Abstract
The expression and secretion of interleukin (IL)-8, the prototype member of the C-X-C subfamily of chemokines, can be induced by diverse inflammatory stimuli in many cells, including endothelial cells (EC). Upon de novo synthesis, IL-8 localizes intracellularly in the Golgi apparatus, from where it is secreted. In addition to this constitutive secretory pathway, we describe a depot storage and separate regulated secretory pathway of IL-8 in EC.
The prolonged stimulation of primary human EC with inflammatory mediators resulted in the accumulation of IL-8 in Weibel-Palade bodies, where it colocalized with von Willebrand factor. IL-8 was retained in these storage organelles for several days after the removal of the stimulus and could be released by EC secretagogues such as phorbol myristate acetate, the calcium ionophore A23187, and histamine. These findings suggest that storage of IL-8 in Weibel-Palade bodies may serve as the EC “memory” of a preceding inflammatory insult, which then enables the cells to secrete IL-8 immediately without de novo protein synthesis.
Keywords: interleukin 8, endothelial cell, inflammation, Weibel-Palade bodies, secretion
The egress of circulating leukocytes into the tissues constitutes a prominent feature of inflammatory diseases. Optimal leukocyte emigration requires the production of leukocyte chemoattractants at the inflammatory sites and the upregulation of different classes of adhesion molecules on the surface of vascular endothelial cells (EC; reference 1). On the one hand, chemoattractants, including members of the chemokine family (for a review, see reference 2), can stimulate the activation of leukocyte integrins (for a review, see reference 3). On the other hand, chemoattractants are postulated to deliver directional cues during the transendothelial migration of leukocytes (1). A prerequisite for the in vivo activity of chemoattractants is that, after production by different cells in the tissues, they diffuse to the vessel wall and are then transported by the EC to the luminal surface (4). Additionally, EC themselves can produce diverse chemoattractants, including platelet-activating factor (PAF), leukotriene B4, S100 chemotactic protein, and several chemokines, such as IL-8, monocyte chemotactic proteins 1 and 3 (MCP-1 and MCP-3), RANTES (regulated upon activation, normal T cell expressed and secreted), and eotaxin (2, 5–7).
The inflammatory EC activation follows two distinct patterns, described as “delayed” (type II) and “immediate” (type I; reference 8). In the delayed (type II) pattern, there is a time delay of several hours between stimulation (e.g., with cytokines) and the EC expression of chemokines, E-selectin, intercellular adhesion molecule (ICAM)-1, and vascular cell adhesion molecule (VCAM)-1, which is due to the requirement of de novo transcription and translation (3). In contrast, in the immediate (type I) pattern, the absence of a time delay between stimulation and expression is attributed to the preformation of adhesion molecules and chemoattractants, which are exocytosed immediately after stimulation from their cytoplasmic storage compartments. In the case of lipid mediators such as PAF, the quick response is explained by rapid synthesis. P-selectin and PAF are a prototype adhesion molecule–chemoattractant pair that appears on the EC surface subsequent to stimulation by secretion from Weibel-Palade bodies and rapid synthesis, respectively (9–11).
Weibel-Palade bodies are Golgi-derived, rod-shaped storage organelles specific for EC (12). In addition to P-selectin, the proteins stored in Weibel-Palade bodies include von Willebrand factor (vWf [13]), endothelin (14), and CD63 (15). Several stimuli, including PMA, calcium ionophore (A23187), thrombin, histamine, and fibrin, induce the fusion of Weibel-Palade bodies with the plasma membrane and the release of the stored contents (16). In this study, we show that, after prolonged inflammatory stimulation, IL-8 accumulates in Weibel-Palade bodies in primary human EC, where it remains stored after the stimulation is discontinued and can be released instantaneously upon restimulation with EC secretagogues.
Materials and Methods
Human umbilical vein EC (HUVEC) were prepared and grown as described (17) and used at passages 3–5. The cells were positive for vWf (>95% of the population). They were seeded into gelatin-coated 96-well plates (Nunc, Inc., Naperville, IL) or 8-well LabTek slides (Nunc, Inc.). After overnight adherence, the cells were stimulated with 1,000 U/ml IL-1β (Genzyme Corp., Cambridge, MA).
Secreted IL-8 was determined from the culture supernatants by a conventional sandwich ELISA, and quantitative and qualitative determinations of intracellular IL-8 were performed using a mouse anti–IL-8 mAb (Novartis Forschungsinstitut [NFI] clone 4G9/A5) and the ELF™ detection system (Molecular Probes, Inc., Eugene, OR), as described elsewhere (17). In the microtiter plate experiments, the values were normalized to cell number, determined by staining of cellular protein with sulforhodamine B (Sigma Chemical Co., St. Louis, MO) as described (17) and calculating cell number on the basis of a calibration curve.
For the double immunofluorescence studies, a rabbit polyclonal anti–IL-8 antibody (NFI) was used in combination with a mouse mAb against a 58-kD Golgi protein (18; Sigma Chemical Co.) and mouse anti–IL-8 mAbs from different sources (NFI clone 4G9/A5; R&D Systems, Inc., Minneapolis, MN, and ImmunoKontact, Frankfurt, Germany) in combination with a rabbit polyclonal antibody against vWf (Dakopatts A/S, Glostrup, Denmark). The primary antibodies were detected with Texas red– labeled goat anti–rabbit IgG (Accurate Chemical and Science Corp., Westbury, NY) and OREGON GREEN™ 500–labeled goat anti–mouse IgG (Molecular Probes, Inc.). Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Sigma Chemical Co.). The slides were examined with a fluorescence microscope (model IX 70; Olympus, Vienna, Austria) or with a confocal laser scanning microscope (MRC 600; Bio-Rad Laboratories Ltd., Hemel Hempstead, UK) in the dual channel mode. Images were merged using Bio-Rad software.
For immunoelectron microscopy, HUVEC were fixed (2% paraformaldehyde, 0.05% glutaraldehyde), cryoprotected in 0.2 M PBS containing 20% polyvinylpyrrolidone and 1.84 M sucrose overnight at 4°C, and processed as described (19). Labeling was performed with anti–IL-8 (NFI) and antioccludin (Zymed Laboratories, Inc., South San Francisco, CA) rabbit polyclonal antibodies, and anti-vWf (Dakopatts A/S) and anti–L-selectin (Dreg 200; gift of T.K. Kishimoto, Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT) mouse mAbs. Second antibodies were goat anti–rabbit 6-nm gold and goat anti–mouse 18-nm gold conjugates (Jackson Immunoresearch Laboratories, Inc., Avondale, PA).
For the study of IL-8 release from Weibel-Palade bodies, cells were stimulated with 1,000 U/ml IL-1β in incubation medium (RPMI 1640 plus 10% FCS) overnight. They were washed three times with PBS and incubated with incubation medium alone for 4 h. The medium was then replaced by fresh incubation medium with or without secretagogues (10 nM PMA, 10 μM A23187, or 5 mM histamine; all from Sigma Chemical Co.) for 1 h. The supernatants were removed, and intracellular and secreted IL-8 were determined.
Results and Discussion
Intracellular Localization of IL-8 in HUVEC after Stimulation with IL-1β.
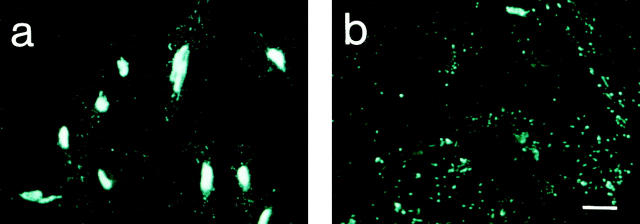
Previously, we determined the levels of secreted and intracellular IL-8 in primary HUVEC compared with EC lines and found them to produce ∼8–10-fold higher amounts of IL-8 (17). Therefore, HUVEC were chosen for cellular localization studies. The optimal stimulus for HUVEC turned out to be IL-1β, followed by IL-1α, TNF-α, and LPS. HUVEC stimulated with IL-1β were used in all experiments described below. As expected for a secreted protein and described previously (17, 20), IL-8 (Fig. 1 a) was found in the Golgi apparatus, where it colocalized with a 58-kD protein that serves as a marker for this organelle (18; Fig. 1 b). Colocalization of the two proteins, red and green in individual images, is shown in yellow in the merged confocal laser scanning image (Fig. 1 c). When HUVEC were exposed to the stimulus for 8 h or more, IL-8 immunoreactivity was also detected in Weibel-Palade bodies, colocalizing with vWf (Fig. 1, d–h). This was demonstrated using two different antibody combinations, a mouse anti–IL-8 mAb (Fig. 1, d and g) and a rabbit polyclonal antibody against vWf (Fig. 1, e and h), as well as a rabbit polyclonal antibody directed against IL-8 in combination with a mouse mAb against vWf (immunofluorescence results not shown, but see Fig. 2). Colocalization of IL-8 (Fig. 1 d) and vWf (Fig. 1 e) is demonstrated by the yellow color in the merged confocal laser scanning image (Fig. 1 f ). Three different anti–IL-8 mAbs gave identical results (not shown). Preincubation of the mouse anti–IL-8 mAb with a 2–5-fold molar excess of recombinant human IL-8 (NFI) completely eliminated staining of both Golgi apparatus and Weibel-Palade bodies, indicating the specificity of the staining reactions (results not shown). To confirm that the rod-shaped staining for IL-8 was indeed intracellular and not cell surface–bound, permeabilized cells (Fig. 1, g–i) were compared with nonpermeabilized cells (Fig. 1, j–l) by conventional fluorescence microscopy. Nonpermeabilized cells did not show any specific staining for IL-8 (Fig. 1 j) or intracellular vWf (Fig. 1 k). The position of the cells in Fig. 1, g, h, j, and k, is indicated by the DAPI stain of the respective nuclei (Fig. 1, i and l). IL-8 localization in Weibel-Palade bodies was also found in IL-1– stimulated primary human microvascular EC (not shown).
Figure 1.
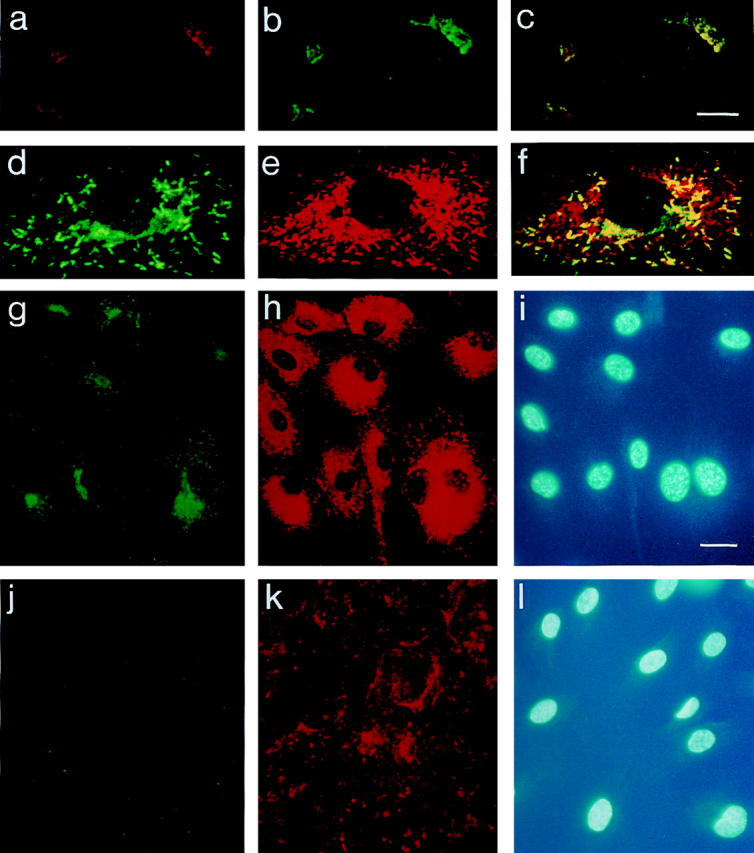
Immunolocalization of IL-8 in HUVEC: Golgi apparatus and Weibel-Palade bodies. HUVEC were stimulated with 1,000 U/ml IL-1β for 4 h (a–c) or overnight (d–l). Cells were fixed and permeabilized (a–i) or fixed only (j–l), and colocalization experiments were performed as described in Materials and Methods. Images (a–f ) were recorded with the confocal laser scanning microscope (scale bar, 10 μm). Localization of IL-8 (a) and the 58-kD Golgi protein (b) is shown in two separate cells and of IL-8 (d) and vWf (e) in one individual cell. Images a and b were merged to yield c, and the merged image of d and e is shown in f; yellow, areas of colocalization. Pictures g–l were photographed from an Olympus IX 70 fluorescence microscope (scale bar, 20 μm): IL-8 (g and j), vWf (h and k), and DAPI (i and l).
Figure 2.
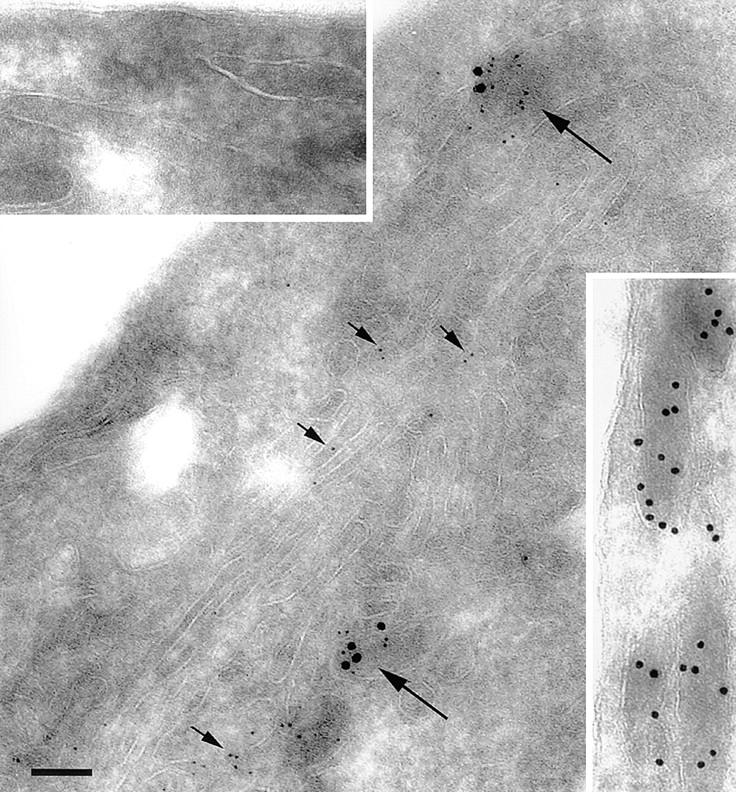
Immunoelectron microscopic colocalization of IL-8 and vWf in Weibel-Palade bodies. HUVEC were stimulated with 1,000 U/ml IL-1β overnight and processed for electron microscopy as indicated in Materials and Methods. Large granules (18-nm gold) represent vWf; small granules (6-nm gold) correspond to IL-8. Short arrows, IL-8 in the Golgi apparatus; long arrows, Weibel-Palade bodies that contain both IL-8 and vWf. Top left inset, The absence of granules from Weibel-Palade bodies stained with the irrelevant antibodies rabbit antioccludin and mouse anti–E-selectin. Bottom right inset, Weibel-Palade bodies in unstimulated HUVEC contained only vWf, but no IL-8. Scale bar, 100 nm.
The colocalization of IL-8 with vWf in Weibel-Palade bodies was confirmed using immunoelectron microscopy (Fig. 2). Nonspecific mouse monoclonal and rabbit polyclonal antibodies did not give any staining (Fig. 2, top left inset), and unstimulated HUVEC did not express IL-8 in Weibel-Palade bodies (Fig. 2, bottom right inset). After stimulation with IL-1β overnight, IL-8 was found both in the Golgi apparatus (short arrows) and in Weibel-Palade bodies (long arrows).
There was considerable heterogeneity between individual HUVEC preparations, with the number of cells containing IL-8–positive Weibel-Palade bodies ranging from 10 to 90%. After overnight stimulation of HUVEC with IL-1β as well as with IL-1α, TNF-α, and LPS (results not shown), three different types of IL-8 staining could be detected: Golgi and Weibel-Palade bodies, Golgi only, and Weibel-Palade bodies only. The percentages of these three types varied between individual HUVEC preparations. Interestingly, not all Weibel-Palade bodies that contained vWf also expressed IL-8. Curiously, in the human microvascular endothelial cell line HMEC-1, which does express vWf, no IL-8 immunoreactivity associated with Weibel-Palade bodies could be detected. These observations may reflect the detection limit for IL-8, since HMEC-1 cells expressed ∼8–10-fold lower levels of IL-8 (17). The minimal concentration of IL-1β required for IL-8 storage in Weibel-Palade bodies varied between individual HUVEC preparations, ranging from 30 to 100 U/ml. Maximal induction of other IL-1–inducible proteins was at 100 U/ml (E-selectin) and 300 U/ml (VCAM-1 and ICAM-1).
Removal of IL-1β Stimulus Led to a Release of IL-8 from the Golgi Apparatus, but not from Weibel-Palade Bodies.
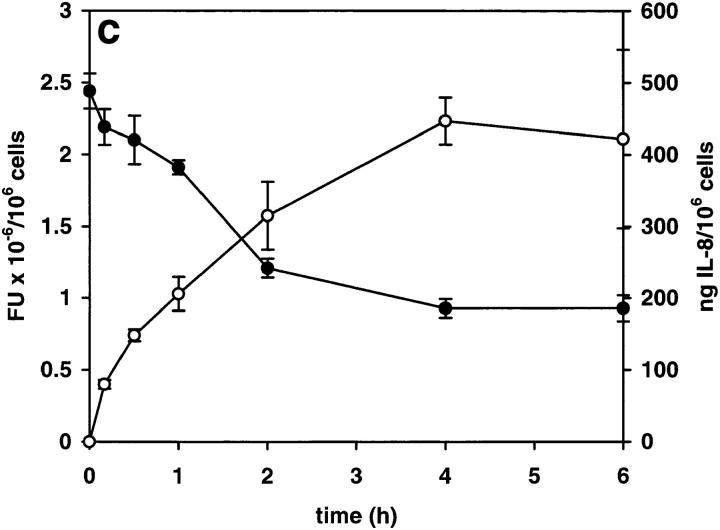
After overnight stimulation of HUVEC with IL-1β, IL-8 was located both in the Golgi apparatus and Weibel-Palade bodies (Fig. 3 a). Upon removal of IL-1, intracellular levels of IL-8 dropped rapidly over a period of 4–8 h, while the levels of secreted IL-8 increased (Fig. 3 c). This secretion occurred only from Golgi, whereas IL-8 associated with Weibel-Palade bodies remained in the cells (Fig. 3 b) and could still be detected in them 96 h after the removal of IL-1.
Figure 3.
Removal of IL-1β from stimulated HUVEC: effect on intracellular and secreted IL-8. HUVEC were stimulated with 1,000 U/ml IL-1β overnight and then further incubated in IL-1–containing medium (a) or IL-1–free medium (b and c) for 4 h (b) or the times indicated (c). Secreted (open circles) and intracellular (filled circles) IL-8 were determined as described in Materials and Methods, and are expressed as nanograms of IL-8 per 106 cells and as arbitrary fluorescence units (FU) × 10−6 per 106 cells, respectively. Results are means ± SD of triplicate samples.
Addition of Secretagogues that Release vWf Induced Secretion of IL-8 from Weibel-Palade Bodies.
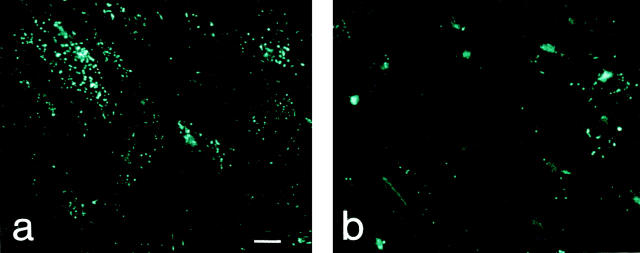
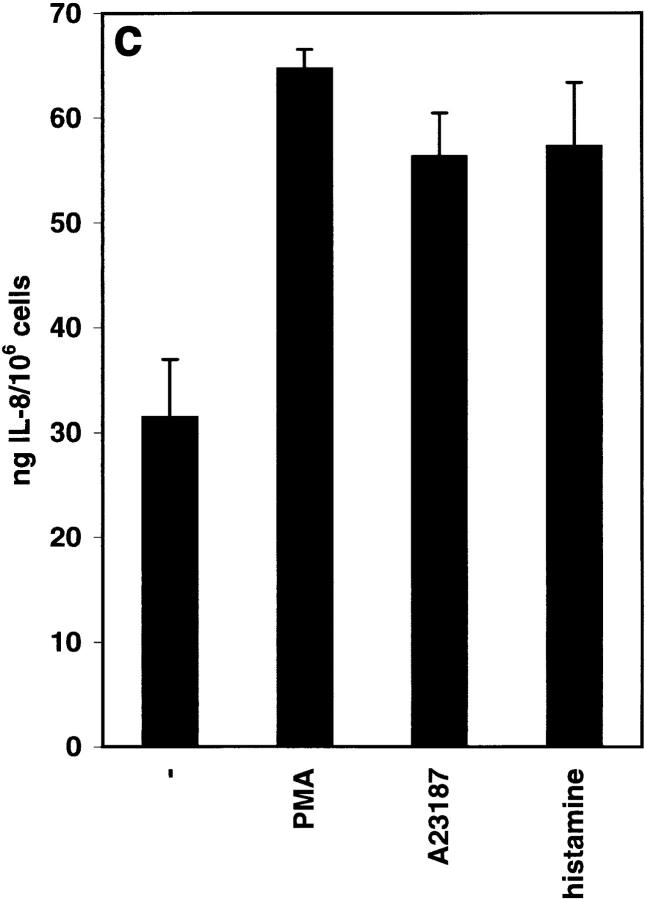
A variety of EC secretagogues, such as PMA, the ionophore A23187, thrombin, histamine, and fibrin, have been shown to induce the release of Weibel-Palade bodies' contents (16). To investigate the release of IL-8 from Weibel-Palade bodies, HUVEC were stimulated with IL-1β overnight and then incubated in fresh, IL-1–free medium for 4 h. By this time, a negligible amount of cell-associated IL-8 immunoreactivity was in the Golgi apparatus, whereas the overwhelming majority of it was present in Weibel-Palade bodies (Fig. 3 b). This pattern did not change upon further incubation of HUVEC in medium for 1 h (Fig. 4 a). After the addition of PMA for 1 h, most of the Weibel-Palade bodies lost their IL-8 immunoreactivity (Fig. 4 b), while, consistent with the anticipated secretion, the amount of IL-8 in the supernatant increased by 33.3 ng/ 106 cells (from 31.4 to 64.7 ng/106 cells; Fig. 4 c). Similar results were obtained with A23187 and histamine (Fig. 4 c). As expected, under the conditions used for IL-8 secretion, the secretagogues also induced the release of vWf from Weibel-Palade bodies (results not shown).
Figure 4.
Release of IL-8 from Weibel-Palade bodies by secretagogues: intracellular and secreted IL-8. HUVEC were stimulated with 1,000 U/ml IL-1β overnight and then released into IL-1–free medium for 4 h. Control cells (a) and cells that were induced to release IL-8 from Weibel-Palade bodies by the addition of 10 nM PMA (b and c), 10 μM A23187 (c), and 5 mM histamine (c) were examined after an additional 1 h. IL-8 was determined as described in Materials and Methods; values are means ± SD of triplicate samples.
Western blot analysis of cell extracts with and without removal of the IL-1 stimulus demonstrated that the IL-8 immunoreactivity seen in Weibel-Palade bodies in HUVEC was identical to the 8.3-kD form of IL-8 (results not shown).
EC and platelets share many molecular and cellular features. Thus, P-selectin and vWf are not only constituents of Weibel-Palade bodies in EC, but also of α-granules in platelets (21). Interestingly, several members of the chemokine family, such as platelet factor 4 (22) and RANTES (23), have been found stored in platelets. Recently, biologically active IL-8 has also been identified in rabbit and human platelets, and a substantial amount can be released upon platelet activation (24). Platelets are functionally specialized for hemostasis but may also have profound effects upon inflammatory reactions. Platelet aggregation may cause the neutrophil infiltration observed at the initial phase of acute inflammation through the release of preformed, stored chemokines (24). The release of stored IL-8 from Weibel-Palade bodies in the EC that have previously experienced inflammatory stimulation may have a similar function: it is rapid and independent of de novo protein synthesis. Thus, IL-8 together with P-selectin, which is constitutively present in Weibel-Palade bodies (9, 10), may be part of the immediate EC response (type I endothelial activation). Previously, the role of IL-8 has been limited to the type II EC activation responses where it may act in synchrony with E-selectin (25).
The EC of the blood vessels in organs and tissues that border the external environment may represent a special case. Due to their anatomical location, these EC may experience chronic inflammatory stimulation and, as a result, constitutively express significant amounts of IL-8 in Weibel-Palade bodies (26). Therefore, EC in some locations may be able to release IL-8 immediately upon stimulation with EC secretagogues.
In conclusion, we describe the storage of IL-8 in Weibel-Palade bodies, which may serve as a substrate of a rudimentary EC memory of a previously suffered inflammatory insult. Such memory may play a previously unrecognized role in disease settings where chronic EC stimulation leads to accumulation of IL-8 in Weibel-Palade bodies and its rapid “recall” is induced by a second stimulus at a later time point. For example, protracted periods of hypoxia in the organs before their transplantation or during cardiopulmonary bypass surgery may induce the production of IL-8 by the EC (27) and its accumulation in Weibel-Palade bodies. After transplantation or during reperfusion at the end of cardiopulmonary bypass, thrombin, histamine (28), or other stimuli which lead to type I EC activation may result in immediate massive release of the stored IL-8. Rapid IL-8 release from an unknown source has been described to take place after pediatric cardiopulmonary bypass surgery, and has been suggested to contribute to leukocyte activation and postoperative pulmonary complications (29). It will be of interest to determine if IL-8 stored in Weibel-Palade bodies plays a pathophysiological role in this case and in other clinically relevant disease situations.
Acknowledgments
The authors would like to thank C. Rabeck, E. Schwarzinger, and M. Zsak for skilled and dedicated technical help, E.S. Brown for assistance with electron microscopy, and Dr. A.P. Winiski for critically reading the manuscript.
Footnotes
A.R. Burns was supported by the Methodist Hospital Foundation, the Chao Fellowship, and the National Institutes of Health (grant HL42550).
J. Middleton's present address is Department of Rheumatology, Orthopaedic and District Hospital, Oswestry S710 7A6, UK.
References
- 1.Hub, E., J. Middleton, and A. Rot. 1996. Mechanism of chemokine-induced leukocyte adhesion and emigration. In Chemoattractant Ligands and Their Receptors. R. Horuk, editor. CRC Press, Inc., Boca Raton, FL. 301–325.
- 2.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 5.Maclouf J, Murphy RC, Henson PM. Transcellular sulfidopeptide leukotriene biosynthetic capacity of vascular cells. Blood. 1989;74:703–707. [PubMed] [Google Scholar]
- 6.Yen T, Harrison CA, Devery JM, Leong S, Iismaa SE, Yoshimura T, Geczy CL. Induction of the S100 chemotactic protein, CP-10, in murine microvascular endothelial cells by proinflammatory stimuli. Blood. 1997;90:4812–4821. [PubMed] [Google Scholar]
- 7.Mantovani A, Bussolino F, Introna M. Cytokine regulation of endothelial cell function: from molecular level to the bedside. Immunol Today. 1997;18:231–240. doi: 10.1016/s0167-5699(97)81662-3. [DOI] [PubMed] [Google Scholar]
- 8.Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation (Baltimore) 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP 140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- 10.McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerman GA, McIntyre TM, Mehra M, Prescott SM. Endothelial cell-associated platelet-activating factor: a novel mechanism for signaling intercellular adhesion. J Cell Biol. 1990;110:529–540. doi: 10.1083/jcb.110.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weibel ER, Palade GE. New cytoplasmic components in arterial endothelia. J Cell Biol. 1964;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner DD, Mayadas T, Marder VJ. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakamoto Y, Doi Y, Ohsato K, Fujimoto S. Immunoelectron microscopy on the localization of endothelin in the umbilical vein of perinatal rabbits. Anat Rec. 1993;237:482–488. doi: 10.1002/ar.1092370407. [DOI] [PubMed] [Google Scholar]
- 15.Vischer UM, Wagner DD. CD63 is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1993;82:1184–1191. [PubMed] [Google Scholar]
- 16.Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- 17.Wolff B, Zsak M, Rabeck C. Immunofluorescence assay for the quantitative and qualitative evaluation of intracellular interleukin-8 in microtiter plates. Anal Biochem. 1997;244:33–39. doi: 10.1006/abio.1996.9881. [DOI] [PubMed] [Google Scholar]
- 18.Bloom GS, Brashear TA. A novel 58-kDa protein associates with the Golgi apparatus and microtubules. J Biol Chem. 1989;264:16083–16092. [PubMed] [Google Scholar]
- 19.Burns AR, Takei F, Doerschuk CM. Quantitation of ICAM-1 expression in mouse lung during pneumonia. J Immunol. 1994;153:3189–3198. [PubMed] [Google Scholar]
- 20.Andersson J, Abrams J, Björk L, Funa K, Litton M, Ågren K. Concomitant in vivoproduction of 19 different cytokines in human tonsils. Immunology. 1994;83:16–24. [PMC free article] [PubMed] [Google Scholar]
- 21.Berman CL, Yeo EL, Wencel-Drake J, Furie BC, Ginsberg ML, Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986;78:130–137. doi: 10.1172/JCI112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deuel TF, Senior KM, Cheng D, Griffin GL, Heinrickson RL, Kaiser ET. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci USA. 1981;78:4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klinger MHF, Wilhelm D, Bubel S, Sticherling M, Schröder J-M, Kühnel W. Immunocytochemical localization of the chemokines RANTES and MIP-1α within human platelets and their release during storage. Int Arch Allergy Immunol. 1995;107:541–546. doi: 10.1159/000237097. [DOI] [PubMed] [Google Scholar]
- 24.Su S-B, Mukaida N, Matsushima K. Rapid secretion of intracellularly pre-stored interleukin-8 from rabbit platelets upon activation. J Leukocyte Biol. 1996;59:420–426. doi: 10.1002/jlb.59.3.420. [DOI] [PubMed] [Google Scholar]
- 25.Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992;13:291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- 26.Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J Exp Med. 1998;188:1751–1756. doi: 10.1084/jem.188.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinsky DJ, Naka Y, Liao H, Oz MC, Wagner DD, Mayadas TN, Johnson RC, Hynes RO, Heath M, Lawson CA, Stern DM. Hypoxia-induced exocytosis of endothelial cell Weibel-Palade bodies: a mechanism for rapid neutrophil recruitment after cardiac preservation. J Clin Invest. 1996;97:493–500. doi: 10.1172/JCI118440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seghaye MC, Duchateau J, Grabitz RG, Mertes J, Marcus C, Buro K, Messmer BJ, von Bernuth G. Histamine liberation related to cardiopulmonary bypass in children: possible relation to transient postoperative arrhythmias. J Thorac Cardiovasc Surg. 1996;111:971–981. doi: 10.1016/s0022-5223(96)70373-2. [DOI] [PubMed] [Google Scholar]
- 29.Finn A, Naik S, Klein N, Levinsky RJ, Strobel S, Elliott M. Interleukin-8 release and neutrophil degranulation after pediatric cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1993;105:234–241. [PubMed] [Google Scholar]