Abstract
Recent evidence indicates that integrin engagement results in the activation of biochemical signaling events important for regulating different cell functions, such as migration, adhesion, proliferation, differentiation, apoptosis, and specific gene expression. Here, we report that β1 integrin ligation on human natural killer (NK) cells results in the activation of Ras/mitogen-activated protein kinase pathways. Formation of Shc–growth factor receptor–bound protein 2 (Grb2) and Shc–proline-rich tyrosine kinase 2–Grb2 complexes are the receptor-proximal events accompanying the β1 integrin–mediated Ras activation. In addition, we demonstrate that ligation of β1 integrins results in the stimulation of interferon γ (IFN-γ) production, which is under the control of extracellular signal–regulated kinase 2 activation. Overall, our data indicate that β1 integrins, by delivering signals capable of triggering IFN-γ production, may function as NK-activating receptors.
Keywords: natural killer cells, integrins, Ras/mitogen-activated protein kinase pathway, interferon γ
Natural killer (NK) cells, a small population of circulating and tissue-resident lymphocytes, play an important role in the early phase of immune responses against certain viruses, parasites, and microbial pathogens by exhibiting cytotoxic functions and secreting a number of cytokines such as IFN-γ, TNF-α, and GM-CSF (1, 2).
It is becoming increasingly clear that the final outcome of NK cell activity results from a balance between triggering and inhibitory receptors and ligands. In the last few years, much attention has been given to the characterization of inhibitory receptors recognizing MHC class I molecules, named killer cell–inhibitory receptors (KIRs), while knowledge about receptors delivering positive signals is less advanced. Activating molecules include receptors belonging to the integrin (LFA-1, α4β1), Ig (CD16, CD2, DNAX accessory molecule 1 [DNAM-1]), and C-type lectin (NKRP-1, CD69) families (3–6). Recently, noninhibitory isoforms of MHC class I receptors have also been described (7–9), as well as the mechanisms by which they transmit stimulatory signals (10).
Integrins are a large family of homologous cell surface receptors that mediate cell–matrix and cell–cell interactions (11, 12) and that are capable of transducing intracellular signals (13–16). The integrin-mediated signaling events include stimulation of phosphoinositide metabolism, elevation of intracellular pH and Ca2+ transients, activation of a number of tyrosine kinases and protein kinase C isoforms, and regulation of the small GTP-binding proteins belonging to the Ras and Rho families. In addition, coupling of integrin receptors to mitogen-activated protein kinase (MAPK)1 pathways has been reported (17), and a role for p21 Ras in the upstream events leading to extracellular signal–regulated kinase (Erk) activation has recently been demonstrated (18, 19). Integrin-induced Ras activation has been shown to involve the Shc-mediated recruitment of the Grb2–mSos complex to the plasma membrane (18, 19). Moreover, a role for p125 focal adhesion kinase (Fak) in the integrin-mediated activation of the Ras-MAPK cascade has been suggested, as formation of the Fak–Grb2–mSos complex parallels activation of Erk kinases (20–22).
Several findings indicate that integrins can affect expression of several genes, including those encoding cytokines. Adhesion of monocytes to extracellular matrix components results in activation of IL-1β, TNF-α, IL-8, Gro-α, Gro-β, and Gro-γ (23–27). In addition, αvβ3 interaction with fibronectin (FN) and vitronectin enhances IL-4 and IL-2 production by murine γ/δ T cells and by a Th cell hybridoma, respectively (28, 29). In human T cells, LFA-1, α4β1, and α5β1 provide a costimulus for the release of multiple cytokines such as IL-2, IL-4, TNF-α, GM-CSF, and IFN-γ (30–34) and similarly, LFA-1 costimulates CD16-triggered TNF-α production in human NK cells (35). Nonetheless, information on the intracellular signaling events leading to the control of gene expression by integrins has been provided so far only in epithelial and mesenchymal cells.
We have previously shown that peripheral blood human NK cells express α4β1 and α5β1 as fibronectin receptors (36) and α6β1 as a laminin receptor (37), and that the expression and function of β1 integrins are modulated upon NK cell activation (37, 38). More recently, we have demonstrated that clustering of β1 integrins on human NK cells transduces intracellular signals leading to activation of the Fak-related nonreceptor tyrosine kinase Pyk-2, tyrosine phosphorylation of paxillin (39), elevation of intracellular calcium, and costimulation of NK cytotoxic functions (40). In this study, we analyzed whether β1 integrin ligation on human NK cells results in the stimulation of the Ras/ MAPK signaling pathways and investigated the role of these events in the production of IFN-γ.
Materials and Methods
Antibodies and Reagents.
The following mouse mAbs were used: anti-CD3 (Leu4), anti-CD16 (Leu11c), and anti-CD56 (Leu19) were purchased from Becton Dickinson (San Jose, CA). Anti-CD16 (B73.1) mAb was provided by Dr. G. Trinchieri (The Wistar Institute, Philadelphia, PA). Anti-CD56 (C218) mAb was provided by Dr. A. Moretta (University of Genoa, Genoa, Italy). Anti-β1 (4B4) was purchased from Coulter Corp. (Hialeah, FL). The antiphosphotyrosine (anti-pTyr) mAb 4G10 was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). The anti-Grb2 and anti-Shc mAbs and the affinity-purified rabbit anti-Shc antiserum were obtained from Transduction Laboratories (Lexington, KY). The affinity-purified antiserum against Erk2 was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The anti-Ras (Y13-259) mAb and the rabbit antiserum against the synthetic peptides corresponding to residues 684– 762 of Pyk-2 (600) were provided by Dr. J. Schlessinger (New York University Medical Center, New York). The affinity-purified rabbit antiserum against mouse Ig was purchased from Zymed Laboratories, Inc. (South San Francisco, CA). Affinity-purified (Fab′)2 fragments of goat anti–mouse Ig (GAM) were purchased from Cappel Laboratories (Organon Teknika, West Chester, PA). Glutathione S-transferase (GST)–Jun fusion protein coupled to glutathione agarose beads was a gift from Dr. C.J. Der (University of North Carolina at Chapel Hill, NC).
Human plasma FN was purchased from GIBCO BRL (Gaithersburg, MD), and the 120- and 40-kD proteolytic fragments of FN were purchased from Chemicon International, Inc. (Temecula, CA).
Human NK Cell Preparation.
Cultured NK cells were obtained by incubating for 10 d nylon nonadherent PBMC (4 × 105 cells) with irradiated (3,000 rads) RPMI 8866 cells (105) as described previously (38). On day 10, contaminating T cells were eliminated by negative panning with anti-CD5 mAb, and the resulting NK cell population was ∼95% CD16+CD56+CD3−CD14− as assessed by immunofluorescence and cytofluorimetric analysis.
Cell Stimulation.
Human NK cells were resuspended in RPMI 1640 serum-free medium (50 × 106 cells/300 μl/tube) and incubated with saturating doses of the appropriate mAb for 30 min at 4°C. After washing off the unbound antibody, cells were resuspended in prewarmed RPMI 1640 medium and incubated for different time periods with polystyrene beads (2.5-μm diameter; Interfacial Dynamics Corporation, Portland, OR) coated with GAM (1.5 μg/106 cells) at 37°C (41). In some experiments, human NK cells were resuspended in RPMI 1640 serum-free medium (50 × 106/300 μl/tube) and incubated for different time periods with polystyrene beads coated with human plasma FN or its proteolytic fragments of 120 and 40 kD or with BSA. When indicated, cells were pretreated (30 min at 37°C) with MEK-1 inhibitor PD 098059 (Calbiochem-Novabiochem, La Jolla, CA).
Immunoprecipitation and Immunoblot Analysis.
To estimate Ras activation, human NK cells were starved for 3 h in phosphate-free DMEM and labeled for 3 h with [32P]orthophosphate (0.5 mCi/ml, 4,500 Ci/mmol; ICN Biomedicals, Inc., Irvine, CA) in phosphate-free DMEM supplemented with 0.1% phosphate-free FCS. After stimulation, the cells were extracted and the immunoprecipitated samples subjected to Ras-GTP loading assay as described previously (19). Nucleotides bound to Ras were analyzed by TLC on polyethyleneimine-cellulose plates in 0.75 M K2HPO4, pH 3.5. Radioactivity in GDP and GTP was estimated by PhosphorImager analysis (Molecular Dynamics, Sunnyvale, CA).
To immunoprecipitate Shc and Pyk-2, stimulated and unstimulated human NK cells were extracted in Triton lysis buffer (50 mM Hepes, pH 7.5, 150 mM NaCl, 1% Triton X-100) containing 1 mM sodium orthovanadate, 2.5 mM sodium pyrophosphate, 25 mM sodium fluoride, 0.01% aprotinin, 4 mg/ml pepstatin A, 10 mg/ml leupeptin, and 1 mM PMSF (all from Sigma Chemical Co., St. Louis, MO) for 30 min on ice. Immunoprecipitation, SDS-PAGE, and immunoblotting analysis were performed as described previously (41). Nitrocellulose-bound antibodies were detected by enhanced chemiluminescence (ECL; Nycomed Amersham plc, Little Chalfont, Bucks, UK).
In Vitro Kinase Assay.
To examine Erk activity, cells were extracted with NP-40 lysis buffer (50 mM Hepes, pH 7.5, 150 mM NaCl, 1% NP-40, 1 mM EDTA) containing phosphatase and protease inhibitors for 30 min on ice. Endogenous Erk2 was immunoprecipitated with anti-Erk2 antibodies and subjected to in vitro kinase assay. The kinase reaction was initiated by adding to the immunoprecipitate 25 μl of kinase buffer (25 mM Tris, pH 7.5, 12.5 mM β-glycerophosphate, 7.5 mM MgCl2, 20 μM cold ATP, 0.5 mM sodium orthovanadate) containing 5 μCi of [γ- 32P]ATP (4,500 Ci/mmol; ICN Biomedicals, Inc.) and 2.5 μg of myelin basic protein (MBP; Sigma Chemical Co.). After 30 min of incubation at 30°C, the samples were boiled in sample buffer and separated by SDS-PAGE. The gels were dried, and the 32P-labeled proteins were made visible by autoradiography.
To analyze the activation of Jnk, cells were extracted for 30 min on ice with modified Triton lysis buffer (25 mM Hepes, pH 7.5, 300 mM NaCl, 0.1% Triton X-100, 0.2 mM EDTA, 20 mM β-glycerophosphate, 1.5 mM MgCl2, 0.5 mM dithiothreitol) containing phosphatase and protease inhibitors. Endogenous Jnk was precipitated with 3 μg of GST–Jun fusion protein coupled to glutathione agarose beads. After washing, the beads were incubated with 25 μl of kinase buffer containing 5 μCi of [γ-32P]ATP (4,500 Ci/mmol; ICN Biomedicals, Inc.). After 30 min of incubation at 30°C, the samples were boiled in sample buffer and separated by SDS-PAGE. The gels were dried, and the 32P-labeled proteins were made visible by autoradiography.
Northern Blot Analysis.
Total RNA was extracted using RNAfast (Molecular Systems, San Diego, CA), size-fractionated in 1% agarose/formaldehyde gels, transferred by capillarity onto nitrocellulose filters, baked at 80°C for 2 h, and hybridized to cDNA probes specific for human IFN-γ and β-actin. cDNA probes were labeled with [α-32P]dCTP (3,000 Ci/mmol; ICN Biomedicals, Inc.) by random priming (Amersham Pharmacia Biotech, Uppsala, Sweden). RNA was visualized in ethidium bromide– stained gels, and the amount of RNA loaded was determined after hybridization to β-actin cDNA. Filters were exposed to X-AR film (Eastman Kodak, Rochester, NY) for autoradiography. Levels of expression of each mRNA species were quantitated as above.
IFN-γ Production Assay.
Unstimulated or stimulated NK cells were seeded in duplicate wells of flat-bottomed plates (107 cells/ well; Costar Corp., Cambridge, MA) in medium containing 1% BSA. After incubation (24 h, 37°C in a 5% CO2 atmosphere), cell-free supernatants were collected. IFN-γ concentration was quantitated with an ELISA kit (EuroClone, Torquay, Devon, UK).
Results
Activation of Ras upon Cross-linking of β1 Integrins on Human NK Cells.
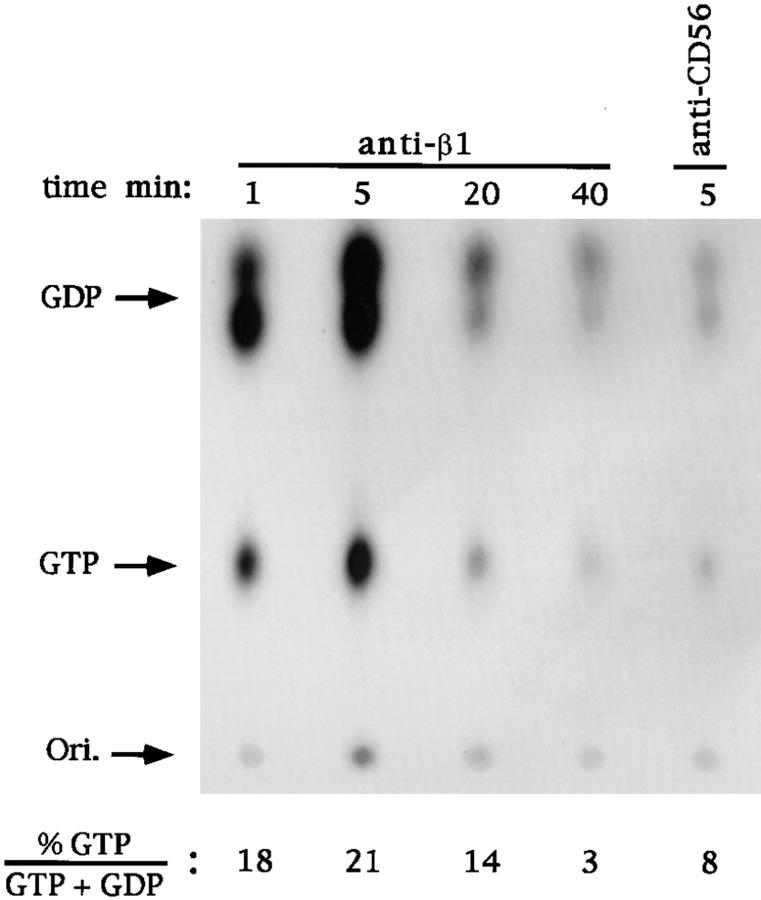
GTP-loading experiments were performed to examine if ligation of β1 integrins on human NK cells results in activation of Ras. After in vivo labeling with [32P]orthophosphate, human NK cells were incubated with saturating concentrations of anti-β1 or anti-CD56 control mAb and stimulated for the indicated times at 37°C with polystyrene beads coated with GAM F(ab′)2 fragments. As shown in Fig. 1, chromatographic analysis of nucleotides bound to Ras indicated that β1 integrin stimulation results in a threefold increase in the proportion of GTP-bound Ras (from 8 to 21%). Ras activation was rapid, peaking at 5 min and returning to basal levels after 40 min. Anti-CD56 mAb–treated control samples showed a p21 Ras GTP to GDP plus GTP ratio comparable to that of the untreated sample (not shown).
Figure 1.
Kinetics of Ras activation by triggering of human NK cells via β1 integrins. TLC of the nucleotides eluted from p21ras immunoprecipitates of [32P]orthophosphate-labeled NK cells. Cells were stimulated for the indicated times with appropriate doses of anti-β1 (4B4) or anti-CD56 (C218) mAb at 37°C before lysis, and immunoprecipitation of p21ras with Y13-259 mAb was performed as described (reference 19). The positions at which GTP and GTP standards run are indicated, as well as origin position (Ori.). Numbers indicate the molar ratio of GTP to total nucleotides from quantitation by direct scanning for β radiation of the same experiment. These results are representative of three independent experiments.
These results indicate that cross-linking of β1 integrins on human NK cells causes activation of Ras.
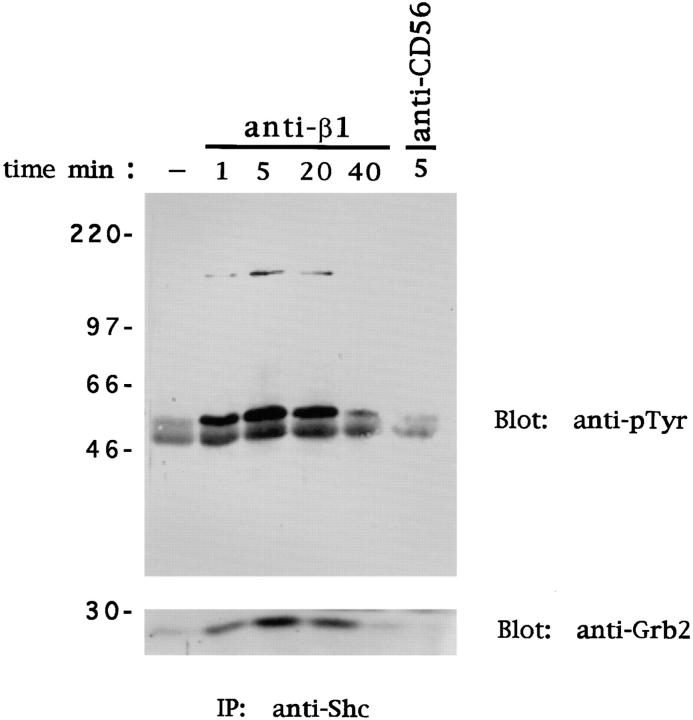
Cross-linking of β1 Integrins on Human NK Cells Induces Shc Tyrosine Phosphorylation and Grb2 Association.
Integrin-induced Ras activation involves tyrosine phosphorylation of the adaptor protein Shc and Shc-mediated recruitment of the adaptor protein Grb2 (18, 19). Since Grb2 is stably associated with the Ras-GTP exchange factor mSos, the association of Grb2 with Shc is likely to bring mSos in close proximity to its target Ras (42). To investigate whether ligation of β1 integrins on NK cells results in tyrosine phosphorylation of Shc, human NK cells were stimulated with anti-β1 or anti-CD56 mAb as above. Anti-Shc immunoprecipitates were examined by immunoblotting with anti-pTyr mAb. Marked tyrosine phosphorylation of both the 46- and 52-kD isoforms of Shc was detected after β1 integrin cross-linking (Fig. 2). β1 integrin–mediated Shc phosphorylation was rapid and persistent, in that it peaked at 5 min and declined at 40 min. No changes in the phosphorylation status of Shc were observed in untreated or anti-CD56 mAb–treated NK cells used as control. Interestingly, an additional tyrosine-phosphorylated protein of ∼145 kD was consistently coimmunoprecipitated with Shc from β1 integrin–stimulated NK cell lysates; tyrosine phosphorylation of Shc and p145 occurred with the same kinetics (Fig. 2). The nature of this protein has not been investigated, but it is likely to correspond to one of the isoforms of the lipid phosphatase Ship, which combines with the Shc phosphotyrosine-binding (PTB) domain upon tyrosine phosphorylation (43). To examine the possibility that tyrosine-phosphorylated Shc associates with the SH2 domain–containing adaptor protein Grb2, Shc immunoprecipitates were immunoblotted with an anti-Grb2 antibody. The results indicate that Grb2 forms a complex with Shc in β1 integrin–stimulated NK cells. No Shc–Grb2 association was observed in NK cells untreated or treated with anti-CD56 mAb used as control (Fig. 2).
Figure 2.
Ligation of β1 integrins on NK cells induces tyrosine phosphorylation of Shc and recruitment of Grb2. Human NK cells were stimulated with control medium or anti-β1 (4B4) or anti-CD56 (C218) mAb for the indicated times at 37°C. Cell lysates (50 × 106 cells/sample) were subjected to immunoprecipitation (IP) with anti-Shc polyclonal antibody. The resulting protein complexes were resolved by 10% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with either anti-pTyr (4G10) or anti-Grb2 antibody. Sizes are indicated in kilodaltons. These results are representative of three independent experiments.
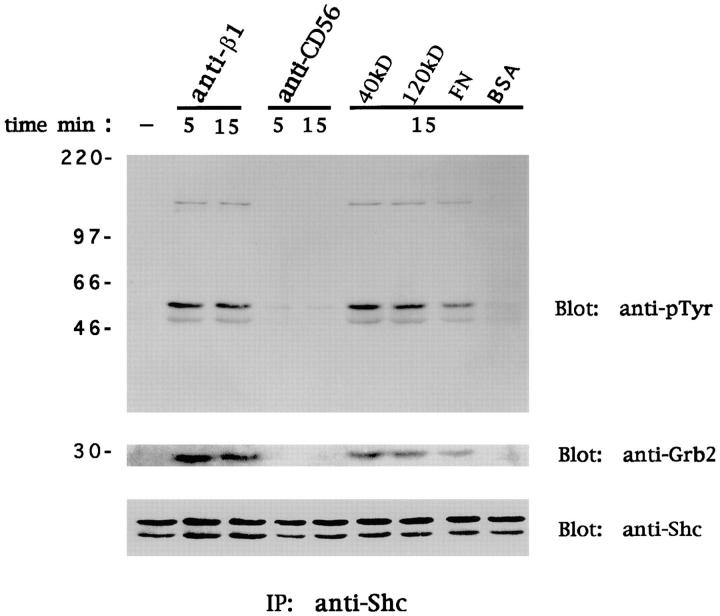
Similar data were obtained upon cross-linking of α4β1 and α5β1 FN receptors with polystyrene beads coated with FN or its 120- and 40-kD proteolytic fragments recognized by α5β1 and α4β1, respectively (Fig. 3). These results indicate that cross-linking of β1 integrin FN receptors on human NK cells induces Shc tyrosine phosphorylation and its association with Grb2. The time course of Shc phosphorylation and Grb2 association strictly parallels the β1 integrin–mediated p21 Ras activation, suggesting that the formation of the Shc-Grb2 complex is important for Ras activation.
Figure 3.
Ligation of β1 integrin FN receptors on NK cells induces tyrosine phosphorylation of Shc and recruitment of Grb2. Human NK cells were stimulated with control medium, anti-β1 (4B4) or anti-CD56 (C218) mAb, FN or its 120- and 40-kD proteolytic fragments, or BSA for the indicated times at 37°C. Cell lysates (50 × 106 cells/sample) were subjected to immunoprecipitation (IP) with anti-Shc polyclonal antibody. The resulting protein complexes were resolved by 10% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-pTyr (4G10), anti-Grb2, or anti-Shc antibody. Sizes are indicated in kilodaltons. These results are representative of three independent experiments.
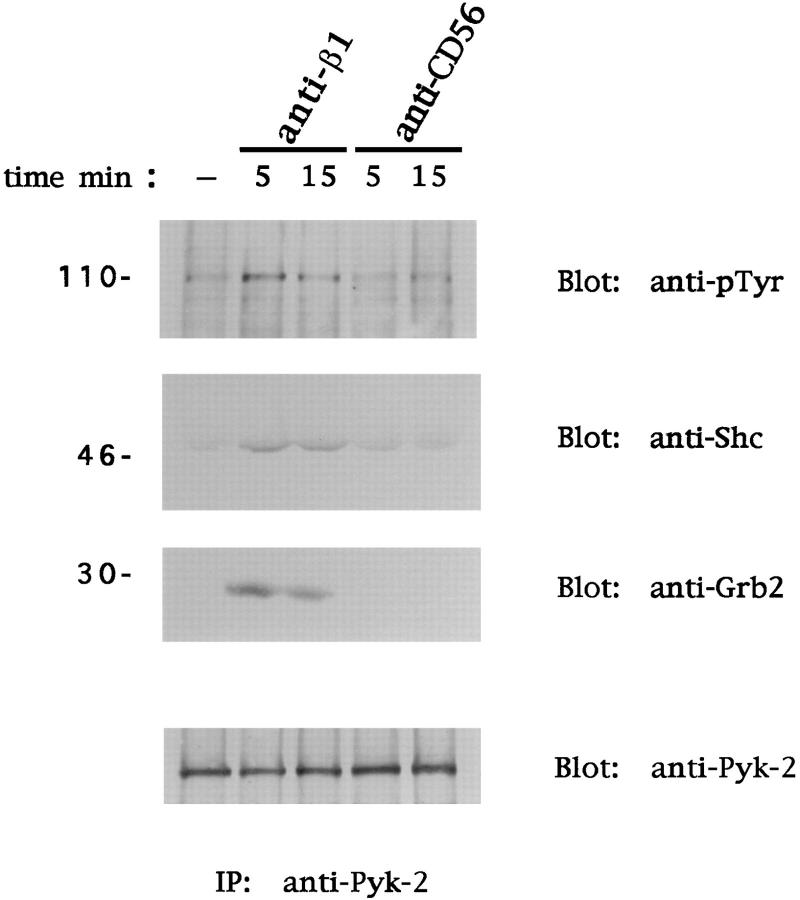
Cross-linking of β1 Integrins on Human NK Cells Induces Shc and Grb2 Association with Pyk-2.
The ability of the Fak family members to combine with Grb2 upon integrin engagement has been shown, and a role for p125Fak in the integrin-mediated activation of the Ras-MAPK cascade has been suggested (20, 21). We reported recently that human peripheral blood NK cells express Pyk-2 and not p125Fak, and that ligation of β1 FN receptors results in Pyk-2 activation (39). To investigate whether Pyk-2 can associate with Shc and Grb2 upon β1 integrin cross-linking, human NK cells were stimulated with anti-β1 or anti-CD56 mAb as above. Pyk-2 immunoprecipitates were then analyzed for the presence of Shc and Grb2 by immunoblotting with specific mAbs. As shown in Fig. 4, the 46-kD isoform of Shc and the adaptor protein Grb2 were detected in the Pyk-2 immunoprecipitates after β1 integrin cross-linking. Shc and Grb2 association to Pyk-2 was observed at 5 min and was still evident at 15 min; formation of this complex parallels Pyk-2 tyrosine phosphorylation. No association of Shc and Grb2 with Pyk-2 was observed in untreated or control mAb–treated NK cells.
Figure 4.
Cross-linking of β1 integrins on human NK cells induces association of Pyk-2 with Shc and Grb2. Human NK cells were stimulated with control medium or anti-β1 (4B4) or anti-CD56 (C218) mAb for the indicated times at 37°C. Cell lysates (50 × 106 cells/sample) were subjected to immunoprecipitation (IP) with anti–Pyk-2 polyclonal antibody. The resulting protein complexes were resolved by 8% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-pTyr (4G10), anti-Shc, anti-Grb2, or anti–Pyk-2 antibody. Sizes are indicated in kilodaltons. These results are representative of three independent experiments.
These results suggest that tyrosine-phosphorylated Pyk-2 forms a complex with Shc and Grb2 upon stimulation of NK cells through β1 integrins, suggesting that Pyk-2 may cooperate with the Shc–Grb2 complex to fully activate Ras in response to integrin ligation.
Ligation of β1 Integrins on Human NK Cells Causes Activation of MAPK Signaling Pathway.
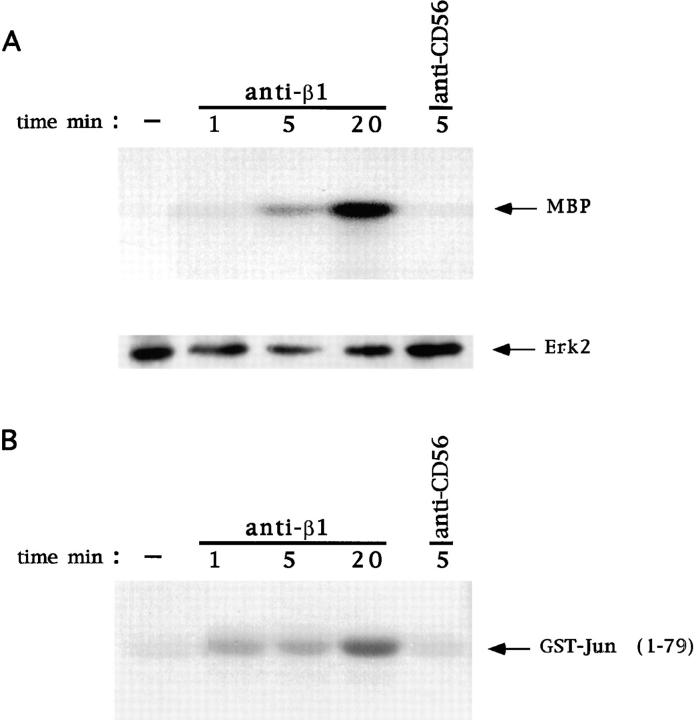
We next examined if ligation of β1 integrins on human NK cells results in activation of Erk and Jnk. Human NK cells were stimulated with anti-β1 or anti-CD56 mAb and subjected to Erk2 and Jnk kinase assay. As shown in Fig. 5, β1 integrin stimulation causes a significant activation of Erk2 (A) and Jnk (B). This activation is already evident at 1 min and still persistent at 20 min. In contrast, anti-CD56 control mAb treatment did not result in any significant activation of Erk2 or Jnk.
Figure 5.
Activation of Erk2 and Jnk by β1 integrin cross-linking on human NK cells. Human NK cells were stimulated with control medium or anti-β1 (4B4) or anti-CD56 (C218) mAb for the indicated times at 37°C. (A) Cell lysates (50 × 106 cells/sample) were immunoprecipitated with anti-Erk2 polyclonal antibody and subjected to in vitro kinase assay using MBP as substrate. The resulting protein complexes were resolved by 15% SDS-PAGE. The position of phosphorylated MBP is indicated. The amount of Erk2 immunoprecipitated from each sample was evaluated by immunoblotting with anti-Erk2 antibody. (B) Jnk was precipitated by using glutathione beads coated with GST–Jun fusion protein and subjected to in vitro kinase assay. The resulting protein complexes were resolved by 10% SDS-PAGE. The position of phosphorylated GST–Jun is indicated. These results are representative of three independent experiments.
These data indicate that ligation of β1 integrins on human NK cells causes a significant and persistent activation of Erk and Jnk.
Ligation of β1 Integrins on Human NK Cells Stimulates IFN-γ Production and Requires Erk2 Activation.
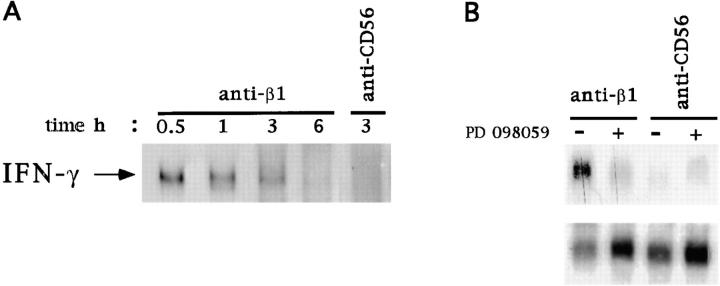
We first examined if ligation of β1 integrins on human NK cells can affect IFN-γ mRNA expression. Northern blot analysis was performed by using total RNA from human NK cells stimulated with anti-β1 or anti-CD56 mAb. Fig. 6 shows that β1 integrin ligation induces IFN-γ mRNA expression, which was maximal at 30 min and declined at 3 h after stimulation. Treatment of human NK cells with anti-CD56 mAb used as control did not influence IFN-γ mRNA expression (Fig. 6 A).
Figure 6.
Erk2 activation controls β1 integrin–induced IFN-γ mRNA expression in human NK cells. (A) Total RNA was extracted from human NK cells stimulated with anti-β1 (4B4) or anti-CD56 (C218) mAb for the indicated times at 37°C. Northern blot analysis was performed using a cDNA probe specific for human IFN-γ. (B) Human NK cells were incubated for 30 min at 37°C with 30 μM PD 098059 (+) or with DMSO (vehicle; −) and then stimulated with anti-β1 (4B4) or anti-CD56 (C218) mAb for 1 h. Total RNA was extracted and subjected to Northern blot analysis using cDNA probes specific for human IFN-γ (top) or β-actin (bottom).
To investigate whether β1 integrin–induced IFN-γ mRNA expression is under the control of Erk activation, we used the synthetic inhibitor PD 098059, which specifically prevents activation of the Erk-activating kinase MEK-1 (44, 45). PD 098059, at the 50 μM concentration that completely inhibits β1 integrin–induced Erk2 activation (not shown), abrogated the stimulation of IFN-γ mRNA expression. The same concentration of PD 098059 did not affect IFN-γ mRNA levels in human NK cells treated with anti-CD56 control mAb (Fig. 6 B).
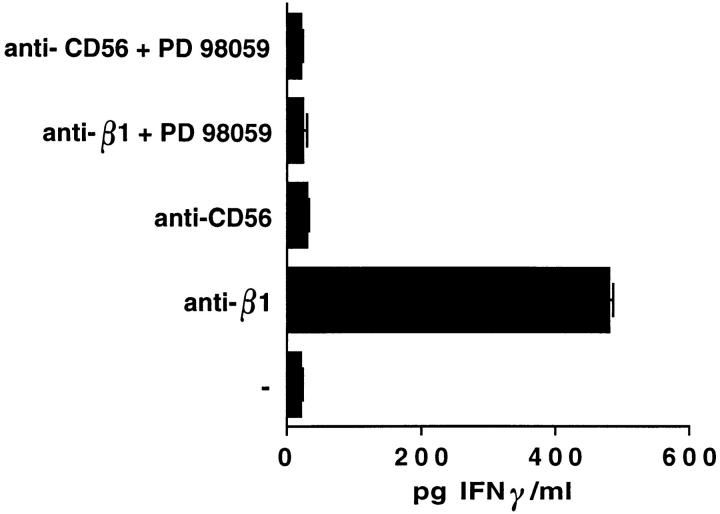
Finally, we evaluated if ligation of β1 integrins on human NK cells stimulates IFN-γ production by testing the presence of this cytokine in the supernatants of NK cells, either untreated or treated for 24 h at 37°C with anti-β1 or anti-CD56 control mAb. As shown in Fig. 7, β1 integrin cross-linking resulted in induction of IFN-γ production at levels comparable to those induced by CD16 engagement (not shown). In contrast, treatment of human NK cells with anti-CD56 mAb used as control did not stimulate IFN-γ production.
Figure 7.
Erk2 activation controls β1 integrin–induced IFN-γ cytokine production in human NK cells. Human NK cells were incubated for 30 min at 37°C with 30 μM PD 098059 (+) or with DMSO (vehicle; −) and then stimulated with anti-β1 (4B4) or anti-CD56 (C218) mAb for 24 h at 37°C in a 5% CO2 atmosphere in duplicate wells of flat-bottomed plates (107 cells/well) in medium containing 1% BSA. After incubation, cell-free supernatants were collected. IFN-γ concentration in the supernatants was quantitated by ELISA.
The induction of IFN-γ synthesis required the activity of Erk2 as shown by the ability of PD 098059 to completely inhibit β1 integrin–induced cytokine production (Fig. 7).
These findings indicate that ligation of β1 integrins on human NK cells stimulates IFN-γ production, and that this event requires Erk2 activation.
Discussion
The ability of integrins to cause activation of Ras has been previously described in several adherent primary cells and cell lines (18, 19). In regard to the immune system, there is only one report showing that α2β1 triggers Ras activation in Jurkat T leukemia cells (46). Here, we demonstrate that β1 integrin ligation on human peripheral blood NK cells results in activation of the Ras/MAPK pathway, which is involved in integrin-triggered IFN-γ production. In addition, we provide information on the receptor-proximal events accompanying the β1 integrin–mediated Ras activation, namely tyrosine phosphorylation of Shc and its association with Grb2, and the association of tyrosine-phosphorylated Pyk-2 with Grb2 and Shc.
Tyrosine phosphorylation of both the 46- and 52-kD isoforms of Shc was observed after mAb-mediated cross-linking of β1 integrins, as well as upon interaction of NK cells with FN and its proteolytic 120- and 40-kD fragments, which are recognized by α5β1 and α4β1, respectively. The time course of β1 integrin–triggered Shc phosphorylation strictly paralleled Ras activation, suggesting that the formation of the Shc–Grb2 complex is important in this event.
These results are in accordance with previous studies indicating the involvement of the Shc–Grb2 complex in Ras activation upon engagement of αvβ3, β4, or several β1 integrins on various adherent cell types (18, 19, 41).
Integrin-mediated activation of Ras has also been suggested to involve the nonreceptor tyrosine kinase p125Fak, and the formation of the Fak–Grb2–mSos complex upon cell adhesion to FN has been described (20, 22). No data are available yet on the involvement of the recently discovered member of the Fak family, Pyk-2, in the activation of Ras through integrins, although the association of Pyk-2 with Grb2 has been observed in FN-activated COS cells (47). However, recent observations indicate that Pyk-2 regulates Ras in response to neurotransmitters, inflammatory cytokines, and cellular stresses by recruiting the Grb2– mSos complex to the plasma membrane (48). We have recently demonstrated that human peripheral blood NK cells express Pyk-2 but not p125Fak, and that tyrosine phosphorylation of Pyk-2 occurs rapidly after ligation of β1 integrins by natural ligands or specific mAbs (39). Here we show that integrin ligation induces association of tyrosine-phosphorylated Pyk-2 with Grb2 and Shc. Interestingly, only the 46-kD isoform of Shc was detected in Pyk-2 immunoprecipitates, suggesting that the p46 and p52 Shc may have different functional properties. In line with this hypothesis, it has been reported that p66 Shc, which is mainly expressed in epithelial cells, mediates opposite effects on the epidermal growth factor (EGF) receptor/MAPK/Fos signaling pathways with respect to the other splicing isoforms (49).
Overall, our results suggest that the formation of the Shc–Grb2 and Shc–Pyk-2–Grb2 complexes may couple β1 integrins to the Ras pathway in human NK cells. The relative contribution of Shc–Grb2 and Shc–Pyk-2–Grb2 complexes in the signaling events leading to integrin-mediated activation of Ras is currently unknown. Upon integrin engagement, both tyrosine-phosphorylated Shc and Pyk-2 may bind to the SH2 domain of Grb2 (22, 48, 50); whether Pyk-2 interacts directly with Shc has not yet been defined. Knowledge of the molecular basis regulating the association of Shc with Grb2-associated Pyk-2 is necessary for a better understanding of whether Shc and Pyk-2 represent additive complementary pathways required to fully activate Ras in response to integrin ligation, or if one of these pathways is dominant and the other redundant.
The results of this study also indicate that ligation of β1 integrins on human NK cells stimulates the enzymatic activities of both Erk2 and Jnk, the last kinases of MAPK cascades controlled by Ras activation.
Activation of Erk and Jnk in response to integrin stimulation has been reported previously (17–19, 51). However, the majority of these studies were performed in adherent cell types, including primary human keratinocytes, endothelial cells, and 3T3 fibroblasts. In the immune system, it has been reported that α4β1 costimulates (pp45) MAPK activation in resting T cells (52), but no evidence is available on the ability of integrins to activate Jnk.
Control of Erk activation has been shown to involve Shc and Ras as indicated by the ability of their dominant negative forms to prevent Erk2 activation in response to cell adhesion to fibronectin or laminin (18, 19, 53). However, Ras-independent signaling events have also been implicated in control of MAPK activation (54). Jnk activation upon integrin ligation involves multiple pathways, since it is prevented by either Ras and Rac dominant negative forms or the phosphatidylinositol 3-kinase inhibitor, wortmannin (19). Overall, it is conceivable that Ras activation may be a crucial event controlling β1 integrin–triggered MAPK cascades in NK cells.
Finally, we provide evidence that ligation of β1 integrins on human NK cells results in the stimulation of IFN-γ mRNA expression and production, which are under the control of Erk2 activation as shown by the use of the specific MEK-1 inhibitor, PD 98059. Similarly, a role for Erk2 activation in the control of IFN-γ synthesis in T cells triggered through the TCR complex has been recently demonstrated (55). The involvement of Erk in the regulation of IFN-γ production may be related to its ability to activate c-Fos transcription and to regulate the formation of the activating protein (AP)-1 heterodimer (56), which is involved in the control of the IFN-γ gene promoter (57, 58). In support of this hypothesis, several studies indicate that integrins activate several transcriptional factors (25, 31, 59, 60); furthermore, recent studies in α4β1-stimulated monocytes have demonstrated an association between Erk activation and nuclear factor (NF)-κB translocation, which leads to the expression of the tissue factor gene (61).
Our data indicate that β1 integrins, by delivering signals capable of triggering IFN-γ production, may function as NK-activating receptors. In a previous study, we have shown that cross-linking of α4β1 and α5β1 by specific mAbs or by the natural ligands vascular cell adhesion molecule 1 (VCAM-1) and FN costimulates but does not trigger natural cytotoxicity (40). Taken together, these results suggest that different receptor signaling thresholds and/or different signaling events are required to elicit distinct NK cell functions: it is possible that β1 integrin–mediated signaling does not reach the threshold for induction of NK cytotoxicity, or that β1 integrins are unable to transmit the signal(s) required for the activation of the NK lytic program. In this regard, ligation of β1 integrins on NK cells does not stimulate phospholipase Cγ (PLC-γ) activation and inositol-1,4,5-triphosphate (PtdIns(1,4,5)P3 generation [Milella, M., personal communication]), a signaling pathway triggered by the NK receptors capable of activating the cytotoxic function. Furthermore, in accordance with this evidence, certain target cell lines resistant to NK cell lysis and unable to stimulate detectable phosphoinositide turnover were found to be capable of inducing IFN-γ production, although the basis of this phenomenon remains unclear (Perussia, B., personal communication).
What is the pathophysiological relevance of IFN-γ produced by NK cells in response to β1 integrin stimulation? β1 integrins have been shown to control NK cell adhesion to endothelial cells and migration of NK cells into the normal and neoplastic tissues (62–64). Based on our data, it can be hypothesized that IFN-γ produced by NK cells upon interaction with activated endothelium or extracellular matrix components may contribute to affect the local inflammatory reaction by regulating several functions of endothelial cells, including expression of ICAM-1 and VCAM-1 adhesion receptors, cytokine release, and nitric oxide production (65, 66). Finally, IFN-γ production by NK cells in response to β1 integrins may be relevant for the antiviral activity exerted by the NK cells at the tissue level, in particular in the liver. It has been recently reported (67) that NK cells control murine CMV infection in the liver by secreting IFN-γ, which exerts its antiviral action through the induction of nitric oxide synthesis; it has also been suggested that unlike in the spleen, NK cell–dependent resistance to murine CMV infection in this organ does not require NK cell contact with virus-modified target cells. Thus, integrins may be indicated as one of the receptors triggering IFN-γ synthesis by liver NK cells which closely interact with the endothelial cells in the hepatic sinusoids.
Acknowledgments
We thank Dina Milana, Anna Maria Bressan, Alessandro Procaccini, Antonio Sabatucci, and Patrizia Birarelli for expert technical assistance, and Ilio Piras for photographic assistance. We also thank Dr. F.G. Giancotti for carefully reviewing the manuscript.
Abbreviations used in this paper
- Erk
extracellular signal–regulated kinase
- FN
fibronectin
- Fak
focal adhesion kinase
- GAM
goat anti–mouse IgG F(ab′)2
- Grb2
growth factor–bound protein 2
- GST
glutathione S-transferase
- Jnk
c-Jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- MEK
MAPK kinase
- Pyk-2
proline-rich tyrosine kinase 2
Footnotes
This work was partially supported by grants from the Italian Association for Cancer Research (AIRC), Istituto Superiore di Sanità Italy-USA “Therapy of Tumors” Program, Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST) 40% and 60%, Ministero della Sanità, and by a Consiglio Nazionale delle Ricerche special project on Biotechnologies.
References
- 1.Scott P, Trinchieri G. The role of natural killer cells in host-parasite interactions. Curr Opin Immunol. 1995;7:34–40. doi: 10.1016/0952-7915(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 2.Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. Natural killer cells: from no receptors to too many. Immunity. 1997;6:371–378. doi: 10.1016/s1074-7613(00)80280-0. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL, Corliss B, Phillips JH. Arousal and inhibition of human NK cells. Immunol Rev. 1997;155:145–154. doi: 10.1111/j.1600-065x.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 5.Long EO, Wagtmann N. Natural killer cell receptors. Curr Opin Immunol. 1997;9:344–350. doi: 10.1016/s0952-7915(97)80080-5. [DOI] [PubMed] [Google Scholar]
- 6.Leibson PJ. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity. 1997;6:655–661. doi: 10.1016/s1074-7613(00)80441-0. [DOI] [PubMed] [Google Scholar]
- 7.Biassoni R, Cantoni C, Falco M, Verdiani S, Bottino C, Vitale M, Conte R, Poggi A, Moretta A, Moretta L. The human leukocyte antigen (HLA)-C–specific “activatory” or “inhibitory” natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J Exp Med. 1996;183:645–650. doi: 10.1084/jem.183.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason LH, Anderson SK, Yokoyama WM, Smith HRC, Winkler-Pickett R, Ortaldo JR. The Ly-49D receptor activates murine natural killer cells. J Exp Med. 1996;184:2119–2128. doi: 10.1084/jem.184.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houchins JP, Lanier LL, Niemi EC, Phillips JH, Ryan JC. Natural killer cell cytolytic activity is inhibited by NKG2-A and activated by NKG2-C. J Immunol. 1997;158:3603–3609. [PubMed] [Google Scholar]
- 10.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 11.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 12.Giancotti FG, Mainiero F. Integrin-mediated adhesion and signaling in tumorigenesis. Biochim Biophys Acta. 1994;1198:47–64. doi: 10.1016/0304-419x(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 14.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 15.Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 16.Defilippi, P., A. Gismondi, A. Santoni, and G. Tarone. 1997. Signal transduction by integrins. In Landes Bioscience. Springer Publishing Company, Austin, TX. 182 pp.
- 17.Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 18.Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:1–11. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 19.Mainiero F, Murgia C, Wary KK, Pepe A, Blumemberg M, Westwick JK, Der CJ, Giancotti FG. The coupling of α6β4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO (Eur Mol Biol Organ) J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlaepfer DD, Hanks SK, Hunter T, Van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 21.Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the GRB2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlaepfer DD, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs? . Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- 23.Eierman DF, Johnson CE, Haskill JS. Human monocyte inflammatory mediator gene expression is selectively regulated by adherence substrates. J Immunol. 1989;142:1970–1976. [PubMed] [Google Scholar]
- 24.Sporn SA, Eierman DF, Johnson CE, Morris J, Martin G, Ladner M, Haskill S. Monocyte adherence results in selective induction of novel genes sharing homology with mediators of inflammation and tissue repair. J Immunol. 1990;144:4434–4441. [PubMed] [Google Scholar]
- 25.Haskill S, Beg AA, Tompkins SM, Morris JS, Yurochko AD, Sampson-Johannes A, Mondal K, Ralph P, Baldwin AS., Jr Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell. 1991;65:1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- 26.Pacifici R, Basilico C, Roman J, Zutter MM, Santori SA, McCracken R. Collagen-induced release of interleukin 1 from human blood mononuclear cells. Potentiation by fibronectin binding to the α5β1 integrin. J Clin Invest. 1992;89:61–67. doi: 10.1172/JCI115586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hershkoviz R, Gilat D, Miron S, Mekori YA, Aderka D, Wallach D, Vlodavsky I, Cohen IR, Lider O. Extracellular matrix induces tumor necrosis factor-α secretion by an interaction between resting rat CD4+T cells and macrophages. J Immunol. 1993;78:50–57. [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts K, Yokoyama WM, Kehn PJ, Shevach EM. The vitronectin receptor serves as an accessory molecule for the activation of a subset of γ/δ T cells. J Exp Med. 1991;173:231–240. doi: 10.1084/jem.173.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi K, Nakamura T, Adachi H, Yagita H, Okumura K. Antigen-independent T cell activation mediated by very late activation antigen-like extracellular matrix receptor. Eur J Immunol. 1991;21:1559–1562. doi: 10.1002/eji.1830210634. [DOI] [PubMed] [Google Scholar]
- 30.Wacholtz MC, Patel SS, Lipsky PE. Leukocyte function–associated antigen 1 is an activation molecule for human T cells. J Exp Med. 1989;170:431–448. doi: 10.1084/jem.170.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada A, Nikaido T, Nojima Y, Schlossman SF, Morimoto C. Activation of human CD4 T lymphocytes. Interaction of fibronectin with VLA-5 receptor on CD4 cells induces the AP-1 transcription factor. J Immunol. 1991;146:53–56. [PubMed] [Google Scholar]
- 32.Van Seventer GA, Newman W, Shimizu Y, Nutman TB, Tanaka Y, Horgan KJ, Gopal TV, Ennis E, O'Sullivan D, Grey H, Shaw S. Analysis of T cell stimulation by superantigen plus major histocompatibility complex class II molecules or by CD3 monoclonal antibody: costimulation by purified adhesion ligands VCAM-1, ICAM-1, but not ELAM-1. J Exp Med. 1991;174:901–913. doi: 10.1084/jem.174.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semnani RT, Nutman TB, Hochman P, Shaw S, Van Seventer GA. Costimulation by purified intercellular adhesion molecule 1 and lymphocyte function–associated antigen 3 induces distinct proliferation, cytokine and cell surface antigen profiles in human “naive” and “memory” CD4+ T cells. J Exp Med. 1994;180:2125–2135. doi: 10.1084/jem.180.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Udagawa T, Woodside DG, McIntyre BW. α4β1 (CD49d/CD29) integrin costimulation of human T cells enhances transcription factor and cytokine induction in the absence of altered sensitivity to anti-CD3 stimulation. J Immunol. 1996;157:1965–1972. [PubMed] [Google Scholar]
- 35.Melero I, Balboa M, Alonso JL, Yague E, Pivel JP, Sanchez-Madrid F, Lopez-Botet M. Signaling through the LFA-1 leukocyte integrin actively regulates intercellular adhesion and tumor necrosis factor-α production in natural killer cells. Eur J Immunol. 1993;23:1859–1865. doi: 10.1002/eji.1830230819. [DOI] [PubMed] [Google Scholar]
- 36.Gismondi A, Morrone S, Humphries MJ, Piccoli M, Frati L, Santoni A. Human natural killer cells express VLA-4 and VLA-5, which mediate their adhesion to fibronectin. J Immunol. 1991;146:384–392. [PubMed] [Google Scholar]
- 37.Gismondi A, Mainiero F, Morrone S, Palmieri G, Piccoli M, Frati L, Santoni A. Triggering through CD16 or phorbol esters enhances adhesion of NK cells to laminin via very late antigen 6. J Exp Med. 1992;176:1251–1257. doi: 10.1084/jem.176.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mainiero F, Gismondi A, Milella M, Morrone S, Palmieri G, Piccoli M, Frati L, Santoni A. Long-term activation of NK cells results in modulation of β1-integrin expression and function. J Immunol. 1994;152:446–454. [PubMed] [Google Scholar]
- 39.Gismondi A, Bisogno L, Mainiero F, Palmieri G, Piccoli M, Frati L, Santoni A. PYK-2 tyrosine phosphorylation by β1 integrin fibronectin receptor cross-linking and association with paxillin in human NK cells. J Immunol. 1997;159:4729–4736. [PubMed] [Google Scholar]
- 40.Palmieri G, Serra A, De Maria R, Gismondi A, Milella M, Piccoli M, Frati L, Santoni A. Cross-linking of α4β1 and α5β1 fibronectin receptors enhances natural killer cell cytotoxic activity. J Immunol. 1995;155:5314–5322. [PubMed] [Google Scholar]
- 41.Mainiero F, Pepe A, Wary KK, Spinardi L, Mohammadi M, Schlessinger J, Giancotti FG. Signal transduction by the α6β4 integrin: distinct β4subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO (Eur Mol Biol Organ) J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 43.Lamkin TD, Walk SF, Liu L, Damen JE, Krystal G, Ravichandran KS. Shc interaction with Src homology 2 domain containing inositol phosphatase (SHIP) in vivo requires the Shc-phosphotyrosine binding domain and two specific phosphotyrosines on SHIP. J Biol Chem. 1997;272:10396–10401. doi: 10.1074/jbc.272.16.10396. [DOI] [PubMed] [Google Scholar]
- 44.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 46.Kapron-Bras C, Fitz-Gibbon L, Jeevaratnam P, Wilkins J, Dedhar S. Stimulation of tyrosine phosphorylation and accumulation of GTP-bound p21rasupon antibody- mediated α2β1 integrin activation in T-lymphoblastic cells. J Biol Chem. 1993;268:20701–20704. [PubMed] [Google Scholar]
- 47.Li J, Avraham H, Rogers RA, Raja S, Avraham S. Characterization of RAFTK, a novel focal adhesion kinase, and its integrin-dependent phosphorylation and activation in megakaryocytes. Blood. 1996;88:417–428. [PubMed] [Google Scholar]
- 48.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+- induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 49.Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti-Furga G, Pawson T, Di Fiore PP, Lanfrancone L, Pelicci PG. Opposite effects of the p52shc/p46shc and p66shcsplicing isoforms on the EGF receptors-MAP kinase-fos signalling pathway. EMBO (Eur Mol Biol Organ) J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato T, Tachibana K, Nojima Y, D'Avirro N, Morimoto C. Role of the VLA-4 molecule in T cell costimulation. J Immunol. 1995;155:2938–2947. [PubMed] [Google Scholar]
- 53.Clark EA, Hynes RO. Ras activation is necessary for integrin-mediated activation of extracellular signal-regulated kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem. 1996;271:14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- 54.Chen Q, Lin TH, Der CJ, Juliano RL. Integrin-mediated activation of MEK and mitogen-activated protein kinase is independent of Ras. J Biol Chem. 1996;271:18122–18127. doi: 10.1074/jbc.271.30.18122. [DOI] [PubMed] [Google Scholar]
- 55.Baumgarth N, Egerton M, Kelso A. Activated T cells from draining lymph nodes and an effector site differ in their responses to TCR stimulation. J Immunol. 1997;159:1182–1191. [PubMed] [Google Scholar]
- 56.Treisman R. Regulation of transcription by Map kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 57.Cippitelli M, Sica A, Viggiano V, Ye J, Ghosh P, Birrer MJ, Young HA. Negative transcriptional regulation of the interferon-γ promoter by glucocorticoids and dominant negative mutants of c-Jun. J Biol Chem. 1995;270:12548–12556. doi: 10.1074/jbc.270.21.12548. [DOI] [PubMed] [Google Scholar]
- 58.Penix LA, Sweetser MT, Weaver WM, Hoeffler JP, Kerppola TK, Wilson CB. The proximal regulatory element of the interferon-γ promoter mediates selective expression in T cells. J Biol Chem. 1996;271:31964–31972. doi: 10.1074/jbc.271.50.31964. [DOI] [PubMed] [Google Scholar]
- 59.Shaw RJ, Doherty DE, Ritter AG, Benedict SH, Clark RA. Adherence-dependent increase in human monocyte PDGF(B) mRNA is associated with increases in c-fos, c-jun, and EGR2 mRNA. J Cell Biol. 1990;111:2139–2148. doi: 10.1083/jcb.111.5.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thieblemont N, Haeffner-Cavaillon N, Haeffner A, Cholley B, Weiss L, Kazatchkine MD. Triggering of complement receptors CR1 (CD35) and CR3 (CD11b/ CD18) induces nuclear translocation of NF-κB (p50/p65) in human monocytes and enhances viral replication in HIV- infected monocytic cells. J Immunol. 1995;155:4861–4867. [PubMed] [Google Scholar]
- 61.McGilvray ID, Lu Z, Bitar R, Dackiw APB, Davreux CJ, Rotstein OD. VLA-4 integrin cross-linking on human monocytic THP-1 cells induces tissue factor expression by a mechanism involving mitogen-activated protein kinase. J Biol Chem. 1997;272:10287–10294. doi: 10.1074/jbc.272.15.10287. [DOI] [PubMed] [Google Scholar]
- 62.Allavena P, Paganin C, Martin-Padura I, Peri G, Gaboli M, Dejana E, Marchisio PC, Mantovani A. Molecules and structures involved in the adhesion of natural killer cells to vascular endothelium. J Exp Med. 1991;173:439–448. doi: 10.1084/jem.173.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Somersalo K, Saksela E. Fibronectin facilitates the migration of natural killer cells. Eur J Immunol. 1991;21:35–42. doi: 10.1002/eji.1830210107. [DOI] [PubMed] [Google Scholar]
- 64.Fogler WE, Volker K, McCormick KL, Watanabe M, Ortaldo JR, Wiltrout RH. NK cell infiltration into lung, liver, and subcutaneous B16 melanoma is mediated by VCAM-1/VLA-4 interaction. J Immunol. 1996;156:4707–4714. [PubMed] [Google Scholar]
- 65.Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990;70:427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 66.Karupiah G, Xie QW, Buller RM, Nathan C, Duarte C, MacMicking JD. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 67.Tay CH, Welsh RM. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]