Abstract
L-selectin binding activity for its ligand expressed by vascular endothelium is rapidly and transiently increased after leukocyte activation. To identify mechanisms for upregulation and assess how this influences leukocyte/endothelial cell interactions, cell-surface dimers of L-selectin were induced using the coumermycin–GyrB dimerization strategy for cross-linking L-selectin cytoplasmic domains in L-selectin cDNA-transfected lymphoblastoid cells. Coumermycin- induced L-selectin dimerization resulted in an approximately fourfold increase in binding of phosphomanan monoester core complex (PPME), a natural mimic of an L-selectin ligand, comparable to that observed after leukocyte activation. Moreover, L-selectin dimerization significantly increased (by ∼700%) the number of lymphocytes rolling on vascular endothelium under a broad range of physiological shear stresses, and significantly slowed their rolling velocities. Therefore, L-selectin dimerization may explain the rapid increase in ligand binding activity that occurs after leukocyte activation and may directly influence leukocyte migration to peripheral lymphoid tissues or to sites of inflammation. Inducible oligomerization may also be a common mechanism for rapidly upregulating the adhesive or ligand-binding function of other cell-surface receptors.
Keywords: L-selectin, dimerization, leukocyte/endothelial interaction, rolling, regulation
Leukocyte accumulation at sites of inflammation is regulated at the level of binding to the vascular endothelium, a multistep process initiated by the selectin family of adhesion molecules (1, 2). Although selective expression is a prominent means of governing selectin function, other mechanisms also regulate adhesive function. With L-selectin, lymphocyte activation through antigen receptors or neutrophil activation by chemokines upregulates its binding affinity for ligand (3). This rapid and transient increase in ligand binding presumably results from intracellular signals involving G proteins, since L-selectin's upregulated binding activity correlates with rapid phosphorylation of conserved cytoplasmic serine residues and upregulated binding activity is blocked by pertussis toxin and protein kinase C inhibitors (4). In addition, L-selectin localization at the tips of leukocyte microvilli (5, 6) regulates leukocyte capture since it facilitates contact with ligand-coated walls of in vitro flow chambers (7). However, the topographical position of L-selectin does not influence rolling velocity or detachment rates once rolling is established (7). Moreover, correct cell surface positioning is not sufficient for L-selectin–mediated adhesion, since deletion of L-selectin's cytoplasmic domain abrogates in vivo and in vitro leukocyte rolling (8) and its interactions with the cytoskeleton, but does not inhibit microvillus localization (9). Thus, the L-selectin cytoplasmic domain critically regulates receptor function. In this study, we assessed whether upregulation of L-selectin binding activity through its cytoplasmic domain could result from the formation of L-selectin dimers on the cell surface and whether receptor dimerization affected receptor function and leukocyte rolling.
Materials and Methods
DNA Constructs and Cell Transfection.
A modified GyrB cDNA fragment was generated as previously described (10) and ligated at the 3′ end of a human L-selectin cDNA (11) via an XbaI site introduced at the L-selectin translation-termination codon. All constructs were verified by DNA sequencing, subcloned into the pMT-2 expression vector (provided by Genetics Institute, Cambridge, MA), and used to transfect 300.19 cells (12). Transfected cells were selected in RPMI 1640 medium containing 10% calf serum and G418 (1 mg/ml; Sigma Chemical Co., St. Louis, MO). Multiple clones of transfected cells expressing similar cell-surface levels of wild-type L-selectin or L-selectin–GyrB fusion proteins were identified by immunofluorescence staining with flow cytometry analysis.
Coumermycin Treatment and Phosphomanan Monoester Core Complex Binding Assay.
Cells were washed once with RPMI 1640 medium before incubation at 37°C for 25 min (unless indicated otherwise) in RPMI 1640 containing either 0.1% DMSO or the indicated amounts of coumermycin and novobiocin (in 0.1% DMSO; Sigma Chemical Co.). After washing with ice-cold PBS without Ca2+/Mg2+, the cells were divided and incubated with biotin-labeled PPME (5 μg/ml) in PBS containing either Ca2+/ Mg2+ or 10 mM EDTA. After a 30-min incubation on ice, FITC-labeled avidin was added to visualize phosphomanan monoester core complex (PPME) binding as previously described (3, 4), with staining assessed immediately by flow cytometry as in Fig. 1. Ca2+-dependent PPME-binding was calculated by subtracting the mean linear fluorescence channel number for background staining (in 10 mM EDTA) from the mean value of fluorescence staining in the presence of Ca2+. Antibiotic treatment did not change the mean fluorescence intensity of background staining.
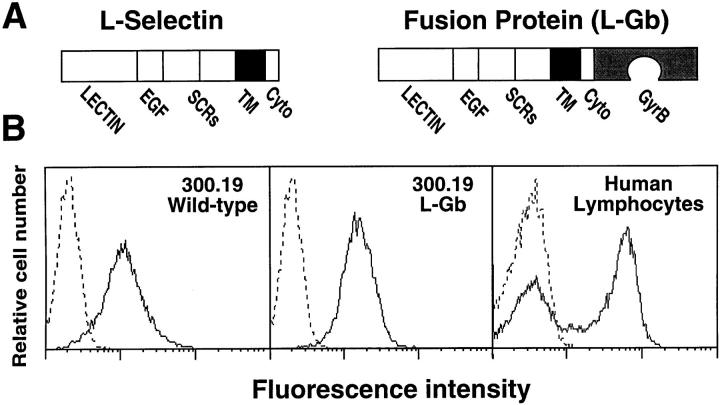
Figure 1.
Generation of L-selectin–expressing cell lines. (A) Structure of L-selectin and the L-selectin–GyrB (L-Gb) fusion protein containing the entire L-selectin protein in-frame with the NH2-terminal 24-kD subdomain of the B subunit of bacterial DNA gyrase (GyrB). Domains: EGF, epidermal growth factor–like; SCR, short consensus repeat; TM, transmembrane; Cyto, cytoplasmic. (B) Cell-surface expression of wild-type L-selectin or L-Gb in stably-transfected 300.19 cells and wild-type L-selectin expression by human blood lymphocytes. Cells were isolated and stained with FITC-conjugated LAM1-116 mAb specific for L-selectin (solid line) or an isotype-matched, nonbinding control mAb (dashed line) as previously described (16). Fluorescence histograms from flow cytometry analysis are on a three-decade log scale and are representative of results from at least five experiments.
Physiologic Shear Flow Assay.
Cells were treated with DMSO, coumermycin, and/or novobiocin as described above. For ligation with antibody, cells (2 × 106 cells/ml in flow medium, PBS containing Ca2+/Mg2+ and 0.5% BSA) were incubated with LAM1-101 or LAM1-118 mAbs (10 μg/ml) at room temperature for 15 min. In vitro rolling experiments were as previously described (13) using a transformed human umbilical vein endothelial cell (HUVEC) line (EA.hy926, provided by Dr. Cora-Jean Edgell, University of North Carolina at Chapel Hill; reference 14) that was transfected with an α1,3fucosyltransferase-VII cDNA (fucosyltransferase VII, provided by Dr. Brent Weston, University of North Carolina at Chapel Hill). Transfected EA.hy926 cells were grown to confluence on 25-mm circular glass coverslips and mounted in a parallel-plate flow chamber. Flow medium was drawn through the chamber at a rate of 804 μl/min with a syringe pump (Harvard Apparatus, Natick, MA), which generates an estimated wall shear stress of 1.85 dynes/cm2. Cells (106 cells/ml) were perfused through the chamber for a 10-min period. Cell rolling was observed using an inverted phase-contrast microscope (Olympus Corporation, Lake Success, NY) and videotaped using a CCD video camera (Hitachi Denshi, Ltd., Tokyo, Japan) with a SuperVHS video recorder (model SVO-9500MD; Sony Corporation of America, New York, NY) and an attached time-date generator (Microimage Video Sales Co., Bechtelsville, PA). Interacting cells (tethering and rolling) were determined by analysis of videotapes in which four fields (0.16 mm2) on a video monitor were counted at 14 random time points throughout the flow period. For calculating velocities, the distance each cell traveled between two time points was measured, converted into actual distance, and divided by the elapsed time.
Results and Discussion
Whether the cytoplasmic domain of L-selectin enhances ligand binding through oligomerization was tested directly by assessing the functional activity of induced cell-surface L-selectin dimers. Although not previously tested for inducing dimerization of transmembrane proteins, the exogenous dimeric antibiotic coumermycin can cross-link and activate cytoplasmic Raf-1–GyrB fusion proteins by simultaneously binding two GyrB subunits (10). cDNAs encoding L-selectin and a GyrB subunit were fused (L-Gb) and expressed in 300.19 cells (Fig. 1), an L-selectin–negative leukemia cell line that positions L-selectin at microvillus tips when expressed (9). The interaction of L-selectin with its ligand was assessed using a multivalent mannose-6 phosphate-rich polysaccharide mimetic, PPME, which can be labeled to assess L-selectin binding activity without the influence of other adhesion receptors (3, 15). Cells expressing L-Gb or wild-type L-selectin bound PPME similarly (Fig. 2 A, top). Coumermycin treatment significantly increased the PPME binding activity of L-Gb expressing cells by three- to five-fold but had no effect on wild-type L-selectin bearing cells (Fig. 2 A, bottom, P < 0.01). EDTA inhibited PPME binding by all cells, consistent with the involvement of L-selectin's calcium-dependent lectin domain. Treatment of cells with the L-selectin function-blocking mAb, LAM1-3, also blocked PPME binding (data not shown). Coumermycin enhancement of PPME binding by L-Gb cells was dose dependent, maximal at 0.9 μM coumermycin, rapid, and sustained (Fig. 2, B and C). Furthermore, pretreatment of L-Gb cells with novobiocin, the monomeric coumermycin analogue (10), completely eliminated the coumermycin-induced effect (Fig. 2 D). Coumermycin-induced dimerization did not increase L-Gb expression levels or induce L-selectin endoproteolytic release from the cell surface (Fig. 2 E), whereas phorbol esters induced L-Gb and wild-type L-selectin endoproteolytic release similarly (data not shown). Coumermycin-induced L-selectin dimerization in L-Gb cells did not generate transmembrane signals leading to upregulated intercellular adhesion over a 24-h time period. Although the molecular explanation for why cross-linking L-selectin with some mAbs induces potent homotypic adhesion while coumermycin does not induce homotypic adhesion is not known, cross-linking L-Gb or wild-type L-selectin on expressing cells with appropriate anti–L-selectin mAbs induced potent homotypic adhesion (data not shown) as previously described (16). Therefore, coumermycin-induced dimerization of L-Gb resulted in enhanced L-selectin binding activity specific for its ligand mimetic, PPME, which provides a mechanistic explanation for leukocyte activation rapidly upregulating L-selectin functional activity (3).
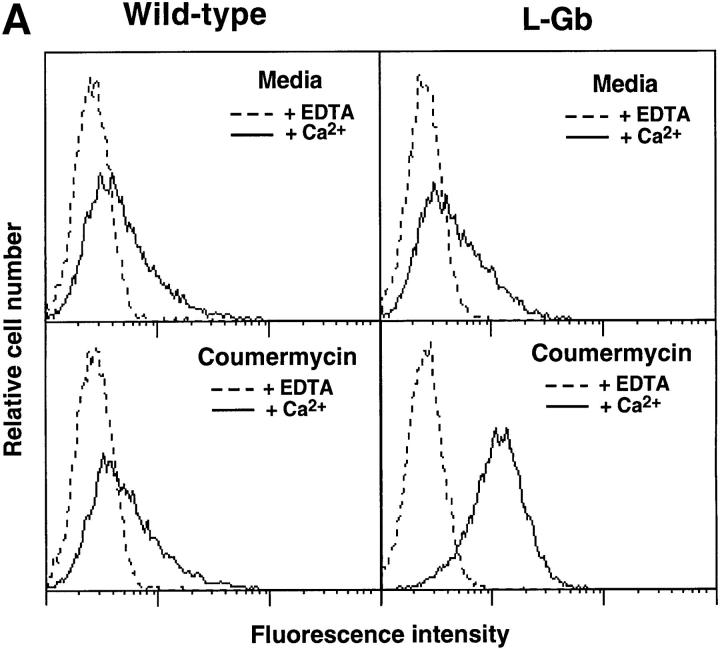
Figure 2.
Coumermycin-induced changes in PPME binding activity by L-Gb–expressing 300.19 cells. (A) Immunofluorescence analysis of PPME binding by cells expressing wild-type L-selectin or L-Gb, before and after treatment with 0.9 μM coumermycin. After coumermycin treatment, the cells were incubated with PPME in the presence of either Ca2+ or EDTA, and PPME binding was assessed by fluorescence staining and flow cytometry analysis with results shown on a three-decade log scale. These results represent those obtained in at least five experiments with the L-Gb clone shown (Fig. 1 B) and are representative of results obtained with two independent clones of L-Gb–transfected cells. (B) Dose–response of coumermycin-induced PPME binding in L-Gb cells. Values represent the mean fold increase in PPME binding relative to untreated cells obtained in four experiments. Asterisk indicates significant differences between treated and untreated samples, P < 0.01, Student's t test. (C) Time kinetics of coumermycin-induced PPME binding by L-Gb transfectants. The cells were treated with 0.9 μM coumermycin for the indicated amounts of time before PPME staining. Asterisk indicates significant differences between treated and untreated samples, P < 0.05. (D) Inhibition of coumermycin-induced PPME binding by novobiocin. Cells were first treated with medium or the indicated amounts of novobiocin at 37°C for 15 min. Coumermycin (0.9 μM final) was then added with PPME binding assessed 25 min later. (E) Effect of coumermycin treatment on L-selectin expression. L-Gb cells were incubated in media containing DMSO (0.1%), 0.9 or 9.0 μM coumermycin at 37°C for the indicated time periods. The cells were washed and L-selectin expression was assessed as in Fig. 1. Values represent mean ± SEM fluorescence channel numbers obtained in three experiments.
The physiological importance of L-selectin dimerization for leukocyte/endothelial interactions was assessed using in vitro flow chambers that mimic vascular flow conditions in vivo. L-selectin supports leukocyte rolling on a monolayer of transformed HUVEC that express L-selectin ligand(s) (14, 17). Both L-Gb and wild-type L-selectin mediated a basal level of 300.19 cell rolling (Fig. 3, A and C), but neither cell type arrested on these HUVEC monolayers due to the absence of other operable adhesion molecules expressed by this cell line (data not shown). L-Gb–mediated rolling of cells on HUVEC monolayers was significantly enhanced (by >700%, P < 0.002, n = 3 experiments) by coumermycin treatment, which was competitively inhibited by novobiocin pretreatment (Fig. 3 A). All rolling on HUVEC monolayers was completely blocked by the LAM1-3 mAb (Fig. 3, A and C). Remarkably, coumermycin treatment also significantly lowered rolling cell velocities by 35%, from a median (±SEM) rolling velocity of 272 ± 9 to 175 ± 12 μm/s (P < 0.001; n = 3, Fig. 3 B). Therefore, L-selectin dimerization markedly increased the number of leukocytes rolling under conditions of physiologic shear stress.
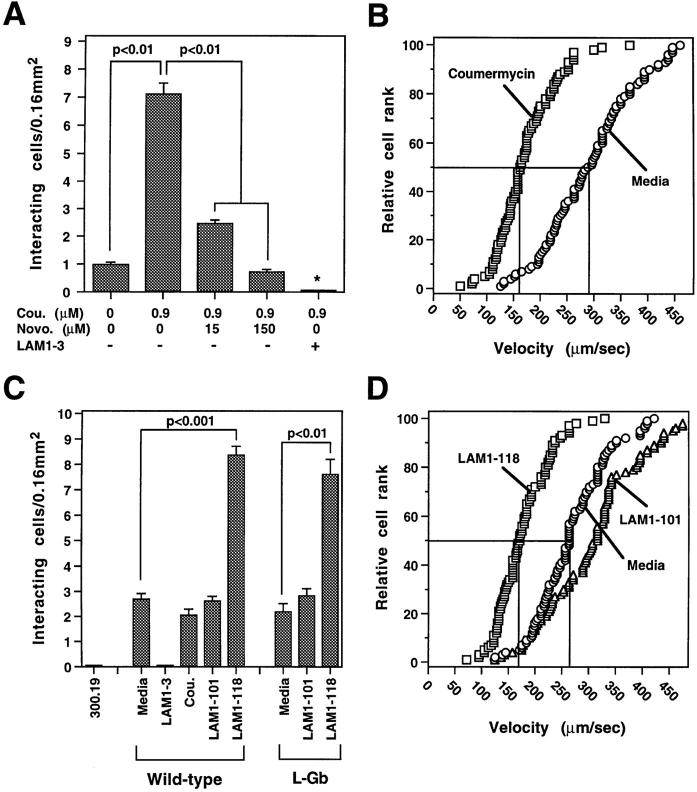
Figure 3.
L-selectin dimerization enhances leukocyte rolling on endothelial cells under physiologic flow. (A) Effect of coumermycin and novobiocin treatments on the number of L-Gb cells rolling on a HUVEC monolayer in an in vitro flow chamber assay. Values represent the number of L-Gb cells interacting with HUVEC monolayers in a 0.16-mm2 field. Asterisk indicates significant differences from all other groups, P < 0.01. (B) Effect of coumermycin (0.9 μM) on rolling velocities of L-Gb cells interacting with a HUVEC monolayer. Each symbol represents the velocity of an individual cell plotted in rank order with median (50%) velocities indicated by horizontal and vertical lines. (C) Effect of anti– L-selectin mAbs on the number of wild-type or L-Gb cells rolling on HUVEC monolayers. (D) Effect of anti–L-selectin mAbs on the rolling velocities of L-Gb cells interacting with HUVEC monolayers. A and C values are mean ± SEM of results obtained in three experiments, and B and D values are representative of results obtained in three experiments.
To further verify that L-selectin dimerization enhances its functional activity, a panel of IgG mAbs was screened to identify those that cross-linked L-selectin extracellular domains in a functionally appropriate configuration while not blocking ligand binding or inducing transmembrane signals (16). Of 32 mAbs screened, the LAM1-118 mAb reactive with the short consensus repeat domains of L-selectin fit these criteria. LAM1-118 mAb treatment of wild-type L-selectin or L-Gb–bearing cells significantly increased the frequency (310 and 350% increase, respectively; P < 0.01) of cells interacting with HUVEC monolayers under physiologic flow conditions (Fig. 3 C). This was consistent with an increase in high endothelial venule binding by lymphocytes pretreated with this mAb (16). As with coumermycin treatment of L-Gb cells, LAM1-118 mAb treatment significantly lowered rolling cell velocities by 35%, from a median (±SEM) of 266 ± 3 to 172 ± 6 μm/s (P < 0.001, n = 3; Fig. 3 D). LAM1-118 mAb treatment of wild-type L-selectin–bearing cells lowered rolling cell velocities similarly (data not shown). LAM1-118 mAb treatment of L-Gb or wild-type L-selectin transfected cells did not increase or decrease receptor expression levels (data not shown), and did not induce homotypic adhesion of 300.19 cells (16). Furthermore, treatment of wild-type or L-Gb L-selectin– bearing cells with LAM1-101, an isotype-matched mAb that binds the epidermal growth factor–like/short consensus repeat domains of L-selectin, had no significant effect on cell attachment or rolling velocities (Fig. 3, C and D). Neither 300.19 cells alone nor wild-type L-selectin– expressing cells treated with the LAM1-3 mAb showed any detectable interaction with the HUVEC monolayers (Fig. 3 C). Therefore, coumermycin-mediated cross-linking of L-selectin cytoplasmic domains and mAb-mediated cross-linking of the L-selectin extracellular domains both generated identical results under physiological flow conditions.
L-selectin binds its ligands with rapid association and dissociation rates, which results in a minimal shear stress threshold requirement for the initiation and maintenance of leukocyte rolling (18–21). Despite the coumermycin- induced increase in L-selectin binding activity, both coumermycin-treated and -untreated L-Gb cells required shear stress for the promotion of L-selectin–dependent interactions since no tethering or rolling was observed below wall shear stresses of 0.75 dynes/cm2 (Fig. 4). Nonetheless, coumermycin-treated L-Gb cells interacted with HUVEC monolayers at a significantly greater frequency compared with untreated L-Gb cells at shear stresses between 0.75 and 3.0 dynes/cm2 (Fig. 4). Thus, L-selectin dimerization influences rolling velocity and receptor detachment rates as opposed to selectin localization to the tips of microvilli, which appears to promote cell capture (5–7). L-selectin dimerization may retard the dissociation of selectin bonds, which would enhance the lifetime of L-selectin binding to its endothelial ligand(s) and promote leukocyte tethering to endothelium during rolling.
Figure 4.
L-selectin dimerization enhances leukocyte attachment and rolling on endothelial cells under physiologic flow. Wall shear stress was varied at 1-min intervals by changing the flow rate through the flow chamber. Asterisk indicates coumermycin-treated cells that were significantly different from untreated cells, P < 0.05. Values represent means ± SEM of results from three experiments.
That inducing L-selectin dimerization by either coumermycin or mAbs can enhance L-selectin adhesion is consistent with a model in which the transient activation- enhanced adhesive function of L-selectin results from receptor dimerization. However, these results do not exclude other mechanisms for transiently enhancing the adhesive function of L-selectin and do not necessarily prove that L-selectin dimerization is a physiologic process. Nonetheless, the formation of cell-surface dimers may also provide a mechanistic explanation of why L-selectin–mediated tethers operate at high shear forces (22). Thus, the rapid upregulation of L-selectin binding activity after leukocyte activation and L-selectin dimerization may stabilize L-selectin bonds under shear force, which facilitates the formation of a second receptor/ligand bond before the first one breaks during rolling. Multivalent L-selectin binding would also distribute the tensile force applied on each tether among several L-selectin/ligand bonds. Therefore, L-selectin's cytoplasmic domain and cytoskeletal associations may be required for its oligomerization within the cell membrane, providing it with strong resistance to shear stresses. Whether dimerization also regulates P- and E-selectin function is unknown, although P-selectin isolated from activated platelets is found in a tetrameric configuration, which facilitates its binding activity in vitro (23).
Since leukocyte rolling may involve multivalent binding (22), rapid oligomerization of L-selectin would favor the formation of multivalent bonds with its low affinity endothelial cell ligands (24). Consistent with this notion, L-selectin ligands consist of multimeric sialylated and sulfated oligosaccharides appropriately presented by mucin scaffolds (1). As such, oligomerized L-selectin molecules may interact cooperatively with ligands presenting multiple low affinity oligosaccharide binding sites that are optimally stabilized by multivalent bonding. This is consistent with the many animal lectins that dramatically increase their affinity for carbohydrate ligands by combining multiple oligosaccharide binding sites in each lectin polypeptide (25). However, in the case of L-selectin the generation of multimeric binding by receptor oligomerization may provide a rapid means for upregulating adhesion receptor function with leukocyte activation. In addition, dimerization may be particularly important when L-selectin or its ligands are expressed at low site densities (26). Therefore, this study supports the notion that selectin oligomerization is of primary physiologic significance and is likely to directly influence leukocyte migration and entry into sites of inflammation. Moreover, the coumermycin–GyrB dimerization strategy is likely to be useful for studying other transmembrane proteins and adhesion molecules that share the property of being functionally upregulated in response to cellular activation.
Acknowledgments
We thank Drs. M.D. Delahunty, S.D. Rosen, F.W. Luscinskas, L. Robinson, M. Inaoki, and X.-Q. Zhang for reagents and help with these experiments.
This work was supported by National Institutes of Health grants AI-26872, CA-54464, and HL-50985.
References
- 1.Rosen SD, Bertozzi CR. The selectins and their ligands. Curr Opin Cell Biol. 1994;6:663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 2.Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB (Fed Am Soc Exp Biol) J. 1995;9:866–873. [PubMed] [Google Scholar]
- 3.Spertini O, Kansas GS, Munro JM, Griffin JD, Tedder TF. Regulation of leukocyte migration by activation of the leukocyte adhesion molecule-1 (LAM-1) selectin. Nature. 1991;349:691–694. doi: 10.1038/349691a0. [DOI] [PubMed] [Google Scholar]
- 4.Haribabu B, Steeber DA, Ali H, Richardson RM, Snyderman R, Tedder TF. Chemoattractant receptor-induced phosphorylation of L-selectin. J Biol Chem. 1997;272:13961–13965. doi: 10.1074/jbc.272.21.13961. [DOI] [PubMed] [Google Scholar]
- 5.Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- 6.Erlandsen SL, Hasslen SR, Nelson RD. Detection and spatial distribution of the beta 2 integrin (Mac-1) and L-selectin (LECAM-1) adherence receptors on human neutrophils by high-resolution field emission SEM. J Histochem Cytochem. 1993;41:327–333. doi: 10.1177/41.3.7679125. [DOI] [PubMed] [Google Scholar]
- 7.von Andrian UH, Hasslen SR, Nelson SL, Erlandsen SL, Butcher EC. A central role for microvillus receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 8.Kansas GS, Ley K, Munro JM, Tedder TF. Regulation of leukocyte rolling and adhesion to HEV through the cytoplasm domain of L-selectin. J Exp Med. 1993;177:833–838. doi: 10.1084/jem.177.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavalko FM, Walker DM, Graham L, Goheen M, Doerschuk CM, Kansas GS. The cytoplasmic domain of L-selectin interacts with cytoskeletal proteins via alpha- actinin: receptor positioning in microvilli does not require interaction with alpha-actinin. J Cell Biol. 1995;129:1155–1164. doi: 10.1083/jcb.129.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrar MA, Alberola-Ila J, Perlmutter RM. Activation of the Raf-1 kinase cascade by coumermycin- induced dimerization. Nature. 1996;383:178–181. doi: 10.1038/383178a0. [DOI] [PubMed] [Google Scholar]
- 11.Tedder TF, Ernst TJ, Demetri GD, Isaacs CM, Adler DA, Disteche CM. Isolation and chromosomal localization of cDNAs encoding a novel human lymphocyte cell-surface molecule, LAM1: homology with the mouse lymphocyte homing receptor and other human adhesion proteins. J Exp Med. 1989;170:123–133. doi: 10.1084/jem.170.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tedder TF, Penta AC, Levine HB, Freedman AS. Expression of the human leukocyte adhesion molecule, LAM1. Identity with the TQ1 and Leu-8 differentiation antigens. J Immunol. 1990;144:532–540. [PubMed] [Google Scholar]
- 13.Luscinskas FW, Kansas GS, Ding H, Pizcueta P, Schleiffenbaum B, Tedder TF, Gimbrone MA., Jr Monocyte rolling, arrest, and spreading on IL-4–activated vascular endothelium under flow is mediated via sequential action of L-selectin, β1-integrins, and β2-integrins. J Cell Biol. 1994;125:1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgell C-J, McDonald CC, Graham JB. Permanent cell line expressing factor VIII–related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glabe CG, Harty PK, Rosen SD. Preparation and properties of fluorescent polysaccharides. Anal Biochem. 1983;130:287–294. doi: 10.1016/0003-2697(83)90590-0. [DOI] [PubMed] [Google Scholar]
- 16.Steeber DA, Engel P, Miller AS, Sheetz MP, Tedder TF. Ligation of L-selectin through conserved regions within the lectin domain activates signal transduction pathways and integrin function in human, mouse and rat leukocytes. J Immunol. 1997;159:952–963. [PubMed] [Google Scholar]
- 17.Spertini O, Luscinskas FW, Kansas GS, Munro JM, Griffin JD, Gimbrone MA, Jr, Tedder TF. Leukocyte adhesion molecule-1 (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J Immunol. 1991;147:2565–2573. [PubMed] [Google Scholar]
- 18.Finger EB, Puri KD, Alon R, Lawrence MB, von Andrian UH, Springer TA. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence MB, Kansas GS, Kunkel EJ, Ley K. Threshold levels of fluid shear promote leukocyte adhesion through selectins (CD62L, P, E) J Cell Biol. 1997;136:717–727. doi: 10.1083/jcb.136.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tözeren A, Ley K. How do selectins mediate leukocyte rolling in venules? . Biophys J. 1992;63:700–709. doi: 10.1016/S0006-3495(92)81660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin–carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 22.Alon R, Chen S, Puri KD, Finger EB, Springer TA. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J Cell Biol. 1997;138:1169–1180. doi: 10.1083/jcb.138.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ushiyama S, Laue TM, Moore KL, Erickson HP, McEver RP. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J Biol Chem. 1993;268:15229–15237. [PubMed] [Google Scholar]
- 24.Nicholson MW, Barclay AN, Singer MS, Rosen SD, van der Merwe PA. Affinity and kinetic analysis of L-selectin (CD62L) binding to glycosylation-dependent cell-adhesion molecule-1. J Biol Chem. 1998;273:763–770. doi: 10.1074/jbc.273.2.763. [DOI] [PubMed] [Google Scholar]
- 25.Weis WI, Drickamer K. Structural basis of lectin-carbohydrate recognition. Annu Rev Biochem. 1996;65:441–473. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- 26.Tang MLK, Steeber DA, Zhang X-Q, Tedder TF. Intrinsic differences in L-selectin expression levels affect T and B lymphocyte subset-specific recirculation pathways. J Immunol. 1998;160:5113–5121. [PubMed] [Google Scholar]