Abstract
We examined the topography of the lateral line primary projection in zebrafish larvae by double labeling. The projections of two identified neuromasts of the posterior lateral line are seen as two separate sets of fibers that show reproducible spatial relationships: the projection of the anterior neuromast is always ventrolateral to that of a more posteriorly located neuromast. The same rule applies to the projection of anterior lateral line neuromasts. The position of the neuromasts along the antero posterior axis of the fish therefore is represented in the central projection of the sensory neurons. This somatotopy is similar to, and may be at the origin of, the tonotopic projection of the cochlear hair cells in mammals.
The coevolution of sense organs and a central processing system that can handle the information they provide is a major problem in developmental neurobiology. One case in point is the development of the auditory system. The generation of an organ capable of discriminating frequencies, such as the mammalian cochlea, would make little sense if the sensory axons were to project indiscriminately in the brain. Indeed there is a marked somatotopy in the central projection of the primary auditory neurons (1). It raises the question of the origin of this somatotopy, and whether it developed before the emergence of a cochlear system. The auditory system is evolutionarily and developmentally related to the lateral line of fishes and amphibians, and indeed it has been suggested that the former is derived from the latter. It therefore is conceivable that the emergence of a topographic representation in the auditory projection finds its roots in a similar representation of the mechanosensory lateral line system.
The mechanosensory lateral line is a water displacement detecting system (2, 3) and is thought to mediate a sense of “distant touch” (4) whereby the animal is able to perceive and localize movements in its vicinity, within a radius of the order of its own body length. Depending on the ecological niche of the animal, the lateral line is associated with various behavioral responses such as prey detection, obstacle or predator avoidance, and schooling behavior.
The peripheral sensory organs of the lateral line, the neuromasts, consist of mechanosensory hair cells surrounded by support cells, much like the organs of the inner ear. They can be enclosed in pouches, canals, or vesicles, but their simplest form is superficial. The base of the hair cells makes synapses on the peripheral process of bipolar sensory neurons located in a cranial ganglion. One ganglion, located anterior to the ear, conveys the sensory information gathered by the head neuromasts (anterior lateral line system), while a second ganglion behind the ear innervates the trunk and tail neuromasts (posterior lateral line system). The axons from the two ganglia enter the hindbrain at different rostro-caudal levels; they then bifurcate and extend anteriorly and posteriorly in the dorsal hindbrain.
In adult bony fishes the central projections of the anterior and posterior lateral lines extend rostro-caudally along most of the hindbrain, forming two adjacent columns of fibers. No somatotopic representation could be documented within the projection of the mechanosensory lateral lines, however, either in fishes or in amphibians (5, 6). This finding is intriguing, not only because it would imply that the emergence of a somatotopic representation in the auditory projection must have its origins elsewhere, but also because this absence of somatotopy does not fit with the behavioral evidence. Mathematical modeling (7) and behavioral experiments on the Mexican blind cave fish, Astyanax fasciatus mexicanus (8), have led to the conclusion that the exquisite spatial discrimination ability of its lateral line cannot be accounted for by the temporal pattern of the stimulus alone, but most probably requires its comparison at different points on the fish body surface (9).
In the zebrafish (Danio rerio), the lateral line apparently is functional as soon as 5 days after fertilization (ref. 10, our personal observation). At this stage the number of neuromasts and sensory neurons is much lower than in the adult, and each neuromast can be individually recognized based on its position. The present study relies on double labeling to examine the topography of the lateral line primary projection in free-swimming, 6-day-old zebrafish larvae. We show that the projections of the anteriormost and posteriormost neuromasts are somatotopically organized both for the anterior and the posterior lines, and that this somatotopy resembles that found in the auditory projection of higher vertebrates.
MATERIALS AND METHODS
Fish Maintenance.
Zebrafish were maintained and a colony was established as described by Westerfield (11). Embryos were obtained from natural spawning and incubated at 28.5°C in tank water. In most experiments, pigment formation was inhibited by adding 200 μM phenylhiourea 24 hr after fertilization.
Vital Staining of the Lateral Line System.
Neuromast hair cells were labeled by incubating live larvae in 5 μM 4-(4-diethylaminostyryl)-N-methylpyridinium iodide (4-Di-2-Asp, Sigma D-3418) (12) in fish Ringer’s solution (11) for about 3 hr. We observed that longer incubation times lead to the labeling of the lateral line nerve, presumably by transynaptic uptake. The whole primary projection could be vitally labeled by incubating larvae in 125 μM 4-Di-2-Asp for 1 hr and returning them to Ringer’s solution for 6 hr.
Labeling of Lateral Line Afferents.
Neuromasts were labeled with 5 μM 4-Di-2-Asp as above, and the larvae were anesthetized in 0.5 mM tricaine (3-aminobenzoic acid ethyl ester, Sigma) in Ringer’s solution and transferred to a glass slide covered with a layer of wet Whatman 105 paper. The neuromasts and lateral line nerve were viewed with a dissection microscope equipped with epifluorescence (Leica, green fluorescent protein filter set). Biotin-dextran, rhodamine-dextran, and fluoresceine-dextran (Mr 3,000, fixable, Molecular Probes) solutions in water were allowed to dry on a glass slide, and minute amounts of dye paste were picked up on sharpened tungsten needles. Branches of the lateral line nerves innervating individual neuromasts were labeled by creating a small lesion with the needle. The fish were left in a humidified chamber for 5 min and transferred to Ringer’s solution. After 6 hr the dyes entirely filled the sensory neurons. The larvae then were anesthetized as above, fixed overnight in 4% paraformaldehyde in PBS, and rinsed in PBS.
Observation of Labeled Fibers.
For single labelings, biotin-labeled samples were processed with the ABC Elite kit and stained with diaminobenzidine as recommended by the manufacturer (Vector). They then were dehydrated, cleared in methylsalicylate, and mounted in Canada balsam. Double labelings initially were performed with biotin-dextran and fluorescein-dextran. Biotin labeling was revealed as above, while fluorescein back-filled neurons were revealed with an antifluorescein antibody coupled to alcaline phosphatase (Boehringer Mannheim), and stained with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium. In our experience, however, the latter staining method results in a coarse blue precipitate, making it difficult to resolve fine structures. Fluorescence analysis of rhodamine- and fluoresceine-labeled samples was done on a Bio-Rad MRC 1024 confocal microscope equipped with a Zeiss plan neofluar 40/1.3 or a Nikon fluor 40/1.3 lens. The samples were incubated in a graded series of PBS/glycerol and mounted in citifluor AF1 (Agar Scientific, Stansted, U.K.). The two channels were imaged simultaneously at a pixel size of 0.5 μm. Each data set consisted of 30–50 images separated by 1 μm. Rotating stereo pairs generated with the laser-sharp processing software (Bio-Rad) were observed with a reflecting stereoscope (VCH, Weinheim, Germany), and selected frames were edited with Photoshop (Adobe, Mountain View, CA). 4-Di-2-Asp vitally labeled neurons were imaged in the same way with a Zeiss achroplan 40/0.75 water immersion lens in live larvae mounted in 1% low melting point agarose in Ringer’s solution.
RESULTS
Vital Labeling of Lateral Line Sensory Neurons.
The hair cells of the neuromasts can be vitally labeled by incubation in dilute solutions of the styryl dye 4-Di-2-Asp (12). In 6-day-old fish, several lines of neuromasts are seen on the head whereas only one is present on the side of the trunk and tail (Fig. 1). Longer incubation times lead to the labeling of nerve fibers that contact each neuromast and presumably are labeled by transynaptic transport of the dye from the hair cells, as no other neurite is labeled (not shown). The signal gets weaker as the distance from the neuromast increases, making it difficult to decide whether these fibers are sensory afferents (in which case the cell bodies would reside in the sensory ganglion) or efferents (with cell bodies in the central nervous system).
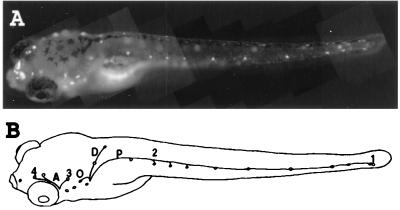
Figure 1.
(A) Vitally labeled neuromast hair cells in a 6-day-old zebrafish larvae. The dye also is taken up by the nasal epithelium. (B) Neuromasts and lateral line nerve branches whose projection was examined in the present work. Neuromasts on one side of the fish are drawn as small circles. The nerve branches that were not back-filled in this work are not shown. A, anterior lines; P, midbody posterior line; D, dorsal line; O, occipital line; 1, last two neuromasts of the posterior midbody line; 2, second neuromast of the posterior midbody line; 3, neuromast located dorso anterior to the ear vesicle; 4, last neuromast of the supra orbital line. Neuromasts 1 and 2 are innervated by the posterior lateral line ganglion, and neuromasts 3 and 4 are innervated by the anterior lateral line ganglion. Magnification: ×20.
Incubating the larvae in more concentrated dye solutions (see Methods) results in a much stronger labeling of the hair cells and the innervating fibers. Although this treatment is toxic to the hair cells, which are extruded within 1 or 2 hr after the incubation, all of the neurons are labeled after a few hours. About 20 labeled cell bodies are observed in the posterior lateral line ganglion (not shown), a figure that corresponds to the number of cells that send an axon in the posterior lateral line nerve (13). The primary projection is also clearly labeled (Fig. 2), showing that the entire extent of the neuromasts sensory neurons can be vitally labeled with 4-Di-2-Asp. As shown in Fig. 2, the posterior and anterior lateral line nerves enter the hindbrain at different rostro-caudal levels. Their projections appear superimposed and form a single column of neuropil that extends longitudinally in the ipsilateral dorsal hindbrain.
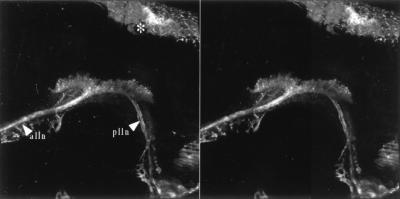
Figure 2.
Vitally labeled lateral line primary projection. Stereo pair of a dorso lateral view seen from the outside of the animal. Anterior is to the left and dorsal to the top. alln, anterior lateral line nerve; plln, posterior lateral line nerve. ∗ indicates dorsal head skin. Magnification: ×160.
Anterior and Posterior Lateral Line Projections.
To see whether the anterior and posterior lateral line nerves of the larva project to different areas, as has been reported for the adult form of other bony fishes, we back-filled the two nerves with different fluorescent dyes. The two projections branch on entering the brain and extend to the same rostral and caudal levels. Fig. 3A shows that the projections from the anterior and posterior lines run parallel but do not mix with each other. The projection of the anterior lateral line forms a ribbon that is ventral to that of the posterior lateral line, as found in the adult bony fishes examined to date (6). The narrow gap, which is seen between the two ribbon-like projections in Fig. 3A but is not seen in Fig. 2, may correspond to the projection of the occipital and dorsal lines as these projections were not back-filled in this experiment. Consistent with this hypothesis, we observed that the projection of the first dorsal neuromast to develop extends between those of the anterior and posterior lines (see below). Because the dorsal and occipital neuromasts are located between those of the head and those of the trunk and tail along the anteroposterior axis of the animal (Fig. 1B), it seems likely that the projections of the different lines segregate as distinct ribbons that are layered in an order that parallels the anteroposterior order of the lines. Thus the overall pattern of the different projections represents a first level of somatotopy.
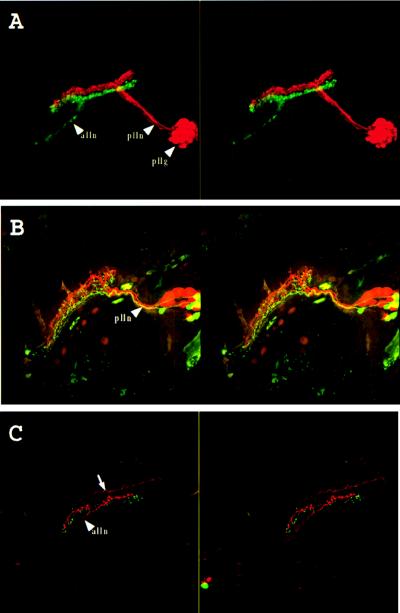
Figure 3.
Double labeling of the lateral line system. (A) Labeling of the midbody posterior lateral line nerve (plln) with rhodamine-dextran (red) and of the anterior lateral line nerve (alln) with fluoresceine-dextran (green). Stereo pair of a side view seen from the outside of the animal. Anterior is to the left and dorsal to the top. pllg, posterior lateral line ganglion. The anterior lateral line ganglion could not be imaged because of the limited working distance of the lens. (B) Labeling of the afferents of the second neuromast of the midbody posterior lateral line (2 in Fig. 1) with fluoresceine-dextran and the last two neuromasts of the same line (1 in Fig. 1) with rhodamine-dextran. Stereo pair of a dorsolateral view seen from the outside of the animal. Anterior is to the left and dorsal to the top. plln, posterior lateral line nerve. (C) Labeling of the afferents of a neuromast located dorso anteriorly to the ear (3 in Fig. 1) with rhodamine-dextran and the last neuromast of the supra orbital line (4 in Fig. 1) with fluoresceine-dextran. Stereo pair of a dorsolateral view seen from the outside of the animal. Anterior is to the left and dorsal to the top. alln, anterior lateral line nerve. The arrow indicates trigeminal descending fibers, which often were labeled in these preparations. Magnification: ×160.
The Projections of Individual Neuromasts.
In fish larvae, most neuromasts are very close to the lateral line nerve that gathers all the sensory fibers, which makes it difficult to backfill the neurons innervating single neuromasts without labeling other sensory fibers that run through the nerve. The neuromasts located at the distal end of a line can, of course, be individually labeled without difficulty, as there are no other neurites running through the nerve at this level. Fortunately, some of the proximal neuromasts are often slightly displaced from the course of the nerve (Fig. 1B), making it possible to label specifically the branch that innervates them. Fig. 4 shows the projection of the second neuromast of the posterior lateral line, which consistently lies ventral to the posterior lateral line nerve. Labeled fibers are not observed posterior to the site of dye application, showing that the labeled projection originates from a single neuromast. Four to five cell bodies typically are labeled in the posterior lateral line ganglion. Their central projection is very similar to that of the whole posterior line, with one interesting difference: instead of extending as a ribbon (as in Figs. 2 and 3A), these fibers form a tightly packed bundle that does not appear to fill the cross-sectional area of the total projection. This finding suggests that the projection of an individual neuromast is confined to one section of the projection of the entire line.
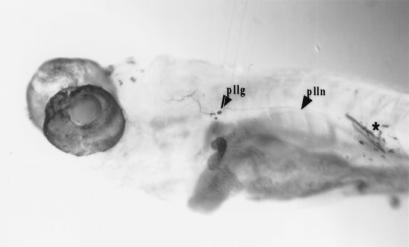
Figure 4.
Labeling of the afferents of the second neuromast of the midbody posterior lateral line (numbered 2 in Fig. 1 and Table 1). ∗ indicates site of dye application. Magnification: ×60.
Ordering of Individual Projections.
To determine whether the projections from different neuromasts are arranged in a reproducible pattern, and whether this pattern somehow reflects their position along the body, we labeled the second neuromast of the posterior line with fluorescein-dextran and the last two neuromasts of the same line with rhodamine-dextran (the last and penultimate neuromasts of this line lie very close to each other at the tip of the tail and cannot be labeled individually). Fig. 3B shows that the corresponding projections are clearly distinct. The ordering of these two projections is constant: in eight successful double labelings, the projection from the posteriormost neuromasts always lies dorsal and medial to the other one (Fig. 5A). The cell bodies also appear to be reproducibly clustered in the ganglion according to the position of the neuromasts they innervate, with cells innervating the posteriormost neuromasts located anterior and dorsal to cells innervating the second neuromast. Approximately twice as many rhodamine-labeled neurons as fluoresceine-labeled neurons are observed, and the rhodamine-labeled projection is often split in two, possibly because two neuromasts were back-filled at the tip of the tail.
Figure 5.
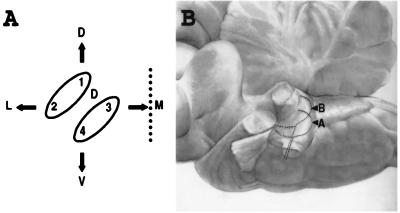
(A) Schematic cross-section of the lateral line somatotopy in the hindbrain. The dotted line represents the midline. 1, 2, 3, 4, and D refer to the neuromasts labeled on Fig. 1B. The upper ellipse (1 and 2) represents the cross-section of the ribbon-like projection of the posterior lateral line; the lower ellipse (3 and 4) that of the anterior lateral line, D stands for the projection of the dorsal neuromast. Arrows: D, dorsal; V, ventral; M, medial; L: lateral. (B) Somatotopic projection of the spiral ganglion neurons in the cochlear complex of the cat. Side view, anterior to the left, dorsal to the top. The projection of hair cells located at the apex (A) of the Corti organ is ventrolateral to that of cells located more basalward (B). After ref. 1. Magnification: ×0.5.
To see whether a similar topography exists in the projection of the anterior lateral line, we did a similar double-labeling experiment. In this case we labeled the neuromast located dorsal and anterior to the ear with rhodamine-dextran and the anteriormost neuromast of the supra-orbital line with fluoresceine-dextran. Fig. 3C shows that the projection from the neuromast close to the ear lies dorsal and slightly medial to that of the most anterior neuromast. Thus for both the anterior and the posterior lines, the projection of anterior neuromasts lies ventrolateral to the projection of posterior neuromasts (Fig. 5A), suggesting the existence of a constant topographical representation of anterior vs. posterior within each lateral line projection.
Finally, we tested the coherence of the two maps, that of the anterior and that of the posterior line, by making double labelings with one neuromast of each line. The results (Table 1) are consistent with the idea that each of the two lines projects as a ribbon where the axons corresponding to individual neuromasts are ordered from ventrolateral to dorsomedial according to the position of the neuromast along the antero-posterior axis of the animal. Double labelings involving one neuromast of either the anterior or posterior lines, and the first neuromast of the dorsal line, show that the projection of the latter extends between the anterior and the posterior line projections (Table 1 and Fig. 5A). It has not been possible to individually label different neuromasts of the dorsal line to determine whether this projection displays the same ordering as the head and trunk lines.
Table 1.
Spatial relationships of the projections from pairs of neuromasts or lines as shown in Fig. 1
| Labeled neuromasts or nerve branches | Relative position of the central projections | Number of successfully labeled specimens |
|---|---|---|
| p vs. A | Dorsolateral | 7 |
| p vs. 3 | Dorsolateral | 3 |
| 1 vs. 2 | Dorsomedial | 8 |
| 1 vs. D | Dorsolateral | 4 |
| 1 vs. 3 | Dorsolateral | 3 |
| 2 vs. D | Ventrolateral | 3 |
| D vs. 3 | Dorsolateral | 2 |
| 3 vs. 4 | Dorsomedial | 5 |
| P vs. 4 | Dorsolateral | 4 |
A, anterior lines; P, midbody posterior line; D, dorsal line; 1, last two neuromasts of the posterior midbody line; 2, second neuromast of the posterior midbody line; 3, neuromast located dorso anterior to the ear vesicle; 4, last (anteriormost) neuromast of the supra orbital line. Neuromasts 1 and 2 are innervated by the posterior lateral line ganglion and neuromasts 3 and 4 are innervated by the anterior lateral line ganglion.
In addition to the main posterior line, three other lines will develop later at different dorso-ventral positions along the trunk and tail. Because these lines develop much later, we have not examined their projections and do not know whether this dorso-ventral organization also is mapped in the central projection of the corresponding neurons.
DISCUSSION
Our experiments show that the primary projection of the lateral line in the hindbrain is doubly somatotopic. First, posterior lines project more dorsally than anterior lines. Second, within a given line posterior neuromasts project more dorsally and medially than anterior neuromasts.
One mechanism that might account for the formation of a somatotopic map would be based on the time of arrival of the axons, as it is known that the successive organs of a line differentiate one after the other (ref. 13 and unpublished observations). The lateral line system originates from ectodermal placodes located close to the ear vesicle. One part of each placode migrates away to form the neuromasts, whereas the static part produces the ganglion. The development of the posterior midbody line has been studied in some detail in zebrafish (13–15). The primordium starts migrating posteriorly at the level of the horizontal myoseptum at 20 hr after fertilization and reaches the caudal fin by 40 hr. During its migration, about seven groups of cells are deposited successively that differentiate to form the primary neuromasts. Thus, the posterior line develops progressively from anterior to posterior, and the progressive shift of the axons from a ventrolateral to a dorsomedial position could reflect their order of arrival in the hindbrain.
In the case of the anterior lateral line, which is formed by a primordium that migrates in the opposite direction, the antero-posterior order of neuromast formation will be opposite to that of the posterior line. Thus one would expect a simple temporal mechanism (acting on a first-come, first-served basis) to generate an inverse organization for the anterior and posterior lines. We observed the same somatotopy for both the anterior and the posterior lines, however. In other words, it appears that the information that is represented in this map is the anteroposterior position of the neuromasts on the body surface and not their proximodistal position relative to the place of origin of the placode. This finding suggests that the building of the map makes use of local cues intrinsic to the hindbrain, rather than of temporal cues related to the order of arrival of the sensory axons.
The establishment of a somatotopic map by the sensory neurons implies that the neurons are somehow able to perceive the position of the neuromasts they innervate. An interesting aspect to the development of this map is that the innervation of a neuromast by a given neuron and the establishment of a central projection by this same neuron may be simultaneous (14). It is tempting to speculate that the somatotopic map is set up at this early time, when the system is developing, rather than a posterori. We could not define when exactly the map is set up, as double-labeling turns out to be exceedingly difficult in younger fish. Our results show, however, that an incipient somatotopy is already present 6 days after fertilization in the zebrafish. Likewise we have documented the existence at this early stage of separate projections for the anterior and posterior lines as previously described in the adults of many species. Further, both the anterior vs. posterior line organization, and the anterior vs. posterior somatotopy within each line, also were observed in larvae of the blind cave fish Astyanax fasciatus (not shown), suggesting that these are general features of larval teleosts. On the other hand, although the neuromasts of 6-day-old zebrafish larvae already contain hair cells of opposite polarity with respect to the position of the kinocilium within the hair bundle (10), we found no obvious feature in the projection of single neuromasts that would suggest that these two cell populations project differentially. In particular, we did not observe the two fascicles that have been described in the central projection of lateral lines in several taxa, and which were proposed to convey the afferents from hair cells of opposite orientations (ref. 16 and references therein). Segregation according to polarity may occur later in development, or alternatively may not occur at all in fish, and be an acquisition of more evolved vertebrates.
The projection of the lateral line system is very similar to the acoustic projection. The inner ear projection in fish lies alongside, and ventral to, the lateral line projection (6). They both bifurcate on entering the hindbrain into two branches, an anterior one and a posterior one, that extend along the entire rhombencephalon. This similarity extends to the organization of the projections: in higher vertebrates, the tonotopic organization of the auditory projection resembles the somatotopic organization documented here for the lateral line. We speculate that both sensory systems use the same guidance mechanisms to organize their projections along the dorsoventral axis in the dorsal hindbrain. For example, a comparison of Fig. 5B with Fig. 3 shows that the projection of the neurons from the spiral (auditory) ganglion resembles the projection of the fish lateral line, with axons extending at a dorsoventral level, which depends on the position of the innervated hair cell. Neurons innervating hair cells located more apically in the cochlea (and therefore tuned to low frequencies) project more ventrally than neurons innervating more basal hair cells (tuned to higher frequencies) (1). In this respect, the cochlea behaves like an anterior lateral line, with proximal, early differentiating sensory cells projecting more dorsally than distal, late-appearing cells.
This striking similarity between the cochlear projection and that of the mechanosensory lateral line contrasts with the primary projection of the electrosensory lateral line system that extends as a third column dorsal to the mechanosensory projections. Within this electrosensory projection, no fewer than four somatotopic maps of the body surface are arranged side by side but in this case the rostro-caudal axis of the animal is represented rostro-caudally in the electrosensory lobe (17, 18) with strong interspecific variations (19). Therefore, the mechanosensory lateral line is more closely related to the ear than to the electrosensory lateral line with respect to its projection pattern. Such a topographic projection probably pre-existed in the primitive mechanosensory system from which the lateral line and the ear evolved.
Coming back to the original question that prompted this work, we can make the following proposal. Originally the mechanosensory organs projected along the hindbrain without any somatotopic organization and provided a “general touch” system. Superficial organs are necessarily aligned along the antero-posterior axis of the animal, however, and the emergence of a central projection reflecting this antero-posterior organization, however roughly, must have improved the efficiency of the system. Once the capability to develop such a somatotopic projection into the hindbrain has evolved, it presumably can be taken advantage of by any part of the mechanosensory system. Thus any incipient invagination of one of the ear sensory maculae instantly would be converted into a primitive tone-discriminating system, thereby providing strong selective pressure for the further amplification and refinement of the auditory system.
Acknowledgments
This paper is dedicated to the memory of Nigel Holder. We thank Nicolas Cubedo for fish care, Claude Dechêne and Nicole Lautrédou for introducing us to the confocal microscope, and Christine Dambly-Chaudière, Olivier Bricaud, and Nicolas Gompel for discussions. This work was supported by the Institut National pour la Santé et la Recherche Médicale (INSERM).
Footnotes
Abbreviation: 4-Di-2-Asp, 4-(4-diethylaminostyryl)-N-methylpyridinium iodide.
References
- 1.Rose J. In: Neural Mechanisms of the Auditory and Vestibular Systems. Rasmussen G, Windle W, editors. Springfield, IL: Thomas; 1960. pp. 116–136. [Google Scholar]
- 2.Hofer B. Ber Kgl Bayer Biol Versuchsstation München. 1908;1:115–164. [Google Scholar]
- 3.Schulze F E. Arch Mikrosk Anat. 1870;6:62–68. [Google Scholar]
- 4.Dijkgraaf S. In: The Mechanosensory Lateral Line: Neurobiology and Evolution. Coombs S, Görner P, Münz H, editors. New York: Springer; 1989. pp. 7–14. [Google Scholar]
- 5.Will U. In: The Mechanosensory Lateral Line: Neurobiology and Evolution. Coombs S, Görner P, Münz H, editors. New York: Springer; 1989. pp. 365–386. [Google Scholar]
- 6.McCormick C A. In: The Mechanosensory Lateral Line: Neurobiology and Evolution. Coombs S, Görner P, Münz H, editors. New York: Springer; 1989. pp. 341–364. [Google Scholar]
- 7.Hassan E-S. Biol Cybernet. 1985;52:23–36. doi: 10.1007/BF00336932. [DOI] [PubMed] [Google Scholar]
- 8.Hassan E-S. J Comp Physiol A. 1986;159:701–710. doi: 10.1007/BF00612042. [DOI] [PubMed] [Google Scholar]
- 9.Hassan E-S. In: The Mechanosensory Lateral line: Neurobiology and Evolution. Coombs S, Görner P, Münz H, editors. New York: Springer; 1989. pp. 217–227. [Google Scholar]
- 10.Nicolson T, Rusch A, Friedrich R, Granato M, Ruppersberg J, Nusslein-Volhard C. Neuron. 1998;20:271–283. doi: 10.1016/s0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- 11.Westerfield M. The Zebrafish Book. Eugene: Univ. of Oregon Press; 1995. [Google Scholar]
- 12.Collazo A, Fraser S, Mabee P. Science. 1994;264:426–430. doi: 10.1126/science.8153631. [DOI] [PubMed] [Google Scholar]
- 13.Metcalfe W, Kimmel C, Schabtach E. J Comp Neurol. 1985;233:377–389. doi: 10.1002/cne.902330307. [DOI] [PubMed] [Google Scholar]
- 14.Metcalfe W. J Comp Neurol. 1985;238:218–224. doi: 10.1002/cne.902380208. [DOI] [PubMed] [Google Scholar]
- 15.Kimmel C, Hatta K, Metcalfe W. Neuron. 1990;4:535–545. doi: 10.1016/0896-6273(90)90111-r. [DOI] [PubMed] [Google Scholar]
- 16.Fritzsch B. In: The Mechanosensory Lateral Line: Neurobiology and Evolution. Coombs S, Görner P, Münz H, editors. New York: Springer; 1989. pp. 99–114. [Google Scholar]
- 17.Heiligenberg W, Dye J. J Comp Physiol. 1982;148:287–296. [Google Scholar]
- 18.Carr C, Maler L, Sas E. J Comp Neurol. 1982;211:139–153. doi: 10.1002/cne.902110204. [DOI] [PubMed] [Google Scholar]
- 19.Lannoo M, Maler L. J Comp Neurol. 1990;294:153–160. doi: 10.1002/cne.902940112. [DOI] [PubMed] [Google Scholar]