thy 1.2 (1:100) and complement (1:10) for 45 min at 37°C. Dead cells were removed by centrifugation through Lympholyte-M (Accurate Chemical & Scientific Corporation) for 20 min at 1,200 g at room temperature.
Cell Proliferation.
Cell proliferation was monitored by [3H] thymidine incorporation. 2 × 105 T cell–depleted splenocytes were cultured in 96-well microtiter plates with various doses of LPS (0–10 μg/ml), IL-4 (5 μl/ml; R&D Systems, Minneapolis, MN), and anti-IgM F(ab′)2 (0–50 μg/ml; Jackson ImmunoResearch Labs., Inc. West Grove, PA) in RPMI 1640 medium containing 10% FCS for 72 h. Cells were pulsed with [3H]thymidine (1 μCi/well) for 18 h before harvesting. Determinations were performed in triplicate and the results are expressed as mean of triplicate. Experiments were repeated at least three times with one to two mice in each group.
Results
Anti-dsDNA B Cells Are Absent, but Anti-ssDNA B Cells Mature in RAG-2− /− Mice.
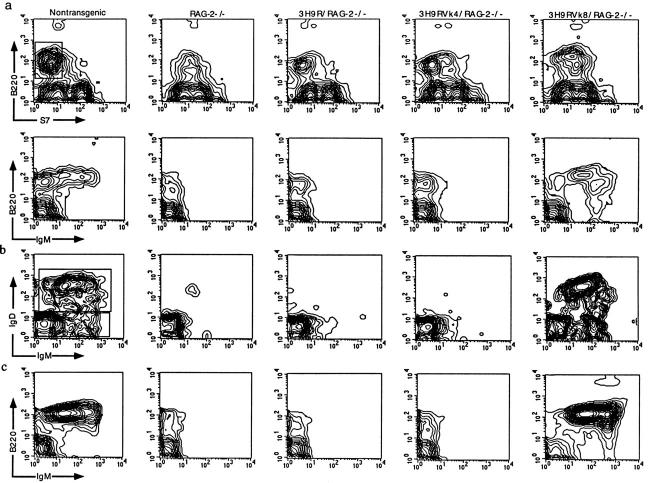
B cell development was assessed in 3H9RVk4/RAG-2−/− or 3H9RVk8/RAG-2−/− mice based on the expression of the cell surface markers B220, CD43, IgM, and IgD. According to B220 and CD43, B cell development was arrested at the pro-B cell stage in RAG-2−/− mice (B220+, CD43low), but addition of the 3H9 heavy chain gene allowed B cells to proceed to the pre-B cell stage (B220+, CD43−, IgM−; Fig. 1 a). These results are similar to those in H chain tg SCID or RAG−/− mice (Table 1). The introduction of both 3H9 H chain and Vk8 L chain genes into RAG-2−/− mice completely rescued B cell development as evidenced by the generation of surface IgM+IgD+ B cells (Fig. 1 b). Mature B cells extensively populated the periphery and expressed normal levels of sIgM (Fig. 1 c). These results show that functional anti-ssDNA antibody genes are able to rescue B cell development in RAG-2−/− mice. Similar results have been reported for 3H9Vk8 transgenes in SCID mice (Table 1).
Figure 1.
Flow cytometric analysis of B cell development. Cells from the bone marrow (a and b) and spleen (c) were resolved by four-color analysis for surface expression of B220/CD45R, CD43/S7, IgM, and IgD. Correlative expression of CD43, B220 (a, top) and IgM (a, bottom) based on lymphocyte gate. Cells from the B220+CD43− window of a (top) were analyzed for expression of IgM and IgD (b). Pre-B (B220+CD43−IgM−), immature B(B220+CD43−IgM+IgD−), and mature B (B220+CD43−IgM+IgD+) cells are shown in different quadrants. Splenic cells were analyzed with anti-B220 and anti-IgM antibodies based on lymphocyte gate (c).
Table 1.
B Cell Development of tg Mice Crossed on RAG− /− or SCID Background
| RAG−/− | Specificity | B cell development | Reference | |||
|---|---|---|---|---|---|---|
| 3H9RVk4 | dsDNA | Deleted at the transition from pre-B to immature B cells | 20 | |||
| 3H9RVk8 | ssDNA | Mature B cells in the periphery | 10 | |||
| Sp6 | TNP/DNA | Arrested at the transition from pre-B to immature B cells | 22 | |||
| Anti–MHC I | H-2k | In H-2b background: deleted at the transition from B220hi to B220bright cells | 17 | |||
| In H-2d background: mature B cells in the periphery | ||||||
| HC186 | NP | Mature B cells in the periphery | 18 | |||
| 3H9Vk8/SCID | ssDNA | Mature B cells in the periphery | 19 |
The H and L chain gene combination 3H9RVk4 that codes for anti-dsDNA failed to promote full maturation of RAG-2−/− B cells. No IgM+ or IgM+IgD+ B cells were detectable in the bone marrow (Fig. 1, a and b) or spleen (Fig. 1 c). We assume that these anti-dsDNA B cells are deleted at the transition from pre-B to immature B cells, at a point at which the BCR density is below our level of detection.
Anti-dsDNA B Cells Are Deleted by Apoptosis.
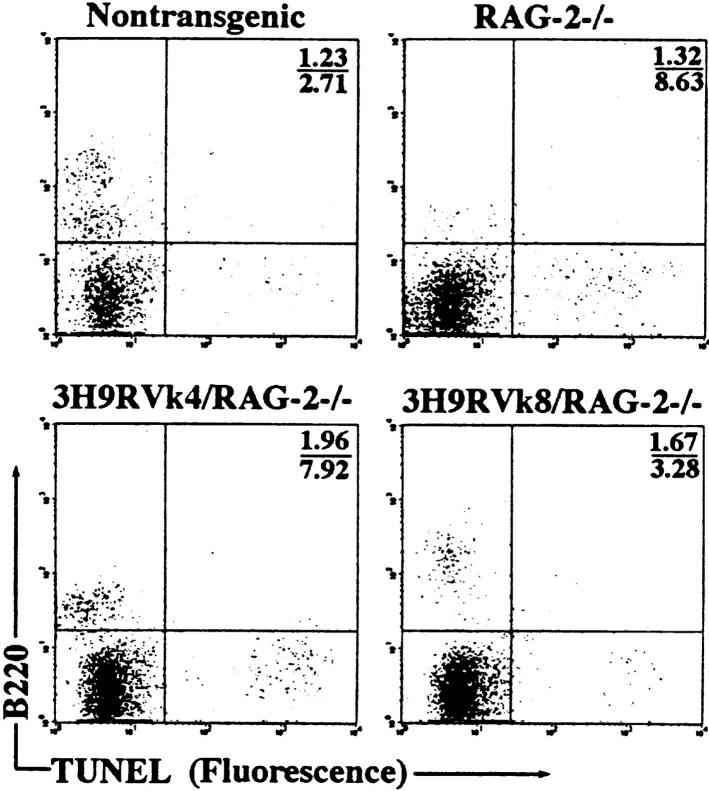
To ask whether anti-dsDNA B cells are eliminated by apoptosis, we assayed for programmed cell death in the bone marrow using the TUNEL (TdT-mediated dUTP nick-end labeling) assay. Apoptotic cells were detected at frequencies of ∼10% in RAG-2−/− and 3H9RVk4/RAG-2−/− mice and 4–5% in non-tg RAG+/+ and 3H9RVk8/RAG-2−/− B cells (Fig. 2). These apparently apoptotic cells had very low levels of B220 on the surface (Fig. 2), which was nevertheless clearly higher than the signal given by nonspecific Ig. Therefore, we believe that these cells are B cell precursors and that the low level of B220 results from the membrane changes of lymphocytes undergoing apoptosis (23, 24). Furthermore, other bone marrow cells such as macrophages and stem cells are unlikely to contribute to the increased apoptosis in 3H9RVk4 RAG−/− tg mice.
Figure 2.
Analysis of apoptotic cells in the bone marrow by flow cytometry. Bone marrow cells were stained with anti-B220 antibody and fixed with 2% paraformaldehyde. Apoptotic cells were labeled and detected using the In Situ Cell Death Detection Kit (Boehringer Mannheim). The numbers shown represent the percentage of apoptotic cells in the upper right and lower right quadrants.
The Regulation of Anti-dsDNA B Cells May Be Initiated by Binding to Apoptotic Cells.
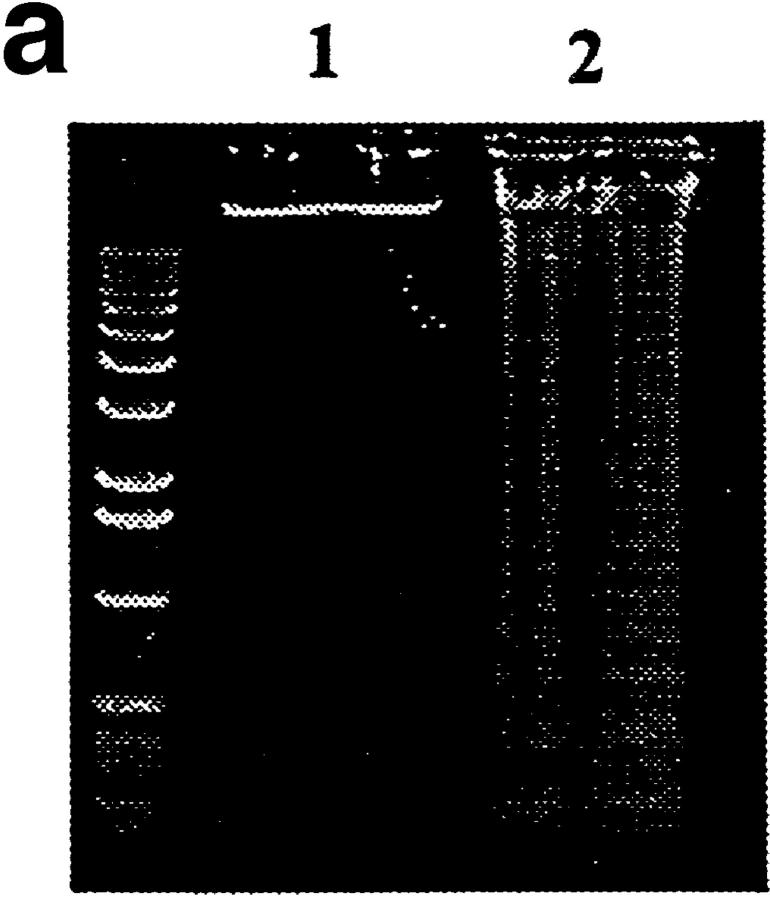
Deletion of anti-dsDNA B cells in the bone marrow implies that the antigen(s) specific for the 3H9RVk4 BCR is present in the bone marrow. A likely source of DNA and/or DNA–protein complexes are the blebs present on the surface of apoptotic cells (6). Therefore, we examined the binding of 3H9Vk4 antibody to apoptotic cells. Spleen cells were treated with ionomycin and apoptosis was confirmed by DNA fragmentation patterns (Fig. 3 a). 3H9Vk4 antibody bound to ∼60% of splenic cells incubated with ionomycin, as compared with 25% of cells without ionomycin. A control antibody of the same isotype, MOPC141, did not bind to apoptotic cells (Fig. 3 b). These results are similar to those observed for SLE sera specific for DNA, Ro, and La antigens (6).
Figure 3.
Binding of 3H9Vk4 antibody to apoptotic cells. (a) DNA fragmentation of apoptotic cells. DNA from freshly isolated splenocytes (lane 1) and cells cultured overnight in the presence of ionomycin (lane 2) was electrophoresed in 1% agar gel. Ionomycin-treated cells show DNA-fragmentation typical of apoptotic cells. (b) Binding of 3H9Vk4 antibody to apoptotic cells. Ionomycin-treated (bold line) and fresh (broken line) spleen cells were incubated with FITC-labeled 3H9Vk4 antibody (left) and the isotype-matched control antibody MOPC141 (right) and analyzed by flow cytometry. (c) Binding of 3H9Vk4 antibody to bone marrow cells. Bone marrow cells of RAG-2−/− (bold line) and control RAG-2+/− (broken line) were isolated, stained with FITC-3H9Vk4 antibody (left) and MOPC 141 (right), and analyzed by flow cytometry.
We also examined the binding of 3H9Vk4 antibody to bone marrow cells of various mice. We found that 3H9Vk4 antibody bound to ∼14% of bone marrow cells from RAG-2−/−, but to only 1% of bone marrow cells from RAG-2+/− mice (Fig. 3 c). The binding of 3H9Vk4 antibody to bone marrow cells was also high in 3H9RVk4/ RAG-2−/− mice (∼13%, data not shown). The comparable level of apoptosis in RAG-2−/− and 3H9RVk4/RAG-2−/− bone marrow suggests that autoreactive B cells in 3H9RVk4/ RAG-2−/− mice are deleted by apoptosis.
Anti-ssDNA B Cells Do Not Secrete Antibodies In Vivo.
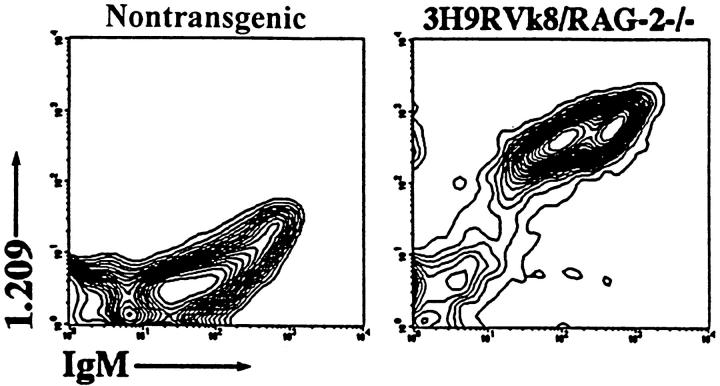
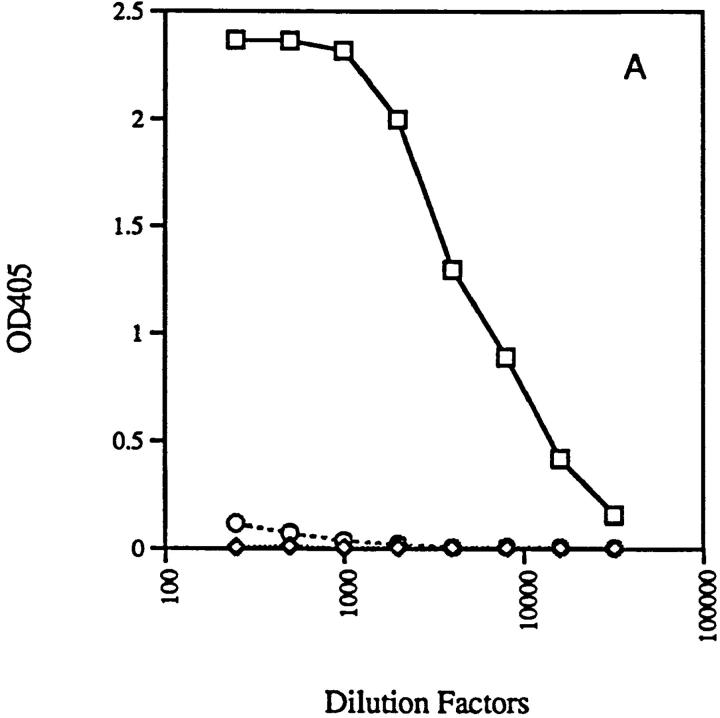
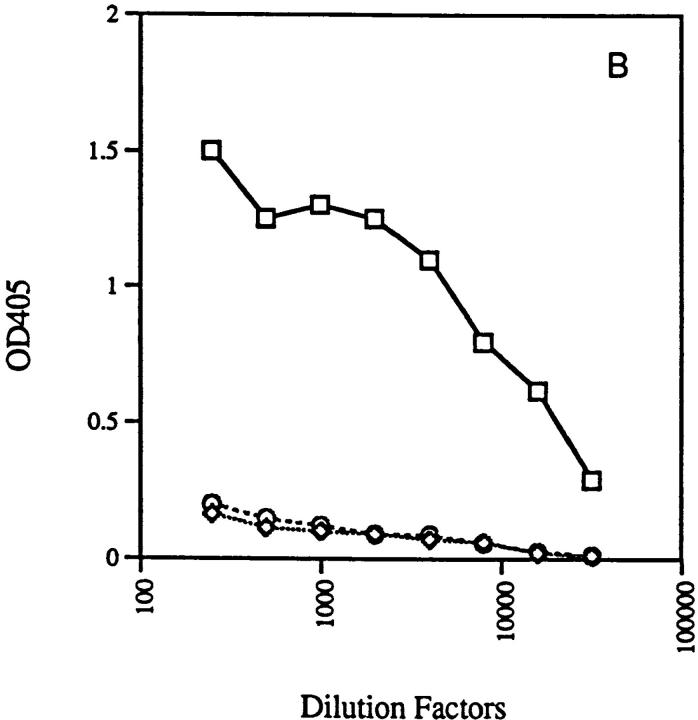
RAG-2−/− mice with anti-ssDNA transgenes (3H9RVk8) have nearly normal numbers of B cells. All these B cells are idiotype positive, as demonstrated by the reaction with 1.209 antibody specific for the 3H9 heavy chain paired with a κ light chain (Fig. 4). These peripheral B cells do not secrete antibody as 3H9RVk8/RAG-2−/− mice have 100–500-fold lower levels of serum Ig than do normal mice (Fig. 5).
Figure 4.
Staining of B cells with antiidiotype antibody 1.209. Splenocytes were stained with the combinations of anti-IgM and antiidiotype 1.209. Lymphocytes were gated for analysis.
Figure 5.
Production of immunoglobulins in 3H9RVk4/RAG-2−/− (○) and 3H9RVk8/RAG-2−/− mice (⋄). Total serum IgM (A) and IgG (B) were determined by ELISA as described in Materials and Methods and compared to normal mice (□).
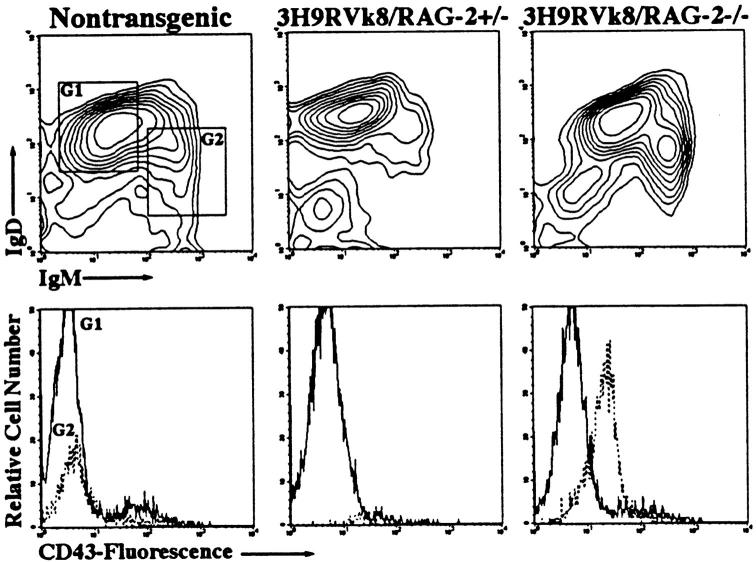
To understand the status of these B cells, we analyzed splenic cells for additional markers. Staining with anti-IgM and anti-IgD showed two populations of B cells, IgMhiIgDlo and IgMloIgDhi B cells, in 3H9RVk8/RAG-2−/− mice, but only IgMloIgDhi B cells in 3H9RVk8/RAG-2+/− mice (Fig. 6, top). The extra IgMhiIgDlo population in the RAG-2−/− mice may either be activated B cells (25) or maturation-blocked B cells (26). To distinguish these alternatives, CD43 expression of the IgMhiIgDlo B cells was examined. CD43 is expressed on pro-B cells and activated B cells (27), but not mature resting B cells (28). We found that IgMhiIgDlo B cells express high levels of CD43 (IgMlo IgDhi B cells had the characteristic low level of CD43) (Fig. 6, bottom). Thus, we can define two populations of B cells in 3H9RVk8/RAG-2−/− mice. One population consists of activated B cells as defined by high expression of IgM and CD43, but low IgD (IgMhiIgDloCD43hi), and a second population consists of B cells with no CD43, low IgM, but high IgD (IgMloIgDhiCD43−). Few, if any, of the activated B cells are found in 3H9RVk8/RAG-2+/− mice or in non-tg littermates (Fig. 6).
Figure 6.
FACS® profile of splenocytes from 3H9RVk8/RAG-2−/− mice. Spleen cells were labeled with anti-B220, anti-IgM, anti-IgD, and anti-CD43 antibodies, and gated B220+ cells were analyzed. Cells were then displayed in IgM versus IgD blots and gated for IgMloIgDhi (G1, solid line) and IgMhiIgDlo (G2, broken line) in the upper panel. Either cell population was then analyzed for CD43 expression in the lower panel.
Are Anti-ssDNA B Cells from RAG-2− /− and RAG-2+ /− Mice Functional?
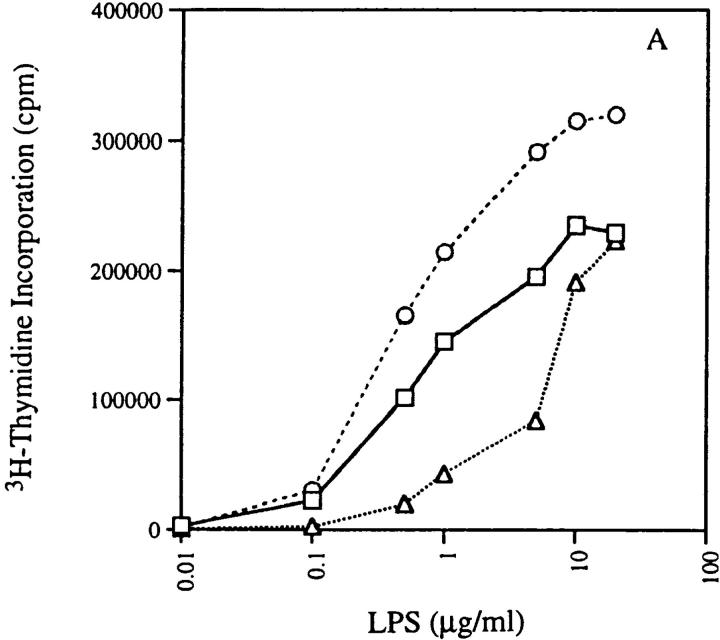
We measured the response of 3H9RVk8 B cells to LPS and found that cells from both 3H9RVk8/ RAG-2+/− and 3H9RVk8/RAG-2−/− mice responded to LPS stimulation in a dose-dependent manner (Fig. 7 a), but 3H9RVk8/RAG-2−/− B cells are hyperresponsive to LPS relative to non-tg RAG-2+/− B cells. This hyperactivity may be derived from the “activated” B cells seen in vivo.
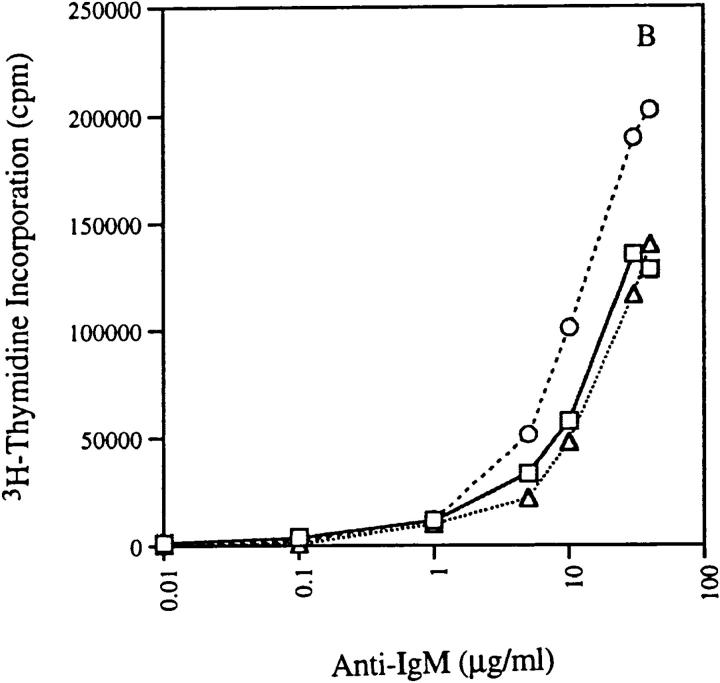
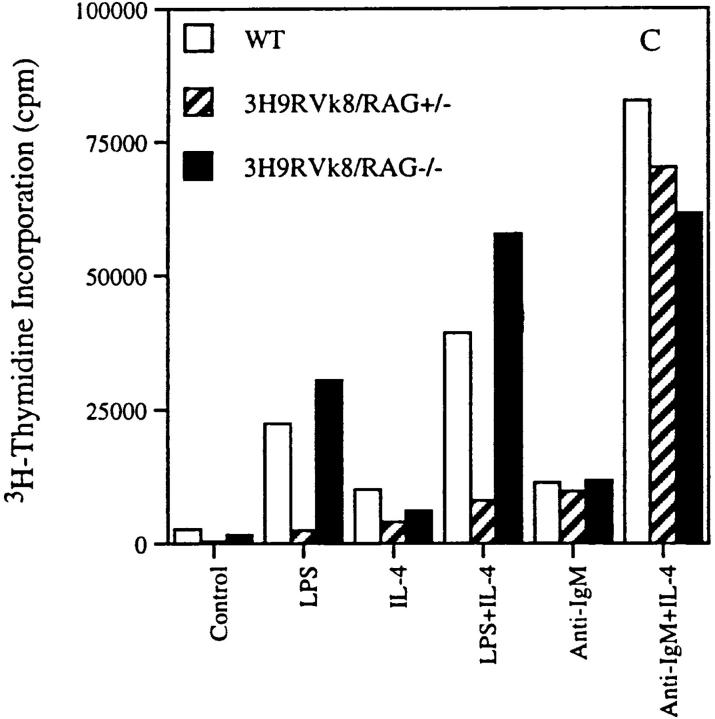
Figure 7.
Proliferation of B cells in response to LPS and anti-IgM in absence and presence of IL-4. 2 × 105 T-depleted splenocytes from non-tg (□), 3H9RVk8/RAG-2+/− (▵), and 3H9RVk8/RAG-2−/− (○) mice were cultured in the presence of various doses of LPS (A) and anti-IgM (B) for 72 h. B cells (2 × 105 T cell–depleted splenic cells) were also cultured with submitogenic concentration of LPS (0.1 μg/ml) or anti-IgM (1 μg/ml) in the presence or absence of IL-4 (5 μg/ml) for 72 h (C). Cells were pulsed with [3H]thymidine (1 μCi/well) for 18 h before harvesting. Data shown are the average of triplicate determinations and are representative of at least three different experiments.
B cells from 3H9RVk8/RAG-2+/− mice had a reduced response to low doses of LPS (Fig. 7 a), consistent with the idea that these B cells are anergic. The difference between RAG-2+/− and RAG-2−/− mice may reflect the presence or absence of T cells. We studied the possible influence of T cells by addition of IL-4 to our in vitro proliferation assay. Proliferation of B cells in response to a submitogenic concentration of LPS (0.1 μg/ml) was enhanced by IL-4 in 3H9RVk8/RAG-2−/− mice (Fig. 7 c), indicating that an IL-4–mediated signal was capable of acting in synergy with LPS. 3H9RVk8/RAG-2+/− B cells responded poorly to low doses of LPS even in the presence of IL-4. The failure of IL-4 to promote 3H9RVk8/RAG-2+/− B cell proliferation in synergy with LPS supports the view that these B cells have a defect in response to LPS and that this defect cannot be overcome by this T cell–derived cytokine.
We also tested the proliferative response of 3H9RVk8 B cells to anti-IgM. 3H9RVk8 B cells from RAG-2−/− mice showed a better response to anti-IgM cross-linking than did those from non-tg RAG-2+/+ mice, providing further evidence for in vivo activation of these anti-ssDNA B cells. Surprisingly, B cells from 3H9RVk8/RAG-2+/− mice responded as well as non-tg RAG-2+/+ mice to anti-IgM stimulation (Fig. 7 b). Why 3H9RVk8/RAG-2+/− B cells have a normal response to anti-IgM cross-linking, but an impaired response to LPS is unclear.
In addition, we determined the effect of IL-4 on B cell proliferation stimulated by anti-IgM. In all three mouse lines, the combination of anti-IgM and IL-4 induced a strong proliferative response that was greater than the sum of that induced by either stimulus alone (Fig. 7 c). This suggests that the 3H9RVk8 B cells have an intact signal transduction pathway through both their Ig and IL-4 receptors.
Discussion
It is generally accepted that of the pool of progenitor B cells, only a few B cells manage to mature and the rest die at various stages of development. Yet it has been difficult to actually detect apoptotic B cells in the bone marrow, probably because of rapid phagocytosis of dying cells by macrophages (29). Recently, cell death has been detected at different stages of B lineage differentiation when precursor B cell proliferation is perturbed, as in Eμ-myc tg (30) and SCID (31) mice. Presumably, phagocytosis cannot keep pace with the rate of cell death in these cases. In keeping with these findings, we detect elevated levels of apoptosis in the bone marrow of RAG-2−/− mice. RAG-2−/− B cell development is arrested at the pro-B stage (Fig. 1), but why cell death should occur at this stage is not known.
Rearrangement and H chain expression is one of the requirements for entry into cell cycle and survival of these cells, and indeed, crossing heavy and light chain transgenes into RAG−/− mice rescues B cell development (17, 18) and reduces apoptosis to wild-type levels (Fig. 2). On the other hand, autoantibody tgs have a limited capacity for restoring functional B cells in RAG−/− mice. 3H9RVk4 transgenes advance B cell development to the B220+CD43−IgM− pre-B stage, but immature B cells (B220+, IgM+) are undetectable. For this autospecificity, a B cell early in the transition from pre-B to immature B cell appears to be the target for deletion, and consequently the level of apoptosis in 3H9RVk4/RAG-2−/− mice is as high as in RAG-2−/− mice. We assume that the expression of the self-reactive IgM on the cell surface initiates signaling events that result in apoptosis. The fact that we cannot detect appreciable levels of IgM or B220 on the apoptotic TUNEL-positive cells — even though they are immature B cells — might be due to membrane disintegration and concomitant loss of cell surface molecules during the apoptotic process (23, 24). A second model for anti-DNA–reactive B cells is the Sp6 tg mouse. Here the anti-TNP/DNA cross-reactive B cells are also deleted at the pre-B to immature B transitional stage (22). Such a short survival time of immature B cells predicts a narrow time window for editing in anti-dsDNA B cells. Indeed, we find that editing in 3H9RVk4 B cells is predominantly on the targeted allele, suggesting that these cells have time for only a few rearrangement attempts (32).
Dying cells display the intracellular targets of SLE autoantibodies (e.g., DNA, Ro, and La) on their surface as shown by the binding of antibody from SLE sera to the blebs on the surface of apoptotic cells (6). Hence, apoptosis in the bone marrow deletes self-reactive B cells and provides an abundant source of autoantigens. We have shown that 3H9Vk4 mAb binds to apoptotic cells in spleen and bone marrow. The close proximity between dying 3H9RVk4 cells and immature 3H9RVk4 B cells may explain the efficient regulation of this autoreactivity. This may also explain why anti-DNA B cells appear to be deleted at an earlier B cell maturation stage than anti-HEL or anti-H2 B cells. The nature of the antigens and the way they are presented to B cells could account for the differences in B cell regulation. Membrane antigens (H-2 and HEL) are presumably distributed evenly on the cell surface. Their interaction with BCR may transduce a moderate signal strong enough to induce editing but not strong enough to initiate an immediate cell death process. These cells may need a more prolonged signal through the BCR to induce the death pathway and therefore are deleted at the immature to mature B cell transition (7, 8, 33). The DNA-containing antigens are presented in the blebs with a high local concentration, which may cause strong local cross-linking of BCRs. This type of interaction may deliver a signal for both editing and deletion. Successful editing within a limited time period may rescue the B cells from the apoptotic pathway. Alternatively, a protective mechanism may have to insure stringent regulation against such harmful autoantigens as dsDNA, e.g., a second type of signal delivered to the B cells during the DNA–BCR interaction.
The anti-ssDNA transgenes, 3H9RVk8, rescue B cell development on the RAG-2−/− background, but yield unexpected results with regard to the phenotype of these cells. About 40% of B cells in the spleen of the tg RAG-2−/− express high levels of IgM and CD43 and low levels of IgD, a phenotype associated with activated B cells (27). In vitro proliferation assays also reveal a B cell population from 3H9RVk8/RAG-2−/− mice that is hyperactive when stimulated with LPS or LPS and IL-4. Taking these two observations together, we conclude that there is a population (IgMhiCD43hiIgDlow) that has been activated in vivo and it is this self-primed population that has an accelerated proliferative response in vitro. These B cells do not secrete autoantibodies in vivo, but that is explainable by the absence of secondary activation signals from T cells. Thus, even though the cells are hyperactive in some assays they are not fully activated for Ig production.
3H9RVk8/RAG-2+/− mice contain few if any B cells with an activation phenotype. Rather they are anergic. We show that they are hyporesponsive to LPS stimulation, whereas strong IgM cross-linking with intact anti-IgM antibody results in normal proliferation of the tg B cells. Similar results have been obtained by Nguyen et al. (34) using conventional VH3H9/Vk8 tg mice. Proliferation to LPS and suboptimal IgM cross-linking with anti-IgM F(ab)′2 antibody were reduced, whereas IgM cross-linking in the presence of T help was normal in most animals (34). Interestingly, the VH3H9/Vk8 anti-ssDNA anergic B cells differ from anti-HEL B cells that are exposed to soluble HEL (9). The latter show a 20-fold reduction of surface IgM (9), whereas the overall IgM levels in conventional and site- directed 3H9/Vk8 tg B cells were similar to non-tg littermates (Fig. 6, reference 34). This finding might also explain the normal Ca2+ flux in the VH3H9/Vk8 mice (34), compared with transient Ca2+ flux in anti-HEL/soluble HEL double tg mice (35). In addition, anti-HEL B cells have a shortened life span in the presence of soluble HEL (36), whereas anti-ssDNA B cells live as long as non-tg B cells (34). In summary, it appears that B cell tolerance not only consists of the two distinct stages of deletion and anergy, but that qualitative differences also exist in the level of anergy. These differences are probably due to variations in the strength and/or quality of antigen–BCR interactions (as discussed earlier for deletion). What mechanism(s) are responsible for the anergy induction? Goodnow (37) discussed that B cell anergy may be induced by (a) the encounter with self-antigen early in development (clonal anergy), (b) interaction with antigen-specific regulatory T cells, or (c) regulatory elements of an idiotype network (37). Our data clearly favor the hypothesis that regulatory T cells silence self-reactive B cells, since self-reactive 3H9RVk8 B cells in RAG-2−/− mice are activated rather than anergized. We presume that the “self” milieu of all mice is similar, although quantitative difference are apparent in tg or mutant mice (see Fig. 2) and self-activated B cells are likely to be a feature of all mice, not just RAG-2−/− tgs. How then do normal mice inactivate 3H9RVk8 or other self-reactive B cells? T cells seem the most likely regulator and the activated status of B cells exposed to antigens at early stages of development may be the target for regulatory T cells. How T cells affect B cell anergy (or accelerated cell death) and whether direct suppression by cell–cell contact occurs or soluble factors produced by T cells are involved is not known. Furthermore, the phenotype of regulatory T cells is also unclear. TCR-γ/δ cells seem to downregulate the autoimmune process in MRL mice since TCR-γ/δ–deficient mice show a much more severe disease (38). In the system of organ-specific autoimmunity, e.g., after neonatal thymectomy, CD4+ IL-2R+ cells (which probably express TCR-α/β) prevent autoimmunity (39). Although it seems likely that the regulatory T cells are activated by the same autoantigen as the self-reactive B cells, the question of specificity of regulatory T cells in either organ-specific or systemic autoimmunity has not yet been resolved. In addition, future work will have to address if B cells become anergized by one single event (antigen–B cell interaction/antigen–B cell–T cell interaction) of if they must constantly receive anergizing signals.
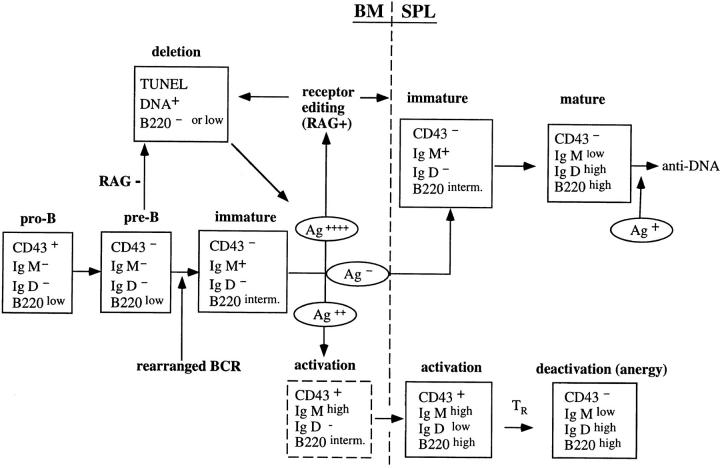
In summary, we propose the following model for B cell selection (Fig. 8): B cells that are unable to express a BCR die by apoptosis at the pre-B cells stage (RAG-2−/− mice). The expression of a functional BCR allows maturation to immature B cells. As soon as IgM is expressed on the cell surface it can receive signals due to antigen binding. If this is a strong binding resulting in a strong signal, then receptor editing is initiated. If a cell continues to express a self-reactive receptor (either because it can not be directly edited in case of a conventional tgs or because the BCR is still autoreactive after editing), the amount of signal will increase and induce apoptosis. Cells that manage to revise their BCR will be released from the death signal and continue to mature. If such mature B cells then encounter antigen in the periphery, they will be stimulated to produce antibody. Alternatively, if B cells bind self-antigen weakly, they will receive a qualitatively different signal that results in cell activation rather than editing/deletion. Such activated B cells persist in the periphery of T cell–deficient mice, but are anergized by regulatory T cells in RAG-competent mice.
Figure 8.
A model for the regulation of anti-DNA B cells. Pre-B cells that have functional rearranged heavy and light chain genes express surface IgM that can bind to autoantigens. The strong IgM cross-linking by dsDNA from blebs of apoptotic cells induces secondary rearrangement (receptor editing) and altered binding specificity. Anti-dsDNA B cells that fail to edit their receptors (e.g., in the absence of RAG) die via apoptosis. These dying cells provide an enriched source of autoantigens to anti-dsDNA B cells. Weaker IgM cross-linking by ssDNA allows the B cells to mature and populate the periphery with an activated phenotype. We propose that these activated B cells are subsequently inactivated or eliminated by T cell–dependent mechanism(s).
Acknowledgments
We thank Andrew J. Beavis for expert technical assistance in flow cytometry.
Abbreviations used in this paper
- ANA
anti-nuclear antibodies
- BCR
B cell receptor
- dsDNA
double-stranded DNA
- RAG
recombination-activating gene
- ssDNA
single-stranded DNA
- tg
transgenic
Footnotes
Dedicated to the memory of Eugenia Spanopoulou, whose work (described in reference 17) was the forerunner of this article.
Martin Weigert is a recipient of a National Institutes of Health grant (GM-20964-24) and Elisabeth Suri-Payer is supported by an Arthritis Investigator Award from the Arthritis Foundation.
References
- 1.Tan EM. Antinuclear antibodies: diagnostic markers and clues to the basis of systemic autoimmunity. Pediatr Infect Dis J. 1988;7:S3–S9. [PubMed] [Google Scholar]
- 2.Hardin JA. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986;29:457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- 3.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein T, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 4.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci USA. 1987;84:9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craft JE, Hardin JA. Linked sets of antinuclear antibodies: what do they mean? . J Rheumatol. 1987;14:106–109. [PubMed] [Google Scholar]
- 6.Casiola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1328. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class-I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 8.Hartley SB, Crosbie J, Brink RA, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 9.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe J, Trent JS, Basten A. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 10.Erikson J, Radic MZ, Camper SA, Hardey RR, Weigert MG. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 11.Gay DT, Saunders S, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radic MZ, Erikson J, Litwin S, Weigert M. B lymphocytes may escape tolerance by revising their antigen receptors. J Exp Med. 1993;177:1165–1173. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luning Prak, E., M. Trounstine, D. Huszar, and M. Weigert. Light chain editing in kappa-deficient animals: a potential mechanism of B cell tolerance. J Exp Med. 1994;180:1805–1815. doi: 10.1084/jem.180.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Nagy Z, Luning E, Prak, Weigert M. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 1995;3:747–755. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 16.Luning Prak, E., and M. Weigert. Light chain replacement: a new model for antibody gene rearrangement. J Exp Med. 1995;182:541–548. doi: 10.1084/jem.182.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spanopoulou E, Roman CAJ, Corcoran LM, Schlissel MS, Silver DP, Nemazee D, Nussenzweig MC, Shinton SA, Hardy RR, Baltimore D. Functional immunoglobulin transgenes guide ordered B-cells differentiation in RAG-1-deficient mice. Genes Dev. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- 18.Young F, Ardman B, Shinkai Y, Landsford R, Blackwell JK, Mendelsohn M, Rolink A, Melchers F, Alt FW. Influence of immunoglobulin heavy-and light-chain expression on B–cell differentiation. Genes Dev. 1994;8:1043–1057. doi: 10.1101/gad.8.9.1043. [DOI] [PubMed] [Google Scholar]
- 19.Chang Y, Bosma GC, Bosma MJ. Development of B cells in scid mice with immunoglobulin transgenes: implications for the control of V(D)J recombination. Immunity. 1995;2:607–616. doi: 10.1016/1074-7613(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Nagy Z, Radic MZ, Hardy RR, Huszar D, Camper SA, Weigert M. The site and stage of anti-DNA B cell deletion. Nature. 1995;373:252–255. doi: 10.1038/373252a0. [DOI] [PubMed] [Google Scholar]
- 21.Li YS, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson J, Melchers F, Rolink A. Stimulation by T cell independent antigens can relieve the arrest of differentiation of immature autoreactive B cells in the bone marrow. Scand J Immunol. 1995;42:21–33. doi: 10.1111/j.1365-3083.1995.tb03621.x. [DOI] [PubMed] [Google Scholar]
- 23.Paramithiotis E, Jacobsen KA, Ratcliffe MJH. Loss of surface immunoglobulin expression precedes B cell death by apoptosis in the bursa of fabricius. J Exp Med. 1995;181:105–113. doi: 10.1084/jem.181.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swat WL, Ignatowicz L, Kisielow P. Detection of apoptosis of immature CD4+CD8+ thymocytes by flow cytometry. J Immunol Methods. 1991;137:79–87. doi: 10.1016/0022-1759(91)90396-w. [DOI] [PubMed] [Google Scholar]
- 25.Thorbecke GJ, Amin AR, Tsiagbe VK. Biology of germinal centers in lymphoid tissue. FASEB (Fed Am Soc Exp Biol) J. 1994;8:832–840. doi: 10.1096/fasebj.8.11.8070632. [DOI] [PubMed] [Google Scholar]
- 26.Rolink A, Ghia P, Grawunder U, Haasner D, Karasuyama H, Kalberer C, Winkler T, Melchers F. In-vitro analyses of mechanisms of B-cell development. Semin Immunol. 1995;7:155–167. doi: 10.1016/1044-5323(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 27.Wells SM, Kantor AB, Stall AM. CD43 (S7) expression identifies peripheral B cell subsets. J Immunol. 1994;153:5503–5515. [PubMed] [Google Scholar]
- 28.Gulley ML, Ogata LC, Thorson JA, Dailey MO, Kemp JD. Identification of a murine pan-T cell antigen which is also expressed during the terminal phases of B cell differentiation. J Immunol. 1988;140:3751–3757. [PubMed] [Google Scholar]
- 29.Osmond DG, Rico-Vargas S, Lu H, Jacobsen K. Apoptosis and macrophage-mediated cell deletion in the regulation of B lymphopoiesis in mouse bone marrow. Immunol Rev. 1994;142:209–230. doi: 10.1111/j.1600-065x.1994.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsen KA, Prasad VS, Sidman CL, Osmond DG. Apoptosis and macrophage-mediated deletion of precursor B cells in the bone marrow of Eμ-myctransgenic mice. Blood. 1994;84:2784–2794. [PubMed] [Google Scholar]
- 31.Osmond GD, Kim N, Manoukian R, Phillips RA, Rico-Vargas SA, Jacobsen K. Dynamics and localization of early B-lymphocyte precursor cells (pro-B cells) in the bone marrow of scid mice. Blood. 1992;79:1695–1703. [PubMed] [Google Scholar]
- 32.Chen C, Luning E, Prak, Weigert M. Editing disease–associated autoantibodies. Immunity. 1997;6:97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- 33.Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, Goodnow C. Elimination of self- reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen K-A, Mandik L, Bui A, Kavaler J, Norvell A, Monroe JG, Roark JH, Erikson J. Characterization of anti-single-stranded DNA B cells in a non-autoimmune background. J Immunol. 1997;159:2633–2644. [PubMed] [Google Scholar]
- 35.Cooke MP, Heath AW, Shokat KM, Zeng Y, Finkelman FD, Linsley PS, Howard M, Goodnow CC. Immunoglobulin signal transduction guides the specificity of B cell–T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulcher DA, Basten A. Reduced life span of anergic self-reactive B cells in a double-transgenic model. J Exp Med. 1994;179:125–134. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodnow CC. B-cell tolerance. Curr Opin Immunol. 1992;4:703–710. doi: 10.1016/0952-7915(92)90049-k. [DOI] [PubMed] [Google Scholar]
- 38.Peng SL, Madaio MP, Hayday AC, Craft J. Propagation and regulation of systemic autoimmunity by γδ T cells. J Immunol. 1996;157:5689–5698. [PubMed] [Google Scholar]
- 39.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]