Abstract
Complement receptor 1–related gene/protein y (Crry) is a potent murine membrane complement regulator that inhibits classical and alternative pathway C3 convertases. In nephrotoxic serum (NTS) nephritis, injected antibodies (Abs) bind to glomeruli, leading to complement activation and subsequent glomerular injury and albuminuria. To study the phenotypic effects of continuous complement pathway blockade, transgenic mice were created that express recombinant soluble (rs) Crry directed by the broadly active and heavy metal-inducible metallothionein-I promoter. One transgenic line expressing high levels of rsCrry was propagated. Serum rsCrry levels were 18.7 ± 2.7 μg/ml (n = 5) at basal level and increased to 118.1 ± 20.6 μg/ml 4 d after addition of zinc to the drinking water. By reverse transcription polymerase chain reaction (RT-PCR), transgene messenger (m)RNA was present in liver, kidney, brain, lung, and spleen, but not in heart. By in situ RT-PCR analysis of kidneys, transgene mRNA was widely expressed both in renal glomeruli and tubules. Urinary excretion of rsCrry was 113.4 ± 22.4 μg/ml with a fractional excretion relative to creatinine of 13.2 ± 2.7%, consistent with local renal production of rsCrry and secretion into urine. The founder and all transgene positive adult animals have remained healthy with no mortality or apparent phenotypic abnormalities, including infection or immune complex disease. To determine whether rsCrry blocked complement-mediated injury, NTS nephritis was induced by injection of NTS immunoglobulin (Ig)G, followed by an 18-h urine collection to quantitate the excretion of albumin as a measure of glomerular injury. In transgene-negative littermates (n = 15), transgene-positive animals (n = 10), and transgene-positive animals fed zinc (n = 10), albuminuria was 4,393 ± 948, 1,783 ± 454, and 1,057 ± 277 μg/mg creatinine, respectively (P < 0.01 by ANOVA). Glomerular C3 was evident by immunofluorescence staining in 12/15 transgene-negative animals, but in none of the transgene-positive animals fed zinc. Thus, we have produced the first transgenic animals that overexpress a soluble C3 convertase inhibitor. rsCrry expression markedly ameliorates an Ab-induced disease model in vivo. These results support the hypothesis that continuous complement inhibition at the C3 convertase step is feasible and effective in complement-mediated injury states.
Keywords: Crry; mice, transgenic; anti-glomerular basement membrane disease; complement inactivators; complement
Activation of the complement pathway occurs after the binding of Ab to local tissue Ags. Products of complement activation can lead to varied inflammatory events, such as recruitment and activation of leukocytes by C3 and C5 cleavage fragments, as well as a number of cellular events mediated by C5b-9 (for reviews see references 1, 2). Using several experimental approaches, the complement system has been shown to play an important role in immune complex disease models. In early studies, this was done through systemic depletion of complement with cobra venom factor (3). More recently, an mAb that blocks the cleavage of C5, as well as neutralizing polyclonal Ab to C5a, has been used successfully (4–6). Rodents that are congenitally deficient in a single component such as C5 or C6, or that have been made deficient in a single component by homologous recombination, such as C3 or C4 (7), have also been studied. In these animals with complement deficiencies, complement activation is blocked distal to the absent component, and tissue damage is ameliorated in several models of Ab-induced and immune complex diseases (8–10).
A further means of inhibiting complement has been to produce intrinsic complement regulators, normally found as membrane proteins, as recombinant soluble (rs)1 proteins. The most widely studied is human rsCR1, which has been used successfully in a variety of immune complex diseases, as well as in diseases not traditionally considered to have an immunological basis, such as myocardial infarction and ischemia/reperfusion injury (11, 12). In rodents, the protein Crry (complement receptor 1–related gene/protein y) functions as a CR1 functional homologue and manifests that same spectrum of inhibitory activities for both the classical and alternative pathways (13). The use of Crry as a recombinant inhibitor in homologous disease models has the advantage that an immune response is not expected to occur, which has limited the use of heterologous proteins such as rsCR1 to short-term disease models. We have produced mouse Crry as a recombinant protein bearing a mouse IgG1 tail (rsCrry-Ig; reference 14) and have shown that as predicted, it can be chronically administered without adverse effects or the generation of a neutralizing Ab response (Kraus, D., and V.M. Holers, unpublished data).
The nephrotoxic serum (NTS) nephritis model has been widely used in several species to illustrate pathogenic events in glomerular inflammation. The complement dependence of this disorder in rats was shown years ago (3, 15). In mice and rabbits, a relative dose dependence is clear, such that when low doses of Ab are used, the complement dependence of this model is apparent, but becomes lost as higher Ab doses are used (16, 17), perhaps because of direct effects of Ab alone (18). Recently, we have successfully used rsCrry-Ig to inhibit NTS nephritis in mice to the same extent as seen with complement depletion using cobra venom factor (14).
In these studies, we tested the hypothesis that mice could be continuously exposed to therapeutically active levels of a complement inhibitor both systemically and locally without untoward effects. To accomplish this, we produced transgenic mice that express rsCrry directed by the metallothionein (MT)-I promoter (19). This promoter, although primarily active in the liver, can direct mRNA synthesis in a heavy metal-inducible fashion in many tissues (20). Hence, we found that Crry is expressed by many cell types including those of the glomerulus. Crry transgenic mice have circulating rsCrry levels that lead to continuous complement inhibition in sera. No evidence of spontaneous clinical complications is apparent in adult transgenic mice, yet they are markedly protected from renal injury in the NTS nephritis model.
Materials and Methods
Construction of MT-Crry–encoding Plasmid.
The cDNA encoding soluble mouse Crry (21) was subcloned into the NruI site of the MT expression vector 2999B4 (gift of Dr. Richard Palmiter, University of Washington). This vector contains the mouse MT-I promoter, the human growth hormone polyadenylation sequence, and the mouse MT gene 5′ and 3′ flanking regions, which serve as locus control regions to confer position-independent and copy number–dependent expression to the transgene (22).
The capacity of the MT-Crry construct to direct production of rsCrry was determined by stable transfection of BSC-1 cells (provided by Dr. Gary Toback, The University of Chicago; reference 23). The MT-Crry plasmid was cotransfected with pWLNeo (Stratagene, La Jolla, CA) by the CaPO4 precipitation method. Stable transfectants were selected by growth in 400 μg/ml G418. After overnight incubation in DMEM/10% FCS with or without 80 μM ZnSO4, the culture medium was harvested for Crry ELISA.
Production of Crry Transgenic Mice
The pBluescript portion of the vector was removed by SalI digestion. The 19.4-kb MT-Crry DNA fragment was gel purified and microinjected into the pronuclei of 1-cell fertilized CD-1 oocytes, and transgenic mice were generated using standard techniques (24). Screening for founders was performed on tail-derived DNA using a PCR strategy designed to specifically detect transgenic Crry, but not endogenous Crry. The 5′ and 3′ primers were 5′-TCG TCC TAT CCG AGC CAG TCG T-3′ and 5′-GGC AGA AGG AAG CTG TGA TGG-3′, which hybridize to the MT-I promoter and Crry, respectively. PCR products were also digested with BamHI or EcoRI. To confirm PCR results, Southern blotting was also performed on SmaI- or BamHI-digested DNA. As a probe, the 650-bp human growth hormone polyadenylation site was cut from the MT-Crry construct with EcoRI and BamHI, gel purified, and 32P labeled.
The Crry transgene was propagated by breeding into normal CD-1 mice (Harlan Sprague Dawley, Inc., Indianapolis, IN). Offspring were followed for the transgene by PCR as described above. Because normal mice do not have circulating Crry, confirmation of the presence of the functional transgene came from identifying rsCrry in sera by ELISA. All animal work was approved and performed under the guidelines set by the University of Chicago and University of Colorado Institutional Animal Care and Use Committees.
ELISA for Determination of Crry Levels.
Dynatech Immulon II (Dynatech, Chantilly, VA) 96-well plates were coated at 4°C overnight with 1 μg/well of IgG purified from rabbit anti–mouse Crry polyclonal Ab (25). Plates were then washed four times with PBS, 0.05% Tween 20 (Sigma Chemical Co., St. Louis, MO) and blocked for 1 h at room temperature with PBS and 1% BSA (Sigma Chemical Co.). The plates were washed four more times and then samples diluted in PBS and 0.1% BSA were added for a 1 h incubation. Plates were then decanted and washed four times before the addition of pretitrated anti-Crry mAb 10A2 coupled with biotin (N-hydroxy succinimidobiotin; Sigma Chemical Co.) in PBS, 0.1% BSA. After a 1-h incubation, the plates were again washed four times. Streptavidin horseradish peroxidase (Sigma Chemical Co.) diluted in PBS was then added for 30 min. After four washes, ABTS (Boehringer Mannheim Corp., Indianapolis, IN) activated with H2O2 was added. Plates were developed in the dark and the OD480 was measured with a Titertek Plus ELISA plate reader (ICN Biomedicals, Costa Mesa, CA).
Western Blot Analysis.
Western blot analysis was performed using urine dialyzed against PBS. After quantification by ELISA, urine containing 50 ng rsCrry was analyzed by 12.5% SDS-PAGE. As a positive control, 50 ng rsCrry-Ig was run in parallel. After transfer to Hybond-ECL nitrocellulose (Amersham Pharmacia Biotech Inc., Piscataway, NJ), membranes were incubated with biotin-conjugated primary mAbs recognizing either Crry (10A2), or mouse CR1 (8C12; reference 26) as a control, diluted in PBS with 10% milk, 1% BSA, and 0.1% Tween 20. After washing four times, membranes were incubated with streptavidin horseradish peroxidase. After four additional washes, blots were developed using the ECL system (Amersham Pharmacia Biotech Inc.).
Reverse Transcription PCR.
RNA was isolated from different tissues using TRIzol reagent (GIBCO BRL, Gaithersburg, MD) followed by phenol-chloroform extraction. cDNA was produced from 5 μg total RNA by reverse transcription (RT) using oligo-dT primers (SuperScript Preamplification System; GIBCO BRL). Subsequent PCR was performed in tubes containing the generated cDNA, 50 mM KCl, 20 mM Tris-HCl, 2.5 mM MgCl2, 2.5% DMSO, 100 μM of each deoxynucleotide triphosphate, 0.1 μM of each primer, and 2.5 U of Taq polymerase. 25 cycles of a 1-min denaturation at 94°C, 1-min annealing at 60°C, and 1-min extension at 72°C were performed. The 5′ primer used in the PCR was 5′-CCT CAC TTA CTC CGT AGC TCC-3′ which hybridizes to bases +33 to +53 of the MT-I promoter and the 3′ primer was 5′-CAG CAC TCG TCC AGG TTG AGT C-3′, which hybridizes to sequence encoding a portion of the first SCR of Crry. Control PCR was performed using primers for glyceraldehyde-3-phosphate dehydrogenase (G3PDH; reference 7).
In Situ RT-PCR.
Kidneys from Crry transgenic animals and paired control transgene negative littermates were snap frozen. 4-μm cryosections were fixed in 4% paraformaldehyde in PBS. Subsequently, sections were treated for 15 min with proteinase K (10 μg/ml) followed by an overnight incubation in RNAse-free DNAse (100 U/ml). RT-PCR was performed on the sections using the same primers described above for solution PCR in 20 mM Tris-HCl, and 2 mM magnesium acetate. Digoxigenin-dUTP was added at 4 μM to the deoxynucleotide triphosphates. RT-PCR was performed with 2.5 U rTth DNA polymerase (Perkin-Elmer Corp., Norwalk, CT). Initial RT was performed for 30 min at 60°C followed by the PCR consisting of 20 cycles of a 45-s denaturation at 94°C, 15-s annealing at 55°C, and 1-min extension at 60°C. The amplified sequences were detected with alkaline phosphatase conjugated anti-digoxigenin Ab, which was developed with NBT/BCIP (nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate).
Purification of Urinary rsCrry.
Five transgene positive animals were fed 25 mM ZnSO4 in drinking water for 1 wk. Animals were then housed for 1 wk in metabolic cages for urine collection. Urine was pooled, dialyzed against PBS, and affinity purified on an anti-Crry mAb 10A2–Sepharose column using conditions shown to retain protein activity (14). Specifically, after washing with PBS, material was eluted with 0.1 M Na2CO3-made 0.5 M NaCl, pH 11. Fractions were neutralized with 0.1 M Tris, pH 6.8. Fractions containing Crry were pooled, concentrated, dialyzed versus PBS, quantitated by ELISA, and then used in assays of complement inhibition.
Analysis of Complement Inhibition.
Measurement of complement activity was performed using previously published quantitative flow cytometric methods (21, 28). For this assay, complement was activated on human K562 cells treated with rabbit anti-K562 polyclonal Ab. Because K562 cells are not lysed by mouse complement, C3 deposition was measured by flow cytometry. The complement inhibitory activity of purified urinary rsCrry added to 10% BALB/c serum was measured and compared with a standard curve generated by adding varying amounts of rsCrry-Ig to sera. In addition, 10% pooled sera from either transgenic mice or control nontransgenic littermates were evaluated in this assay. The negative controls were sera containing 10 mM EDTA, and the positive control was BALB/c serum with no Crry. Samples were incubated at 37°C for 20 min in 2 mM MgCl2 and 0.15 mM CaCl2-containing buffer after which the reaction was stopped with 10 mM EDTA. K562 cells were then washed twice with PBS, 1% BSA and incubated with FITC-conjugated goat anti– mouse C3 (Cappel Laboratories, Malvern, PA) for 1 h on ice. After washing with the same buffer and fixation using 1% paraformaldehyde, cells were analyzed for the level of C3 binding by flow cytometry using an EPICS cytometer (Coulter Immunology, Hialeah, FL). Percentage inhibition was calculated using the formula (1 − [sample MCF − background {10 mM EDTA condition}]/ [positive control MCF {no Crry} − background]) × 100.
Induction of NTS Nephritis.
NTS raised in a single sheep was supplied by Dr. David Salant (Boston University Medical Center; reference 29). The complement-fixing IgG1 subclass was isolated by anion-exchange chromatography on DEAE-Sephacel (30). NTS nephritis was induced in 10 transgene positive mice and 15 transgene negative littermates. An additional 10 transgene positive mice were fed 25 mM ZnSO4 in drinking water before disease induction. Disease was induced at the same time in the three groups. An equal number of males and females were studied. NTS IgG was injected at a dose of 0.5 mg i.v., after which animals were housed for 18 h in metabolic cages to collect urine. Mice were then killed to harvest blood and renal tissue. Identical studies were performed in which mice were injected intravenously with either 1.0 or 2.0 mg NTS IgG. For these, three transgene positive males and four transgene negative littermate males were studied at each dose. To examine the transient neutrophil influx observed in this model (31), another group of studies was performed in which three transgene positive males and four transgene negative males were injected with 0.5 mg i.v. NTS IgG. Animals were killed 2 h later for tissue harvest.
Urinary albumin concentration was measured by ELISA as previously described (10), whereas urinary creatinine was measured with a Beckman Autoanalyzer. To normalize albumin excretion, data are expressed as μg albumin/mg creatinine (10, 31). Normal mice excrete <25 μg albumin/mg creatinine, similar to the case in humans. Direct immunofluorescence (IF) microscopy for sheep IgG and mouse C3 was performed on snap-frozen renal tissue (32). The number of neutrophils stained with mAb 7/4 (Serotec Ltd., Kidlington, Oxford, UK) in at least 60 glomeruli/ animal was counted (10). Glomerular IF scoring and neutrophil counts were accumulated in a blinded fashion.
Statistical analyses were performed with Minitab Software (College Park, PA). Data are expressed as mean ± SEM. Statistical significance was determined by one-way analysis of variance followed by Tukey's pairwise comparisons, using a family error rate of 0.05 to indicate statistical significance.
Results
Production and Characterization of Crry Transgenic Mice.
The construct shown in Fig. 1 was created by subcloning cDNA encoding the signal peptide and extracellular domain of mouse Crry, without the transmembrane or intracellular domains, into the expression vector MT-2999B4, containing the mouse MT-I promoter and 5′/3′ MT gene flanking sequences. BSC-1 cells were stably transfected with this construct. When cells were grown in medium alone, there was no detectable rsCrry in the culture medium, but with inclusion of 80 μM ZnSO4 in culture medium, there was 2.21 μg/ml rsCrry after a 12-h culture. Thus, this construct directs production of rsCrry that is under control of a heavy metal-inducible promoter.
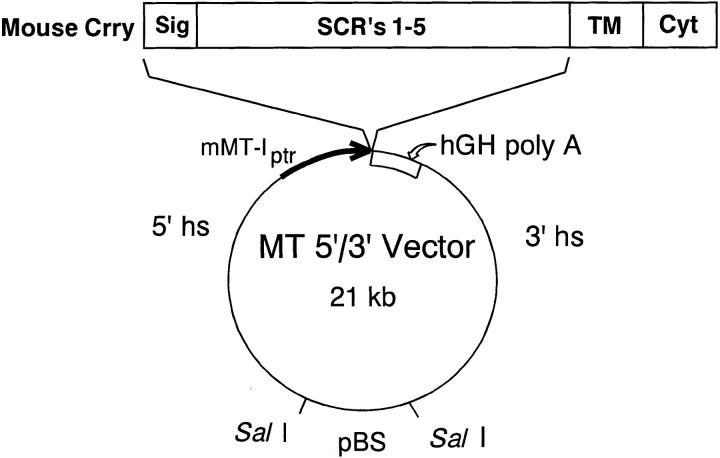
Figure 1.
Schematic representation of the plasmid construct used to generate Crry transgenic mice. cDNA for the signal sequence and five short consensus repeats (SCR's) of mouse Crry was subcloned into the MT 5′/3′ vector. This vector uses the mouse MT-I promoter, the human growth hormone polyadenylation site, and the mouse MT gene locus control regions contained within 5′ and 3′ hypersensitive sites. The pBluescript portion of the plasmid was removed by SalI digestion.
Transgenic mice were generated with the SalI-SalI microinjection fragment. Transgene positive animals were identified by PCR (Fig. 2). These PCR products contained the expected single BamHI and EcoRI cleavage sites. Southern blot analysis with the 650-bp human growth hormone fragment confirmed the presence of the transgene (not shown). One founder line was propagated that had desired serum levels and tissue expression of Crry. The Crry transgene was perpetuated in CD-1 mice and all studies described in this report were performed with F2 animals. In 184 F2 animals tested after weaning, 76 were positive for the transgene (41.3%). This departs from a Mendelian mode of inheritance (P < 0.02 by chi-square goodness-of-fit test). The reason for this is unexplained, but several possibilities exist. rsCrry may partially interfere with fertilization or gestation, or lead to an unobserved increased perinatal or neonatal mortality. These issues were not addressed in this study. However, after weaning of transgene positive animals, no phenotypic abnormalities were present, and no mortality was observed (discussed further below).
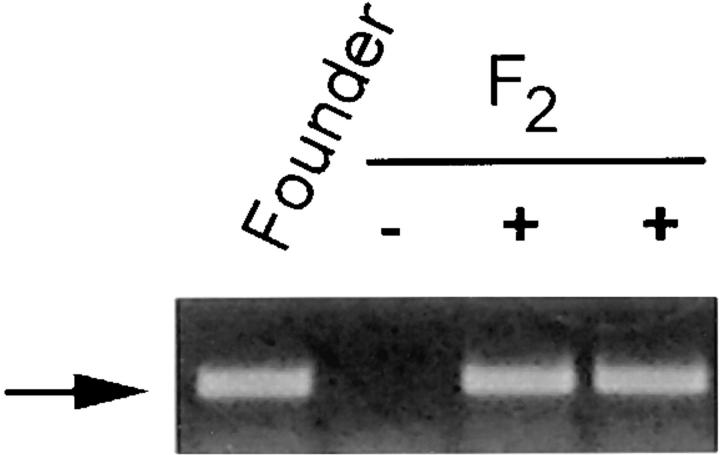
Figure 2.
Detection of the Crry transgene by PCR. PCR was performed on tail- derived DNA from the founder and selected F2 offspring using 5′ and 3′ primers derived from the MT-I promoter and Crry, respectively. The arrow indicates the 464-bp PCR product.
Serum rsCrry levels were 18.7 ± 2.7 μg/ml (n = 5) in the basal state, which increased to 118.1 ± 20.6 μg/ml 4 d after addition of 25 mM ZnSO4 to drinking water. As expected, none of the transgene negative littermate animals with or without zinc feeding had detectable serum Crry, as there is no intrinsic soluble serum Crry. Urinary excretion of rsCrry was also measured. As with sera, there was no detectable Crry in the urine of Crry transgene negative littermate animals. In contrast, Crry transgene animals had high urinary levels of rsCrry (111.3 ± 22.4 μg/ml; n = 4). The fractional excretions of rsCrry and albumin relative to creatinine, which is freely filtered at the glomerulus, were 13.2 ± 2.7% and 0.00012 ± 0.00002%, respectively. Since the size (45 kD) and charge (pI 5.6) of rsCrry (21) are near those of albumin, this ∼100,000-fold difference between the two supports the conclusion that rsCrry is actively produced in the kidney.
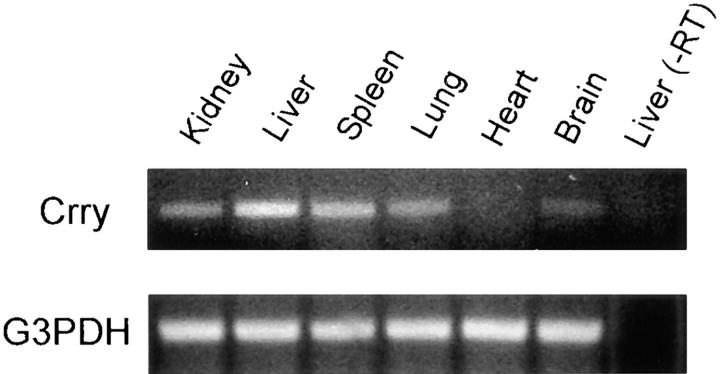
To further evaluate the expression of the Crry transgene, RT-PCR was performed. RNA from kidney, liver, spleen, lung, heart, and brain were isolated. RT-PCR was performed using a 5′ primer from the 5′ untranslated region of the MTH promoter construct and a 3′ primer from mouse Crry. This strategy allows PCR amplification of the transgene derived mRNA only. As shown in Fig. 3, after 25 cycles of PCR, Crry mRNA from the tg was present in all organs but heart. As a control for RNA integrity, RT-PCR for G3PDH showed equivalent intensities of PCR product. To verify that RNA was not contaminated with genomic DNA, controls for each organ were performed in which RNA was treated identically, except that reverse transcription was not performed (in Fig. 3, the control for liver is shown). Thus, the Crry transgene mRNA is present in many tissues, with liver expression being the most prominent, as predicted (19, 33).
Figure 3.
RT-PCR showing the tissue localization of the Crry transgene mRNA. PCR was performed for 25 cycles using a 5′ primer derived from the 5′ untranslated region of the mRNA derived from the MTH promoter construct and a 3′ primer from mouse Crry sequence. As a positive control, RT-PCR for G3PDH was performed, and a negative control included RNA which was treated identically, except that reverse transcription was not performed. This is demonstrated for liver and designated −RT.
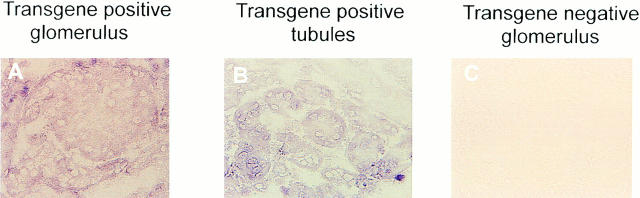
The localization of Crry transgene mRNA in kidney was evaluated by in situ RT-PCR. mRNA for the transgene was strongly positive in glomeruli (Fig. 4 A) and tubules (Fig. 4 B). As also illustrated, kidney from a transgene negative littermate was negative (Fig. 4 C). These RT-PCR data showing prominent renal expression are consistent with the appearance of substantial quantities of rsCrry in the urine. Furthermore, they show that we have achieved the desired local production of rsCrry in the glomerulus.
Figure 4.
In situ RT-PCR on renal tissue from Crry transgene positive (A and B) and negative (C) animals. The primers were the same as used in Fig. 3.
Functional Characterization of rsCrry in Crry Transgenic Mice.
Complement activity in Crry transgenic mice was evaluated. Five male Crry transgenic and control transgene negative animals were killed and sera were pooled. The percent inhibition of C3 deposition on Ab-sensitized K562 cells relative to pooled sera from male transgene negative littermates was determined. In this assay, complement activation was inhibited by 71.6% in sera from transgene positive animals.
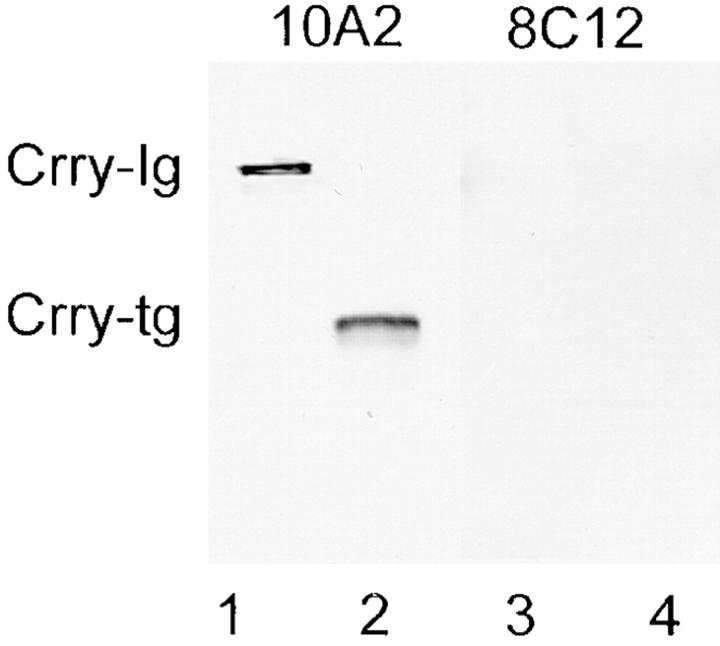
To evaluate rsCrry appearing in the urine, five animals were zinc fed and housed in metabolic cages. Urine was collected, pooled, dialyzed against PBS, and then subjected to Western blotting with mAb 10A2. By this approach, a single 45-kD band was identified (Fig. 5, lane 2). In this analysis, rsCrry-Ig was included as a positive control for reactivity with mAb 10A2 (lane 1). As a negative control, mAb 8C12, specific for mouse CR1, did not react with urinary proteins from Crry transgenic animals (lane 4), nor with rsCrry-Ig (lane 3).
Figure 5.
Western blot analysis showing the presence of intact rsCrry in the urine of Crry transgenic animals. Urine from Crry transgenic animals was electrophoresed in lanes 2 and 4, while purified rsCrry-Ig was in lanes 1 and 3 as a positive control. Western blotting was performed with anti-Crry mAb 10A2 (lanes 1 and 2), and with anti-mouse CR1 mAb 8C12 as a negative control (lanes 3 and 4). No urinary Crry is detectable in nontransgenic mice (data not shown).
rsCrry was purified from dialyzed urine by affinity chromatography using mAb 10A2-Sepharose. The activity of this rsCrry preparation was compared with rsCrry-Ig and shown to have equivalent complement inhibitory activity on a molar basis (data not shown). Therefore, as expected using this Crry cDNA (21) functional rsCrry is produced in Crry transgenic animals.
Chronic Complement Inhibition in Crry Transgenic Mice Is Not Detrimental.
Given the propensity to develop immune complex disease in humans with complement deficiencies (34) and the demonstrated abnormalities in immune complex handling in complement deficient animals hyperimmunized with foreign Ag (10), it was important to exclude spontaneous immune complex disease in Crry transgenic animals. All Crry transgenic animals identified after weaning have remained healthy with no mortality in all mice studied, including the 18-mo-old founder. Urinary albumin excretion is normal (15.6 ± 2.7 μg albumin/mg creatinine; n = 4), and no cryoprecipitates have been noted in any serum samples (10).
To see whether chronic intraperitoneal injections affected Crry transgenic animals (which is relevant to ongoing studies of chronic immune complex disease), seven transgenic animals were given daily intraperitoneal injections of sterile PBS for 7 wk. Three of the seven animals were fed 25 mM ZnSO4 in drinking water for the duration of the protocol. Glomeruli were then examined for immune complexes. By IF microscopy for IgG, six of seven animals had no detectable IgG, while the remaining animal had trace IgG staining. All animals had detectable glomerular IgM (average staining score = 1.1 ± 0.2), but this is no different than that found in normal mice (1.0 ± 0.1; reference 10). Thus, there are no apparent phenotypic abnormalities in adult mice related to continuous complement inhibition by Crry.
Crry Transgenic Mice Are Protected from Ab-induced Glomerular Injury.
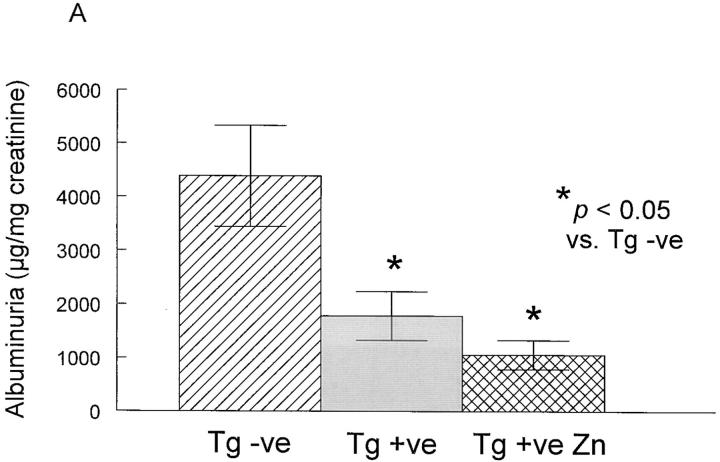
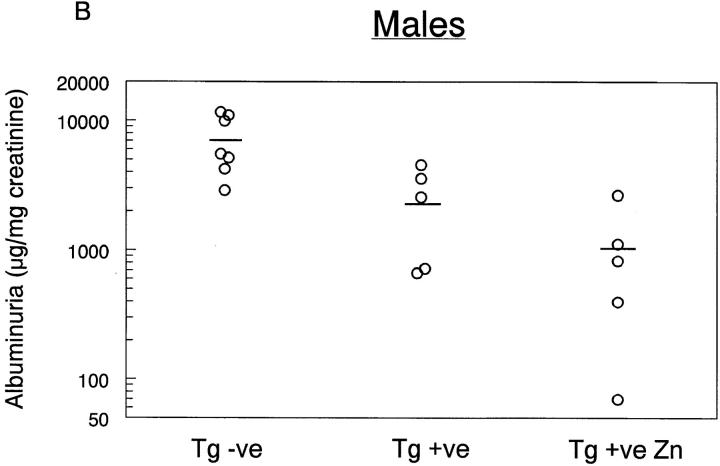
Studies were then performed to evaluate whether these animals were protected from glomerular injury in NTS nephritis (Fig. 6). Crry transgenic mice (Tg + ve) were studied in parallel with littermates that were negative for the transgene (Tg − ve). A second group of Crry transgenic animals was fed 25 mM ZnSO4 in drinking water (Tg + ve Zn). NTS nephritis was induced in all animals by intravenous injection of NTS IgG followed by an 18-h urine collection. As shown in Fig. 6 A, there was a significant reduction in urinary albumin excretion in Crry transgenic animals compared with transgene negative controls, and this effect was more pronounced in animals fed zinc (P < 0.05 by one-way analysis of variance with Tukey's multiple comparisons). The effect of sex was evaluated statistically, and individual data points are shown in Fig. 6 B (males) and C (females). A distinct difference among sexes was noted, with albuminuria of 7,195 ± 1,344 and 1,941 ± 415 μg/mg creatinine in transgene negative males (n = 7) and females (n = 8), respectively. However, albuminuria was reduced to a comparable extent in Crry transgenic animals fed zinc (1,014 ± 449 and 1,100 ± 360 μg albumin/mg creatinine in males and females, respectively; n = 5 each).
Figure 6.
Effects of complement inhibition on NTS IgG-induced albuminuria. NTS nephritis was induced in Crry transgenic mice (Tg + ve) and in Crry transgenic animals in which the MT-I promoter was stimulated with zinc feeding (Tg + ve Zn). Transgene negative littermate animals (Tg − ve) were studied in parallel. In A, the data are the mean ± SEM of all animals, while in B and C individual data points are shown from males (B) and females (C), with the average in each group depicted as a horizontal line.
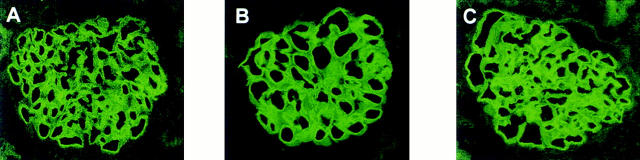
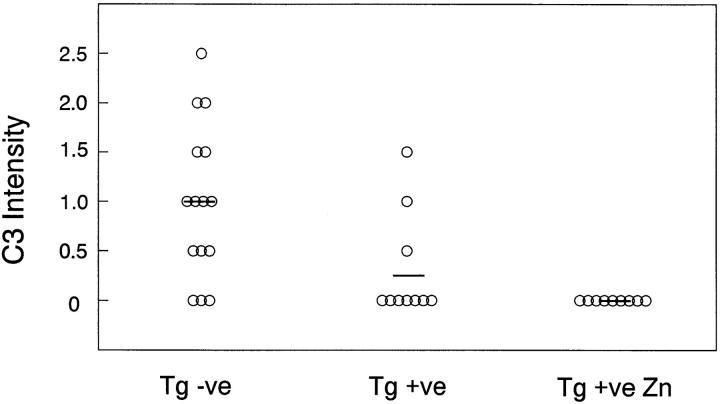
Glomerular neutrophil counts 2 h after NTS IgG injection were 0.34 ± 0.14 and 0.38 ± 0.12 cells per glomerulus in transgene positive and negative male mice, respectively. At 18 h this mild neutrophil infiltration had completely subsided, consistent with the transient nature of the neutrophil influx in this model (31). By IF microscopy, all animals had intense linear staining for sheep IgG that was indistinguishable between the three groups (Fig. 7), suggesting that the presence of rsCrry in the Crry transgenic mice did not affect glomerular binding of the NTS IgG. C3 staining by IF was significantly different between transgene negative and positive animals (shown in graphical form for all animals in Fig. 8). No zinc-fed Crry transgenic animal had detectable C3 staining in glomeruli, illustrating the effectiveness of rsCrry to limit complement activation. Albuminuria positively correlated with C3 staining in males (r = 0.692, P = 0.002) and in females (r = 0.605, P = 0.008), supporting the conclusion that complement activation and C3 deposition were related to glomerular injury as measured by albuminuria in this analysis.
Figure 7.
IF staining for sheep IgG in representative glomeruli from mice given NTS IgG. Shown are glomeruli from transgene negative (A), transgene positive (B), and zinc-fed transgene positive (C) animals.
Figure 8.
Effects of complement inhibition on NTS-IgG induced C3 deposition in glomeruli. IF scoring intensity for C3 for individual animals is shown, with the mean of each group as a horizontal line. The legends are the same as in Fig. 6.
Further studies examined the effect of Ab dose on NTS nephritis disease manifestations. As occurred with the lower dose of NTS IgG, Crry transgenic animals had significantly less glomerular complement activation than transgene negative controls as assessed by IF microscopy (Table 1). However, Crry transgenic mice developed heavy albuminuria that was comparable to transgene negative littermates. Again consistent with the evanescent nature of the neutrophil influx, neutrophil accumulation at 18 h was unremarkable, with <0.3 cells/glomerulus in all groups. Thus, despite the retained ability to inhibit complement activation at the higher doses of injected Ab, Crry transgenic animals developed albuminuria at these quantities of NTS IgG Ab.
Table 1.
Disease Variables in Mice Given Higher Doses of NTS IgG
| 1 mg | 2 mg | |||||||
|---|---|---|---|---|---|---|---|---|
| Tg + ve | Tg − ve | Tg + ve | Tg − ve | |||||
| Albuminuria (μg/mg creatinine) | 31,342 ± 6,631 | 28,361 ± 9,962 | 29,661 ± 10,296 | 23,667 ± 5,514 | ||||
| IF staining intensity for C3 | 0.3 ± 0.2* | 1.8 ± 0.5* | 0.3 ± 0.2* | 1.4 ± 0.2* | ||||
Transgene positive and littermate negative mice were injected intravenously with 1 or 2 mg NTS IgG. Urine was collected for 18 h for albumin and creatinine determinations, after which animals were killed and renal tissue was processed to determine glomerular C3 binding by IF microscopy. Data represent the mean ± SEM.
P < 0.02 by ANOVA.
Discussion
We have produced transgenic mice expressing rsCrry, a potent inhibitor of complement C3 convertases (13, 21). Whereas normal mice have endogenous Crry on cell membranes (25), as well as circulating factor H and C4 binding protein (35, 36), it was our goal to overexpress Crry as a soluble protein to achieve chronic complement inhibition to a substantially greater extent than that provided by these intrinsic regulators. Several lines of evidence support our conclusion that transgenic mice manifest the desired complement inhibitory effect. First, we have shown in previous studies that the form of rsCrry used here retains its inhibitory activity (21), and that the addition of soluble forms of Crry at comparable levels blocks complement activation in normal mouse serum in the fluid phase and onto target surfaces (14, 21). Second, rsCrry isolated from the urine of these Crry transgenic animals demonstrated the expected complement inhibitory activity in vitro. Most importantly, though, sera obtained from Crry transgenic animals were less active in a classical pathway activation assay compared with sera from transgene negative littermate controls, illustrating that transgene-derived rsCrry interfered with complement activation.
In addition to having biologically significant levels of rsCrry in sera, a secondary aim was to produce rsCrry in tissue sites, such as the glomerulus, where it would exert an effect on complement activation occurring locally. We clearly show that such widespread tissue distribution was achieved using the strategy we have chosen. Glomerular expression of the Crry transgene derived mRNA was readily apparent, and urinary levels of rsCrry relative to creatinine were sufficiently high to assure that this pool of protein was primarily produced locally.
Despite the capacity of rodent glomerular cells to inhibit complement activation using endogenous membrane-anchored Crry and CD59 (25, 37, 38), in several Ab-induced disorders complement activation occurs which apparently overwhelms this intrinsic complement regulation (15, 39–42). In the NTS nephritis model, NTS IgG first binds to glomerular Ags, which is then followed by complement activation (3, 15, 17). Here we show that glomerular complement activation in NTS nephritis, as assessed by glomerular C3 staining by IF, was significantly reduced in Crry transgenic mice despite similar localization of IgG anti-glomerular Abs. Furthermore, the most important effect of complement activation, which is the relative loss of the glomerular barrier to protein passage manifested by the development of albuminuria, was significantly diminished in Crry transgenic animals. In our studies, males had greater injury than females. It is known that the hemolytic activity of male sera is higher than that from females, which is due to a relative deficiency of C5, C6, and C7 in female mice (43). This disparity in complement activity likely underlies the differences we observed in these studies.
As has been shown previously, the complement dependence of the NTS nephritis model becomes lost at higher Ab doses (14, 16, 17). The mediator systems in NTS nephritis have been dissected by Boyce and Holdsworth (17). In their careful experiments, as the dose of NTS IgG was raised, the disease first became neutrophil independent and subsequently neutrophil and complement independent. In our studies, NTS IgG doses of 1 mg and above led to apparent complement- and neutrophil-independent glomerular damage. Such injury is believed to be due to direct reactivity of NTS IgG with glomerular cell Ags (18). The complement dependence of NTS nephritis has recently been examined by Sheerin et al. (44) using mice made deficient in C3 and C4. In these studies, injection of 0.5 mg of NTS IgG led to albuminuria in wild-type mice, which was reduced by 90 and 98% in C4- and C3-deficient mice, respectively. Even with 1.0 mg NTS IgG, there was minimal albuminuria in C3-deficient mice, and this was 13 times less than wild-type mice. Despite this, neutrophil infiltration within 2 h of Ab injection was no different between wild-type and C3-deficient animals, consistent with such a dose leading to complement-dependent but neutrophil-independent glomerular injury.
The effect of increasing rsCrry expression through stimulation of the MT-I promoter with zinc feeding appeared to have an additional effect on both C3 staining and albuminuria. However, since both of these outcome measures were already significantly reduced in Crry transgenic animals not fed zinc, these differences did not reach statistical significance between these two groups of Crry transgenic animals. In these studies, we also could not separate out the effects of systemic rsCrry from those of locally produced rsCrry. To do so, either comparisons between Crry transgenic and normal mice given comparable amounts of rsCrry systemically, or the generation of Crry transgenic mice expressing rsCrry only outside of the target organ, will be required.
In sum, this is the first biological example where long-term complement inhibition has been achieved using transgenic techniques, and establishes the principle that chronic therapy directed at the C3 activation step can be effective in ameliorating disease. Proof of its utility will come from studying a disorder such as the autologous NTS nephritis model, in which injury is of a more chronic nature and mediated by autologous IgG (29, 45). Given the current availability of functionally comparable human inhibitors such as rsCR1 (46) and a decay-accelerating factor-membrane cofactor protein chimera (47), our findings support the potential utility of these compounds in patients with Ab-mediated renal diseases, as well as those affecting other organs.
Our results are also relevant to several other questions. One potential problem with chronic complement inhibition is that the beneficial effects of complement to resist infectious agents and to process naturally generated immune complexes may be diminished. However, our accumulated experience with >100 animals has shown that adult animals remain healthy in standard housing conditions, and there is no apparent susceptibility to infections with environmental pathogens. Furthermore, we have carefully studied transgenic mice for the development of immune complex disease of a similar nature as may occur with deficiencies of individual complement components in humans (34) and animals (48, 49), but have failed to identify such a predisposition. We believe that two possible reasons account for the lack of deleterious effects. The first is that we have not completely inhibited complement activation, and sufficient activity may remain to resist normal environmental pathogens and process immune complexes. Second, complement components may be synthesized and directly secreted onto pathogens by macrophages (50), and rsCrry may not significantly affect this process. Whatever the explanation, though, we believe our results prove the principle that chronic complement inhibition in adult animals is safe.
Our results are also relevant to current uncertainties related to the relative role of complement versus Fc receptors (FcR) in initiating and amplifying acute and chronic inflammatory tissue damage in vivo. This issue has been studied by ourselves and others in several ways. Some results have suggested that signals through FcR are the most important regulators of tissue injury (51–53), while others have suggested that complement plays a central role (14, 54, 55). Certainly some of the differences in these results are based on variations in the models studied, the target organ, and/or the doses of disease-inducing reagents used. Several studies have clearly demonstrated that the complement dependence of the tissue damage induced by lower doses of disease-inducing Ab is lost when higher doses of Ab are used (16, 17, 56). However, we believe that it is also likely that FcR and complement activation fragments each engage nonoverlapping inflammatory and immune pathways. Recent studies of the effects of FcR γ chain deficiency on glomerulonephritis in the (NZB × NZW)F1 female model, in which glomerular C3 deposition is still qualitatively present at similar levels and autoantibody levels are unchanged, but there is less renal damage (53), are consistent with this hypothesis. Relevant to this, a similar decrease in renal disease was also reported using the same model system in mice treated with inhibitory anti-C5 mAb (5). The apparent lack of effect of FcR γ chain deficiency on humoral responses (57) as compared with the marked decrease in C3-deficient (58) and C4/C3 receptor (Cr2)- deficient mice (59, 60) also highlights the differences between inhibition of complement versus FcR. Indeed, if there were inflammatory pathways differentially regulated by each effector mechanism, blockade of both complement activation and FcR effects at the same time should lead to additive or synergistic effects. With our development of these Crry transgenic mice and rsCrry-Ig as an exogenously administered inhibitor (14) this hypothesis can now be experimentally tested.
Finally, previous studies in mice have shown that inhibition of complement activation at the generation of C5a ameliorates arthritis in the CIA model (4) and glomerulonephritis in (NZB × NZW)F1 female model of lupus (5). However, it is possible that inhibition at the C3 activation step would be more effective than C5 inhibitors. An inhibitor such as rsCrry (or rsCR1), because it decreases C3 activation and C3a liberation, may lead to decreased influx of inflammatory cells that express the C3a receptor (61). Alternatively, because C3 is required for a normal Ab response to foreign Ag (58, 62, 63), pathogenic Ab generation may be decreased in the setting of a C3 convertase inhibitor such as rsCrry. Each of these questions can now be specifically addressed in relevant disease models by direct comparison of the two strategies.
Acknowledgments
We thank Dr. Richard Palmiter (Howard Hughes Medical Institute, University of Washington) for providing the MT-2999B4 vector, Dr. Gary Toback (The University of Chicago) for supplying BSC-1 cells, and Dr. David Salant (Boston University) for providing NTS IgG. We wish to recognize the outstanding skills of Linda Degenstein (Transgenic Mouse Facility, The University of Chicago Cancer Research Center) used in generating the mice described here.
This work was supported by National Institutes of Health (NIH) grants DK41873, DK42086, DK20595, and AI31105; a chapter grant from the Arthritis Foundation, Greater Chicago Chapter; a Biomedical Sciences Grant from the National Arthritis Foundation; and a grant-in-aid from the National Kidney Foundation of Illinois. J.J. Alexander was supported by NIH training grant DK07510, and D. Kraus by NIH training grant AI07405.
Abbreviations used in this paper
- Crry
complement receptor 1–related gene/protein y
- G3PDH
glyceraldehyde-3-phosphate dehydrogenase
- IF
immunofluorescence
- MT
metallothionein
- NTS
nephrotoxic serum
- rs
recombinant soluble
- RT
reverse transcription
References
- 1.Dalmasso AP. Complement in the pathophysiology and diagnosis of human diseases. Crit Rev Clin Lab Sci. 1986;24:123–183. doi: 10.3109/10408368609110272. [DOI] [PubMed] [Google Scholar]
- 2.Holers, V.M. 1995. Complement. In Principles and Practices of Clinical Immunology. R. Rich, editor. Mosby, St. Louis, MO. 363–391.
- 3.Cochrane CG, Müller-Eberhard HJ, Aikin BS. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970;105:55–69. [PubMed] [Google Scholar]
- 4.Wang Y, Rollins SA, Madri JA, Matis LA. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc Natl Acad Sci USA. 1995;92:8955–8959. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Hu Q, Madri JA, Rollins SA, Chodera A, Matis LA. Amelioration of lupus-like autoimmune disease in NZB/W F1mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc Natl Acad Sci USA. 1996;93:8563–8568. doi: 10.1073/pnas.93.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulligan MS, Schmid E, Beck-Schimmer B, Till GO, Friedl HP, Brauer RB, Hugli TE, Miyasaka M, Warner RL, Johnson KJ, Ward PA. Requirement and role of C5a in acute lung inflammatory injury in rats. J Clin Invest. 1996;98:503–512. doi: 10.1172/JCI118818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falk RJ, Jennette JC. Immune complex induced glomerular lesions in C5 sufficient and deficient mice. Kidney Int. 1986;30:678–686. doi: 10.1038/ki.1986.240. [DOI] [PubMed] [Google Scholar]
- 9.Brandt J, Pippin J, Schulze M, Hänsch GM, Alpers CE, Johnson RJ, Gordon K, Couser WG. Role of the complement membrane attack complex (C5b-9) in mediating experimental mesangioproliferative glomerulonephritis. Kidney Int. 1996;49:335–343. doi: 10.1038/ki.1996.50. [DOI] [PubMed] [Google Scholar]
- 10.Quigg RJ, Lim A, Haas M, Alexander JJ, He C, Carroll MC. Immune complex glomerulonephritis in C4- and C3-deficient mice. Kidney Int. 1998;53:320–330. doi: 10.1046/j.1523-1755.1998.00723.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalli KR, Hsu P, Fearon DT. Therapeutic uses of recombinant complement protein inhibitors. Springer Semin Immunopathol. 1994;15:417–431. doi: 10.1007/BF01837368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore FD., Jr Therapeutic regulation of the complement system in acute injury states. Adv Immunol. 1994;56:267–299. doi: 10.1016/s0065-2776(08)60454-x. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y-U, Kinoshita T, Molina H, Hourcade D, Seya T, Wagner LM, Holers VM. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J Exp Med. 1995;181:151–159. doi: 10.1084/jem.181.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quigg RJ, Kozono Y, Berthiaume D, Lim A, Salant DJ, Weinfeld A, Griffin P, Kremmer E, Holers VM. Blockade of antibody-induced glomerulonephritis with Crry-Ig, a soluble murine complement inhibitor. J Immunol. 1998;160:4553–4560. [PubMed] [Google Scholar]
- 15.Unanue E, Dixon FJ. Experimental glomerulonephritis. IV. Participation of complement in nephrotoxic nephritis. J Exp Med. 1964;119:965–982. doi: 10.1084/jem.119.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrijver G, Assmann KJ, Bogman MJ, Robben JC, de Waal RM, Koene RA. Antiglomerular basement membrane nephritis in the mouse. Study on the role of complement in the heterologous phase. Lab Invest. 1988;59:484–491. [PubMed] [Google Scholar]
- 17.Boyce NW, Holdsworth SR. Anti-glomerular basement membrane antibody-induced experimental glomerulonephritis: Evidence for dose-dependent direct antibody and complement-induced, cell-independent injury. J Immunol. 1985;135:3918–3921. [PubMed] [Google Scholar]
- 18.O'Meara YM, Natori Y, Minto AWM, Goldstein DJ, Manning EC, Salant DJ. Nephrotoxic antiserum identifies a β1-integrin on rat glomerular epithelial cells. Am J Physiol. 1992;262:F1083–F1091. doi: 10.1152/ajprenal.1992.262.6.F1083. [DOI] [PubMed] [Google Scholar]
- 19.Palmiter RD, Norstedt G, Gelinas RE, Hammer RE, Brinster RL. Metallothionein-human GH fusion genes stimulate growth of mice. Science. 1983;222:809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- 20.Palmiter RD. Molecular biology of metallothionein gene expression. Exper Suppl (Basel) 1987;52:63–80. doi: 10.1007/978-3-0348-6784-9_4. [DOI] [PubMed] [Google Scholar]
- 21.Foley S, Li B, Dehoff M, Molina H, Holers VM. Mouse Crry/p65 is a regulator of the alternative pathway of complement activation. Eur J Immunol. 1993;23:1381–1384. doi: 10.1002/eji.1830230630. [DOI] [PubMed] [Google Scholar]
- 22.Palmiter RD, Sandgren EP, Koeller DM, Brinster RL. Distal regulatory elements from the mouse metallothionein locus stimulate gene expression in transgenic mice. Mol Cell Biol. 1993;13:5266–5275. doi: 10.1128/mcb.13.9.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hopps HE, Bernheim BC, Nisalak A, Tjio JH, Smadel JE. Biologic characteristics of a continuous kidney cell line derived from the African green monkey. J Immunol. 1963;91:416–424. [PubMed] [Google Scholar]
- 24.Hogan, B., R. Beddington, F. Constantini, and E. Lacy. 1994. Manipulating the Mouse Embryo. A Laboratory Manual. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 25.Li B, Sallee C, Dehoff M, Foley S, Molina H, Holers VM. Mouse Crry/p65: characterization of monoclonal antibodies and the tissue distribution of a functional homologue of human MCP and DAF. J Immunol. 1993;151:4295–4305. [PubMed] [Google Scholar]
- 26.Kinoshita T, Takeda J, Hong K, Kozono H, Sakai H, Inoue K. Monoclonal antibodies to mouse complement receptor type 1 (CR1). Their use in a distribution study showing that mouse erythrocytes and platelets are CR1-negative. J Immunol. 1988;140:3066–3072. [PubMed] [Google Scholar]
- 27.Foss RD, Guha-Thakurta N, Conran RM, Gutman P. Effects of fixative and fixation time on the extraction and polymerase chain reaction amplification of RNA from paraffin-embedded tissue. Comparison of two housekeeping gene mRNA controls. Diagn Mol Pathol. 1994;3:148–155. doi: 10.1097/00019606-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Molina H, Wong W, Kinoshita T, Brenner C, Foley S, Holers VM. Distinct receptor and regulatory properties of recombinant mouse complement receptor 1 (CR1) and Crry, the two genetic homologues of human CR1. J Exp Med. 1992;175:121–129. doi: 10.1084/jem.175.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salant DJ, Cybulsky AV. Experimental glomerulonephritis. Methods Enzymol. 1988;162:421–461. doi: 10.1016/0076-6879(88)62096-9. [DOI] [PubMed] [Google Scholar]
- 31.Mayadas TN, Mendrick DL, Brady HR, Tang T, Papayianni A, Assmann KJM, Wagner DD, Hynes RO, Cotran RS. Acute passive anti-glomerular basement nephritis in P-selectin-deficient mice. Kidney Int. 1996;49:1342–1349. doi: 10.1038/ki.1996.190. [DOI] [PubMed] [Google Scholar]
- 32.Quigg RJ, Abrahamson DR, Cybulsky AV, Badalamenti J, Minto AWM, Salant DJ. Studies with antibodies to cultured rat glomerular epithelial cells: Subepithelial immune deposit formation after in vivo injection. Am J Pathol. 1989;134:1125–1133. [PMC free article] [PubMed] [Google Scholar]
- 33.Palmiter RD. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moulds JM, Krych M, Holers VM, Liszewski MK, Atkinson JP. Genetics of the complement system and rheumatic diseases. Rheum Dis Clin North Am. 1992;18:893–914. [PubMed] [Google Scholar]
- 35.Ferreira A, Takahashi M, Nussenzweig V. Purification and characterization of mouse serum protein with specific binding affinity for C4 (Ss protein) J Exp Med. 1977;146:1001–1008. doi: 10.1084/jem.146.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinoshita T, Nussenzweig V. Regulatory proteins for the activated third and fourth components of complement (C3b and C4b) in mice. I. Isolation and characterization of factor H: the serum cofactor for the C3b/C4b inactivator (factor I) J Immunol Methods. 1984;71:247–257. doi: 10.1016/0022-1759(84)90071-1. [DOI] [PubMed] [Google Scholar]
- 37.Quigg RJ, Holers VM, Morgan BP, Sneed AE. Crry and CD59 regulate complement in rat glomerular epithelial cells and are inhibited by the nephritogenic antibody of passive Heymann nephritis. J Immunol. 1995;154:3437–3443. [PubMed] [Google Scholar]
- 38.Quigg RJ, Morgan BP, Holers VM, Adler S, Sneed AE, Lo CF. Complement regulation in the rat glomerulus: Crry and CD59 regulate complement in glomerular mesangial and endothelial cells. Kidney Int. 1995;48:412–421. doi: 10.1038/ki.1995.309. [DOI] [PubMed] [Google Scholar]
- 39.Salant DJ, Belok S, Madaio MP, Couser WG. A new role for complement in experimental membranous nephropathy in rats. J Clin Invest. 1980;66:1339–1350. doi: 10.1172/JCI109987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto T, Wilson CB. Complement dependence of antibody-induced mesangial cell injury in the rat. J Immunol. 1987;138:3758–3765. [PubMed] [Google Scholar]
- 41.Johnson RJ, Alpers CE, Pruchno C, Schulze M, Baker PJ, Pritzl P, Couser WG. Mechanisms and kinetics for platelet and neutrophil localization in immune complex nephritis. Kidney Int. 1989;36:780–789. doi: 10.1038/ki.1989.263. [DOI] [PubMed] [Google Scholar]
- 42.Couser WG, Johnson RJ, Young BA, Yeh CG, Toth CA, Rudolph AR. The effects of soluble recombinant complement receptor 1 on complement-mediated experimental glomerulonephritis. J Am Soc Nephrol. 1995;5:1888–1894. doi: 10.1681/ASN.V5111888. [DOI] [PubMed] [Google Scholar]
- 43.Nilsson UR, Müller-Eberhard HJ. Deficiency of the fifth component of complement in mice with an inherited complement defect. J Exp Med. 1967;125:1–16. doi: 10.1084/jem.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheerin NS, Springall T, Carroll MC, Hartley B, Sacks SH. Protection against anti-glomerular basement membrane (GBM)-mediated nephritis in C3- and C4-deficient mice. Clin Exp Immunol. 1997;110:403–409. doi: 10.1046/j.1365-2249.1997.4261438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikolic-Paterson DJ, Lan HY, Hill PA, Vannice JL, Atkins RC. Suppression of experimental glomerulonephritis by the interleukin-1 receptor antagonist: inhibition of intercellular adhesion molecule-1 expression. J Am Soc Nephrol. 1994;4:1695–1700. doi: 10.1681/ASN.V491695. [DOI] [PubMed] [Google Scholar]
- 46.Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 47.Higgins PJ, Jone-Long K, Lobell R, Sardonini C, Alessi MK, Yeh CG. A soluble chimeric complement inhibitory protein that possesses both decay-accelerating and factor I cofactor activities. J Immunol. 1997;158:2872–2881. [PubMed] [Google Scholar]
- 48.Bottger EC, Hoffmann T, Hadding U, Bitter-Suermann D. Guinea pigs with inherited deficiencies of complement components C2 or C4 have characteristics of immune complex disease. J Clin Invest. 1986;78:689–695. doi: 10.1172/JCI112628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cork LC, Morris JM, Olson JL, Krakowka S, Swift AJ, Winkelstein JA. Membranoproliferative glomerulonephritis in dogs with a genetically determined deficiency of the third component of complement. Clin Immunol Immunopathol. 1991;60:455–470. doi: 10.1016/0090-1229(91)90101-f. [DOI] [PubMed] [Google Scholar]
- 50.Colten HR. Tissue-specific regulation of inflammation. J Appl Physiol. 1992;72:1–7. doi: 10.1152/jappl.1992.72.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 52.Sylvestre D, Clynes R, Ma M, Warren H, Carroll MC, Ravetch JV. Immunoglobulin G-mediated inflammatory responses develop normally in complement-deficient mice. J Exp Med. 1996;184:2385–2392. doi: 10.1084/jem.184.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 54.Colten HR. Drawing a double-edged sword. Nature. 1994;371:474–475. doi: 10.1038/371474a0. [DOI] [PubMed] [Google Scholar]
- 55.Hopken UE, Lu B, Gerard NP, Gerard C. Impaired inflammatory responses in the reverse arthus reaction through genetic deletion of the C5a receptor. J Exp Med. 1997;186:749–756. doi: 10.1084/jem.186.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ben-Efraim S, Cinader B. The role of complement in the passive cutaneous reaction of mice. J Exp Med. 1964;120:925–942. doi: 10.1084/jem.120.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 58.Fischer MB, Ma M, Goerg S, Zhou X, Xia J, Finco O, Han S, Kelsoe G, Howard RG, Rothstein TL, et al. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J Immunol. 1996;157:549–556. [PubMed] [Google Scholar]
- 59.Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, Carroll MC. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 60.Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin U, Bock D, Arseniev L, Tornetta MA, Ames RS, Bautsch W, Kohl J, Ganser A, Klos A. The human C3a receptor is expressed on neutrophils and monocytes, but not on B or T lymphocytes. J Exp Med. 1997;186:199–207. doi: 10.1084/jem.186.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pepys MB. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974;140:126–145. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ochs HD, Nonoyama S, Zhu Q, Farrington M, Wedgwood RJ. Regulation of antibody responses: the role of complement and adhesion molecules. Clin Immunol Immunopathol. 1993;67:S33–40. [PubMed] [Google Scholar]