Abstract
During αβ thymocyte development, the clonotypic αβ–T cell receptor (TCR) is preceded by sequentially expressed immature versions of the TCR–CD3 complex: the pre-TCR, containing a clonotypic TCR-β chain and invariant pre-Tα, is expressed on pre-T cells before rearrangement of the TCR-α locus. Moreover, clonotype-independent CD3 complexes (CIC) appear on pro-T cells before VDJ rearrangements of TCR-β genes. The pre-TCR is known to mediate TCR-β selection, the prerequisite for maturation of CD4−8− double negative (DN) thymocytes to the CD4+8+ double positive stage. A developmental function of CIC has so far not been delineated. In mice single deficient and double deficient for CD3ζ/η and/or p56lck, we observe a pronounced reduction in the proportions of CD25+ DN thymocytes that express intracellular TCR-β chains. TCR-β transcripts are reduced in parallel with TCR-β polypeptide chains whereas no reduction in TCR-β locus rearrangements could be detected. Wild-type levels of TCR-β transcripts and of cells expressing TCR-β polypeptide chains are induced by treatment with anti-CD3ε mAb. The data suggest that the initial expression of rearranged TCR-β VDJ genes in pro-T cell to pre-T cell progression is dependent on CD3 complex signaling, and thus define a putative developmental function for CIC.
Keywords: TCR-β gene expression, CD3 complex, pro-TCR, thymus, signaling
During T cell ontogeny, the complete αβ-TCR first appears on the cell surface during the CD4+8+ double positive (DP)1 stage of thymocyte development, after VDJ rearrangements of both the TCR-β and the TCR-α gene loci. Its initial function is the screening of DP cells for proper recognition of MHC–peptide complexes in the thymic microenvironment, thus selecting the MHC- restricted, self-tolerant repertoire of peripheral T lymphocytes (reviewed in references 1, 2). The mature αβ-TCR is preceded by the pre-TCR, expressed on CD4−8− double negative (DN) thymocytes that have rearranged TCR-β VDJ genes and before rearrangement of the TCR-α locus. Accordingly, the pre-TCR consists of a TCR-β chain, a surrogate TCR-α chain termed pre-Tα, and components of the CD3 complex. It serves as a screening device for productive rearrangement of a TCR-β VDJ gene, a process termed TCR-β selection. Successful DN cells survive, proliferate, and mature to the DP stage (for review see references 3–6).
Expression of the pre-TCR on the thymocyte surface is preceded by expression of CD3 complexes that lack clonotypic components, so-called clonotype-independent CD3 complexes (CIC). This has first been recognized in experiments in which immature DN thymocytes were exposed to anti-CD3ε mAb, either in thymic organ culture (7, 8) or in vivo (9, 10). In mice genetically unable to generate a TCR-β chain (8–10), treatment with anti-CD3ε induced most known pre-TCR–dependent developmental responses. In wild-type (wt) mice treatment with anti-CD3ε induced premature shutdown of TCR-β gene rearrangement, equivalent to allelic exclusion (7). The latter experiments suggested that CD3ε is expressed at the surface of thymocytes of wt mice before TCR-β VDJ rearrangements (3, 7). The biochemical composition of CIC has first been studied by Wiest et al. (11) who reported the existence of CD3εγ and CD3εδ dimers that are brought to the surface of TCR-β negative immature thymocytes together with calnexin, due to a leaky endoplasmic reticulum retention mechanism in these cells (12). The CD3δ chain is physically present in CIC (11) but not required for pre-TCR function, as indicated by the undisturbed TCR-β selection in CD3δ-deficient mutant mice (13). CD3ζ is functionally associated with the pre-TCR (14) but its involvement with CIC has not been obvious (15). CD3ε cross-linking studies have suggested a role for the src family protein tyrosine kinase p56lck (Lck) in CIC signaling (15).
A function for CIC in normal thymocyte differentiation has so far not been recognized. On the contrary, the nature of the block in thymic development in CD3ε-deficient mice (16) has argued against a functional role of CIC: the DN stage of thymocyte development can be divided by the marker antigens CD44 and CD25 into four consecutive subsets: CD44+CD25−, CD44+CD25+, CD44−CD25+, and CD44−CD25− (17). TCR-β locus VDJ rearrangement begins during the CD44+CD25+ stage and peaks at the CD44−CD25+ stage (18, 19). TCR-β polypeptide chains are first detected in CD44−CD25+ DN cells (20, 21). TCR-β positive cells proceed to the CD44−CD25− stage and subsequently become DP cells (20–23). The block in thymocyte development in CD3ε-deficient mice lies between the CD44−CD25+ and the CD44−CD25− stages and has thus been indistinguishable from that in mice deficient for RAG1/2 or for several other components required for a functional pre-TCR (reviewed in reference 24). This has been taken as evidence against a functional role of CD3 complexes before the expression of the TCR-β chain and the formation of the pre-TCR.
In this paper, we report experiments on several strains of mutant mice with graded defects in CD3 complex signaling, suggesting a possible functional role for CIC. In mice deficient for Lck (25), or for CD3ζ/η (26–28), the generation of up to 15% of the wt number of DP thymocytes suggests residual pre-TCR/CD3 signaling activities. A drastic further reduction in DP cells in mice double deficient for CD3ζ/η and Lck suggests a more pronounced impairment in pre-TCR function with both defects combined. In addition, mice single deficient or double deficient (sd or dd, respectively) for CD3ζ/η and/or Lck have moderately or severely reduced numbers, respectively, of DN thymocytes with intracellular TCR-β polypeptide chains. TCR-β VDJ transcripts are reduced in parallel with TCR-β polypeptide chains whereas no reduction in TCR-β locus rearrangements could be detected. Stimulation with anti-CD3ε mAb restores TCR-β expression in thymocytes of mice double deficient for CD3ζ/η and Lck to the wt level. The results suggest that expression of rearranged TCR-β VDJ genes in pro-T cells requires signals from a functional CD3 complex, possibly assigning a developmental function to CIC.
Materials and Methods
Mice.
BALB/c mice, mice deficient of p56lck (25), and mice deficient of CD3ζ/η (28) were bred in the specific pathogen-free animal facilities of the Max-Planck-Institute. The latter two strains were back-crossed at least five times to C57Bl/6. Crosses of the p56lck and CD3ζ/η mutations were monitored by PCR analysis of DNA isolated from tissue obtained by earpunching, using primers described in the original papers (25, 28).
mAbs and Flow Cytometry.
The following mAbs, unlabeled or labeled with either FITC, phycoerythrin or biotin, were purchased from PharMingen (San Diego, CA): anti-CD4 (H129.19), anti-CD8 (53-6.7), anti-TCR-β (H57-597), anti-CD44 (IM7), anti-Ly9.1 (30C7), and anti-CD3ε (500A2). Anti-CD25 (5A2) was purified and labeled with FITC in our own laboratory. Red-670 coupled to streptavidin was used for B-labeled antibodies. Thymocytes were preincubated with supernatant of anti-FcR mAb 2.4G2 before analysis. Intracellular staining was done on cells fixed with paraformaldehyde and permeabilized with Saponin (Sigma, Heidelberg, Germany) as previously described (7, 20). Controls for cell surface stainings employed isotype-matched mAb labeled with the same fluorochrome; controls for intracellular stainings employed blocking with an excess of the same unlabeled mAb. Three-color FCM analysis employed a FACScan®; sorting employed a FACStar® plus (Becton Dickinson & Co., Sparks, MD).
Treatment with Anti-CD3ε mAb.
Mice were injected with 15 μg purified anti-CD3ε mAb 500A2 i.p. and their thymi were analyzed by FCM at various time points thereafter. To analyze the degree of thymocyte proliferation after injection of anti-CD3ε, the mice were injected at the same time with 250 μg 5-bromodeoxyuridine (BrdU; Boehringer Mannheim GmbH, Mannheim, Germany) i.p., and the drinking water was supplemented with 1 mg/ml BrdU. Thymocytes were analyzed for BrdU incorporation as previously described (21). Of note, cells were heated to 95°C for 10 min to melt DNA, before staining with anti-BrdU mAb. BrdU staining by this procedure is stoichiometric, and allows investigators to distinguish cells with incomplete labeling, i.e., after 1 S phase, from cells with maximal labeling, i.e., after 2 or more S phases (21, 29). Cell cycle analyses employed DNA staining with 7-amino-actinomycin D (7AAD; Sigma) and FCM analysis according to the method described in Rabinovitch et al. (30).
Reaggregate Thymic Organ Culture.
Reaggregate thymic organ culture (RTOC) were set up essentially as previously described (31, 32). To test the effect of anti-CD3ε mAb on thymocytes in RTOC, 1–2 × 105 thymocytes were incubated in suspension for 2 h with 10 μg/ml mAb 500A2, washed three times with culture medium, reaggregated with an equal number of thymic stroma cells, and incubated for 3 d. Thymic stroma cells were obtained from BALB/c fetal thymi at day of gestation 15 by treatment of thymic lobes for 5 d with desoxyguanine (31). Thymocytes derived from the mutant mice used in this study were of C57Bl/6 background that are, in contrast to BALB/c, negative for the genetic marker Ly9.1 (33). FCM analyses after 3 d of culture included gating for Ly9.1 negative cells.
Analysis of TCR-β Gene Rearrangements by Semiquantitative PCR.
DNA was prepared from thymocytes as described (34). DNA was amplified in 25 μl reaction buffer containing 0.1 μg DNA, 0.5 μM 5′ and 3′ primers, 200 μM of each dNTP (Pharmacia, Freiburg, Germany), and 0.2 U Supertaq (HT Biotechnology, Cambridge, UK), for 31 cycles (40 s at 94°C, 60 s at 63°C, 120 s at 72°C). Primers were 5′ Dβ2, 5′ Vβ8, 3′ Jβ2 (35), and 5′ Vβ5 (22). DNA samples were adjusted to similar concentrations according to the strength of the signals for the insulin gene that displays as two PCR products using the following primers: 5′ CCACCCAGGCTTTTGTCAA, 3′ ATGCTGGTGCAGCACTGATC. The insulin PCR was performed in serial dilutions (undiluted, 1:4, and 1:16). After adjustment of DNA concentrations, TCR-β PCR reactions were also done in 1:4 dilution steps. PCR products were subjected to gel electrophoresis in Tris-acetate-EDTA buffer with 1.6% agarose and visualized by staining with ethidium bromide as described (35, 36).
Estimation of TCR-β VDJC mRNAs by Semiquantitative Reverse Transcription PCR.
Total RNA was isolated using TRISOLV (Biotex, Houston, TX) as directed by the manufacturer. To eliminate remaining genomic DNA, RNA preparations were subjected to DNAse I (Boehringer Mannheim GmbH) digestion for 20 min at 37°C. Oligo(dt)-primed cDNA was prepared from total RNA using RNaseH-Reverse Transcriptase (Gibco, Eggenstein, Germany) according to the recommendations of the manufacturer. Concentrations of cDNAs were adjusted by competitive PCR between hypoxanthine phosphoribosyltransferase (HPRT, 249 bp) and a known amount of a control fragment (200 bp) using the primers described in Keller et al. (37). PCR was performed in a volume of 25 μl using 0.5 U SUPER TAQ and reagents obtained from HT Biotechnology. For reverse transcription (RT)-PCR of Vβ5DJCβ and Vβ8DJCβ products we used oligonucleotides and cycle conditions as previously described (35). 10 μl of the resulting amplified material was subjected to gel electrophoresis in Tris-borate-EDTA buffer with 1% agarose and visualized by staining with ethidium bromide.
Results
Reduced Proportions of Cells with Intracellular TCR-β Chains Among CD25+ DN Thymocytes in Mice Single Deficient and Double Deficient for CD3ζ/η and Lck.
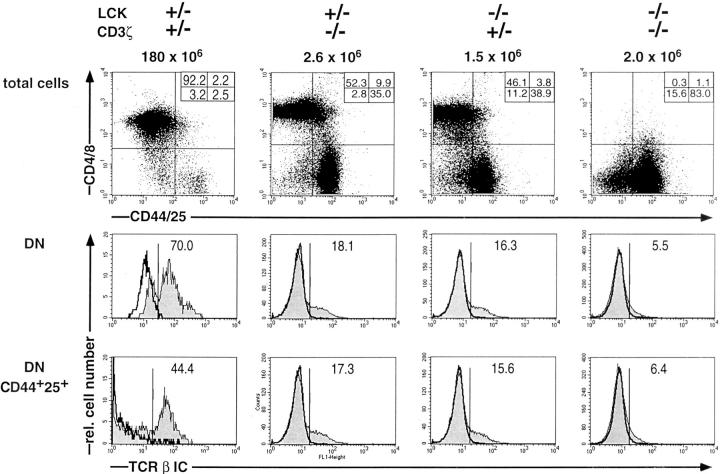
Previous results have shown that mice single deficient for CD3 ζ/η (ζ-sd) or Lck (Lck-sd) generate ∼5–15% of the wt number of DP cells (25–28). As shown by the data in Fig. 1 (top), the development of DP thymocytes is virtually completely blocked in mice double deficient of CD3ζ/η and Lck (ζ/ Lck-dd). As previously shown, CD25 is maintained on some of the DP cells in the single deficient mice (24, 25). A detailed characterization of the block in pre-TCR–dependent thymic development in ζ/Lck-dd mice has recently been reported (Würch, A., J. Biro, I. Falk, and K. Eichmann, manuscript submitted for publication).
Figure 1.
Three-color flow cytometric analyses of total thymocytes of adult wt, ζ-sd, Lck-sd, and ζ/Lck-dd mice: first color, CD4/CD8; second color, CD44/CD25; third color, intracellular (IC) TCR-β. (Top) Two parameter dot plots of CD4/CD8 vs. CD44/CD25; (middle) single parameter histograms of TCR-β IC (shaded profiles) in gated CD4−8− DN cells; (bottom) single parameter histograms for TCR-β IC (shaded profiles) in gated DN CD44+/ CD25+ cells. Genotypes and absolute numbers of total thymocytes are given on top, percentages of subpopulations are given within each panel. Negative controls for intracellular TCR-β stainings employed blocking with an excess of the same, unlabeled mAb (bold open profiles). The wt mouse was analyzed separately so that differences in fluorescence intensities are experimentally determined.
This paper is concerned with events before the formation of the pre-TCR, i.e., with factors that control the initial expression of TCR-β VDJ genes. The experiments were stimulated by the unexpected observation that total DN thymocytes of single deficient and in particular of ζ/Lck-dd mice contain reduced proportions of cells with intracellular TCR-β chains (Fig. 1, middle). As previously shown, thymocytes harboring intracellular TCR-β polypeptide chains first appear among CD44−CD25+ DN cells (20, 21). Whereas typically 40–50% of CD44−CD25+ cells are TCR-β+, this proportion increases to >90% in the CD44−CD25− subset, due to TCR-β selection. Accordingly, with considerable individual variation, ≤50% of the DN thymocytes of adult wt mice may consist of CD44−CD25− cells. In contrast, as the formation of this subset is pre-TCR–dependent, it is drastically reduced in single deficient mice and in ζ/Lck-dd mice (Fig. 1, top, and Würch, A., J. Biro, I. Falk, and K. Eichmann, manuscript submitted for publication). Therefore, we compared TCR-β expression of wt and mutant mice by excluding CD44−CD25− DN cells from the analysis, i.e., by gating on DN cells positive for CD44 and/or CD25 (Fig. 1, bottom). For the sake of convenience, cells gated in this way will be referred to as CD25+ DN cells, accounting for >85% of this subset in wt and mutant mice. In both kinds of single deficient mice, the proportions of TCR-β+ cells among CD25+ DN cells are <20% compared with >40% in wt mice. A more pronounced reduction of TCR-β+ cells is seen in the CD25+ DN cells of ζ/Lck-dd mice, ranging from 4–10% in individual mice. In addition, the intensity of intracellular TCR-β staining is decreased, particularly in thymocytes of ζ/Lck-dd mice. These results suggest that mice with compromised CD3 signaling possess reduced numbers of TCR-β+ CD25+ DN thymocytes. The extent of the reduction appears to be proportional to the severity of the defect in CD3 signaling.
Analyses of TCR-β Locus DJ and VDJ Rearrangements in Thymocytes of Mice Single-deficient and Double-deficient for CD3ζ/η and/or Lck.
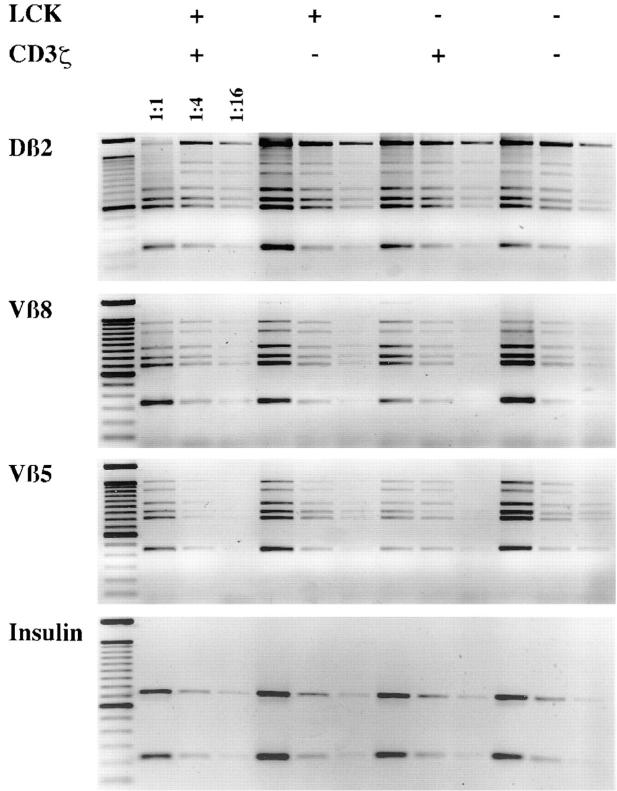
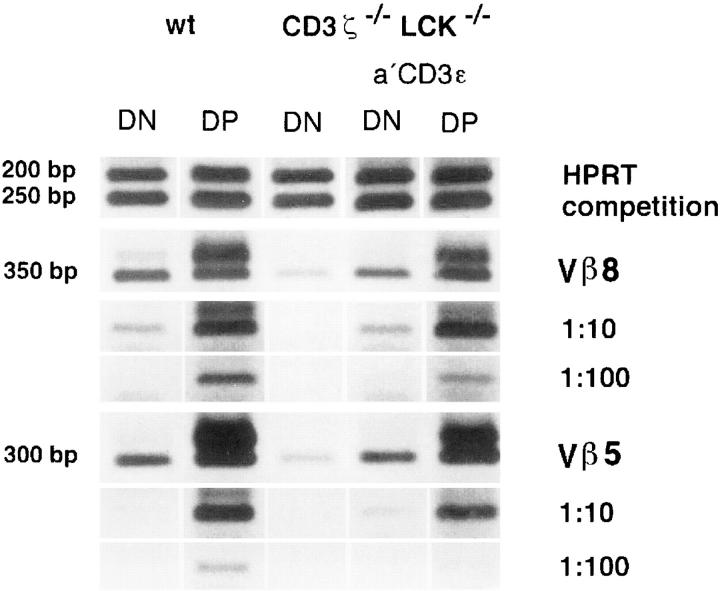
The reduced number of TCR-β+ CD25+ DN cells in ζ/Lck-dd mice may in principle be due to a reduced generation rate, a reduced proliferation rate, or an increased death rate of cells in this subset, or any combination of these factors. A reduced rate of generation of TCR-β+ thymocytes in mice deficient in CD3 complex signaling may be due to compromised TCR-β locus VDJ rearrangement. Semiquantitative analyses of DJ and VDJ rearrangements in purified CD25+ DN thymocytes of wt, ζ-sd, Lck-sd, and ζ/Lck-dd mice are shown in Fig. 2. If reduced TCR-β rearrangements were responsible for the low numbers of TCR-β+ CD25+ DN cells in single deficient and ζ/Lck-dd mice, this should be reflected in weaker PCR signals. In particular, signal intensities of ζ/Lck-dd thymocytes should be 5–10-fold weaker than that of wt CD25+ DN thymocytes, i.e., shifted by at least one dilution step. The data do not support this possibility, as they fail to reveal significant quantitative differences between the four strains of mice. There are minor quantitative variations, but these vary between experiments and may reflect individual and/or technical variability. Within the limits of this semiquantitative method, TCR-β DJ and VDJ rearrangements appear to proceed normally in thymocytes of mice deficient in CD3 complex signaling.
Figure 2.
Analyses of TCR-β locus rearrangements by semiquantitative PCR of sorted CD25+ DN cells of adult wt, ζ-sd, Lck-sd, and ζ/Lck-dd mice. DNA was adjusted to similar amounts according to the strength of the insulin PCR signals from fourfold dilutions. DJ (Dβ2Jβ2) and VDJ (Vβ5DJβ2, Vβ8DJβ2) PCRs were done in fourfold dilutions of the adjusted DNA samples as indicated. Amplified materials were subjected to gel electrophoresis in Tris-acetate-EDTA buffer with 1.6% agarose and visualized by staining with ethidium bromide. In the DJ rearrangements, the top band represents the germline configuration. The six bands underneath and in the VDJ rearrangements represent the Jβ21-6 elements from top to bottom.
Induction of Expression of Intracellular TCR-β Polypeptide Chains in Thymocytes of Mice Double-deficient for CD3ζ/η and Lck by Anti-CD3ε mAb.
If the paucity of TCR-β+ DN cells in single deficient and ζ/Lck-dd mice was in any way related to the deficiency in CD3 signaling it should be possible to increase the number of TCR-β+ DN cells by stimulating the thymocytes with anti-CD3ε mAb. Previously we and others have shown that treatment of thymocytes of various pre-TCR mutant mice with anti-CD3ε mAb leads to thymocyte proliferation and differentiation to the DP stage (8–10, 15, 38). Although in these previous experiments no attempts were made to separate proliferation from differentiation, it seemed desirable for the present experiments to distinguish the putative anti-CD3ε induced differentiation to TCR-β+ cells from the expansion of the preexisting TCR-β+ population through anti-CD3ε induced proliferation. If an increase in the size of the TCR-β+ subset depended on proliferation, the paucity of TCR-β+ cells would most likely be due to insufficient expansion. An increase in the size of the TCR-β+ subset independent of proliferation would support the possibility that CD3-mediated signals were involved in the control of TCR-β expression.
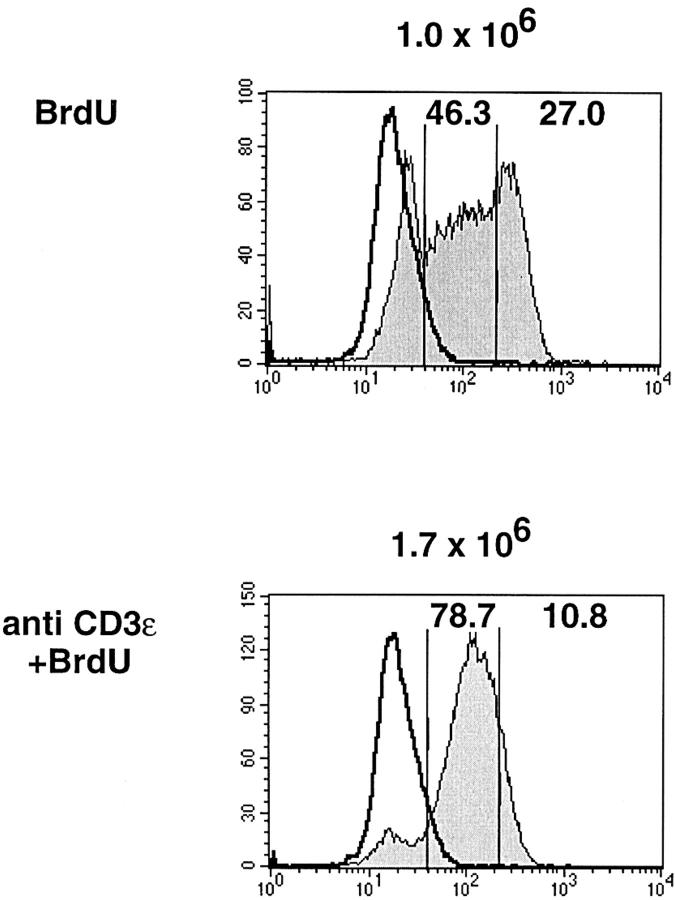
To distinguish these possibilities, we titrated the amounts of anti-CD3ε injected per ζ/Lck-dd mouse (data not shown) and identified a low dose that induces differentiation without increasing the spontaneous proliferation of thymocytes. The absence of additional proliferation is documented in Fig. 3, which compares the BrdU incorporation into thymocytes of ζ/Lck-dd mice during 3 d, untreated or after injection of 15 μg anti-CD3ε. We observe three distinct populations in untreated mice: cells with maximal BrdU labeling have passed two or more S phases, cells with submaximal labeling have passed one S phase, and cells without label have not divided. In mice injected with anti-CD3ε the vast majority of cells show homogeneous submaximal label. The data suggest that thymocytes of untreated mice divide heterogeneously and asynchronously with a mean division rate of approximately once per 3 d. Injection of 15 μg anti-CD3ε synchronizes cell division such that almost all of the cells divide once during the 3 d. Accordingly, total thymocyte numbers of injected mice remain within the range observed for untreated mice.
Figure 3.
BrdU incorporation (shaded profiles) into thymocytes of ζ/Lck-dd mice injected 3 d previously with 250 μg BrdU (top) or BrdU plus 15 μg anti-CD3ε mAb i.p. BrdU (1 mg/ml) was included in the drinking water of all injected mice. Total thymocyte numbers given at the top of each panel, percentages of gated subpopulations within each panel. Staining with anti-BrdU mAb was done after heating of the cells to melt DNA, allowing stoichiometric labeling of DNA. Controls (open profiles, bold) employed thymocytes from untreated mice subjected to the same staining protocol.
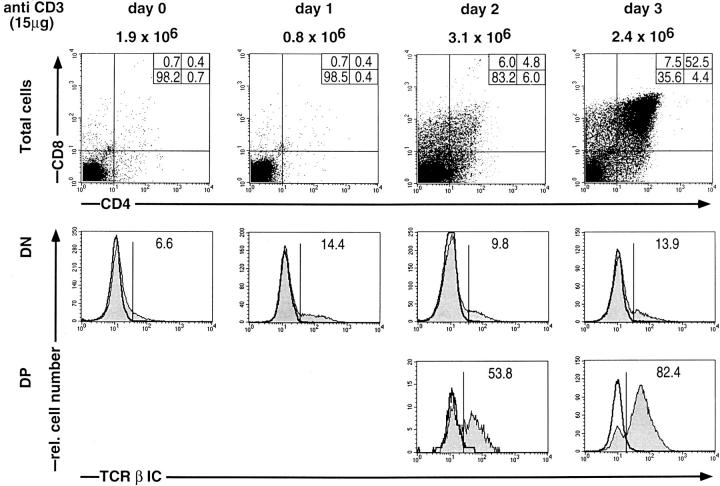
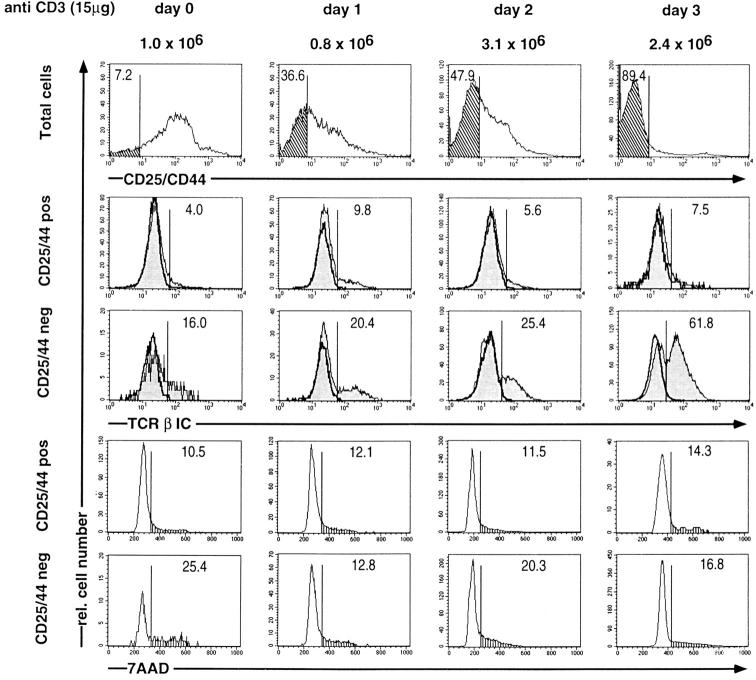
Fig. 4 shows experiments in which ζ/Lck-dd mice were injected with 15 μg anti-CD3ε on day 0 and their thymocytes analyzed for CD4 and CD8 and for intracellular TCR-β on each of three subsequent days. The proportion of TCR-β+ DN cells increases slightly but not significantly above the range observed in untreated mice. However, beginning with day 2 we observe the appearance of DP cells containing increasing proportions of TCR-β+ cells, reaching >80% on day 3. Since DP thymocytes on day 3 account for ∼50%, >40% of all thymocytes are now TCR-β+. As virtually all cells have divided once during the 3 d, this increased proportion of TCR-β+ cells cannot have arisen by proliferation of the preexisting TCR-β+ population. These data therefore suggest that cross-linking of CD3ε induces de novo expression of intracellular TCR-β polypeptide chains in ζ/Lck-dd thymocytes.
Figure 4.
Three-color FCM analyses of thymocytes of ζ/Lck-dd mice before (day 0) or on days 1, 2, and 3, after injection of 15 μg anti-CD3ε. Two parameter dot plots for CD4 and CD8 are shown on top, single parameter histograms of intracellular TCR-β expression (shaded profiles) in gated DN and DP cells below. Absolute thymocyte numbers given at the top. Percentages of subpopulations indicated in each frame. Negative controls for intracellular stainings employed blocking with an excess of the same, unlabeled mAb (bold profiles). DN thymocytes have a slightly higher background than DP thymocytes.
From the data in Fig. 4 it appears that induction of TCR-β expression by anti-CD3ε in ζ/Lck-dd thymocytes occurs mostly after the cells acquire the DP phenotype. Since this would indicate a reversal of the physiological order of events, it was of interest to test TCR-β induction by anti-CD3ε in subpopulations of DN thymocytes. As shown in Fig. 5, untreated ζ/Lck-dd mice generate very few CD44−CD25− cells. However, TCR-β+ cells are enriched in this population suggesting rudimentary TCR-β selection (Würch, A., J. Biro, I. Falk, K. Eichmann, manuscript submitted for publication). The CD44−CD25− population increases rapidly on days 1 and 2 after anti-CD3ε, i.e., before the appearance of significant numbers of DP cells (compare to Fig. 4, data on the same individual mice). These CD44−CD25− DN cells contain enhanced proportions of TCR-β+ cells, suggesting an induction of TCR-β expression before acquisition of the DP stage, at least in a proportion of cells. Nevertheless, little TCR-β induction is seen in CD25+ DN cells, suggesting that stimulation with anti-CD3ε induces TCR-β expression and subsequent differentiation in rapid succession.
Figure 5.
Three-color FCM analyses of thymocytes of ζ/Lck-dd mice before (day 0) or on days 1, 2, and 3, after injection of 15 μg anti-CD3ε. First color, CD44/CD25; second color, intracellular TCR-β; third color, DNA staining by 7AAD. Single parameter histograms for total thymocytes, and for gated CD44+/ CD25+, and CD44−CD25− subsets shown as indicated. Percentages of CD44−CD25− subsets (top, cross-hatched), for TCR-β+ subsets (middle, shaded profiles; blocking controls in bold), and cells in S/G2/M phase of cell cycle (vertical hatch, bottom) given in each frame. The mice injected with anti-CD3ε are the same as those shown in Fig. 4, to facilitate comparisons of the proportions of DP cells (Fig. 4) and CD44−CD25− cells on each day.
The experiment in Fig. 5 was complemented by DNA staining in order to assess anti-CD3ε induced alterations in cell cycle in DN subpopulations, as well as putative apoptotic effects. Thymocytes with subdiploid DNA content are not detected in either untreated or treated mice, arguing against the possibility that the observed effects are influenced by massive cell death. The CD25+ DN population contains a small proportion of cells in S/G2/M that does not vary after injection of anti-CD3ε. The few CD44− CD25− cells in untreated ζ/Lck-dd mice contain more than twice as many cells in S/G2/M, consistent with the maintenance of limited TCR-β selection. Whereas the number of CD44−CD25− cells increases after injection of anti-CD3ε, a transient decrease in the proportion of CD44− CD25− cells in S/G2/M is seen on day 1. This may indicate a transient cell cycle arrest, possibly the basis for the anti-CD3ε induced synchronization of proliferation suggested by the data in Fig. 3. These data exclude the possibility that injection of 15 μg anti-CD3ε selectively stimulates proliferation in the CD44−CD25− population and further support our conclusion that induction of TCR-β expression in ζ/Lck-dd thymocytes is independent of proliferation.
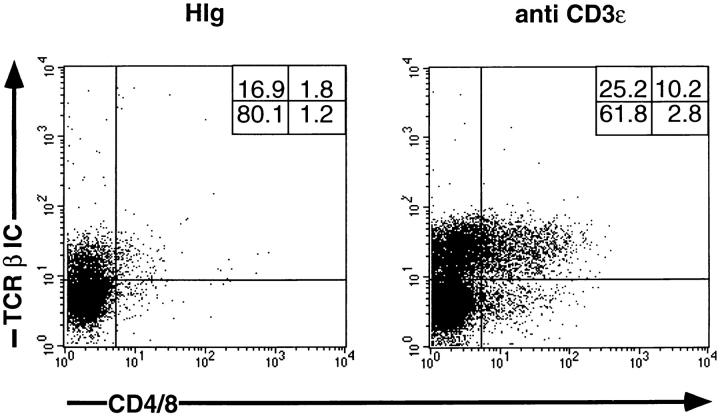
Even though ζ/Lck-dd mice generate minimal numbers of peripheral CD3 positive cells (data not shown), it was possible that in vivo injection of anti-CD3ε mAb induces such cells to release cytokines that influence thymocytes (39), possibly including the induction of TCR-β in the thymus. To exclude this possibility, RTOC were set up with ζ/Lck-dd thymocytes preincubated with anti-CD3ε or HIg for 2 h in suspension, then extensively washed, and thereafter reaggregated with thymic stroma cells. As shown in Fig. 6, after 3 d of RTOC such anti-CD3ε–treated ζ/Lck-dd thymocytes contain increased proportions of TCR-β+ cells, some of which have acquired CD4/CD8 expression. Induction is less efficient than in vivo, most likely owing to the brief exposure to the antibody. After RTOC, HIg-treated (and untreated, not shown) thymocytes also have a slightly enhanced TCR-β expression compared with ex vivo cells, perhaps due to experimental handling. The data suggest that TCR-β induction occurs by direct ligation of CD3ε on the surface of ζ/Lck-dd thymocytes.
Figure 6.
Three-color FCM analysis of thymocytes of ζ/Lck-dd mice on day 3 of RTOC. On day 0, 1.2 × 105 thymocytes were incubated for 2 h in suspension culture with either 10 μg/ml of HIg or of anti-CD3ε. Thymocytes were extensively washed before reaggregation with 1.2 × 105 thymic stroma cells derived from day 15 BALB/c fetal thymic lobes incubated for 5 d with desoxyguanine. First color, CD4/CD8; second color, TCR-β IC; third color, Ly9.1. Two parameter dot plots are on gated Ly9.1 negative cells, i.e., cells originating from ζ/Lck-dd mice.
TCR-β Expression in ζ/Lck-dd Thymocytes Is Controlled at the mRNA Level.
The levels of TCR-β VDJC mRNAs were estimated by semiquantitative RT-PCR. Purified DN and DP thymocytes of wt mice, DN cells of untreated ζ/Lck-dd mice, and DN and DP thymocytes of ζ/Lck-dd mice 3 d after injection of 15 μg anti-CD3ε mAb, were analyzed. The results in Fig. 7 reveal ∼10-fold lower Vβ8 transcript levels in DN cells of ζ/Lck-dd mice compared with wt DN cells. Slightly less pronounced differences are seen for Vβ5 transcripts. Injection of anti-CD3ε induces Vβ8 and Vβ5 mRNAs in DN cells nearly to wt levels, and the DP cells induced in anti-CD3ε treated ζ/Lck-dd mice display wt levels of Vβ8 and Vβ5 mRNAs as well. The data exclude the possibility that TCR-β expression in ζ/Lck-dd mice is limited by some form of translational or posttranslational regulation. The good correlations between levels of TCR-β VDJC mRNAs and the proportions of cells expressing TCR-β polypeptide chains suggest that TCR-β expression in the thymus of ζ/Lck-dd mice is impaired at the mRNA level.
Figure 7.
Semiquantitative RT-PCR of Vβ5DJCβ and Vβ8DJCβ mRNAs in isolated DN and DP thymocytes of wt mice, in DN cells of untreated ζ/Lck-dd mice, and in DN and DP cells of ζ/Lck-dd mice injected 3 d previously with anti-CD3ε mAb. cDNAs were adjusted to equal concentrations by competitive PCR between HPRT (249 bp) and a control fragment (200 bp) added in known amounts. cDNA concentrations equivalent to the standard amount of HPRT control fragment shown on top. 10 μl of the resulting amplified material was subjected to gel electrophoresis in Tris-borate-EDTA buffer with 1% agarose and visualized by staining with ethidium bromide.
Discussion
The work presented in this paper was stimulated by the phenotype of ζ/Lck-dd thymocytes which features a drastic reduction in the numbers of TCR-β+ CD25+ DN cells and reduced TCR-β mRNA levels in this population. This was unexpected as a pre-TCR–associated block in thymocyte development should leave maturation up to the TCR-β+ CD25+ DN stage unperturbed. Experiments were performed to link this phenotype to the defect in CD3 signaling in ζ/Lck-dd mice, and to distinguish among three nonmutually exclusive mechanisms that could account for this phenotype: a block in the generation, a block in the proliferation, or a shorter lifetime of TCR-β+ cells.
A causal relationship between defective CD3 complex signaling and the paucity of TCR-β+ cells in the mutant mice is suggested by two lines of evidence. First, the extent in reduction of TCR-β+ cells appears to reflect the severity in the malfunction of the CD3 complex. Whereas mice single-deficient for CD3ζ/η or for Lck possess ∼50% of the wt number of TCR-β+ CD25+ DN cells, the number further drops to ∼10% in ζ/Lck-dd mice. It is not excluded that Lck and perhaps also the CD3ζ/η module may have roles in other signaling pathways in immature thymocytes, but the aggravation associated with the combined deficiency strongly suggests the involvement of the CD3 complex. Second, the reduction in TCR-β+ cells including TCR-β mRNA levels is corrected by treating ζ/Lck-dd mice with anti-CD3ε mAb. We excluded that the induction is mediated by long range in vivo effects, suggesting a direct induction via CD3ε on the thymocyte surface.
A defect in the generation of TCR-β+ cells in ζ/Lck-dd mice would suggest a role for CD3 complexes before the appearance of TCR-β chains, i.e., clonotype-independent CD3 components. In contrast, a block in proliferation or a shortened survival time of TCR-β+ cells would indicate regulation by the pre-TCR–associated CD3 complex. Therefore, it is important to discriminate between these alternatives. Thymocytes of untreated ζ/Lck-dd mice proliferate asynchronously such that about one-fourth divides more than once, one-half divides just once, and one-fourth does not divide during 3 d. The mean rate of division thus appears to be once in 3 d, i.e., 3 d appears to be the mean turnover period for thymocytes of untreated ζ/Lck-dd mice. By titration of anti-CD3ε we identified a low dose at which proliferation and turnover appear to be synchronized but not quantitatively altered. The subset of TCR-β+ cells increases selectively ∼10-fold in ζ/Lck-dd mice treated with this dose of anti-CD3ε, whereas the total thymic cellularity remains within the range of untreated mice. Synchronization extends to the CD44−CD25− cells, excluding selective proliferation in the induced cell population. These data prove that the numerical increase of the TCR-β+ subset after injection of anti-CD3ε is independent of proliferation. As a second possibility, the increased proportion of the TCR-β+ subset after anti-CD3ε may be caused by a selective increase in longevity of the TCR-β+ cells in ζ/Lck-dd mice. As outlined above, cells turnover once during 3 d so that full survival of all TCR-β+ cells may result in an increase of merely twofold within 3 d after injection of anti-CD3ε. Thus, in order to account for the observed proportion of >40% TCR-β+ cells after 3 d, the numbers of TCR-β negative cells would have to be reduced at least fourfold, which would require substantial cell death and result in a significant decrease in thymic cellularity. Such a scenario is not consistent with our data which therefore argues against the possibility that anti-CD3ε increases the number of TCR-β+ cells by increasing their longevity. Assuming that the anti-CD3ε treatment corrects the physiological defects associated with CD3 malfunction, these results argue against the possibility that the paucity of TCR-β+ cells in ζ/Lck-dd mice is mainly caused by impaired proliferation or a reduced survival time. Hence, the paucity of TCR-β+ cells in ζ/Lck-dd mice appears to be mainly a consequence of the impaired generation of cells that express intracellular TCR-β polypeptide chains.
Among the biosynthetic levels at which the generation of TCR-β+ cells could be compromised we considered the rearrangement of TCR-β VDJ genes and their expression at the mRNA levels. An astounding fact in lymphopoiesis has been the striking parallelism in the early phases of T and B cell development, including the molecular design and the selection processes mediated by the pre-TCR and the pre-BCR (40). Recent data by Gong and Nussenzweig (41) have shown that the Igβ signaling component of the BCR is required for rearrangement of the Igμ chain genes in immature B cells. In contrast, we could not detect evidence for impaired TCR-β rearrangements in the CD3 signaling-deficient mice studied in this report. It should be pointed out that we used a semiquantitative method to estimate the frequencies of TCR-β rearrangement, so that moderate quantitative differences might have been missed. Nevertheless, using similar semiquantitative technology, we see striking quantitative differences between TCR-β VDJC mRNA levels of wt and ζ/Lck-dd mice. Indeed, the mRNA levels fully account for the differences in the proportions of TCR-β+ cells in wt and ζ/Lck-dd mice. Therefore, we suggest that the paucity of TCR-β+ cells in ζ/Lck-dd mice is predominantly caused by a block in the expression of rearranged TCR-β VDJ genes at the mRNA level. So far we do not know whether regulation takes place at a transcriptional or posttranscriptional level. Other examples for a control of mRNA levels by CD3 signaling in immature thymocytes include the RAG genes (42) and germline transcripts of TCR-β (43) and TCR-α genes (42).
It cannot be excluded that TCR-β chains are expressed in most ζ/Lck-dd thymocytes at a level below detection by intracellular staining. If this were the case, a role for the pre-TCR in TCR-β expression could be rescued by assuming that a low basal level of TCR-β chains is synthetized CD3-independently, giving rise to the formation of a few pre-TCR complexes on the cell surface; subsequent full TCR-β expression requires positive feedback by signaling through the pre-TCR. In the absence of experimental support for such a scenario, we think that a more plausible interpretation of the present experiments is that the CD3-dependent signals inducing TCR-β expression in immature thymocytes are generated by CIC. CIC may thus represent developmentally relevant CD3 complexes. As CIC are expressed on thymocytes at the pro-T cell stage we suggest the term pro-TCR, in analogy to the pre-TCR. It remains to be elucidated in which way the pro-TCR generates the signals required for TCR-β expression. Speculations with regard to a ligand for the pre-TCR have been discussed at length (40, 44) and the arguments, which apply to the pro-TCR as well, shall not be repeated here. At present the evidence appears to favor the possibility that the immature forms of the TCR may generate signals without a requirement for ligand recognition (45). Additional open questions include whether the pro-TCR contains other components, in addition to those of the CD3 complex. An obvious candidate is pre-Tα (46), which has been shown to be present at the mRNA level in pro-T cells (47). A surrogate TCR-β chain may also be considered, as well as other proteins that have been found associated with the pre-TCR (48, 49).
In ζ/Lck-dd mice, a small residual CD3 signaling activity is suggested by a small amount of pre-TCR–dependent TCR-β selection spontaneously taking place. Recent work on the characterization of mice double deficient for the src-family protein tyrosine kinases Lck and Fyn has revealed that both are involved in pre-TCR signaling (50, 51). Although Lck seems to be part of the major pathway, Fyn can partially replace Lck in its absence. Therefore, it is reasonable to assume that the small residual CD3 signaling activity, as well as the activation of ζ/Lck-dd thymocytes by anti-CD3ε, is mediated by a cooperation between CD3ε and Fyn. Although this activity is insufficient for the generation of significant numbers of DP cells, it suffices to mediate ∼10% of wt TCR-β expression. This finding indicates a relatively low signaling requirement of TCR-β expression, and is consistent with a hypothesis in which successive CD3-dependent steps in thymocyte development require stepwise increasing signal intensities (52). It would be of interest to study TCR-β expression in the thymus of mice in which CD3 signaling is totally abolished.
What could be the biological purpose of a mechanism that places TCR-β expression under the control of CD3 signaling? Optimal CD3 signaling requires expression of multiple gene products including those of the CD3 complex proper, downstream elements such as protein tyrosine kinases of the src and syk families, as well as other known and unknown factors. It is possible that the correct timing in the activation of the large array of involved genes is not free of errors. Placing TCR-β expression under the control of CD3 signaling would provide an elegant mechanism for a selective recruitment of signal-competent cells into the developmental process, and for excluding signal-incompetent cells from this process. Future studies will have to test the hypothesis that ordered and complete expression of the CD3-associated signaling system may be error prone. If so, TCR-β expression would define a novel CD3-dependent checkpoint in early thymocyte development, preceding TCR-β selection.
Acknowledgments
We thank Drs. B. and M. Malissen for mice; Drs. A. Singer, P. Love, and I. Haidl for critical reading of the manuscript; and Mr. H. Kohler and Ms. P. Wehrstedt for able technical assistance.
Abbreviations used in this paper
- BrdU
5-bromodeoxyuridine
- CIC
clonotype-independent CD3 complex
- dd
double deficient
- DN
double negative
- DP
double positive
- HPRT
hypoxanthine phosphoribosyltransferase
- Lck
p56lck
- RT
reverse transcription
- RTOC
reaggregate thymic organ culture
- sd
single deficient
- wt
wild-type
Footnotes
This paper was written while K. Eichmann was a Scholar-in-Residence at the Fogarty International Center for Advanced Study in the Health Sciences, National Institutes of Health, Bethesda, Maryland.
A. Würch and J. Biro contributed equally to this work.
References
- 1.Kisielow P, von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- 2.Robey E, Fowlkes BJ. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 3.Levelt CN, Eichmann K. Receptors and signals in early thymic selection. Immunity. 1995;3:667–672. doi: 10.1016/1074-7613(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 4.Fehling HJ, von Boehmer H. Early αβ T cell development in the thymus of normal and genetically altered mice. Curr Opin Immunol. 1997;9:263–275. doi: 10.1016/s0952-7915(97)80146-x. [DOI] [PubMed] [Google Scholar]
- 5.Zunicka-Pflücker JL, Lenardo MJ. Regulation of thymocyte development from immature progenitors. Curr Opin Immunol. 1996;8:215–224. doi: 10.1016/s0952-7915(96)80060-4. [DOI] [PubMed] [Google Scholar]
- 6.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 7.Levelt CN, Ehrfeld A, Eichmann K. Regulation of thymocyte development through CD3. I. Timepoint of ligation of CD3ε determines clonal deletion or induction of developmental program. J Exp Med. 1993;177:707–716. doi: 10.1084/jem.177.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levelt CN, Mombaerts P, Iglesias A, Tonegawa S, Eichmann K. Restoration of early thymocyte differentiation in T-cell receptor β-chain-deficient mutant mice by transmembrane signaling through CD3ε. Proc Natl Acad Sci USA. 1993;90:11401–11405. doi: 10.1073/pnas.90.23.11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs H, Vandeputte D, Tolkamp L, De Vries E, Borst J, Berns A. CD3 components at the surface of pre-T cells can mediate pre-T cell development in vivo. . Eur J Immunol. 1994;24:934–939. doi: 10.1002/eji.1830240423. [DOI] [PubMed] [Google Scholar]
- 10.Shinkai Y, Alt FW. CD3ε-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2−/−mice in the absence of TCR-β chain expression. Int Immunol. 1994;6:995–1001. doi: 10.1093/intimm/6.7.995. [DOI] [PubMed] [Google Scholar]
- 11.Wiest DL, Burgess WH, McKean D, Kearse KP, Singer A. The molecular chaperone calnexin is expressed on the surface of immature thymocytes in association with clonotype-independent CD3 complexes. EMBO J. 1995;14:3425–3433. doi: 10.1002/j.1460-2075.1995.tb07348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiest DL, Bhandoola A, Punt J, Kreibich G, McKean D, Singer A. Incomplete endoplasmic-reticulum (ER) retention in immature thymocytes as revealed by surface expression of ER-resident molecular chaperones. Proc Natl Acad Sci USA. 1997;94:1884–1889. doi: 10.1073/pnas.94.5.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dave VP, Cao ZS, Browne C, Alarcon B, Fernandezmiguel G, Lafaille J, Delahera A, Tonegawa S, Kappes DJ. CD3-δ deficiency arrests development of the αβ but not the γδ T-cell lineage. EMBO J. 1997;16:1360–1370. doi: 10.1093/emboj/16.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Oers NSC, von Boehmer H, Weiss A. The pre-T cell receptor (TCR) complex is functionally coupled to the TCR-ζ subunit. J Exp Med. 1995;182:1585–1590. doi: 10.1084/jem.182.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levelt CN, Mombaerts P, Wang B, Kohler H, Tonegawa S, Eichmann K, Terhorst C. Regulation of thymocyte development through CD3: functional dissociation between p56lckand CD3ζ in early thymic selection. Immunity. 1995;3:215–222. doi: 10.1016/1074-7613(95)90091-8. [DOI] [PubMed] [Google Scholar]
- 16.Malissen M, Gillet A, Ardouin L, Bouvier G, Tracy J, Ferrier P, Vivier E, Malissen B. Altered T cell development in mice with a targeted mutation of the CD3β gene. EMBO J. 1995;14:4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8−triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 18.Godfrey DI, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. Onset of TCRβ gene rearrangement and role of TCRβ expression during CD3−CD4−CD8−thymocyte differentiation. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- 19.Petrie HT, Livak F, Burtrum D, Mazel S. T cell receptor gene recombination patterns and mechanisms: cell death, rescue, and T cell production. J Exp Med. 1995;182:121–127. doi: 10.1084/jem.182.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levelt CN, Carsetti R, Eichmann K. Regulation of thymocyte development through CD3. II. Expression of T cell receptor β CD3ε and maturation to the CD4+CD8+stage are highly correlated in individual thymocytes. J Exp Med. 1993;178:1867–1875. doi: 10.1084/jem.178.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falk I, Biro J, Kohler H, Eichmann K. Proliferation kinetics associated with T cell receptor-β chain selection of fetal murine thymocytes. J Exp Med. 1996;184:2327–2339. doi: 10.1084/jem.184.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallick CA, Dudley EC, Viney JL, Owen MJ, Hayday AC. Rearrangement and diversity of T cell receptor β chain genes in thymocytes: a critical role for the β chain in development. Cell. 1993;73:513–519. doi: 10.1016/0092-8674(93)90138-g. [DOI] [PubMed] [Google Scholar]
- 23.Dudley EC, Petri HT, Shah LM, Owen MJ, Hayday AC. T cell receptor β chain gene rearrangement and selection during thymocyte development in adult mice. Immunity. 1994;1:83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka Y, Ardouin L, Gillet A, Lin SY, Magnan A, Malissen B, Malissen M. Early T cell development in CD3-deficient mice. Immunol Rev. 1995;148:171–199. doi: 10.1111/j.1600-065x.1995.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 25.Molina TJ, Kishihara K, Siderovski DP, Van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A, Davidson D, Mak T. Profound block in thymocyte development in mice lacking p56lck . Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 26.Liu CP, Ueda R, She J, Sancho J, Wang B, Weddell G, Loring J, Kurahara E, Dudley EC, Hayday A, et al. Abnormal T cell development in CD3-ζ−/−mutant mice and identification of a novel T cell population in the intestine. EMBO J. 1993;12:4863–4875. doi: 10.1002/j.1460-2075.1993.tb06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Love PE, Shores EW, Johnson MD, Tremblay ML, Lee EJ, Grinberg A, Huang SP, Singer A, Westphal H. T cell development in mice that lack the ζ chain of the T cell antigen receptor complex. Science. 1993;261:918–921. doi: 10.1126/science.7688481. [DOI] [PubMed] [Google Scholar]
- 28.Malissen M, Gillet A, Rocha B, Trucy J, Vivier E, Boyer C, Kontgen F, Brun N, Mazza G, Spanopoulou E, et al. T cell development in mice lacking the CD3-ζ/η gene. EMBO J. 1993;12:4347–4355. doi: 10.1002/j.1460-2075.1993.tb06119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoy CA, Seamer LL, Schimke RT. Thermal denaturation of DNA for immunochemical staining of incorporated bromodeoxyuridine (BrdU): critical factors that affect the amount of fluorescence and the shape of BrdUrd/DNA histogram. Cytometry. 1989;10:718–725. doi: 10.1002/cyto.990100608. [DOI] [PubMed] [Google Scholar]
- 30.Rabinovitch PS, Torres RM, Engel D. Simultaneous cell cycle analysis and two-color surface immunofluorescence using 7-amino-actinomycin D and single laser excitation: applications to study of cell activation and the cell cycle of murine Ly-1 B cells. J Immunol. 1986;136:2769–2775. [PubMed] [Google Scholar]
- 31.Anderson S, Owen JJ, Moore NC, Jenkinson EJ. Characteristics of an in vitrosystem of thymocyte positive selection. J Immunol. 1994;153:1915–1920. [PubMed] [Google Scholar]
- 32.Barthlott T, Kohler H, Eichmann K. Asynchronous coreceptor downregulation after positive thymic selection; prolonged maintenance of the double-positive state in CD8 lineage differentiation due to sustained biosynthesis of the CD4 coreceptor. J Exp Med. 1997;185:357–362. doi: 10.1084/jem.185.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ledbetter JA, Herzenberg LA. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 34.Gross-Bellard M, Oudet P, Chambon P. Isolation of high molecular weight DNA from mammalian cells. Eur J Biochem. 1973;36:32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 35.Anderson SJ, Abraham KM, Nakayama T, Singer A, Perlmutter RM. Inhibition of T-cell receptor β chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck . EMBO J. 1992;11:4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace VA, Kawai K, Levelt CN, Kishihara K, Molina T, Timms E, Pircher H, Penninger J, Ohashi P, Eichmann K, Mak TW. T lymphocyte development in p56lckdeficient mice: allelic exclusion of the TCR β locus is incomplete but thymocyte development is not restored by TCRβ or TCRαβ transgenes. Eur J Immunol. 1995;25:1312–1318. doi: 10.1002/eji.1830250527. [DOI] [PubMed] [Google Scholar]
- 37.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fehling HJ, Iritani BM, Krotkova A, Forbush KA, Laplace C, Perlmutter RM, von Boehmer H. Restoration of thymopoiesis in pTα-/- mice by anti-CD3ε antibody treatment or with transgenes encoding activated Lck or tailless pTα. Immunity. 1997;6:703–714. doi: 10.1016/s1074-7613(00)80446-x. [DOI] [PubMed] [Google Scholar]
- 39.Rueff-Juy D, Libermann I, Drapier AM, Guillon JC, Leclerc C, Cazenave PA. Cellular basis of the resistance of newborn mice to the pathogenic effects of anti-CD3 treatment. Int Immunol. 1990;3:683–690. doi: 10.1093/intimm/3.7.683. [DOI] [PubMed] [Google Scholar]
- 40.Borst J, Jacobs H, Brouns G. Composition and function of T-cell receptor and B-cell receptor complexes on precursor lymphocytes. Curr Opin Immunol. 1996;8:181–190. doi: 10.1016/s0952-7915(96)80056-2. [DOI] [PubMed] [Google Scholar]
- 41.Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Ig beta. Science. 1996;272:411–414. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- 42.Levelt CN, Wang B, Ehrfeld A, Terhorst C, Eichmann K. Regulation of T cell receptor (TCR)-β locus allelic exclusion and initiation of TCR-α locus rearrangement in immature thymocytes by signaling through the CD3 complex. Eur J Immunol. 1995;25:1257–1265. doi: 10.1002/eji.1830250519. [DOI] [PubMed] [Google Scholar]
- 43.Senoo M, Shinkai Y. Regulation of Vβ germline transcription in RAG-deficient mice by CD3ε-mediated signals: implication of Vβ transcriptional regulation in TCRβ allelic exclusion. Int Immunol. 1998;10:553–560. doi: 10.1093/intimm/10.5.553. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs H, Iacomini J, Van de Ven M, Tonegawa S, Berns A. Domains of the TCRβ chain required for early thymocyte development. J Exp Med. 1996;184:1833–1843. doi: 10.1084/jem.184.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irving BA, Alt FW, Killeen N. Thymocyte development in the absence of pre-T cell receptor extracellular immunoglobulin domains. Science. 1998;280:905–909. doi: 10.1126/science.280.5365.905. [DOI] [PubMed] [Google Scholar]
- 46.Fehling JJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T cell receptor α gene in the development of αβ but not of γδ cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 47.Wilson A, MacDonald HR. Expression of genes encoding the pre-TCR and CD3 complex during thymus development. Int Immunol. 1995;7:1659–1664. doi: 10.1093/intimm/7.10.1659. [DOI] [PubMed] [Google Scholar]
- 48.Takase K, Wakizaka K, von Boehmer H, Wada I, Moriya H, Saito T. A new 12-kilodalton dimer associated with pre-TCR complex and clonotype-independent CD3 complex on immature thymocytes. J Immunol. 1997;159:741–747. [PubMed] [Google Scholar]
- 49.Wakizaka K, Masuda Y, Saito T. A novel 90-kda tyrosine-phosphorylated protein associated with tcr complex in thymocytes. Eur J Immunol. 1998;28:636–645. doi: 10.1002/(SICI)1521-4141(199802)28:02<636::AID-IMMU636>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 50.Van Oers NSC, Lowin-Kropf B, Finlay D, Conolly K, Weiss A. αβ T cell development is abolished in mice lacking both lck and fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 51.Groves T, Smiley P, Cooke MP, Forbush K, Perlmutter RM, Guidos CJ. Fyn can partially substitute for lck in T lymphocyte development. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 52.Eichmann K. A signal strength hypothesis of thymic selection—preliminary considerations. Immunol Lett. 1995;44:87–90. doi: 10.1016/0165-2478(94)00197-y. [DOI] [PubMed] [Google Scholar]