Abstract
The interaction of the T cell receptor (TCR) with its cognate peptide–major histocompatibility complex (MHC) on the surface of antigen presenting cells (APCs) is a primary event during T cell activation. Here we used a dimeric IEk-MCC molecule to study its capacity to activate antigen-specific T cells and to directly analyze the role of CD4 in physically stabilizing the TCR–MHC interaction. Dimeric IEk-MCC stably binds to specific T cells. In addition, immobilized dimeric IEk-MCC can induce TCR downregulation and activate antigen-specific T cells more efficiently than anti-CD3. The potency of the dimeric IEk-MCC is significantly enhanced in the presence of CD4. However, CD4 does not play any significant role in stabilizing peptide-MHC–TCR interactions as it fails to enhance binding of IEk-MCC to specific T cells or influence peptide-MHC–TCR dissociation rate or TCR downregulation. Moreover, these results indicate that dimerization of peptide-MHC class II using an IgG molecular scaffold significantly increases its binding avidity leading to an enhancement of its stimulatory capacity while maintaining the physiological properties of cognate peptide–MHC complex. These peptide-MHC–IgG chimeras may, therefore, provide a novel approach to modulate antigen-specific T cell responses both in vitro and in vivo.
Keywords: T cell stimulation, CD4 coreceptor, major histocompatibility complex multimerization, T cell receptor downregulation
The critical initiating event in T cell activation involves delivery of signal 1 through the TCR. It is now appreciated that TCR signaling involves multiple components occurring at the interface between the APC and T cell membranes in order to initiate optimal intracellular signaling cascades. First and foremost, TCR signaling is critically dependent on the affinity of the TCR–peptide-MHC interaction. Surface plasmon resonance studies of TCR's interactions with peptide-MHC molecules indicate that while the overall range of affinities of stimulatory ligands is low relative to antibody–antigen interactions, it needs to be sufficiently high (>10−5 M) to induce T cell activation. MHC-peptide ligands with affinities <10−5 M can function as antagonists or may fail to generate any response in T cells at all (1).
It is well known that multimerization of TCR and CD3 molecules can trigger T cell activation. The experiments demonstrating this include cross-linking with antibodies against TCR or cross-linking chimeric molecules consisting of a non-TCR extracellular domain linked to the CD3ζ cytoplasmic dimer (2, 3). In addition, work with T cells specific for fluorescein or arsonate has shown that activation of these T cells requires at least dimerization of the TCR molecules (4–6). The mechanism by which TCRs are cross-linked under natural circumstances of peptide-MHC recognition is unclear although several studies suggest an intrinsic capability of MHC molecules to multimerize under certain conditions (7, 8).
Recent evidence has shown that individual peptide-MHC complexes on APC can serially engage multiple TCRs, thereby allowing a relatively small number of appropriate peptide-MHC complexes to engage a large proportion of TCRs on cognate T cells (9). Serial TCR engagement presumably allows for the accumulation of signal towards a critical threshold for T cell activation. This threshold has been estimated to require serial engagement of 8,000 TCRs in the absence of CD28 engagement and roughly 1,500 TCRs in the presence of CD28 engagement (10). According to this model, there is no strict requirement for TCR multimerization during T cell activation. Further work is needed to clarify the role of TCR cross-linking and serial engagement in T cell activation.
Association of the CD4 and CD8 coreceptors with engaged TCR represents an additional process that dramatically affects TCR signaling and T cell activation (2). Recruitment of the coreceptor to the occupied TCR complex allows cytoplasmically associated Lck to tyrosine phosphorylate CD3ζ and/or ζ-associated proteins, thereby helping to initiate a kinase signaling cascade (11). Indirect evidence has implicated the coreceptors in modulating additional components of signal 1 generation including stabilization and/or multimerization of the peptide-MHC–TCR complex as well as TCR downregulation associated with serial TCR engagement (11–14). Defining the interaction between the coreceptors and these different parameters of TCR signaling will provide an enhanced ability to efficiently manipulate antigen-specific T cell responses.
Production of soluble MHC and TCR molecules has markedly increased our understanding of the basic mechanism of T cell recognition through crystallographic and biophysical studies (1). However, the low affinity of these molecules for cognate ligands has limited their usefulness in studying the interactions of the TCR with other surface molecules that act in concert to produce immune responses or in tracking antigen-specific T cells in vivo. To overcome the low-affinity problem, multivalent peptide–MHC class I ligands have been generated recently and have been used successfully for visualization of antigen-specific T cells in several different systems (15–17).
In this study we examined the capacity of a dimeric peptide–MHC class II–IgG chimera to bind to and activate cognate T cell lines. We found that, in contrast to monomeric peptide–MHC class II, the dimeric peptide–MHC class II–IgG complexes bound stably and specifically to cognate T cells. Further analyses showed that peptide-MHC class II–IgG was significantly more potent than anti-CD3 at stimulating cognate T cells due to its ability to recruit CD4 into the engaged TCR complex as well as to more effectively induce TCR downregulation. Analysis of matched CD4-positive and CD4-negative subclones using these peptide-MHC class II–IgG molecules allowed us to directly evaluate the role of the CD4 coreceptor in T cell activation. In contrast to previous suggestions, our data showed that the CD4 coreceptor played no apparent role in either enhancing the stability of TCR–peptide-MHC interactions or in mediating TCR downregulation.
Materials and Methods
Mice.
C57BL/6 × SJL founder AND transgenic (Tg)1 mice, specific for moth cytochrome C peptide (MCC 91–103) in the context of IEk, backcrossed to B10.BR (H-2k) mice for six to eight generations, were bred and maintained in the animal care facility at the Cancer Center of the Johns Hopkins School of Medicine (18, 19).
T Cell Hybridomas and IL-2 Production.
The two hybridomas used in this study were a generous gift of Drs. Kappler and Marrack (National Jewish Medical and Research Center, Denver, CO). 5KC73.8 is specific for MCC 91–103 in the context of IEk (20). DO11.10 is specific for chicken ovalbumin peptide (cOVA 327–339) in the context of IAd (21). Since 5KC73.8 cells have a tendency to lose expression of the CD4 molecule, limiting dilutions were used to obtain a CD4-positive (No. 9) and a CD4-negative (No. 10) clone of the hybridoma. These two clones expressed similar levels of TCR.
For stimulation assays, wells of Immulon4 microtiter plates (Dynatech Laboratories Inc., Chantilly, VA) were coated with different proteins in PBS overnight at 4°C. Plates were then washed and coated with 10% FCS for 1 h. Wells coated with monomeric IEk-MCC, dimeric IEk-MCC (described below), or anti-CD3 mAb were then used to activate T cell hybridomas at a density of 5 × 104 cells per well. After overnight incubation at 37°C, IL-2 was measured using an ELISA minikit (Endogen, Cambridge, MA).
Production of Soluble Dimeric MHC Class II IEk-MCC Protein.
Soluble monomeric IEk covalently linked to the MCC 91–103 peptide was produced and purified as described before (22). Dimeric IEk-MCC was produced by inserting the cDNA encoding the extracellular domains of the IEα and β chain upstream of the IgG light and heavy chains, respectively. A Kpn1 restriction site and a linker were inserted immediately 3′ to the interface between the extracellular and transmembrane domains of IEβ polypeptide. The 5′ regions of the gene had been modified already to encode the MCC peptide and an EcoR1 restriction site. The IEα chain was modified by introduction of a HindIII restriction site immediately 3′ to the codon at the interface between the extracellular and transmembrane domains. Soluble proteins were produced in a baculovirus expression system and purified on 14.4.4.S (anti-IEαk) affinity column as described previously (22).
Antibodies and Flow Cytometric Analysis.
The following antibodies were used in this study: H597 and 2C11 mAbs specific for murine TCR Cβ chain and CD3ε, respectively, were produced following standard techniques. Fluoresceinated RM4-5 specific for mouse CD4 and biotin-A85-1 specific for murine IgG1 were purchased from PharMingen (San Diego, CA) whereas PE- labeled goat anti–mouse IgG1 was purchased from Southern Biotech. Association, Inc. (Birmingham, AL). 14.4.4.S mAb specific for the α chain of IEk molecule was purified from culture supernatant using a protein A affinity column.
Cells (2 × 105) were incubated with a saturating dose of monomeric IEk-MCC or dimeric IEk-MCC in 100 μl of staining buffer on ice for 2 h. Cells were then washed, and bound dimeric IEk-MCC was detected by PE-conjugated goat anti–mouse IgG1, or biotin-A85-1 mAb followed by PE-streptavidin conjugate, respectively. For detection of TCR and CD4 levels, cells were incubated with biotinylated H597 for 30 min. Cells were then washed and incubated with PE-conjugated streptavidin and fluoresceinated RM4-5 for 20 min on ice followed by analysis by FACScan® (Becton Dickinson, Mountain View, CA).
In Vitro Proliferation Assays.
Single cell suspensions from spleens of AND × B10.BR Tg mice were harvested and cultured at 2.5 × 105 in 96-well Immulon4 microtiter plates that contained various concentrations of immobilized monomeric IEk-MCC, dimeric IEk-MCC, or anti-CD3. Cultures were incubated in 200 μl of CTM (complete tissue culture medium) at 37°C for 72 h and then pulsed with 1 μCi of 3H-TdR for 6–12 h. Cultures were then harvested and 3H-TdR uptake was determined by scintillation counting. Results are expressed as the mean of triplicate cultures (background subtracted).
Measurement of TCR Downregulation.
The CD4-positive and CD4-negative clones of the 5KC73.8 T cell hybridoma were incubated with varying concentrations of immobilized monomeric IEk-MCC, dimeric IEk-MCC, or anti-CD3 in CTM for 5 h at 37°C. Control cells were incubated in parallel cultures in PBS. All cultures were then recovered and stained, as described above, with anti-Cβ mAb H597 to determine the level of TCRs that remained on the cell surface after stimulation. Results were expressed as percentages of TCRs on surfaces of the PBS-stimulated control clones.
Effect of CD4 on Binding of IEk-MCC Dimer to 5KC73.8 Clones.
Various concentrations of IEk-MCC dimer were incubated with 2 × 105 cells on ice for 2 h. Cells were washed and bound dimer was detected by PE-labeled goat anti–mouse IgG1 antibodies. As a control, tested clones were stained with anti-TCRβ (H597) and anti-CD4 (RM4-5) to confirm the level of TCR and CD4 at the time of each experiment.
To study the effect of CD4 on dissociation of the dimeric IEk-MCC from 5KC 73.8 cells, various concentrations of soluble dimer were incubated with the CD4-positive (No. 9) and the CD4-negative (No. 10) clones for 2 h on ice. All cultures were then placed at 4°C, washed once, and resuspended in 180 μl of staining buffer for the indicated period of time at 4°C. Cells were immediately washed at 4°C and the bound dimer was detected using PE-conjugated goat anti–murine IgG1, as described above.
Results
Stable and Specific Binding of Dimeric IEk-MCC to Specific T Cells.
We used the well characterized I-Ek restricted MCC system to study T cell activation by dimeric peptide– MHC complexes. Using IgG as a molecular scaffold we expressed a soluble IEk-MCC dimer. For the production of this chimeric molecule, two chimeric genes were constructed and introduced into a baculovirus transfer vector. The first chimeric gene linked DNA encoding the extracellular domains of IEk α gene to the 5′ end of a gene encoding the mature Ig light chain. The second chimeric gene linked DNA encoding the extracellular domains of IEk β gene to the 5′ end of a gene encoding the mature Ig heavy chain. The 5′ sequences of the β chain had been altered already by the addition of DNA sequences encoding the antigenic peptide MCC 91–103 via an alanine/glycine encoded linker (22). The constructs were cloned into the dual promoter baculovirus expression vector and used to infect Sf9 cells. Infected cells produced ∼0.5 mg of chimeric protein per liter of culture. A monomeric IEk-MCC, which utilized the same MCC 91–103 peptide and a linker sequence as the dimeric IEk-MCC, was used for comparative purposes (22).
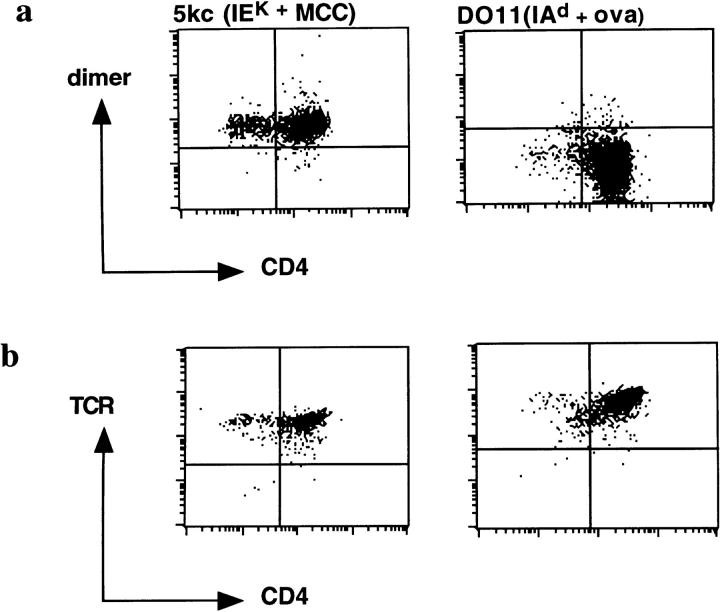
We first sought to determine whether dimeric IEk-MCC would bind stably and specifically to MCC-specific T cells. For these purposes, we used the IEk + MCC-specific T cell hybridoma, 5KC73.8, and, as a specificity control, the IAd + cOVA-specific T cell hybridoma, DO11.10. Analysis by flow cytometry showed significant binding of dimeric IEk-MCC to 5KC73.8 T cell hybridoma. No binding of the dimeric IEk-MCC to control DO11.10 T cell hybridoma was detected although it expressed a high level of TCR, comparable to those expressed by the 5KC73.8 T cell hybridoma (Fig. 1, a and b). Not surprisingly, there was no stable binding of monomeric IEk-MCC to either 5KC73.8 or to DO11.10 T cell hybridomas at all doses tested (data not shown). Therefore, dimerization of IEk-MCC on the IgG scaffold augmented affinity significantly, allowing for binding to cognate T cells that was detectable by flow cytometry.
Figure 1.
Specific binding of the dimeric IEk-MCC to 5KC73.8 T cell hybridoma. (a) Binding of the IEk-MCC dimer to the 5KC73.8 T cell hybridoma but not to the negative control, DO11.10 T cell hybridoma. Cells were incubated with 100 μl of 100 μg/ml of the dimer for 2 h on ice. Cells were then washed, and bound dimer was detected using biotin-A85-1 followed by PE-conjugated streptavidin. (b) Both 5KC37.8 and DO11.10 T cell hybridomas express high levels of TCR. Cells were stained with a saturating amount of biotin-H597 for 2 h on ice and bound antibody was detected with PE-streptavidin conjugate.
Dimeric IEk-MCC Is a Potent T Cell Stimulatory Molecule.
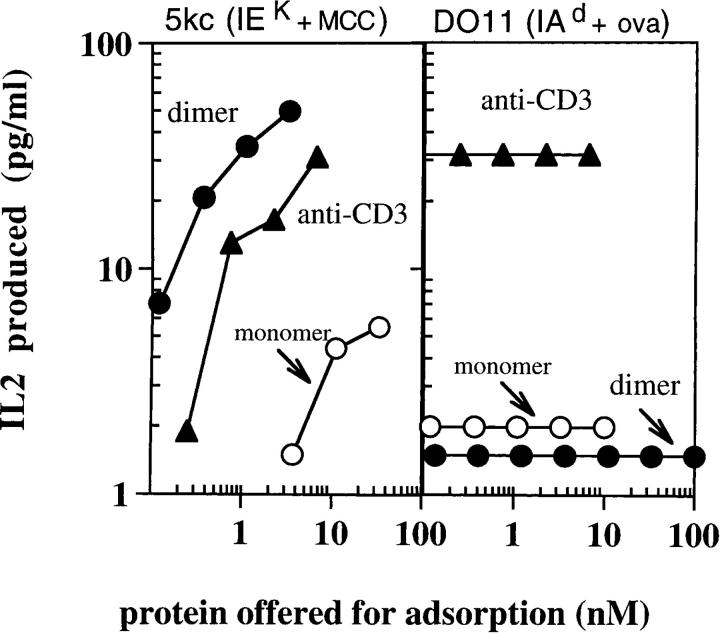
We next compared the ability of monomeric IEk-MCC, dimeric IEk-MCC, and anti-CD3 to activate MCC-specific T cells. To assay T cell stimulation, the different molecules were coated at varying concentrations onto wells of Immulon4 plates and used to stimulate IL-2 release by the MCC-specific T cell hybridoma. Both monomeric IEk-MCC and dimeric IEk-MCC stimulated specific IL-2 release by 5KC73.8 T cells, but with considerably different potency (Fig. 2). Dimeric IEk-MCC was significantly better at stimulating the 5KC73.8 T cell hybridoma than monomeric IEk-MCC. At least 40-fold, or more, immobilized monomeric IEk-MCC was required to achieve a similar response as that elicited by the dimer. Interestingly, dimeric IEk-MCC was even more potent than divalent anti-CD3 mAb, by ∼5–10-fold. As a negative control, we showed that DO11.10 T cell hybridoma responded to anti-CD3 mAb but not to either monomeric IEk-MCC or dimeric IEk-MCC, confirming the specificity of these molecules for their cognate TCR.
Figure 2.
Specific and vigorous response of 5KC73.8 T cell hybridoma to the dimeric IEk-MCC. 5KC73.8 cells (left) and DO11.10 cells (right) were incubated overnight at 37°C with various concentrations of immobilized monomeric IEk-MCC, dimeric IEk-MCC, or anti-CD3. IL-2 produced was measured as described in Materials and Methods. Results are expressed as the mean of duplicate cultures. This experiment is representative of at least three experiments with similar results.
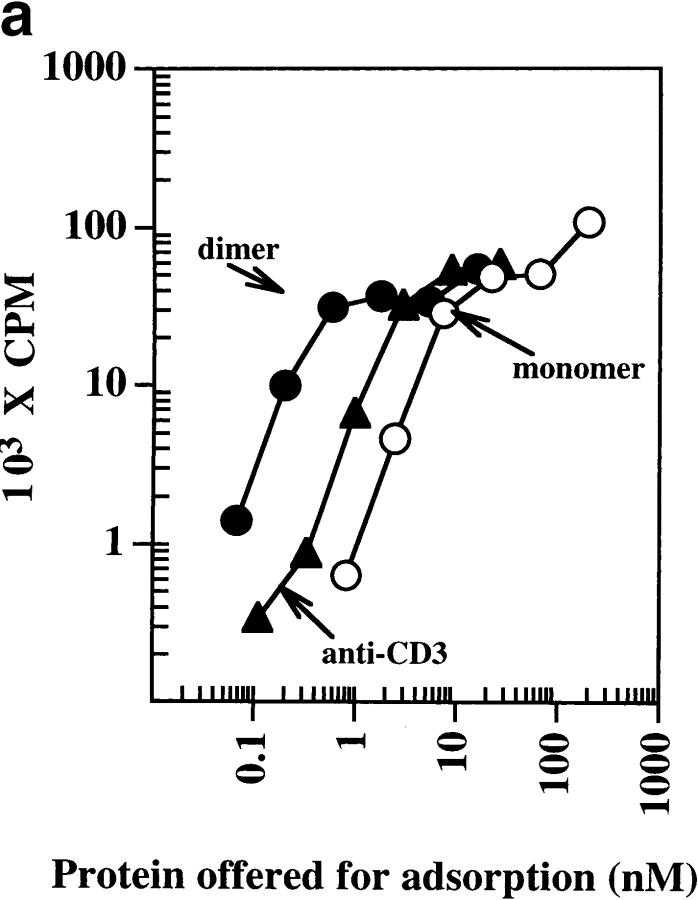
The stimulatory capacity of the dimeric IEk-MCC was also evident using naive untransformed T cells from MCC-specific AND transgenic mice (Fig. 3 a). Under these conditions, immobilized monomeric IEk-MCC was the least stimulatory for T cells, while dimeric IEk-MCC was roughly 10-fold more stimulatory than anti-CD3. No significant activation of splenocytes from naive B10.BR mice was observed using monomeric or dimeric IEk-MCC, indicating the response was antigen specific (data not shown). An additional advantage of the dimeric IEk-MCC molecules is their ability to bind through the Fc portion of the IgG to FcR+ splenocytes and activate specific T cells. As expected, the soluble dimer added to splenocytes from AND × B10.BR Tg mice elicited strong IL-2 response, comparable to that induced by anti-CD3, whereas a small amount of IL-2 was produced in response to the soluble monomer (Fig. 3 b).
Figure 3.
Stimulation of splenocytes from AND Tg mice with immobilized and soluble dimeric IEK-MCC. (a) Splenocytes from AND × B10.BR Tg mice were incubated for 72 h at 37°C with various concentrations of immobilized monomeric IEK-MCC, dimeric IEK-MCC, or anti-CD3. Cultures were pulsed with 3H-TdR for 12 h at 37°C and uptake was measured by scintillation counting. Results are expressed as the mean of triplicate cultures (background was subtracted). (b) Strong response of splenocytes from AND Tg mice to soluble IEK-MCC dimer. Splenocytes from AND × B10.BR Tg mice were incubated for 48 h at 37°C with various concentrations of soluble monomeric IEK-MCC, dimeric IEK-MCC, or anti-CD3. IL-2 present in culture supernatants was measured using ELISA. Results are expressed as the mean of triplicate cultures. One of three experiments with similar results is shown.
Recent experiments indicate that T cell response correlates in magnitude and kinetics with the downregulation of the TCR (10, 23). To further compare the physiological properties of monomeric IEk-MCC, dimeric IEk-MCC, and anti-CD3, the capacity of immobilized forms of these complexes to induce TCR downregulation of the CD4-positive clone of 5KC73.8 T cell hybridoma was tested. We found that dimeric IEk-MCC was more efficient than anti-CD3 in triggering TCR downregulation (Fig. 4 a). However, we did not observe any significant downregulation of TCRs in response to the immobilized monomer at concentrations sufficient to induce IL-2 production by 5KC73.8 T cell hybridoma. Whether failure of monomeric IEk-MCC to induce TCR downregulation is due to reduced sensitivity of the assay or multimerization is needed for this process requires further investigation. On the other hand, only anti-CD3, but not monomeric IEk-MCC or dimeric IEk-MCC, was able to effectively induce TCR downregulation of the specificity control DO11.10 T cell hybridoma (Fig. 4 b). Thus, the enhanced stimulatory capacity of dimeric IEk-MCC correlates very well with its ability to downregulate TCR associated with serial triggering.
Figure 4.
Dimeric IEk-MCC triggers TCR downregulation more efficiently than anti-CD3. (a) The CD4-positive clone of 5KC73.8 T cell hybridoma was incubated with various concentrations of immobilized monomeric IEK-MCC, dimeric IEK-MCC, or anti-CD3 in CTM for 5 h at 37°C. Cells were then washed and stained with biotin-H597 as described in Materials and Methods. It is noteworthy to mention that binding of dimeric IEk-MCC to 5KC73.8 T cell hybridoma does not block binding of H597 mAb. Results are expressed as a percentage of TCR that remained on surface of cells stimulated with PBS-coated wells. (b) Anti-CD3, but not dimeric IEk-MCC, induced downregulation of the TCR of the control D011.10 hybridoma. Experiments were performed as described in a. This experiment is representative of at least three experiments with similar results.
The Enhanced T Cell Stimulation by Dimeric IEk-MCC Is Partly Mediated by the CD4 Molecule.
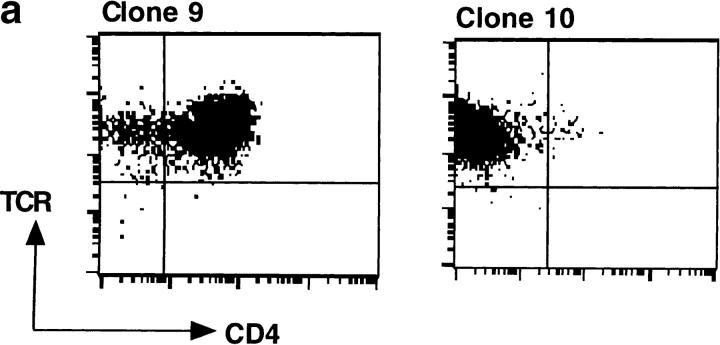
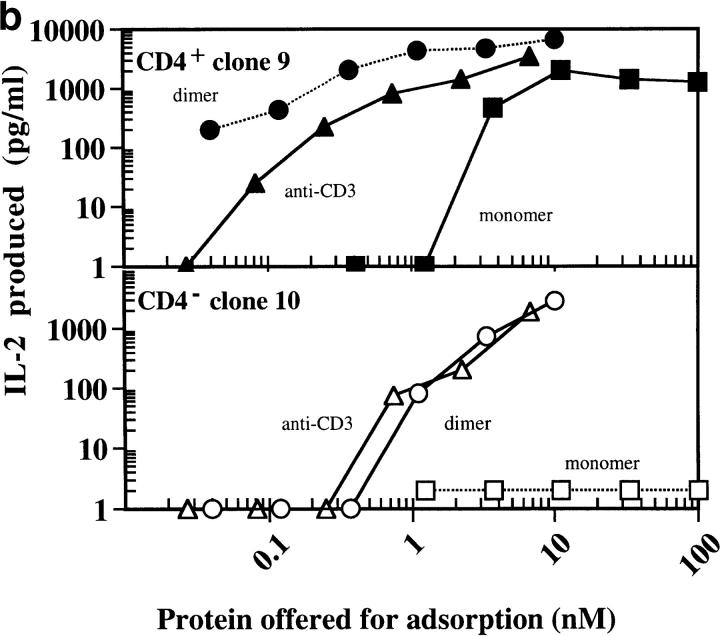
Engagement of CD4 by the MHC class II molecules is necessary for optimum T cell response to specific antigens. If this is the reason behind the enhanced potency of IEk-MCC relative to anti-CD3, then responses of the 5KC73.8 T cell hybridoma to dimeric IEk-MCC, but not to anti-CD3, should significantly diminish when the CD4 effect is removed. To test this hypothesis, CD4-positive (No. 9) and CD4-negative (No. 10) clones of the 5KC73.8 T cell hybridoma that expressed identical levels of TCRs were isolated (Fig. 5 a). Dose–response curves of these clones to all immobilized antigens (monomeric IEk-MCC, dimeric IEk-MCC, and anti-CD3) were significantly shifted to the right in the absence of CD4, indicating that the 5KC73.8 T cell hybridoma response to MCC antigen is CD4 dependent (Fig. 5 b). Interestingly, dimeric IEk-MCC was more potent than anti-CD3 only when used to stimulate the CD4-positive clone. However, when the CD4-negative clone was stimulated, the dimeric IEk-MCC and anti-CD3 elicited equivalent responses. This result also indicates that dimerization of MHC class II by IgG scaffold does not interfere with CD4/ MHC binding as manifested by the major impact of CD4 on the ability of dimeric IEk-MCC, but not anti-CD3, to activate specific T cells. Thus, it appears that the enhanced stimulatory capacity of dimeric IEk-MCC, relative to anti-CD3, depends on its ability to recruit the CD4 coreceptor to engaged TCR complexes.
Figure 5.
(a) Isolation of CD4-positive and CD4-negative clones of 5KC73.8 T cell hybridoma. 5KC73.8 T cell hybridoma was cloned by limiting dilution. A CD4-positive (No. 9) and a CD4-negative (No. 10) clone of 5KC73.8 T cell hybridoma that expressed almost identical levels of TCRs were selected. (b) CD4 expression significantly enhances the response of 5KC73.8 T cell hybridoma to dimeric IEK-MCC but not to anti-CD3. IL-2 produced by the CD4-positive clone No. 9 (top) and the CD4-negative clone No. 10 (bottom) incubated with various concentrations of immobilized monomeric IEK-MCC, dimeric IEK-MCC, or anti-CD3 was measured as described in the legend for Fig. 2. Monomeric IEK-MCC elicited an IL-2 response from only the CD4-positive but not the CD4-negative clone, whereas dimeric IEK-MCC induced IL-2 responses by both the CD4-positive and CD4-negative clones. However, dimeric IEK-MCC was ∼10-fold better than anti-CD3 at stimulating the CD4-positive clone. Results are expressed as the mean of duplicate cultures. A representative of three experiments with similar results is shown.
CD4 Does Not Stabilize TCR–peptide-MHC Complex Interactions or Alter TCR Downregulation.
The ability to directly measure binding of dimeric IEk-MCC to the T cell surface, as well as the finding that 5KC73.8 T cell hybridoma activation is CD4 dependent, allowed us to examine the role of CD4 in stabilizing TCR–MHC class II–peptide binding. A potential adhesion role for CD4 has been derived from indirect functional data and from cell–cell adhesion assays using B cells expressing high levels of MHC class II and fibroblasts transfected with supraphysiological levels of CD4 (12, 13, 24). In addition, some structural models have implicated the CD4 molecule in binding and multimerizing TCR–MHC-peptide complexes (7).
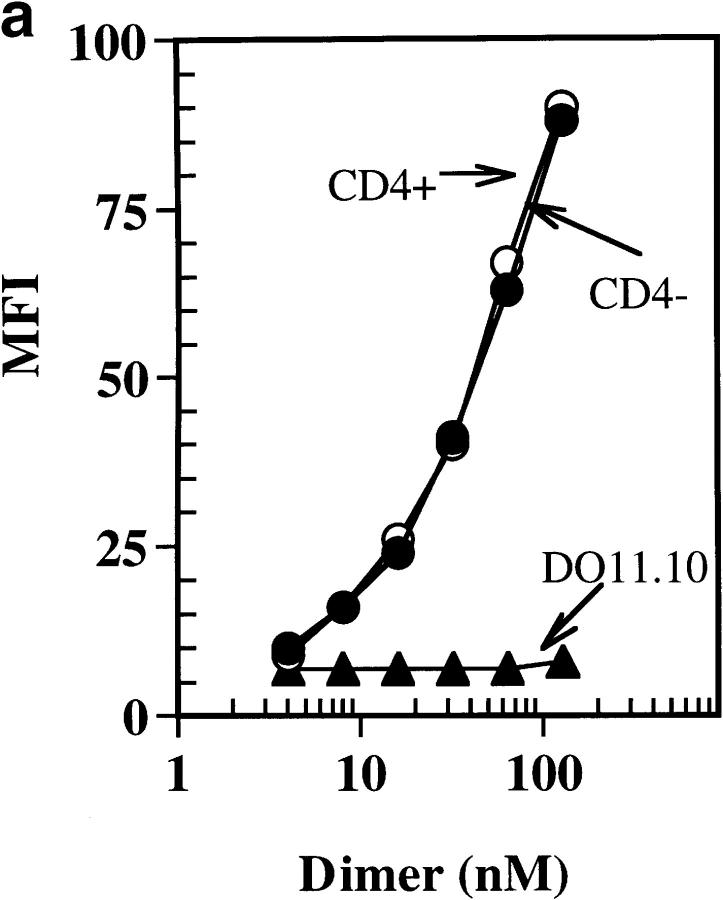
To determine if CD4 can enhance binding of soluble dimeric IEk-MCC to 5KC73.8 cells, we compared binding of various concentrations of dimeric IEk-MCC to the CD4-positive and CD4-negative 5KC73.8 clones. The binding of IEk-MCC to both the CD4-positive and CD4-negative 5KC73.8 clones was virtually identical at all concentrations tested (Fig. 6 a). The binding specificity was confirmed by the failure of the dimer to bind to the negative control, DO11.10 T cell hybridoma. Similar results were obtained with cells stained with dimeric IEk-MCC at 37°C (data not shown). Consistent with this binding data, we did not see any significant difference in the rate of dissociation of the dimer from the CD4-positive and CD4-negative clones of 5KC73.8 (Fig. 6 b). These results demonstrated that CD4 does not play any significant role in physically stabilizing TCR–MHC-peptide complexes even though it is required for optimum T cell activation.
Figure 6.
(a) CD4 does not enhance the binding of soluble dimeric IEK-MCC to 5KC73.8 T cell hybridoma. The CD4-positive and the CD4-negative clones of 5KC73.8 T cell hybridoma, as well as the control DO11.10 T cell hybridoma were incubated with various doses of soluble dimeric IEK-MCC for 2 h at 4°C. Cells were then washed, and bound dimer was detected using PE-conjugated goat anti–mouse IgG1. This experiment is representative of at least three experiments with similar results. (b) The CD4 molecule does not significantly influence dissociation of the IEk-MCC dimer from 5KC73.8 T cell hybridoma. Various concentrations of soluble dimer were incubated with CD4-positive and CD4-negative clones for 2 h on ice. Cells were then washed once and resuspended in 180 μl of staining buffer and incubated for the indicated period of time at 4°C. Cells were then immediately washed at 4°C and a saturating dose of PE-conjugated streptavidin goat anti–mouse IgG1 was used to detect bound dimer by FACScan®.
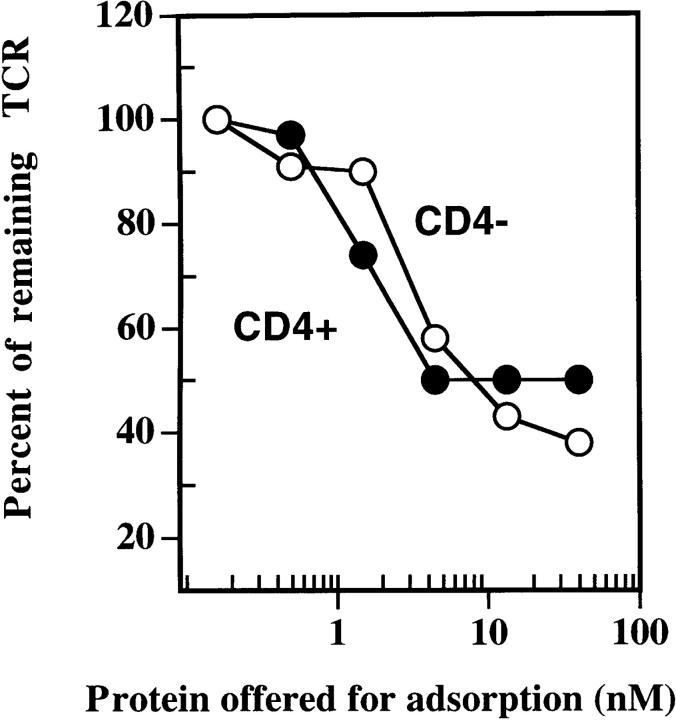
We next examined the influence of the CD4 molecule on TCR downregulation, a phenomenon that has been used recently to measure T cell activation (9). We reasoned that if CD4 binding to TCR–peptide-MHC complex is required, then removal of CD4 should delay or inhibit antigen-induced TCR downregulation. Therefore, the ability of dimeric IEk-MCC to induce TCR downregulation in the presence or absence of the CD4 molecule was determined. The results in Fig. 7 showed that both the CD4-positive and CD4-negative 5KC73.8 clones downregulated their TCR to the same extent, regardless of the level of CD4 expression. We conclude that antigen-induced TCR downregulation, at least in this system, can occur in a CD4-independent manner.
Figure 7.
TCR downregulation induced by the immobilized dimer is not influenced by CD4 molecule expression. The CD4-positive and CD4-negative clones of 5KC73.8 T cell hybridoma were incubated with various concentrations of the immobilized dimer for 5 h. The percentage of TCR that remained on the cell surface after stimulation was determined as described in the legend to Fig. 4. This experiment is representative of at least three experiments with similar results.
Discussion
In this study, a dimeric peptide–MHC class II molecule was made, its capacity to bind and activate antigen-specific T cells was analyzed, and then it was used to directly examine the adhesion role of the CD4 coreceptor. We demonstrated that dimeric IEk-MCC could stably bind to cognate T cells and efficiently stimulate them due to its ability to cross-link TCR, engage CD4 molecule, and induce serial TCR triggering. In addition, we found that CD4 did not play a significant adhesion function as it failed to enhance binding of IEk-MCC to specific T cell hybridoma, influence MHC-TCR dissociation rate, or influence TCR downregulation.
The MCC system has been used widely to study peptide-MHC class II interactions with specific T cells. Numerous T cell hybridomas that recognize MCC in the context of IEk have been made and affinity of their soluble TCRs for IEk-MCC has been measured. Generally, the affinity of specific TCRs for IEk-MCC complex is weak, with Kds in between 30 and 90 μM (1, 25). This low affinity has made it difficult to study and visualize binding of soluble peptide–MHC class II complexes to specific T cells. Dimerization of IEk-MCC by the immunoglobulin scaffold significantly increased the avidity of the complex resulting in stable binding detectable by flow cytometry. Affinity measurements of dimeric MHC class I complexed with several peptides recognized by the dimeric 2C TCR indicate that dimerization increase avidity by ∼50-fold, both in cases of alloreactive and cognate ligands (15). Analogous to our IEk-MCC binding results presented here, peptide-MHC class I–IgG dimers bind stably and specifically to CD8 T cells and have been used to detect HTLV-1–specific T cells from the peripheral blood of HTLV-1–associated myelopathy patients (16). Therefore, it is conceivable that the stable binding we see with our IEk-MCC dimers reflects geometric features of the immunoglobulin hinge region that confer flexibility to the MHC arms that allow it to both bind and activate antigen-specific T cells.
Dimeric IEk-MCC was more efficient than anti-CD3 at inducing serial engagement presumably because, as previously suggested, the high binding avidity of anti-CD3 hampers its ability to serially engage the TCR (10). Interestingly, dimerization of MHC class II with an IgG scaffold increases MHC-peptide avidity for the TCR without effectively interfering with serial engagement. More importantly, the dimerization of IEk-MCC does not appear to block the MHC–CD4 interaction as manifested by the ability of CD4 to increase the T cell response to the dimeric IEk-MCC but not to anti-CD3. Consequently, dimeric IEk-MCC are more potent than monomeric IEk-MCC and anti-CD3 to activate specific T cells.
A potential role for CD4 in stabilizing the binding of peptide-MHC complexes with the TCRs on the T cell surface has been derived from indirect functional data and cell–cell adhesion assays (12, 13, 24). In this report we showed that stable binding of the dimer to specific T cells was independent of CD4 expression. Furthermore, the dissociation of peptide-MHC class II from T cell is CD4 independent. The fact that the response of 5KC73.8 to IEk-MCC is CD4 dependent, especially at low antigen dose, and the generally low affinity of T cells for MCC antigen provide stringent conditions that should have allowed us to unmask any significant adhesion property of the CD4 molecule.
Our observation that TCR downregulation induced by dimeric IEk-MCC is CD4 independent contrasts with the result of Viola et al., who found that peptide-MHC–mediated TCR downregulation was inhibited by the addition of antibodies against CD4 (14). However, interpretation of the anticoreceptor antibody blocking studies is probably complicated by the potential direct effect of the antibody-mediated coreceptor cross-linking. In addition, in the same study the authors found that inhibitors of p56lck kinase blocked T cell activation but not TCR downregulation, indicating that TCR downregulation can be dissociated from T cell activation. This latter result is consistent with our observation that lack of CD4 expression significantly impaired the ability of 5KC73.8 T cell hybridoma to produce IL-2 (Fig. 5 b), but not to downregulate its TCR (Fig. 7).
Several previous reports have concluded indirectly that CD4 has a minimal adhesion effect. For example, Weber and Karjalainen (26) had estimated the affinity of CD4 for MHC to be <104 M−1 and concluded that it was too low to mediate any adhesion function. Glaichenhaus et al. (27) reported that CD4 plays a minor adhesion function, based on their finding that only a small amount of IL-2 was produced by T cells expressing mutant CD4 molecule that could not bind p56lck. In addition, Davis et al. (1), using BIAcore analysis, were not able to detect any increase in the affinity of soluble TCR for its cognate peptide–MHC complex in the presence of soluble CD4 molecule. Nevertheless, the failure to detect enhancement of TCR–peptide-MHC binding by CD4 does not rule out a potential role for it in facilitating cross-linking of TCR–peptide-MHC complexes through lateral interactions at the T cell– APC interface or in cases of other less potent antigens such as partial agonist and antagonists peptides.
In contrast to the CD4 molecule, the adhesion function of the CD8 molecule has been demonstrated recently by two different groups. Luescher et al. (28) showed that expression of CD8 could significantly enhance binding of soluble MHC class I to specific T cells as measured by flow cytometry. On the other hand Garcia et al. (29) showed that the addition of soluble CD8 molecule increased the affinity of soluble TCR for its cognate peptide–MHC class I complex by ∼10-fold. This functional difference between the CD4 and CD8 molecules should not be that surprising given the fundamental structural and biochemical difference between these two molecules. The CD8 molecule is a heterodimer and usually is associated with only small numbers of p56lck molecules. On the other hand, CD4 is monomeric and usually is associated with as many as 10 times the amount of p56lck as that associated with the CD8 molecule (30). Therefore, it is possible that CD4 functions as a signal transducing molecule more than as an adhesion molecule and the opposite may be true for the CD8 molecule.
The experimental system described here outlines a general approach for using divalent high affinity ligands to dissect out the role of MHC class II engagement in activation of antigen-specific T cells. We found an enhanced potency of the peptide-MHC class II–IgG chimera, relative to anti-CD3, in stimulating T cells. This enhanced potency appears to be due to both the ability of dimeric IEk-MCC to recruit the coreceptor to engaged TCR and to more efficiently mediate serial engagement and cross-linking of TCR. The ability to efficiently stimulate antigen-specific T cells, even when added to cultures in soluble rather than a plate-bound form, attests to the multifaceted usage of such molecules. These features make peptide-MHC–IgG chimeras exciting new tools to modulate antigen-specific T cell responses.
Acknowledgments
The authors would like to thank Dr. John Kappler, Dr. Philippa Marrack, Mrs. Janice White, and Mrs. Frances Crawford for the kind gift of reagents, helpful advice, and encouragement. We also thank Dr. Susan Swain (Trudeau Institute, New York) for the generous gift of AND × B10.BR mice. Critical review of the manuscript by Dr. X. Zhou, T. Greten, K. Kraemer, and T. Armstrong is appreciated.
This work was supported in part by the Janey Fund and gifts from the Topercer family and Mrs. Dorothy Needle.
Abbreviation used in this paper
- Tg
transgenic
Footnotes
Note added in proof. Recently, Crawford et al. observed no effect of CD4 on binding of tetrameric MHC class II MHC covalent peptide complexes to cognate T cells. (Crawford, F., H. Kozono, J. White, P. Marrack, and J. Kappler. 1998. Immunity. 8:675–682.)
References
- 1.Davis M, Boniface J, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by αβ T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 2.Janeway C. The T cell receptor as a multicomponent signaling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645–674. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- 3.Davis MM. Serial engagement proposed. Nature. 1995;375:104. doi: 10.1038/375104a0. [DOI] [PubMed] [Google Scholar]
- 4.Rao A, Ko WW, Faas S, Cantor H. Analogs that compete for antigen binding to an arsonate reactive T cell clone inhibit the functional response to arsonate. Cell. 1984;36:879–888. doi: 10.1016/0092-8674(84)90038-2. [DOI] [PubMed] [Google Scholar]
- 5.Symer D, Dintzis R, Diamond D, Dintzis H. Inhibition or activation of human T cell receptor transfectants is controlled by defined, soluble antigen arrays. J Exp Med. 1992;176:1421–1430. doi: 10.1084/jem.176.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abastado J, Lone Y, Casrouge A, Boulot G, Kourilsky P. Dimerization of soluble major histocompatibility complex–peptide complexes is sufficient for activation of T cell hybridoma and induction of unresponsiveness. J Exp Med. 1995;182:439–447. doi: 10.1084/jem.182.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H, Kwong P, Hendrickson W. Dimeric association and segmental variability in the structure of human CD4. Nature. 1997;387:527–530. doi: 10.1038/387527a0. [DOI] [PubMed] [Google Scholar]
- 8.Reich Z, Boniface J, Lyons D, Borochov N, Wachtel E, Davis M. Ligand specific oligomerization of T cell receptor molecules. Nature. 1997;387:617–620. doi: 10.1038/42500. [DOI] [PubMed] [Google Scholar]
- 9.Valitutti S, Muller S, Celia M, Padovan E, Lanzavecchia A. Serial triggering of many T cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–161. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 10.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 11.Madrenas J, Chau LA, Smith J, Bluestone J, Germain RN. The efficiency of CD4 recruitment to ligand engaged TCR controls the agonist/partial agonist properties of peptide-MHC molecule ligands. J Exp Med. 1997;185:219–229. doi: 10.1084/jem.185.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrack P, Endres R, Shimonkevitz R, Zlotnik A, Dialynas D, Fitch F, Kappler J. The major histocompatibility complex restricted antigen receptor on T cells. II. Role of the L3T4 product. J Exp Med. 1983;158:1077–1091. doi: 10.1084/jem.158.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gay D, Maddon P, Sekaly R, Talle M, Godfrey M, Long E, Goldstein G, Chess L, Axel R, Kappler J, Marrack P. Functional interaction between human T cell protein CD4 and the major histocompatibility complex HLA-DR antigen. Nature. 1987;328:626–629. doi: 10.1038/328626a0. [DOI] [PubMed] [Google Scholar]
- 14.Viola A, Salio M, Tuosto L, Linkert S, Acuto O, Lanzavecchia A. Quantitative contribution of CD4 and CD8 to T cell antigen receptor serial triggering. J Exp Med. 1997;186:1775–1779. doi: 10.1084/jem.186.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Herrin S, Lebowitz M, Bieler J, al-Ramadi B, Utz U, Bothwell A, Schneck J. Analysis of the expression of peptide–major histocompatibility complexes using high affinity soluble divalent T cell receptors. J Exp Med. 1997;186:1333–1345. doi: 10.1084/jem.186.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greten T, Slansky J, Kubota R, Soldan S, Jaffee E, Leist T, Pardoll D, Jacobson S, Schneck J. Direct visualization of antigen specific T cells: HTLV-1 Tax11-19 specific CD8+ T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc Natl Acad Sci USA. 1998;95:27568–27573. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman J, Moss P, Goulder P, Barouch D, Mcheyzer-Williams M, Bell J, McMichael A, Davis M. Phenotypic analysis of antigen specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 18.Kaye J, Hsu M-L, Sauron M-E, Jameson SC, Gascoigne NR, Hedrick S. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 19.Cauley L, Cauley K, Shub F, Huston G, Swain S. Transferable anergy: superantigen treatment induces CD4+ T cell tolerance that is reversible and requires CD4− CD8−cells and interferon γ. J Exp Med. 1997;186:71–81. doi: 10.1084/jem.186.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White J, Pullen A, Choi K, Marrack P, Kappler J. Antigen recognition of mutant Vβ 3+ T cell receptor are consistent with an immunoglobulin like structure for the receptor. J Exp Med. 1993;177:119–125. doi: 10.1084/jem.177.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gay D, Coeshott C, Golde W, Kappler J, Marrack P. The major histocompatibility complex restricted antigen receptor on T cells. IX. Role of accessory molecules in recognition of antigen plus isolated IA. J Immunol. 1986;136:2026–2032. [PubMed] [Google Scholar]
- 22.Kozono H, White J, Clements J, Marrack P, Kappler J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature. 1994;369:151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- 23.Rothenberg E. How T cells count. Science. 1996;273:78–79. doi: 10.1126/science.273.5271.78. [DOI] [PubMed] [Google Scholar]
- 24.Dolye C, Strominger J. Interaction between CD4 and class II MHC molecules mediated cell adhesion. Nature. 1987;330:256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Crawford F, Marrack P, Kappler J. T cell positive selection by a high density, low affinity ligand. Proc Natl Acad Sci USA. 1998;95:4522–4526. doi: 10.1073/pnas.95.8.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber S, Karjalainen K. Mouse CD4 binds MHC class II with extremely low affinity. Int Immunol. 1992;5:695–698. doi: 10.1093/intimm/5.6.695. [DOI] [PubMed] [Google Scholar]
- 27.Glaichenhaus N, Shastri N, Littman D, Turner J. Requirement for association of P56lckwith CD4 in antigen specific signal transduction. Cell. 1991;64:511–520. doi: 10.1016/0092-8674(91)90235-q. [DOI] [PubMed] [Google Scholar]
- 28.Luescher IF, Vivier E, Layer A, Mahlou J, Godeau F, Malissen B, Romero P. CD8 modulation of T cell antigen receptor ligand interactions on living cytotoxic T lymphocytes. Nature. 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 29.Garcia K, Scott C, Brunmark A, Carbone F, Peterson P, Wilson I, Teyton L. CD8 enhances formation of stable T cell receptor/MHC class I molecule complexes. Nature. 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 30.Maroun C, Juluis M. Distinct roles of CD4 and CD8 as coreceptors in T cell receptor signalling. Eur J Immunol. 1994;24:959–966. doi: 10.1002/eji.1830240427. [DOI] [PubMed] [Google Scholar]