Abstract
Vitiligo is an autoimmune condition characterized by loss of epidermal melanocytes. Using tetrameric complexes of human histocompatibility leukocyte antigen (HLA) class I to identify antigen-specific T cells ex vivo, we observed high frequencies of circulating MelanA-specific, A*0201-restricted cytotoxic T lymphocytes (A2–MelanA tetramer+ CTLs) in seven of nine HLA-A*0201–positive individuals with vitiligo. Isolated A2–MelanA tetramer+ CTLs were able to lyse A*0201-matched melanoma cells in vitro and their frequency ex vivo correlated with extent of disease. In contrast, no A2–MelanA tetramer+ CTL could be identified ex vivo in all four A*0201-negative vitiligo patients or five of six A*0201-positive asymptomatic controls. Finally, we observed that the A2–MelanA tetramer+ CTLs isolated from vitiligo patients expressed high levels of the skin homing receptor, cutaneous lymphocyte-associated antigen, which was absent from the CTLs seen in the single A*0201-positive normal control. These data are consistent with a role of skin-homing autoreactive melanocyte-specific CTLs in causing the destruction of melanocytes seen in autoimmune vitiligo. Lack of homing receptors on the surface of autoreactive CTLs could be a mechanism to control peripheral tolerance in vivo.
Keywords: human, skin, cytotoxic T lymphocytes, fluorescence-activated cell sorter, autoimmunity
itiligo is a common progressive depigmentary condition that is believed to be due to the autoimmune-mediated destruction of epidermal melanocytes. Melanocyte-specific autoantibodies have been described in vitiligo patients, but their pathogenic role remains uncertain (1–5). Titers of such autoantibodies in individuals with melanoma-associated hypopigmentation are similar to those in normal controls, suggesting that, in these patients, other mechanisms may lead to melanocyte loss (4). The observation that melanocyte proteins are targets for antimelanoma CTLs (6–8) raises the possibility that destruction of epidermal melanocytes in vitiligo patients could be due to a melanocyte-specific CTL response. Indeed, the spontaneous appearance of vitiligo has been associated with an improved prognosis in individuals with metastatic melanoma (9–10). Histology of the advancing margins of vitiligo reveals a lymphocytic infiltrate predominantly composed of CD8+ T cells expressing the skin homing receptor, cutaneous lymphocyte-associated antigen (CLA),1 which is a modified form of P selectin–binding glycoprotein 1 (11–14). Recently, dendritic cell-based vaccination strategies for treatment of melanoma have been accompanied by the acquisition of tumor-specific CTL responses and cutaneous depigmentation, suggesting that melanocyte-specific CTLs can play a role in melanocytic destruction (15). Animal models of vitiligo are also consistent with a role for autoreactive CD8+ T cells (16), but it has remained unclear whether antigen-specific CD8+ T cells are involved in vitiligo or indeed any other human autoimmune condition. Here, we demonstrate the presence of high frequencies of skin-homing melanocyte-specific CTLs in the peripheral blood of patients with autoimmune vitiligo compared with healthy volunteers.
Materials and Methods
HLA–Peptide Tetrameric Complexes and Flow Cytometry.
HLA– peptide tetrameric complexes were synthesized as previously described (17). In brief, purified HLA heavy chain and β2 microglobulin were synthesized using a prokaryotic expression system (pET; Novagen, Milwaukee, WI). The heavy chain was modified by deletion of the transmembrane/cytosolic tail and COOH-terminal addition of a sequence containing the BirA enzymatic biotinylation site. Heavy chain, β2 microglobulin, and peptide were refolded by dilution. A*0201-binding peptides were tyrosinase 369–377 YMDGTMSQV (18–20) and MelanA 26-35 ELAGIGILTV (7, 8, 21). The 45-kD refolded product was isolated using fast protein liquid chromatography (FPLC), biotinylated by BirA (Avidity, Denver, CO) in the presence of biotin (Sigma Chemical Co., St. Louis, MO), ATP (Sigma Chemical Co.) and Mg2+ (Sigma Chemical Co.). The biotinylated product was separated from free biotin by gel filtration and ion exchange using FPLC. Streptavidin–PE conjugate (Sigma Chemical Co.) was added in a 1:4 molar ratio and the tetrameric product was concentrated to 1 mg/ml. Analysis of cells for the expression of cell surface markers was performed using a FACScan® (Becton Dickinson & Co., Mountain View, CA) and CellQuest software (Becton Dickinson & Co.). Frozen PBMC were thawed and incubated for 24–48 h in RPMI 1640 supplemented with 10% FCS to allow recovery of cell viability. 106 cells were centrifuged at 300 g for 5 min and resuspended in 50 μl cold PBS. Anti–CLA antibody (rat IgM; PharMingen, San Diego, CA) was added and the samples incubated on ice for 20 min. After two washes with PBS, anti-rat IgM-FITC (PharMingen) was added and the cells left for a further incubation on ice for 20 min. After two washes with PBS, the tetramer and anti–CD8-Tricolor (Caltag Laboratories, Burlingame, CA) were added and incubated for another 20 min. The samples were washed two more times with PBS before formaldehyde fixation. Triple-color analysis was performed with tetramer-PE, anti–CD8-tricolor, and anti–CLA. Controls for the tetramers included staining A*0201-negative individuals and the use of an irrelevant A*0201-tetramer (SLYNTVATL p17Gag 77-85; reference 22).
Subjects.
13 individuals with vitiligo were recruited through the Vitiligo Society (London, UK) and nine were found to be HLA-A*0201 positive when screened using allele-specific PCR. Six patients had associated autoimmune conditions (four were HLA-A*0201 positive) including thyroid disease and pernicious anaemia, but the remaining seven (five were HLA-A*0201 positive) had no other clinical disorders. None of the patients were on immunosuppressive therapy. The negative control individuals were healthy asymptomatics with no history of autoimmune disease.
Cytotoxicity Assays.
A*0201-positive EBV-transformed B cell lines, T2 cells, or melanoma cells (provided by Dr. P. van der Bruggen, Ludwig Institute for Cancer Research, Bruxelles, Belgium) were incubated at 37°C for 1 h in the presence of Na2 51CrO4 (Amersham Pharmacia Biotech, Inc., Piscataway, NJ) at 2 μCi/μl. The cells were washed once in medium containing 10% FCS and incubated either with or without peptide at 1 μM for 1 h. The targets were washed a further two times before plating into 96-well round-bottomed plates at 2,500 cells per well. 100 μl of medium containing cells at documented E/T ratios were added to each well after counting in the presence of trypan blue. The plates were incubated at 37°C for 4 h before harvesting 20 μl of supernatant. Percentage lysis was estimated from (experimental counts − media control) × 100/(detergent counts − media control). Media controls were between 10 and 15% of detergent controls.
Cell Culture.
PBMCs were incubated with 100 μM peptide for 1 h before dilution at 2 × 106 cells/ml in RPMI 1640 supplemented with 5% human serum and 25 ng/ml IL-7 (23). Lymphocult-T (Biotest, Dreieich, Germany) was added at day 4 to a final concentration of 10%. The cells were harvested at day 12 for flow cytometry analysis or cytotoxicity assays.
Results and Discussion
To test the hypothesis that high frequencies of melanocyte-specific CTLs correlate with vitiligo, we quantified the frequency of ex vivo melanocyte-specific CTLs by constructing HLA-A*0201–peptide tetrameric complexes (17, 24) based on two A*0201-binding peptides derived from melanocyte proteins MelanA (MelanA 26–35) and tyrosinase (tyrosinase 369–377) (7, 8, 18–20). A modified MelanA 26-35 peptide (alanine to leucine at position 2) has previously been shown to increase the binding affinity for A*0201 without affecting CTL recognition (21). HLA heavy chain was expressed in Escherichia coli with an engineered COOH-terminal signal sequence containing a biotinylation site for the enzyme BirA. After the refolding of heavy chain, β2 microglobulin, and peptide, the complex was biotinylated and tetramer formation induced by the addition of streptavidin. By using fluorescently labeled streptavidin, the tetramer was used to stain and sort antigen-specific cells by flow cytometry. HLA–peptide tetrameric complexes bind to antigen-specific CTLs with high specificity such that CTL clones and lines directed to different epitope peptides bound to the same HLA molecule do not stain. Tetramer binding is known to correlate well with both peptide-specific cytolytic activity and IFN-γ production (24–26), and even down to low frequencies of antigen-specific T cells (1 in 5,000 CD8+ T cells or less) it is possible to directly isolate tetramer-positive cells by FACS® (26).
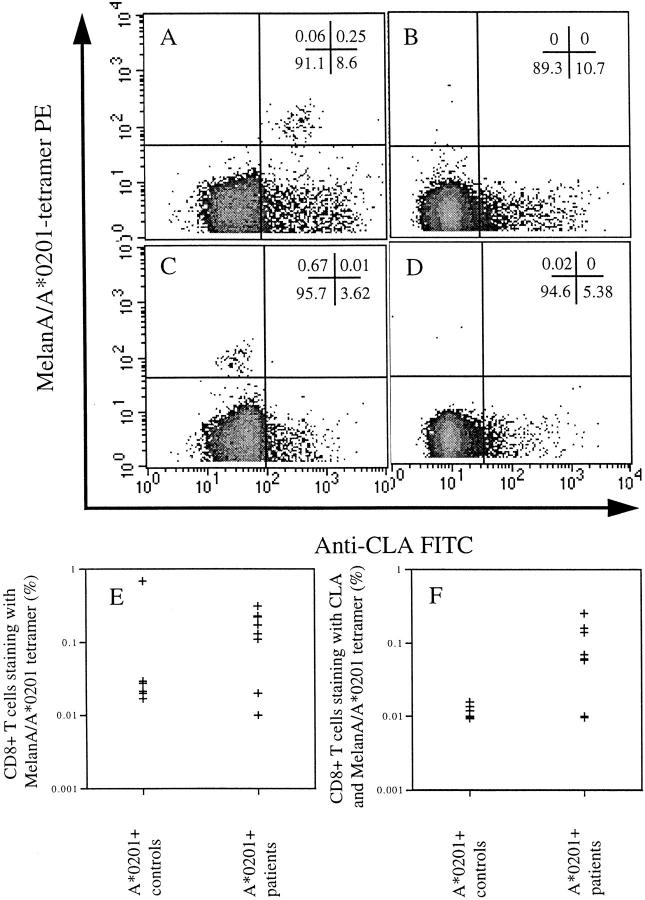
By staining of PBMC directly ex vivo, without any antigen-specific stimulation, we observed high frequencies of MelanA-specific CTLs in seven of nine A*0201-positive vitiligo patients, but in only one of six A*0201-positive asymptomatic controls (Fig. 1, A–D). Frequencies were similar to those previously obtained for A*0201-restricted influenza A matrix protein in asymptomatic patients (26). The identification of A*0201-restricted MelanA-specific CTLs was dependent on the presence of HLA-A*0201, as none of four A*0201-negative vitiligo controls had detectable CTLs ex vivo. No tyrosinase (369–377)-specific CTLs were observed directly ex vivo in any patient group. It is certainly possible that melanocyte-specific CTLs exist to epitopes other than those tested with the HLA-tetrameric complexes. The one A*0201-positive asymptomatic control with a frequency of MelanA-specific CTLs similar to that observed in the vitiligo patients had no personal or family history suggestive of autoimmune disease. To explain such a high frequency of MelanA-specific CTLs in the absence of disease, we examined the skin-homing capacity of CTLs from the cohort. The majority of MelanA-specific CTLs from the vitiligo patients expressed the skin-homing receptor CLA, in contrast to those from the asymptomatic control who had detectable CTLs, in whom the CTLs were all negative for CLA (Fig. 1, A–F). There was a statistically significant difference between the number of CLA-positive A2–MelanA tetramer+ CTLs between patients and controls (P < 0.05). These data confirmed that high frequencies of autoreactive CTLs could be detected directly ex vivo in patients with vitiligo, but were only associated with disease if they were able to home to the skin.
Figure 1.
Uncultured CD8+ T cells stained with A2–MelanA tetramer and anti–CLA antigen from: (A) an A*0201-positive patient with vitiligo in whom the majority of MelanA-specific CTLs are positive for CLA (percentages given in each quadrant); (B) an A*0201-positive normal control individual in whom there were no detectable MelanA-specific CTLs ex vivo (pattern observed in five of six normal controls); (C) the A*0201- positive normal control individual in whom there were detectable MelanA-specific CTLs ex vivo, but which were all negative for CLA (pattern observed in one of six normal controls); (D) an A*0201-negative patient with vitiligo in whom there were no detectable A2–MelanA tetramer+ CTLs (observed in all A*0201-negative vitiligo patients). E shows the percentage of CD8+ T cells staining with A2–MelanA tetramer in the A*0201-positive normal controls and A*0201-positive vitiligo patients. F confirms a significant difference between the percentage of CLA-positive MelanA-specific CTLs in the PBMC of A*0201-positive patients and controls (P < 0.05).
Through the use of repeated antigen-specific stimulation in vitro, it has previously been possible to generate tyrosinase- and MelanA-specific CTLs, from normal controls, that have the capacity to lyse melanoma target cells (27, 28). We examined whether it would be possible to use the HLA–peptide tetrameric complexes to compare the frequency of melanocyte-specific CTLs generated by peptide-specific stimulation from our cohort of vitiligo patients and asymptomatic controls. Using an optimized peptide-specific stimulation protocol in the presence of IL-7 (23), we screened peptide-stimulated cultures at 2 wk for staining with the A2–MelanA and A2/tyrosinase tetramers. High frequencies of melanocyte-specific CTLs were observed in cultures from the A*0201-positive vitiligo patients, but only low frequencies were observed in five of the six asymptomatic controls (Fig. 2, A–D). The asymptomatic control with detectable MelanA-specific CTLs ex vivo had a similar frequency of CTLs generated by peptide stimulation as the vitiligo patients. No A2–MelanA tetramer+ CTLs were observed in cultures from A*0201-negative vitiligo patients. We were also able to generate tyrosinase-specific CTLs from A*0201-positive vitiligo patients and normal controls, but these were consistently at least an order of magnitude lower than the MelanA-specific CTL frequencies (data not shown). The rank order of frequencies of MelanA-specific CTLs generated by peptide stimulation was identical to the order obtained directly ex vivo, indicating that all individuals had cells with a relatively consistent ability to proliferate and survive in culture for 2 wk. We looked at whether CLA was expressed by the peptide-stimulated melanocyte-specific CTLs (Fig. 2 F) and observed a significant difference between patients and controls (P < 0.05), namely high expression on CTLs generated from vitiligo patients and low or absent staining from controls. Although the cultures from vitiligo patients maintained CLA expression at levels above those observed in normal controls, there was evidence of a reduction in CLA staining compared with uncultured MelanA-specific CTLs consistent with previous observations (13). These data showed that it was possible through optimized stimulation protocols to generate MelanA- and tyrosinase-specific CTLs from A*0201-positive vitiligo patients and normal controls, but the frequencies of such CTLs are significantly higher in the patients and, in addition, the cells are able to home to the skin. Therefore, it was possible to isolate autoreactive CTLs from asymptomatic controls, but these were maintained at a low frequency (in five of six asymptomatic controls) and lacked the expression of CLA, a marker associated with the ability to home to skin. Lack of tissue homing receptors on the surface of potentially autoreactive CTLs may be a mechanism to prevent autoreactivity in vivo and to control peripheral tolerance.
Figure 2.
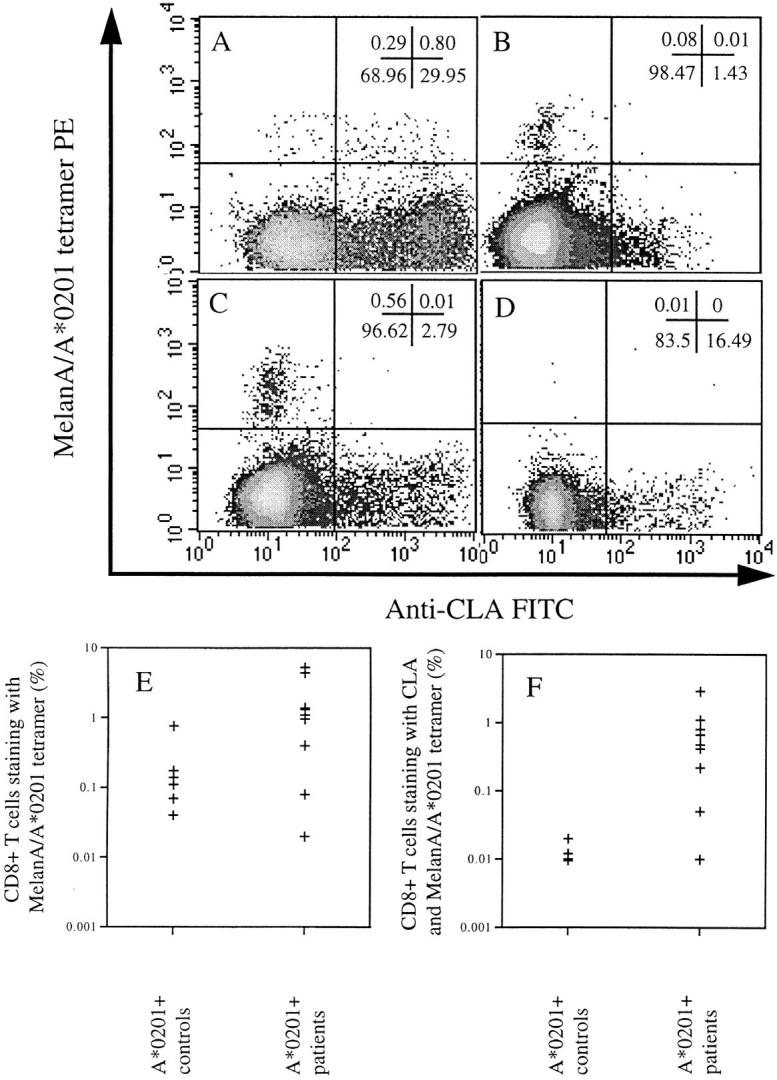
Optimized peptide-stimulated IL-7–based cultures showing the CD8+ T cells stained with A2–MelanA tetramer and anti–CLA from: (A) an A*0201-positive patient with vitiligo in whom the majority of cultured MelanA-specific CTLs are positive for CLA (percentages given in each quadrant); (B) an A*0201-positive normal control individual in whom there was a low frequency of cultured MelanA-specific CTLs that were predominantly negative for CLA (pattern observed in five of six normal controls); (C) the A*0201-positive normal control individual in whom there were detectable MelanA-specific CTLs ex vivo, and in whom it was possible to culture high levels of MelanA-specific CTLs that were all persistently negative for CLA (pattern observed in one of six normal controls); (D) an A*0201-negative patient with vitiligo in whom there were no detectable A2–MelanA tetramer+ CTLs (observed in all A*0201-negative vitiligo patients) after a period of stimulation in vitro. E shows the percentage of CD8+ T cells from the cultures staining with A2–MelanA tetramer in the A*0201-positive normal controls and A*0201-positive vitiligo patients. F shows a significant difference between the percentage of CLA-positive MelanA-specific CTLs in the cultures from A*0201-positive patients and controls (P < 0.05).
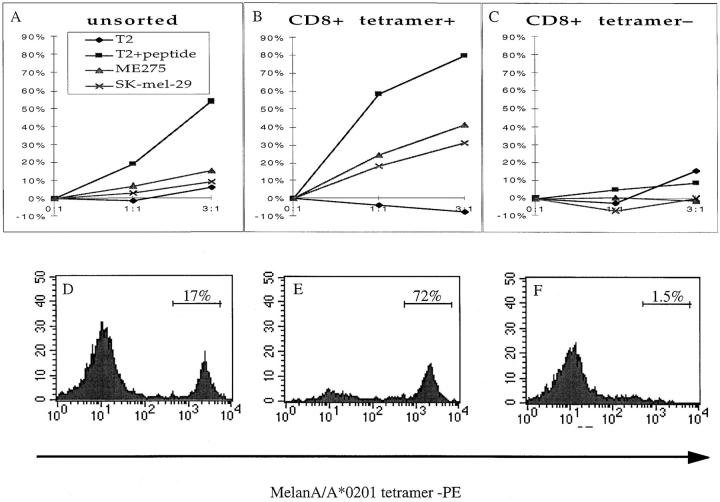
We examined whether the melanocyte-specific CTLs were capable of causing melanocytic destruction by sorting tetramer-positive cells from polyclonal lines generated by multiple rounds of peptide stimulation from one vitiligo patient, using FACS®, and then assessed the ability of the cells to lyse both peptide-pulsed target cells and A*0201-positive melanoma cells (Fig. 3, A–F). CD8+ A2–MelanA tetramer+ cells were able to lyse both peptide-pulsed targets and melanoma cells (Fig. 3, B and E), in contrast to CD8+ A2–MelanA tetramer− cells, which had no detectable cytolytic activity (Fig. 3, C and F). These data confirmed that tetramer-binding cells were able to recognize and lyse melanoma cells that present the epitope peptide through endogenous processing pathway.
Figure 3.
(A–C) Cytolytic activity of MelanA/A*0201-specific CTL lines derived from an A*0201-positive vitiligo patient either unsorted (A), enriched for CD8+ A2–MelanA tetramer+ cells (B) or enriched for CD8+ A2–MelanA tetramer− cells (C). The unsorted and CD8+-tetramer+ cells were both able to lyse peptide-pulsed targets, but only the CD8+ A2–MelanA tetramer+ population showed significant lysis of A*0201-matched melanoma cells (ME 275 and SK-mel-29). (D–E) The corresponding percentages of CD8+ T cells staining with the A2–MelanA tetramer are shown: the unsorted population contained 17% antigen-specific cells (D); the CD8+ A2–MelanA tetramer+ population contained 72% antigen-specific cells (E); and the CD8+ A2–MelanA tetramer− population contained 1.5% antigen-specific cells.
If melanocyte-specific CTLs are important in causing the tissue damage observed in vitiligo, there may be an association between the frequency of CTLs and the extent of disease. We measured the area involved by vitiligo in all patients and, in those with <20% of skin surface involved by vitiligo, there were significantly less MelanA-specific CTLs identified directly ex vivo (P < 0.05). Furthermore, in the two vitiligo patients with the lowest frequencies of MelanA-specific CTLs, the vitiligo had been stable for many years and was not progressing.
Using HLA–peptide tetrameric complexes, we have shown an association between high frequencies of skin-homing melanocyte-specific CTLs and autoimmune vitiligo. Furthermore, the frequency of such CTLs correlates with the extent of disease. Therefore, in addition to the previously documented melanocyte-specific autoantibodies (1–5), these data are consistent with a role for skin-homing melanocyte-specific CTLs in vitiligo. The ability of such cells to home to sites of potential tissue damage may be a means to control peripheral tolerance in vivo. This raises the possibility that CD8-dependent cell-mediated immunity may be an important generalized effector mechanism in autoimmune conditions and therefore a potential target for therapeutic intervention.
Acknowledgments
We are grateful to the patients of the Vitiligo Society for blood samples.
This work was supported by the Medical Research Council, UK, and the Cancer Research Campaign.
Abbreviation used in this paper
- CLA
cutaneous lymphocyte-associated antigen
References
- 1.Song YH, Connor E, Li Y, Zorovich B, Balducci P, Maclaren N. The role of tyrosinase in autoimmune vitiligo. Lancet. 1994;344:1049–1052. doi: 10.1016/s0140-6736(94)91709-4. [DOI] [PubMed] [Google Scholar]
- 2.Kemp EH, Gawkrodger DJ, Watson PF, Weetman AP. Immunoprecipitation of melanogenic enzyme autoantigens with vitiligo sera: evidence for cross-reactive autoantibodies to tyrosinase and tyrosinase-related protein-2 (TRP-2) Clin Exp Immunol. 1997;109:495–500. doi: 10.1046/j.1365-2249.1997.4781381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara I, Takechi Y, Houghton AN. Implicating a role for immune recognition of self in tumor rejection: passive immunization against the brown locus protein. J Exp Med. 1995;182:1609–1614. doi: 10.1084/jem.182.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merimsky O, Shoenfeld Y, Baharav E, Altomonte M, Chaitchik S, Maio M, Ferrone S, Fishman P. Melanoma-associated hypopigmentation: where are the antibodies? . Am J Clin Oncol. 1996;19:613–618. doi: 10.1097/00000421-199612000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Naughton GK, Eisinger M, Bystryn JC. Antibodies to normal human melanocytes in vitiligo. J Exp Med. 1983;158:246–251. doi: 10.1084/jem.158.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulie PG, Brichard V, Van Pel A, Wolfel T, Schneider J, Traversari C, Mattei S, De Plaen E, Lurquin C, Szikora J-P, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins P, Rivoltini L, Yannelli J, Appella E, Rosenberg S. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2–restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordlund JJ, Kirkwood JM, Forget BM, Milton G, Albert DM, Lerner AB. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689–696. doi: 10.1016/s0190-9622(83)70182-9. [DOI] [PubMed] [Google Scholar]
- 10.Duhra P, Ilchyshyn A. Prolonged survival in metastatic malignant melanoma associated with vitiligo. Clin Exp Dermatol. 1991;16:303–305. doi: 10.1111/j.1365-2230.1991.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 11.Badri AM, Todd PM, Garioch JJ, Gudgeon JE, Stewart DG, Goudie RB. An immunohistological study of cutaneous lymphocytes in vitiligo. J Pathol. 1993;170:149–155. doi: 10.1002/path.1711700209. [DOI] [PubMed] [Google Scholar]
- 12.Le Poole I, van den Wijngaard R, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol. 1996;148:1219–1228. [PMC free article] [PubMed] [Google Scholar]
- 13.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 14.Borges E, Pendl G, Eytner R, Steegmaier M, Zollner O, Vestweber D. The binding of T cell-expressed P-selectin glycoprotein ligand-1 to E- and P-selectin is differentially regulated. J Biol Chem. 1997;272:28786–28792. doi: 10.1074/jbc.272.45.28786. [DOI] [PubMed] [Google Scholar]
- 15.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 16.Erf GF, Trejo SA, Smyth JJ. T cells in regenerating feathers of Smyth line chickens with vitiligo. Clin Immunol Immunopathol. 1995;76:120–126. doi: 10.1006/clin.1995.1105. [DOI] [PubMed] [Google Scholar]
- 17.Altman JD, Moss P, Goulder P, Barouch DH, McHeyzer WM, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 18.Wolfel T, Van Pel A, Brichard V, Schneider J, Seliger B, Meyer zum Buschenfelde KH, Boon T. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 19.Cox A, Skipper J, Chen Y, Henderson R, Darrow T, Shabanowitz J, Engelhard V, Hunt D, Slingluff C. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 20.Skipper JC, Hendrickson RC, Gulden PH, Brichard V, Van Pel A, Chen Y, Shabanowitz J, Wolfel T, Slingluff CL, Jr, Boon T, et al. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valmori D, Fonteneau JF, Lizana CM, Gervois N, Lienard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini JC, Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- 22.Parker KC, Bednarek MA, Hull LK, Utz U, Cunningham B, Zweerink HJ, Biddison WE, Coligan JE. Sequence motifs important for peptide binding to the human MHC class I molecule, HLA-A2. J Immunol. 1992;149:3580–3587. [PubMed] [Google Scholar]
- 23.Lalvani A, Dong T, Ogg G, Patham AA, Newell H, Hill AV, McMichael AJ, Rowland JS. Optimization of a peptide-based protocol employing IL-7 for in vitro restimulation of human cytotoxic T lymphocyte precursors. J Immunol Methods. 1997;210:65–77. doi: 10.1016/s0022-1759(97)00177-4. [DOI] [PubMed] [Google Scholar]
- 24.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao Y, Rowland JS, Cerundolo V, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 25.Murali KK, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 26.Dunbar PR, Ogg GS, Chen J, Rust N, van der Bruggen P, Cerundolo V. Direct isolation, phenotyping and cloning of low frequency antigen-specific CTL from peripheral blood. Curr Biol. 1998;8:413–416. doi: 10.1016/s0960-9822(98)70161-7. [DOI] [PubMed] [Google Scholar]
- 27.Visseren M, van Elsas A, van der Voort E, Ressing M, Kast M, Schrier P, Melief C. CTL specific for the tyrosinase can be induced from healthy donor blood to lyse melanoma cells. J Immunol. 1995;154:3991–3998. [PubMed] [Google Scholar]
- 28.van Elsas A, van der Burg S, van der Minne C, Borghi M, Mourer JS, Melief CJ, Schrier PI. Peptide-pulsed dendritic cells induce tumoricidal cytotoxic T lymphocytes from healthy donors against stably HLA-A*0201-binding peptides from the Melan-A/MART-1 self antigen. Eur J Immunol. 1996;26:1683–1689. doi: 10.1002/eji.1830260803. [DOI] [PubMed] [Google Scholar]