Abstract
A basic principle of immunology is that prior immunity results in complete protection against a homologous agent. In this study, we show that memory T cells specific to unrelated viruses may alter the host's primary immune response to a second virus. Studies with a panel of heterologous viruses, including lymphocytic choriomeningitis (LCMV), Pichinde (PV), vaccinia (VV), and murine cytomegalo (MCMV) viruses showed that prior immunity with one of these viruses in many cases enhanced clearance of a second unrelated virus early in infection. Such protective immunity was common, but it depended on the virus sequence and was not necessarily reciprocal. Cell transfer studies showed that both CD4 and CD8 T cell populations from LCMV-immune mice were required to transfer protective immunity to naive hosts challenged with PV or VV. In the case of LCMV-immune versus naive mice challenged with VV, there was an enhanced early recruitment of memory phenotype interferon (IFN) γ–secreting CD4+ and CD8+ cells into the peritoneal cavity and increased IFN-γ levels in this initial site of virus replication. Studies with IFN-γ receptor knockout mice confirmed a role for IFN-γ in mediating the protective effect by LCMV-immune T cell populations when mice were challenged with VV but not PV. In some virus sequences memory cell populations, although clearing the challenge virus more rapidly, elicited enhanced IFN-γ–dependent immunopathogenesis in the form of acute fatty necrosis. These results indicate that how a host responds to an infectious agent is a function of its history of previous infections and their influence on the memory T cell pool.
Keywords: memory T cells, virus, heterologous viruses, protective immunity, immunopathogenesis
It had been previously thought that memory T cells were present at relatively low frequencies and were essentially dormant, but recent studies have shown that, subsequent to infections of mice with lymphocytic choriomeningitis virus (LCMV),1 Pichinde virus (PV), or vaccinia virus (VV), virus-specific pCTL frequencies (pCTL/f) are maintained at very high levels for the lifetime of the animal (1, 2). In the well-characterized LCMV model a subpopulation of these memory cells at any given time consists of cytolytically active cycling cells (3–6) expressing IL-2 receptors (4, 7) and high levels of adhesion molecules (8–10). When the immune system is biased by a high frequency of precursors specific for a given virus, some of these T cells originally stimulated by one virus infection will cross-react with and be stimulated by a second heterologous virus (11, 12). Infections of LCMV-immune mice with PV, VV, or MCMV result in the activation of blast-size, LCMV-specific CTL that are easily detectable in cytotoxicity assays without additional in vitro stimulation (11, 12). Limiting dilution analyses demonstrated that splenocytes from LCMV- immune mice acutely infected with PV or VV contained many T cell clones cross-reactive between LCMV and PV or between LCMV and VV, whereas T cell clones from nonimmune mice infected with these viruses did not show this cross-reactivity to LCMV (12). The consequence of this cross-reactive stimulation of memory T cells is that a second virus infection will reactivate memory cells specific for the first virus and help prime a T cell response to the second unrelated virus. Given their high frequency (1, 2), state of activation (3–6), and ease of further activation (13– 16), as well as their propensity for cross-reactivity with heterologous agents, we questioned whether memory cells generated in response to one infectious agent would contribute significantly to the progression of disease elicited by a heterologous agent. In this study, we demonstrate that prior immunity to one virus can significantly enhance clearance of a second unrelated virus early in infection, before the development of the high affinity virus-specific T cell response that occurs after stimulation of naive T cells. In some selective virus sequences we demonstrate that prior immunity to one virus not only clears the second unrelated virus more rapidly but also results in enhanced immunopathology due to an altered T cell response.
Materials and Methods
Mice.
C57BL/6 (H-2b) male mice and 129/SEV and IFN-γ receptor knockout (IFN-γR KO; reference 17) mice of both sexes were either purchased from The Jackson Laboratory (Bar Harbor, ME) or else bred in our facility and used at 2–12 mo of age.
Viruses.
LCMV, strain Armstrong, an RNA virus in the Old World arenavirus family, was propagated in BHK21 baby hamster kidney cells (18). The WR strain of VV, a DNA virus in the poxvirus family (11), and the AN3739 strain of PV, an RNA virus in the New World arenavirus family only distantly related to LCMV, were propagated in L929 or BHK21 cells (11, 18, 19). MCMV, strain Smith, a DNA virus in the herpesvirus family, was obtained from salivary glands of infected BALB/c mice (20). For acute virus infections, mice were injected intraperitoneally with 4 × 104 PFU of LCMV, 2 × 104, 4 × 105, or 106 PFU of VV, 106 PFU of PV, or 104 or 105 PFU of MCMV.
Cell Lines.
KO (H-2b), an SV40-transformed kidney cell line derived from a C57BL/6 mouse (21) and provided to us by Dr. Satvir Tevethia (Pennsylvania State Medical Center, Hershey, PA), was propagated in DMEM (GIBCO BRL, Gaithersburg, MD) supplemented with 5 × 10−5 M 2-ME. L929 (H-2k), a continuous liver cell line derived from C3H mice, MC57G (H-2b), a methylcholanthrene-induced fibroblast cell line from C57BL/6 mice, early passage mouse embryo fibroblast (MEF) cells from C57BL/6 mice, and ATCC Vero cells were propagated in Eagle's MEM (GIBCO BRL). KO and MC57G cells were infected with LCMV and PV at a multiplicity of infection (MOI) of 0.1–0.2 PFU/cell and incubated for 2 d at 37°C. All cell lines were supplemented with 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate, 2 mM l-glutamine, 10 mM Hepes, and 10% heat-inactivated (56°C, 30 min) fetal bovine serum (FBS; Sigma Chemical Co., St. Louis, MO).
Infection Protocol with Heterologous Viruses.
Mice were immunized with a sublethal dose of one virus. After the rise and fall of the acute T cell response and when the immune system had returned to homeostasis (usually 6 wk or longer), the mouse was challenged with the second virus. At 3–5 d after the second virus infection, the spleen, fat pads, and/or liver were harvested, homogenized, and titrated for virus plaques, as previously described (22). In mice infected with LCMV only the spleens were titrated, as this strain of LCMV does not replicate in the liver or fat pads. These viruses were used either in crude tissue culture supernatants or were purified over sucrose gradients and diluted in PBS. Control naive mice were either left uninjected or injected with tissue culture media or with culture media sedimented like virus over a sucrose gradient, and there was no significant difference in virus titer when any one of these methods was compared with another. The control mice were always age matched to the experimental group and housed under exactly the same pathogen-free conditions as the experimental group for the identical time period. All mice used were healthy with no evidence of any underlying disease.
Intracellular IFN-γ Staining.
Peritoneal cells from individual mice were stained for intracellular IFN-γ based on a method previously described (23). Peritoneal cells at 2 × 106 per tube were stimulated with PMA (Sigma Chemical Co.) at 50 ng/ml and ionomycin (Sigma Chemical Co.) at 500 ng/ml for 2 h at 37°C. Then brefeldin A (Sigma Chemical Co.) was added at 10 μg/ml for 2 h at 37°C enabling intracellular proteins to accumulate. After the cells were washed with FACS® buffer (PBS with 2% FBS and 0.02% sodium azide) they were incubated with anti-Fc γII receptor antibody (PharMingen, San Diego, CA) as a blocking antibody, for 10 min at 4°C. Then tricolor-conjugated anti– mouse CD4 (clone CT-CD4; Caltag Laboratories, San Francisco, CA) or CD8 (clone CT-CD8α; Caltag Laboratories) was added for 30 min at 4°C. After two washes with FACS® buffer the cells were fixed with 200 μl of 2% paraformaldehyde for 20 min at room temperature. They were then washed with FACS® buffer containing 0.5% saponin (Sigma Chemical Co.), a permeabilizing agent, and then incubated in this buffer for another 10 min at room temperature before adding PE-conjugated rat anti–mouse anti-IFN-γ mAb (PharMingen) or control PE-conjugated rat IgG1 isotype for 30 min at room temperature. They were then washed two more times with FACS® buffer with saponin and then one time with FACS® buffer before analysis on either a FACS® 440 (Becton Dickinson & Co., Sparks, MD) or FACStar® Plus (Becton Dickinson & Co.). The total number of CD8+ and CD4+ or the total number of CD8+ and CD4+ cells secreting IFN-γ was determined by multiplying the percentage times the total cell yield from each mouse. The total CD4+ percentages may be an underestimate as these cells tend to downregulate the CD4+ receptor on stimulation with PMA and ionomycin. That was not a problem with the CD8+ cells.
LDA for Virus-specific CTL Precursors.
Adult 129/SEV and IFN-γ receptor knockout mice (IFN-γR KO) mice were inoculated intraperitoneally with LCMV, and at the indicated times after infection spleens were harvested. The LCMV-specific precursor frequency (p/f) per CD8+ cell was quantified by LDAs of unsorted cells (12, 24). The percentage of CD8+ T cells was determined by fluorescent antibody staining and FACS® analysis, as previously described (12, 24). The limiting dilution assays used a previously described method (12, 24). In brief, splenic lymphocytes from infected mice were harvested and titrated in U-bottomed, 96-well plates with 24 replicates at each dilution. They were stimulated with virus-infected PECs (3–4 × 104/well) and supplemented with irradiated splenic feeders (1–2 × 105/well) and growth factors provided by using a 16% culture supernatant from IL-2–secreting, gibbon lymphoma tumor cell line MLA.144 (American Type Culture Collection, Rockville, MD; reference 25). After 4 d, the cultures were fed with 104 irradiated, virus-infected PEC.
On days 7–8 of culture, individual wells were split twofold and assayed for cytolytic function on LCMV-infected or uninfected syngeneic target cells (KO) using a modified 51Cr-release assay. 51Cr-labeled targets (5 × 103) were added to all wells, and after an 8–10 h incubation at 37°C the supernatant was harvested. Positive wells were defined as those wells whose 51Cr-release exceeded the mean spontaneous release by >3 standard deviations. All wells that lysed uninfected syngeneic targets were eliminated from the analysis. Frequencies were calculated using χ2 analysis according to Taswell (26) on a computer program kindly provided by Dr. Richard Miller (University of Michigan, Ann Arbor, MI).
Cytotoxicity Assays.
Cell-mediated cytotoxicity was determined using a standard microcytotoxicity (CTL) assay (19). Varying numbers of effector leukocytes were plated in triplicate to achieve the desired E/T ratio. 51Cr-labeled MC57G target cells (5 × 103), either uninfected or infected with virus, were added to all wells, and after a 6 h incubation at 37°C the supernatant was harvested and counted. Data are expressed as percent specific 51Cr-release = 100 × ([experimental cpm − spontaneous cpm]/[maximum release cpm − spontaneous release cpm]). Lytic units were calculated using exponential fit method (27) provided by software from Proteins International (Rochester Hills, MI). One lytic unit was defined as the number of effector cells required to lyse 5% of a population of 5 × 103 targets in a 6 h CTL assay. A low percentage lysis was used for these calculations because some of the samples examined had relatively low levels of killing, and we wished to make quantitative comparisons within a wide range of very low and high CTL activity. The spontaneous release for each target used in these assays was <20%.
Adoptive Cell Transfers.
C57BL/6 mice were injected intravenously with one spleen equivalent (∼5–10 × 107 splenocytes) of immunologically naive (nonimmune) leukocytes or LCMV- immune (>6 wk after infection) leukocytes and challenged I.P. with PV (106 PFU) or VV (106 PFU). In some experiments LCMV-immune leukocytes were treated with anti-CD8 (rat anti–mouse CD8 mAb, clone Lyt 2.43; reference 28) and complement (rabbit serum) to remove CD8+ T cells (29) or with anti-CD4 (rat anti–mouse CD4 mAb, clone GK1.5) and complement (guinea pig serum) to remove CD4+ T cells before transfer. Spleens were harvested, homogenized, and plaque-assayed for virus titer at 3–4 d after infection. The effectiveness of the CD4+ or CD8+ T cell depletion was monitored by staining with anti– mouse CD4 (clone L3T4 RM4-4; PharMingen) or anti–mouse CD8 (clone CD8a Ly-2(53-6.7); PharMingen; reference 3). FACS® analyses showed that the CD4+ or CD8+ T cell contamination was <2%.
Isolation of Fat Pad Lymphocytes.
The infiltrating leukocytes of VV-challenged LCMV-immune mice were isolated from the fat pads by mincing and digesting with collagenase B (200 mg/ml) in MEM media plus 4% BSA for 1 h at 37°C, and then the lymphocytes were separated over Lympholite M (Cedarlane Labs., Hornby, Ontario, Canada) and analyzed.
Virus Titration.
The number of PFU was determined by plaque assay using a 10% homogenate of tissue taken from individual mice and 10-fold dilutions of this homogenate on the appropriate cell line, ATCC vero cells for LCMV, PV, VV, and MEF cells for MCMV. Results were expressed as the geometric mean titers, i.e., the arithmetic averages of the logs for four or five separate animals titrated for virus individually plus or minus the SEM. Titers reported are log10 PFU per whole spleen, liver, and both abdominal fat pads.
Scoring the Level of AFN.
The level of acute fatty necrosis was scored visually based on the severity of disease from level 0 to 7. Levels 1 and 2 represent very mild to mild disease with a few white necrotic spots on one or both lower abdominal fat pads; levels 3 and 4 represent mildly moderate and moderate with larger patches of necrosis of the lower abdominal fat pads and extension into the upper left quadrant fat pad around the spleen; and levels 5 and 6 represent moderately severe to severe with very extensive large patches of necrosis on the lower abdominal fat pads and spotty fatty necrosis throughout omental fat pads as well as the splenic fat pad; level 7 represents very severe disease with such severe fatty necrosis that the organs are adherent to each other.
Results
Prior Infection with a Heterologous Virus Induces Protective Immunity to Unrelated Viruses.
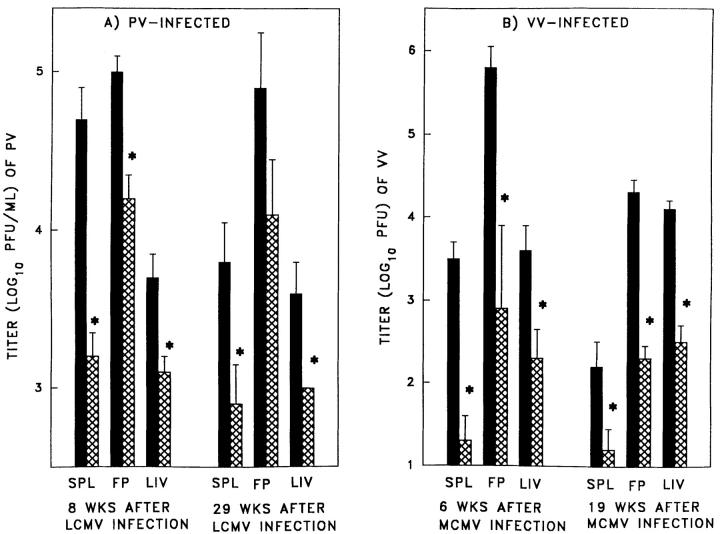
Table 1 shows viral titration data from experiments in which mice immune to one virus were challenged with any of a panel of heterologous viruses. Prior immunity to LCMV, PV or MCMV resulted in significant 10–300-fold lower VV titers in three major organs (spleen, fat pads, and liver) at 3–5 d after VV infection. Prior immunity to LCMV also resulted in a significant 5–13-fold lower PV titers 3–4 d after PV infection, while immunity to MCMV provided a less dramatic but still significant 2.5-fold decrease in PV titer of the fat pads. Immunity to MCMV resulted in a significant sixfold lower virus titer in the spleen of LCMV-infected mice; immunity to PV lowered the splenic LCMV titer only 2.5-fold (the Armstrong strain of LCMV does not replicate in the liver or fat pad). Immunity to LCMV and PV resulted in a four- and fivefold decrease, respectively, in liver titers in MCMV-infected mice. In contrast, immunity to VV did not significantly lower LCMV, PV, or MCMV titers. Prior immunity to LCMV or PV significantly lowered VV titers over a 2 log10 range of challenge virus dose (Table 2). This protective effect of prior immunity lasted for long periods of time after the original virus infection, as mice either 8 or 29 wk after LCMV infection demonstrated equal efficiency at resisting PV challenge (Fig. 1 A) as did mice either 6 or 19 wk after MCMV infection challenged with VV (Fig. 1 B). These studies suggest that protective immunity between these four heterologous viruses is a common occurrence, but it is not universal to all virus combinations, as there is a selective protective effect dependent on the sequence of virus infections.
Table 1.
Heterologous Immunity Between Viruses
| Mean (± SEM) Log10 Change in Challenge Virus Titer* in the Immune versus the Naive Spleen‡ | ||||||||
|---|---|---|---|---|---|---|---|---|
| Immunizing virus | Challenge virus (range of mean log10 of naive mice challenged with virus)§ | |||||||
| LCMV (5.5–6.0) | PV (3.7–4.0) | MCMV (3.4) | VV (3.2–4.1) | |||||
| LCMV | ND | −1.1 ± 0.2 (3)‖ | −0.4 (1) | −1.5 ± 0.4 (4)‖ | ||||
| PV | −0.4 ± 0.1 (3) | ND | UND | −1.8 ± 0.4 (4)‖ | ||||
| MCMV | −0.8 ± 0.1 (3)‖ | −0.5 ± 0 (2) | ND | −1.5 ± 0.4 (3)‖ | ||||
| VV | −0.3 ± 0.2 (3) | −0.1 ± 0.2 (3) | UND | ND | ||||
| Mean (± SEM) Log10 Change in Challenge Virus Titer* in the Immune versus the Naive Fat Pad‡ | ||||||||
| Immunizing virus | Challenge virus | |||||||
| LCMV | PV (4.3–5.1) | MCMV (3.7) | VV (4.4–5.8) | |||||
| LCMV | ND | −0.7 ± 0.1 (3)‖ | −0.2 (1) | −1.8 ± 0.3 (5)‖ | ||||
| PV | ND | ND | UND | −2.4 ± 0.4 (4)‖ | ||||
| MCMV | ND | −0.4 ± 0.06 (3)‖ | ND | −2.5 ± 0.3 (3)‖ | ||||
| VV | ND | −0.2 ± 0.1 (4) | UND | ND | ||||
| Mean (± SEM) Log10 Change in Challenge Virus Titer in the Immune versus the Naive Liver‡ | ||||||||
| Immunizing virus | Challenge virus | |||||||
| LCMV | PV (2.7–3.6) | MCMV (3.9–4.7) | VV (3.6–4.6) | |||||
| LCMV | ND | −0.7 ± 0.06 (3)‖ | −0.6 ± 0.1 (4)¶ | −1.0 ± 0.3 (5)‖ | ||||
| PV | ND | ND | −0.7 ± 0.3 (2) | −1.6 ± 0.05 (2)‖ | ||||
| MCMV | ND | −0.3 ± 0 (2) | ND | −1.5 ± 0.1 (3)‖ | ||||
| VV | ND | −0.1 ± 0.1 (3) | 0.0 (1) | ND | ||||
Prior immunity to one virus can lower viral titer after infection with a heterologous virus. UND, the titer of MCMV was undetectable in both naive and immunized mice. MCMV titer was detectable in the spleen and fat pad of mice in only one experiment. As these experiments were done over a number of years a new stock of virus was used for the later experiments, and this virus stock cleared very rapidly from the spleens and fat pads of older mice even when using the maximal dose of virus which could be given without being lethal in this time frame. Therefore we only have liver titer data available with these later experiments.
VV, PV, and LCMV were titrated on ATCC vero cell monolayers using 3-, 4-, and 5-d plaque assays, respectively. MCMV was titrated on mouse embryo fibroblasts using a 5-d plaque assay.
Data are presented as average of mean reduction of titers in multiple experiments ± SEM. The number in parentheses represents the number of experiments that were done to derive the mean log10 decrease in virus titer. There were four to five mice per treatment group. The infection of mice was done as described in Materials and Methods.
The number in parentheses after each challenging virus represents the range of mean log10 of naive mice challenged with the virus indicated.
Statistically significant difference between naive and immunized groups of mice (two-way Analysis of Variance with replication, P < 0.0001).
Statistically significant difference between naive and immunized groups of mice (two-way Analysis of Variance, P = 0.02).
Table 2.
Prior Immunity to LCMV or PV Can Lower VV Titer Independently of the Challenge Dose
| Virus infections* Immune | VV Titer (mean log10 PFU/ml ± SEM)‡ | |||||||
|---|---|---|---|---|---|---|---|---|
| Acute VV infection virus dose | Spleen | Fat pads | Liver | |||||
| PFU | ||||||||
| NAIVE | 2 × 104 | <1.4 ± 0.2 | 3.4 ± 0.7 | 2.9 ± 0.2 | ||||
| LCMV | 2 × 104 | <1.1 ± 0.05 | <2.1 ± 0.1§ | <2.3 ± 0.2§ | ||||
| PV | 2 × 104 | <1.0 ± 0 | <2.6 ± 0.4§ | <2.2 ± 0.1§ | ||||
| NAIVE | 4 × 105 | 4.0 ± 0.3 | 4.8 ± 0.3 | 4.9 ± 0.3 | ||||
| PV | 4 × 105 | 1.7 ± 0.3§ | 2.5 ± 0.5§ | 2.1 ± 0.2§ | ||||
| NAIVE | 1 × 106 | 3.5 ± 0.2 | 5.8 ± 0.3 | 3.6 ± 0.3 | ||||
| LCMV | 1 × 106 | <1.0 ± 0§ | <2.9 ± 0.05§ | <2.0 ± 0.4§ | ||||
| PV | 1 × 106 | <1.4 ± 0.3§ | <2.4 ± 0.5§ | <2.0 ± 0§ | ||||
The infection of mice was done as described in Materials and Methods.
VV was titrated on ATCC vero cell monolayers using a 3-d plaque assay.
Statistically significant difference between naive and immunized mice (Student's t test, P < 0.05).
Figure 1.
Prior immunity to one virus can lower virus titer to second virus independently of the time after infection with the first virus. The infection of mice was done as described in Materials and Methods. (A) Titers of PV in spleen, fat pad, and liver of age matched naive mice (black bars) and LCMV-immune mice (cross-hatched bars) challenged with PV either 8 or 29 wk after the initial LCMV infection. (B) Titers of VV in spleen, fat pad, and liver of age-matched naive mice (black bars) and MCMV-immune mice (cross-hatched bars) challenged with VV 6 or 19 wk after the initial MCMV infection. *Represents statistically significant difference between naive and immunized mice (Student's t test, P < 0.05).
These experiments were initially done with standard virus stocks derived from serum-containing tissue culture supernatants but then were performed with purified virus stocks to determine if cross-reactive responses to cellular contaminants or FBS were influencing the results. Impurities such as FBS and cellular debris were removed by purification of the viruses over sucrose gradients, followed by substantial 20–100-fold dilutions in PBS before inoculation of mice. Use of purified and diluted viruses eliminated any anamnestic response to FBS or cellular contaminants. With elispot assays we did detect FBS-specific responses of CD4+ cells to IFN-γ and IL-4 when inoculations were done with virus prepared in media containing 10% FBS (data not shown), but no such responses were detected when purified or diluted virus preparations were used. For the protective immunity experiments, however, the conventional virus stocks and purified viruses produced similar results, so all the results were pooled for the analyses in Table 1. It should be further noted that the virus stocks were grown in different cell lines; LCMV was grown in BHK cells, PV was grown in BHK or L929 cells, and VV was grown in NCTC929 cells or L929 cells (11 and 12). MCMV was derived directly from mouse salivary glands (11). Also, serum from LCMV-, PV-, and VV-immune mice infected with the unpurified virus stocks did not demonstrate any antibody production against L929 or BHK cell surface molecules by FACS® analysis (data not shown). All this information makes it highly unlikely that a cellular contaminant influenced the protective immunity.
T Cell Dependence of Heterologous Immunity.
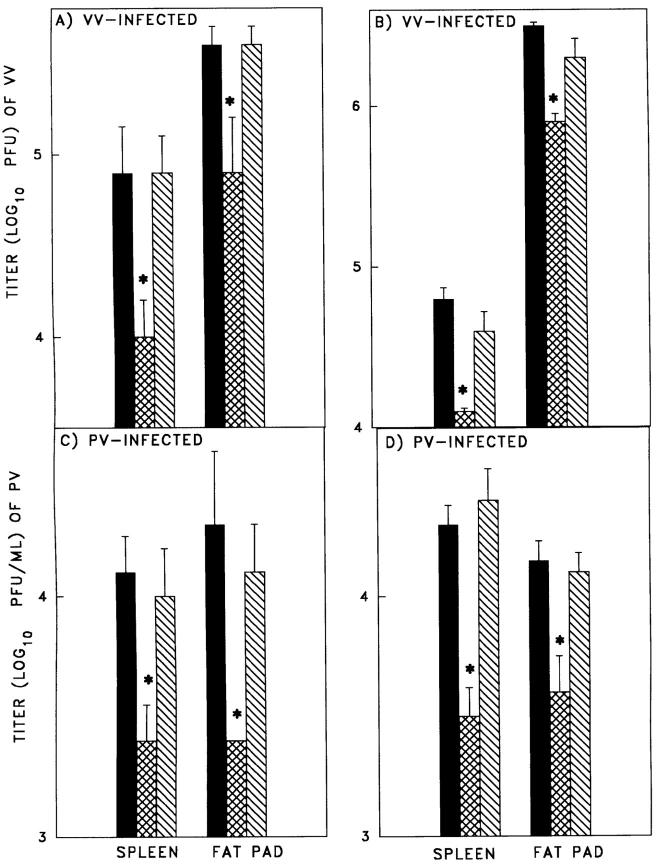
Adoptive cell transfer of LCMV-immune splenocytes into naive mice resulted in five- to eightfold more rapid virus clearance in the spleen and fat pads, on VV or PV challenge than in mice adoptively reconstituted with naive splenocytes (Fig. 2). These results supported the concept that the protective heterologous immunity against acute VV and PV infections was dependent on immune lymphocytes. Depletion of either CD8+ (Fig. 2, A and C) or CD4+ (Fig. 2, B and D) T cells before the cell transfer resulted in a loss of this protective effect, suggesting that both memory T cell types were required. Studies were done to examine whether CD4+ memory T cells were required for reactivation of the LCMV-specific memory CTL in mice challenged acutely with PV. Depletion of CD4+ T cells in vivo by inoculation with anti-CD4 mAb did not have a significant effect on PV-specific CTL induction in PV-infected control or LCMV-immune mice. In LCMV-immune mice challenged with PV, the reactivated LCMV-specific bulk CTL activity (3) in the control group was 6.3 ± 1.4 lytic units (LU; calculated as 5% lysis of 5 × 103 targets) per 106 splenocytes (n = 18) in bulk assays, but in the CD4+ T cell– depleted mice it was dramatically lower, at <1.2 ± 0.1 LU per 106 splenocytes (n = 6). All of these mice were depleted of NK cells with mAb to NK1.1 in order to reduce background cytotoxicity to better resolve the virus-specific killing (12). These results collectively suggest that CD4+ T cells were required for the heterologous activation of CD8+ CTL and that both CD4+ and CD8+ memory T cells were required for protective immunity.
Figure 2.
Protective effect of adoptively transferred LCMV-immune splenocytes mediating heterologous T cell–mediated immunity against unrelated viruses. C57BL/6 mice were injected intravenously with naive or LCMV-immune leukocytes and challenged with VV (A and B) or PV (C and D). LCMV-immune leukocytes were depleted of CD8+ (A and C) or CD4+ (B and D) T cells before cell transfer. Spleens and fat pads were harvested, homogenized, and plaque-assayed for viruses at 3–4 d after infection. The solid bars represent mice receiving adoptively transferred naive cells; the cross-hatched bars represent mice who received adoptively transferred LCMV-immune cells; and the diagonally striped bars represent mice who received adoptively transferred LCMV-immune cells in vitro depleted of either CD4 or CD8 T cells. Each experiment is representative of three similar experiments. *Represents statistically significant difference between naive and immunized groups of mice (Student's t test, P < 0.05).
Requirement of IFN-γ for Heterologous Immunity to VV.
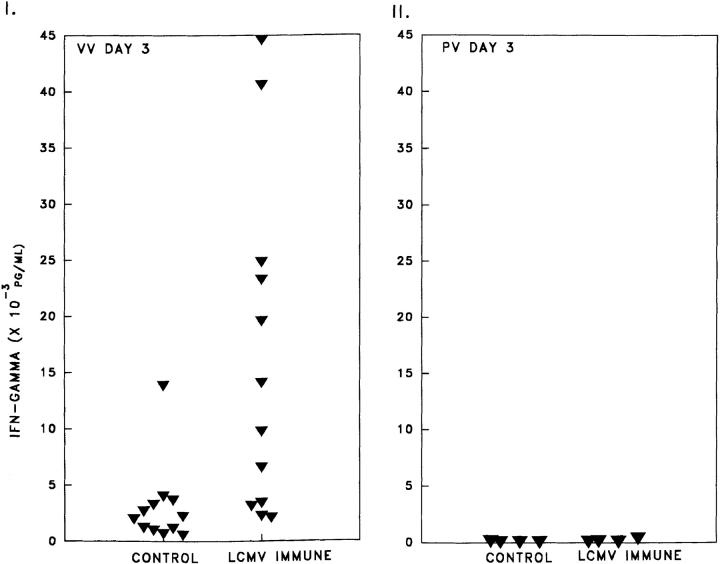
A property of memory T cells is their ability to produce much higher levels of cytokines than do naive cells after stimulation (14). Therefore, we hypothesized that a heterologous virus that stimulated memory T cells would elicit a potent IFN-γ response, a cytokine to which VV is known to be extremely sensitive (17, 30). Analysis by ELISA assay of IFN-γ concentrations (Fig. 3 I) in the peritoneal fluid of LCMV-immune mice at day 3 after VV infection (16,115 ± 4,262 pg/ml, n = 12) revealed >1,074-fold higher titers of IFN-γ than in uninfected naive mice (<15 ± 0 pg/ml, n = 8) or LCMV-immune mice (<15 ± 0 pg/ml, n = 8) and 5.4-fold (2,979 ± 1,039 pg/ml; n = 12; Student's t test, P = 0.007) more than control mice challenged with VV. LCMV-immune mice challenged with PV did not show this enhanced production of IFN-γ (Fig. 3 II) nor did VV-immune mice challenged with LCMV (data not shown).
Figure 3.
(I) Increased IFN-γ levels in peritoneal lavage fluid of LCMV immune mice versus naive mice 3 d after challenge with VV (8 × 105 PFU), unlike challenge with PV (II). An Endogen mouse IFN-γ ELISA assay was done as per manufacturer's protocol.
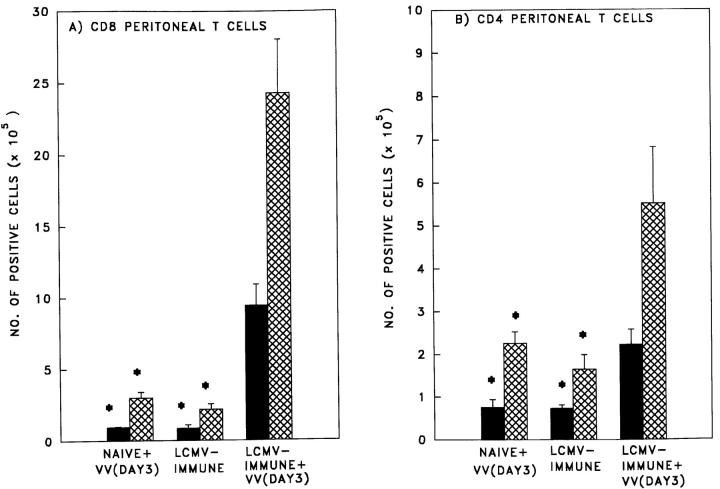
We then examined the peritoneal cavity more closely, as this is the site of initial VV replication where the most significant impact on early virus replication could occur. FACS® staining of PECs resolved a significant 8- and 2.7-fold enhanced recruitment of CD8+ and CD4+ T cells, respectively, in the LCMV-immune versus naive mice 3 d after challenged with VV (Fig. 4, A and B). Furthermore, the peritoneal T cells in the LCMV-immune mice 3 d after challenge with VV were predominantly of the memory phenotype, CD44hi. Basing our cutoff value for CD44hi (FITC-conjugated anti-CD44; PharMingen) at a mean fluorescence of 12.6 units as determined on naive splenocytes, it was found that 92 ± 5% (n = 3) of the peritoneal CD8+ cells and 75 ± 19% (n = 3) of the peritoneal CD4+ cells were CD44hi memory phenotype. The CD8+ cells had a mean fluorescence of 182 ± 40 and the CD4+ cells had a mean fluorescence of 177 ± 58. These cells were examined for expression of intracellular IFN-γ after treatment with PMA and ionomycin. There was a significant 10- and 2.7-fold increase in the number of IFN-γ–producing CD8+ and CD4+ T cells, respectively, in LCMV-immune mice versus naive mice challenged with VV (Fig. 4, A and B).
Figure 4.
Increased numbers of IFN-γ–secreting (A) CD8+ and (B) CD4+ T cells in the peritoneum of LCMV-immune mice challenged with VV (Day 3). Peritoneal cells were harvested from age-matched naive and LCMV-immune C57BL/6 mice challenged with purified VV at day 3 of infection or from LCMV-immune mice challenged with control purified cell supernatant (day 3). The total number of CD8+ and CD4+ cells (cross-hatched bars) and total number of IFN-γ secreting CD8+ and CD4+ cells (solid bars) was determined as described in Materials and Methods. *Represents a statistically significant difference from the LCMV-immune+VV group (P < 0.01).
To further examine whether IFN-γ was playing a role in the protective effect against heterologous viruses, we initially treated LCMV-immune mice with anti-IFN-γ antibodies, as previously described (31). In brief, we gave 0.2 ml of a 1/5 dilution of anti-IFN-γ ascites intraperitoneally daily, in combination with a similar intravenous dose on day 0 and day 3 of VV infection. In the first experiment at 4 d after VV infection there was a 20-fold increase in the VV fat pad titer in the anti-IFN-γ–treated group (LCMV-immune + VV, 2.77 ± 0.23 PFU/ml vs. LCMV-immune + VV + anti-IFN-γ, 4.07 ± 0.45 PFU/ml; n = 5; P = 0.04, Student's t test). In the second experiment at day 6 after VV infection there was a 13-fold increase in the anti-IFN-γ–treated group (LCMV-immune + VV, 4.23 ± 0.61 PFU/ml vs. LCMV-immune + VV + anti–IFN-γ, 5.31 ± 0.22; n = 5; P = 0.006, Student's t test).
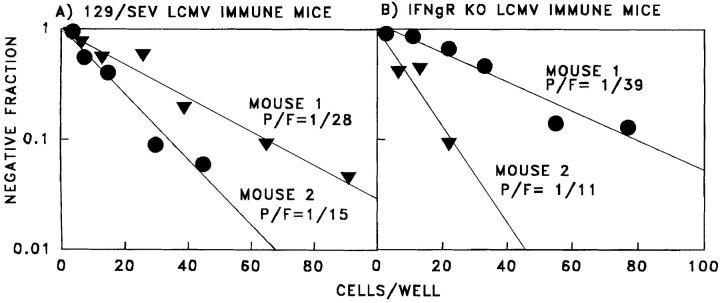
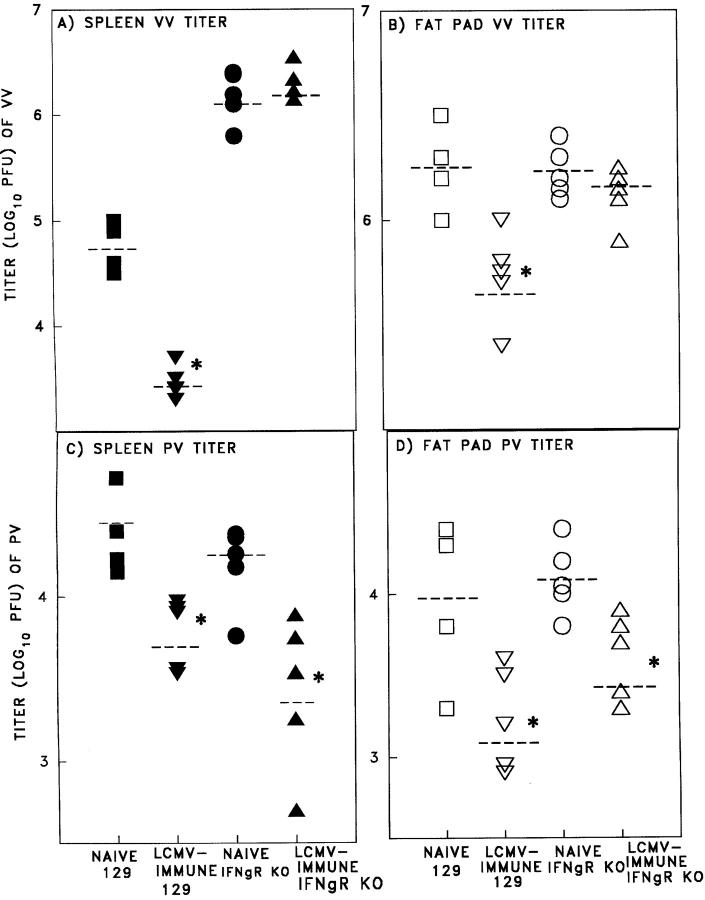
To further confirm this observation, we extended these studies into IFN-γ receptor knockout (IFN-γR KO) mice. Since the IFN-γR KO mice were on the 129/SEV background instead of the C57BL/6 background, normal 129/ SEV mice were used as controls (Fig. 5). The 129/SEV and 129 IFN-γR KO mice developed equivalent high frequency LCMV-specific memory pCTL, as shown by limiting dilution assays (Fig. 5, A and B). 129/SEV mice immune to LCMV developed protective heterologous immunity to PV and VV, similar to that in the C57BL/6 mice. However, IFN-γR KO mice immune to LCMV did not resist VV infection, confirming a role for IFN-γ in the control of VV by LCMV-induced memory T cells (Fig. 6, A and B). Similar experiments demonstrated that IFN-γ was not required for the protective effect against PV (Fig. 6, C and D), suggesting the possibility that other cytokines or even direct cytotoxicity by the cross-reactive CTL may be effective. A role for cytotoxicity could not be easily addressed in these systems, as cytotoxicity-deficient perforin KO mice develop persistent LCMV infections instead of memory T cell responses (32).
Figure 5.
Normal CD8+ T cell memory responses to LCMV in 129/ SEV (A) and IFN-γR KO (B) (reference 17) LCMV-immune mice as determined by LDA. 129/SEV mice 1 and 2 were previously infected with LCMV for 9 and 29 wk, respectively. IFN-γR KO mice 1 and 2 were previously infected with LCMV for 29 and 52 wk, respectively.
Figure 6.
Role for IFN-γ in T cell–mediated heterologous immunity against VV but not PV after challenge of LCMV-immune mice. Naive 129/SEV mice or mice immunized (>6 wk) I.P. with LCMV (4 × 104 PFU) were challenged with VV (106 PFU; A and B) or PV (106 PFU; C and D). In the same experiment, naive IFN-γR KO mice or mice which were immunized with LCMV were challenged with VV (A and B) or PV (C and D). The spleen (A and C) and fat pads (B and D) were harvested, homogenized, and plaque-assayed for virus titers (22) at day 4 after the second virus infection. (□, ▪) naive 129/SEV mice; (▿,▾) LCMV- immune mice; (○, •) naive IFN-γR KO mice; (▵, ▴) LCMV-immune IFN-γR KO mice. The closed symbols demonstrate splenic virus titers and the open symbols demonstrate fat pad virus titers. *Represents a statistically significant difference between naive and immunized groups of mice (Student's t test, P < 0.05). Each experiment is representative of three (for VV) and two (for PV) experiments with similar results.
Heterologous Immunity between Viruses Predisposes a Host to Immunopathogenesis.
Substantial evidence has been provided that T cells can not only be mediators of protective immunity, but also of immunopathology (10, 33–37). Our studies described below of heterologous immunity between viruses indicate that a previous virus infection may predispose a host to a much more severe disease upon infection with an unrelated heterologous virus. In general accordance with an earlier report (22), mice immunized with LCMV, unlike naive mice, developed severe immunopathology consisting of extensive acute fat necrosis (AFN) with a cellular infiltrate in visceral fat pads 5–7 d after VV challenge (Fig. 7). In contrast, prior immunity to PV or MCMV, although protective against VV challenge, did not result in AFN on VV infection. The leukocyte infiltrate within the abdominal fat pads contained 19% ± 7 (SEM; n = 2) CD4+ T cells and 23% ± 5 (SEM; n = 2) CD8+ T cells and mediated CTL activity against both VV- (8 ± 4 lytic units per 106 cells; n = 5) and LCMV- (9.4 ± 6.9 lytic units per 106 cells; n = 5) infected targets. The CTL activity segregated to the CD8+ T cell population on sorting (data not shown). Therefore, these fat pads contained infiltrates of activated CTL specific not only for VV but also for LCMV. A reactivation of the LCMV-specific memory splenic CTL by VV has previously been documented (11, 12). Adoptive transfer studies showed that splenocytes from LCMV-immune mice not only provided some protective immunity against VV synthesis, but also induced rather severe acute fatty necrosis (Fig. 8 A). This immunopathology did not occur in mice lacking IFN-γ receptors (Fig. 8 B). Further, LCMV-immune mice acutely infected with VV and treated with anti-IFN-γ daily had decreased evidence of AFN (level of AFN: LCMV-immune + VV, 4 ± 1.2; LCMV-immune + VV + anti-IFN-γ, 1 ± 0.5; n = 5). As shown above, LCMV-immune mice challenged with VV (Fig. 3 I) had higher levels of IFN-γ in the peritoneal fluid than did naive mice challenged with VV. This enhanced IFN-γ response in immune mice may thus play a role in protective immunity as well as in the induction of immunopathology. In this sequence of viruses the immunological contribution of the LCMV-specific memory cells may result in an overwhelming delayed-type hypersensitivity-like cell-mediated immune response, resulting in enhanced pathogenesis, even though the VV is cleared more rapidly.
Figure 7.
Acute fatty necrosis (AFN) in an LCMV-immune mouse challenged with VV. Absence of AFN in (I) nonchallenged LCMV-immune mice and in (II) naive mice challenged with VV (day 5); (III) severe AFN in LCMV-immune mice challenged with VV (day 5).
Figure 8.
(A, I) Increased level of AFN in mice on day 4 after adoptive transfer of LCMV-immune splenocytes and challenge with VV. (II) Decreased VV titer in fat pads of these same mice as described above. (B, I) AFN was not present in LCMV-immune IFN-γR KO mice when challenged with VV (day 3). (II) No protective effect on fat pad VV titer in these same mice. *Represents a statistically significant difference between naive and immunized groups of mice (Student's t test, P < 0.05). Each experiment is representative of three experiments with similar results.
Discussion
Most models for the immune response to viral infections have used infected animals sheltered from other environmental pathogens, a rather unnatural circumstance. In this study, we show that these sheltered mice responded quite differently to infection than did mice previously exposed to other pathogens, as early protective immunity can be mediated by memory T cells generated by a previous heterologous viral infection. Our results would suggest that both memory CD4+ and CD8+ T cells can be reactivated, as both populations were required for the protective immunity to occur in the virus combinations tested. For instance, in LCMV-immune versus a naive host challenged with VV, both memory cell types were recruited early in infection into the peritoneal cavity, the site of virus challenge, and were producing significantly more IFN-γ, a cytokine to which VV is very sensitive (17, 30). We have not examined the role of memory B cells in this system but clearly memory T cells are required for this type of protective immunity.
Although T cells are generally thought to be highly specific for peptide–MHC complexes, more extensive investigations have shown that a single T cell clone can recognize two unrelated peptide sequences on the same protein, two different proteins of the same virus, and two different proteins from two unrelated viruses (38–40). It is not surprising that peptides from a second viral infection could cross-react with and stimulate some of the many clones of T cells comprising the memory pool specific for another virus. We have reported that memory CD8+ T cells can often cross-react between heterologous viruses (12), and such cross- reactive T cells may contribute to this more rapid early resistance to a second infection. The T cell repertoire to each new infection would be altered as cross-reactive memory cells join in the response, resembling in some ways the original antigenic sin network of antibody responses to different strains of influenza virus, where primary immunity is boosted not by a homologous but, by a cross-reacting vaccine of a different influenza virus serotype, and some of the newly formed antibodies react better with the primary virus than with the virus actually eliciting the response (41). Memory T cells are known to express high levels of adhesion molecules (8–10) and to be easier to stimulate (13–16) than naive cells, making them susceptible to low affinity cross-reactive TcR triggering. Some reports have suggested that memory T cells can be stimulated nonspecifically by cytokines (5, 6, 42), and may be cross-activated by an infection that does not require stimulation through their T cell receptor by cross-reactive viral peptides. However, data from our laboratory suggests that memory T cells do not nonspecifically proliferate in response to an antigen to which there are no cross-reactive TcR epitopes detectable (43), and our experiments on heterologous immunity between viruses argue on behalf of cross-reactive memory cells rather than nonspecific activation effects, because the specific sequence of virus infections determines if there is any protective immunity. Nevertheless, it is possible there is some as yet unknown selective mechanism for cross-activation of different memory T cell populations. The contribution of memory cells specific for one virus to the natural resistance against another virus is presently not predictable and is not necessarily reciprocal. If the immunodominant memory T cell response to an earlier virus could recognize a cross-reactive determinant on the challenge virus, the challenge virus would find a large pool of memory T cells capable of a rapid response. Should the challenge virus not be recognized by part of the immunodominant memory T cell repertoire, then less of a protective effect may ensue.
Individuals vary considerably in their responses to viral infections, ranging from subclinical to severe. There are many factors that contribute to this variation in responsiveness, including the dose and route of infection, as well as the physiological state and genetic background of the host. In this study, we show that memory T cells specific to unrelated viruses may also contribute to the host's primary response to a second virus. The beneficial effect of these early protective memory T cells is to slow down the spread of infection, much like a natural immune-mediated response, allowing time for more suitable high affinity antigen-specific T and B cell responses to develop. It is reasonable to expect that this level of resistance may be the difference between clinical and subclinical infections or lethal and nonlethal infections. The detrimental immunopathological effect of memory T cells leads us to question whether they may play a role in human infections like with Epstein-Barr virus or varicella-zoster virus that lead to much more severe disease in young adults than in young children, who presumably have a more restricted memory T cell repertoire (44, 45). Reactivation of memory T cells may also play a role in exacerbation of autoimmune diseases, such as multiple sclerosis or diabetes. For example, in an experimental model for chronic virus-induced CNS autoimmunity, disease may be induced by infection with a virus sharing epitopes with a protein expressed in oligodendrocytes, and this T cell–dependent disease could be exacerbated by a second infection with an unrelated virus (46). Thus, alterations in acute responses caused by the memory T cell modulations induced by the host's history of prior infections may have the paradoxical effect of producing both beneficial protective heterologous immunity and/or immunopathology.
Acknowledgments
We thank Dr. R. Fujinami (University of Utah, Salt Lake City) for supplying us with the Lyt 2.34 hybridoma cell line; K. Vergilis, C. O'Donnell, and P. Santolucito for their excellent technical assistance; and E. Szomolanyi-Tsuda, F.A. Ennis and H. Robinson for useful discussions concerning the manuscript.
This work was supported by United States Public Health Service, National Institutes of Health (NIH) research grants AR-35506, AI-17672, and CA-34461 to Raymond Welsh, Clinical Investigator Award AI01362 to Liisa K. Selin, and training grants AI-07272 and AI-07349. Its contents are solely the responsibilities of the authors and do not necessarily represent the official views of the NIH.
Abbreviations used in this paper
- AFN
acute fat necrosis
- FBS
fetal bovine serum
- IFN-γR KO
interferon γ receptor knockout mice
- LCMV
lymphocytic choriomeningitis virus
- MCMV
murine cytomegalovirus
- MEF
mouse embryo fibroblast
- MOI
multiplicity of infection
- PEC
peritoneal exudate cells
- p/f
precursor frequency
- PV
Pichinde virus
- VV
vaccinia virus
References
- 1.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 2.Selin LK, Vergilis K, Welsh RM, Nahill SR. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J Exp Med. 1996;183:2489–2499. doi: 10.1084/jem.183.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selin LK, Welsh RM. Cytolytically active memory CTL present in lymphocytic choriomeningitis virus-immune mice after clearance of virus infection. J Immunol. 1997;158:5366–5373. [PubMed] [Google Scholar]
- 4.Razvi ES, Welsh RM, McFarland HI. In vivo state of antiviral CTL precursors: characterization of a cycling population containing CTL precursors in immune mice. J Immunol. 1995;154:620–632. [PubMed] [Google Scholar]
- 5.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sprent J, Tough DF. Lymphocyte life-span and memory. Science. 1996;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 7.Akbar AN, Salmon M, Janossy G. The synergy between naive and memory T cells during activation. Immunol Today. 1991;12:184–188. doi: 10.1016/0167-5699(91)90050-4. [DOI] [PubMed] [Google Scholar]
- 8.McFarland HI, Nahill SR, Maciaszek JW, Welsh RM. CD11b (Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. J Immunol. 1992;149:1326–1333. [PubMed] [Google Scholar]
- 9.Zimmermann C, Brduscha-Riem K, Blaser C, Zinkernagel RM, Pircher H. Visualization, characterization, and turnover of CD8+memory T cells in virus- infected hosts. J Exp Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson EC, Christenson JP, Marker O, Thomsen AR. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J Immunol. 1994;152:1237–1245. [PubMed] [Google Scholar]
- 11.Yang H, Dundon PL, Nahill SR, Welsh RM. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989;142:1710–1718. [PubMed] [Google Scholar]
- 12.Selin LK, Nahill SR, Welsh RM. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabi Z, Lynch F, Ceredig R, Allan JE, Doherty PC. Virus-specific memory T cells are Pgp-1+ and can be selectively activated with phorbol ester and calcium ionophore. Cell Immunol. 1988;113:268–277. doi: 10.1016/0008-8749(88)90026-3. [DOI] [PubMed] [Google Scholar]
- 14.Bradley LM, Croft M, Swain SL. T-cell memory: new perspectives. Immunol Today. 1993;14:197–199. doi: 10.1016/0167-5699(93)90161-D. [DOI] [PubMed] [Google Scholar]
- 15.Tough DL, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 16.Pihlgren M, Dubois PM, Tomkowiak M, Sjogren T, Marvel J. Resting memory CD8+T cells are hyperactive to antigenic challenge in vitro. J Exp Med. 1996;184:2141–2151. doi: 10.1084/jem.184.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller U, Steinhoff U, Reis LFL, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 18.Welsh RM, Lampert PW, Burner PA, Oldstone MBA. Antibody-complement interactions with purified lymphocytic choriomeningitis virus. Virol. 1976;73:59–71. doi: 10.1016/0042-6822(76)90060-x. [DOI] [PubMed] [Google Scholar]
- 19.Welsh RM. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978;148:163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131:1531–1538. [PubMed] [Google Scholar]
- 21.Tanaka Y, Tevethia SS. In vitroselection of SV40 antigen epitope loss variants by site-specific cytotoxic T lymphocyte clones. J Immunol. 1988;140:4348–4354. [PubMed] [Google Scholar]
- 22.Yang H, Joris I, Majno G, Welsh RM. Necrosis of adipose tissue induced by sequential infections with unrelated viruses. Am J Pathol. 1985;120:173–177. [PMC free article] [PubMed] [Google Scholar]
- 23.Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahill SR, Welsh RM. High frequency of cross-reactive cytotoxic T lymphocytes elicited during the virus-induced polyclonal cytotoxic T lymphocyte response. J Exp Med. 1993;177:317–327. doi: 10.1084/jem.177.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabin H, Hopkins RF, III, Ruscetti FW, Neubauer RH, Brown RL, Kawakami TG. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981;127:1852–1856. [PubMed] [Google Scholar]
- 26.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 27.Pross HF, Baines MG, Rubin P, Shragge P, Paterson MS. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. J Clin Immunol. 1981;1:51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- 28.Sarmiento M, Glasebrook AL, Fitch FW. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 29.Szomolanyi-Tsuda E, Welsh RM. T cell–independent antibody-mediated clearance of polyoma virus in T cell–deficient mice. J Exp Med. 1996;183:403–411. doi: 10.1084/jem.183.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsh, R.M., and G.C. Sen. 1996. Non-specific host responses to viral infections. In Viral Pathogenesis. N. Nathanson, editor. Lippincott-Raven Publishers, New York. 109–142.
- 31.Tay CH, Welsh RM. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kagi D, Seiler P, Pavlovic J, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. The roles of perforin- and Fas-dependent cytotoxocity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 33.Doherty PC, Zinkernagel RM. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19:89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 34.Cole GA, Nathanson N, Prendergast RA. Requirement for Φ-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature. 1972;238:335–337. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- 35.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiological study of altered clinical reactivity to respiratory syncitial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 36.Cannon MJ, Openshaw PJM, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson RW, Bennick JR, Yewdell JW, Maloy WL, Coligan JE. Influenza basic polymerase 2 peptides are recognized by influenza nucleoprotein-specific cytotoxic T lymphocytes. Mol Immunol. 1992;29:1089–1096. doi: 10.1016/0161-5890(92)90041-u. [DOI] [PubMed] [Google Scholar]
- 39.Shimojo N, Maloy WL, Anderson RW, Biddison WE, Coligan JE. Specificity of peptide binding by the HLA-A2.1 molecule. J Immunol. 1989;143:2939–2947. [PubMed] [Google Scholar]
- 40.Kuwano K, Reyes RE, Humphreys RE, Ennis FA. Recognition of disparate HA and NS1 peptides by an H-2Kd-restricted, influenza specific CTL clone. Mol Immunol. 1991;28:1–7. doi: 10.1016/0161-5890(91)90080-4. [DOI] [PubMed] [Google Scholar]
- 41.Fazekas de St. Groth S, Webster RG. Disquisitions on original antigenic sin II. Proof in lower creatures. J Exp Med. 1966;124:347–361. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehl S, Hombach J, Aichele P, Hengartner H, Zinkernagel RM. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J Exp Med. 1997;185:1241–1251. doi: 10.1084/jem.185.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarozinski CC, Welsh RM. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response. J Exp Med. 1997;185:1629–1639. doi: 10.1084/jem.185.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinstein L, Meade RH. Respiratory manifestations of chickenpox. Arch Intern Med. 1956;98:91–99. doi: 10.1001/archinte.1956.00250250097013. [DOI] [PubMed] [Google Scholar]
- 45.Rickinson, A.B., and E. Kieff. 1996. Epstein-Barr virus. In Virology Volume 2. B.N. Fields, D.M. Knipe, P.M. Howley, R.M. Chanock, J.L. Melnick, T.P. Monath, B. Roizman, and S.S. Straus, editors. Lippincott-Raven Publishers, Philadelphia. 2397–2446.
- 46.Evans CF, Horwitz MS, Hobbs MV, Oldstone MBA. Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease. J Exp Med. 1996;184:2371–2384. doi: 10.1084/jem.184.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]