Abstract
Interleukin (IL)-8, a C-X-C chemokine, activates integrin-mediated adhesion of neutrophils. Presentation of IL-8 on the endothelial cell surface may promote leukocyte extravasation. We found that cultured human microvascular endothelial cells from the intestine (HIMEC) and from nasal polyps (PMEC), but not human umbilical vein endothelial cells (HUVEC), contained IL-8 in intracellular granules that coexpressed von Willebrand factor (vWf ). This observation was corroborated by the immunohistochemical observation of double-positive granules (IL-8+vWf+) in vessels of small and large intestine, nasal mucosa, and skin, whereas umbilical cords revealed no endothelial IL-8. After treatment of HIMEC or PMEC with histamine or thrombin, a dramatic increase in supernatant IL-8 concentration was observed within 3 min, whereas no increase in IL-8 was detected in supernatants of identically treated HUVEC cultures. Histamine or thrombin treatment also caused IL-8–containing granules to rapidly disappear from HIMEC. In HUVEC, IL-8–containing granules were inducible by treatment with recombinant human IL-1β for 24 h; additional histamine treatment doubled IL-8 secretion from HUVEC in the same rapid manner observed for mucosal EC. These data suggested that IL-8 prestored in microvascular endothelial cells may provide a rapid pathway for specific activation of neutrophil adhesion at sites of acute inflammation.
Keywords: chemokine, inflammation, leukocyte adhesion, neutrophil recruitment, secretion
Adhesion of neutrophils to endothelial cells (EC) is an early, requisite event in the acute inflammatory response. Such binding of neutrophils can be rapid and transient, occurring within minutes under some conditions, or developing over hours depending on the factors that incite inflammation or tissue damage. According to the current paradigm, these interactions involve a stepwise engagement of juxtaposed neutrophil- and EC-adhesion molecules, first selectins and their counterreceptors, which mediate neutrophil tethering and rolling, then β2-integrins and immunoglobulin-like intercellular adhesion molecules, which mediate firm neutrophil adhesion (1–3). The most rapid recruitment of neutrophils may be initiated by P-selectin, a transmembrane glycoprotein constitutively present in the Weibel-Palade bodies of EC (4, 5); this selectin is translocated within minutes to the cell surface membrane after stimulation with a number of secretagogues, including thrombin or histamine (6). On the EC surface, P-selectin supports the initial rolling of neutrophils both in vitro (7) and in vivo (8, 9). However, this rolling is of a transient nature, and firm adhesion through integrin binding requires neutrophil activation through Gαi-linked receptors (7, 10, 11). Such activation can be accomplished by broadly acting leukocyte activators (3, 12) such as platelet-activating factor (PAF [13, 14]) or by activators that are more specific for neutrophils, such as the chemotactic cytokine (chemokine) IL-8 (15–18).
In fact, the entire process of inflammatory neutrophil recruitment can be emulated in vivo by injection of IL-8 into the tissues (19–22). Moreover, neutrophil influx is severely impaired after intraperitoneal injection of thioglycollate in mice deficient in the IL-8 receptor homologue (23). However, it has been noted that soluble chemoattractant gradients cannot persist on the blood–EC interface; they are likely to be washed away by the blood flow (24–26). Therefore, the observation of in situ binding of IL-8 to EC in human skin (27), and the enhanced ability of immobilized chemokines to attract and activate leukocytes in vitro (28), led to the theory that chemokines bound to the EC membrane may effectively promote leukocyte–EC adhesion in vivo. Furthermore, tissue-derived IL-8 can be transcytosed and presented on the surface of EC (29), but such translocation of IL-8 requires its release or production in the tissue and its subsequent transport to the EC surface.
Here we describe that IL-8 can also be released within minutes from Weibel-Palade bodies in resting (i.e., not previously exposed to proinflammatory reagents in vitro) cultured human intestinal microvascular EC (HIMEC) and nasal polyp–derived microvascular EC (PMEC) after stimulation with histamine or thrombin. Such storage of a chemokine may be a novel principle in the biology of leukocyte adhesion that would serve to rapidly increase local chemokine concentrations at the EC surface and enable a higher level of specificity than that provided by classical chemoattractants such as PAF.
Materials and Methods
Reagents
Cytokines and Cell Culture Reagents.
Recombinant human (rh)IL-1β and rh basic fibroblast growth factor were obtained from R&D Systems Ltd. (Abingdon, UK). MCDB 131, cycloheximide, dibutyryl cAMP, histamine, thrombin, epidermal growth factor, and hydrocortisone were from Sigma Chemical Co. (St. Louis, MO), and Endothelial–SFM, FCS, and gentamicin from Life Technologies Ltd. (Paisley, UK).
Primary Antibodies and Secondary Conjugates.
mAbs to human IL-8 (clone NAP-1, IgG1; and clone LS04/2A2, IgG1) were gifts from Dr. C. Svanborg (University of Lund, Lund, Sweden) and Dr. C. Mackay (LeukoSite, Inc., Cambridge, MA), respectively; rabbit anti–human von Willebrand factor (vWf, IgG fraction) and FITC- labeled swine anti–rabbit IgG conjugate were purchased from Dakopatts A/S (Glostrup, Denmark); biotinylated horse anti–mouse IgG was from Vector Laboratories, Inc. (Burlingame, CA), and streptavidin–Texas Red conjugate from GIBCO BRL (Gaithersburg, MD).
Cell Culture
HIMEC and PMEC were isolated from segments of small intestine or from nasal polyps and cultured as described (30, 31). In brief, cells were dispersed from minced tissue by collagenase/dispase treatment, plated, and grown to confluence in Endothelial– SFM containing 2.5% FCS, 1 μg/ml hydrocortisone, 0.5 mM dibutyryl cAMP, 50 μg/ml gentamicin, and 0.25 μg/ml amphotericin-B. EC were selected from primary cultures by using paramagnetic beads armed either with mAb to CD31 (positive selection) or with mAb to CD44 (negative selection [31]). HIMEC and PMEC were subsequently maintained in MCDB 131 as described for HUVEC (see below).
HUVEC were isolated as described by Jaffe et al. (32) and cultured in MCDB 131 containing 7.5% FCS, 10 ng/ml epidermal growth factor, 1 μg/ml hydrocortisone, 50 μg/ml gentamicin, and 0.25 μg/ml amphotericin-B. All cultures were used at passage levels 1–8, were uniformly positive for vWf, and contained <1% contaminating cell types determined as described elsewhere (30, 31).
ELISA Experiments
HIMEC, PMEC, and HUVEC were grown to confluence in 96-well trays (Becton Dickinson, San Jose, CA). At the start of the experiment, resting or IL-1β–activated (100 U/ml, 24 h) EC monolayers were washed twice. Cells were subsequently incubated at 37°C in culture medium with or without histamine (0.1 mM) or thrombin (3 U/ml). Cell culture supernatant fluids were analyzed for IL-8 with an ELISA kit according to the recommendations of the manufacturer (R&D Systems Ltd.). In our hands, this assay provided an analytical sensitivity of 51 pg/ml.
Immunocyto/histochemistry
Confluent monolayers of EC grown on LabTek chamber slides (Nunc, Inc., Roskilde, Denmark) were fixed (10 min, pH = 7.4) in 0.5% periodate-lysine-paraformaldehyde (33) or in 4% paraformaldehyde (PFA). Tissue specimens from histologically normal small (n = 2) and large (n = 2) intestine, skin (n = 2), nasal mucosa (n = 3), and umbilical cord (n = 1) were snap frozen and stored at −70°C. Cryosections were cut at 4 μm and fixed in 4% PFA for 5 min at 23°C. All subsequent incubation steps except the last washing step were performed with 0.1% saponin for permeabilization. Cell monolayers or tissue sections were first incubated with mAb to IL-8 (10 μg/ml) for 1 h or overnight, respectively; then with rabbit IgG anti–human vWf (1:1,400) combined with biotinylated horse anti–mouse IgG (1:250) for 1 h; and finally with streptavidin–Texas red conjugate (1:200) combined with FITC-labeled swine anti–rabbit IgG (1:50) for 0.5 h. Negative controls were incubated with irrelevant isotype- and concentration-matched primary antibodies. All fixations and incubations took place at room temperature.
The immunostained slides were examined and analyzed with a confocal laser scanning microscope (MRC 600; Bio-Rad Laboratories Ltd., Hemel Hempstead, UK) or a fluorescence microscope (model E800; Nikon, Tokyo, Japan) equipped with a cooled CCD camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan).
Results
Resting Microvascular EC but not HUVEC Contain IL-8+ Granules.
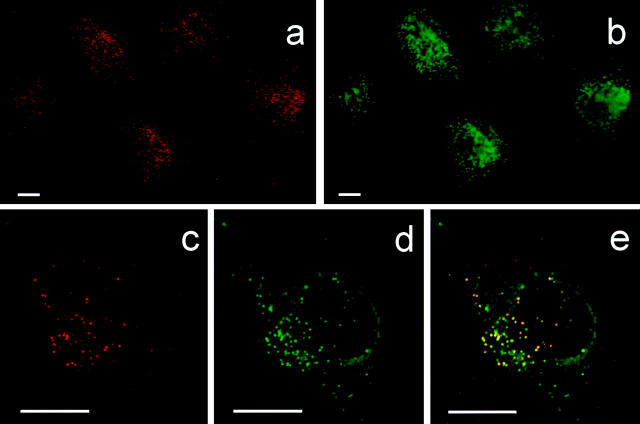
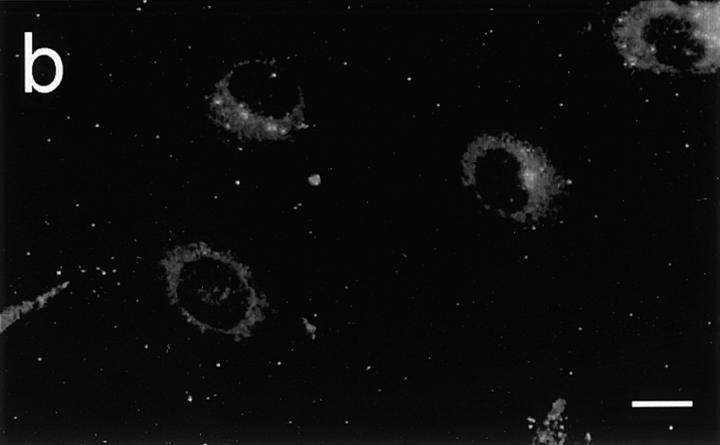
In contrast to HUVEC, unstimulated HIMEC (Fig. 1, a, c, and e) and PMEC (data not shown) contained IL-8 in intracellular vesicle–like granules as revealed by staining with mAbs LS04/2A2 and NAP-1. Staining of nonpermeabilized monolayers revealed no reactivity (data not shown). The observed subcellular localization of IL-8 was similar to that described for vWf (34) and P-selectin (4, 5), which both are stored in Weibel-Palade bodies. Therefore, we performed paired immunostaining with an antibody to vWf (Fig. 1, b, d, and e) and found coexpression of the two proteins in most granules. In addition, mAb NAP-1 revealed perinuclear IL-8 reactivity compatible with its presence in the Golgi apparatus (data not shown), as also reported for other cytokines (35), but this staining pattern was not observed with mAb LS04/2A2.
Figure 1.
Expression of IL-8 and vWf in resting HIMEC. Two-color immunofluorescence staining for IL-8 (a, c, and e) and vWf (b, d, and e) on monolayer culture. Red, IL-8 staining; green, vWf staining; yellow, colocalized IL-8 and vWf staining. Scale bars, 10 μm.
Rapid Secretion of IL-8 from Microvascular EC Is Induced by Histamine or Thrombin.
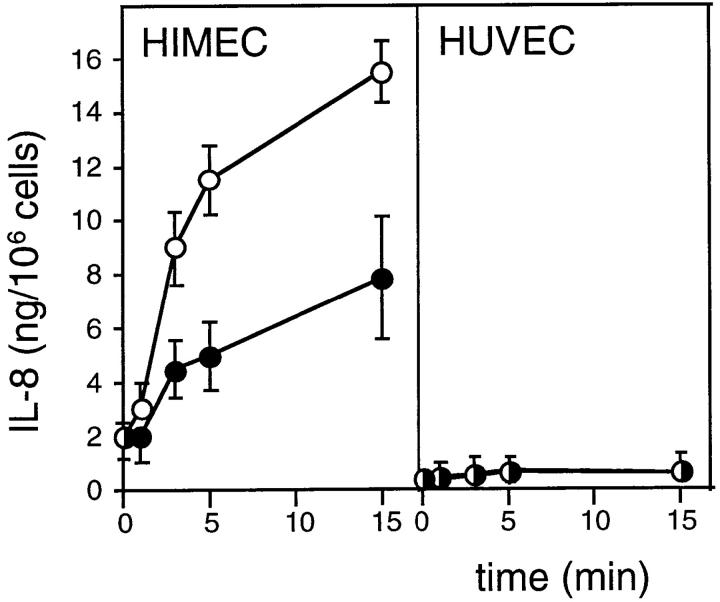
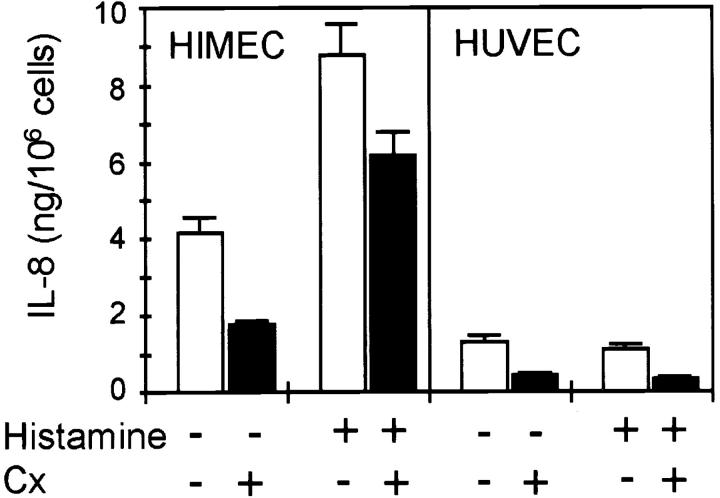
We observed a higher constitutive secretion of IL-8 into the culture supernatant in unstimulated HIMEC and PMEC than in HUVEC (Fig. 2, and data not shown). However, treatment of HIMEC (Fig. 2) and PMEC (data not shown) monolayers with histamine (0.1 mM) or thrombin (3 U/ml, data not shown) more than doubled the level of IL-8 over unstimulated cultures within 5 min (Fig. 2). Consistent with the lack of detectable granule-associated IL-8 in HUVEC, these agents had no effect on IL-8 levels (Fig. 2). To exclude enhanced protein synthesis as a possible explanation for the increased levels of IL-8 in the supernatant, HIMEC and HUVEC were incubated with the protein synthesis inhibitor cycloheximide (1 mg/ml) for 24 h before histamine stimulation. Despite further reduction of the low level of constitutive IL-8 secretion observed in both cell types, cycloheximide had no effect on the amount of IL-8 released in response to histamine (Fig. 3).
Figure 2.
Effect of histamine on release of IL-8. Time course of IL-8 release from confluent EC cultures in the presence (open circles) or absence (filled circles) of histamine (0.1 mM). ELISA measurements in supernatant fluid, representative results from one of four similar experiments (mean ± SD of triplicate wells).
Figure 3.
Effect of cycloheximide on histamine-induced IL-8 secretion. Confluent EC were incubated in the absence (white bars) or presence (black bars) of cycloheximide (Cx; 1 mg/ml, 24 h) and washed twice in fresh medium before histamine stimulation (0.1 mM, 15 min). ELISA measurements of IL-8 in EC culture supernatants. Representative results from one of three similar experiments (mean ± SD of triplicate wells).
IL-8+ Granules Disappear Rapidly after Stimulation with Histamine.
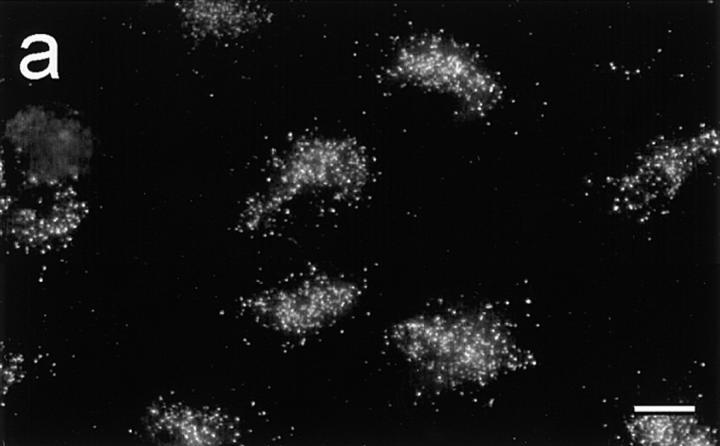
Immunocytochemical staining of resting and histamine-stimulated HIMEC (Fig. 4) revealed that the observed increase of secreted IL-8 was accompanied by disappearance of IL-8+ granules, most apparent after 15 min (Fig. 4 b). Double staining also revealed a reduction of vWf+ granules after histamine stimulation (data not shown).
Figure 4.
IL-8+ vesicles disappear rapidly after activation with histamine. IL-8 expression in the absence (a) or presence (b) of histamine (0.1 mM, 15 min). Immunofluorescence staining (clone NAP-1) of fixed and permeabilized HIMEC culture (scale bars, 10 μm).
Microvascular EC, but not HUVEC, Contain IL-8+ Granules In Situ.
This disparity between small vessel–derived EC and HUVEC observed in vitro was substantiated by immunohistochemical detection of IL-8 in small vessels of small intestine (Fig. 5, a and c) but not in EC of the umbilical vein (data not shown). The IL-8 staining of these vessels was observed in granules that costained for vWf (Fig. 5, b and c). Small vessels of the normal skin, nasal mucosa, and large intestine also contained IL-8+ granules (data not shown).
Figure 5.
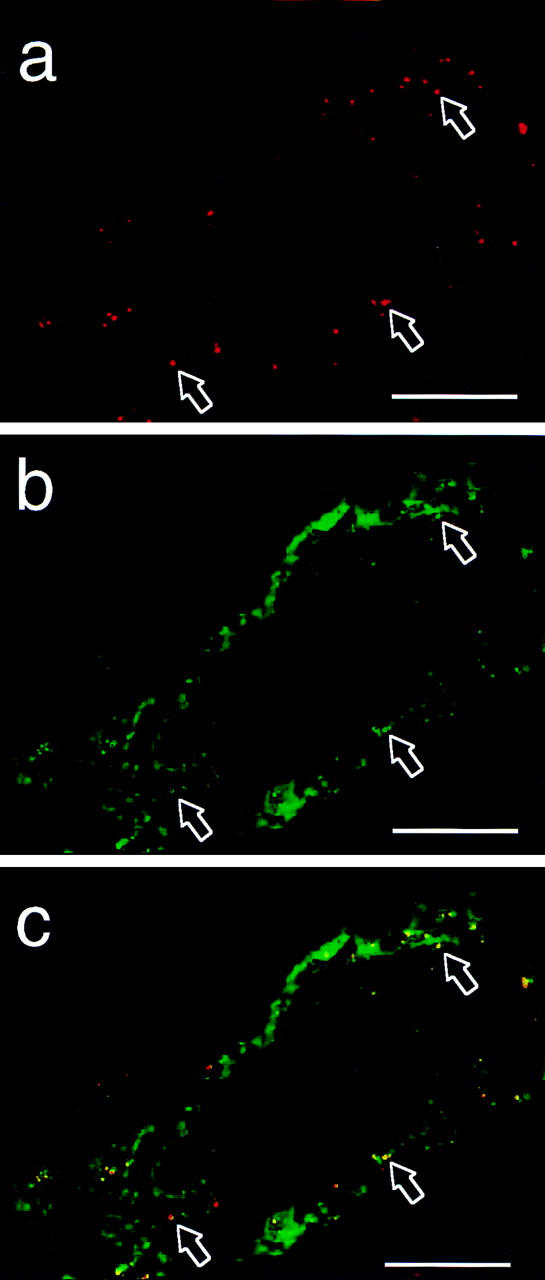
In situ detection of IL-8 in human small intestine. EC in submucosal vein of human jejunum stained for IL-8 (a and c) and vWf (b and c). Red, IL-8 staining; green, vWf staining; yellow, colocalized IL-8 and vWf staining. Arrows, Double-positive granules; note variability of staining intensity for the two proteins. Two-color immunofluorescence staining of fixed and permeabilized tissue sections (scale bars, 20 μm).
IL-8+ Granules Are Inducible in HUVEC.
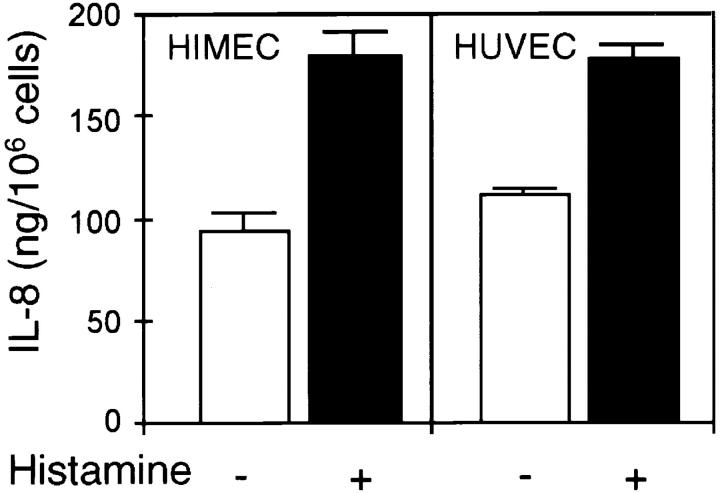
After activation with rhIL-1β (100 U/ml) for 24 h, IL-8+ granules coexpressing vWf appeared in HUVEC (data not shown), and strongly elevated IL-8 secretion levels were observed for both HIMEC and HUVEC after such treatment (Fig. 6). In HIMEC, IL-1 treatment enhanced the staining intensity of IL-8+ granules (data not shown). We next tested if histamine would effect the release of IL-8 from cytokine-activated EC and found almost doubling of the level of released IL-8 in both cell types within 15 min (Fig. 6).
Figure 6.
Effect of histamine on release of IL-8 from IL-1β– activated EC. Confluent EC monolayers were activated with rhIL-1β (100 U/ml, 24 h) and washed twice in fresh medium before histamine stimulation (0.1 mM, 15 min). ELISA measurements of IL-8 in EC culture supernatants, representative results from one of four similar experiments (mean ± SD of triplicate wells).
Discussion
This study reports that the chemokine IL-8 is stored in resting microvascular EC and can be rapidly released to the luminal EC surface after activation by secretagogues such as histamine and thrombin. This availability may play a crucial role in providing rapid recruitment of neutrophils because prestored P-selectin, which is also made available at the endothelial surface after such activation (6), merely permits transient rolling of neutrophils (7). Subsequent firm adhesion appears to require the activation of β2-integrins, which can be achieved by stimulation of leukocytes with broadly acting classical chemoattractants or with leukocyte type-specific chemokines affording a higher level of specificity (7, 10, 11). The classical chemoattractant PAF is the only known adhesion-activating factor that can be made available at the endothelial surface within minutes of EC stimulation (18). However, PAF activates several types of leukocytes that can both roll on P-selectin and adhere firmly to integrin counterreceptors on EC (3, 12, 14). On the other hand, the ability of IL-8 to activate integrin- mediated adhesion appears restricted to neutrophils, and its rapid secretion from previously resting microvascular EC as described here may provide a higher level of specificity in the earliest phases of neutrophil recruitment.
Our data convincingly show that human microvascular EC in intestinal and airway mucosa, as well as in skin, contain IL-8 in intracellular granules that also contain vWf, clearly indicating that these granules are Weibel-Palade bodies (34). Indeed, immunoelectron microscopy of IL-1– activated HUVEC revealed IL-8 in the Weibel-Palade bodies (36), thus supporting our conclusion. Moreover, histamine or thrombin induced a rapid release of IL-8 from HIMEC and PMEC, in a time course virtually identical to that described for translocation of P-selectin to the EC surface (6), whereas resting HUVEC failed to release IL-8 above background levels. The observed level of IL-8 released to the culture supernatant probably underestimated the true concentration obtainable in the microenvironment between the surface membranes of EC and adhering leukocytes. First, EC in vivo possess binding sites for IL-8 (24) that are grossly lost upon culture of HIMEC (our unpublished data) or EC from other tissue sources (37, 38). Second, the recently described presentation of transcytosed IL-8 on EC microvilli (29), paralleling that of α4β7 and L-selectin on leukocytes (39, 40), raises the interesting question as to whether prestored endothelial IL-8 may be presented in the same manner, thereby enhancing the availability of IL-8 to its leukocyte receptors. On the other hand, in vitro studies have suggested that as few as 300 molecules of IL-8 per μm2 are sufficient for activation of adhesion to occur (Campbell, J.J., personal communication).
The constitutive secretion of IL-8 measured at various time points also indicated a difference in the resting state between microvascular EC and HUVEC, possibly pointing to a fundamental biological difference between small and large vessels. However, after activation with rhIL-1β, both HUVEC and HIMEC secreted elevated levels of IL-8; in this case, IL-8+ vesicles appeared in HUVEC, as also described by Wolff et al. (36). The biological significance of the latter finding is uncertain, but it may reflect a common sorting signal in the IL-8 sequence, whereas the absence of IL-8 inside unstimulated HUVEC might merely reflect the low constitutive level of IL-8 synthesis. To this end, it is interesting to note that the microvascular EC described in this study all derive from organs or tissues that border the external environment and may thus experience chronic inflammatory stimulation, possibly resulting in a higher level of constitutive IL-8 expression. The molecular mechanisms that allow such constitutive differences to persist over weeks in vitro remain to be elucidated.
In conclusion, we describe a novel mechanism by which IL-8 can be made available at the EC surface within minutes of activation. Such presentation may provide a rapid pathway for specific activation of neutrophil adhesion at sites of acute inflammation.
Acknowledgments
We thank Drs. Eugene C. Butcher, James J. Campbell, and Finn-Eirik Johansen for helpful discussions, Drs. C. Svanborg and C. Mackay for providing mAbs, and Dr. Henrik Huitfeldt for assistance with the confocal microscope. Drs. Edward P. Bowman and Michael Delay kindly commented on the manuscript, and Ms. Gunn Jamne, Ms. Liv Mangschau, and Ms. Inger Johanne Ryen are gratefully acknowledged for their expert technical assistance. We are also grateful to the staffs at the Maternity Unit, Department of Gynecology, and at Surgical Department B, Rikshospitalet, for their kind assistance in obtaining tissue specimens.
This study was supported by the Norwegian Cancer Society, the Research Council of Norway, and Anders Jahre's Fund. G. Haraldsen and F.L. Jahnsen are Postdoctoral Fellows of the Norwegian Cancer Society and the Research Council of Norway, respectively.
References
- 1.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 2.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.McEver RP, Beckstead JH, Moore KL, Marshall CL, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP 140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- 6.Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- 7.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 8.Bienvenu K, Granger DN. Molecular determinants of shear rate-dependent leukocyte adhesion in postcapillary venules. Am J Physiol. 1993;264:H1504–H1508. doi: 10.1152/ajpheart.1993.264.5.H1504. [DOI] [PubMed] [Google Scholar]
- 9.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P-selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 10.von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Andrian UH, Hansell P, Chambers JD, Berger EM, Torres I, Filho, Butcher EC, Arfors KE. L-selectin function is required for beta 2-integrin-mediated neutrophil adhesion at physiological shear rates in vivo. Am J Physiol. 1992;263:H1034–H1044. doi: 10.1152/ajpheart.1992.263.4.H1034. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman GA. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992;13:93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]
- 13.Rainger GE, Fisher AC, Nash GB. Endothelial-borne platelet-activating factor and interleukin-8 rapidly immobilize rolling neutrophils. Am J Physiol. 1997;41:H114–H122. doi: 10.1152/ajpheart.1997.272.1.H114. [DOI] [PubMed] [Google Scholar]
- 14.Simon HU, Tsao PW, Siminovitch KA, Mills GB, Blaser K. Functional platelet-activating factor receptors are expressed by monocytes and granulocytes but not by resting or activated T and B lymphocytes from normal individuals or patients with asthma. J Immunol. 1994;153:364–377. [PubMed] [Google Scholar]
- 15.Carveth HJ, Bohnsack JF, McIntyre TM, Baggiolini M, Prescott SM, Zimmerman GA. Neutrophil activating factor (NAF) induces polymorphonuclear leukocyte adherence to endothelial cells and to subendothelial matrix proteins. Biochem Biophys Res Commun. 1989;162:387–393. doi: 10.1016/0006-291x(89)92009-3. [DOI] [PubMed] [Google Scholar]
- 16.Lo SK, Detmers PA, Levin SM, Wright SD. Transient adhesion of neutrophils to endothelium. J Exp Med. 1989;169:1779–1793. doi: 10.1084/jem.169.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Detmers PA, Lo SK, Olsen EE, Walz A, Baggiolini M, Cohn ZA. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990;171:1155–1162. doi: 10.1084/jem.171.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorant DE, Patel KD, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991;115:223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colditz IG, Zwahlen RD, Baggiolini M. Neutrophil accumulation and plasma leakage induced in vivo by neutrophil-activating peptide-1. J Leukocyte Biol. 1990;48:129–137. doi: 10.1002/jlb.48.2.129. [DOI] [PubMed] [Google Scholar]
- 20.Leonard EJ, Yoshimura T, Tanaka S, Raffeld M. Neutrophil recruitment by intradermally injected neutrophil attractant/activation protein-1. J Investig Dermatol. 1991;96:690–694. doi: 10.1111/1523-1747.ep12470612. [DOI] [PubMed] [Google Scholar]
- 21.Rot A. Chemotactic potency of recombinant human neutrophil attractant/activation protein-1 (interleukin-8) for polymorphonuclear leukocytes of different species. Cytokine. 1991;3:21–27. doi: 10.1016/1043-4666(91)90006-y. [DOI] [PubMed] [Google Scholar]
- 22.Swensson O, Schubert C, Christophers E, Schroder JM. Inflammatory properties of neutrophil-activating protein-1/interleukin 8 (NAP-1/IL-8) in human skin: a light- and electronmicroscopic study. J Investig Dermatol. 1991;96:682–689. doi: 10.1111/1523-1747.ep12470606. [DOI] [PubMed] [Google Scholar]
- 23.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 24.Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992;13:291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y, Adams DH, Shaw S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol Today. 1993;14:111–115. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- 26.Colditz IG. Margination and emigration of leucocytes. Surv Synth Pathol Res. 1985;4:44–68. doi: 10.1159/000156964. [DOI] [PubMed] [Google Scholar]
- 27.Rot A. Binding of neutrophil attractant/activation protein-1 (interleukin 8) to resident dermal cells. Cytokine. 1992;4:347–352. doi: 10.1016/1043-4666(92)90077-5. [DOI] [PubMed] [Google Scholar]
- 28.Webb LM, Ehrengruber MU, Clark-Lewis I, Baggiolini M, Rot A. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc Natl Acad Sci USA. 1993;90:7158–7162. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 30.Haraldsen G, Rugtveit J, Kvale D, Scholz T, Muller WA, Hovig T, Brandtzaeg P. Isolation and longterm culture of human intestinal microvascular endothelial cells. Gut. 1995;37:225–234. doi: 10.1136/gut.37.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jahnsen FL, Brandtzaeg P, Haye R, Haraldsen G. Expression of functional VCAM-1 by cultured nasal polyp-derived microvascular endothelium. Am J Pathol. 1997;150:2113–2123. [PMC free article] [PubMed] [Google Scholar]
- 32.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 34.Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson U, Matsuda T. Human interleukin 6 and tumor necrosis factor α production studied at a single cell level. Eur J Immunol. 1989;19:1157–1160. doi: 10.1002/eji.1830190629. [DOI] [PubMed] [Google Scholar]
- 36.Wolff B, Burns AR, Middleton J, Rot A. Endothelial cell “memory” of inflammatory stimulation: human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J Exp Med. 1998;188:1757–1762. doi: 10.1084/jem.188.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petzelbauer P, Watson CA, Pfau SE, Pober JS. IL-8 and angiogenesis: evidence that human endothelial cells lack receptors and do not respond to IL-8 in vitro. Cytokine. 1995;7:267–272. doi: 10.1006/cyto.1995.0031. [DOI] [PubMed] [Google Scholar]
- 38.Watson CA, Camerabenson L, Palmercrocker R, Pober JS. Variability among human umbilical vein endothelial cultures. Science. 1995;268:447–448. doi: 10.1126/science.7716553. [DOI] [PubMed] [Google Scholar]
- 39.von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 40.Berlin C, Bargatze RF, Campbell JJ, von Andrian U, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. Alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]