Abstract
Apoptosis induced by Fas (CD95) ligation is frequently lost during tumor progression; however, there is no direct evidence to support an association of Fas loss-of-function with metastatic tumor behavior. To determine whether Fas loss-of-function is critical for acquisition of the metastatic phenotype, we have compared the ability of Fas-sensitive K1735 murine melanomas to form spontaneous lung metastases in wild-type and Fas ligand–deficient mice. Fas-sensitive melanoma clones are highly tumorigenic but rarely metastatic in wild-type syngeneic mice. However, in Fas ligand–deficient mice, both the incidence and number of metastases are increased. These findings provide the first evidence that Fas–Fas ligand interactions can suppress metastasis and that tumor Fas loss-of-function may be causally linked to metastatic progression.
Keywords: Fas ligand, metastasis, melanoma, apoptosis, lung microenvironment
Apoptosis fundamentally regulates tissue homeostasis. Impairment of this process can result in aberrant cellular accumulation and increased tumor incidence (1). Fas (CD95) and Fas ligand (FasL)1 are complementary receptor–ligand proteins that induce apoptosis in many cell types. Fas is constitutively expressed in many tissues, whereas FasL is largely restricted to the spleen, lung, large and small intestine, uterus, testis, and structures of the eye (2, 3). In the lymphoid population, Fas-induced apoptosis has been shown to preserve immunologic tolerance, limit clonal expansion, and maintain immunologic privilege (4). The potential involvement of Fas in the control of malignant disease has been recognized only recently (5–7). Relevant to the current studies, we (8) and others (9, 10) have reported that Fas-mediated apoptosis can be lost during malignant progression as a consequence of Fas downregulation or impaired Fas signaling. Metastasis is the ultimate step in the process of tumor progression and the principal cause of death in cancer patients (11). In this report, we identify Fas loss-of-function as a parameter both necessary and sufficient for acquisition of the metastatic phenotype.
To produce a clinically significant metastasis, tumor cells must undergo a step-by-step sequence of events including tissue invasion at the site of the primary lesion, entrance into blood and lymphatics, avoidance of host immune defenses, survival in the circulation, adhesion to the vascular endothelium, extravasation, and growth at a distant site (12). During the metastatic process, tumor cells are subjected to intense selection pressures such that the majority die (11, 12). Because each step in the metastatic cascade is potentially rate limiting, failure to complete a single step will render a tumor cell nonmetastatic. To determine potential restriction points in the metastatic process, we have previously investigated the K1735 melanoma system comprised of a parental tumor with low metastatic capacity (13) and clonally derived variants displaying enhanced or reduced metastatic properties compared to the parental tumor (14, 15). Using this tumor model, we have shown that viable, genetically tagged nonmetastatic K1735 tumor cells can disseminate from the subcutaneous site, survive transit in the circulation and lymphatics, and extravasate into the lung microenvironment (16). However, once in the lung, the nonmetastatic K1735 tumor cells fail to survive and proliferate, with the subsequent failure to form metastatic foci (16). An expansive body of literature has correlated successful metastatic growth with tumor responsiveness to paracrine and endocrine growth factors (11, 17, 18). However, in light of more recent evidence implicating apoptosis in the control of tumor behavior (1, 5–7), we were led to consider the potential role of tumor responsiveness to apoptosis-inducing factors in the microenvironment. Three lines of circumstantial evidence led us to query whether FasL regulated K1735 metastatic outgrowth in the lung. First, FasL is present in the lung (2) and has been shown to induce apoptosis in activated, Fas+ lymphocytes in vivo in this organ microenvironment (19); second, nonmetastatic and metastatic K1735 variants differ significantly in their survival rate in lung conditioned media in vitro (20); and third, the rate limiting step for K1735 metastasis has been documented to be outgrowth in the lung (16). Taken together, these results point toward a potential role for lung-derived FasL in the control of K1735 tumor growth in the lung by a process involving Fas-mediated apoptosis.
Materials and Methods
Fas Expression on Murine Melanoma Clones.
Hamster anti– murine Fas (Jo2) was purchased from PharMingen (San Diego, CA). PE-conjugated goat anti–hamster antibody was purchased from Caltag Labs. (Burlingame, CA). Indirect staining was carried out as previously described (21) using 106 cells and 0.5 μg anti-Fas or isotype-matched control and a 1:50 dilution secondary antibody. Fas staining was determined on cells that had been fixed overnight in 1% paraformaldehyde in PBS using a FACScan® (Becton Dickinson, Mountain View, CA) with the windows set to exclude dead cells and debris. 10,000 cells were examined for each determination.
Fas Sensitivity of Murine Melanoma Clones.
Fas sensitivity was determined using the CD4+ T hybridoma line, 3A9 (22). 3A9 cells were activated by exposure to 10 ng/ml PMA (Sigma Chemical Co., St. Louis, MO) and 3 μg/ml ionomycin (Sigma Chemical Co.) for 4 h to upregulate FasL. After activation, 3A9 cells were washed three times and plated in quadruplicate in 96-well microtiter plates with 51Cr-labeled K1735 targets (5 × 103 cells/well) at a 50:1 effector/target ratio in a final volume of 200 μl calcium-free medium. Target cells were 51Cr labeled as previously reported (23). After 16–18 h incubation at 37°C in a 5% CO2 atmosphere, 100-μl aliquots of cell-free supernatant were harvested and radioactivity was measured using an LKB gamma counter (Amersham Pharmacia Biotech, Piscataway, NJ). Maximum lysis was determined by incubating 51Cr-labeled targets in 2 N HCL. Percentage specific target cell lysis was calculated as follows: 100 × [(experimental lysis − spontaneous lysis)/(maximum lysis − spontaneous lysis)]. Lytic activity of activated 3A9 cells was blocked by the inclusion of Fas-Fc (data not shown); unactivated 3A9 cells demonstrated no lytic activity. Anti-Fas killing was carried out by plating tumor cells at 5 × 103/well in triplicate and adding 100 ng/ml biotinylated anti-Fas (Jo-2 clone; PharMingen) or biotinylated isotype-matched control antibody (PharMingen) for 30 min at 37°C then adding 200 ng/ml streptavidin (Jackson ImmunoResearch Labs., West Grove, PA). After a 48-h culture cell viability was determined using a tetrazolium reduction (MTT) assay as described previously (8). Values shown represent the percentage of specific cytotoxicity in anti-Fas treated wells after subtracting that of the biotinylated, isotype-matched controls [(1 − mean anti-Fas)/(mean isotype-matched control)] × 100.
Bioassay for FasL in Lung-conditioned Medium and FasL Detection by Western Blotting.
Lung conditioned medium (LCM) was prepared according to the method of Szaniaswska et al. (24) with modifications. In brief, murine lungs were surgically excised, washed in cold PBS, cut into 1–2 mm3 pieces, washed in cold HBSS, and suspended in serum-free MEM with 1% BSA and rotated at 37°C for 6 h. Initial medium was discarded and replaced with MEM with 0.01% BSA, and the lung fragments were rotated at 37°C for an additional 24 h. After the final rotation, lung fragments were removed and discarded and the remaining medium was spun at 15,000 g for 15 min. The cell-free supernatants were collected (LCM) and their protein concentration was determined. For the determination of bioactivity, K1735 cell lines and clones were plated in media supplemented with 0.3% fetal bovine serum and 65 μg/ml LCM at 106 cells/ml and cultured at 37°C, 5% CO2 for 48 h. After culture, relative cellular viability was determined using the tetrazolium reduction (MTT) assay as previously described by our laboratory (21). Background cell death was determined by culturing cells in media supplemented with 0.3% fetal bovine serum alone. Specificity of LCM killing was shown by inclusion of either Fas-Fc (25) or isotype control–Fc (LTβR-Fc) at 200 ng/ml for the 48-h culture period. The presence of FasL protein in LCM was verified by Western blotting. For this procedure, 10-μg aliquots of LCM were separated by 12.5% SDS-PAGE gel and transferred to nitrocellulose membrane. FasL was visualized by hybridization with a monoclonal anti-FasL (clone 33; Transduction Laboratories, Lexington, KY) and anti–mouse horseradish peroxidase was detected using the ECL system (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK).
Detection of FasL in Murine Lung Sections by Immunohistochemistry.
Frozen sections of murine lung were fixed with 2% formaldehyde in PBS for 10 min and washed with PBS. Endogenous peroxidase was blocked with 3% H2O2 in methanol for 12 min and the sections were incubated with blocking solution (PBS supplemented with 5% horse serum and 1% goat serum) for 20 min. Sections were then incubated with FasL-specific antibody, N-20 (Santa Cruz Biotechnologies, Santa Cruz, CA), at a 1:800 dilution in blocking solution overnight at 4°C. After washing, antibody staining was visualized using peroxidase-conjugated goat anti–rabbit IgG, F(ab′)2 (Jackson ImmunoResearch Labs.) at 1:500 in blocking solution and stable diaminobenzidine (Research Genetics, Inc., Huntsville, AL). All sections were counterstained with hematoxylin. Control sections were exposed to blocking solution alone.
Tumor Growth and Metastasis In Vivo.
C3H/gld and C3H/wt mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Single cell suspensions of K1735 melanoma cells were prepared by brief incubation of monolayer cultures in 0.02% EDTA and 0.25% trypsin. Before injection, cells were resuspended in PBS. For the determination of experimental metastases, 105 cells were injected intravenously into the lateral tail vein and metastatic lung tumor growth was enumerated by visual inspection using light microscopy 3–5 wk after injection. For the determination of spontaneous metastases, mice were injected with 5 × 105 cells subcutaneously in the flank. Tumors were aseptically removed when their mean diameter reached 1.5 cm. Metastatic lung growth was determined by visual inspection using light microscopy 4–6 wk later. Animal experimentation was performed in accordance with institutional guidelines and the regulations and standards of the USDA. Statistical analyses of metastatic incidence in C3H and C3H/gld mice were carried out using a 2 × 2 contingency table of the number of mice with metastases versus the number of mice without metastases using a one-sided χ2 analysis. Statistical analyses of the number of metastases in C3H versus C3H/gld mice were carried out using a Mann-Whitney test.
Results and Discussion
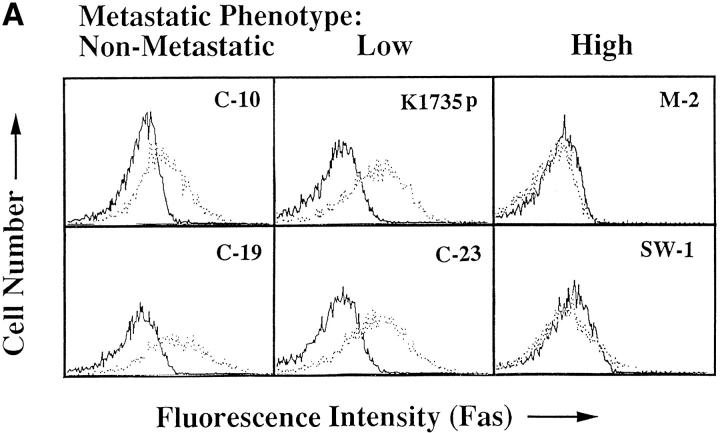
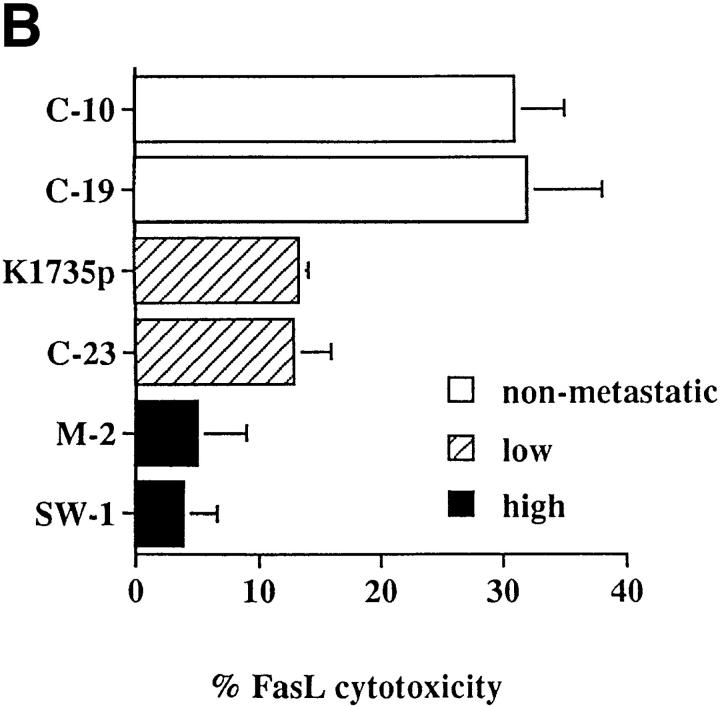
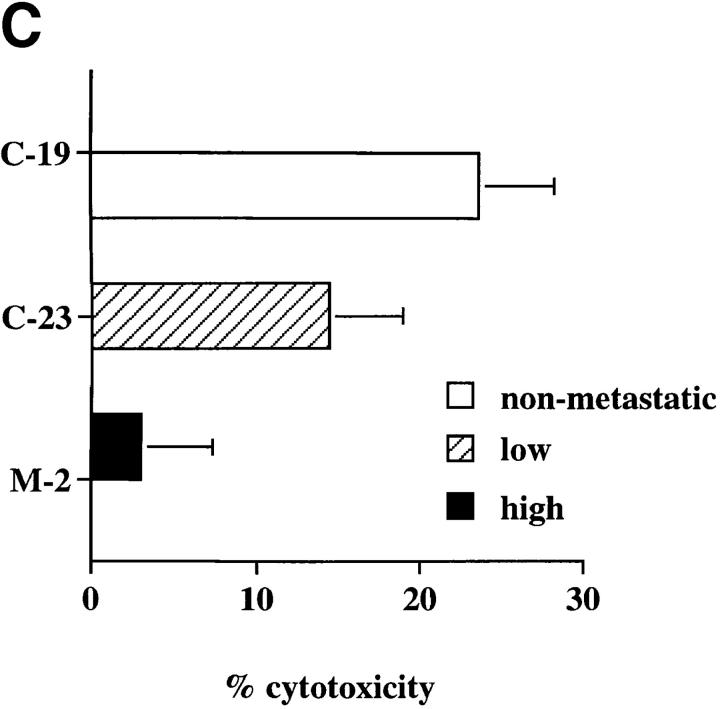
If lung-derived FasL elicits the apoptosis of nonmetastatic K1735 tumors in vivo, then metastatic K1735 tumors should lack Fas expression or demonstrate a resistance toward Fas-mediated apoptosis while their nonmetastatic counterparts should retain Fas sensitivity. To test this premise, we examined Fas expression and the ability to induce FasL-mediated cell death in vitro in the parental K1735 tumor (K1735p) and five clonally derived tumor variants with well-defined metastatic properties. K1735p (13) and the K1735 C-23 variant (14) rarely metastasize from a subcutaneously growing tumor site and are considered to have low metastatic properties. In contrast, the K1735 clones designated SW-1 and M-2 (15) are highly metastatic to the lung, whereas their counterparts K1735 C-10 and C-19 are essentially nonmetastatic (14). As shown in Fig. 1 A, Fas is highly expressed on both the low and nonmetastatic K1735 tumors (K1735p, C-23, C-10 and C-19), whereas the highly metastatic clones SW-1 and M-2 lack Fas expression. Although a striking correlation existed between metastatic behavior and loss of Fas for the highly metastatic K1735 clones, Fas was equivalently expressed on both the low and nonmetastatic K1735 tumors. However, Fas expression does not necessarily predict cellular sensitivity to FasL-induced apoptosis (8, 9). To determine whether metastatic behavior in the low and nonmetastatic K1735 clones correlated with Fas sensitivity, tumor targets were 51Cr-labeled and incubated with a FasL-expressing effector cell (22), and cytotoxicity was determined as described in Materials and Methods. As shown in Fig. 1 B, FasL was cytotoxic for all Fas-positive K1735 tumors. The nonmetastatic clones C-19 and C-10 were most sensitive to FasL-induced death, whereas the poorly metastatic tumors K1735p and C-23 were significantly more resistant to such cytotoxicity. In three out of three experiments, the low metastatic K1735 tumors were three- to fivefold more resistant to FasL-induced cytotoxicity than were their metastatic counterparts. As expected, the highly metastatic M-2 and SW-1 clones lacking Fas expression were insensitive to FasL-mediated killing. An identical sensitivity profile was observed when anti–Fas-mediated killing was determined. Representative clones are shown in Fig. 1 C. Therefore, Fas sensitivity rather than Fas expression appears to uniquely differentiate among those tumors that are highly metastatic, poorly metastatic, and nonmetastatic. The observation that Fas sensitivity and metastatic tumor behavior are inversely correlated suggested a causal effect of Fas loss-of-function on tumor progression.
Figure 1.
Fas expression and sensitivity of K1735 melanoma clones. (A) Flow cytometric analyses were carried out as described in Materials and Methods using a Fas-specific antibody (stippled line) and an isotype-matched control (solid line). Results represent two to five independent determinations for each clone. (B) FasL-mediated lysis of K1735 clones in overnight 51Cr-release assay as described in Materials and Methods. Values represent mean ± SEM of quadruplicate determinations within a single experiment. C10 and C19 killing were shown to be statistically different from that of K1735p and C-23 using Student's t test (C-10 versus K1735p, P = 0.017; C-10 versus K1735p, P = 0.003; C-10 versus C-23, P = 0.010; C-19 versus C-23, P = 0.016) for the data shown. (C) Anti-Fas mediated killing of K1735 clones as described in Materials and Methods. Values represent mean ± SEM of triplicate determinations within a single experiment. Data are representative of two to five independent determinations for FasL and anti-Fas–mediated killing of K1735 clones.
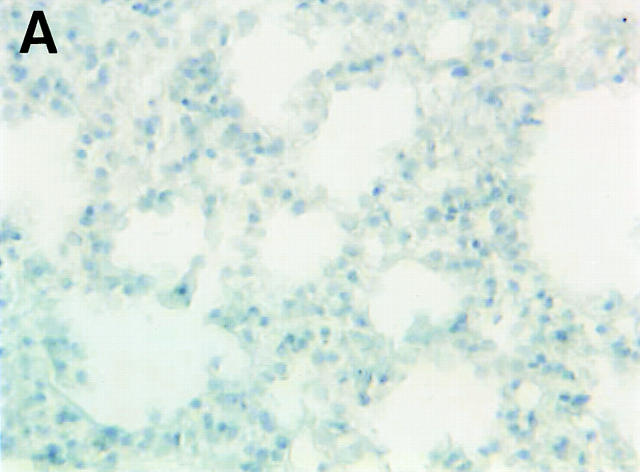
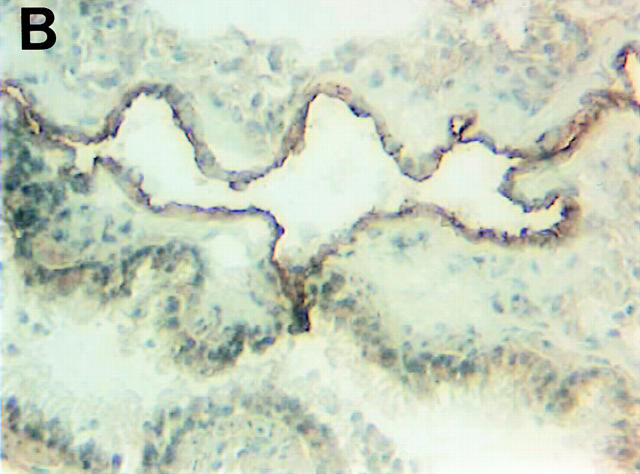
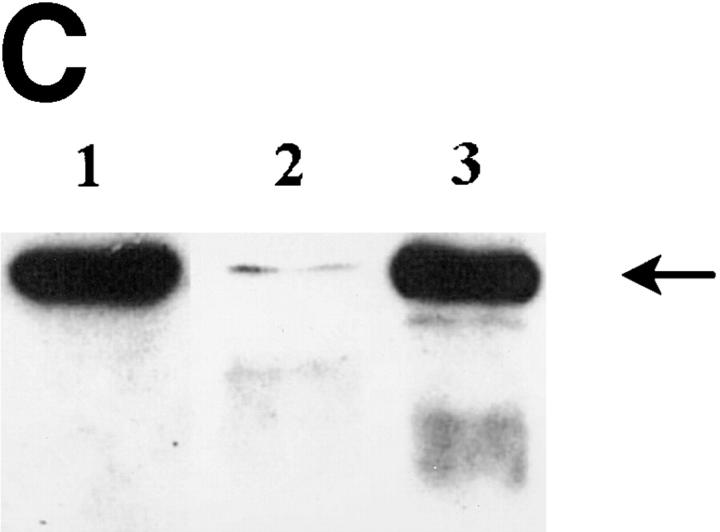
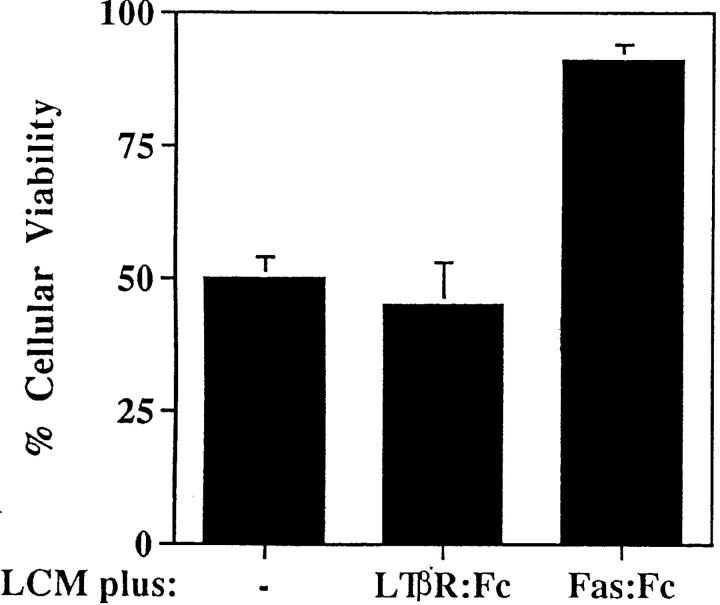
Although FasL mRNA is expressed in the lung (2), there is scant knowledge about the distribution of FasL in this organ. To this end, we carried out immunohistochemical staining of normal murine lung using a FasL-specific antibody. As shown in Fig. 2, A and B, the majority of FasL is expressed on lung endothelial cells, with rare staining observed in type II pneumocytes as previously reported for human lung tissue (26). To determine the bioactivity of lung-expressed FasL, LCM was prepared as previously described (24). We have previously reported that LCM contains a cytotoxic factor that differentially induces cell death in nonmetastatic K1735 tumors while sparing highly metastatic tumor variants (20). To ascertain whether such cytotoxicity was attributable to FasL, we examined LCM bioactivity against the nonmetastatic K1735 variant C-19 (14). As shown in Fig. 3, potent, specific FasL-mediated cytotoxicity was observed in bioactive LCM preparations. Approximately 50% of control K1735 C-19 cells were nonviable after a 48-h culture in LCM. Fas-Fc, a competitive inhibitor of FasL binding (25), almost completely inhibited LCM-mediated killing of the K1735 C-19 cells. However, in the presence of a non-FasL binding control such as the lymphotoxin β receptor–Fc (LTβR-Fc), no such reversal of LCM cytotoxicity was observed. LCM-derived FasL was additionally verified by immunoblotting. An intense band of ∼40 kD and an additional 32-kD mol mass form representing the nonglycosylated form of FasL (27) were consistently observed in bioactive LCM preparations (Fig. 2 C, lane 3). Other immunoreactive lower molecular weight bands, possibly FasL degradation products, were observed in some preparations (Fig. 2 C, lane 3). In nonbioactive LCM preparations subjected to multiple freeze–thaw cycles, FasL was virtually undetectable by immunoblotting (Fig. 2 C, lane 2), substantiating a direct relationship between LCM bioactivity and FasL protein.
Figure 2.
FasL expression in the lung. Shown are staining of murine lung using isotype-matched control (A) and FasL-specific antibody (B) visualized by peroxidase-labeled secondary antibodies as described in Materials and Methods. (C) Western blot analysis of FasL protein in LCM. LCM was subjected to Western blotting using a FasL-specific antibody as described in Materials and Methods. Lane 1, control lysate from endothelial cells (Santa Cruz Biotechnologies, Santa Cruz, CA); lane 2, LCM preparation subjected to multiple freeze–thaw cycles lacking FasL bioactivity; lane 3, LCM preparation used for the bioactivity assay shown in Fig. 3. The blot shown is representative of four independent analyses of LCM.
Figure 3.
Loss of K1735 C-19 viability after LCM culture is reversed by Fas-Fc. Cells were plated in media supplemented with 0.3% fetal bovine serum and 65 μg/ml LCM. Fas-Fc or isotype control–Fc (LTβR-Fc) were added at 200 ng/ml and relative cellular viability was determined at 48 h using the tetrazolium reduction (MTT) assay. Values shown were normalized using values of cells cultured in media supplemented with 0.3% fetal bovine serum alone as background. Results are representative of two independent experiments.
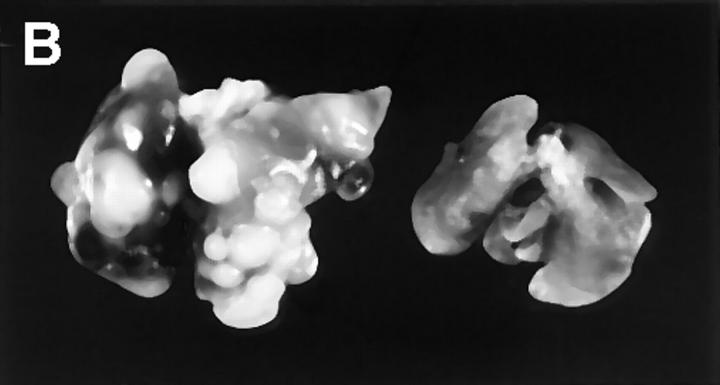
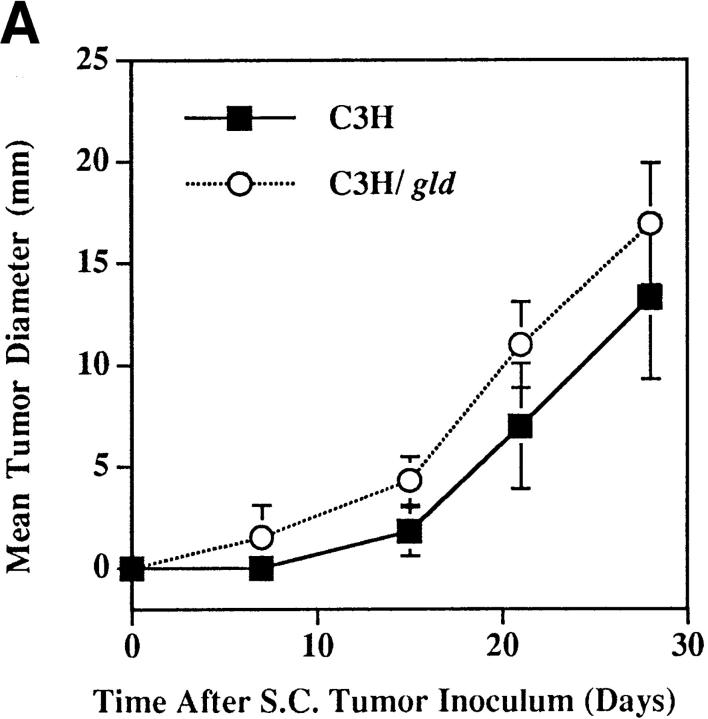
If tumor Fas loss-of-function contributes to the development of the metastatic phenotype, then uncoupling Fas– FasL interactions should permit the metastatic outgrowth of Fas-sensitive tumors in the lung. To this end, we evaluated the metastatic behavior of the Fas-sensitive K1735 tumor and clones K1735p, C-23, and C-19 (Fig. 1) in normal (C3H) and FasL-deficient mice (C3H/gld). K1735 tumors were initially injected into the subcutis of both C3H or C3H/gld mice and regional growth was monitored. Although palpable tumors were usually detected earlier in C3H/gld mice than in C3H control mice, measurable tumor growth rates were nearly identical between the two strains. Fig. 4 A shows one such growth curve using the K1735p tumor. Furthermore, the mean tumor weights at the time of surgical resection 28 d after injection were not statistically different between C3H and C3H/gld mice for any of the tumors (Table 1). However, the incidence and number of spontaneous lung metastases were increased for all three tumors in mice lacking host-derived FasL. The most striking enhancement of metastatic incidence and number of metastases were observed using the K1735p tumor (Fig. 4 B, Table 1). This tumor showed macroscopic lung metastases in 9 out of 10 C3H/gld mice (median 18, range 0–49), whereas only 3 out of 10 wild-type C3H mice showed evidence of metastatic deposits (median 0, range 0–25). The increased incidence and number of metastases using the K1735p tumor was highly significant in C3H/gld mice compared to C3H controls (difference in incidence between C3H and C3H/gld P = 0.0003 using a one-sided χ2 test; difference in number of lung metastases P < 0.0001 using a Mann-Whitney test). We next examined the metastatic growth of the clonally derived C-23 variant that, like K1735p, is considered to have low metastatic properties in C3H mice. As shown in Table 1, C-23 was metastatic in five out of seven C3H/gld mice (median 2, range 0–10), but only one out of five C3H mice (median 0, range 0–20), reflecting a significantly increased incidence of metastasis in C3H/gld mice compared with wild-type controls (difference in incidence between C3H and C3H/ gld mice P = 0.039, a one-sided χ2 test). To address the effects of FasL loss-of-function on a nonmetastatic tumor, we determined lung metastasis of the C-19 clone. As shown in Table 1, the C-19 clone was spontaneously metastatic in two out of five C3H/gld mice (range 0–16), whereas one out of five C3H mice showed evidence of lung deposits (range 0–1). Although not statistically significant with the number of animals used for this experiment, these findings again support a trend for increased incidence and number of metastases in FasL-deficient animals. Moreover, these data suggest that the loss of FasL is sufficient for acquisition of the metastatic phenotype in a nonmetastatic tumor. A potential criticism of such experiments is that the gld host microenvironment is more permissive for metastatic tumor growth irrespective of the loss of FasL. If this were the case, then Fas-insensitive, metastatic tumors such as M-2 might also demonstrate increased metastatic capacity in C3H/gld animals. To test this premise, lung metastatic growth of M-2 was evaluated in C3H and C3H/gld mice. As shown in Table 1, lung metastases were not increased in FasL-deficient mice compared with wild-type mice (difference in lung metastases P = 0.421, Mann-Whitney test) documenting that the gld lung environment per se is not inherently more permissive for metastatic tumor outgrowth. Taken together, these results demonstrate that uncoupling of Fas– FasL interactions can enhance tumor metastasis to organ sites constitutively expressing FasL (K1735p, C-23) and may be sufficient to confer metastatic phenotype in a nonmetastatic clone (C-19).
Figure 4.
Enhanced metastasis of K1735p melanoma cells in FasL-deficient (C3H/gld) mice. (A) 5 × 105 K1735p cells were injected subcutaneously into the flank of C3H and C3H/gld mice. At indicated times, tumor diameters were determined by caliper measure. Results shown represent mean ± SEM, n = 10, for each strain. (B) Gross metastatic tumor growth in two representative lungs from C3H/gld (left) and C3H control mice (right) subcutaneously injected with K1735p cells.
Table 1.
Metastasis of K1735 Clones in FasL-deficient (C3H/gld) Mice
| Tumor clone | Mice | Tumor weight (g) | Lung metastasis | |||||
|---|---|---|---|---|---|---|---|---|
| Incidence | (No. of metastases/animal) | |||||||
| mean ± SEM | ||||||||
| K1735p | C3H control | 2.63 ± 1.33 | 3/10 | (0, 0, 0, 0, 0, 0, 0, 2, 11, 25) | ||||
| C3H/gld | 2.58 ± 1.38 | 9/10* | (0, 1, 4, 8, 17, 18, 20, 34, 42, 49)‡ | |||||
| C-23 | C3H control | 2.30 ± 0.90 | 1/5 | (0, 0, 0, 0, 20) | ||||
| C3H/gld | 2.47 ± 0.65 | 5/7* | (0, 0, 1, 2, 3, 8, 10) | |||||
| C-19 | C3H control | 2.20 ± 0.70 | 1/5 | (0, 0, 0, 0, 1) | ||||
| C3H/gld | 1.52 ± 0.50 | 2/5 | (0, 0, 0, 4, 16) | |||||
| M-2 | C3H control | 1.74 ± 0.30 | 5/5 | (1, 2, 2, 15, 74) | ||||
| C3H/gld | 1.87 ± 0.80 | 5/5 | (3, 4, 12, 16, 50) | |||||
Cells were injected subcutaneously into mice and subcutaneous tumors were removed at 1.5-cm diameter and weighed. Incidence is the number of mice with macroscopic metastases per mice injected. All mice injected had local tumor growth; lung metastatic growth was determined by light microscopy at 5 wk after removal of subcutaneous tumor or when mice were moribund.
Significant difference in incidence of metastases between C3H and C3H/gld mice by χ2 test (P = 0.003 for K1735p; P = 0.039 for C23).
Significant difference in number of metastases between C3H and C3H/gld, P < 0.0001, Mann-Whitney test.
A large body of evidence supports the concept that tumor cell growth and metastasis are dependent upon the interactions of the malignant cell and the host organ environment (17, 18). Our studies are commensurate with the interpretation that the interactions of Fas and FasL on the tumor and host environment, respectively, are critical determinants of tumor cell survival and growth in the metastatic lung site. Although the successful establishment of metastatic lesions has been largely correlated with tumor responsiveness to paracrine growth factors released in specific organ sites (17, 18), our data indicate an additional role for apoptosis-inducing factors present in the specific microenvironment. As Fas-sensitive tumor cells would undergo apoptosis in organ sites constitutively expressing high levels of FasL, we expect a priori that metastatic K1735 tumors preferentially colonizing the lung would be resistant to Fas-mediated apoptosis. In this report, we document that four out of four metastatic K1735 melanoma clones show a loss of Fas expression and/or function compared with two nonmetastatic clones. We have observed a similar loss of Fas expression and function in the highly metastatic K1735 variants C-4 and C-2 (reference 14 and data not shown). The highly metastatic lung-derived clones SW-1 and M-2 demonstrated a complete loss of both Fas expression and function, whereas the intermediate metastatic clones K1735p and C-23 showed a significantly decreased Fas sensitivity (Fig. 1). That Fas sensitivity was diminished but not entirely absent in K1735p and C-23 clones with low metastatic capacity implies that even a partial loss of Fas function may result in significant biologic consequences, as has been reported for tumor development in lpr mice where Fas expression is diminished (5). When Fas–FasL interactions are completely disrupted, such tumors may become significantly more metastatic (Table 1).
Initiation of Fas-mediated apoptosis is complex and involves, at a minimum, levels of Fas sufficient to insure optimal receptor cross-linking, the assembly of critical intracellular Fas-associated proteins necessary to initiate the apoptotic cascade, and the presence or absence of “resistance” proteins such as bcl-xl, Fas-associated phosphatase 1 (FAP-1), and Flice inhibitory protein (FLIP) (9, 28–30). In this respect, a balance between functional tumor-expressed Fas and host-derived FasL may be critical parameters regulating the capacity of a particular tumor to grow at a specified site. For example, Fas expression is absent on the highly metastatic tumors K1735 SW-1 and M-2, precluding initiation of the apoptotic cascade by FasL cross-linking. Although Fas is highly expressed on low metastatic clones K1735p and C-23, postreceptor alterations in Fas signaling may prevent optimal initiation of the apoptotic cascade in response to FasL (Fig. 1, A and B). Similarly, the relative abundance of FasL in the microenvironment may influence the capacity of a given tumor to survive or undergo apoptosis in disparate organ sites. In support of this premise, constitutive FasL expression has been reported to be considerably higher in the murine lung than in the skin (3), providing a cogent explanation for the apparent conundrum that the Fas-sensitive K1735 tumors grew comparably in the subcutis of C3H and C3H/gld mice but were significantly more metastatic in the lung microenvironment. Although we favor the interpretation that an absence of FasL in the lung microenvironment permitted the selective outgrowth of the Fas-sensitive K1735 tumors in C3H/ gld mice, it is conceptually possible that an absence of FasL-positive immune effector cells encountered in the blood and lymphatics was also contributory (31–33). Although the latter possibility seems unlikely in view of the fact that viable, nonmetastatic tumor cells reach the lung microenvironment in normal mice (16), we are formally testing this premise using bone marrow chimeras.
In summary, our studies provide the first evidence that host-derived FasL can inhibit the malignant potential of Fas-sensitive tumor cells and suppress organ-specific metastasis. The critical involvement of Fas–FasL in regulation of the metastatic cascade further underscores the importance of these proteins in homeostasis and prompts consideration of strategies aimed toward modification of the tumor–host environment to prevent metastatic outgrowth.
Acknowledgments
Supported in part by a National Institutes of Health (NIH) Cancer Center Support Core Grant 16672, an institutional Physicians Referral Grant (to L.B. Owen-Schaub), a grant from the Skin Cancer Foundation at the University of Texas M.D. Anderson Cancer Center (to L.B. Owen-Schaub), American Cancer Society Grant CIM 88929 (to L.B. Owen-Schaub), and NIH fellowship F32 AI09351 (to L.L. Hill).
Abbreviations used in this paper
- FasL
Fas ligand
- LCM
lung-conditioned medium
Footnotes
We would like to acknowledge and thank C.F. Ware (La Jolla Institute for Allergy and Immunology, La Jolla, CA) for providing Fas-Fc and LTβR-Fc; C.D. Bucana for providing expert advice with immunohistochemistry; G. Kiriakova and E. Kruzel for technical assistance; Karen Rameriz for providing technical expertise with flow cytometric analyses; and K. Dunner for assistance with photography.
K.L. van Golen's present address is Department of Internal Medicine, The University of Michigan, 1150 West Medical Center Drive, Ann Arbor, MI 48109.
Reference
- 1.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 2.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 3.French LE, Hahne M, Viard I, Radlgruber G, Zanome R, Becker K, Müller C, Tschopp J. Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 5.Peng SL, Robert ME, Hayday AC, Craft J. A tumor-suppressor function for Fas (CD95) revealed in T cell– deficient mice. J Exp Med. 1996;184:1149–1154. doi: 10.1084/jem.184.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zörnig M, Grzeschiczek A, Kowalski M-B, Hartmann K-U, Möröy T. Loss of Fas/APO-1 receptor accelerates lymphomagenesis in Eμ L-MYCtransgenic mice but not in animals infected with MoMuLV. Oncogene. 1995;10:2397–2401. [PubMed] [Google Scholar]
- 7.Takeuchi T, Sasaki Y, Ueki T, Kaziwara T, Moriyama N, Kawabe K, Kakizoe T. Modulation of growth and apoptosis response in PC-3 and LNCAP prostate-cancer cell lines by Fas. Int J Cancer. 1996;67:709–714. doi: 10.1002/(SICI)1097-0215(19960904)67:5<709::AID-IJC20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Owen-Schaub LB, Radinsky R, Kruzel E, Berry K, Yonehara S. Anti-Fas on nonhematopoietic tumors: levels of Fas/APO-1 and bcl-2 are not predictive of biological responsiveness. Cancer Res. 1994;54:1580–1586. [PubMed] [Google Scholar]
- 9.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J-L, Schröter M, Burns K, Mattmann C, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 10.Möller P, Koretz K, Leithhäuser F, Brüderlein S, Henne C, Quentmeir A, Krammer PH. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer. 1994;57:371–377. doi: 10.1002/ijc.2910570314. [DOI] [PubMed] [Google Scholar]
- 11.Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 12.Price JE, Aukerman SL, Fidler IJ. Evidence that the process of murine melanoma metastasis is sequential and selective and contains stochastic elements. Cancer Res. 1986;46:5172–5178. [PubMed] [Google Scholar]
- 13.Kripke ML. Speculations on the role of ultraviolet radiation in the development of malignant melanoma. J Natl Cancer Inst. 1979;81:541–548. doi: 10.1093/jnci/63.3.541. [DOI] [PubMed] [Google Scholar]
- 14.Fidler IJ, Gruys E, Cifone MA, Barnes Z, Bucana C. Demonstration of multiple phenotypic diversity in a murine melanoma of recent origin. J Natl Cancer Inst. 1981;67:947–956. [PubMed] [Google Scholar]
- 15.Talmadge JE, Fidler IJ. Enhanced metastatic potential of tumor cells harvested from the spontaneous metastasis of heterogenous murine tumors. J Natl Cancer Inst. 1982;69:975–980. [PubMed] [Google Scholar]
- 16.van Golen KL, Risin S, Staroselsky A, Berger D, Tainsky MA, Pathak S, Price JE. Predominance of the metastatic phenotype in hybrids formed by fusion of mouse and human melanoma clones. Clin Exp Metastasis. 1996;14:95–106. doi: 10.1007/BF00121206. [DOI] [PubMed] [Google Scholar]
- 17.Fidler IJ. Modulation of the organ environment for treatment of cancer metastasis. J Natl Cancer Inst. 1995;87:1588–1592. doi: 10.1093/jnci/87.21.1588. [DOI] [PubMed] [Google Scholar]
- 18.Rusciano D, Burger MM. Why do cancer cells metastasize to particular organs? . Bioessays. 1992;14:185–194. doi: 10.1002/bies.950140309. [DOI] [PubMed] [Google Scholar]
- 19.Milik AM, Buechner-Maxwell VA, Sonstein J, Kim S, Seitzman GD, Beals TF, Curtis JL. Lung lymphocyte elimination by apoptosis in the murine response to intratracheal particulate antigen. J Clin Invest. 1997;99:1082–1091. doi: 10.1172/JCI119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Golen KL, Owen-Schaub LB, Price JE. Metastatic and nonmetastatic K1735 melanoma cells differ in Fas-mediated apoptosis and growth response to lung-conditioned medium. Proc Am Assoc Cancer Res. 1996;37:A551. . (Abstr.) [Google Scholar]
- 21.Owen-Schaub LB, Meterissian S, Ford RJ. Fas/APO-1 expression and function on malignant cells of hematologic and nonhematologic origin. J Immunother. 1993;14:234–241. doi: 10.1097/00002371-199310000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Allen PM, Unanue ER. Differential requirement for antigen processing by macrophages for lysozyme-specific T-cell hybridomas. J Immunol. 1984;132:1077–1079. [PubMed] [Google Scholar]
- 23.Hill LL, Perussia B, McCue PA, Korngold R. Effect of human natural killer cells on the metastatic growth of human melanoma xenografts in mice with severe combined immunodeficiency. Cancer Res. 1994;54:763–770. [PubMed] [Google Scholar]
- 24.Szaniawska B, Majewski S, Kaminski MJ, Noremberg K, Swierz M, Janik P. Stimulatory and inhibitory activities of lung-conditioned medium on the growth of normal and neoplastic cells in vitro. J Natl Cancer Inst. 1985;75:303–306. [PubMed] [Google Scholar]
- 25.Crowe PD, Van Arsdale TL, Walter BN, Dahms KM, Ware CF. Production of lymphotoxin (LT alpha) and a soluble dimeric form of its receptor using the baculovirus expression system. J Immunol Methods. 1994;168:79–89. doi: 10.1016/0022-1759(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 26.Niehans GA, Brunner T, Frizelle SP, Liston JC, Salerno CT, Knapp DJ, Green DR, Kratze RA. Human lung carcinomas express Fas ligand. Cancer Res. 1997;57:1007–1012. [PubMed] [Google Scholar]
- 27.Mariani SM, Matiba B, Bäumler C, Krammer PH. Regulation of cell surface APO-1/Fas (CD95) ligand expression by metalloproteases. Eur J Immunol. 1995;25:2303–2307. doi: 10.1002/eji.1830250828. [DOI] [PubMed] [Google Scholar]
- 28.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/ Ced-3-like protease, is recruited to the CD95 (Fas/APO-1) death inducing signalling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 29.Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995;268:411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- 30.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xl. . Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 31.Arase H, Arase N, Saito T. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J Exp Med. 1995;181:1235–1238. doi: 10.1084/jem.181.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keiner PA, Davis PM, Starling GC, Mehlin C, Klebanoff SJ, Ledbetter JA, Liles WC. Differential induction of apoptosis by Fas–Fas ligand interactions in human monocytes and macrophages. J Exp Med. 1997;15:1511–1516. doi: 10.1084/jem.185.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]