Abstract
Characterization of cytolytic T lymphocyte (CTL) responses to tumor antigens has been impeded by a lack of direct assays of CTL activity. We have synthesized reagents (“tetramers”) that specifically stain CTLs recognizing melanoma antigens. Tetramer staining of tumor-infiltrated lymph nodes ex vivo revealed high frequencies of tumor-specific CTLs which were antigen-experienced by surface phenotype. In vitro culture of lymph node cells with cytokines resulted in very large expansions of tumor-specific CTLs that were dependent on the presence of tumor cells in the lymph nodes. Tetramer-guided sorting by flow cytometer allowed isolation of melanoma-specific CTLs and confirmation of their specificity and their ability to lyse autologous tumor cells. Our results demonstrate the value of these novel reagents for monitoring tumor-specific CTL responses and for generating CTLs for adoptive immunotherapy. These data also indicate that strong CTL responses to melanoma often occur in vivo, and that the reactive CTLs have substantial proliferative and tumoricidal potential.
Keywords: melanoma, Melan-A/MART-1, tyrosinase, immunotherapy, tumor immunity
he recent molecular characterization of tumor antigens recognized by MHC class I–restricted human CTLs has opened up new possibilities for the immunotherapy of cancer. It is now well established that human tumor cells, notably cutaneous melanoma, may express multiple CTL-defined antigens that are shared among tumors, providing the prospect for generic vaccines applicable to large subsets of cancer patients (1, 2). Hence, numerous clinical trials are underway aimed at inducing vigorous CTL responses against defined tumor antigens. However, further progress in understanding natural or vaccine-induced CTL responses to tumor antigens has been prevented by the lack of direct assays of CTL activity. Previous assays for detection of antigen-specific CTLs have depended on their ability to proliferate extensively and acquire lytic activity, or to release relatively large amounts of cytokine (3), such that accurate quantification of these cells has not been possible. The necessity of stimulating CTLs with antigen in order to detect them has also prevented characterization of their phenotype in vivo. Recently, tetrameric arrays of soluble class I MHC–peptide complexes (“tetramers”) have been used to identify antigen-specific CTLs (4), and techniques for detecting and isolating low-frequency CTLs using these reagents have been developed and validated (5). We report here the first analyses of patient samples using tetramers incorporating melanoma-derived antigenic peptides. These reagents were used to directly enumerate and phenotype melanoma-specific CTLs ex vivo from both metastatic lesions and peripheral blood, using multiparameter flow cytometry.
Materials and Methods
Tissues and Cells.
Melanoma patients subjected to therapeutic surgical LN resection were selected for this study on the basis of HLA-A2 antigen expression as assessed by flow cytometry of PBMC stained with allele-specific mAb BB7.2 (6). Individual LNs collected by surgical dissection were dissociated to single cell suspensions in sterile RPMI 1640 supplemented with 10% FCS. Melanoma cell lines were obtained from the adherent fraction of LN cells from all patients in Table 1, except LAU 132, LAU 181, and LAU 240, resulting after 2 h incubation at 37°C of an aliquot of LN cell suspension. The rest of the LN cell suspension was cryopreserved in RPMI 1640, 40% FCS, 10% DMSO. Vials containing 5–10 × 106 cells were stored in liquid nitrogen. Control LNs (a total of 12 LNs) were obtained from 8 nonmelanoma cancer patients (Table 2). Half of each control LN was processed as described above to generate LN cell suspensions. The rest of each control LN was submitted to anatomopathological examination. T2 cells were maintained in IMDM supplemented with 10% FCS. Cells derived from each LN were kept as separate, individual batches of cryopreserved cells. PBMC were separated from heparinized blood by centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden), washed three times, and cryopreserved as described above. CTL clones specific for Melan-A and tyrosinase were derived from patients LAU 203 and LAU 132, respectively, and the influenza matrix–specific CTL clone was from a healthy donor and maintained in continuous culture as described elsewhere (7, 8).
Table 1.
Clinical Parameters of Metastatic Melanoma Patients
| Patient* | Age | Tumor stage (AJCC) | LN dissection | Disease interval‡ | Previous treatment | Survival status 1998 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| yr | yr | yr | ||||||||||
| LAU 50 | 65 | pT4aN2cM0 | 1 N+/14 N | 5 | ILP (TIM) | 8, tumor free | ||||||
| LAU 56 | 39 | pT4aN2cM0 | 2 N+/17 N | 10 | ILP (TIM) | 11, tumor free | ||||||
| LAU 132 | 35 | pT3aN2aM0 | 15 N+/16 N | 1.3 | ILP (M) IFN-α, 1 yr§ | 3, tumor free | ||||||
| LAU 181 | 42 | pT4N2M0 | 2 N+/14 N | 2 | Surgery | 3, tumor free | ||||||
| LAU 203 | 66 | pTxN2cM0 | ≥3 N+ | 5 | Surgery | 7, tumor free | ||||||
| LAU 233 | 75 | pT4N2cM0 | 6 N+/10 N | 2 | None | 4, tumor free | ||||||
| LAU 240 | 65 | pTxN2cM0 | 11 N+/15 N | <1 | None | 1, PD | ||||||
| LAU 253 | 61 | pT3aN2M0 | 18 N+/27 N | 1.3 | Surgery | 4, PD | ||||||
| LAU 267 | 45 | pT4aN2cM0 | 1 N+ | <1 | IFN-α, <1 yr | 1, PD |
AJCC, American Joint Committee on Cancer. ILP (TIM), Isolated limb perfusion with TNF-α (4 mg), IFN-γ (0.2 mg), and melphalan. ILP (M), Isolated limb perfusion with melphalan alone. N, Node. PD, Progressive disease.
All metastatic nodes included in this series, except that from LAU 56, were found to express Melan-A and tyrosinase mRNA by RT-PCR.
Time elapsed between diagnosis of primary melanoma and surgical resection of LNs analyzed in this study.
IFN-α administered as adjuvant therapy intermediate high dose regimen.
Table 2.
Enumeration of A2/Tumor Antigen Tetramer Binding Lymphocytes in Ex Vivo LN Cells Draining Nonmelanoma Tumors
| LN no. | Patient | Age | Carcinoma | Stage | Type of LN* | HLA-A2‡ | % CD8+§ | % CD8+ tetramer+∥ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| yr | ||||||||||||||||
| 1 | LAU 292 | 75 | Bladder | BCGitis¶ | Right ilioobturator NLN | + | 2.2 2.7 | 0.18 0.08 | ||||||||
| 2 | LAU 293 | 46 | Breast | pT2N0Mx | Low axillary NLN | − | 9.7 7.2 | 0.18 0.13 | ||||||||
| 3 | LAU 293 | External axillary NLN | − | 9.1 8 | 0.17 0.2 | |||||||||||
| 4 | LAU 295 | 61 | Renal cell | pT2N0Mx | Right pararenal NLN | − | 5.6 6 | 0.11 0.14 | ||||||||
| 5 | LAU 296 | 75 | Lung | pT1N1Mx | Intertracheal NLN | − | 8.5 8.2 | 0.09 0.18 | ||||||||
| 6 | LAU 296 | Right paratracheal NLN | − | 6.5 7.3 | 0.11 0.11 | |||||||||||
| 7 | LAU 297 | 75 | Colon | pT4N0M1 | Left colon NLN | − | 3.5 4.6 | 0.08 0.06 | ||||||||
| 8 | LAU 297 | Paraaortic NLN | − | 5.4 5.8 | 0.04 0.04 | |||||||||||
| 9 | LAU298 | 49 | Bladder | pT4aN2 | Left TILN | + | 3.2 3.6 | 0.1 0.14 | ||||||||
| 10 | LAU 298 | Right NLN | + | 6 | 0.11 | |||||||||||
| 11 | LAU 299 | 68 | Prostate | pT3bN0 | Left ilioobturator NLN | + | 4.4 | 0.11 | ||||||||
| 12 | LAU 301 | Gastric | pT3N0Mx | Satellite NLN | + | 3.1 3.3 | 0.08 0.04 |
A cut-off value of 0.25 was calculated as the mean of a total of 22 determinations (0.11) of CD8+ tetramer+ LN cells from 12 LNs analyzed from nonmelanoma tumors, plus 3 SD (0.14).
Based on pathology examination of a fragment of the same LN.
An aliquot of the LN cell suspension was stained with HLA-A2–specific mAb BB7.2.
LN cell suspensions were cultured overnight in complete medium supplemented with rIL-2 and rIL-7. Aliquots of the cell suspension were analyzed by two-color flow cytometry with anti-CD8PerCP and A2/Melan-A tetramers. The percentage of CD8+ cells was calculated relative to all cells included in the live cell gate.
Live CD8+ LN cells were electronically gated, and the percentage of tetramer+ cells was calculated by setting a region similar to those used in Fig. 2 A. The determinations were generally performed in duplicate except in those LNs in which there were insufficient numbers of cells.
Subacute granulomatous cystitis after therapy with BCG. No residual tumor was found upon anatomopathological examination of resected tissues. The primary tumor had been resected 2 yr earlier by endoscopy.
Tetramers.
Complexes were synthesized as described (4, 9). In brief, purified HLA heavy chain and β2-microglobulin (β2M)1 were synthesized using a prokaryotic expression system (pET; R&D Systems, Inc., Minneapolis, MN). The heavy chain was modified by deletion of the transmembrane cytosolic tail and COOH-terminal addition of a sequence containing the BirA enzymatic biotinylation site. Heavy chain, β2M, and peptide were refolded by dilution. The 45-kD refolded product was isolated by fast protein liquid chromatography and then biotinylated by recombinant BirA (Avidity, Denver, CO) in the presence of biotin, adenosine 5′-triphosphate, and Mg2+ (all from Sigma Chemical Co., St. Louis, MO). Streptavidin–PE conjugate (Sigma Chemical Co.) was added in a 1:4 molar ratio, and the tetrameric product was concentrated to 1 mg/ml.
mAbs.
Anti-CD8FITC,PerCP, anti-CD3PerCP, and anti-CD45RAFITC were obtained from Becton Dickinson (San Jose, CA). Anti-CD45ROFITC was from DAKO Corp. (Carpinteria, CA).
Flow Cytometry Immunofluorescence Analysis.
Thawed LN cell suspensions were cultured individually for 16–20 h in IMDM supplemented with 0.55 mM Arg, 0.24 mM Asn, 1.5 mM Gln, 10% pooled human A+ serum (complete medium), supplemented with recombinant human (rh)IL-2 (100 U/ml) and rhIL-7 (10 ng/ml). Cells (0.5–1 × 106) were stained with tetramers and FITC and PerCP mAb conjugates in 50 μl of PBS, 2% BSA, and 0.2% azide for 40 min at 4°C. Cells were washed once in the same buffer and analyzed immediately in a FACSCalibur® (Becton Dickinson). Data acquisition and analysis were performed using CellQuest™ software. CD8+ lymphocytes were purified from PBMC in two rounds of positive selection by magnetic cell sorting using a miniMACS® device (Miltenyi Biotec Inc., Sunnyvale, CA). The resulting cells which were >98% CD3+CD8+ were stained for flow cytometry analysis as described above.
Chromium-release Assay.
Antigen recognition was assessed using target cells (T2 or melanoma) labeled with 51Cr for 1 h at 37°C and washed two times. Labeled target cells (103 cells in 50 μl) were then added to varying numbers of effector cells (50 μl) in V-bottomed microwells in the presence or absence of 1 μg/ml of antigenic peptide (50 μl). The effector cells were preincubated for at least 20 min at 37°C with unlabeled K562 cells (5 × 104/ well) to eliminate nonspecific lysis due to NK-like effectors. Chromium release was measured in supernatant (100 μl) harvested after 4 h incubation at 37°C. The percent specific lysis was calculated as described (7).
Analysis of mRNA Expression.
Total RNA was extracted from frozen LNs by the guanidinium isothiocyanate/cesium chloride procedure. cDNA was synthesized as described (10). Aliquots corresponding to 100 ng of starting RNA were amplified by 30 cycles of PCR using Melan-A–specific primers (10) and the tyrosinase-specific primers HTYR1 and HTYR2 (11). Aliquots of each reaction were run on a 2% agarose gel and visualized by ethidium bromide fluorescence. To verify RNA integrity, a 21-cycle PCR assay with primers specific for β-actin was carried out in each case. To increase sensitivity in the detection of tumor cells in those LNs macroscopically uninfiltrated, the PCR products were further analyzed by Southern blotting using internal cDNA fragments as probes. Alternatively, PCR was performed with 40 cycles. The detection limit was approximately equivalent to 10 tumor cells per 106 lymphocytes, as determined from titration experiments with the melanoma cell line SK23-MEL.
Results
Generation and Characterization of A2/Melanoma Peptide Tetramers.
Tetramers were synthesized around two antigenic peptides recognized by HLA-A*0201–restricted CTLs, both of which derive from melanocyte lineage–specific proteins. One peptide was the natural tyrosinase 368–376 epitope (the N370D variant, YMDGTMSQV, generated by antigen processing [12]); the other was a modification of the Melan-A 26–35 epitope (7). This modified epitope, ELAGIGILTV, carrying a substitution of Ala for Leu at position 2 from the NH2 terminus (hereafter A27L), forms relatively stable complexes with HLA-A2 and is a more potent immunogen than the natural Melan-A peptide (8). MHC class I–peptide complexes were prepared essentially as described (4, 5, 9), by refolding expressed HLA-A*0201 heavy chain (hereafter A2) and β2M around the peptides. The heavy chain had been engineered with a COOH-terminal signal sequence for the biotinylating enzyme BirA, so complexes purified by FPLC were biotinylated and bound to PE-labeled streptavidin at a 4:1 ratio to form A2/Melan-A and A2/tyrosinase tetramers. A2/matrix tetramer based on the HLA-A*0201–restricted influenza matrix protein peptide 58–66 (GILGFVFTL) was used as a control reagent (5).
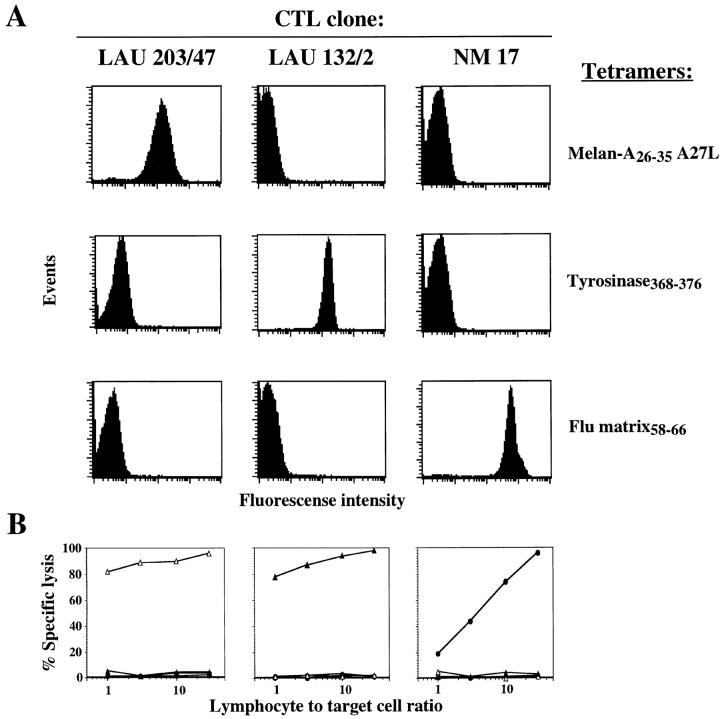
Monoclonal CTL populations were used to test the ability of these tetramers to specifically label cells expressing the appropriate cognate TCR. As shown in Fig. 1 A, tetramers uniformly stained CTL clones in a specific fashion, generating fluorescent signal two orders of magnitude higher than background. The specificity of staining correlated perfectly with the specificity of lytic activity displayed by the CTL clones against chromium-labeled target cells pulsed with their cognate peptide (Fig. 1 B). It is noteworthy that the Melan-A–specific CTL clone used in these experiments recognized both the unmodified peptide (data not shown) and the A27L-modified peptide, indicating that the A2/ Melan-A tetramers carrying the modified A27L peptide stain cells recognized the natural peptide. Further confirmation that the two peptides are interchangeable was obtained by demonstrating that a tetramer synthesized around the unmodified peptide also stained the CTL clone (data not shown).
Figure 1.
Fluorescent soluble HLA-A2/ antigenic peptide tetramers specifically stain cloned CTLs. (A) Three HLA-A2/peptide tetramers, incorporating Melan-A26–35 A27L analogue, tyrosinase368–376, and influenza matrix58–66 peptides, were tested for their ability to stain CTL clones LAU 203/47 specific for Melan-A26–35, LAU 132/2 specific for tyrosinase368–376, and NM 17 specific for influenza matrix58–66. (B) The specificity of peptide antigen recognition by the three CTL clones was assayed on chromium-labeled T2 target cells sensitized with either Melan-A26–35 (open triangles), tyrosinase368–376 (filled triangles), or influenza matrix58–66 (filled circles) at the indicated lymphocyte to target cell ratios.
Enumeration of Melan-A– and Tyrosinase-specific CTLs in Tumor-infiltrated LNs.
Tissue and/or blood samples were obtained from nine HLA-A*0201 patients with metastatic melanoma, whose clinical characteristics are presented in Table 1. Tumor-infiltrated LNs (TILNs) were surgically excised and separated to single cell suspensions which were immediately stained or cryopreserved. In two cases, LNs which were not macroscopically infiltrated with tumor (NLNs) were also surgically excised from the same anatomical region, and processed to single cells. TILNs and NLNs were examined for the expression of tumor antigens by reverse transcription (RT)-PCR. All TILNs except one (from patient LAU 56) contained tumor cells expressing the Melan-A and tyrosinase antigens (Table 3). One of the NLN preparations (LAU 267) was negative for both tumor antigens, whereas the second (LAU 181) was weakly positive for Melan-A, suggesting the presence of a small number of tumor cells infiltrating the node (Table 3).
Table 3.
Enumeration of A2/Tumor Antigen Tetramer Binding Lymphocytes in Ex Vivo or Short-term Cultured LN Cells from HLA-A2+ Melanoma Patients
| Melanoma patient | Type of LN | PCR | Exp. no. | Days in culture* | % CD8+ in lymphocyte gate‡ | % tetramer+ in CD8+ LN cells | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melan-A | Tyrosinase | Melan-A | Tyrosinase | |||||||||||||
| LAU 50 | TILN | + | + | 21.2 | 1 | 12.3 | 0.94 | 0.08 | ||||||||
| LAU 56 | TILN | − | − | 21.2 | 1 | 4.8 | 0.64 | 0 | ||||||||
| LAU 132 | TILN | + | + | 21.2 | 21 | 93 | 3.67 | 0.63 | ||||||||
| LAU 181 | NLN | + | − | 3.3 | 1 | 11.8 | 0.29 | |||||||||
| 3.3 | 1 | 11.8 | 0.23 | |||||||||||||
| 18.5 | 1 | 13.7 | 0.35 | |||||||||||||
| 18.5 | 1 | 13.8 | 0.30 | |||||||||||||
| TILN | + | + | 3.3 | 1 | 41 | 0.51 | ||||||||||
| 3.3 | 1 | 41.9 | 0.48 | |||||||||||||
| 18.5 | 1 | 41.2 | 0.46 | |||||||||||||
| 18.5 | 1 | 43.3 | 0.49 | |||||||||||||
| LAU 203 | TILN | + | + | 21.2 | 16 | 89 | 12.00 | 0.05 | ||||||||
| LAU 233 | TILN | + | + | 21.2 | 12 | 76 | 21.10 | 0.48 | ||||||||
| LAU 240 | TILN-1 | + | + | 21.2 | 1 | 15.6 | 0.82 | 0.03 | ||||||||
| TILN-2 | + | + | 21.2 | 1 | 4.3 | 0.52 | 0 | |||||||||
| LAU 253 | TILN-1 | + | + | 23.7 | 1 | 13.5 | 1.05 | 0.14 | ||||||||
| 23.7 | 1 | 14.6 | 1.21 | |||||||||||||
| TILN-2 | + | + | 23.7 | 1 | 10.2 | 0.78 | ||||||||||
| 23.7 | 1 | 10.2 | 0.81 | |||||||||||||
| LAU 267 | NLN | − | − | 4.3 | 0 | 11.2 | 0.22 | |||||||||
| 4.3 | 0 | 11.4 | 0.12 | |||||||||||||
| 18.5 | 1 | 12.6 | 0.21 | |||||||||||||
| 18.5 | 1 | 12.9 | 0.30 | |||||||||||||
| TILN-1 | + | + | 4.3 | 0 | 9.7 | 0.45 | ||||||||||
| 4.3 | 0 | 9.9 | 0.40 | |||||||||||||
| 18.5 | 1 | 10.4 | 0.45 | |||||||||||||
| 18.5 | 1 | 10.1 | 0.47 | |||||||||||||
| 1 | 10.4 | 0.62 | ||||||||||||||
| TILN-2 | + | + | 4.3 | 0 | 10.3 | 3.27 | ||||||||||
| 4.3 | 0 | 10.8 | 3.52 | |||||||||||||
| 18.5 | 1 | 11.8 | 3.53 | |||||||||||||
| 18.5 | 1 | 11.9 | 3.14 | |||||||||||||
| 18.5 | 1 | 11.8 | 3.70 | |||||||||||||
NLN, Macroscopically normal LN. Exp., Experiment.
LN cell suspensions were cultured in complete medium supplemented with rIL-2 and rIL-7 at 37°C for the indicated number of days. In the assays where d = 0, fresh LN cells were analyzed immediately after cell suspension preparation. In the assays where d = 1, thawed LN cells were cultured for 16–20 h before analysis.
Aliquots of TILNs or NLNs were analyzed by three-color flow cytometry after staining with anti-CD3 or anti-CD45RA, or anti-CD45RO, anti-CD8, and either A2/Melan-A or A2/tyrosinase tetramers as in Fig. 2 A. CD8+ LN cells were gated, and the percentage of tetramer+ cells was determined by setting a region as in Fig. 2 A. CD3+CD8− LNs were also analyzed on a separate gate, and the percentage of tetramer+ cells varied between 0.00 and 0.06% of gated cells, with one single exception where this percentage was 0.22% for cells of patient LAU 233 stained with the A2/ Melan-A tetramers (not shown).
Initially, four TILNs from three patients were thawed, cultured overnight in cytokine-supplemented medium, then triple-stained with anti-CD8 mAb, anti-CD3 mAb, and either the A2/Melan-A or the A2/tyrosinase tetramer. Tetramer+ cells were confined to the CD8+ compartment of CD3+ cells, as expected for CTLs (Fig. 2 A). When cells were gated for staining similar to that of Melan-A–specific clones, frequencies of A2/Melan-A tetramer+ cells were surprisingly high, 1.8, 0.42, 0.92, and 0.22%, respectively, of CD3+CD8+ cells, i.e., a frequency of CTLs between 1 in 50 and 1 in 500 CD8+ LN cells. The frequency of A2/ Melan-A tetramer+ CD8+ cells in LN cells from a normal individual stained under similar conditions was <0.05% (not shown). To determine more precisely the levels of nonspecific tetramer staining of CD8+ LN cells, a series of 12 LNs draining randomly selected nonmelanoma tumors were also analyzed in a similar fashion. The levels of CD8/ tetramer double positives were in average 0.11% of CD8+ LN cells, with a standard deviation of 0.05% (Table 2). When these LNs were segregated into HLA-A2+ (5 LNs and a total of 8 stainings) and HLA-A2− (7 LNs and a total of 14 stainings), the mean percentages of CD8/tetramer double positive LN cells detected were not significantly different (0.11 ± 0.04 and 0.12 ± 0.05, respectively). It can be concluded from this analysis that the lower detection limit with A2/Melan-A tetramers is ∼0.2% of CD8+ LN cells (mean + 3 SD = 0.25; Table 2). Finally, frequencies of A2/tyrosinase tetramer+ CTLs in the melanoma TILNs were <0.05% in the four TILNs (illustrated for LAU 50, Fig. 2 A).
Figure 2.

Direct identification of HLA-A2/tumor peptide antigen tetramer binding lymphocytes in TILNs. (A) Four TILNs obtained from three HLA-A2 melanoma patients were stained, after overnight culture, with either A2/Melan-A26–35 A27L or A2/tyrosinase368–376 tetramers together with anti-CD3PerCP mAb and anti-CD8FITC mAb. Dot plots are shown for gated CD8+ LN cells. (B) Ex vivo A2/Melan-A tetramer+ TILNs have an activated phenotype. Cell suspensions prepared from normal (NLN) or metastatic (TILN) LNs from patient LAU 267 were directly analyzed by three-color flow cytometry using anti-CD8PerCP mAb, A2/Melan-A tetramers, and either anti-CD45RAFITC mAb (top) or anti-CD45ROFITC mAb (bottom). Dot plots are shown for gated CD8+ LN cells. (C) Enumeration and phenotype of tetramer+ cells in ex vivo circulating lymphocytes. Highly homogeneous CD8+ lymphocyte populations (>98%) were obtained from PBMC of patient LAU 267, or from a healthy donor (HD), by two rounds of positive selection with magnetic cell sorting. The lymphocyte preparation was then stained with A2/Melan-A or A2/influenza matrix tetramers and anti-CD45RACychrome and analyzed immediately by flow cytometry. The histograms on top of dot plots show the intensity of the fluorescence signal on the FL-3 channel of gated Melan-A/tetramer+ or influenza tetramer+ lymphocytes. (D) Summary of phenotyping data obtained from NLNs, TILNs, and PBMC from patients LAU 181 and LAU 267. The LN cell suspensions were stained either immediately after cell suspension preparation (LAU 267) or after overnight culture of previously cryopreserved uncultured LN cells (LAU 181) with either anti-CD45RAFITC or anti-CD45ROFITC in combination with anti-CD8PerP and A2/Melan-A tetramers. The results for the CD45RA and CD45RO triple staining of NLNs and TILN-2 from LAU 267 are illustrated in B. Additionally, PBMC available from the same time of the LN dissection from patient LAU 267 were stained as illustrated in C. The percentages of either CD45RO+ or CD45RA− in gated A2/Melan-A tetramer+ LN cells were calculated with CellQuest™ software.
Frequencies of antigen-specific CTLs derived from tetramer staining were compared with frequencies generated using the limiting dilution assay (LDA), which detects CTLs with high proliferative potential. LDA of selected TILNs showed that the frequencies of Melan-A–specific CTLs were consistently lower (5–20-fold) than those obtained through direct staining with tetramers (data not shown). This discrepancy is consistent with data obtained for influenza- and HIV-specific CTLs, where frequencies reported using tetramer staining have been approximately one order of magnitude higher than those reported for LDA (5, 9), and confirms that tetramers can stain CTLs which are potent antigen-specific effector cells despite limited proliferative potential (5).
Phenotype Analysis of Melan-A–specific CTLs in TILNs and Peripheral Blood.
To establish whether the A2/Melan-A tetramer+ cells in infiltrated LNs had previously been exposed to antigen, LN cells were triple stained ex vivo with A2/Melan-A tetramer, anti-CD8 mAb, and anti-CD45RO mAb, the latter recognizing a stable marker of antigen-experienced cells (13). Two melanoma patients were studied, and one or two TILNs were compared with adjacent NLNs. Compared with the latter, the frequencies of A2/Melan-A tetramer+ CD8+ cells were increased (up to 11-fold) in all three TILNs (Fig. 2 B, and Table 3). In the TILNs from patient LAU 181 and TILN-2 from patient LAU 267, 95% of the A2/Melan-A tetramer+ CD8+ cells were CD45RO+ and CD45RA− (Fig. 2, B and D). By contrast, in the NLNs from patient LAU 267, the vast majority of A2/Melan-A tetramer+ CD8+ cells detected were neither CD45RO+ nor CD45RA− (Fig. 2, B and D). Hence, an expanded population of A2/Melan-A tetramer+ CD8+ cells was identified in TILNs compared with NLNs, and these cells had the phenotype of antigen-experienced cells, providing direct evidence that an antigen-specific immune response had been triggered in these patients. Interestingly, fewer tetramer+ CD8+ cells in TILN-1 from patient LAU 267 were CD45RO+/CD45RA− than in TILN-2 (∼55 vs. 95%, Fig. 2 D). TILN-2 showed a much larger expansion of A2/Melan-A tetramer+ CD8+ cells than TILN-1 (11-fold vs. 1.8-fold); therefore, a greater expansion in these CTLs correlated with a more substantial shift towards the CD45RO+/CD45RA− phenotype. Similarly, the relatively higher proportion of CD45RO+/ CD45RA− tetramer+ cells in the NLNs from patient LAU 181 compared with the NLNs from patient LAU 267 (Fig. 2 D) may reflect the presence of small numbers of tumor cells in the LAU 181 NLNs which were absent from the LAU 267 NLNs, as determined by RT-PCR.
The results of multiple flow cytometry analyses of individual LNs performed with Melan-A or tyrosinase tetramers are compiled in Table 3. It is noteworthy that the proportions of CD8+ lymphocytes staining positively with A2/Melan-A tetramers calculated from independent determinations, in the same assay or even in two independent assays, using the same batch of cryopreserved LN cells were generally in good agreement (LAU 181, LAU 253, and LAU 267; Table 3). Moreover, the short period of overnight culture in the presence of cytokines used before staining of cryopreserved LN cells did not seem to significantly alter the numbers of tetramer+ CD8+ LN cells or their CD45 isoform phenotype (LAU 267; Table 3, and data not shown).
To see whether the presence of a strong Melan-A– specific CTL response in TILNs could be detected in the periphery, PBMC from patient LAU 267 were stained with tetramers. A2/Melan-A tetramer+ CD8+ cells could be detected in peripheral blood after enrichment for CD8+ cells, but only a minority (∼16%) of these cells were CD45RA− (Fig. 2 C). In contrast, essentially all A2/influenza matrix tetramer+ CD8+ cells in the blood of a normal individual were CD45RA− (Fig 2 C), consistent with previous antigen exposure. Hence, the marked bias towards the CD45RO+/CD45RA− phenotype observed in Melan-A– specific CTLs in TILNs was specific to the site of tumor contact.
Expansion of Melan-A– and Tyrosinase-specific CTLs from TILNs.
Since CTLs which have recently been exposed to their cognate antigen proliferate when exposed to cytokines such as IL-2, it might be predicted that some melanoma-specific CD45RO+/CD45RA− CTLs in TILNs would proliferate during in vitro culture in the presence of such cytokines. Indeed, when TILN cell suspensions were cultured for 2–3 wk in the presence of rhIL-2 and rhIL-7, substantial populations of A2/Melan-A tetramer+ CD3+CD8+ cells emerged, accounting for up to one CTL in five CD8+ cultured cells, while smaller populations of A2/tyrosinase tetramer+ cells were also seen (Table 3). The massive expansion of A2/Melan-A tetramer+ cells was followed over time in samples from a fifth patient, LAU 181. TILN and NLN cells from this patient were cultured in parallel with cytokines for 21 d, and stained at three time points during culture. A large expansion of A2/Melan-A tetramer+ CD3+CD8+ cells was observed in the cultures of TILN cells, but not in cultures of NLN cells (Fig. 3 A). Calculations of the absolute numbers of tetramer+ CD3+CD8+ cells indicated that these cells had expanded 860-fold by day 15 (equivalent to at least 9 rounds of cell division), and 2,560-fold by day 21 (at least 11 cell divisions). In contrast, in the parallel culture of NLN cells, A2/Melan-A tetramer+ CD3+CD8+ cells had expanded at most fourfold by day 21. The possibility that small numbers of tumor cells may have infiltrated this sample, as suggested by RT-PCR for Melan-A mRNA, may explain the presence of small numbers of Melan-A–specific CTLs in this NLN culture.
Figure 3.

Functional activity of TILNs sorted according to their tetramer staining phenotype correlates with antigen specificity. (A) Cell suspensions prepared from a metastatic (TILN) and a normal (NLN) LN excised from the same anatomical region of patient LAU 181 were cultured in complete medium supplemented with rIL-2 and rIL-7, and aliquots of cells were triple stained with anti-CD8FITC mAb, anti-CD3PerCP mAb, and A2/Melan-A tetramers either after overnight (top), 15 d (middle), or 21 d (bottom) of culture. A2/Melan-A tetramer versus CD3 dot plots are shown for gated CD8+ live lymphocytes, and the percentage of cells staining positively with the tetramers is indicated. (B) The TILN population from patient LAU 233 was analyzed by three-color cytometry, after 12 d in culture with recombinant cytokines only, using the same reagents as in A, and the staining profiles are shown (top, Unsorted TILN). CD3+ CD8+ TILNs were sterile sorted into A2/ Melan-A tetramer+ and tetramer− populations. After expansion for 2 wk in the presence of irradiated allogeneic PBMC and PHA, the sorted cells were stained as above, and results are shown for the tetramer− cell fraction (middle) and the tetramer+ cell fraction (bottom). (C) Each cell fraction was tested for its lytic activity against chromium-labeled T2 cells which were sensitized separately with the following HLA-A2– restricted melanoma-associated peptides: T2 cells alone (open circles, broken lines), T2 + gp100 154– 162 (filled circles, broken lines), T2 + gp100 209– 217 (open triangles, broken lines), T2 + gp100 280–288 (filled triangles, broken lines), T2 + gp100 457–466 (open squares), T2 + gp100 476–485 (filled squares), T2 + tyrosinase 1–9 (open circles), T2 + tyrosinase 368–376 (filled circles), T2 + Melan-A 26–35 (open triangles), T2 + MAGE-3 271–279 (filled triangles). (D) The same cell fractions assayed above, unsorted population (open circles), tetramer+ (filled triangles), and tetramer− (open triangles), were also assayed for their lytic activity against the autologous tumor cell line, Me 305, derived from the same TILN cell population.
Functional Evidence that Tetramer+ CD3+CD8+ Cells from TILNs Are Antigen-specific CTLs.
TILNs cultured in cytokines were used to confirm the functional specificity of tetramer+ cells. Cultured populations containing a substantial proportion of A2/tetramer+ CD3+CD8+ cells were sorted according to tetramer staining, to produce highly homogeneous subpopulations that were either tetramer+ or tetramer− (as shown in Fig. 3 B). These subpopulations were subsequently tested for their ability to lyse target cells pulsed with various HLA-A*0201–restricted melanoma antigen peptides. A2/Melan-A tetramer+ CD3+CD8+ cells lysed targets pulsed with the natural Melan-A peptide, but not with peptides from other antigens, whereas the A2/ Melan-A tetramer− cells were not able to lyse targets pulsed with any of the peptides, including Melan-A (Fig. 3 C). These data provide direct evidence that A2/Melan-A tetramer+ CD3+CD8+ cell populations derived from TILNs contain HLA-A2–restricted CTLs recognizing peptide Melan-A 26–35. In addition, the cells were able to lyse an autologous melanoma cell line generated from the same TILN (Fig. 3 D), thus confirming their tumoricidal capacity. Interestingly, the Melan-A tetramer+ subpopulation displayed higher lytic capacity per cell than the unsorted population, although tumoricidal CTLs (which presumably recognize other antigens) were also detected in the tetramer− subpopulation (Fig. 3 D).
Discussion
This study demonstrates, for the first time, the quantification and phenotyping of tumor-specific CTLs in metastatic tumors directly ex vivo. Our results show that tumor-specific CTLs are often present in high numbers in TILNs, are antigen-experienced, and are capable of massive expansion when exposed to the appropriate cytokines, generating highly tumoricidal CTL populations.
Previous studies (7, 8, 14, 15) have provided indirect evidence for the presence of CTLs infiltrating tumor sites, but it had not previously been possible to directly identify and enumerate such cells. Prolonged tissue culture of cells from melanoma metastases produced cell lines that were tumoricidal in vitro, could be administered to patients with some clinical benefit (16), and were eventually instrumental in the identification of molecularly defined tumor antigens. However, little was known about the nature of any immune response generated in patients, since CTLs specific for tumor antigens could not be identified ex vivo, precluding accurate estimates of their frequency or assessment of their phenotype. Even in long-term polyclonal CTL lines generated from patients, accurate assessment of antigen-specific cytolytic activity by chromium-release assay has often been confounded by high nonspecific lysis due to lymphokine activation of other lymphocytes.
The use of fluorescent class I MHC–peptide complexes allowed us to confirm what has long been suspected but technically impossible to address, namely that at least some patients with metastatic melanoma develop a substantial CTL response against their tumor. Indeed, the frequency of Melan-A–specific CTLs in freshly isolated TILNs ex vivo was found to be as high as 1 in 30 CD3+CD8+ cells, a frequency much higher than that reported previously using less sensitive techniques. Given that we have only analyzed two known tumor epitopes from a list of tens of defined epitopes, and perhaps many more epitopes as yet unknown, it seems likely that tumor-specific CTLs may account for a substantial proportion of the total CD3+CD8+ cells present in TILNs.
It is also noteworthy that Melan-A–specific CTL populations purified from TILNs by flow cytometry sorting were functionally extremely effective in killing both peptide-pulsed cells and autologous tumor cells. Similar results were obtained with sorted A2/tyrosinase tetramer+ TILN cells (data not shown). In contrast to previous methods used for generating CTLs for adoptive immunotherapy, tetramer-based sorting methods do not require peptide stimulation for generation and thus may circumvent the production of low-avidity CTLs due to prolonged exposure to cognate peptide (17). Clearly, isolation of melanoma-specific CTLs using tetramers may provide new opportunities in adoptive immunotherapy.
What Is the Clinical Significance of Strong CTL Responses to Tumor Antigens?
Some patients with high numbers of Melan-A–specific CTLs have lived for many years free of tumor, despite evidence of the infiltration of multiple nodes at surgery. However, it is clear from at least one patient (LAU 267) that melanoma cells may continue to grow in the presence of infiltrating tumoricidal CTLs, resulting in progressive disease. Several mechanisms have already been proposed for this “tumor escape,” and may pertain to our data. One possibility is that tumor cells can simply divide faster than CTLs, so that the balance between tumor progression and immune attack is eventually tilted in the tumor's favor. Another well-documented mechanism of tumor escape is the result of deletions or mutations affecting the genes encoding proteins along the MHC class I pathway, or the antigen itself (18). Hence, a strong Melan-A–specific CTL response may be ineffective because the appropriate MHC class I–peptide complexes are no longer expressed on the melanoma cell surface. This seems an unlikely mechanism in many of the cases we studied, since where autologous melanoma lines could be generated, these were efficiently lysed by the Melan-A–specific CTLs. Nevertheless, heterogeneity of tumor cells has been documented in vivo (19, 20), and escape loss variants may be selected by strong CTL pressure. In one patient (LAU 56), Melan-A mRNA was not detectable in TILNs which contained significant numbers of Melan-A–specific CTLs, although the node was clearly infiltrated with tumor, suggesting Melan-A–negative tumor cells may have been selected for their resistance to CTL lysis. In support of this interpretation, two subcutaneous metastases resected from the same patient 4 yr before LN excision were positive for both Melan-A and tyrosinase mRNA.
Other tumor escape mechanisms have been proposed, including altered TCR signal transduction or expression (21), deficient cytokine milieu, and triggering of Fas receptors on CTLs by Fas ligand expressed by tumor cells (22). In our study, the intense burst of proliferation exhibited by tumor-specific CTLs upon in vitro culture in the presence of cytokines, as well as the rapid elimination of autologous tumor cells from these cultures, suggest that inhibition of CTL proliferation and/or function in vivo rather than killing of CTLs by tumor cells could explain the coexistence of tumor cells and tumor-specific CTLs in TILNs.
Finally, it is worth considering whether various treatment regimes may have contributed to the varying levels of CTL responses to Melan-A observed in our study, since several of our patients had been treated with adjuvant therapy, such as isolated limb perfusion with TNF-α and melphalan, or systemic IFN-α. Clearly, although chemotherapy may have an immunosuppressive effect, adjuvant therapy with IFNs or TNF-α may have enhanced CTL responses. However, no simple correlation between treatment modalities and Melan-A–specific CTL responses is apparent in our study. In this respect, it is conceivable that longitudinal analyses using tetramers, particularly before and after therapeutic intervention, may identify effects of treatment regimens on tumor-specific CTL responses.
Acknowledgments
We thank Nicole Montandon, Renate Milesi, and Pierre Zaech for technical assistance. We would like to give special thanks to all of the patients for their enthusiastic collaboration, and to the personnel of the Centre Pludisciplinaire d'Oncologie for collecting tissue samples. Dr. J. Wellinger, Dr. V. Bettschart (General Surgery), Dr. H.-J. Leisinger, Dr. C. Gygi, Dr. M. Wisard (Urology), Dr. L. Guillou, Dr. E.-P. Saraga, and Dr. R. Lemoine (Pathology) from the Centre Hospitalier Universitaire Vaudois are gratefully acknowledged for providing and characterizing nonmelanoma tumor–draining LNs. We also thank Dr. D. Speiser for his critical reading of the manuscript.
Abbreviations used in this paper
- β2M
β2-microglobulin
- LDA
limiting dilution assay
- NLN
non–tumor-infiltrated lymph node
- rh
recombinant human
- RT
reverse transcription
- TILN
tumor-infiltrated lymph node
Footnotes
This work is partly funded by the United Kingdom Medical Research Council and Cancer Research Campaign. P.R. Dunbar is the Girdlers Research Fellow at Green College.
P. Romero and P.R. Dunbar contributed equally to this work.
References
- 1.Robbins PF, Kawakami Y. Human tumor antigens recognized by T cells. Curr Opin Immunol. 1996;8:628–636. doi: 10.1016/s0952-7915(96)80078-1. [DOI] [PubMed] [Google Scholar]
- 2.Romero P. Cytolytic T lymphocyte responses of cancer patients to tumor-associated antigens. Springer Semin Immunopathol. 1996;18:185–198. doi: 10.1007/BF00820665. [DOI] [PubMed] [Google Scholar]
- 3.Romero P, Cerottini JC, Waanders G. Novel methods to monitor antigen-specific cytotoxic T cell responses in cancer immunotherapy. Mol Med Today. 1998;4:305–312. doi: 10.1016/s1357-4310(98)01280-5. [DOI] [PubMed] [Google Scholar]
- 4.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 5.Dunbar PR, Ogg GS, Chen J, Rust N, van der Bruggen P, Cerundolo V. Direct isolation, phenotyping and cloning of low frequency antigen-specific cytotoxic T lymphocytes from peripheral blood. Curr Biol. 1998;8:413–416. doi: 10.1016/s0960-9822(98)70161-7. [DOI] [PubMed] [Google Scholar]
- 6.Parham P, Brodsky FM. Partial purification and some properties of BB7.2, a cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981;3:277–284. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- 7.Romero P, Gervois N, Schneider J, Escobar P, Valmori D, Pannetier C, Steinle A, Wölfel T, Liénard D, Brichard V, et al. Cytolytic T lymphocyte recognition of the immunodominant HLA-A*0201 restricted Melan-A/ MART-1 antigenic peptide in melanoma. J Immunol. 1997;159:2366–2374. [PubMed] [Google Scholar]
- 8.Valmori D, Fonteneau JF, Marañón C, Lizana, Gervois N, Liénard D, Rimoldi D, Jongeneel CV, Jotereau F, Cerottini JC, Romero P. Enhanced generation of specific tumor-reactive CTL in vitroby selected Melan-A/ MART-1 immunodominant peptide analogs. J Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- 9.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Cerundolo V, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 10.Mulcahy KA, Rimoldi D, Brasseur F, Rodgers S, Liénard D, Marchand M, Rennie IG, Murray AK, McIntyre CA, Platts KE, et al. Infrequent expression of the MAGE gene family in uveal melanomas. Int J Cancer. 1996;66:738–742. doi: 10.1002/(SICI)1097-0215(19960611)66:6<738::AID-IJC5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Smith B, Selby P, Southgate J, Pittman K, Bradley C, Blair GE. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet. 1991;338:1227–1229. doi: 10.1016/0140-6736(91)92100-g. [DOI] [PubMed] [Google Scholar]
- 12.Skipper JCA, Hendrickson RC, Gulden PH, Brichard V, Van Pel A, Chen Y, Shabanowitz J, Wölfel T, Slingluff CL, Jr, Boon T, et al. An HLA-A2–restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young JL, Ramage JM, Gaston JSH, Beverley PCL. In vitro responses of human CD45RObright RA− and CD45RO− RAbrightT cell subsets and their relationship to memory and naive T cells. Eur J Immunol. 1997;27:2383–2390. doi: 10.1002/eji.1830270937. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- 15.Mazzocchi A, Storkus WJ, Traversari C, Tarsini P, Maeurer MJ, Rivoltini L, Vegetti C, Belli F, Anichini A, Parmiani G, Castelli C. Multiple melanoma-associated epitopes recognized by HLA-A3-restricted CTLs and shared by melanomas but not melanocytes. J Immunol. 1996;157:3030–3038. [PubMed] [Google Scholar]
- 16.Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997;18:175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 17.Yee C, Riddell SR, Greenberg PD. Prospects for adoptive T cell therapy. Curr Opin Immunol. 1997;9:702–708. doi: 10.1016/s0952-7915(97)80052-0. [DOI] [PubMed] [Google Scholar]
- 18.Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995;16:487–494. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 19.de Vries TJ, Fourkour A, Wobbes T, Verkroost G, Ruiter DJ, van Muijen GN. Heterogeneous expression of immunotherapy candidate proteins gp100, MART-1, and tyrosinase in human melanoma cell lines and in human melanocytic lesions. Cancer Res. 1997;57:3223–3229. [PubMed] [Google Scholar]
- 20.Cormier JN, Huazi YM, Abati A, Fetsch P, Bettinotti M, Steinberg SM, Rosenberg SA, Marincola FM. Heterogeneous expression of melanoma-associated antigens and HLA-A2 in metastatic melanoma in vivo. Int J Cancer. 1998;75:517–524. doi: 10.1002/(sici)1097-0215(19980209)75:4<517::aid-ijc5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Mizoguchi H, O'Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 22.Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Liénard D, et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]