Abstract
Mast cells have long been believed to be the central effector cells in the development of immunoglobulin (Ig)E-dependent anaphylaxis. In this study, we investigated the role of mast cells in IgE-dependent hapten-induced active fatal anaphylaxis using mast cell–deficient WBB6F1- W/Wv (W/Wv) and congenic normal (+/+) mice. Although a 5-min delay in shock signs and death were observed in W/Wv mice, 100% fatal reactions to penicillin V (Pen V) occurred in both +/+ and W/Wv mice. Administration of monoclonal anti–IL-4 antibody completely prevented the fatal reactions, and the effect of anti–IL-4 was associated with its suppressive activity on Pen V–specific serum levels of IgE, but not IgG. The platelet-activating factor (PAF) antagonist, BN 50739, completely prevented the fatal reactions in both strains of mice. Our kinetic study revealed, in contrast to no elevation of plasma histamine level in W/Wv mice, high levels of PAF in the circulation after challenge in both +/+ and W/Wv mice, albeit to a lesser degree in the latter case. These data indicate that cells other than mast cells are sufficient to induce an IgE-dependent active fatal anaphylaxis by elaborating PAF, which is the critical mediator for fatal murine anaphylaxis.
Keywords: mast cells, W/Wv mice, immunoglobulin E, platelet-activating factor, penicillin V
Mast cells express receptors on their cell surface that bind IgE Abs with high specificity and affinity (FcεRI [1–3]). Antigen-mediated cross-linkage of the FcεRI triggers mast cells to release a wide spectrum of mediators critical for the development of immediate hypersensitivity reactions, including anaphylaxis.
Understanding the role of mast cells in anaphylaxis has been aided by the availability of mast cell–deficient W/Wv and SI/SId mice. Several groups of investigators have established that W/Wv and SI/SId mice that are actively sensitized to protein antigens such as OVA or chicken gammaglobulin (CGG),1 can exhibit active fatal anaphylaxis (4–8). Thus, mast cells may not contribute importantly to protein-induced anaphylaxis. Some evidence indicates that protein-induced anaphylaxis can be elicited by IgG Abs (9, 10) even in the absence of IgE Abs (11), suggesting that cells other than mast cells that bind IgG Abs elaborate sufficient mediators leading to fatal reactions. Nevertheless, mast cells have long been believed to be the central effector cells in the development of IgE-dependent anaphylaxis. However, the in vivo extent to which the reactions are mast cell–dependent remains to be elucidated due to the lack of a suitable animal model of IgE-dependent anaphylaxis.
We have recently developed a murine model of IgE- dependent, penicillin V (Pen V)–induced active fatal anaphylaxis (12). The reaction was 100% fatal in C57BL/6 mice and was exclusively IgE dependent, since (a) IgE, but not IgG, Abs against Pen V passively sensitized normal mice to develop severe anaphylactic reactions; (b) anti–IL-4 mAb completely prevented the fatal reaction; and (c) the effect of anti–IL-4 was associated with its suppressive activity on Pen V–specific serum IgE, but not IgG, levels. This model allowed us to investigate the role of mast cells in IgE-dependent anaphylaxis. In this study, the role of mast cells in IgE-dependent Pen V–induced anaphylaxis using genetically mast cell–deficient WBB6F1-W/Wv (W/Wv) and congenic normal (+/+) mice was investigated. It was found that virtually identical Pen V–induced fatal anaphylaxis occurred in mast cell–deficient mice, indicating that mast cells are not essential for the development of IgE- dependent anaphylaxis.
Materials and Methods
Animals.
Female mast cell–deficient WBB6F1-W/Wv (W/ Wv) and mast cell–sufficient control WBB6F1 +/+ mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and were kept in our animal facility for at least 2 wk before use. All mice were used at 8–9 wk of age.
Reagents.
Pen V, OVA (grade V), and BSA (fraction V) were purchased from Sigma Chemical Co. (St. Louis, MO). Platelet-activating factor (PAF) antagonist, BN 50739, was provided by Dr. P. Braquet (Institut Henri Beaufour, Le Plessis-Robinson, France). Bordetella pertussis was obtained from the National Institute of Health (Seoul, Korea).
Abs.
The rat–mouse hybridoma, 11B11, which secretes rat IgG1 specific for murine IL-4 (13), was purchased from American Type Culture Collection (Rockville, MD). As a control mAb, J4-1 (14), which secretes rat IgG1 with specificity for the hapten, nitrophenol (NP), was provided by Dr. F.D. Finkelman, Uniformed Services University of the Health Sciences (Bethesda, MD). Both mAbs were prepared as ascites in pristane-primed nude mice. Preparations were precipitated in 45% ammonium sulfate and dialyzed against PBS (pH 7.2), and protein was quantitated before use. The IgG1 mAb specific for Pen V (12) was used to measure Pen V–specific serum IgG1 levels (see below).
Pen V–Protein Conjugates.
Conjugates (Pen V–OVA and Pen V–BSA) were prepared as described previously (12). In brief, 20 mg of OVA or BSA in 5 ml of 50 mM veronal buffer (pH 8.5) was added to 100 mg of Pen V in 5 ml veronal buffer and stirred overnight at 37°C. The pH was maintained between 8.5 and 9.0 by adding 1 N NaOH. The reaction mixture was centrifuged, and the supernatant was dialyzed for 7 d against 0.01 M PBS (pH 7.2). Aliquots of the dialyzed supernatant (5 mg/ml) were stored at −20°C.
Induction of Active Systemic Anaphylaxis to Pen V.
Mice were sensitized by intraperitoneal injection of 500 μg of Pen V–OVA conjugate plus 2 × 109 B. pertussis and 1.0 mg of alum. Challenge was given as an injection of 100 μg i.v. of Pen V–BSA conjugate 14 d later (12).
Measurement of Pen V–specific Serum Levels of IgE and IgG1.
Pen V–specific serum IgE levels were determined by a passive cutaneous anaphylaxis (PCA) reaction as described previously (12). In brief, serial dilutions of individual sera from mice immunized with Pen V–OVA were injected intracutaneously into the shaved backs of male Wistar rats. After 24 h, 1 ml of 1% Evan's blue dye in PBS containing 4 mg of Pen V–BSA was injected intravenously. The rats were killed 30 min later, skins were removed, and a blue spot with a diameter >5 mm was regarded as a positive reaction. Pen V–specific serum IgG1 levels were determined by an ELISA as described previously (12). Microtiter plates (Irvine Scientific, Santa Ana, CA) were coated with 50 μl of Pen V–BSA (10 μg/ml) in 50 mM sodium carbonate buffer, pH 9.6, overnight at 4°C. After the plates were washed, a serial dilution of sera and Pen V–specific IgG1 mAb in 2.7% gelatin was added and incubated at 37°C for 1 h. After washing, peroxidase-conjugated rabbit anti–mouse IgG1 (1:4,000; Organon-Teknika Capell, Durham, NC) was added and incubated at 37°C for 45 min. After a final washing, 75 μl of O-phenylenediamine in phosphate- citrate buffer (pH 5.0) was added, and color was developed. Absorbance was measured at 492 nm, and results were expressed as concentrations, from a standard curve of known concentrations of Pen V–specific IgG1 mAb.
Determination of Plasma PAF and Histamine.
The postchallenge blood was taken from the heart, which had been cut open, and was mixed with a 0.1 vol of 3.8% ice-chilled citrate solution, then centrifuged immediately using an Eppendorf microfuge. The plasma was stored at −20°C until use. Plasma PAF was measured using the method described by Sugatani et al. (15). In brief, 20– 50 μl of plasma was vortexed with 4 vol of 2-propanol and centrifuged. Extraction of lipids from the samples was repeated three times with 2-propanol. The supernatants were applied to a reverse phase, 100-mg octadecyl column (Amprep minicolumn; Nycomed Amersham plc, Little Chalfont, Bucks, UK). The column was washed with 2 ml of 30% 2-propanol solution, followed by 2 ml of 55% ethanol solution, and PAF was eluted with 2 ml of 67% ethanol solution. The eluates were evaporated under nitrogen and separated by thin-layer chromatography on silica gel G plates (250 μm, 4 × 20 cm, Uniplate; Analtech, Inc., Newark, DE) with a solvent system of CHCl3/CH3OH/H2O (65:35:6, vol/vol/vol). The PAF, which was located on the thin layer plate between the areas corresponding to sphingomyelin and lysophosphatidylcholine, was detected by UV fluorescence after spraying the plate with 1 mM 6-p-toluidine-2-naphthalenesulfonic acid. The PAF fraction was then scraped off and extracted by the method of Bligh and Dyer (16). The extract was evaporated under nitrogen, and PAF was quantified using a SPRIA kit (Nycomed Amersham plc) according to the manufacturer's protocol. Results were expressed as concentrations, from a standard curve of known concentrations of PAF. Plasma histamine level was assayed by the method of Harvima et al. (17). 10 μl of plasma was mixed with 1.5 μl of S-adenosyl (methyl-14C) methionine, 40 μl of 300 mM Tris-glycine buffer (pH 8.3), and 5 μl of histamine N-methyl transferase, and incubated for 90 min at 37°C. The reaction was stopped by adding 20 μl of 10 N NaOH. The mixture was extracted with 1 ml of toluene-isoamyl alcohol. The radioactivity of the supernatant was measured using a beta counter. Results were expressed as concentrations, from a standard curve of known concentrations of histamine.
Results
Occurrence of Pen V–induced Fatal Anaphylaxis in W/Wv Mice.
As shown in Table 1, 100% (9/9) of mast cell–sufficient +/+ mice died of shock 15–20 min after the challenge injections. Typical protein-induced murine anaphylaxis symptoms (5, 6), including itching, increased activity, and excitability, for the first 20–30 s after the challenge, then ruffling of fur, dyspnea, sluggish gait, paresis, prostration, and convulsion, followed by death were observed. Mast cell–deficient W/Wv mice exhibited the same 100% (14/14) death rate as control mice. However, the shock signs and death were somewhat delayed, for ∼4–5 min, in W/Wv mice.
Table 1.
Occurrence of Pen V–induced Active Fatal Anaphylaxis in W/Wv Mice
| Mice | Mortality | |
|---|---|---|
| no. of dead/total | ||
| +/+ | 9/9 | |
| W/Wv | 14/14 |
Mice were sensitized by intraperitoneal injection of 500 μg of Pen V–OVA plus 2 × 109 B. pertussis and 1.0 mg of alum. Challenge was given as an injection of 100 μg i.v. of Pen V–BSA 14 d later.
Anti–IL-4 mAb Prevents Pen V–induced Fatal Anaphylaxis in W/Wv Mice.
To confirm that the fatal reactions in W/Wv mice are IgE-dependent, we examined whether anti–IL-4 mAb, which is effective to suppress IgE but not IgG production (12, 14), could prevent Pen V–induced fatal anaphylaxis in W/Wv mice. Administration of 1 mg of anti–IL-4 on days 0 (30 min before sensitization), 2, and 4 completely prevented the fatal reactions, but the control anti-NP mAb did not show any protective effect (Table 2). Blood samples were taken 1 h before challenge from the mice, and serum levels of IgE and IgG were determined by PCA and ELISA, respectively. Anti–IL-4 mAb significantly suppressed (by >90%) the Pen V–specific IgE responses, but did not suppress the IgG1 response. The IgE response was not suppressed in mice treated with anti-NP mAb. These findings confirmed our previous observation (12) that IgE Abs play a major role in Pen V–induced active anaphylaxis. Taken together, these data indicated that IgE-dependent anaphylaxis did occur in mast cell–deficient mice.
Table 2.
Anti–IL-4 mAb Prevents Pen V–induced Active Fatal Anaphylaxis in W/Wv Mice
| Ig level‡ | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mice | Anti–IL-4* | Mortality | IgE | IgG1 | ||||
| no. of dead/total | PCA titer | μg/ml | ||||||
| +/+ | − | 8/8 | 70 ± 22 | 2.5 ± 0.4 | ||||
| +/+ | + | 0/8 | <4§ | 2.4 ± 0.6 | ||||
| +/+ | Anti-NP | 7/7 | 64 ± 12 | 1.9 ± 0.2 | ||||
| W/Wv | − | 8/8 | 64 ± 16 | 2.3 ± 0.4 | ||||
| W/Wv | + | 0/10 | <4§ | 1.9 ± 0.4 | ||||
| W/Wv | Anti-NP | 6/7 | 60 ± 12 | 2.0 ± 0.3 | ||||
1 mg i.p. of mAbs was injected on days 0 (30 min before sensitization), 2, and 4.
Mice were partially bled 1 h before challenge, and serum levels of Pen V–specific IgE and IgG1 were determined by a PCA reaction and ELISA, respectively. The results are expressed as mean ± SEM.
Statistical differences analyzed by Student's t test were considered significant at P < 0.05 compared with saline-injected control.
PAF Is Critical for the Fatal Reactions in W/Wv Mice.
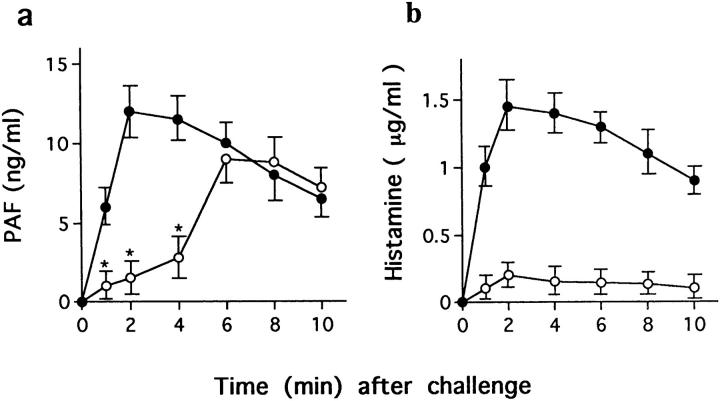
PAF is thought to be critical for murine fatal anaphylaxis, as PAF antagonist can prevent death (18–20). Pretreatment with the PAF antagonist BN 50739 (200 μg/mouse) 10 min before challenge injections completely blocked the fatal reactions in both +/+ and W/Wv mice (Table 3), indicating that PAF is critical for the fatal reaction in both strains of mice. To confirm the association of PAF with fatal reactions in W/Wv mice, levels of plasma PAF, together with histamine levels, were determined. As shown in Fig. 1 a, PAF was released immediately upon challenge injection and reached a peak in the circulation within 2 min in +/+ mice. In contrast, a significant level of PAF was not detected by 3–4 min in W/Wv mice, but it increased thereafter to a peak level that was 70–80% that of control mice, at 6–7 min. The postchallenge plasma histamine levels in +/+ mice were similar to PAF, but histamine levels barely increased in W/Wv mice (Fig. 1 b).
Table 3.
PAF Antagonist Prevents Pen V–induced Active Fatal Anaphylaxis in W/Wv Mice
BN 50739 (200 μg i.p.) was injected 10 min before challenge.
Statistical differences analyzed by Student's t test were considered significant at P < 0.05 compared with saline-injected control.
Figure 1.
Postchallenge plasma PAF and histamine levels in +/+ (filled circles) and W/Wv (open circles) mice. After challenge, blood was taken from the heart, which was cut open at the time indicated, and was mixed with a 0.1 vol of 3.8% ice-chilled citrate solution, then centrifuged immediately. Plasma PAF and histamine levels were determined as described in Materials and Methods. The results were expressed as mean ± SEM from one of two representative experiments (n = 2–4 animals for each time point), and statistical significance was determined by the Mann-Whitney U test (*P < 0.05; ‡ P < 0.01 compared with +/+ mice).
Discussion
Jacoby et al. (4) and Ha and colleagues (5, 6) reported that fatal anaphylaxis can occur in mast cell–deficient mice upon challenge with specific antigens. Some physiological responses, such as hypotension and cardiopulmonary alteration associated with anti-IgE– and specific antigen– induced active anaphylaxis (7, 8), developed to a similar degree in both normal and mast cell–deficient mice. However, these data are from studies using protein antigens such as OVA, BSA, or CGG. In protein-induced anaphylaxis, IgE Abs are not absolutely required, since (a) fatal anaphylaxis occurs in IgE-deficient mice (11), and (b) antigen-specific polyclonal and monoclonal IgG Abs are capable of inducing a fatal reaction in normal as well as mast cell–deficient mice (9, 10). The implications of IgG Abs in human fatal anaphylaxis (21) clearly indicate that IgG, in addition to IgE, Abs are critical for protein-induced fatal anaphylaxis. Furthermore, recent works have demonstrated that anaphylactic reactions can be induced in normal or mast cell– deficient mice that have received passive transfer of antigen-specific IgG1 Abs, and that these reactions reflect the binding of these Abs to FcγRIII, which can be expressed on the surface of mast cells as well as other cell types in the mouse (22, 23). Thus, it is likely that, in addition to mast cells, other cells which bind to IgG Abs, such as macrophages, platelets, and eosinophils (24), represent the source of the mediators in protein-induced fatal anaphylaxis. However, the role of mast cells in an exclusively IgE-dependent anaphylaxis has never been convincingly demonstrated because of the lack of a suitable experimental animal model. The recent development of a murine model of IgE-dependent anaphylaxis induced by the hapten Pen V (12) has made it possible to investigate the role of mast cells in IgE-dependent anaphylaxis.
In this study, we have shown that genetically mast cell– deficient W/Wv mice expressed active fatal reactions to Pen V that were indistinguishable from those in control +/+ mice in terms of death rate. Consistent with our previous report (12), 100% (9/9) of congenic normal +/+ mice died of shock upon challenge injections. The same mortality was elicited in mast cell–deficient W/Wv mice, although signs of shock and death were delayed by 5 min. Administration of anti–IL-4 mAb, as low as 3 mg from the day of sensitization, completely prevented fatality and suppressed (by >90%) Pen V–specific serum IgE, but not IgG, responses. Despite the presence of 2–3 μg/ml of Pen V–specific circulating IgG1 Abs, anti–IL-4–treated mice did not show any signs of shock, suggesting that IgG1 Abs do not contribute importantly to Pen V–induced fatal anaphylaxis. In contrast to these findings, IgG1-dependent active or passive anaphylaxis can be elicited in W/Wv mice (10, 22). These differences may be attributed to the different types of IgG1 Abs, i.e., Ab against protein or hapten. This hypothesis was further strengthened by our unpublished observations that induction of passive anaphylaxis by administration of 2 mg of anti–Pen V IgG1 (12) mAb resulted in eliciting only mild shock signs, such as itching, whereas the same concentration of anti-CGG IgG1 mAb (9) induced 40% fatal reactions in C57BL/6 mice. Taken together, our data confirmed our previous results (12) that IgE Abs were found to play a major role in Pen V–induced fatal anaphylaxis, and indicate that IgE-dependent anaphylaxis occurred in mast cell–deficient mice. In this study, we have also examined whether passive transfer of IgE from immunized normal mice can transfer anaphylactic reactivity to naive mast cell–deficient mice. Both +/+ and W/Wv mice were intraperitoneally injected three times at 10-min intervals with 0.4 ml of sera taken from Pen V–sensitized C57BL/6 mice and were challenged 24 h later (12). Upon the challenge injections, four out of four +/+ mice developed severe anaphylactic signs, such as prostration and paresis, whereas six out of six W/Wv mice showed only mild signs, such as itching and slight limitation of movement (data not shown). Although a weaker passive anaphylactic reaction occurred in W/Wv mice, these findings further support our conclusion that mast cell–deficient mice can express IgE-dependent anaphylactic reactions.
PAF is produced by a variety of cells involved in inflammatory reactions, including neutrophils, basophils, mast cells, monocytes/macrophages, platelets, and endothelial cells (25, 26). Recent studies have demonstrated that a PAF antagonist prevents murine fatal anaphylaxis (18–20), indicating that PAF is critical for murine fatal anaphylaxis. Pretreatment with the PAF antagonist BN 50739 10 min before challenge completely blocked the Pen V–induced fatal anaphylaxis in both +/+ and W/Wv mice. BN 50739 also completely blocked other signs of shock in both strains of mice, suggesting that PAF is critical for IgE-dependent anaphylaxis even in mast cell–deficient mice. The PAF antagonist BN 50739 is a synthetic PAF analogue counteracting the effects of endogenous PAF by inhibiting PAF binding to its receptor and the subsequent cellular responses. We have proved the efficacy of this reagent as a PAF blocker in other studies (27, 28). Our time–kinetic study revealed that the initial rapid increase in plasma PAF level and the peak PAF levels were delayed by 4–5 min in W/Wv mice compared with +/+ mice. This finding suggests that mast cells represent the source of PAF released immediately upon challenge in +/+ mice, and may explain the 5-min delay of shock signs and death in W/Wv mice. The peak level of plasma PAF in W/Wv mice was 70–80% that of control mice. In contrast, little histamine was detected in W/Wv mice, confirming that histamine is a major vasoactive mediator released by activated mast cells (29, 30). Taken together, these data indicate that cells other than mast cells are enough to elaborate a sufficient amount of PAF to cause death.
It was not clear which cell populations represent the source of PAF in the W/Wv mice in this study. As Pen V–induced anaphylaxis is an IgE-dependent reaction, the cells which express IgE receptors on their surfaces would be involved in the reaction. In humans, the FcεRI is expressed on mast cells, basophils, Langerhans cells, eosinophils, some monocytes, and platelets (31–35). So far, however, there is no evidence that FcεRI can be expressed on cells other than mast cells and basophils in the mouse (36). Furthermore, it has been reported that FcεRI is more widely distributed on hematopoietic cells than had previously been suspected (37). Given these considerations, the main candidate for a source of PAF in IgE-dependent anaphylaxis in W/Wv mice would appear to be basophils.
In summary, these results show that IgE-dependent fatal anaphylaxis can be elicited in mast cell–deficient mice, indicating that mast cells are not essential for the development of IgE-dependent immediate hypersensitivity reactions.
Acknowledgments
We thank Dr. Amy Terhune for discussion and critical reading of the manuscript.
This work was supported by Korea Science and Engineering Foundation (KOSEF) Project 971-0705-043-1 and Ministry of Education Basic Science Research Institute Program Project BSRI-97-4426.
Abbreviations used in this paper
- CGG
chicken gammaglobulin
- NP
nitrophenol
- PAF
platelet-activating factor
- PCA
passive cutaneous anaphylaxis
- Pen V
penicillin V
References
- 1.Kinet JP. The high affinity receptor for IgE. Curr Opin Immunol. 1989;2:499–505. doi: 10.1016/0952-7915(90)90002-x. [DOI] [PubMed] [Google Scholar]
- 2.Metzger H, Alcaraz G, Hohman R, Kinet JP, Pribluda V, Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- 3.Ishizaka T, Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- 4.Jacoby WP, Cammarata V, Findlay S, Pincus S. Anaphylaxis in mast cell-deficient mice. J Investig Dermatol. 1984;83:302–304. doi: 10.1111/1523-1747.ep12340431. [DOI] [PubMed] [Google Scholar]
- 5.Ha TY, Reed ND, Crowle PK. Immune response potential of mast cell-deficient W/Wvmice. Int Arch Allergy Appl Immunol. 1986;80:85–94. doi: 10.1159/000234031. [DOI] [PubMed] [Google Scholar]
- 6.Ha TY, Reed ND. Systemic anaphylaxis in mast cell-deficient mice of W/Wv and SI/SIdgenotypes. Exp Cell Biol. 1987;55:63–68. doi: 10.1159/000163399. [DOI] [PubMed] [Google Scholar]
- 7.Takeishi T, Martin TR, Katona IM, Finkelman FD, Galli SJ. Differences in the expression of the cardiopulmonary alteration associated with anti-immunoglobulin E–induced or active anaphylaxis in mast cell–deficient and normal mice. J Clin Invest. 1991;88:598–608. doi: 10.1172/JCI115344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin TR, Ando A, Takeishi T, Katona IM, Drazen JM, Galli SJ. Mast cells contribute to the changes in heart rate, but not hypotension or death, associated with active anaphylaxis. J Immunol. 1993;151:367–374. [PubMed] [Google Scholar]
- 9.Lee HK, Lee HH, Park YM, Park HJ, Lee JH, Ha TY. Anti-IL-4 antibody inhibits antigen-specific IgE response but fails to prevent chicken gamma globulin- induced active systemic anaphylaxis: evidence for the involvement of IgG antibodies. J Korean Med Sci. 1996;11:111–117. doi: 10.3346/jkms.1996.11.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arimura A, Nagata M, Watanabe A, Nakamura K, Takeuchi M, Harada M. Production of active and passive anaphylactic shock in the WBB6F1 mouse, a mast cell-deficient strain. Experientia. 1990;46:739–742. doi: 10.1007/BF01939952. [DOI] [PubMed] [Google Scholar]
- 11.Oettgen H, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Science. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 12.Park JS, Choi IH, Lee DG, Han SS, Ha TY, Lee JH, Lee WH, Park YM, Lee HK. Anti-IL-4 monoclonal antibody prevents antibiotics-induced active fatal anaphylaxis. J Immunol. 1997;158:5002–5006. [PubMed] [Google Scholar]
- 13.Ohara J, Paul WE. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985;315:333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- 14.Finkelman FD, Katona IM, Urban JF, Holmes J, Ohara J, Tung AS, Sample JG, Paul WE. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- 15.Sugatani J, Miwa M, Komiyama Y, Murakami T. Quantitative analysis of platelet-activating factor in human plasma: application to patients with liver cirrhosis and disseminated intravascular coagulation. J Immunol Methods. 1993;166:251–261. doi: 10.1016/0022-1759(93)90366-f. [DOI] [PubMed] [Google Scholar]
- 16.Bligh EG, Dyer WA. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 17.Harvima RJ, Harvima IT, Fraki JE. Optimization of histamine radioenzymatic assay with purified histamine N-methyltransferase. Clin Chim Acta. 1988;171:247–251. doi: 10.1016/0009-8981(88)90150-7. [DOI] [PubMed] [Google Scholar]
- 18.Braquet P, Etienne A, Touvay C, Bourgain RH, Lefort J, Vargaftig BB. Involvement of platelet activating factor in respiratory anaphylaxis, demonstrated by PAF-acether inhibitor BN 52021. Lancet. 1985;1:1501. doi: 10.1016/s0140-6736(85)92269-x. [DOI] [PubMed] [Google Scholar]
- 19.Vilain B, Lagente V, Touvay C, Desquand S, Rendon J, Lefort J, Braquet P, Vargaftig BB. Pharmacological control of the in vivo passive anaphylactic shock by the PAF-acether antagonist compound BN 52021. Pharmacol Res Commun. 1986;18(Suppl.):119–126. doi: 10.1016/0031-6989(86)90044-5. [DOI] [PubMed] [Google Scholar]
- 20.Hervert JM, Lespy L, Maffrand JP. Protective effect of SR27417, a novel PAF antagonist, on lethal anaphylactic and endotoxin-induced shock in mice. Eur J Pharmacol. 1991;205:271–276. doi: 10.1016/0014-2999(91)90909-a. [DOI] [PubMed] [Google Scholar]
- 21.Weiss ME, Nyhan D, Peng Z, Horrow JC, Lowenstein E, Hirshman C, Adkinson F. Association of protamine IgE and IgG antibodies with life-threatening reactions to intravenous protamine. N Engl J Med. 1989;320:886–892. doi: 10.1056/NEJM198904063201402. [DOI] [PubMed] [Google Scholar]
- 22.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and FcγRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dombrowicz D, Flamand V, Miyajima I, Ravetch JV, Galli SJ, Kinet JP. Absence of FcεRI α chain results in upregulation of FcγRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between FcεRI and FcγRIII for limiting amounts of FcR β and γ chains. J Clin Invest. 1997;99:915–925. doi: 10.1172/JCI119256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 25.Camussi G, Bussolono G, Salvidio G, Baglioni C. Tumor necrosis factor/cachectin stimulates peritoneal macrophages, polymorphonuclear neutrophils, and vascular endothelial cells to synthesize and release platelet-activating factor. J Exp Med. 1987;166:1390–1404. doi: 10.1084/jem.166.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chignard M, Le Couedic JP, Tence M, Vargaftig BB, Benveniste J. The role of platelet-activating factor in platelet aggregation. Nature. 1989;279:799–800. doi: 10.1038/279799a0. [DOI] [PubMed] [Google Scholar]
- 27.Choi IH, Ha TY, Lee DG, Park JS, Lee JH, Park YM, Lee HK. Occurrence of disseminated intravascular coagulation (DIC) in active systemic anaphylaxis: role of platelet-activating factor. Clin Exp Immunol. 1995;100:390–394. doi: 10.1111/j.1365-2249.1995.tb03711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Im SY, Han SJ, Ko HM, Choi JH, Chun SB, Lee DG, Ha TY, Lee HK. Involvement of nuclear factor-κB in platelet-activating factor-mediated tumor necrosis factor-α expression. Eur J Immunol. 1997;27:2800–2804. doi: 10.1002/eji.1830271109. [DOI] [PubMed] [Google Scholar]
- 29.Silvers WS. Exercise-induced allergies: the role of histamine release. Ann Allergy. 1992;68:58–63. [PubMed] [Google Scholar]
- 30.Sun S, Weil MH, Tang W, Gazmuri RJ, Bisera J, Greenberg SR, Kim TB. Cardiac anaphylaxis in the Sprague-Dawley rat. J Lab Clin Med. 1992;120:589–596. [PubMed] [Google Scholar]
- 31.Rieger A, Wang B, Kilgus O, Ochiai K, Mauerer D, Fodinger D, Kinet JP, Stingl G. Fc epsilon RI mediates IgE binding to human epidermal Langerhans cells. J Investig Dermatol. 1992;99:30S–32S. doi: 10.1111/1523-1747.ep12668293. [DOI] [PubMed] [Google Scholar]
- 32.Bieber T, de la Salle H, Wollenberg A, Hakimi J, Chizzonite R, Ring J, de la Salle DH. Human epidermal Langerhans cells express the high affinity receptor for immunoglobulin E (FcεRI) J Exp Med. 1992;175:1285–1290. doi: 10.1084/jem.175.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gounni AS, Lamkhioued B, Ochiai K, Tanaka Y, Delaporte E, Capron A, Kinet JP, Capron M. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 34.Maurer D, Fiebiger E, Reininger B, Wolff-Winiski B, Jouvin MH, Kilgus O, Kinet JP, Stingl G. Expression of functional high affinity immunoglobulin E receptors (FcεRI) on monocytes of atopic individuals. J Exp Med. 1994;179:745–750. doi: 10.1084/jem.179.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph M, Gounni AS, Kusnierz JP, Vorng H, Sarfati M, Kinet JP, Tonnel AB, Capron A, Capron M. Expression and functions of the high-affinity IgE receptor on human platelets and megakaryocyte precursors. Eur J Immunol. 1997;27:2212–2218. doi: 10.1002/eji.1830270914. [DOI] [PubMed] [Google Scholar]
- 36.de Andres B, Rakasz E, Hagen M, McCormik ML, Mueller AL, Elliot D, Metwali A, Sandor M, Britigan BE, Weinstock JV, Lynch RG. Lack of Fc-epsilon receptors on murine eosinophils: implication for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood. 1997;89:3826–3836. [PubMed] [Google Scholar]
- 37.Capron M, Morita M, Woerly G, Lengrand F, Gounni AS, Delaporte E, Capron A. Differentiation of eosinophils from cord blood cell precursors: kinetics of FcεRI and FcεRII expression. Int Arch Allergy Immunol. 1997;113:48–50. doi: 10.1159/000237505. [DOI] [PubMed] [Google Scholar]