Abstract
The development of a normal T cell repertoire in the thymus is dependent on the interplay between signals mediating cell survival (positive selection) and cell death (negative selection or death by neglect). Although the CD28 costimulatory molecule has been implicated in this process, it has been difficult to establish a role for the other major costimulatory molecule, cytotoxic T lymphocyte antigen (CTLA)-4. Here we report that in vivo stimulation through the T cell receptor (TCR)–CD3 complex induces expression of CTLA-4 in thymocytes and leads to the association of CTLA-4 with the SH2 domain–containing phosphatase (SHP)-2 tyrosine phosphatase. Moreover, intrathymic CTLA-4 blockade dramatically inhibits anti-CD3–mediated depletion of CD4+CD8+ double positive immature thymocytes. Similarly, anti-CD3–mediated depletion of CD4+CD8+ double positive cells in fetal thymic organ cultures could also be inhibited by anti–CTLA-4 antibodies. Thus, our data provide evidence for a role of CTLA-4 in thymic selection and suggest a novel mechanism contributing to the regulation of TCR-mediated selection of T cell repertoires.
Keywords: cytotoxic T lymphocyte antigen 4 (CD152), CD28, costimulation, T cell development, tyrosine phosphatase SHP-2
During thymocyte differentiation, progenitor T cells proceed through a process of positive and negative selection upon ligation of the TCR (1). The outcome of TCR ligation is believed to be dependent on the avidity of the interaction between the TCR and the MHC (2, 3), and on the differentiation status of the T cell progenitor (4). This process has also been suggested to include a role for T cell costimulatory molecules, notably the CD28 receptor.
The CD28/CTLA-4/B7 cell surface molecules are essential for the control of T cell responses regulating a complex pathway of costimulatory signals (5, 6). Although signaling through CD28 leads to the enhancement and maintenance of T cell activation and proliferation, accumulating data suggest that CTLA-4 functions as a negative regulator of T cell activity (7–10). CTLA-4 and CD28 also differ in their expression pattern and in avidity to their common ligands, B7-1 (CD80) and B7-2 (CD86). CD28, which binds the B7 molecules with a relatively low avidity, is constitutively expressed on the cell surface of resting and activated T cells (11). On the other hand, CTLA-4 is transiently expressed at the cell surface only after T cell activation. The avidity for interaction with the B7 molecules is ∼10–100-fold higher than for CD28 (12–14). Recent studies have demonstrated that after T cell activation the majority of CTLA-4 is localized in cytoplasmic vesicles rather than on the cell surface, and that once expressed on the membrane it is rapidly internalized (15, 16). These data suggest that CTLA-4 intracellular trafficking is tightly regulated, and could explain the low levels of expression detected at the cell surface.
The observation that the ligands for the CD28/CTLA-4 receptors, the B7 molecules, could be expressed in the thymic epithelium (17, 18) suggested a functional role of these costimulatory receptors during thymocyte differentiation. In line with this, the CD28 receptor has been reported to be involved in the rescue of CD4+CD8+ double positive (DP)1 thymocytes from death by neglect, and to be required for induction of apoptosis during negative selection (19–21). During T cell differentiation, CD28 is expressed on 90–95% of thymocytes and can be detected already at the CD4−CD8− double negative (DN) pre-T cell stage. The expression level is upregulated in DP thymocytes and decreases again in mature CD4 and CD8 single positive (SP) T cells (11).
Although CTLA-4 has been suggested to play a major role in the establishment of peripheral self-tolerance as well as in the regulation of normal T cell immunity (6, 22, 23), its potential role during thymocyte development is more controversial (24–28). Evidence for basal expression of CTLA-4 in non activated thymocytes comes from the original report by Brunet et al. where a weak but still detectable RNA expression was seen by Northern blot analysis (29). Low expression of CTLA-4 protein on the surface of fresh thymocytes has also been reported (26).
We have addressed the role of CTLA-4 in T cell development by analyzing CTLA-4 expression and function in thymocytes activated in vivo through CD3 stimulation. We report that CTLA-4 is induced and associates with the SHP-2 tyrosine phosphatase upon in vivo anti-CD3 administration. Intrathymic CTLA-4 blockade dramatically inhibits anti-CD3–mediated depletion of DP immature thymocytes. Furthermore, CTLA-4 blockade was also found to inhibit anti-CD3–mediated depletion of DP thymocytes in fetal thymic organ cultures (FTOCs), demonstrating that the observed effect is not due to an indirect effect executed upon peripheral T cells. Together, these results provide evidence for a role of CTLA-4 in thymic selection and suggest a novel mechanism contributing to the regulation of TCR-mediated selection of T cell repertoires.
Materials and Methods
Mice.
C57BL/6 (B6) mice were purchased from Bomholtgård Breeding & Research Centre (Ry, Denmark) and maintained in our own facility. All mice used in the experiments were 6–10 wk old.
Antibodies.
Hamster anti-CD3 mAb (clone 145-2C11) was purified in our laboratory from hybridoma culture supernatant by affinity chromatography on Protein G column (Amersham Pharmacia Biotech, Inc., Uppsala, Sweden). Supernatant from hybridoma producing hamster anti-FcR (clone 2.4G2) was used to block FcRs during thymocyte staining. Anti-CTLA-4–PE (clone UC10-4F10-11), control hamster IgG anti-TNP–PE (clone G155-178), anti-CD4–FITC or –PE (clone H129.19), and anti-CD8–biotin or –FITC (clone 53-6.7) were all purchased from PharMingen (San Diego, CA).
In Vivo Anti-CD3 Treatment.
Groups of two to three mice were injected with 100 μg (unless otherwise stated) of anti-CD3 intraperitoneally in 200 μl total volume, or with saline as control. After different time points, thymi were explanted and single cell suspension was prepared by passage through nylon mesh.
Intrathymic Injection.
Groups of three mice were intrathymically injected using a technique originally described by Scollay et al. (30). In brief, mice were anesthetized, and after thymus exposure between 5 and 8 μg of purified soluble anti–CTLA-4 mAb (clone UC10-4F10-11, low endotoxin, sodium azide–free; PharMingen) in a total volume of 10 μl was injected in each thymus lobe. Control mice received either an equal amount of hamster IgG anti-TNP (clone G155-178, low endotoxin, sodium azide–free, PharMingen) or only saline. After mice recovered from surgery, generally between 2 and 4 h, anti-CD3 was injected intraperitoneally at the indicated doses. Mice were killed 24 h later and thymocytes were stained for flow cytometry analysis as described below.
Staining and Flow Cytometry.
For CTLA-4 surface expression, freshly isolated thymocytes were washed twice in staining buffer (3% FCS and 0.01% sodium azide in PBS) before the stainings were performed. In brief, 106 cells were first incubated with 100 μl of anti-FcR mAb supernatant for 30 min on ice to reduce unspecific staining due to FcR binding of mAbs. After washing with staining buffer, cells were triple stained with saturating concentrations of anti-CD4–FITC, anti-CD8–biotin, and anti-CTLA-4–PE for 30 min on ice followed by a final incubation with streptavidin Cy-Chrome (PharMingen) for 30 min on ice to reveal biotinylated mAb. For CTLA-4 intracellular staining, after FcR blocking, cells were first stained for surface markers, fixed in 2% paraformaldehyde, washed twice in saponin buffer (0.03% saponin in PBS), and then permeabilized with 0.3% saponin in PBS for 5 min on ice. Cells were then stained with anti-CTLA-4–PE in the presence of 0.3% saponin for an additional 30 min, washed extensively in saponin buffer, once in staining buffer, and finally resuspended in PBS for FACS® (Becton Dickinson, San Jose, CA) analysis. Flow cytometry was performed with a FACScalibur® cytometer (Becton Dickinson), and CellQuest® software (Becton Dickinson) was used for data collection and analysis. At least 2 × 104 viable cells were acquired based on their morphology using FCS/SSC parameters.
For detection of apoptosis by propidium iodide (PI) staining and DNA content analysis, 106 thymocytes recovered from FTOCs were fixed in 70% ethanol for 2 h on ice, washed twice with PBS, and resuspended in hypotonic PI solution containing 0.1% sodium citrate, 0.1% Triton X-100, 50 μg/ml PI (Sigma Chemical Co., St. Louis, MO), and 20 μg/ml RNAse (Sigma Chemical Co.). Cells were kept at 4°C in the dark overnight before FACS® analysis was performed. Apoptotic cells were identified by hypodiploid DNA content.
FTOC.
Fetal thymi were isolated from B6 embryos at day 18.5 of gestational age under sterile conditions. Organ cultures were set up as originally described (31) with slight modifications. In brief, explants were divided into single lobes and put onto 25-mm Nucleopore® membranes (0.8 μm polycarbonate; Costar Corp., Cambridge, MA) floating on 5 ml IMDM complete medium (GIBCO BRL, Gaithersburg, MD), containing 1× nonessential amino acids (GIBCO BRL), penicillin/streptomycin, 5 × 10−5 M 2-ME, 10% FCS, and 1 mM sodium pyruvate in six-well culture plates (Costar Corp.), and incubated at 37°C with 5% CO2. FTOCs were analyzed after 24 h of incubation in the presence or absence of the indicated soluble antibodies (10 μg/ml anti-CD3, 10 μg/ml anti–CTLA-4).
RNA Extraction and Reverse Transcriptase PCR.
Total RNA was isolated using the Ultraspec RNA isolation system (Biotecx Labs., Houston, Texas) according to the manufacturer's indications. In brief, thymi from anti-CD3 and saline-treated mice were explanted and quickly frozen in liquid nitrogen. The thymi were then homogenized in lysis buffer and RNA was extracted with chloroform and precipitated with isopropanol. For cDNA synthesis, 300 ng of total RNA from each sample was incubated with oligo(dT) primer (Amersham Pharmacia Biotech, Inc.) for 10 min at 70°C and chilled on ice for 5 min. Samples were then incubated with 0.1 M dithiothreitol, 500 μM dNTPs, 20 U RNase inhibitors (Boehringer Mannheim, Mannheim, Germany) for 2 min at room temperature before 1.5 μl (300 U) of SuperScript RNaseH-free reverse transcriptase (RT; GIBCO BRL) was added. Samples were then incubated for 2 min at room temperature followed by 1 h at 37°C and finally 5 min at 95°C. Amplification of synthetized cDNA from each sample was carried out with Dynazyme DNA polymerase (Finnzymer OY, Espoo, Finland) in 50 μl reaction under the following conditions: 95°C for 2 min, 55°C for 1 min, and 72°C for 1 min for a total of 32 cycles. Specific primers were designed to amplify the CTLA-4 coding region (sense primer: 5′-TCCTGTTGGGTTTTACTCTAC-3′; antisense primer: 5′-GAAATTGCCTCAGCTTTAG-3′) and hypoxanthine-guanine phosphoribosyl transferase (HPRT) primers (sense: 5′-CACAGGACTAGAACACCTGC-3′; antisense: 5′-GCTGGTGAAAAGGACCTCT-3′) were used as a control for cDNA input and RT-PCR.
Immunoprecipitation of CTLA-4.
Sixty million fresh thymocytes were resuspended in CHAPS lysis buffer (50 mM Tris, pH 7.2, 300 mM NaCl, 1 mM EDTA, 6 mM CHAPS, 2 mM Pefabloc SC, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mM sodium vanadate). Lysis was allowed to proceed for 2 h at 4°C and insoluble debris was removed by centrifugation at 25,000 g for 20 min at 4°C. Supernatants were diluted 1:2 with TNB buffer (50 mM Tris, pH 7.2, 300 mM NaCl, and 2 mg/ml BSA) and then precleared by mixing with 4 μl each of protein A–sepharose and Gammabind G–sepharose (Amersham Pharmacia Biotech, Inc.) for 45 min at 4°C, followed by centrifugation at 500 g for 3 min at 4°C. Cleared supernatants were then incubated for 18 h at 4°C with 2 μg each of anti-CTLA-4 antibody. Immune complexes were recovered by incubation with 4 μl each of protein A–sepharose and Gammabind G–sepharose for 45 min at 4°C, followed by centrifugation at 500 g for 3 min at 4°C. Pellets were washed twice in dilution buffer (10 mM Tris, pH 8.0, 140 mM NaCl, 0.1% Triton X-100, 0.1% bovine hemoglobin, and 0.025% sodium azide), once in TSA buffer (10 mM Tris, pH 8.0, 140 mM NaCl, and 0.025% sodium azide), and once in 50 mM Tris, pH 6.8, and then the proteins were solubilized in 2× SDS loading buffer (100 mM Tris, pH 6.8, 4% SDS, 0.2% Bromophenol blue, 200 mM dithiothreitol, and 20% glycerol). Samples were boiled for 5 min before resolution by 8% SDS-PAGE.
Gel Electrophoresis and Western Blotting.
Polyacrylamide gels were run using a Mini Protean II gel apparatus (Bio-Rad, Hercules, CA) and then transferred to PVDF membrane (Pierce Chemical Co., Rockford, IL) using a Sammy D Semi-dry blotter (Schleicher and Schuell, Dassel, Germany). Blots were blocked overnight in TBS-T (20 mM Tris, pH 7.5, 137.5 mM NaCl, and 0.1% Tween 20) containing 10% horse serum (TBS-T/HS). Incubations were in TBS-T/HS as follows: anti–SHP-2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) 1:250, 2 h; biotinylated donkey anti–rabbit (Amersham Pharmacia Biotech) 1:5,000, 2 h; and streptavidin-horseradish peroxidase (Pierce Chemical Co.) 1:5,000, 1 h. Filters were washed overnight with several changes of TBS-T, developed with Supersignal chemiluminescent substrate (Pierce Chemical Co.), and exposed to Cronex4 X-Ray film (Du Medical Scandinavia, Sollentua, Sweden).
Results
CTLA-4 Is Induced in Thymocytes after In Vivo CD3 Stimulation.
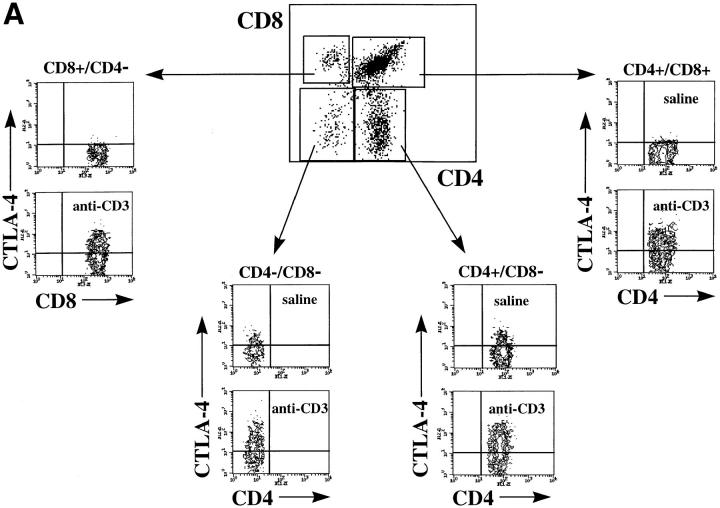
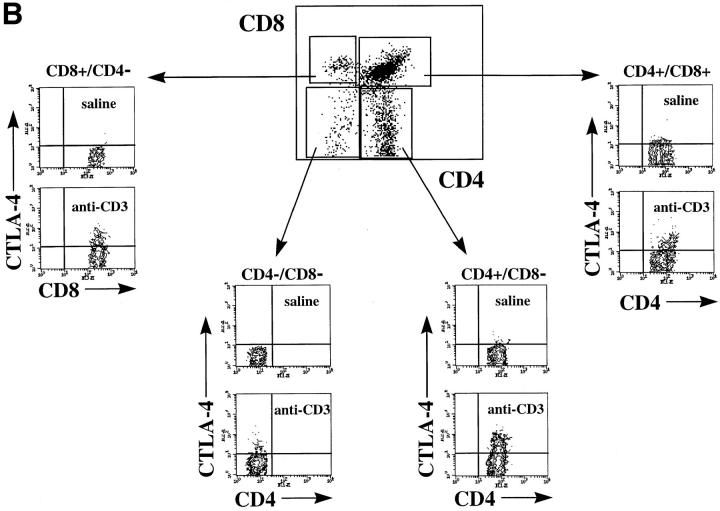
To investigate whether CTLA-4 expression could be induced on thymocytes by in vivo activation, we analyzed the intracellular and membrane expression of CTLA-4 on thymocytes before and after in vivo administration of anti-CD3 mAbs. Mice were killed at different time points after administration of anti-CD3 and thymocytes were analyzed for intracellular and cell surface expression of CTLA-4 by flow cytometry (Fig. 1, A and B). Before in vivo activation by anti-CD3, no significant surface expression of CTLA-4 was observed in any of the main thymocyte subpopulations (Fig. 1 B). However, low levels of CTLA-4 expression were observed intracytoplasmatically in the DN and the CD4 SP thymocyte subpopulations (Fig. 1 A). Upon in vivo activation, an upregulation of CTLA-4 expression was observed in all thymocyte subpopulations (Figs. 1 and 2). This upregulation of expression occurred more rapidly in the intracytoplasmatic pool, where it was already evident at 16 h after stimulation (Fig. 2). At the level of membrane expression, the increase in CTLA-4 expression was slower, and significant upregulation was observed in DP and SP thymocytes only 24 h after stimulation (Figs. 1 B and 2). In the DN subpopulation, the upregulation of membrane expression was less pronounced than in the other thymocyte subpopulations (Figs. 1 B and 2).
Figure 1.
CTLA-4 expression is induced upon in vivo anti-CD3 administration. Mice were injected intraperitoneally with 100 μg of anti-CD3 mAbs or with saline as control. After 24 h mice were killed and thymocytes were analyzed for CTLA-4 expression by three-color flow cytometry. (A) Intracellular expression of CTLA-4: thymocytes were first double stained with anti-CD4–FITC and anti-CD8–biotin surface markers, fixed, permeabilized, and finally incubated with anti–CTLA-4–PE. (B) Cell surface expression of CTLA-4: thymocytes were triple stained with anti-CD4–FITC, anti-CD8–biotin, and anti–CTLA-4–PE. In all experiments biotin has been revealed by incubation with streptavidin Cy-Chrome. Contour dot-plots represent CTLA-4 expression evaluated on viable thymocyte subsets by differential gating based on CD4 and CD8 expression (boxes). These results are representative of at least three independent experiments.
Figure 2.
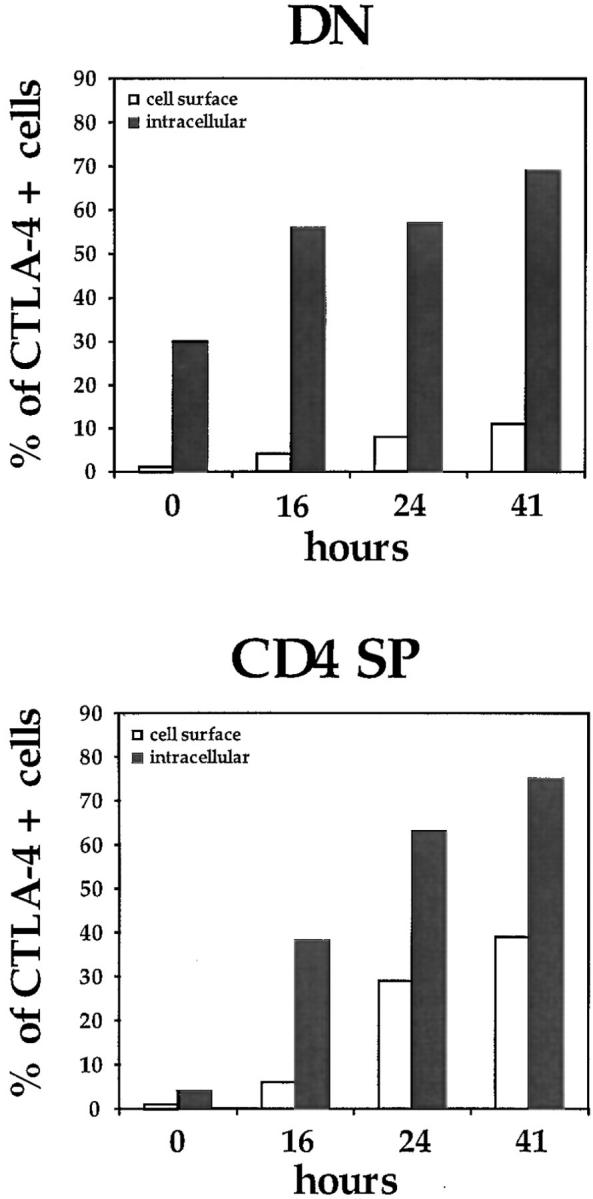
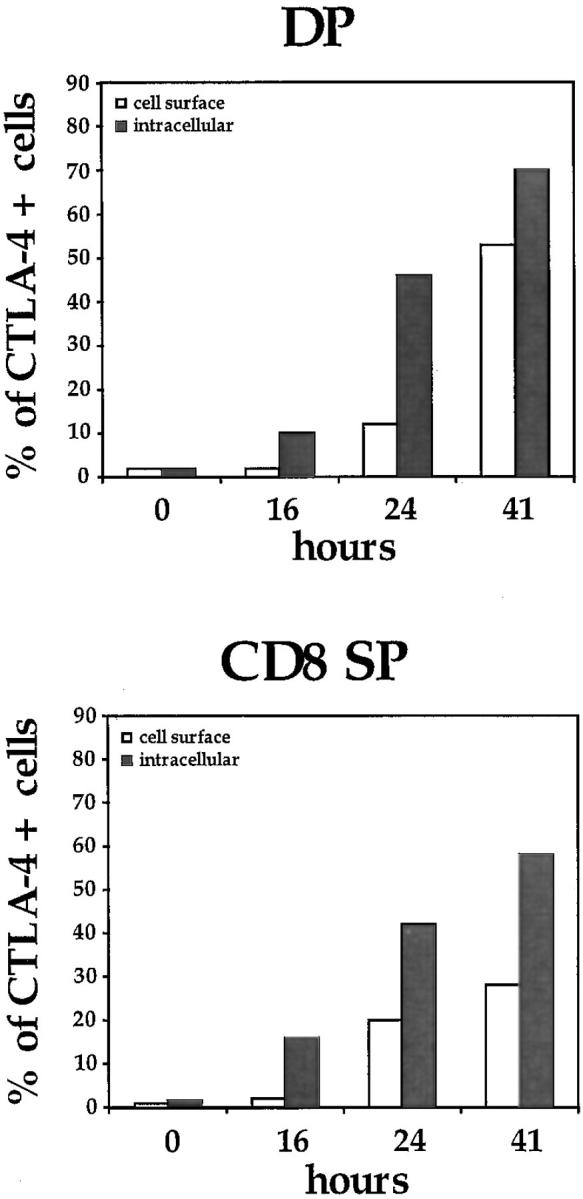
Kinetics of CTLA-4 expression in the major thymocyte populations. Thymocytes were stained and analyzed by flow cytometry at different time points after in vivo administration of anti-CD3 as described in Fig. 1 and in Materials and Methods. DP, CD4+CD8+ double positives; SP, CD4+ or CD8+ single positives; DN, CD4−CD8− double negatives. One experiment is shown of at least three independent experiments with similar results.
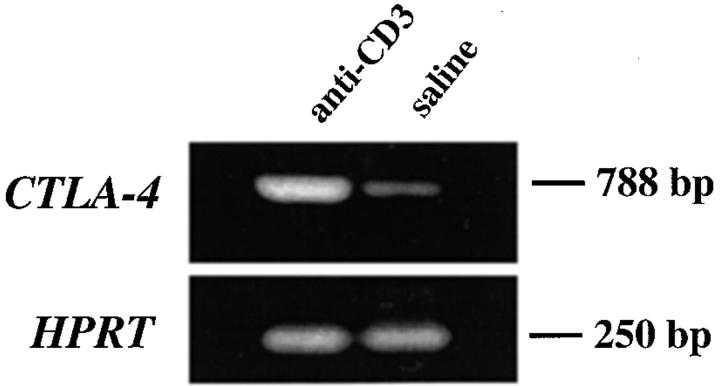
To confirm that the induced expression of CTLA-4 protein was accompanied by an increase in the transcription rate of the Ctla-4 gene, the levels of specific mRNA were estimated using an RT-PCR approach. As illustrated in Fig. 3, Ctla-4–specific mRNA was increased in thymocytes upon in vivo anti-CD3 stimulation compared with thymocytes recovered from mice injected with only saline (Fig. 3). Together, these results demonstrated that the transcription and protein expression of CTLA-4 can indeed be induced in thymocytes upon in vivo stimulation with anti-CD3.
Figure 3.
Expression of Ctla-4 mRNA in thymocytes upon in vivo anti-CD3 treatment. RNA was isolated from thymus after 24 h of treatment with 100 μg of anti-CD3 or saline and analyzed by RT-PCR for Ctla-4 RNA expression. Hypoxanthine-guanine phosphoribosyl transferase (HPRT) RNA expression is shown in the lower panel as control for cDNA loading and RT-PCR. This experiment is representative of two independent RT-PCRs.
CTLA-4 Associates with SHP-2 Phosphatase after Anti-CD3 Treatment.
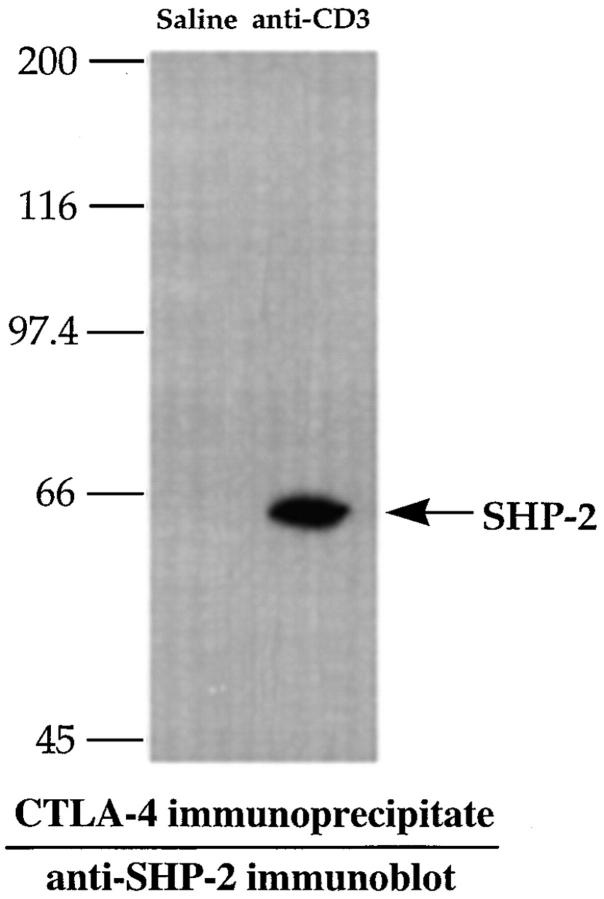
Recently, immunoprecipitation studies have shown that the SH2 domain–containing protein tyrosine phosphatase SHP-2 specifically associates with CTLA-4 in peripheral T cells (32). These results have been suggested to indicate a role for CTLA-4 in regulating TCR signaling through recruitment of SHP-2 and interference with the phosphorylation of TCR-associated kinases. To determine whether a similar recruitment of SHP-2 could be detected in in vivo activated thymocytes, we determined the levels of SHP-2 associated with CTLA-4 in thymocytes upon in vivo stimulation with anti-CD3. As illustrated in Fig. 4, SHP-2 could be readily immunoprecipitated with CTLA-4 from thymocytes after, but not before, in vivo stimulation with anti-CD3 mAbs. Thus, in vivo stimulation of thymocytes through the TCR–CD3 receptor complex appears to result in an increase in the SHP-2 associated with the CTLA-4 receptor. In contrast, no recruitment of the homologous phosphatase SHP-1 was found in thymocytes after anti-CD3 administration (data not shown).
Figure 4.
CTLA-4 associates with SHP-2 in in vivo activated thymocytes. Thymocytes from mice treated for 24 h with 100 μg of anti-CD3 or with saline only were lysed and CTLA-4 was immunoprecipitated with anti–CTLA-4. Proteins were subjected to SDS-PAGE, transferred to PVDF membrane, and immunoblotted with anti–SHP-2 antibody as described in Materials and Methods.
Intrathymic CTLA-4 Blockade Prevents Anti-CD3–mediated Depletion of Immature Thymocytes.
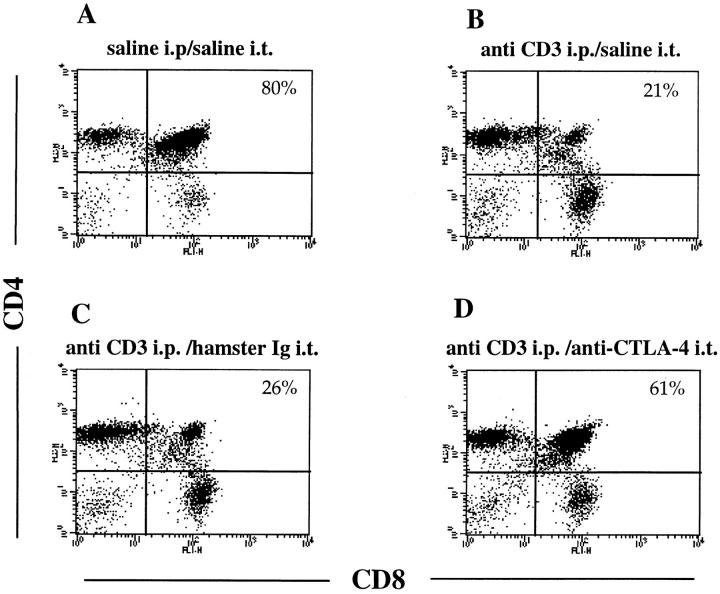
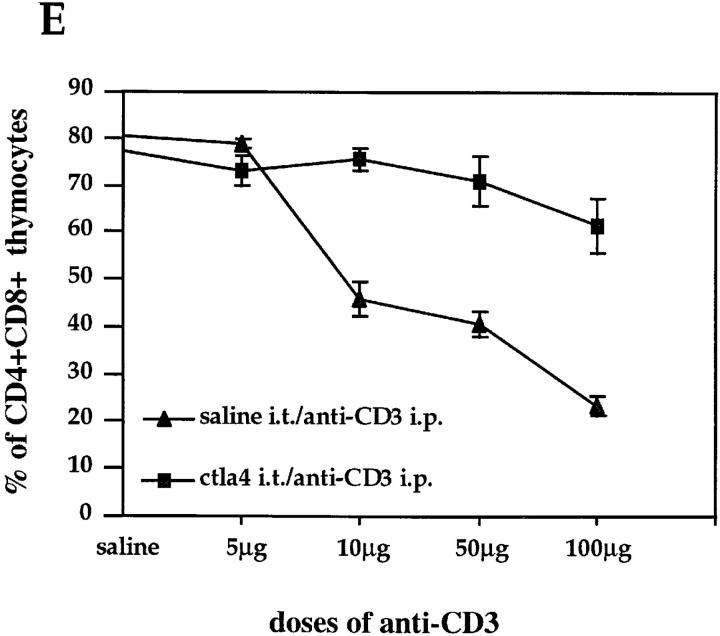
To examine the possibility that the induced CTLA-4 expression would indeed mediate a functional effect on thymocytes, we tested whether blockade of the CTLA-4 receptor by intrathymic injection of soluble anti-CTLA-4 mAbs could interfere with the depletion of DP thymocyte mediated by anti-CD3 treatment. Mice were first injected intrathymically with soluble anti-CTLA-4, then treated with 100 μg of anti-CD3 intraperitoneally. After 24 h, thymocytes were analyzed for CD4 and CD8 expression (Fig. 5). Control mice received saline intraperitoneally and/or saline or anti-TNP hamster Ig intrathymically. In vivo administration of anti-CD3 led to a rapid depletion of immature thymocytes (Fig. 5, A and B). As illustrated in Fig. 5 D, intrathymic injection of anti–CTLA-4 before anti-CD3 administration significantly inhibited the anti-CD3–mediated depletion of DP thymocytes, whereas control hamster Ig or saline had no effect (Fig. 5, B and C). The observed effect was dose dependent and primarily involved the compartment of DP thymocytes (Fig. 5 E). Interestingly, the effect of CTLA-4 blockade was dramatic at doses of anti-CD3 up to 50 μg, where the depletion of DP thymocytes was almost completely inhibited by intrathymic CTLA-4 blockade. However, the inhibitory effect of anti–CTLA-4 appears to be overcome at increased doses of anti-CD3 (Fig. 5 E).
Figure 5.
Intrathymic CTLA-4 blockade inhibits anti-CD3– mediated depletion of DP thymocytes. Groups of three mice were intrathymically injected with soluble anti–CTLA-4, with only saline or with anti-TNP hamster Ig as controls before administration of anti-CD3. Thymocytes were double stained with anti-CD4–PE and anti-CD8–FITC and analyzed by flow cytometry. Dot-plots represent CD4/CD8 profiles of thymocytes from (A) mice injected with saline intraperitoneally (i.p.) and intrathymically (i.t.), (B) mice injected with 100 μg anti-CD3 intraperitoneally and saline intrathymically, (C) mice injected with 100 μg anti-CD3 intraperitoneally and hamster Ig intrathymically, (D) mice injected with 100 μg anti-CD3 intraperitoneally and anti–CTLA-4 intrathymically. Percentages of DP thymocytes are shown on the upper right quadrant of each dot-plot. (E) Thymocytes recovered from mice (three mice each group) treated with increasing amounts of anti-CD3 and injected intrathymically either with anti-CTLA-4 or only saline were double stained with anti-CD4 and anti-CD8 as above. These results are expressed as the mean value ± SD and are representative of at least three independent experiments.
In Vitro CTLA-4 Blockade Prevents Anti-CD3–induced Apoptosis of DP Thymocytes in FTOCs.
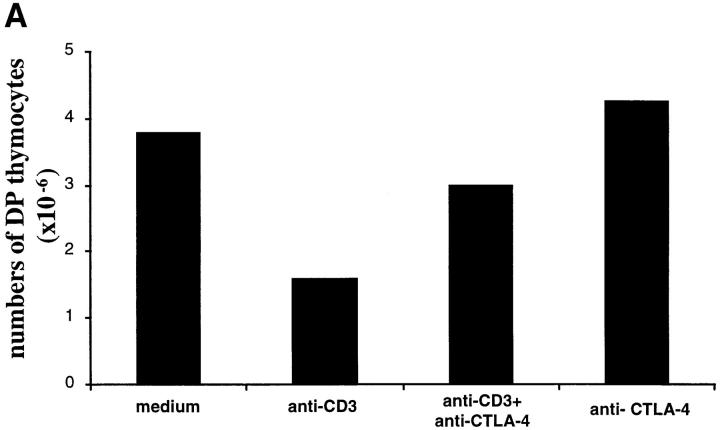
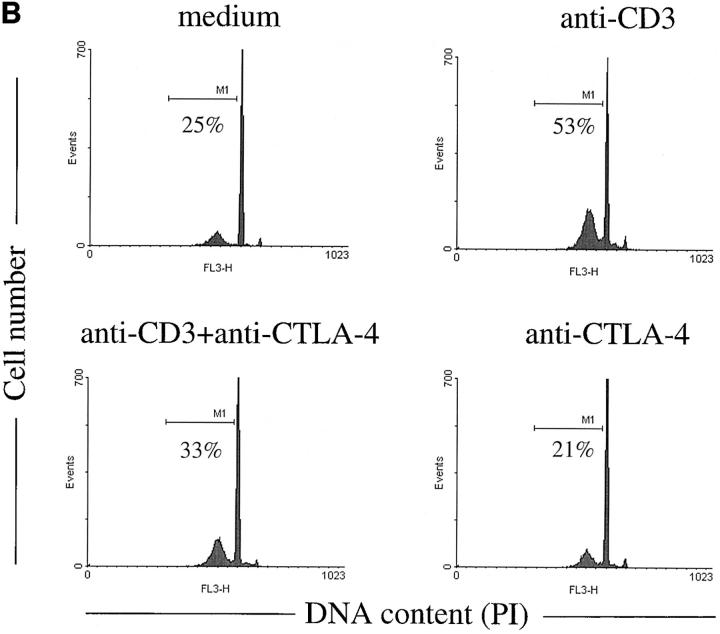
It could be argued that the effect of in vivo anti-CD3 administration on thymocytes is an indirect effect mediated through the activation of peripheral T cells. Thus, it is not ruled out that the inhibitory effect obtained by intrathymic injections of anti– CTLA-4 could be the result of anti–CTLA-4 inhibition of anti-CD3–activated peripheral T cells. To address this, we investigated if the effect of CTLA-4 blockade could be directly observed on thymocytes in FTOCs. As illustrated in Fig. 6, anti-CD3 stimulation of FTOCs increased the number of apoptotic cells and decreased the number of DP cells. This effect was inhibited by adding anti–CTLA-4 antibodies to the culture, demonstrating a direct effect of CTLA-4 blockade on thymocytes.
Figure 6.
Anti–CTLA-4 antibodies inhibit anti-CD3–mediated apoptosis and depletion of DP thymocytes in FTOCs. Fetal thymi were maintained in organ culture in the presence of the indicated antibodies for 24 h. (A) Total number of DP thymocytes was calculated multiplying the percentage of CD4+CD8+ thymocytes by the total number of cells recovered from each lobe. (B) FACS® profiles of DNA content of thymocytes harvested after 24 h of organ culture under the indicated conditions. Apoptotic cells are represented by the hypodiploid peak to the left of the G0/G1 peak.
Discussion
We report here that expression of the costimulatory receptor CTLA-4 in thymocytes can be induced upon in vivo stimulation through the TCR–CD3 receptor complex. Cell surface expression of CTLA-4 was clearly observed in DP as well as CD4 and CD8 SP thymocytes, but cell surface expression had slower kinetics compared with the induction of intracellular expression. Although surface expression does not per se demonstrate functional relevance, the inhibition of anti-CD3–mediated depletion of DP thymocytes through the blockade of CTLA-4 provided evidence for a functional role for CTLA-4 during thymocyte differentiation. The observation that CTLA-4 blockade inhibits the anti-CD3–mediated depletion of thymocytes in FTOCs further supports this notion. This observation also provides evidence for a direct functional effect of CTLA-4 blockade on thymocytes, escaping the possibility that the observed effect was mediated through inhibiting peripheral T cells activated by the anti-CD3 treatment.
Accumulating data have suggested that CTLA-4 functions as a negative regulator of T cell activity in the periphery. An association of SHP-2 with CTLA-4 in mature peripheral T cells has been demonstrated previously (32), and here we demonstrate this association in thymocytes. The association of SHP-2 with CTLA-4 was suggested to mediate a negative signal imparted by CTLA-4. However, previous data have implicated SHP-2 in a positive signaling role (33, 34), and the inhibition of CD3–mediated depletion of DP thymocytes by blockade of CTLA-4 observed here may indicate that CTLA-4 signaling in fact is potentiating the signaling through the TCR–CD3 receptor complex. The view of CTLA-4 as a negative regulator has been recently challenged by others, who suggest that CTLA-4 could act as a receptor mediating costimulatory signals along with the signals mediated by the TCR–CD3 receptor complex and the CD28 receptor (35).
The suggested function of CTLA-4 in thymic development is in agreement with the initial reports of the Ctla-4−/− mice demonstrating an impairment in thymocyte development (24, 25). However, they are in apparent contrast to more recently presented analyses of these mice reporting no major alterations in cell numbers, kinetics of development, or distribution of subpopulations (27, 28). These recent papers indicate that the CTLA-4 is primarily involved in the regulation of peripheral T cell activation. Reinterpreted within the frame of a suggested positive signaling through CTLA-4, these data may still be compatible with a functional role of CTLA-4 in the thymus. Current views hold that the signaling of positive versus negative selection mediated by the TCR– MHC complex is determined on the basis of the overall avidity of this interaction (1). If, as suggested, CTLA-4 mediates a positive costimulatory signal, the interaction of CTLA-4 with its B7 ligands would produce an additive effect to the TCR-MHC interaction during thymic selection, thus strengthening the signals mediated through the TCR. The CD28 and the CTLA-4 costimulatory receptors would, at least in this respect, have similar modes of action.
Due to the difference in avidity for their ligands, different kinetics of expression, and association with different signaling molecules (6, 11, 15), CD28 and CTLA-4 may still play fundamentally different roles in the thymic selection process. Thus, the higher avidity of CTLA-4 to B-7 and the fact that it is only expressed upon activation is compatible with a mainly negative regulatory function, mediated through the surpassing of a threshold of T cell signaling leading to cell death rather than activation and further differentiation. In this way, a defective CTLA-4 expression is likely to result in the skewing of TCR affinities rather than in largely distorted cell numbers or alterations in the various subpopulations that represent unique stages of the differentiation process. Thus, the suggested function of CTLA-4 during thymocyte differentiation may not be incompatible with relatively normal cell numbers and subset distribution in Ctla-4−/− mice (27, 28).
Our data suggest a model that predicts a skewing towards increased mean affinities for self-MHC–peptide complexes in mice with defective CTLA-4 function or expression. In the Ctla-4−/− mice the predicted bias towards self-reactivity could, together with a defective peripheral control of T cell activity, contribute to the massive lymphoproliferation observed in these mice. This scenario also provides a plausible mechanism for the contribution of CTLA-4 in mice and humans with autoimmune disorders that have been genetically linked to the Ctla-4 gene (36–41). The predicted repertoire skewing in recent thymic emigrants in these cases could be tested.
Acknowledgments
This work was supported by grants from the European Commission, the Swedish Medical Research Council (nos. 07929, 10324, 11598, and 11324), the Swedish Cancer Society, and Umeå Biotechnology Foundation. C.M. Cilio is supported by a fellowship from the foundation “Istituto Pasteur-Fondazione Cenci Bolognetti” and A. Malashicheva is the recipient of a fellowship from the Swedish Institute.
Abbreviations used in this paper
- DN
double negative
- DP
double positive
- FTOC
fetal thymic organ culture
- RT
reverse transcriptase
- SHP
SH2 domain–containing phosphatase
- SP
single positive
Footnotes
A. Malashicheva's present address is Institute of Cytology, Russian Academy of Science, Tichozetzky p2.4, 194064 St. Petersburg, Russia.
References
- 1.von Boehmer H. Thymic selection: a matter of life and death. Immunol Today. 1992;13:454–458. doi: 10.1016/0167-5699(92)90075-I. [DOI] [PubMed] [Google Scholar]
- 2.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 3.Kisielow P, Miazek A. Positive selection of T cells: rescue from programmed cell death and differentiation require continual engagement of the T cell receptor. J Exp Med. 1995;181:1975–1984. doi: 10.1084/jem.181.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kisielow P, von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- 5.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 6.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 7.Waterhouse P, Marengere LE, Mittrucker HW, Mak TW. CTLA-4, a negative regulator of T-lymphocyte activation. Immunol Rev. 1996;153:183–207. doi: 10.1111/j.1600-065x.1996.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 8.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 9.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 12.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 14.Kariv I, Truneh A, Sweet RW. Analysis of the site of interaction of CD28 with its counter-receptors CD80 and CD86 and correlation with function. J Immunol. 1996;157:29–38. [PubMed] [Google Scholar]
- 15.Alegre ML, Noel PJ, Eisfelder BJ, Chuang E, Clark MR, Reiner SL, Thompson CB. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- 16.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 17.Nelson AJ, Hosier S, Brady W, Linsley PS, Farr AG. Medullary thymic epithelium expresses a ligand for CTLA4 in situ and in vitro. J Immunol. 1993;151:2453–2461. [PubMed] [Google Scholar]
- 18.Degermann S, Surh CD, Glimcher LH, Sprent J, Lo D. B7 expression on thymic medullary epithelium correlates with epithelium-mediated deletion of V beta 5+thymocytes. J Immunol. 1994;152:3254–3263. [PubMed] [Google Scholar]
- 19.Amsen D, Kruisbeek AM. CD28-B7 interactions function to co-stimulate clonal deletion of double-positive thymocytes. Int Immunol. 1996;8:1927–1936. doi: 10.1093/intimm/8.12.1927. [DOI] [PubMed] [Google Scholar]
- 20.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+thymocytes by T cell receptor–induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185:263–271. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 23.Lane P, Haller C, McConnell F. Evidence that induction of tolerance in vivo involves active signaling via a B7 ligand-dependent mechanism: CTLA4-Ig protects V beta 8+T cells from tolerance induction by the superantigen staphylococcal enterotoxin B. Eur J Immunol. 1996;26:858–862. doi: 10.1002/eji.1830260420. [DOI] [PubMed] [Google Scholar]
- 24.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 25.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 26.Wagner DH, Jr, Hagman J, Linsley PS, Hodsdon W, Freed JH, Newell MK. Rescue of thymocytes from glucocorticoid-induced cell death mediated by CD28/ CTLA-4 costimulatory interactions with B7-1/B7-2. J Exp Med. 1996;184:1631–1638. doi: 10.1084/jem.184.5.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers CA, Cado D, Truong T, Allison JP. Thymocyte development is normal in CTLA-4–deficient mice. Proc Natl Acad Sci USA. 1997;94:9296–9301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waterhouse P, Bachmann MF, Penninger JM, Ohashi PS, Mak TW. Normal thymic selection, normal viability and decreased lymphoproliferation in T cell receptor-transgenic CTLA-4–deficient mice. Eur J Immunol. 1997;27:1887–1892. doi: 10.1002/eji.1830270811. [DOI] [PubMed] [Google Scholar]
- 29.Brunet JF, Denizot F, Luciani MF, Roux M, Dosseto, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily—CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 30.Scollay R, Wilson A, Shortman K. Thymus cell migration: analysis of thymus emigrants with markers that distinguish medullary thymocytes from peripheral T cells. J Immunol. 1984;132:1089–1094. [PubMed] [Google Scholar]
- 31.Smith CA, Williams GT, Kingston R, Jenkinson EJ, Owen JJ. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989;337:181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 32.Marengere LE, Waterhouse P, Duncan GS, Mittrucker HW, Feng GS, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 33.Qu CK, Shi ZQ, Shen R, Tsai FY, Orkin SH, Feng GS. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol Cell Biol. 1997;17:5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y. Is CTLA-4 a negative regulator for T-cell activation? . Immunol Today. 1997;18:569–572. doi: 10.1016/s0167-5699(97)01170-5. [DOI] [PubMed] [Google Scholar]
- 36.Garchon HJ, Bedossa P, Eloy L, Bach JF. Identification and mapping to chromosome 1 of a susceptibility locus for periinsulitis in non-obese diabetic mice. Nature. 1991;353:260–262. doi: 10.1038/353260a0. [DOI] [PubMed] [Google Scholar]
- 37.Nistico L, Buzzetti R, Pritchard LE, Van der Auwera B, Giovannini C, Bosi E, Larrad MT, Rios MS, Chow CC, Cockram CS, et al. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Belgian Diabetes Registry. Hum Mol Genet. 1996;5:1075–1080. doi: 10.1093/hmg/5.7.1075. [DOI] [PubMed] [Google Scholar]
- 38.Badenhoop K, Donner H, Braun J, Siegmund T, Rau H, Usadel KH. Genetic markers in diagnosis and prediction of relapse in Graves' disease. Exp Clin Endocrinol Diabetes. 1996;4:98–100. doi: 10.1055/s-0029-1211712. [DOI] [PubMed] [Google Scholar]
- 39.Marron MP, Raffel LJ, Garchon HJ, Jacob CO, Serrano-Rios M, Martinez MT, Larrad, Teng WP, Park Y, Zhang ZX, Goldstein DR, et al. Insulin-dependent diabetes mellitus (IDDM) is associated with CTLA4 polymorphisms in multiple ethnic groups. Hum Mol Genet. 1997;6:1275–1282. doi: 10.1093/hmg/6.8.1275. [DOI] [PubMed] [Google Scholar]
- 40.Kotsa K, Watson PF, Weetman AP. A CTLA-4 gene polymorphism is associated with both Graves disease and autoimmune hypothyroidism. Clin Endocrinol. 1997;46:551–554. doi: 10.1046/j.1365-2265.1997.1710996.x. [DOI] [PubMed] [Google Scholar]
- 41.Donner H, Rau H, Walfish PG, Braun J, Siegmund T, Finke R, Herwig J, Usadel KH, Badenhoop K. CTLA4 alanine-17 confers genetic susceptibility to Graves' disease and to type 1 diabetes mellitus. J Clin Endocrinol Metab. 1997;82:143–146. doi: 10.1210/jcem.82.1.3699. [DOI] [PubMed] [Google Scholar]