Abstract
B cell antigen receptor (BCR) cross-linking activates three distinct families of nonreceptor protein tyrosine kinases (PTKs): src-family kinases, Syk, and Btk; these PTKs are responsible for initiating downstream events. BCR cross-linking in the chicken DT40 B cell line also activates three distinct mitogen-activated protein kinases (MAPKs): extracellular signal–regulated kinase (ERK)2, c-jun NH2-terminal kinase (JNK)1, and p38 MAPK. To dissect the functional roles of these PTKs in MAPK signaling, activation of MAPKs was examined in various PTK-deficient DT40 cells. BCR-mediated activation of ERK2, although maintained in Lyn-deficient cells, was abolished in Syk-deficient cells and partially inhibited in Btk-deficient cells, indicating that BCR-mediated ERK2 activation requires Syk and that sustained ERK2 activation requires Btk. BCR-mediated JNK1 activation was maintained in Lyn-deficient cells but abolished in both Syk- and Btk-deficient cells, suggesting that JNK1 is activated via a Syk- and Btk-dependent pathway. Consistent with this, BCR-mediated JNK1 activation was dependent on intracellular calcium and phorbol myristate acetate–sensitive protein kinase Cs. In contrast, BCR-mediated p38 MAPK activation was detected in all three PTK-deficient cells, suggesting that no single PTK is essential. However, BCR-mediated p38 MAPK activation was abolished in Lyn/Syk double deficient cells, demonstrating that either Lyn or Syk alone may be sufficient to activate p38 MAPK. Our data show that BCR-mediated MAPK activation is regulated at the level of the PTKs.
Keywords: B cell antigen receptor, mitogen-activated protein kinase, Lyn, Syk, Btk
Bcells recognize and respond to foreign antigens through their surface-expressed B cell antigen receptor (BCR)1 complexes composed of antigen-binding membrane Ig and disulfide-linked heterodimer Igα/Igβ, which are the signaling subunits of the BCR (1, 2). The signaling cascade initiated by Igα/Igβ after BCR cross-linking can promote different biological outcomes, depending on the differentiation stage of the B cell and the nature of additional signals received by the cell. Immature B cells become anergized or undergo apoptosis upon antigen binding, whereas mature B cells enter the cell cycle after BCR ligation and can then be induced by other signals to differentiate into antibody-secreting plasma cells. A key early event of BCR signaling is the activation of protein tyrosine kinases (PTKs), which initiates most if not all of the subsequent signaling events (3, 4). Since the BCR has no intrinsic PTK activation, for signal transduction it uses three distinct families of nonreceptor cytoplasmic PTKs: Lyn, Blk, Fyn, Lck, and Fgr of the Src family, Syk of the Syk/ ZAP-70 family, and Btk of the Tec family (5–8).
Mitogen-activated protein kinases (MAPKs) play a central role in transducing extracellular signals from the cytosol to the nucleus (9, 10). Three structurally related MAPK subfamilies have been identified in mammalian cells: the p42 and p44 kinases (extracellular signal-regulated kinases [ERKs]; references 11, 12), the c-jun NH2-terminal kinases (JNKs)/stress activated protein kinases (SAPKs) (13–15), and the p38 MAPK family (16, 17). Different patterns of activation of MAPKs may well direct B cells to different cell fates such as proliferation, differentiation, and apoptosis. In a human B cell line B104, activation of JNK and p38 MAPK, but not ERK, is correlated with induction of apoptosis by surface IgM stimulation (18). Both ERKs and JNK1 are activated during positive signaling in naive B cells, whereas only ERKs but not JNK1 are activated in tolerant cells upon BCR stimulation by the same ligand (19). However, the mechanisms that lead to the activation of these distinct MAPKs have not been elucidated.
The chicken DT40 B cell line expresses only specific members of the Src, Syk/ZAP-70, and Tec PTK families: Lyn of the Src-family, Syk of the Syk/ZAP70 family, and Btk of the Tec family (20, 21). Using Syk-, Lyn-, and Btk-deficient DT40 cells, we investigated the participation of PTKs in MAPK signaling. We report that BCR cross-linking in DT40 cells activates three distinct MAPKs: ERK2, JNK1, and p38 MAPK. Whereas Syk and Btk regulate the activation of ERK2 and JNK1, Lyn does not. Furthermore, either Syk or Lyn is sufficient for the activation of p38 MAPK upon BCR ligation, whereas Btk does not appear to regulate this MAPK family member. Thus, the BCR uses different PTKs to regulate members of distinct MAPKs in B cells.
Materials and Methods
Reagents.
The mouse anti–chicken IgM mAb M4 was prepared as previously described (22); PMA, ionomycin, and 4α-phorbol were purchased from Calbiochem, Inc. (San Diego, CA). Thapsigargin was purchased from Sigma Chemical Co. (St. Louis, MO). Polyclonal rabbit antisera against JNK1 (C-17), ERK2 (C-14), ERK1 (C-16), and p38 MAPK (C-20) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyclonal rabbit antiphospho-MAPK (ERK2) was purchased from Promega Corp. (Madison, WI). Purified mouse anti–human JNK1 monoclonal antibody was purchased from PharMingen (San Diego, CA). Myelin basic protein (MBP) was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Glutathione S-transferase (GST)-c-jun (5–89) and GST-activating transcription factor (ATF)2 were expressed in Escherichia coli, and the fusion proteins were purified as previously described (23). Protein A–sepharose was obtained from Amersham Pharmacia Biotech, Inc. (Piscataway, NJ). BAPTA-AM [the acetoxymethyl ester of bis-(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid] and Pluronic F-127 were purchased from Molecular Probes, Inc. (Eugene, OR).
Cell Lines.
The generation of Syk-, Lyn-, and Btk-deficient, and Lyn/Syk double deficient, DT40 cell lines was carried out as previously described (20, 21). DT40 wild-type and PTK-deficient cell lines were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 2 mM glutamine, 10 U/ml penicillin, 10 μg/ml streptomycin, 1 mM pyruvate and nonessential amino acids, 50 μM mercaptoethanol, and 1% heat-inactivated chicken serum. The cells were maintained below a density of 3 × 106 cells/ml.
Measurement of Intracellular Free Calcium.
Cells (2.5 × 107 cell per sample) were collected and resuspended to 107 cells/ml in fresh complete RPMI 1640 medium. After incubation at 37°C for 15 min, indo-1/AM (7 μg/ml final concentration) was added, and the cells were incubated for a further 45 min with resuspension every 15 min. The cells were subsequently centrifuged and resuspended in complete RPMI 1640 medium at 2 × 106 cells/ml. The fluorescence intensity of intracellular indo-1 was monitored and analyzed using a FACStar® plus flow cytometer (Becton Dickinson, Mountain View, CA) as previously described (24).
Western Blot Analysis.
Unstimulated cells or anti-IgM stimulated cells (5 × 106 cells per sample) were lysed in 100 μl RIPA lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 10 mM Na4P2O7, 25 mM sodium β-glycerophosphate, 1 mM EDTA, 1% (wt/vol) NP-40, 0.5% (wt/vol) sodium deoxycholate, 0.1% (wt/ vol) SDS, 1 mM PMSF, 1 mM Na3VO4, 10 μM E-64, 1 μg/ml pepstatin, 10 μg/ml aprotinin, 10 μg/ml leupeptin). After incubation on ice for 15 min, cell lysates were centrifuged at 16,000 g for 15 min at 4°C. Lysates were subsequently denatured in an equal volume of 2× SDS sample buffer, resolved by 10% SDS-PAGE and electrotransferred to nitrocellulose membranes in non-SDS-containing transfer buffer (25 mM Tris, 0.2 M glycine, 20% methanol, pH 8.5). Western blotting was performed with the following primary antibodies and accompanying dilutions: antiphospho ERK2 (1:20,000), anti-MAPK (ERK1 or ERK2, 1:2,000), anti-p38 (1:1,000), and mouse monoclonal anti-JNK1 (1:500) followed by 1:2,000 anti–rabbit or anti–mouse horseradish peroxidase–conjugated IgG (Santa Cruz Biotechnology, Inc.) and were developed with an ECL Western blotting kit (Amersham Corp., Arlington Heights, IL).
Immunoprecipitation and In Vitro Kinase Assay.
For JNK1, ERK2, and p38 MAPK in vitro kinase assays, cells (5–10 × 106 cells per sample) were resuspended in complete RPMI 1640 medium to a density of 2 × 106 cells/ml and stimulated with either anti-IgM (10 μg/ml) or PMA (50 ng/ml) with or without ionomycin (250 ng/ml) for the indicated times. Incubations were terminated by addition of 10 vol of ice-cold PBS and were subsequently centrifuged at 500 g for 8 min at 4°C. The supernatants were aspirated and cells were lysed by resuspension in 0.5 ml RIPA lysis buffer. After incubation on ice for 15 min, the cell lysates were centrifuged at 16,000 g for 15 min at 4°C. For JNK1 and ERK2 in vitro kinase assays, supernatants were added to 20 μl packed protein A–sepharose beads and 0.5 μg polyclonal rabbit anti-JNK or anti-ERK2 antibody, followed by overnight incubation at 4°C. For p38 MAPK assays, the lysates were first added to 1 μg polyclonal rabbit anti-p38 MAPK antiserum with an additional 0.1% SDS. After constant mixing by inversion for 3 h or overnight at 4°C, the mixtures were added to 20 μl packed protein A-sepharose beads for another 1 h. The immune complexes were pelleted at 4°C by centrifugation at 16,000 g for 10 min. The beads were washed twice each with (a) 1 ml of RIPA buffer with 0.1 mM Na3VO4 and (b) 1 ml of p38 MAPK assay buffer (25 mM Hepes, pH 7.4, 25 mM sodium β-glycerophosphate, 25 mM MgCl2, 2 mM dithiothreitol, and 0.1 mM Na3VO4). The pellets were resuspended to 90 μl p38 MAPK assay buffer with 20 μM ATP containing 10 μCi γ-[32P]ATP and 50 μg/ml substrate (MBP for ERK2, GST–c-jun for JNK1, or GST-ATF2 for p38 MAPK). After incubation for 20 min at 30°C with mixing every 2 min, the reactions were terminated by adding 45 μl 2× SDS sample buffer. After boiling for 5 min followed by brief centrifugation, samples were resolved by SDS-PAGE (12% for JNK1 and p38 MAPK assays and 14 or 12% for ERK2 assays). The gels were stained for 30 min with 40% methanol, 10% acetic acid, and 0.005% Coomassie brilliant blue G-250, destained for 30 min with 40% methanol, 10% acetic acid, and 5% glycerol, and dried before autoradiography at −70°C for various times.
Results
BCR-mediated Activation of ERK2 Requires Syk and Btk.
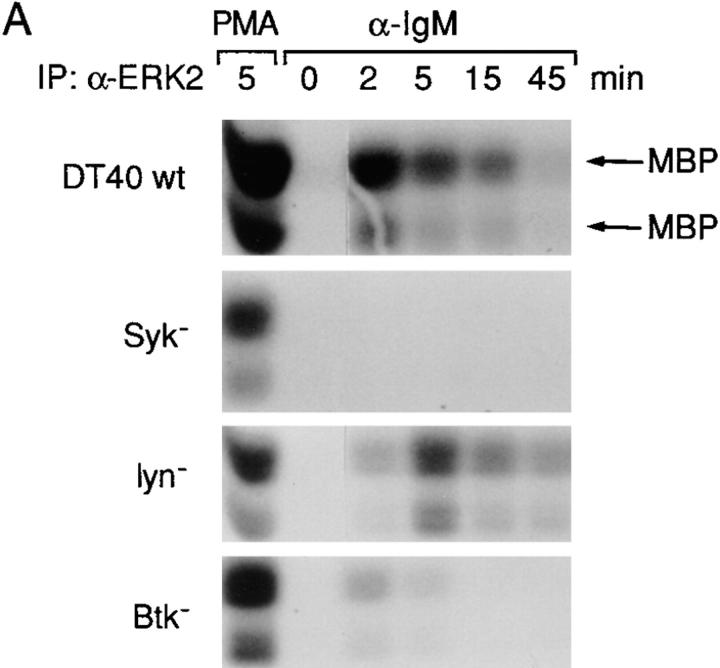
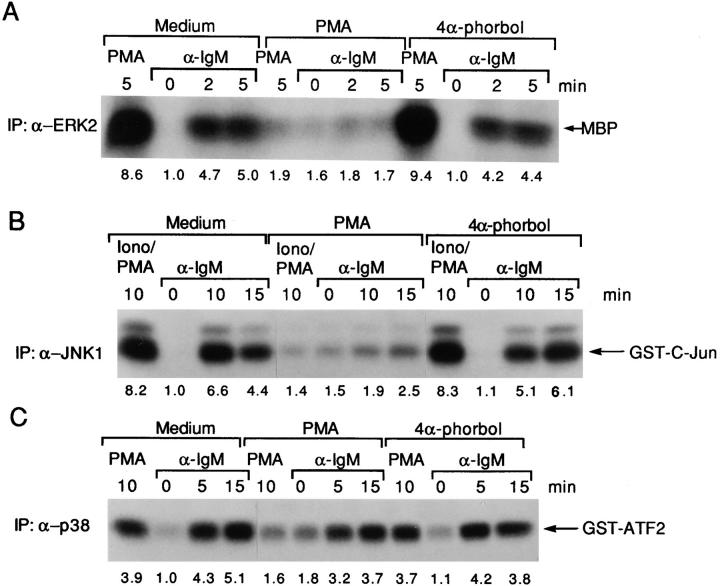
Since engagement of the BCR complex strongly activates p42 ERK2 in other B cell lines and in normal B cells (25, 26), we tested whether BCR cross-linking in DT40 B cells could also lead to ERK2 activation. In vitro kinase assays were performed with anti-ERK2 immunoprecipitates to assess induction of ERK2 activation. Consistent with previous studies, ERK2 activation was detected shortly after BCR cross-linking, with a maximal fourfold activation compared with unstimulated cells evident at 2 min and sustained for at least 15 min (Fig. 1 A). To determine the PTK requirement for BCR-mediated ERK2 activation, we compared ERK2 activation in wild-type versus mutant DT40 cell lines deficient in Lyn, Syk, or Btk that expressed comparable levels of surface IgM (20, 21). BCR-mediated ERK2 activation was completely abolished in Syk-deficient cells even though ERK2 was activated by PMA to a similar extent compared with wild-type DT40 cells (Fig. 1 A); thus, Syk is required for BCR-mediated ERK2 activation. In contrast, BCR-mediated ERK2 activation was largely maintained in Lyn-deficient cells with a maximal threefold activation at 5 min (Fig. 1 A), indicating that ERK2 can be activated via the BCR in the absence of Lyn. In contrast to either Syk- or Lyn-deficient cell lines, BCR ligation in Btk-deficient cells led to a transient and weak ERK2 activation (less than twofold), suggesting that a Btk-independent ERK2 activation pathway exists and that Btk is required for sustained ERK2 activation (Fig. 1 A).
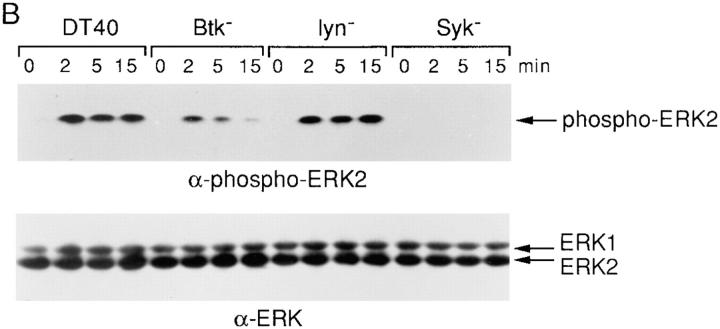
Figure 1.
ERK2 is activated after anti-IgM stimulation in DT40 wild-type, Lyn-, and Btk-deficient cells but not in Syk-deficient cells. (A) Cells (5 × 106 per sample) were stimulated with either anti-IgM mAb M4 (5 μg/ml) for 0–45 min or PMA (50 ng/ ml) for 5 min, and cell lysates were immunoprecipitated with polyclonal anti-ERK2 antiserum (0.5 μg per sample). In vitro kinase assays were performed on the immune complexes using MBP (50 μg/ml) as a substrate; kinase reaction products were resolved by 14% SDS-PAGE, and their activation was quantified by autoradiography and scanning densitometry. MBP appeared as two separated bands under these conditions, reflecting some degradation. One of three similar independent experiments is shown. Note that the ERK2 activation after BCR engagement for 2 min in Lyn-deficient cells was atypically lower than that of DT40 wild-type cells but was similar to that of DT40 wild-type cells in two other experiments. (B) Phosphorylation of ERKs in wild-type and various PTK-deficient DT40 cells. Cells (5 × 106 per sample) were stimulated with anti-IgM mAb M4 (10 μg/ml) for 0–15 min. Cell lysates (equal to 106 per sample) were subsequently analyzed by immunoblotting with anti-phospho-MAPK (top) or anti-ERK1 (bottom) Abs. The anti-ERK1 antibody recognized both ERK1 and ERK2, whereas the anti-phospho-MAPK (ERK2) only detected phosphorylated ERK2 in these DT40 cells. The anti-ERK2 antibody we used for both ERK2 immunoprecipitation and in vitro kinase assays recognized only ERK2 not ERK1 (data not shown).
As an alternative assay for ERK2 activation, we measured the phosphorylation of ERK2 using Western blotting and a phospho-MAPK antiserum, which only detected phosphorylated ERK2 in DT40 cells (Fig. 1 B). Consistent with our in vitro kinase assays, phosphorylated ERK2 was detected in DT40 cells between 2 and 15 min after anti-IgM stimulation (Fig. 1 B). No BCR-mediated phosphorylation of ERK2 was detected in Syk-deficient cells; in Lyn-deficient cells phosphorylation of ERK2 was comparable with wild-type DT40 cells, whereas weak and transient ERK2 phosphorylation was observed in Btk-deficient cells (Fig. 1 B). In summary, BCR-mediated ERK2 activation requires Syk, whereas sustained ERK2 activation requires Btk.
BCR-mediated JNK1 Activation Requires both Syk and Btk but not Lyn.
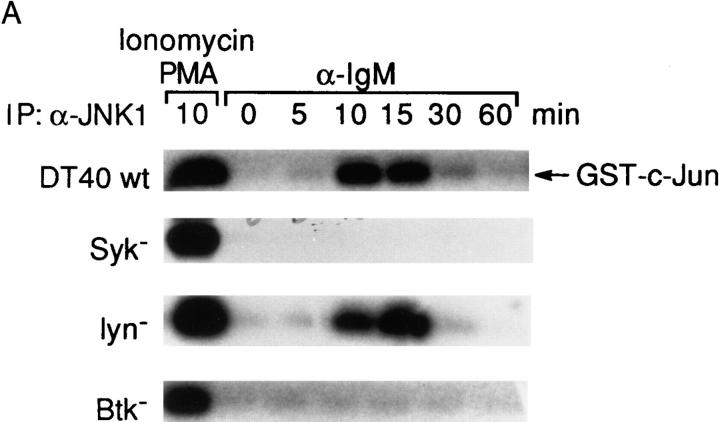
JNKs are activated preferentially by a variety of extracellular stresses, and in most circumstances they are activated through a different pathway from ERKs (10, 13– 15). BCR cross-linking also led to JNK1 activation in DT40 cells as determined by its ability to phosphorylate GST–c-jun. JNK1 was maximally activated (fourfold) between 10 and 15 min and then decreased by 30 min (Fig. 2 A). To test whether BCR-mediated JNK1 activation has the same PTK requirement as BCR-mediated ERK2 activation, we compared the ability of the BCR to activate JNK1 in Syk-, Lyn-, and Btk-deficient DT40 cells. The expression levels of JNK1 were not affected either by the deletion of various PTK genes or anti-IgM stimulation (Fig. 2 B). BCR-mediated JNK1 activation was maintained in Lyn-deficient cells with a maximal fivefold JNK1 activation at 15 min (Fig. 2 A), suggesting that Lyn was not essential for BCR-mediated JNK1 activation. However, in both Syk- and Btk-deficient cells, the anti-IgM–induced JNK1 activation was completely abolished (Fig. 2 A), indicating that both Syk and Btk were essential for JNK1 activation. Thus, BCR-mediated JNK1 activation requires both Syk and Btk but not Lyn.
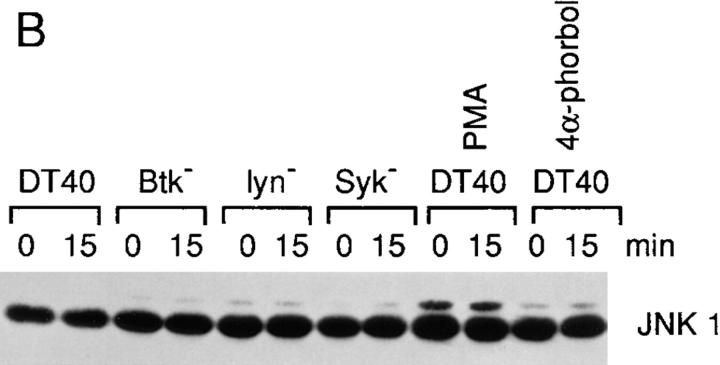
Figure 2.
BCR-induced JNK1 activation requires both Syk and Btk. (A) Activation of JNK1 in wild-type and PTK-deficient DT40 cells. Cells (5 × 106/sample) were stimulated with 5 μg/ ml anti–chicken IgM mAb M4 for 0–60 min or with PMA (50 ng/ml) and ionomycin (250 ng/ ml) for 10 min at 37°C. Cells were then lysed in RIPA buffer and immunoprecipitated with rabbit polyclonal anti-JNK1 antiserum (0.5 μg/sample). In vitro kinase assays were performed with GST–c-jun as a specific exogenous substrate; kinase reaction products were resolved by 12% SDS-PAGE, and their activation was quantified by autoradiography and scanning densitometry. (B) Western blot analysis of JNK1 protein expression. DT40 cells were incubated with PMA (100 ng/ml), the inactive derivative 4α-phorbol (100 ng/ml) or DMSO solvent vehicle control for 24 h before anti-IgM stimulation. Cell lysates (equal to 106/sample) were denatured in SDS sample buffer, resolved by 10% SDS-PAGE, and subsequently were analyzed by Western blot analysis using a mouse anti–human JNK1 monoclonal antibody.
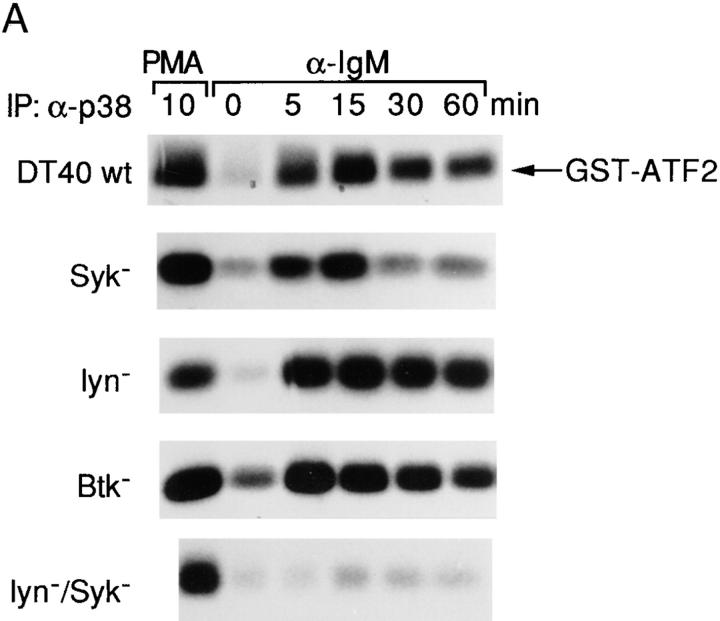
BCR-mediated p38 MAPK Activation Occurs via a Different Pathway from both ERK2 and JNK1.
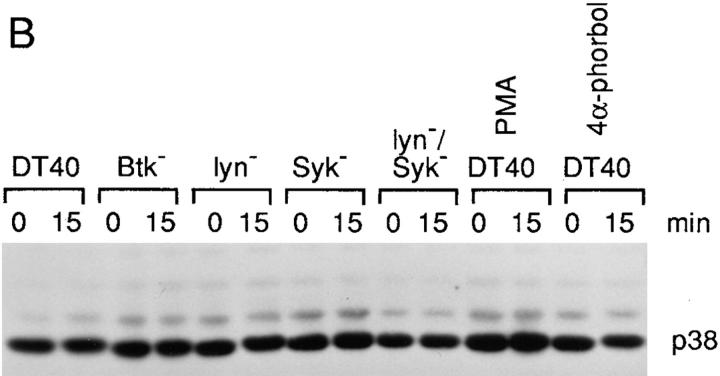
As a relatively new member of the mammalian MAPKs, p38 MAPK has both a different substrate specificity and different upstream activating kinases (MKKs) and appears to have a distinct transducing pathway and function (9, 10, 16, 17). p38 MAPK activation was examined by the ability of anti-p38 MAPK immunoprecipitates to phosphorylate GST-ATF2. p38 MAPK reached a maximal fourfold activation at 15 min after anti-IgM stimulation, and, distinct from both ERK2 and JNK1 activation, persisted for at least 12 h (Fig. 3 A and data not shown). To investigate whether p38 MAPK has different requirements for upstream PTKs, we examined p38 MAPK activation in various PTK-deficient DT40 cells. There was no significant difference in p38 MAPK expression levels between the parental DT40 cells and various PTK-deficient DT40 cells including the Lyn/Syk double mutant cells (Fig. 3 B). In addition, the recovery of p38 MAPK after immunoprecipitation was also not significantly different in DT40 wild-type and mutant cell lines (data not shown). To our surprise, BCR-mediated p38 MAPK activation in Lyn-deficient cells (sixfold maximal activation), Syk-deficient cells (threefold), and Btk-deficient cells (threefold) was similar to that in wild-type DT40 cells (Fig. 3 A). The fold activations of p38 MAPK in Syk- and Btk-deficient cells were lower in part because these cells had a higher basal level of p38 MAPK activation (Fig. 3 A). Thus, these data suggest that none of these PTKs alone is essential for the BCR to activate p38 MAPK. Previous studies have shown that both Lyn and Syk associate with the BCR and account for most of the initial tyrosine phosphorylation including activation of Btk (21). Our data suggested either that there was another PTK required for p38 MAPK activation, or alternatively that Syk and Lyn had a redundant role in BCR-mediated p38 MAPK activation. To test these possibilities, a Lyn/Syk double deficient DT40 cell line was examined. BCR-mediated p38 MAPK activation was lost in Lyn/Syk double deficient cells (Fig. 3 A), indicating that either Lyn or Syk was sufficient for BCR-mediated p38 MAPK activation.
Figure 3.
Either Lyn or Syk is sufficient for BCR-mediated p38 MAPK activation. (A) BCR-mediated p38 MAPK activation was only abolished in Lyn/syk double deficient cells. Cells (5 × 106 per sample) were stimulated with 10 μg/ml anti–chicken IgM mAb M4 for 0–60 min or with PMA (100 ng/ml) for 10 min at 37°C. Cells were then lysed in RIPA lysis buffer and immunoprecipitated with polyclonal anti-p38 MAPK antiserum (1 μg/sample) in the presence of an additional 0.1% SDS. In vitro kinase assays were performed using GST-ATF2 as a specific exogenous substrate and analyzed as described in the legend to Fig. 2 A. (B) Western blot analysis of p38 MAPK expression. Cell lysates were prepared as described in the legend to Fig. 2 B. The lysates (equal to 106/ sample) were resolved by 10% SDS-PAGE and subsequently were analyzed by Western blot analysis using a polyclonal anti-p38 MAPK antiserum.
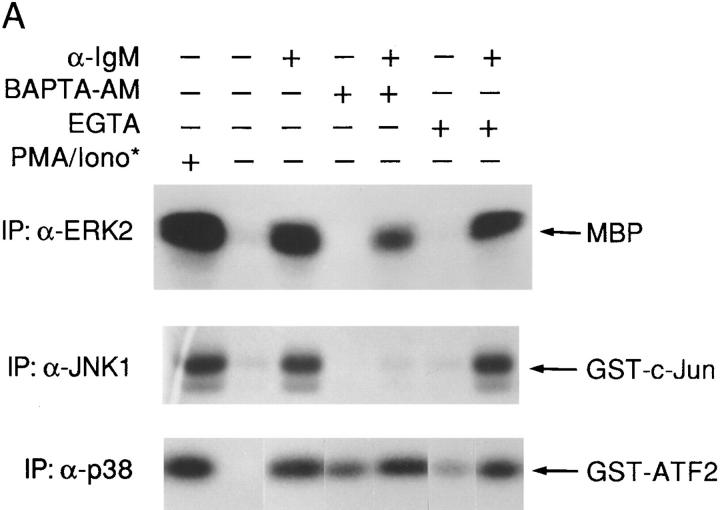
Activation of JNK1, but not ERK2 and p38 MAPK, via the BCR Is Dependent on Intracellular Calcium.
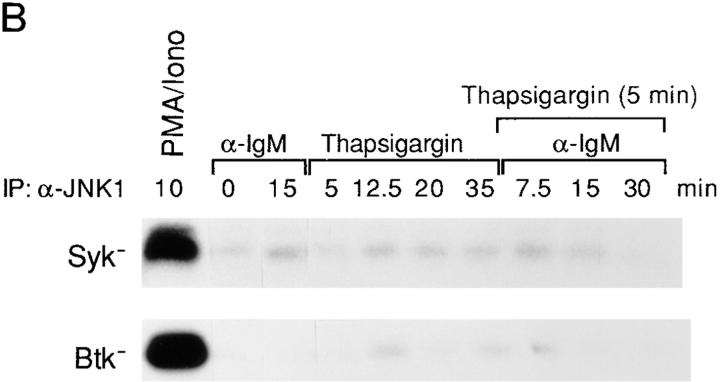
Anti-IgM stimulation leads to a rapid increase of intracellular calcium [Ca2+]i composed of both Ca2+ release from intracellular pools and extracellular calcium influx (27). Previous studies have demonstrated that loss of either Btk or Syk but not Lyn resulted in abrogation of calcium mobilization upon BCR cross-linking of DT40 cells (20, 21). Since the pattern of BCR-mediated JNK1 activation correlated precisely with that of BCR-mediated calcium mobilization, we hypothesized that a calcium signal may modulate BCR-mediated JNK1 activation. Furthermore, we could activate JNK1 only with a combination of both ionomycin and PMA (Fig. 2 A and data not shown), also supporting the idea that a calcium signal was required for BCR-mediated JNK1 activation. To define the requirement for calcium in BCR-mediated JNK1 activation and specifically determine whether intracellular or extracellular calcium was required, cells were treated with either BAPTA-AM, a cell permeable calcium chelator, or EGTA, which chelates extracellular calcium before anti-IgM stimulation. The concentrations of BAPTA-AM and EGTA were titrated to prevent calcium release and extracellular calcium influx, respectively (data not shown). BAPTA-AM pretreatment completely blocked BCR-induced JNK1 activation, whereas EGTA pretreatment did not (Fig. 4 A), suggesting that BCR-mediated JNK1 activation is dependent on intracellular rather than extracellular calcium. We also asked whether a calcium signal alone was sufficient for BCR- mediated JNK1 activation. To directly address this question, we pretreated DT40 mutant cells with thapsigargin, a pharmacological reagent that prevents reuptake of intracellular calcium into storage vesicles and thus mimics the receptor-dependent increase of [Ca2+]i while bypassing phosphatidylinositol 4,5-bisphosphate hydrolysis (28). The concentrations of thapsigargin were carefully titrated to give a similar peak calcium signal as anti-IgM stimulation (data not shown). JNK1 activation after BCR ligation was not reconstituted in either Syk- or Btk-deficient DT40 cells (Fig. 4 B), indicating that a calcium signal alone was not sufficient to activate JNK1. Other BCR-mediated signals, which require Syk and/or Btk, are also needed for JNK1 activation.
Figure 4.
BCR-mediated ERK2, JNK1, and p38 MAPK activation have different requirements of calcium. (A) Anti-IgM–induced activation of JNK1, but not of ERK2 and p38 MAPK, is dependent on intracellular calcium. Cells were preincubated with BAPTA-AM (10 μM) for 10 min or EGTA (2 μM) for 30 min and then stimulated with anti-IgM mAb M4 (10 μg/ml). The concentrations of BAPTA-AM and EGTA were titrated to prevent calcium release or extracellular calcium influx, respectively (data not shown). In vitro kinase assays were performed to measure the activation of ERK2, JNK1 and p38 MAPK as described in the legends to Figs. 1–3, except that 12% SDS-PAGE was used for all these assays. The times of stimulation of cells were as follows: ERK2, PMA for 5 min or anti-IgM for 2 min; JNK1, PMA and ionomycin or anti-IgM for 10 min; and p38 MAPK, PMA for 10 min or anti-IgM for 15 min. (B) A calcium signal is not sufficient for anti-IgM–induced JNK1 activation. Btk- and Syk-deficient cells were pre-incubated with thapsigargin (10 nM) for 5 min and then stimulated with anti-IgM mAb M4 (10 μg/ml) or incubated with thapsigargin alone for the indicated times. JNK1 activation was quantified by in vitro kinase assays as described in the legend to Fig. 2 A.
Since partial BCR-mediated ERK2 activation was detectable in Btk-deficient DT40 cells (Fig. 1), which are incapable of calcium mobilization after BCR ligation (21), it seemed unlikely that BCR-mediated ERK2 activation was calcium dependent. Indeed, pretreatment of DT40 cells with either BAPTA-AM or EGTA did not block BCR-mediated ERK2 activation (Fig. 4 A), indicating that a BCR-mediated calcium signal was not required for ERK2 activation.
Both Syk- and Btk-deficient DT40 cells maintained the ability to activate p38 MAPK via the BCR, suggesting that BCR-mediated p38 MAPK activation was independent of calcium. Indeed, neither BAPTA-AM nor EGTA pretreatment blocked BCR-mediated p38 MAPK activation, showing that BCR-mediated p38 MAPK activation was independent of calcium (Fig. 4 A). Furthermore, BAPTA-AM alone activated p38 MAPK (Fig. 4 A). Together with the observation that the Btk- and Syk-deficient DT40 cells, two mutants that lose their ability to mobilize calcium upon BCR ligation, also exhibited higher basal levels of p38 MAPK activation (Fig. 3), our results suggest that free calcium may play a negative role in p38 MAPK activation.
BCR-induced Activation of ERK2 and JNK1 Is, but p38 MAPK Is Not, PKC Dependent.
PMA treatment of wild-type DT40 cells leads to p38 MAPK and ERK2 activation, whereas both PMA and ionomycin are required for JNK1 activation (Fig. 1–3). Since both BCR cross-linking and PMA treatment lead to PKC activation, it was interesting to examine the role of PKCs in BCR-mediated MAPK signaling.
DT40 cells were pretreated for 24 h with PMA (which leads to depletion of PMA-dependent PKCs), an inactive derivative of PMA, 4α-phorbol, or medium, before stimulation with either anti-IgM or PMA. ERK2, JNK1, or p38 MAPK activation of the cells was then analyzed by in vitro kinase assays. Neither PMA nor 4α-phorbol pretreatment of DT40 cells had a significant effect on the expression levels of JNK1, p38 MAPK, and ERK2 (Figs. 2 B and 3 B and data not shown). BCR ligation did not significantly increase ERK2 activation above basal levels in PKC-depleted cells (Fig. 5 A), suggesting that BCR-mediated ERK2 activation is dependent on PMA-depletable PKCs. JNK1 activation was also strongly decreased in PKC-depleted cells (Fig. 5 B), suggesting that PMA-depletable PKCs are required in addition to a calcium signal (Fig. 4) for BCR-mediated JNK1 activation. The requirement of both a calcium signal and PKC activation is consistent with the observation that only a combination of PMA and ionomycin, but not PMA or ionomycin alone, could lead to significant JNK1 activation in DT40 cells (Fig. 5 B and data not shown). For p38 MAPK, significant BCR-dependent activation (greater than threefold increase) was still observed in PKC-depleted cells (Fig. 5 C), suggesting that BCR-mediated p38 MAPK activation is largely independent of PMA-depletable PKCs. However, our results cannot rule out the participation of other PMA-insensitive PKCs such as the atypical PKCζ in BCR-mediated p38 MAPK activation.
Figure 5.
ERK2, JNK1, and p38 MAPK have different requirements for PMA- depletable PKCs. Cells (5 × 106/sample) were incubated with PMA (100 ng/ml), the inactive derivative 4α-phorbol (100 ng/ml), or DMSO solvent vehicle control for 24 h before anti-IgM stimulation. Cell lysates (5 × 106/sample) were then prepared, and ERK2, JNK1, and p38 MAPK activation was measured as described in the legends to Figs. 1–3, except that 12% SDS-PAGE was used in all cases.
Discussion
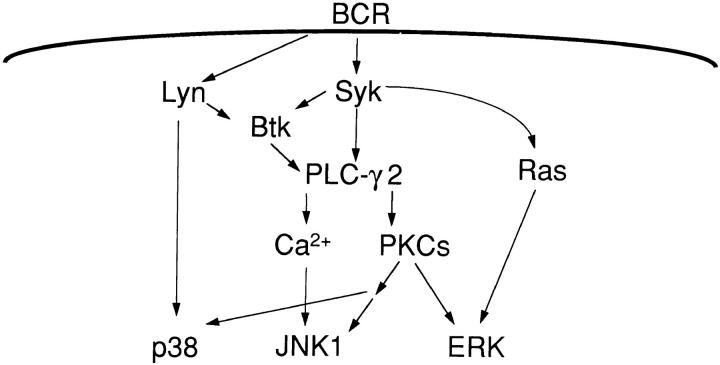
In this report, we show that each of the three major mammalian MAPK family members activated through the BCR requires a different set of upstream protein tyrosine kinases (Figs. 1–3 and 6).
Figure 6.
A model for the different pathways leading to BCR-mediated ERK2, JNK1, and p38 MAPK activation in DT40 cells. BCR cross-linking leads to activation of the protein tyrosine kinases Lyn, Syk, and Btk. Loss of either Syk or Btk leads to abrogation of calcium mobilization, although only Syk is essential for PLC-γ2 phosphorylation. PLC-γ2 phosphorylation leads to calcium mobilization and PKC activation. Syk but not Lyn is essential for BCR-mediated ERK2 activation and Btk may function as a regulator of the ERK2 pathway since partial BCR-mediated ERK2 activation is observed in Btk-deficient cells. The requirement for Syk and Btk may reflect the requirement of PKCs for ERK2 activation. In contrast, BCR-mediated JNK1 activation depends on both Syk and Btk but not Lyn, via a calcium- and PKC-dependent pathway. For p38 MAPK, a distinct pathway is used for BCR-mediated activation of p38 MAPK, since either Syk or Lyn is sufficient for BCR-mediated p38 MAPK activation. A Lyn-dependent pathway for p38 MAPK activation may not require PKCs, whereas the Syk-dependent pathway does. Neither a calcium signal nor PMA-depletable PKCs are absolutely essential for BCR-mediated p38 MAPK activation, although PMA-insensitive PKCs may be required.
Although it has been reported that ERKs are activated upon anti-IgM stimulation via a Ras/Raf-1/MEK signaling pathway (26, 29), the participation of proximal PTKs in BCR-mediated MAPK activation is not well understood. Our data demonstrate that Syk but not Lyn is absolutely essential for BCR-mediated activation of ERK2 (Fig. 1). Supporting this notion, recent studies using the Lck-negative JCaM1.6 variant of the Jurkat T cell line have shown that Syk enabled the TCR to cause a full activation of ERKs in the absence of the Src family kinase Lck (30). In contrast, BCR-mediated ERK2 activation is only partially dependent on Btk, suggesting that Btk has a regulatory role rather than being an essential component in the ERK2 signaling pathway. This possibility is also supported by the fact that Syk is essential for phosphorylation of PLC-γ2, whereas the loss of Btk only reduces PLC-γ2 phosphorylation (20, 21). Previous studies suggest that BCR-mediated activation of Ras and PKCs may converge at the level of Raf-1 for activation of ERKs (26, 29, 31). In a parallel study (32), we found that BCR-mediated activation of ERK2 was incompletely blocked in PLC-γ2–deficient DT40 cells or in DT40 cells transfected with a dominant negative form of Ras (Ras N17). A complete block of ERK2 activation was seen in PLC-γ2–deficient cells transfected with Ras N17, indicating that both Ras- and PLC-γ2–regulated PKCs are involved in BCR-mediated ERK2 activation. Taken together, our data suggest that Syk is essential for both Ras-dependent activation of ERK2 and the PLC-γ2– and PKC-dependent, Btk-regulated pathway leading to ERK2 activation (Fig. 6). Lyn was reported to play a negative role in ERK activation (33), and Lyn-deficient DT40 cells showed augmented BCR-mediated PKC activation (34), leading to the speculation that Lyn-deficient DT40 cells have augmented ERK2 activation. The fact that we did not detect augmented ERK2 activation in Lyn-deficient cells may reflect different roles of Lyn and/or involvement of different PKCs for ERK activation at different stages of B cell development. Interestingly, a novel PKC isoform, PKCμ, which coprecipitates with both Syk and PLC-γ1/2 (35), showed the same pattern of BCR- mediated activation as did ERK2. PKCμ was activated in wild-type and Lyn-deficient DT40 cells but not in Syk- deficient DT40 cells, and was only partially activated in Btk-deficient cells. Furthermore, PKCμ is both calcium independent and PMA inducible, suggesting that PKCμ may participate directly in the activation of ERK2 upon BCR ligation. A possible role of PKCμ in MAPK signaling would not be surprising since other novel PKC isoforms are also capable of activating ERKs (36). Further studies are required to elucidate the precise role of PKCμ and other PKC isoforms in BCR-mediated ERK2 activation.
Both JNK and p38 MAPK are preferentially activated by inflammatory cytokines and cellular stresses instead of classic mitogenic stimuli (37, 38). The JNK activation pathway is well studied, and Rac1/cdc42, MEKK1, and MKK4/ MKK7 contribute to JNK activation (39–41). Consistent with our finding that BCR-mediated JNK1 activation requires both a calcium signal and PMA-sensitive PKCs, JNK activation in a mouse B cell line also requires both PMA and ionomycin (42). In T lymphocytes, JNK activation requires engagement of both the TCR and CD28 or both PMA and ionomycin in contrast to the full activation of ERK1 and ERK2 by either PMA or TCR engagement alone (43). Our data also reveal that BCR-mediated JNK1 activation is dependent on Syk and Btk, which regulate PLC-γ2, but is not dependent on the Src family kinase Lyn (Fig. 6). Consistent with our data, a recent study showed that Syk but not Src family kinases in cooperation with Rac led to enhanced JNK activation in Jurkat T cells (44). Similarly, both a calcium signal and PKCs seem to account for the requirement of Syk for JNK activation. Since Syk- induced JNK activation is potentiated by anti-CD3, anti-CD28, or PMA, it is likely that the requirement of Syk could be at least partly due to a requirement for calcium (44); the fact that JNK activation is not solely activated by ionomycin or calcineurin, and that calcineurin does not synergize with Rac to enhance JNK activation, suggests that PKCs are the second component involved in JNK activation by Syk (45). We also found that PLC-γ2–deficient cells exhibited impaired JNK1 activation and, interestingly, a dominant negative Rac1 (Rac1 N17) blocked activation of JNK1, suggesting a model where cooperation of PKCs and Rac1 leads to JNK1 activation (32). Evidence from studies of Jurkat cells also supports this model. A recent study revealed that a particular PKC isoform, PKCθ, but not PKCα or PKCε, participated in JNK activation; moreover, specific cooperation between calcineurin and PKCθ converged on Rac leading to potent JNK activation (45).
Btk is of interest since Btk is essential for BCR-mediated JNK1 activation but not for p38 MAPK or ERK2 activation. A study using mast cells from Btk-defective mice also had a similar result: the lack of Btk only led to a loss of FcεRI-mediated JNK1 activation but not ERKs or p38 MAPK activation (46). Recently, a novel MKK kinase, MEKK4, which specifically activates the JNK pathway but not ERKs or p38 MAPK, was identified (47); MEKK4 has a Rac/cdc42 interacting binding motif, a putative pleckstrin homology (PH) domain, and a proline-rich region that could provide the binding site for the Src homology (SH)3 domain. Since Btk has a PH domain, a proline-rich region, and SH2 and SH3 domains (48, 49), and since PH domains can interact with PKCs (50), one attractive model is that the interaction of Btk and MEKK4 defines the specificity of JNK signaling pathway (Fig. 6).
Less is known concerning the p38 MAPK signaling pathway. p38 MAPK can be turned on independently of JNKs since both MKK3 and MKK6 activate p38 MAPK specifically (51, 52); however, the upstream activators of MKK3 and MKK6 are still not fully defined. One potential upstream activator may be a STE20/PAK homologue such as Mst1 (53, 54). It is also not clear at what point p38 MAPK and JNK pathways diverge and what the mechanisms are that lead to specific activation of p38 MAPK versus JNKs. Although our data do not entirely solve the puzzle, they do indicate that in DT40 cells p38 MAPK is activated by BCR ligation via a distinct pathway from JNK1: either Syk or Lyn is sufficient and Btk is not required for BCR-mediated p38 MAPK activation; in contrast, anti-IgM–induced JNK activation requires both Syk and Btk but not Lyn (Fig. 6). BCR-mediated p38 MAPK was only partially affected in Syk-deficient (Fig. 3 A) and PKC-depleted cells (Fig. 5 C), again suggesting that both Syk- and Lyn-dependent signals may contribute to p38 MAPK activation independently (Fig. 6). Just how the two p38 MAPK signaling pathways interact and are regulated remains to be determined.
Activation of MAPK family members may contribute to the induction of programmed cell death or apoptosis. Apoptosis plays an important role in B cell development and the generation of immune responses (55), and DT40 cells undergo apoptosis upon BCR cross-linking. Our studies using the B104 B cell line show that p38 MAPK plays a role in BCR-mediated apoptosis (56) and that activation of JNK1 correlates with induction of cell death (18). In DT40 cell lines, BCR-mediated apoptosis is blocked in both Syk- and Btk-deficient cells (57, 58), which also do not activate JNK1; cells in which JNK1 is activated via the BCR (wild-type and Lyn-deficient DT40 cells) also undergo apoptosis (53); in addition, BAPTA-AM blocks both apoptosis and JNK1 activation but not BCR-mediated ERK2 or p38 MAPK activation (Fig. 4 and data not shown). Thus, activation of JNK1 correlated best with the induction of apoptosis in DT40 cells. We also tested the role of p38 MAPK in apoptosis and found that only a relatively high dose of SB203580 (20 μM), a highly specific and cell-permeable inhibitor of p38 MAPK, completely blocked BCR-mediated apoptosis in DT40 cells (Jiang, A., A. Craxton, and E.A. Clark, unpublished data). Thus, it is not presently clear whether JNK1 alone or in cooperation with p38 MAPK may be needed to activate a death program in DT40 cells. Further work with knockout mice and primary B cells is needed to assess the function of distinct MAPKs in B cell development and activation.
Acknowledgments
We would like to thank M. Domenowske for preparation of figures, G. Shu and K.E. Draves for technical assistance, Y. Sun for reading the manuscript, and other members of the Clark laboratory for helpful discussions.
This work was supported by National Institutes of Health grants GM-42508 and GM-37905 (to E.A. Clark) and by grants from the Japanese Ministry of Education, Science, Sports and Culture (to T. Kurosaki).
Abbreviations used in this paper
- ATF
activating transcription factor
- BAPTA-AM
the acetoxymethyl ester of bis-(o-aminophenoxy) ethane-N,N,N′, N′-tetraacetic acid
- BCR
B cell antigen receptor
- [Ca2+]i
intracellular cytoplasmic free Ca2+ concentration
- ERK
extracellular signal-regulated kinase
- GST
glutathione S-transferase
- JNK
c-jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- PKC
protein kinase C
- PLC-γ2
phospholipase C-γ2
- PTK
protein tyrosine kinase
References
- 1.Campbell KS, Cambier JC. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO (Eur Mol Biol Organ) J. 1990;9:441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- 3.Bolen JB. Protein tyrosine kinases in the initiation of antigen receptor signaling. Curr Opin Immunol. 1990;7:306–311. doi: 10.1016/0952-7915(95)80103-0. [DOI] [PubMed] [Google Scholar]
- 4.Sefton BM, Campbell MA. The role of tyrosine protein phosphorylation in lymphocyte activation. Annu Rev Cell Biol. 1991;7:257–274. doi: 10.1146/annurev.cb.07.110191.001353. [DOI] [PubMed] [Google Scholar]
- 5.Law CL, Craxton A, Otipoby KL, Sidorenko SP, Klaus SJ, Clark EA. Regulation of signalling through B-lymphocyte antigen receptors by cell–cell interaction molecules. Immunol Rev. 1996;153:123–154. doi: 10.1111/j.1600-065x.1996.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 6.Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309–318. doi: 10.1016/s0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 7.DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 8.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 9.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 10.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 11.Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/theonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 12.Ahn NG, Seger R, Bratlien RL, Diltz CD, Tonks NK, Krebs EG. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991;266:4220–4227. [PubMed] [Google Scholar]
- 13.D'Erijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 14.Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 15.Kallunki T, Su B, Tsigelny I, Sluss HK, D'Erijard B, Moore G, Davis R, Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 16.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 17.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 18.Graves JD, Draves KE, Craxton A, Saklatvala J, Krebs EG, Clark EA. Involvement of stress-activated protein kinase and p38 mitogen-activated protein kinase in mIgM-induced apoptosis of human B lymphocytes. Proc Natl Acad Sci USA. 1996;93:13814–13818. doi: 10.1073/pnas.93.24.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, Lewis RS, Goodnow CC. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 20.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+mobilization through distinct pathways. EMBO (Eur Mol Biol Organ) J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takata M, Kurosaki T. A role for Bruton's tyrosine kinase in B cell antigen receptor–mediated activation of phospholipase C-γ2. J Exp Med. 1996;184:31–40. doi: 10.1084/jem.184.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CL, Lehmeyer JE, Cooper MD. Evidence for an IgD homologue on chicken lymphocytes. J Immunol. 1982;129:2580–2585. [PubMed] [Google Scholar]
- 23.Berberich I, Shu G, Siebelt F, Woodgett JR, Kyriakis JM, Clark EA. Cross-linking CD40 on B cells preferentially induces stress-activated protein kinases rather than mitogen-activated protein kinases. EMBO (Eur Mol Biol Organ) J. 1996;15:92–101. [PMC free article] [PubMed] [Google Scholar]
- 24.Rabinovitch PS, June CH, Grossmann A, Ledbetter JA. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986;137:952–961. [PubMed] [Google Scholar]
- 25.Casillas A, Hanekom C, Williams K, Katz R, Nel AE. Stimulation of B-cells via the membrane immunoglobulin receptor or with phorbol myristate 13-acetate induces tyrosine phosphorylation and activation of a 42-kDa microtubule-associated protein-2 kinase. J Biol Chem. 1991;266:19088–19094. [PubMed] [Google Scholar]
- 26.Gold MR, Sanghera JS, Stewart J, Pelech SL. Selective activation of p42 mitogen-activated protein (MAP) kinase in murine B lymphoma cell lines by membrane immunoglobulin cross-linking. Evidence for protein kinase C–independent and –dependent mechanisms of activation. Biochem J. 1992;287:269–276. doi: 10.1042/bj2870269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson HA, Greenblatt D, Poenie M, Finkelman FD, Tsien RY. Cross-linkage of B lymphocyte surface immunoglobulin by anti-Ig or antigen induces prolonged oscillation of intracellular ionized calcium. J Exp Med. 1987;166:601–606. doi: 10.1084/jem.166.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, Bjerrum PJ, Christensen SB, Hanley MR. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989;27:17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- 29.Tordai A, Franklin RA, Patel H, Gardner AM, Johnson GL, Gelfand EW. Cross-linking of surface IgM stimulates the Ras/Raf-1/MEK/MAPK cascade in human B lymphocytes. J Biol Chem. 1994;269:7538–7543. [PubMed] [Google Scholar]
- 30.Williams S, Couture C, Gilman J, Jascur T, Deckert M, Altman A, Mustelin T. Reconstitution of T cell antigen receptor-induced Erk2 kinase activation in Lck-negative JCaM1 cells by Syk. EurJ Biochem. 1997;245:84–90. doi: 10.1111/j.1432-1033.1997.00084.x. [DOI] [PubMed] [Google Scholar]
- 31.Kawauchi K, Lazarus AH, Sanghera JS, Man GL, Pelech SL, Delovitch TL. Regulation of BCR- and PKC/Ca(2+)-mediated activation of the Raf1/MEK/MAPK pathway by protein-tyrosine kinase and -tyrosine phosphatase activities. Mol Immunol. 1996;33:287–296. doi: 10.1016/0161-5890(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto A, Okada H, Jiang A, Kurosaki M, Greenberg S, Clark EA, Kurosaki T. Involvement of guanosine triphosphatases and phospholipase C-γ2 in extracellular signal–regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J Exp Med. 1998;188:1287–1295. doi: 10.1084/jem.188.7.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 34.Katsuta H, Tsuji S, Niho Y, Kurosaki T, Kitamura D. Lyn-mediated down-regulation of B cell antigen receptor signaling: inhibition of protein kinase C activation by Lyn in a kinase-independent fashion. J Immunol. 1998;160:1547–1551. [PubMed] [Google Scholar]
- 35.Sidorenko SP, Law CL, Klaus SJ, Chandran KA, Takata M, Kurosaki T, Clark EA. Protein kinase C mu (PKC mu) associates with the B cell antigen receptor complex and regulates lymphocyte signaling. Immunity. 1996;5:353–363. doi: 10.1016/s1074-7613(00)80261-7. [DOI] [PubMed] [Google Scholar]
- 36.Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 38.Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 39.Moriguchi T, Toyoshima F, Masuyama N, Hanafusa H, Gotoh Y, Nishida E. A novel SAPK/JNK kinase, MKK7, stimulated by TNFalpha and cellular stresses. EMBO (Eur Mol Biol Organ) J. 1997;16:7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 41.Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 42.Sutherland CL, Heath AW, Pelech SL, Young PR, Gold MR. Differential activation of the ERK, JNK, and p38 mitogen-activated protein kinases by CD40 and the B cell antigen receptor. J Immunol. 1996;157:3381–3390. [PubMed] [Google Scholar]
- 43.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben N-Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 44.Jacinto E, Werlen G, Karin M. Cooperation between Syk and Rac1 leads to synergistic JNK activation in T lymphocytes. Immunity. 1998;8:31–41. doi: 10.1016/s1074-7613(00)80456-2. [DOI] [PubMed] [Google Scholar]
- 45.Werlen G, Jacinto E, Xia Y, Karin M. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. EMBO (Eur Mol Biol Organ) J. 1998;17:3101–3111. doi: 10.1093/emboj/17.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawakami Y, Miura T, Bissonnette R, Hata D, Khan WN, Kitamura T, Maeda Y-M, Hartman SE, Yao L, Alt FW, Kawakami T. Bruton's tyrosine kinase regulates apoptosis and JNK/SAPK kinase activity. Proc Natl Acad Sci USA. 1997;94:3938–3942. doi: 10.1073/pnas.94.8.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerwins P, Blank JL, Johnson GL. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J Biol Chem. 1997;272:8288–8295. doi: 10.1074/jbc.272.13.8288. [DOI] [PubMed] [Google Scholar]
- 48.Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, Sparkes RS, Kubagawa H, Mohandas T, Quan S, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 49.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein- tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 50.Yao L, Kawakami Y, Kawakami T. The pleckstrin homology domain of Bruton's tyrosine kinase interacts with protein kinase C. Proc Natl Acad Sci USA. 1994;91:9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D'Erijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 52.Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 53.Graves JD, Gotoh Y, Draves KE, Ambrose D, Han DKM, Wright M, Chernoff J, Clark EA, Krebs EG. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO (Eur Mol Biol Organ) J. 1998;17:2224–2234. doi: 10.1093/emboj/17.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rudel T, Zenke FT, Chuang TH, Bokoch GM. p21-activated kinase (PAK) is required for Fas-induced JNK activation in Jurkat cells. J Immunol. 1998;160:7–11. [PubMed] [Google Scholar]
- 55.VanParijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: Turning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 56.Graves JD, Draves KE, Craxton A, Krebs EG, Clark EA. A comparison of signaling requirements for apoptosis of human B lymphocytes induced by the B cell receptor and CD95/Fas. J Immunol. 1998;161:168–174. [PubMed] [Google Scholar]
- 57.Takata M, Homma Y, Kurosaki T. Requirement of phospholipase C-γ2 activation in surface immunoglobulin M-induced B cell apoptosis. J Exp Med. 1995;182:907–914. doi: 10.1084/jem.182.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uckun FM, Waddick KG, Mahajan S, Jun X, Takata M, Bolen J, Kurosaki T. BTK as a mediator of radiation-induced apoptosis in DT-40 lymphoma B cells. Science. 1996;273:1096–1100. doi: 10.1126/science.273.5278.1096. [DOI] [PubMed] [Google Scholar]