Abstract
T cell activation and clonal expansion is the result of the coordinated functions of the receptors for antigen and interleukin (IL)-2. The protein tyrosine kinase p56lck is critical for the generation of signals emanating from the T cell antigen receptor (TCR) and has also been demonstrated to play a role in IL-2 receptor signaling. We demonstrate that an IL-2–dependent, antigen-specific CD4+ T cell clone is not responsive to anti-TCR induced growth when propagated in IL-2, but remains responsive to both antigen and CD3ε-specific monoclonal antibody. Survival of this IL-2–dependent clone in the absence of IL-2 was supported by overexpression of exogenous Bcl-xL. Culture of this clonal variant in the absence of IL-2 rendered it susceptible to anti-TCR–induced signaling, and correlated with the presence of kinase-active Lck associated with the plasma membrane. The same phenotype is observed in primary, resting CD4+ T cells. Furthermore, the presence of kinase active Lck associated with the plasma membrane correlates with the presence of ZAP 70–pp21ζ complexes in both primary T cells and T cell clones in circumstances of responsive anti-TCR signaling. The results presented demonstrate that IL-2 signal transduction results in the functional uncoupling of the TCR complex through altering the subcellular distribution of kinase-active Lck.
Keywords: Lck, kinase, interleukin 2, regulation
T cell activation and subsequent growth is dependent upon the coordinated expression and function of the α/β-TCR and the IL-2 receptor (1–6). Expression of the high affinity receptor for IL-2, as well as the de novo transcription and translation of IL-2, is induced upon T cell antigen recognition (7–10). The control of T cell growth in these circumstances is regulated through the autocrine production and use of IL-2, which are interrelated processes in that the loss of high levels of membrane IL-2Rα chain expression and the production of IL-2 are coordinated (11–15). IL-2 has also been shown to predispose T cells to apoptosis upon subsequent interaction with antigen (16). Furthermore, the exposure of T cell clones to exogenous IL-2 can result in their inability to respond to antigen (17). Thus, IL-2 is essential in supporting T cell clonal expansion, and is also involved in a feedback regulatory mechanism limiting cellular expansion and/or function.
The JAK/STAT pathway is central in the transduction of IL-2R signaling (18–23). However, a role for src family tyrosine kinases has also been demonstrated. Specifically, stimulation of human T cell clones with exogenous IL-2 results in the activation of p56lck, which is independent of its association with either CD4 or CD8 (24, 25). Lck has also been shown to associate with the β chain of the IL-2 receptor (25), which is critical for IL-2–mediated Lck activation (26).
We have developed a T cell clonal system that provides further insight into the mechanisms underlying the coordinated function of the TCR and the IL-2R. The antigen receptor signaling phenotypes of CD4+ and CD4− variants of an IL-2–dependent, ovalbumin-specific T cell clone have been described previously (27). Specifically, although both variants respond to antigen and mAbs specific for CD3ε, only the CD4− variant responds to mAbs specific for TCRCβ or Vβ. Expression of exogenous wild-type CD4, but not mutated CD4 unable to bind Lck, in CD4− variants restored their TCR–CD3 signaling phenotype to that of cells expressing endogenous CD4. Thus, the refractoriness of clones expressing wild-type CD4 to TCR-specific mAbs correlates with the sequestration of cellular Lck by CD4 (27).
The role of Lck in T cell antigen receptor signaling is well established. Biochemical (28–38), and genetic (39–46) analyses demonstrate that its presence and activity are required for the most proximal signaling events initiating through the TCR. Thus, it is plausible that the sequestration of Lck by CD4 in the above described clonal system disables the transmission of the most proximal signals emanating from the TCR.
However, the above observations in the CD4+ T cell clones do not correspond well with TCR–CD3 signaling phenotypes observed in primary CD4+ T cells. Specifically, the latter do respond to mAb specific to TCRCβ, albeit less robustly than they respond to mAb specific for CD3ε (47–49). An obvious difference within this comparison is the state of activation of the cells involved. Primary CD4+ lymph node T cells are in the majority resting, with >95% in the G0 phase of the cell cycle. In contrast, the CD4+ T cell clones are maintained in exogenous IL-2 before assay, and are in the majority, cycling. Although a significant proportion of these cycling cells contain a diploid content of DNA, these cells cannot be equated with noncycling cells in G0. Since IL-2 has been shown to affect the physiology of Lck (24, 25), coupled with the obligate role of Lck in TCR signaling, the observed TCR signaling phenotype in CD4+ IL-2–dependent clones may reflect an IL-2–mediated physical and/or functional redistribution of cellular Lck.
This study was undertaken to characterize the molecular basis of IL-2–mediated perturbation of TCR signaling. We demonstrate that exogenous IL-2 disables anti-TCR–, but not anti-CD3–induced T cell growth, and that the permissive anti-TCR signaling phenotype correlates with the presence of kinase-active Lck associated with the plasma membrane. Primary CD4+ T cells, which are also responsive to anti-TCR–induced growth, are also shown to have kinase-active Lck at the plasma membrane. Furthermore, permissive anti-TCR signaling is shown to correlate with the presence of ZAP-70–pp21ζ complexes in both primary T cells and clones deprived of IL-2.
Materials and Methods
Mice.
C57BL/6 male mice, 6–8 wk old, were purchased from Charles River Canada, Inc. (Laval, Quebec, Canada) or The Jackson Laboratory (Bar Harbor, ME), and maintained in a pathogen-free animal facility at the Ontario Cancer Institute (Toronto, Ontario, Canada).
Antibodies.
Hamster mAbs H57.597 (anti-TCRCβ) and 145.2C11 (anti-CD3ε), and rat mAbs KT410.1 (anti-Vβ4) and H.129 (anti-CD4) were purified as previously described (27). Both rabbit polyclonal anti-Lck (50) and rabbit polyclonal anti– ZAP-70 (51) were prepared by Dr. A. Veillette. The phosphotyrosine-specific mouse mAb 4G10 (52) was supplied by Dr. B. Druker (Oregon Health Sciences University, Portland, OR), and the ζ chain–specific mouse mAb G3 (53) was supplied by Dr. H.-S. Teh (UBC, Vancouver, British Columbia, Canada). Purified polyclonal rabbit anti–Bcl-x (54) was prepared by Dr. L. Boise. 4G10 immunoblots were developed with horseradish peroxidase (HRP)1-conjugated polyclonal goat anti–mouse IgG (Sigma Chemical Co., St. Louis, MO), in 1% gelatin (Bio- Rad, Hercules, CA). Immunoblots probed with rabbit anti-lck, rabbit anti–Zap-70, or rabbit anti–Bcl-x were developed with HRP-conjugated Protein A (ICN Biomedicals, Inc., Irvine, CA) in 5% nonfat dry milk. Biotinylated CD4 was revealed with HRP-conjugated streptavidin (Amersham Pharmacia Biotech, Piscataway, NJ) in 0.5% nonfat dry milk. Polyclonal rabbit anti–rat Ig was purchased from Bio/Can Scientific (Mississauga, Ontario, Canada).
Cell Preparation and Culture.
Clones were maintained at 0.5– 1.0 × 106 cells/ml in serum-free IMDM supplemented with 10 U/ml (1%) rIL-2 as previously described (27). Experiments involving rescue of responsive anti-TCR signaling in the presence of low concentrations of IL-2 required that CD4+ clones be antigen pulsed, which increased their sensitivity to IL-2. Specifically, clone 2.5 was cocultured with irradiated C57BL/6 spleen cells and 1–10 μg/ml of the ovalbumin-derived peptide143–157 for 48 h in the absence of rIL-2. Cultures were harvested and irradiated filler cells were removed by centrifugation through Lympholyte-M (Cedarlane Labs. Ltd., Hornby, Ontario, Canada). Viable T cell blasts were washed and seeded ≥0.5 × 106 cells/ml in serum-free IMDM supplemented with 10, 3, or 1 U/ml rIL-2. Clones were incubated at 37°C in 5% CO2 for 4 d, after which cells were harvested and proliferation assays were set up as previously described (27).
For overexpression of Bcl-xL in clone 2.5, 107 cells in 0.5 ml IMDM received either 15 μg of pSFFV-Neo empty vector or pSFFV–bcl-xL (55, 56). A 0.8-kb EcoRI fragment of Bcl-xL cDNA containing one asymmetric HindIII site was cloned into the EcoRI site of pSFFV-Neo. The plasmid was not deliberately linearized before electroporation. Cells were incubated with plasmid for 10 min at room temperature and were transferred to 0.4-cm electroporation cuvettes (Bio-Rad) and electroporated (Bio-Rad Gene Pulser) at 260 V, 960 μF. Cells were then incubated for an additional 10 min, harvested into IMDM containing 2.5% heat-inactivated FCS, and washed once. Cells were resuspended and cultured for 48 h in IMDM supplemented with 1% rIL-2. G418 (0.5 mg/ml) was added at 48 h, and a drug-resistant population was selected over 2 wk and subsequently cloned by micromanipulation. Clones were then assessed for the expression of Bcl-xL by immunoblotting. Clone 2.5.2 was selected for all subsequent studies.
To “rest” clone 2.5.2, cells were expanded in IMDM containing 0.3% rIL-2 to high density. Cells were harvested, washed twice, and seeded at 2 × 106 cells/well in multiple 24-well culture plates (Costar Corp., Cambridge, MA) along with 2 × 106 freshly prepared, irradiated, syngeneic C57BL/6 spleen cells. Cultures were incubated in IMDM with no rIL-2 at 37°C in 5% CO2 for 24 h. Cells were harvested and dead cells and fillers were removed by centrifugation over Lympholyte-M. Cell cycle status of these “rested” populations were assessed for each experiment as described below.
Primary CD4+ lymph node T cells were prepared as previously described (48). In brief, axillary nodes, brachial nodes, inguinal nodes, and superficial cervical nodes were isolated from 6–8-wk-old C57BL/6 mice. Cell suspensions were washed, counted, incubated on ice with rat anti-CD8, and passed over mouse “T cell columns” (BioTex Labs., Edmonton, Alberta, Canada) pretreated with goat anti–rat IgG to remove CD8+ T cells and goat anti–mouse IgG to remove B cells. Effluent cells from these columns were typically >98% CD4+.
Cell cycle status of lymphocyte populations was assessed using propidium iodide. In brief, 2 × 105 cells were pelleted, aspirated dry, and resuspended by dropwise addition of ice-cold Vindelov's low salt solution containing 3.4 mM Tris, pH 8.0, 75 μM propidium iodide, 0.1% vol/vol NP-40, 10 mM NaCl, and 700 U/liter ribonuclease (Boehringer Mannheim, Indianapolis, IN) (59). Suspensions were incubated on ice for 10 min and revortexed before analysis on a FACScan® (Becton Dickinson, San Jose, CA). Data were acquired at a flow rate of 20 events/s using the FL2 channel and doublet discrimination. Analysis was carried out using Lysis II software (Becton Dickinson) on FL2 (A).
Cell Fractionation.
Preparation of membranes and cytosol used procedures previously described in detail (57). In brief, cells were pelleted and resuspended in ice-cold calcium- and magnesium-free PBS in chilled borosilicate tubes (Fisher Scientific Co., Pittsburgh, PA). After two more washes in ice-cold PBS, the cells were resuspended to a final volume not exceeding 0.5 ml at 2 × 107 cells/ml in ice-cold hypotonic extraction buffer containing 25 mM Hepes, pH 7.0, 1 mM MgCl2, 1 mM EGTA, and 100 μg/ml each of leupeptin and aprotinin. Cells were incubated on ice for 40 min, then subjected to 20 “shears” per tube (avoiding foaming) using a 30-gauge needle on a 1-ml syringe barrel. Sheared material was examined microscopically to verify that no intact cells remained and nuclear material was removed by centrifugation. The volume of the final supernatant was measured, transferred to Eppendorf tubes, and centrifuged at 12,000 g for 10 min. The pellet from this spin contains the heavy membrane fraction (HMF) and the supernatant contains the cytosol. The cytosol fraction was centrifuged at 100,000 g for 1 h at 4°C, the supernatant of which was resolubilized in 0.1% Triton X-100 for 45 min with gentle agitation before immunoprecipitation. The HMF was washed once in 200 μl of ice-cold extraction buffer containing 0.1% delipidated BSA (Boehringer Mannheim), and resuspended to 4 × 107 cell equivalents/ml in ice-cold extraction buffer containing 0.1% Triton X-100. This suspension was solubilized with gentle agitation and then centrifuged at 12,000 rpm for 10 s to remove detergent-insoluble material. The supernatant of this spin contains the detergent-soluble HMF.
Biotinylation of cell surface proteins was achieved as follows. Cells were harvested and washed in ice-cold calcium- and magnesium-free PBS and resuspended to 107 cells/ml in borate buffer containing 10 mM anhydrous sodium borate and 150 mM NaCl, pH 8.8. Long chain biotin stock (Pierce Chemical Co., Rockville, IN) was dissolved in DMSO to a concentration of 10 mg/ml, and the appropriate volume was added to the cell suspension to achieve a final concentration of 50 μg/ml. Cells were incubated with biotin for 10 min at room temperature with frequent, gentle vortexing. The reaction was stopped by the addition of 10 μl/ml of 1 M NH4Cl. Cells were washed three times in room temperature PBS containing 10 mM Tris and 1mM EDTA and was examined microscopically for viability, which was typically >95%. An aliquot of cells was stained with PE-conjugated streptavidin (Southern Biotechnology Associates, Huntington, AL) to determine efficiency of cell biotinylation.
Immunoprecipitation and Immunoblotting.
Precipitation of Lck, CD4, and Bcl-xL for immunoblotting was achieved by lysing either T cell clones or primary CD4+ lymph node T cells at 4 × 107 cells/ml in hypotonic TNE lysis buffer (50 mM Tris, pH 8.0, 20 mM EDTA, 100 μM oxidized sodium orthovanadate, 50 mM sodium fluoride, 20 μg/ml each of leupeptin and aprotinin [Boehringer Mannheim], 100 μg/ml Pefabloc [Boehringer Mannheim], 50 μg/ml l-1-chloro-3-(4-tosylamido)-7-amino-2-heptanone hydrochloride [TLCK; Boehringer Mannheim], and 1% NP-40). Cells were lysed in prechilled Eppendorf tubes on ice for 40 min and centrifuged at 4°C at 12,000 g for 10 min. The supernatant was transferred to fresh Eppendorf tubes and maintained on ice until ready for use.
Immunoblotting for Bcl-xL from whole cell lysates was achieved by adding 12.5 μl of lysate (0.5 × 106 cell equivalents) to 12.5 μl of 2× Laemmli sample buffer plus 2.5 μl β-2-ME; samples were boiled for 5 min, and proteins were resolved by 12.5% SDS-PAGE for 900 V h and processed as described below. Antibodies used for quantitative immunoprecipitation were affinity purified and covalently coupled to activated cyanogen bromide Sepharose 4B (Amersham Pharmacia Biotech, Piscataway, NJ). Quantitative immunoprecipitations from total cell lysates or membrane/cytosol preparations from CD4+ cells was accomplished by four consecutive immunoprecipitations from lysate containing 6 × 106 cell equivalents with Sepharose-coupled anti-CD4, followed by four consecutive precipitations with Sepharose-coupled anti-Lck. Precipitates were subsequently washed four times in ice-cold TNE wash buffer, as described above but without protease inhibitors, and resuspended in 10 μl of wash buffer, to which 10 μl of 2× Laemmli sample buffer and 2 μl of β-2-ME was added. Samples were boiled for 5 min, and electrophoresed on 8% SDS-PAGE for 850 V h. Material was transferred to nitrocellulose (Schleicher & Schuell, Keene, NH) at 55 V for 2 h. Nitrocellulose was blocked for 2 h in 5% (for Lck and Bcl-xL) or 0.5% (for CD4) lowfat milk dissolved in wash buffer containing 50 mM NaCl, 10 mM Tris, 2.5 mM EDTA, and 0.1% Tween 20, pH 7.5. The latter buffer was also used as diluent for developing antibodies and/or reagents.
CD4 immunoblots were developed by addition of HRP-conjugated streptavidin (see above) in wash buffer (as above) containing 0.5% milk, and revealed with Renaissance ECL Reagents (NEN, Boston, MA). CD4 blots were stripped by washing in buffer containing 10 mM Tris-HCl, pH 2.3, and 150 mM NaCl for 15 min at room temperature, and then reblocked in 5% milk. These immunoblots were then reprobed with polyclonal rabbit anti-Lck in wash buffer containing 5% milk, developed with HRP-conjugated Protein A in 5% milk, and revealed with DuPont ECL reagents.
Immunoblots probed with antiphosphotyrosine, anti-ζ, or anti–ZAP-70 were performed essentially the same way as above. ZAP-70 was precipitated, nonquantitatively, from lysate containing 5 × 106 cell equivalents. Cells were lysed in pH 7.4 isotonic lysis buffer containing 140 mM NaCl, 10 mM Tris, 0.4 mM sodium orthovanadate, 10 mM sodium fluoride, 10 mM anhydrous sodium pyrophosphate, and 1% Triton X-100. This buffer was supplemented with protease inhibitors as for TNE buffer (see above). The corresponding wash buffer contained 0.1% Triton X-100 and no protease inhibitors. Samples were electrophoresed on 12.5% SDS-PAGE gels, transferred to nitrocellulose, and blocked in 5% milk (for anti–ZAP-70) or 3% gelatin (for anti-phosphotyrosine) at 30°C. Immunoblots were developed with HRP-conjugated Protein A in 5% milk (for anti–ZAP-70) or polyclonal goat anti-mouse IgG in 1% gelatin (for antiphosphotyrosine), and subsequently revealed with DuPont ECL reagents.
Samples were processed for Lck kinase assays as follows. Immunoprecipitations from membrane/cytosol preparations were done in Triton X-100, which was used throughout the kinase assay. Otherwise, the detergent was NP-40. Immunoprecipitates were washed once in ice-cold TNE wash buffer containing 100 μM sodium orthovanadate, once in the same buffer without sodium orthovanadate but containing 1M NaCl, once in ice-cold TNE wash buffer without sodium orthovanadate or NaCl, and finally in ice-cold 1× Lck kinase buffer containing 20 mM 3-(N-morpholino) propane sulfonic acid (MOPS), pH 7.0, 5 mM MgCl2, and 3 mM MnCl2 supplemented with 0.1% NP-40. Tubes were placed on ice and each received 10 μl of 2× Lck kinase buffer supplemented with 20 μg/ml each of aprotinin and leupeptin, 100 μg/ml Pefabloc, 50 μg/ml TLCK, and 0.1% NP-40. The reaction mixture was supplemented with 1 μg of freshly acid-denatured enolase (Boehringer Mannheim), cold ATP (Boehringer Mannheim) to yield a final concentration of 50 μM, and 2.5 μl (25 μCi/reaction final) γ-[32P]ATP. The reaction was allowed to proceed at room temperature for 30 min and was terminated by the addition of 25 μl of 2× Laemmli sample buffer containing 20% β-2-ME. Samples were boiled for 5 min and electrophoresed on 8% SDS-PAGE for 800 V h. Gels were de-stained with multiple changes of a solution containing 50% ddH2O, 40% methanol, and 10% glacial acetic acid over a 24-h period, and then dried and exposed.
CD4 aggregation followed by assessment of phosphotyrosyl content and kinase activity of CD4-associated Lck was achieved as follows. Clone 2.5.2 propagated in 1.0% rIL-2 was harvested, washed, and resuspended to 107/ml in RPMI (GIBCO BRL, Gaithersburg, MD) containing 50 μg/ml of the CD4-specific rat mAb, H129. After a 30-min incubation on ice, cells were pelleted, resuspended to 2 × 107/ml and prewarmed for 3 min at 37°C. Polyclonal rabbit anti–rat was added to some tubes to a final concentration of 50 μg/ml, and cells were mixed, pelleted, and immediately (within 5–10 s) lysed. CD4 immunoprecipitates were collected using Protein G–Sepharose. Proteins in these immunoprecipitates were resolved by SDS-PAGE transferred to nitrocellulose membranes and immunoblotted with the phosphotyrosine-specific mAb 4G10 as described above. These membranes were then stripped as described above and re-probed with anti-Lck. Alternatively, CD4 immunoprecipitates were subjected to immune complex kinase assays in the presence of enolase as an exogenous substrate after the procedure described above.
Results
IL-2–mediated Inhibition of Anti-TCR–induced Growth in CD4+ and CD4− Clonal Variants.
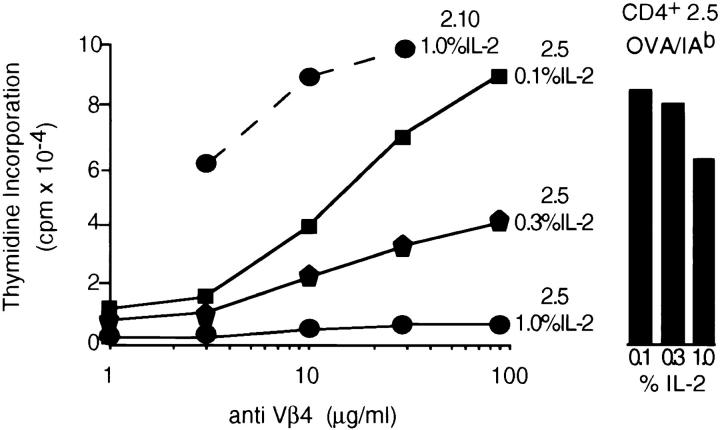
Both the CD4+ (2.5) and CD4− (2.10) variants of the ovalbumin-specific T cell clone were propagated in varying concentrations of rIL-2 for 1 wk. Viable cells were harvested and their capacity to respond to anti-Vβ4 and antigen was assessed. As illustrated in Fig. 1, there is an inverse correlation between the amount of exogenous IL-2 used to propagate the CD4+ variant and its capacity to subsequently respond to anti-Vβ4. This IL-2–dependent refractoriness to anti-TCR stimulation is also observed using an mAb specific for TCRCβ (data not shown). The inability to respond to anti-TCRVβ or anti-TCRCβ is not due to the compromised expression of the antigen receptor complex at the plasma membrane. Direct assessment of the membrane levels of TCR and CD3 by immunofluorescence revealed no differences in cells grown over the range of IL-2 concentrations used in this study (data not shown). The highest concentration of IL-2 used in this experiment (1% = 10 U/ml) results in a 20-fold inhibition of the anti-TCR response of CD4+ variants. However, the antigen responses of cells cultured in 10 vs. 1 U/ml of IL-2 were within a factor of two of one another (Fig. 1). Thus, IL-2 is not predisposing cells to die upon subsequent ligation of the TCR–CD3 complex as previously described (16). Rather, it is profoundly affecting their capacity to respond to TCR-specific mAbs.
Figure 1.
IL-2–mediated inhibition of anti-TCRVβ–induced growth. Clones 2.5 and 2.10 were stimulated with peptide (OVA143–157) at 10 μM in the presence of irradiated syngeneic splenocytes for 48 h. Activated cells were harvested and expanded in medium supplemented with the indicated concentrations of rIL-2. After 4 d, cells were harvested from these cultures and stimulated with the indicated concentrations of anti-Vβ4 or OVA143–157 in the presence of irradiated syngeneic splenocytes (see Materials and Methods). At 40 h, cultures were pulsed with 1 μCi of [3H]TdR and harvested 6 h later, and levels of thymidine incorporation were assessed by liquid scintillation spectroscopy. Counts represent the mean of triplicate cultures; 1 SE was within 15% of the indicated means for all cultures.
The involvement of cellular Lck in this signaling phenotype is derived from the observation that CD4− clonal variants are not susceptible to this IL-2–mediated effect. Thus, whether clone 2.10 was propagated in 0.1 or 1.0% IL-2, the subsequent response to anti-TCR was comparable. Shown in Fig. 1 is the anti-Vβ4 response of clone 2.10, which was propagated in 1.0% IL-2. To elucidate the role of Lck, both membrane and cytosolic fractions of clones 2.5 and 2.10 were prepared and the content and enzymatic activity of Lck contained within these fractions was assessed quantitatively. This analysis was performed using cells propagated in 1% IL-2, circumstances in which the CD4+ clone 2.5 does not respond to anti-TCR.
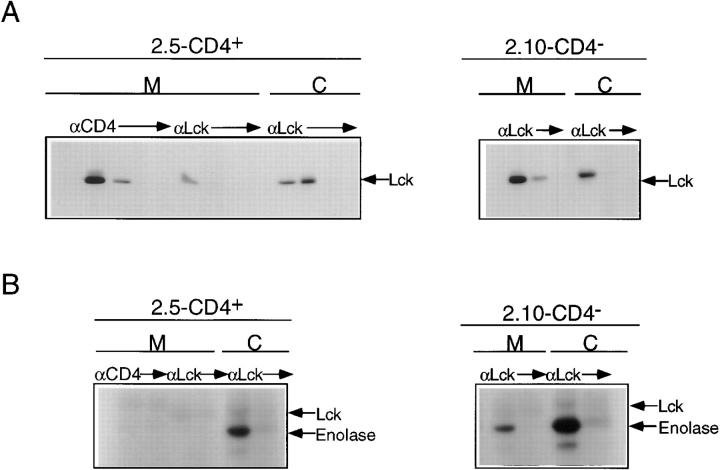
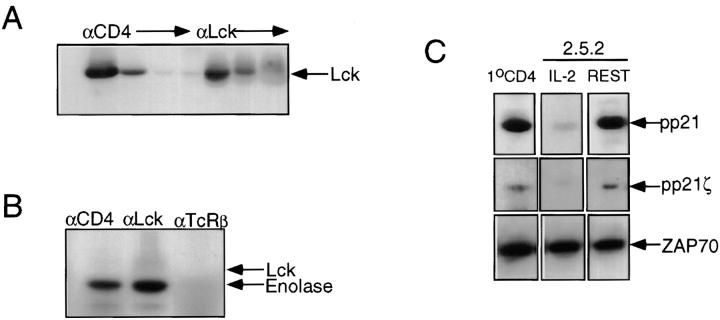
As illustrated in Fig. 2 A, quantitative precipitation of Lck from membrane and cytosolic fractions of clone 2.5 followed by immunoblotting revealed three pools, two of which were membrane associated, and the third of which was cytosolic. Phosphorimaging revealed that ∼70% of cellular Lck in clone 2.5 is associated with the membrane fraction, and that within this fraction >85% is associated with CD4. A comparable membrane/cytosol distribution of Lck is observed in clone 2.10 despite the absence of CD4 (Fig. 2 A). Immune complex kinase assays were performed in parallel with Lck protein analyses within each of the isolated pools, from each of the clonal variants. As shown in Fig. 2 B, the cytosolic pools from both clonal variants contained kinase-active Lck, revealing a robust phosphorylation of the exogenous substrate, enolase, coupled with a weak autophosphorylation signal. Neither the CD4-associated nor the non-CD4-associated pools of membrane Lck of clone 2.5 exhibited detectable kinase activity (Fig. 2 B). In marked contrast, membrane-associated Lck derived from clone 2.10 was kinase active (Fig. 2 B).
Figure 2.
Cellular distribution and activity of Lck in clones 2.5 and 2.10. (A) Membrane (M) and cytosol (C) fractions of clones 2.5 (CD4+) and 2.10 (CD4−) were prepared as described in Materials and Methods (also see legend to Fig. 4). Solubilized membrane fraction from clone 2.5 (6 × 106 cell equivalents) was subjected to four sequential precipitations with CD4-specific mAb followed by four sequential precipitations with polyclonal anti-Lck. Solubilized cytosol from clone 2.5 (6 × 106 cell equivalents) was subjected to four sequential precipitations with polyclonal anti-Lck. Solubilized membrane (M) and cytosol (C) fractions from clone 2.10 (6 × 106 cell equivalents) were each subjected to three sequential precipitations with polyclonal anti-Lck. Precipitates were resolved by 8% SDS-PAGE, transferred to nitrocellulose, blocked, and subsequently probed with polyclonal anti-Lck, followed by HRP-conjugated Protein A, and developed using DuPont ECL reagents as described in Materials and Methods. (B) Solubilized membrane (M) and cytosol (C) fractions (6 × 106 cell equivalents) from each of clones 2.5 (CD4+) and 2.10 (CD4−) were prepared as described in Materials and Methods. Solubilized membranes from clone 2.5 (6 × 106 cell equivalents) were subjected to four sequential precipitations with CD4-specific mAb, followed by four sequential precipitations with polyclonal anti-Lck and solubilized cytosol (6 × 106 cell equivalents) was subjected to four sequential precipitations with polyclonal anti-Lck. Solubilized membrane and cytosol fractions from clone 2.10 (6 × 106 cell equivalents each) were precipitated as for cytosol from clone 2.5. The first three precipitates were pooled from each of the above series, and the fourth precipitation of each series was assayed separately to ensure clearance. Immune complexes were washed and processed for in vitro Lck kinase activity as described in Materials and Methods. Shown here is a 24-h exposure.
Of note is that, based on the enolase signals observed, the specific activity of cytosolic Lck derived from clone 2.10 appears higher than that of the analogous Lck pool derived from clone 2.5 (Fig. 2 B). Densitometric analysis of these enolase signals, normalized to the content of Lck within the respective precipitates, reveals that the signal derived from clone 2.10 is 2.3-fold higher than that derived from clone 2.5. Thus, the differences in the kinase activities observed in these clonal variants may not be restricted to the plasma membrane-associated pools of Lck, exclusively. The comparison of these clonal variants suffers from what may be more complicated affects mediated by CD4 on cellular Lck in general. Although the distinct subcellular distribution of Lck kinase activity in the CD4+ and CD4− clonal variants described above could reflect the underlying differences in their susceptibility to anti-TCR–mediated growth, a potential caveat relates to the role of CD4 in altering the function of cellular Lck. A direct comparison of the subcellular distribution and enzymatic activity of Lck in anti-TCR permissive and nonpermissive CD4+ variants is required to obviate this concern. The low number of cells rescued after culturing CD4+ clone 2.5 in concentrations of exogenous IL-2 that supported subsequent responses to anti-TCR (Fig. 1) precluded our analyses. Therefore, we took advantage of the recent demonstration showing that forced expression of exogenous Bcl-xL was able to maintain the viability of IL-2–dependent T cells after withdrawal of IL-2 (58).
Overexpression of Bcl-xL Enables IL-2 Withdrawal and Rescue of Permissive TCR-α/β Signaling.
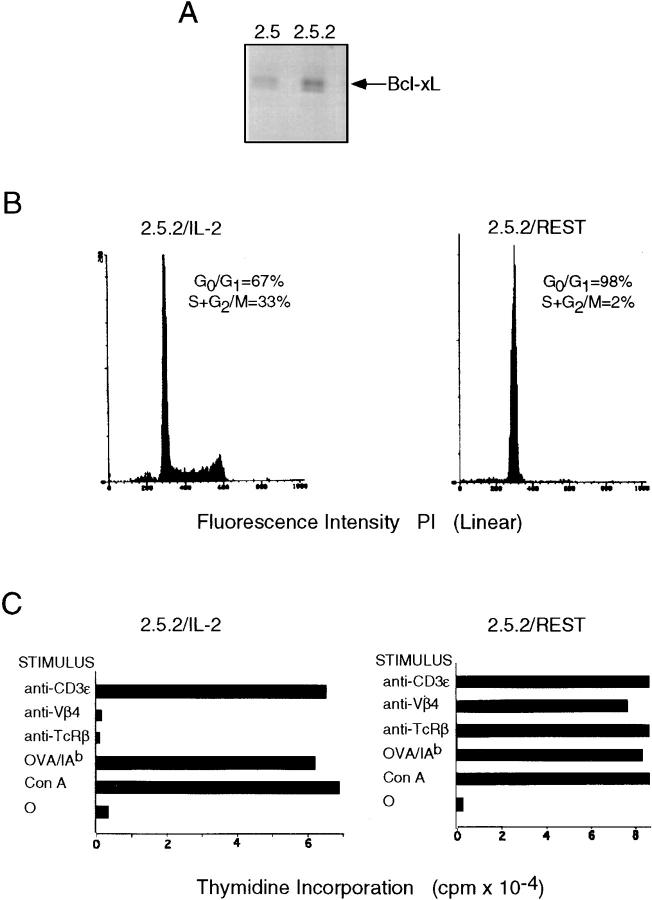
Bcl-xL was cloned into the expression vector pSFFV-neo (55, 56) and electroporated into clone 2.5. G418 resistant cells were propagated and clones were derived from this expanded drug resistant population. The level of Bcl-xL expressed in clone 2.5.2, in comparison to that expressed by clone 2.5, is illustrated in Fig. 3 A. Quantitation of this immunoblotting analysis revealed that the transfected clone 2.5.2 expressed ∼2.5-fold more Bcl-xL than the endogenous levels expressed by clone 2.5.
Figure 3.
IL-2 dependence of anti–TCR-α/β signaling. (A) Clone 2.5 was electroporated with 15 μg of pSFFV-Neo–Bcl-xL as described in Materials and Methods, followed by selection in the presence of 0.5 mg/ml active G418, and G418-resistant cells were subsequently cloned by micromanipulation. Illustrated is a comparison of the levels of Bcl-xL in whole cell lysates (0.5 × 106 cell equivalents) of clone 2.5 (wild-type) and clone 2.5.2 (Bcl-xL). Phosphorimaging revealed that clone 2.5.2 expresses roughly 2.2× the level of endogenous Bcl-xL present in clone 2.5. (B) Clone 2.5.2 was propagated in 10 U/ml rIL-2, or rested in the absence of rIL-2 for 24 h in the presence of irradiated syngeneic splenocytes, as described in Materials and Methods. 2 × 105 cells cultured in each of the two conditions were pelleted and resuspended in ice-cold Vindelov's solution, incubated for 10 min on ice, and analyzed for cell cycle status flow cytometrically using a FACScan® equipped with doublet discrimination software. (C) Clone 2.5.2 cultured either in the presence or absence of rIL-2, as for Fig. 3 B, was harvested and analyzed for its capacity to respond to various stimuli. 5 × 104 cells from either of the two culture conditions were cocultured with 5 × 105 irradiated syngeneic splenocytes in 0.2 ml of serum-free IMDM containing the indicated stimuli: Con A at 1 μg/ml; OVA143–157 at 10 μM; mAb H57 specific for TCRCβ at 0.1 μg/ml; mAb KT4.10 specific for TCRVβ4 at 0.1 μg/ml; and mAb 145.2C11 specific for CD3ε at 0.1 μg/ml. Cultures were pulsed with 1 μCi of [3H]TdR at 40 h, harvested 6 h later, and levels of thymidine incorporation were assessed by liquid scintillation spectroscopy. Counts represent the mean of triplicate cultures; 1 SE was within 15% of the indicated means for all cultures.
Clone 2.5.2 was then cultured in the presence of irradiated syngeneic splenocytes in medium containing either 10 U/ml of rIL-2 or no IL-2. Viable cells harvested 24 h after initiation of these cultures were assessed for cell cycle status using propidium iodide (59). As shown in Fig. 3 B, of those cells expressing ≥2n DNA content, roughly one-third of the cells derived from IL-2–containing cultures were in the S and G2/M phases of cycle, compared with 2% of the cells that were harvested from cultures containing no IL-2. Of note is that levels of expression of TCRCβ, CD3ε, and CD4, as assessed by immunofluorescence analysis of clone 2.5.2, were unaffected by exposure to IL-2 (data not shown).
The question followed whether clone 2.5.2 cultured in the absence of exogenous IL-2 was now responsive to anti-TCR mediated growth. As depicted in Fig. 3 C, this was indeed the case. Thus, once “rested” for 24 h in the absence of exogenous IL-2, clone 2.5.2 responded as robustly to anti-TCR as it did to anti-CD3ε (Fig. 3 C). In contrast, and as for clone 2.5, clone 2.5.2 was unresponsive to anti-TCR if derived from cultures containing 10 U/ml rIL-2. However, consistent with the phenomenon of IL-2–mediated functional uncoupling of TCR and CD3 signaling, these cells responded robustly to anti-CD3ε (Fig. 3 C).
Permissive Anti-TCR Signaling Correlates with the Presence of Membrane-associated, Kinase-active Lck.
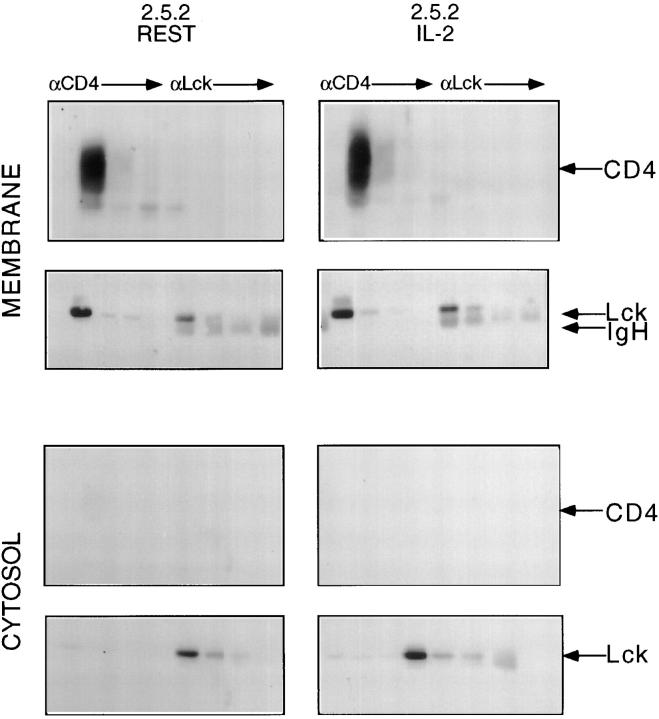
The availability of anti-TCR responsive and nonresponsive CD4+ clonal variants enabled a direct comparison of the cellular distribution and activity of Lck in these two circumstances. Towards this end, CD4+ clone 2.5.2 was propagated in 10 U/ml IL-2, or rested in the absence of exogenous IL-2. Viable cells were rescued from each of the two culture conditions, followed by the preparation of membrane and cytosol fractions. To control the cell fractionation technique used, cells derived from the two conditions were biotinylated and CD4 was used as a membrane marker. As illustrated in Fig. 4, immunoprecipitation from “membrane” but not “cytosolic” fractions with anti-CD4 revealed the appropriate signal. Sequential precipitation with anti-CD4, from biotinylated membrane fractions of both resting and IL-2– propagated 2.5.2 was followed by sequential precipitation with anti-Lck. Precipitates were fractionated by SDS-PAGE, immunoblotted and developed with enzyme-linked streptavidin.
Figure 4.
Fractionation of membranes and cytosol from clone 2.5.2. Clone 2.5.2 was cultured in the presence or absence of exogenous rIL-2, harvested, and labeled with “long chain” biotin as described in Materials and Methods. Membrane and cytosol fractions were then prepared from each of these two biotinylated populations, as described in Materials and Methods. Membrane and cytosol fractions prepared from 6 × 106 cell equivalents were subjected to four sequential precipitations with mAb specific for CD4, followed by four sequential precipitations with polyclonal anti-Lck. Precipitates were resolved by 8% SDS-PAGE, transferred to nitrocellulose, blocked, and probed with HRP-conjugated streptavidin to reveal CD4, then stripped, reblocked, and reprobed with polyclonal anti-Lck, and developed with HRP-conjugated Protein A to reveal the accompanying Lck signal. Immunoblots were visualized with DuPont ECL reagents.
As illustrated in Fig. 4, the CD4 signal was cleared by anti-CD4 and not detectable in subsequent anti-Lck precipitates. Stripping the immunoblot followed by probing with anti-Lck revealed that CD4 associated Lck had been cleared, and that membrane Lck not associated with CD4 was subsequently rescued, quantitatively, with anti-Lck (Fig. 4). The cytosolic fractions from the two 2.5.2 populations were sequentially precipitated with anti-CD4, followed by anti-Lck, as described above for the membrane fractions. As shown in Fig. 4, developing the immunoblot with enzyme-linked streptavidin did not reveal a CD4 signal, consistent with this fraction being devoid of material derived from the plasma membrane. However, non-CD4-associated cytoplasmic Lck was evident (Fig. 4).
This analysis revealed that the cellular distribution of Lck was not significantly altered in cycling and noncycling populations of clone 2.5.2. Thus, as observed in clone 2.5 (Fig. 2), the majority of cellular Lck was associated with the plasma membrane fraction, and of the two pools comprising this fraction, the majority of Lck was found to be associated with CD4.
In marked contrast to the unaltered cellular distribution of Lck, its enzymatic activity was profoundly affected by exogenous IL-2 (Fig. 5). Immune complex kinase assays were performed on membrane and cytosolic fractions of clone 2.5.2 cultured in the presence and absence of exogenous IL-2 as described above. As illustrated in Fig. 5, the cytosolic fractions of 2.5.2 cultured in either condition contained kinase-active Lck. As shown, the enolase signal is easily detectable, and although the position of autophosphorylated Lck is indicated, this signal is barely detectable. Thus, if kinase-active Lck is required for responsiveness to anti-TCR stimulation, cytosolic Lck is probably not playing a role.
Figure 5.
Kinase active Lck is associated with membranes of quiescent clone 2.5.2. Membrane and cytosol fractions were prepared from clone 2.5.2 cultured in the presence or absence of rIL-2, as described in Materials and Methods. Membrane fractions derived from 6 × 106 2.5.2 clones cultured in each of the two conditions were subjected to four sequential precipitations with mAb specific for CD4, followed by four sequential precipitations with polyclonal anti-Lck. Cytosolic fractions from 6 × 106 2.5.2 clones cultured in each of the two conditions were subjected to four sequential precipitations with polyclonal anti-Lck. Immune complex kinase assays were performed on each of the precipitates as described in Materials and Methods.
Assessment of the kinase activities contained within the two pools of membrane-associated Lck from clone 2.5.2 cultured in the presence and absence of exogenous IL-2 revealed striking differences (Fig. 5). Clone 2.5.2 cultured in the absence of IL-2 and responsive to anti-TCR–induced growth (Fig. 3 C) contained membrane-associated Lck that was kinase active. Both Lck associated with CD4 and that which was not gave detectable enolase signals, and for the cytosolic Lck described above, autophosphorylation signals were virtually undetectable in precipitates from the number of cell equivalents assayed (Fig. 5). In marked contrast, kinase activity within the analogous pools of membrane-associated Lck derived from clone 2.5.2 cultured in 10 U/ml IL-2 was undectable. Thus, as concluded from the results obtained with clones 2.5 and 2.10 (Fig. 2), the presence of membrane-associated, kinase-active Lck correlates with a permissive anti-TCR signaling phenotype.
CD4-Associated Lck in Clone 2.5.2 Propagated in IL-2 can be Acutely Activated.
There is an obligate requirement for Lck in the generation of the most proximal signals emanating from the TCR complex. The observation that clone 2.5.2 propagated in IL-2 cannot respond to TCRVβ-specific mAb raises the question of how the involvement of Lck is manifest in response to antigen. An obvious difference between anti-TCR and antigen-induced T cell activation is the latter's capacity to recruit CD4, and thus the CD4-associated pool of Lck, to the activation complex. However, as shown in Fig. 5, the CD4-associated pool of Lck is kinase inactive in clone 2.5.2 propagated in IL-2. The question is whether the activity of this pool may be altered during the T cell response to antigen. Although it has not been reported that antigen-mediated coaggregation of CD4 and TCR–CD3 results in the activation of CD4-associated Lck, it has been demonstrated that mAb-mediated aggregation of CD4 does (50, 60). Therefore, we determined whether mAb-mediated aggregation of CD4 resulted in an alteration in the phosphotyrosyl content and kinase activity of associated Lck derived from clone 2.5.2 propagated in IL-2.
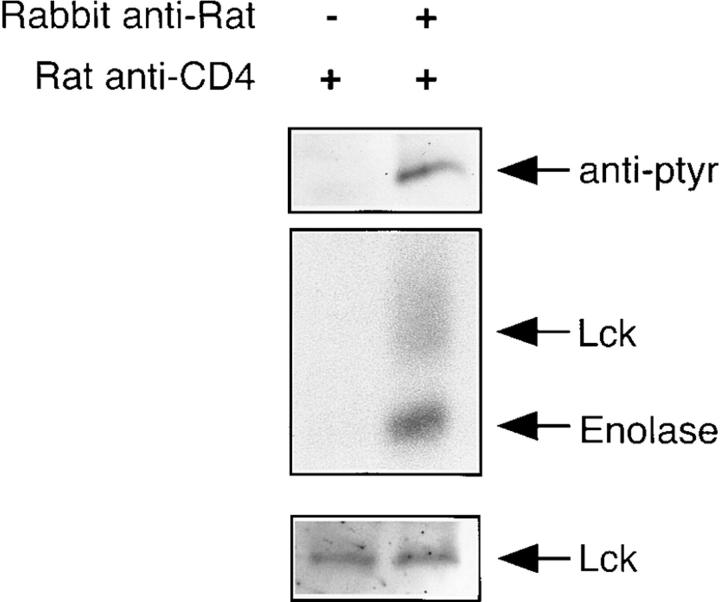
As illustrated in Fig. 6, this was indeed the case. Specifically, and corresponding with the results shown in Fig. 5, when CD4 is not ligated, the associated pool of Lck is not kinase active (Fig. 6, middle), nor does it contain detectable levels of phosphotyrosine (Fig. 6, top). However, upon mAb-mediated aggregation of CD4, both the phosphotyrosyl content and the kinase activity of the pool of associated Lck is elevated (Fig. 6). Thus, the IL-2–mediated downregulation of the activity of the CD4-associated pool of Lck in clone 2.5.2 propagated in IL-2 is not irreversible. Furthermore, these results are consistent with the capacity of antigen, but not anti-TCR, to recruit and use the CD4-associated pool of Lck.
Figure 6.
CD4-associated Lck in clone 2.5.2 propagated in IL-2 can be acutely activated. Clone 2.5.2 propagated in 1.0% IL-2 was harvested, washed, and resuspended to 107/ml in RPMI containing 50 μg/ml of the CD4-specific rat mAb, H129. After a 30-min incubation on ice, cells were pelleted, resuspended to 2 × 107/ml, and prewarmed for 3 min at 37°C. Polyclonal rabbit anti–rat was added to some tubes to a final concentration of 50 μg/ml, cells were mixed, pelleted immediately (within 5–10 s), and lysed. CD4 immunoprecipitates were collected using Protein G–Sepharose as described in Materials and Methods. The top panel shows the phosphotyrosyl content of CD4-associated Lck from cells in which CD4 had been aggregated or not. The middle panel shows the kinase activity associated with this pool of Lck from cells in which CD4 had been aggregated or not. The bottom panel shows the levels of Lck associated with CD4 in these two circumstances.
Permissive Anti-TCR Signaling Correlates with the Presence of Kinase-active Membrane Lck and Constitutive ZAP-70– pp21ζ Complexes in Primary T Cells and Clones.
To determine whether the observations described above did or did not reflect the idiosyncratic behavior of a T cell clone, we assessed the distribution and enzymatic activity of cellular Lck in primary CD4+ T cells. However, although the partitioning of membrane and cytosolic fractions was as efficient as that observed with the T cell clones, we were unable to detect kinase activity associated with Lck derived from any of the fractions. Cells were therefore lysed using standard technique and immune complex kinase assays were performed with anti-CD4 and anti-Lck precipitates from total cell lysate.
As illustrated in Fig. 7 A, sequential precipitation from lysate derived from primary CD4+ lymph node T cells with anti-CD4 followed by anti-Lck revealed that the majority of cellular Lck is associated with CD4. The remaining Lck would be partitioned between the membrane and cytosol. Immune complex kinase assays were performed on both total cellular Lck and on CD4-associated Lck to ensure that we were assessing the activity of some membrane-associated Lck. As shown in Fig. 7 B, both anti-Lck and anti-CD4 precipitates mediated robust enolase phosphorylation. An Lck autophosphorylation signal was not observed in anti-CD4 precipitates, and only marginally in anti-Lck precipitates (Fig. 7 B). Anti-TCRCβ precipitates derived from the same lysate were kinase negative (Fig. 7 B). These results support the conclusion that membrane Lck is kinase active in primary, resting CD4+ T cells.
Figure 7.
Kinase-active Lck is associated with the presence of a ZAP-70–pp21ζ complex and permissive anti–TCR-α/β signaling. (A) CD4+ primary lymph node T cells were purified as described in Materials and Methods. Lysate derived from 4 × 106 cell equivalents was subjected to four sequential precipitations with mAb specific for CD4, followed by three sequential precipitations with polyclonal anti-Lck. Precipitates were resolved by 8% SDS-PAGE, transferred to nitrocellulose, blocked, probed with polyclonal anti-Lck, followed with HRP-conjugated Protein A, and developed using DuPont ECL reagents. (B) CD4+ lymph node T cells were purified as described in Materials and Methods. Immune complex kinase assays were performed as described in Materials and Methods, on precipitates derived from lysate containing 4 × 106 cell equivalents with mAbs specific for CD4, TCRCβ, and polyclonal anti-Lck. (C) The top row shows the pp21 signal associated with anti–ZAP-70 precipitates from lysates (5 × 106 cell equivalents) derived from primary CD4+ lymph node T cells, and clone 2.5.2 cultured in the presence (IL-2) or absence (REST) of rIL-2. Anti–ZAP-70 precipitates were resolved on 12.5% SDS-PAGE, transferred to nitrocellulose, probed with 4G10 mAb specific for phosphotyrosine, developed with goat anti–mouse HRP-conjugated IgG, and revealed with DuPont ECL reagents. The middle row shows the pp21ζ signal associated with the anti–ZAP-70 precipitates. The blot shown in the top row was stripped and reprobed with ζ chain–specific mAb. The bottom row shows the same series of precipitations as in the top row, but immunoblotted with polyclonal anti–ZAP-70, developed with HRP-conjugated Protein A, and revealed with DuPont ECL reagents.
One of the roles of Lck in TCR signaling is to mediate the phosphorylation of the ζ chain (33, 34, 36, 60), thus enabling the recruitment and subsequent activation of ZAP-70 (32, 33, 35–37). Given that kinase-active Lck is present in resting primary T cells and T cell clones, it was of interest to determine the phosphorylation status of the ζ chain and whether it was associated with ZAP-70. Towards this end, three populations of T cells were analyzed: clone 2.5.2 cultured in the presence of IL-2; clone 2.5.2 cultured in the absence of IL-2; and primary CD4+ lymph node T cells. Anti-ZAP precipitates were isolated from lysates of each of these three populations, resolved on SDS-PAGE, and immunoblotted with phosphotyrosine-specific mAb 4G10 (52) with ζ chain–specific mAb, G3 (53), and anti–ZAP-70 (51). The top panel in Fig. 7 C depicts the pp21 signal that coprecipitated with anti–ZAP-70 in each of the three populations, revealed with phosphotyrosine-specific mAb. The middle panel of Fig. 7 C illustrates that the pp21 signals were also revealed with the ζ chain–specific mAb. The bottom panel of Fig. 7 C demonstrates that equivalent amounts of ZAP-70 were present in precipitates from each of the three populations.
The results presented in Fig. 7 C indicate that the ζ chain is at least partially phosphorylated in primary resting T cells and in clonal populations that exhibit a permissive anti-TCR signaling phenotype. Notably, pp21ζ is present at much reduced levels in clonal populations derived from cultures containing IL-2, which are nonpermissive to anti-TCR. Furthermore, this pp21ζ is constitutively associated with ZAP-70 in resting primary T cells, as previously demonstrated (34), and in resting T cell clones. Thus, both primary resting T cells, and resting clones exhibit a basal level of pp21ζ, at least some of which is constitutively associated with ZAP-70. This phenotype correlates with both the presence of kinase-active Lck at the plasma membrane and responsiveness to anti-TCR–mediated growth.
Discussion
To gain insight into the mechanism through which IL-2 can affect TCR–CD3 function, we have analyzed the signaling capacity of elements of the antigen receptor complex in a CD4+, IL-2 dependent clone. We demonstrate that IL-2, in a dose dependent fashion, inhibits the capacity of TCR, but not CD3 specific mAbs to induce T cell proliferation. The forced expression of exogenous Bcl-xL enabled the withdrawal of exogenous IL-2 from clone 2.5.2, and the rescue of cells, >98% of which were in G0 phase of cycle. This resting clonal population is responsive to anti-TCR. However, responsiveness to anti-TCR is not strictly a cell cycle dependent phenomenon. Clone 2.5 propagated in low concentrations of exogenous IL-2 (1 and 3 U/ml), do contain a significant proportion of cycling cells, ranging from 5 to 20%, and yet are responsive to anti-TCR. Thus, IL-2 appears to function in two domains, one in which it supports T cell growth without disabling anti-TCR signaling, and another in which IL-2 in excess prohibits the induction of the most membrane proximal signaling events induced by TCRVβ- and TCRCβ-specific mAbs (27).
Responsiveness to TCR-specific mAbs in clone 2.5.2 and primary CD4+ T cells correlates with the presence of detectable kinase active Lck at the plasma membrane, and this compartment of Lck is modulated in response to exogenous IL-2. Specifically, IL-2–mediated refractoriness to anti-TCR in clone 2.5.2 correlates with the absence of detectable kinase-active, membrane-associated Lck. In contrast, the cytosolic pool of Lck derived from clone 2.5.2 was kinase active whether or not the cells were responsive to anti-TCR. Thus, kinase-active cytosolic Lck is probably not involved in anti-TCR–mediated T cell growth. Consistent with this tenet is previous work demonstrating that forced expression of exogenous constitutively active Lck results in a hypersensitive TCR signaling phenotype (29) only when Lck can tether to the inner leaflet of the plasma membrane (30). These results support the conclusion that plasma membrane-associated and cytosolic pools of Lck support distinct cellular functions (61), and the results presented here demonstrate that IL-2 may induce a redistribution of activity within these pools.
Our study extends those first describing the capacity of IL-2 to alter Lck function (24, 25), with some notable differences. The latter studies demonstrated an IL-2–mediated transient activation of cellular Lck. Mitogen-activated T cells were propagated in IL-2, then starved of IL-2 before assessing the effects of subsequent IL-2 exposure on Lck activity. Neither the kinase activity of Lck in the initial resting T cell population, nor the subcellular distribution of the kinase active Lck induced by IL-2, was assessed. In contrast, we have observed an IL-2–mediated downregulation of Lck kinase activity, specifically of that Lck associated with the plasma membrane. The high basal level of cytosolic Lck kinase activity observed in both resting and cycling T cell clones may have precluded our detection of an IL-2– mediated increase in Lck activity in the cytosolic pool. However, this basal level of Lck kinase activity appears to be physiological, as it is observed in primary resting T cells.
No differences in the subcellular distribution of Lck protein could be correlated with IL-2–induced alterations in the cellular distribution of Lck kinase activity. It is possible that, once activated by IL-2, Lck is dislodged from the plasma membrane to the cytosol, and replaced by kinase-inactive Lck. This Lck would in turn become activated and redistribute to the cytosol under continuing IL-2–mediated pressure. Thus, we posit that use of Lck in IL-2 receptor signaling (26) results in the redistribution of kinase-active protein, and thus impairs subsequent anti-TCR–mediated responses.
According to this paradigm, IL-2–mediated inhibition of anti-TCR–induced growth would be ameliorated in the absence of membrane CD4 expression. Anti-TCR and antigen-mediated T cell activation would not engage the CD4-associated pool of Lck in similar fashions. mAb-mediated T cell activation may predominantly engage that pool of membrane-associated Lck that is not bound to CD4. In the absence of membrane CD4 expression, the available pool of membrane-associated Lck required for anti-TCR signaling would be larger and therefore proportionally less depleted by IL-2. This would explain the anti-TCR responsiveness of clone 2.10, the CD4− clonal variant of clone 2.5. Thus, when cultured in concentrations of IL-2 that inhibit anti-TCR–induced growth of the CD4+ clone 2.5, clone 2.10 is still responsive to anti-TCR–induced growth (Fig. 1 and reference 27). Furthermore, kinase-active Lck associated with the plasma membrane is observed in clone 2.10 (Fig. 2). The prediction follows that increasing the concentration of IL-2 used to propagate clone 2.10 should ultimately result in the inhibition of anti-TCR–induced growth, and indeed antigen-induced growth. Anti-TCR– mediated growth of clone 2.10 propagated in 50–100 U/ml IL-2 is inhibited, as is the antigen response of both clones 2.5 and 2.10 propagated in these conditions (data not shown). Similar IL-2–mediated inhibition of the antigen response of CD4+ T cell clones have been reported by other investigators (17). It is possible that these high concentrations of exogenous IL-2 have predisposed cells to antigen receptor–induced apoptosis (16).
The observed differences in the sensitivity of anti-TCR and antigen induced responses to exogenous IL-2 may reflect the differential involvement of the CD4 associated compartment of membrane Lck in these two modes of activation. Thus, doses of IL-2 that virtually ablate the anti-TCR responses of clones 2.5 and 2.5.2 do not impair their responses to antigen (Figs. 1 and 3). Membrane-associated Lck is nonetheless kinase inactive in these cells before antigen stimulation (Figs. 2 and 5). As previously described for clone 2.5, antibody-mediated coaggregation of CD4 with TCR does result in robust responses, but only if CD4 is associated with Lck (27). Thus, as with coaggregation of mAbs, antigen-mediated juxtaposition of CD4–Lck with the TCR–CD3 complex (62) obviates the requirement for preexisting membrane-associated kinase-active Lck associated with anti-TCR responsiveness in resting clones and primary T cells.
Consistent with these notions is the demonstration in this study that mAb-mediated aggregation of CD4 on clone 2.5.2 propagated in IL-2 results in the induction of kinase activity within the CD4-associated pool of Lck (Fig. 6). This result supports the conclusion that the capacity of antigen, but not anti-TCR, to induce the growth of clone 2.5.2 propagated in these conditions reflects the differential recruitment of the CD4-associated pool of Lck to the antigen receptor complex. Furthermore, the inactivity of “membrane” Lck in clone 2.5.2 propagated in IL-2 correlates with undetectable levels of phosphotyrosyl content, suggesting that phosphorylation of Y394, demonstrated by others to predicate Lck activation, is a probable basis for this IL-2–mediated Lck phenotype. Thus, IL-2–mediated downregulation of the basal kinase activity of membrane-associated Lck in clone 2.5.2 is not irreversible, and can presumably be rescued by antigen-mediated coaggregation of CD4 with the TCR–CD3 complex.
The functional uncoupling of mAb-mediated TCR and CD3 signaling observed in this study is not unprecedented. The first demonstration involved the characterization of the differential capacity of anti-TCRCβ and anti-CD3ε to induce calcium flux in thymocytes and lymph node T cells (63). Subsequent studies extended this signaling phenotype to include mature primary T cells (47–49). The central role of Lck in this uncoupling phenotype is supported by results obtained using Lck-deficient T cells. Studies using Lck− variants of the T cell line, Jurkat, demonstrated that signaling mediated by Ti-specific mAb was profoundly reduced in comparison to that mediated by CD3ε-specific mAb (42). Furthermore, mAb-mediated signaling through the TCR in primary T cells from Lck-deficient animals is ablated, whereas that through CD3ε is only partially affected (43). The results presented in this study demonstrate that IL-2, through altering the subcellular distribution of kinase-active Lck, mediates the same uncoupling phenotype. Taken together, the results support the conclusion that mAb-mediated signaling through TCR and CD3 has differential requirements for Lck.
Although the role of Lck in generating proximal signals emanating from the TCR has been established, there is a paucity of information correlating its cellular location with its delivery of function. A plausible function of the kinase-active, membrane-associated Lck described in this study is the maintenance of a basal level of TCR-ζ phosphorylation. In vitro observations have demonstrated that the ζ chain can be a substrate for Lck (35, 36). In this study we demonstrate that the presence of kinase-active Lck at the plasma membrane correlates with the presence of pp21ζ and its constitutive association with ZAP-70. This result supports and extends the recently published observation that thymocytes isolated from mice containing a targeted disruption of the Lck locus do not contain pp21ζ, whereas those thymocytes from Lck-sufficient animals do (36). The pp21ζ observed in this study represents a hypophosphorylated form of the molecule. As previously described (64), it is accompanied by a pp23 form, induced upon aggregation of the antigen receptor. Furthermore, immune complex kinase assays indicate that the ZAP-70 in pp21ζ–ZAP 70 complexes is inactive and is activated only upon aggregation of the antigen receptor complex (34). Thus, resting T cells are poised to respond through TCR, and the basal configuration of the antigen receptor complex supporting this phenotype is mediated at least in part through the presence of kinase-active, membrane-associated Lck.
Recent results provide a possible mechanism through which the basal level of Lck kinase activity reported here could be induced and maintained in primary resting T cells, and by extension in resting T cell clones cultured in the presence of syngeneic irradiated splenocytes. The survival of mature CD4+ and CD8+ naive T cells is profoundly compromised in the absence of MHC class II and MHC class I expression, respectively, in the periphery (65, 66). Thus, in MHC null mice, the half-life of mature T cells of either lineage is markedly reduced. These results support the conclusion that continuous, and probably low affinity, interaction of TCR with self-MHC molecules in the absence of nominal peptide is providing critical survival signals. The basal configuration of the TCR–CD3 complex described in this study, comprised of pp21ζ and its complexes with ZAP-70, could reflect the biochemical consequences of these interactions on the activity of Lck.
Acknowledgments
We thank C. Cantin and Denis Bouchard and the flow cytometry unit at the Ontario Cancer Institute; Mina Marmor for help in the isolation of primary CD4+ lymph node T cells; and Philippe Poussier and Robert Rottapel for helpful discussion and critical review of the manuscript.
Abbreviations used in this paper
- HMF
heavy membrane fraction
- HRP
horseradish peroxidase
Footnotes
L. Haughn was supported by a fellowship from the Cancer Research Society, Inc. B. Leung was supported by a Medical Research Council studentship. A. Veillette is the recipient of an MRC Scientist Award. This work was supported by grants from the Medical Research Council of Canada, the National Cancer Institute, and the National Institutes of Health.
L. Haughn and B. Leung contributed equally to this paper.
References
- 1.Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993;73:5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- 2.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 3.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor γ chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 4.Wange RL, Samelson LE. Complex complexes: signaling at the TCR. Immunity. 1996;5:197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 5.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 6.Alberola-Ila J, Takaki S, Kerner JD, Perlmutter RM. Differential signaling by lymphocyte antigen receptors. Annu Rev Immunol. 1997;15:125–154. doi: 10.1146/annurev.immunol.15.1.125. [DOI] [PubMed] [Google Scholar]
- 7.Cantrell DA, Smith KA. Transient expression of interleukin 2 receptors. J Exp Med. 1983;158:1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durand DB, Shaw J-P, Bush MR, Replogle RE, Belagaje R, Crabtree GR. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988;8:1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serfling E, Barthelmas R, Pfeuffer I, Schenk B, Zarius S, Swoboda R, Mercurio F, Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO (Eur Mol Biol Organ) J. 1989;8:465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crabtree GR, Clipstone NA. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 11.Meuer SC, Hussey RE, Cantrell DA, Hodgdon JC, Schlossman SF, Smith KA, Reinherz EL. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci USA. 1984;81:1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith KA, Cantrell DA. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci USA. 1985;82:864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Depper JM, Leonard WJ, Drogula C, Kronke M, Waldmann TA, Greene WC. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci USA. 1985;82:4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gullberg M, Smith KA. Regulation of T cell autocrine growth T4+cells become refractory to interleukin 2. J Exp Med. 1986;163:270–284. doi: 10.1084/jem.163.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantrell DA, Collins MKL, Crumpton MJ. Autocrine regulation of T lymphocyte proliferation: differential induction of IL-2 and IL-2 receptor. Immunology. 1988;65:343–349. [PMC free article] [PubMed] [Google Scholar]
- 16.Lenardo MJ. Interleukin-2 programs mouse αβT lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 17.Otten G, Wilde DB, Prystowsky MB, Olshan JS, Rabin H, Henderson LE, Fitch FW. Cloned helper T lymphocytes exposed to interleukin 2 become unresponsive to antigen and concanavalin A but not to calcium ionophore and phorbol ester. Eur J Immunol. 1986;16:217–225. doi: 10.1002/eji.1830160302. [DOI] [PubMed] [Google Scholar]
- 18.Johnston JA, Kawamura M, Kirken RA, Chen Y-Q, Blake TB, Shibuya K, Ortaldo JR, McVicar DW, O'Shea JJ. Phosphorylation and activation of the Jak-3 janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 19.Witthuhn BA, Silvennoinen O, Miura O, Lai KS, Cwik C, Liu ET, Ihle JN. Involvement of the Jak-3 janus kinase in signaling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu Z-J, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, Taniguchi T. Functional activation of Jak 1 and Jak 3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 21.Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, et al. Interaction of IL-2Rβ and γ chains with Jak 1 and Jak 3: implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 22.Ihle JN. Cytokine receptor signaling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe S, Arai K-I. Roles of the JAK-STAT system in signal transduction via cytokine receptors. Curr Opin Gen Dev. 1996;6:587–596. doi: 10.1016/s0959-437x(96)80088-8. [DOI] [PubMed] [Google Scholar]
- 24.Horak ID, Gress RE, Lucas PJ, Horak EM, Waldmann TA, Bolen JB. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves activation of p56lck . Proc Natl Acad Sci USA. 1991;88:1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatakeyama M, Kono T, Kobayashi N, Kawahara A, Levin SD, Perlmutter RM, Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991;252:1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- 26.Minami Y, Kono T, Yamada K, Kobayashi N, Kawahara A, Perlmutter RM, Taniguchi T. Association of p56lck with IL-2 receptor β chain is critical for the IL-2- induced activation of p56lck . EMBO (Eur Mol Biol Organ) J. 1993;12:759–768. doi: 10.1002/j.1460-2075.1993.tb05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haughn L, Gratton S, Caron L, Sekaly R-P, Veillette A, Julius M. Association of tyrosine kinase p56lckwith CD4 inhibits the induction of growth through the αβT-cell receptor. Nature. 1992;358:328–331. doi: 10.1038/358328a0. [DOI] [PubMed] [Google Scholar]
- 28.Marth JD, Lewis DB, Wilson CB, Gearn ME, Krebs EG, Perlmutter RM. Regulation of pp56lckduring T cell activation: functional implications for the src-like protein tyrosine kinases. EMBO (Eur Mol Biol Organ) J. 1987;6:2727–2734. doi: 10.1002/j.1460-2075.1987.tb02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham N, Miceli MC, Parnes JR, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase, p56lck . Nature. 1991;350:62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- 30.Caron L, Abraham N, Pawson T, Veillette A. Structural requirements for enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck . Mol Cell Biol. 1992;12:2720–2729. doi: 10.1128/mcb.12.6.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karnitz L, Sutor SL, Torigoe T, Reed JC, Bell MP, McKean DJ, Leibson PJ, Abraham RT. Effects of p56lckdeficiency on the growth and cytolytic effector function of an interleukin-2-dependent cytotoxic T-cell line. Mol Cell Biol. 1992;12:4521–4530. doi: 10.1128/mcb.12.10.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan AC, Iwashima M, Turck CW, Weiss A. Zap 70: a 70 kD protein-tyrosine kinase that associates with the TCRζ chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 33.Iwashima M, Irving BA, van Oers NSC, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 34.van Oers NSC, Killeen N, Weiss A. Zap-70 is constitutively associated with tyrosine-phosphorylated TCRζ in murine thymocytes and lymph node T cells. Immunity. 1994;1:675–685. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 35.Chan AC, Dalton M, Johnson R, Kong G-H, Wang T, Thoma R, Kurosaki T. Activation of zap-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO (Eur Mol Biol Organ) J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Oers NSC, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and Zap-70 in murine thymocytes. J Exp Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamasaki S, Takamatsu M, Iwashima M. The kinase, SH3, and SH2 domains of lck play critical roles in T-cell activation after zap-70 membrane localization. Mol Cell Biol. 1996;16:7151–7160. doi: 10.1128/mcb.16.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiest DL, Ashe JM, Abe R, Bolen JB, Singer A. TCR activation of zap 70 is impaired in CD4+CD8+ thymocytes as a consequence of intrathymic interactions that diminish available p56lck . Immunity. 1996;4:495–504. doi: 10.1016/s1074-7613(00)80415-x. [DOI] [PubMed] [Google Scholar]
- 39.Teh H-S, Garvin AM, Forbush KA, Carlow DA, Davis CB, Littman DR, Perlmutter RM. Participation of CD4 coreceptor molecules in T-cell repertoire selection. Nature. 1991;349:241–243. doi: 10.1038/349241a0. [DOI] [PubMed] [Google Scholar]
- 40.van Oers NSC, Garvin AM, Davis CB, Forbush KA, Carlow DA, Littman DR, Perlmutter RM, Teh H-S. Disruption of CD8-dependent negative and positive selection of thymocytes is correlated with a decreased association between CD8 and the protein tyrosine kinase, p56lck . Eur J Immunol. 1992;22:735–743. doi: 10.1002/eji.1830220317. [DOI] [PubMed] [Google Scholar]
- 41.Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann K-U, Veillette A, et al. Profound block in thymocyte development in mice lacking p56lck . Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 42.Strauss DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 43.Poussier P, Julius MH. T cell development and selection in the intestinal epithelium. Semin Immunol. 1995;7:321–334. doi: 10.1016/1044-5323(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 44.Anderson SJ, Perlmutter RM. A signaling pathway governing early thymocyte maturation. Immunol Today. 1995;16:99–105. doi: 10.1016/0167-5699(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 45.Groves T, Smiley P, Cooke MP, Forbush K, Perlmutter RM, Guidos CJ. Fyn can partially substitute for lck in T lymphocyte development. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 46.van Oers NSC, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. αβ T cell development is abolished in mice lacking both lck and fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 47.Newell MK, Haughn LJ, Maroun CR, Julius MH. Death of mature T cells by separate ligation of CD4 and the T-cell receptor for antigen. Nature. 1990;347:286–289. doi: 10.1038/347286a0. [DOI] [PubMed] [Google Scholar]
- 48.Maroun CR, Julius M. Distinct roles for CD4 and CD8 as co-receptors in T cell receptor signalling. Eur J Immunol. 1994;24:959–966. doi: 10.1002/eji.1830240427. [DOI] [PubMed] [Google Scholar]
- 49.Maroun CR, Julius M. Distinct involvement of CD45 in antigen receptor signalling in CD4+ and CD8+ primary T cells. Eur J Immunol. 1994b;24:967–973. doi: 10.1002/eji.1830240428. [DOI] [PubMed] [Google Scholar]
- 50.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck . Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 51.Fournel M, Davidson D, Weil R, Veillette A. Association of tyrosine protein kinase Zap-70 with the protooncogene p120c-cbl in T lymphocytes. J Exp Med. 1996;183:301–306. doi: 10.1084/jem.183.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrison DK, Kaplan DR, Escobedo JA, Rapp UR, Roberts TM, Williams LT. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGFβ-receptor. Cell. 1989;58:649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- 53.van Oers NSC, Teh S-J, Irving BA, Tiong J, Weiss A, Teh H-S. Production and characterization of monoclonal antibodies specific for the murine T cell receptor ζ chain. J Immunol Methods. 1994;170:261–268. doi: 10.1016/0022-1759(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 54.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 55.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 56.Fuhlbrigge RC, Fine SM, Unanue ER, Chaplin DD. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1a cDNA. Proc Natl Acad Sci USA. 1988;85:5649–5653. doi: 10.1073/pnas.85.15.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsemeyer SJ. Bcl-2 functions in an anti-oxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 58.Boise LH, McShan CL, Thompson CB. Introduction of the cell survival gene bcl-xL improves the viability of CTLL-2 cells without affecting their IL-2 proliferative response. Implications for the development of bioassays. J Immunol Methods. 1996;191:143–148. doi: 10.1016/0022-1759(96)00011-7. [DOI] [PubMed] [Google Scholar]
- 59.Vindelov LL. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and staining of nuclei. Virchows Arch B Cell Pathol. 1977;24:227–242. [PubMed] [Google Scholar]
- 60.Veillette A, Bookman MA, Horak EM, Samelson LE, Bolen JB. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck . Nature. 1989;338:257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- 61.Xu H, Littman DR. A kinase-independent function of lck in potentiating antigen-specific T cell activation. Cell. 1993;74:633–643. doi: 10.1016/0092-8674(93)90511-n. [DOI] [PubMed] [Google Scholar]
- 62.Kupfer A, Singer SJ, Janeway CA, Jr, Swain SL. Coclustering of CD4 (L3T4) molecule with the T-cell receptor is induced by specific direct interaction of helper T cells and antigen-presenting cells. Proc Natl Acad Sci USA. 1987;84:5888–5892. doi: 10.1073/pnas.84.16.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finkel TH, Marrack P, Kappler JW, Kubo RT, Cambier JC. αβT cell receptor and CD3 transduce different signals in immature T cells. Implications for selection and tolerance. J Immunol. 1989;142:3006–3012. [PubMed] [Google Scholar]
- 64.Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. ζ phosphorylation without zap 70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 65.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 66.Rooke R, Waltzinger C, Benoist C, Mathis D. Targeted complementation of MHC Class II deficiency by intrathymic delivery of recombinant adenoviruses. Immunity. 1997;7:123–134. doi: 10.1016/s1074-7613(00)80515-4. [DOI] [PubMed] [Google Scholar]