Abstract
Members of the tumor necrosis factor (TNF) family induce pleiotropic biological responses, including cell growth, differentiation, and even death. Here we describe a novel member of the TNF family designated APRIL (for a proliferation-inducing ligand). Although transcripts of APRIL are of low abundance in normal tissues, high levels of mRNA are detected in transformed cell lines, and in human cancers of colon, thyroid, and lymphoid tissues in vivo. The addition of recombinant APRIL to various tumor cells stimulates their proliferation. Moreover, APRIL-transfected NIH-3T3 cells show an increased rate of tumor growth in nude mice compared with the parental cell line. These findings suggest that APRIL may be implicated in the regulation of tumor cell growth.
Keywords: tumor necrosis factor, tumorigenesis, cell survival, ligand, protein
Members of the TNF cytokine family are critically involved in the regulation of infections, inflammation, autoimmune diseases, and tissue homeostasis (1). These ligands can act in a membrane-bound form or as proteolytically processed, soluble cytokines in an autocrine, paracrine, or endocrine manner (1). Binding to their respective receptors leads to the triggering of diverse signaling pathways, including the activation of caspases, the translocation of nuclear factor-κB (NF-κB), or the activation of mitogen-activated kinases such as c-Jun NH2-terminal kinase (JNK) or extracellular signal–regulatory kinase (ERK). Thus, TNF-related ligands can lead to either apoptosis, differentiation, or proliferation (1). To date, 13 members of the TNF cytokine family have been described: TNF-α, lymphotoxin (LT)α, LTβ, CD40L, CD30L, CD27L, 4-1BBL, OX40L, FasL, TRAIL/APO-2L (2, 3), TRANCE/ RANKL (4, 5), LIGHT (6), and TWEAK (7).
The ligand members are type II membrane molecules. Their extracellular domains have β jelly roll topography (8), and are important in ligand trimerization. Intrinsic to oligomerization is the formation of the receptor binding site at the junction between neighboring subunits, creating a multivalent ligand. The binding of the ligands to their respective receptors induces oligomerization, initiating downstream signaling events.
Here we characterize the structural and functional properties of a new ligand of the TNF cytokine family. The new ligand, termed APRIL (for a proliferation-inducing ligand), is primarily expressed in tumor tissues and can accelerate the growth of transformed cells in vitro and in vivo.
Materials and Methods
Materials.
The anti-FLAG M2 mAb and the anti-FLAG M2 antibody coupled to agarose were purchased from Eastman Kodak Co. (Rochester, NY). Protein A–Sepharose and CNBr-activated Sepharose were purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). Cell culture reagents were obtained from Life Sciences (Basel, Switzerland). Human TWEAK cDNA was provided by Dr. J. Browning (Biogen, Inc., Cambridge, MA). FLAG-tagged soluble human TWEAK (residues 141–284) and FLAG-tagged soluble FasL were produced in 293 cells as described (9, 10).
Cells.
Murine B lymphoma A20 and human embryonic kidney cells 293 T (11) were maintained in DMEM containing 10% heat-inactivated FCS. The human T lymphoblastoma Jurkat cells, colon adenocarcinoma HT-29 cells, Raji Burkitt lymphoma, and human MCF-7 cells were grown in RPMI supplemented with 10% FCS. All media contained antibiotics (penicillin and streptomycin at 5 μg/ml each and neomycin at 10 μg/ml). Other cell lines referred to in this paper are deposited in and described by the American Type Culture Collection (Rockville, MD).
Northern Blot Analysis and In Situ Hybridization.
Northern blot analysis was carried out using the following membranes: human multiple tissue Northern blots I and II (7760-1 and 7759-1; Clontech, Palo Alto, CA), human cancer cell line MTN blot (7757-1; Clontech), and human tumor panel blot V (D3500-01; Invitrogen Corp., Carlsbad, CA). The membranes were incubated in ExpressHyb hybridization solution (8015-1; Clontech) for at least 1 h at 62°C. The random-primed cDNA probe (Boehringer Mannheim Corp., Indianapolis, IN) was synthesized using cDNA corresponding to the extracellular domain of APRIL as template. The heat-denatured cDNA probe was added at 1.5 × 106 cpm/ml in fresh ExpressHyb. The membrane was hybridized 12– 24 h at 62°C, washed three times in 2× SSC containing 0.05% SDS, and exposed at −70°C to x-ray films.
For in situ hybridization, cryostat sections (6–8 μm) of primary colon carcinomas (three patients) were air dried, fixed in 4% (wt/ vol) paraformaldehyde in PBS, and used immediately for immunofluorescence or stored in 70% ethanol at 4°C before in situ hybridization. In situ hybridization and immunofluorescence were performed as reported previously (12). After in situ hybridization, slides were directly exposed to x-ray films. Specificity controls included the systematic use of sense cRNA probes in each experiment.
Characterization of APRIL cDNA and Expression of Recombinant Soluble APRIL.
The full-length APRIL gene (sequence data available from EMBL/GenBank/DDBJ under accession no. AF046888) was contained in the expressed sequence tag (EST) clones AA292304 and AA292358. EST clone AA292304 was used to amplify the coding region of APRIL using a specific 5′ forward primer flanked by an EcoRI site (5′-CCAGCCTCATCTCCTTTCTTGC-3′) and a specific 3′ reverse primer flanked by an XbaI site (5′-TCACAGTTTCACAAACCCCAGG-3′). The amplified fragment was cut with EcoRI/XbaI and cloned into a modified version of pCRIII (Invitrogen Corp.), in frame with an NH2-terminal FLAG peptide (9). The soluble form of APRIL (sAPRIL) was generated using the two primers 5′-AAACAGAAGAAGCAGCACTCTG-3′ and 5′-TCACAGTTTCACAAACCCCAGG-3′ containing a PstI and XbaI site, respectively, and subsequently cloned into a modified pCRIII vector containing both a hemagglutinin signal for protein secretion in eukaryotic cells and an NH2-terminal FLAG epitope (9). Purification of FLAG-tagged APRIL was affinity-purified on anti-FLAG M2 antibody coupled to agarose.
Proliferation Assays.
The proliferation of cells was determined by incubating 5 × 104 cells/well in 100 μl medium with the indicated concentrations of recombinant sAPRIL, sTWEAK, and sFasL using the Celltiter 96 AQ proliferation assay (Promega Corp., Madison, WI) after 24 h, following the manufacturer's instructions. Alternatively, cells were pulsed for 4 h with [3H]thymidine (0.5 μCi/well), exposed to three cycles of freezing and thawing, and harvested. [3H]Thymidine incorporation was monitored by liquid scintillation counting. For the immunodepletion of FLAG-APRIL, anti-FLAG antibodies coupled to agarose (Eastman Kodak Co.) were used.
Cell Lines Stably Expressing APRIL.
The full-length FLAG-tagged APRIL containing pCRIII expression vector was transfected into NIH-3T3 cells using the calcium phosphate method of transfection. 48 h after transfection, cells were seeded at 104 cells/well in flat-bottomed 96-well plates under selection with 800 μg/ml G418 (Sigma Chemical Co., St. Louis, MO). Cell extracts of stable clones were electrophoretically separated by SDS-PAGE under reducing conditions and subsequently transferred to nitrocellulose. Immunoblots of FLAG-tagged APRIL were probed using 5 μg/ml of anti-FLAG M2 mAb and the ECL system (Amersham Pharmacia Biotech).
Tumor Growth in Mice.
NIH-3T3 fibroblasts (American Type Culture Collection) and the various transfectants (105 cells) were suspended in 50 μl PBS and injected subcutaneously into the flank region of BALB/c nude mice (Harlan Nederland, Zeist, The Netherlands). Mice were sex- and age-matched.
Results and Discussion
APRIL Is a Novel Ligand of the TNF Family.
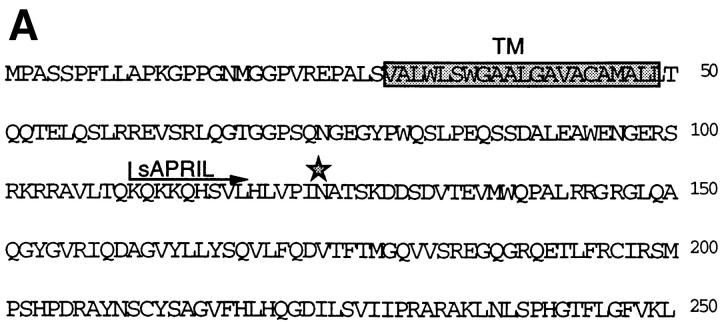
All TNF ligand/receptor family members characterized to date are biologically important. Therefore, we screened public databases using an improved profile search (13) based on an optimal alignment of all currently known TNF ligand family members. Several candidate clones were found coding for a unique, novel TNF-α–related ligand which we termed APRIL (for a proliferation-inducing ligand). Two of these cDNA clones (AA292358 and AA292304) contained full-length sequences (1.5 and 1.7 kb, respectively) encoding a protein of 250 amino acids, with a predicted cytoplasmic domain of 28 amino acids, a hydrophobic transmembrane region, and an extracellular domain of 201 amino acids (Fig. 1 A). The absence of a signal peptide suggested that APRIL was a type II membrane protein which is typical of the members of the TNF ligand family. The single N-linked glycosylation site (N124) predicted for this protein lies within the first of several β strands which are folded into an antiparallel β sandwich structure (14). The sequence of the extracellular domain of APRIL showed highest homology with FasL (21% amino acid identity), TNF-α (20%), and LTβ (18%), followed by TRAIL, TWEAK, and TRANCE (15%) (Fig. 1 B).
Figure 1.
(A) Predicted amino acid sequence of human APRIL. The predicted transmembrane region (TM, boxed), the potential N-linked glycosylation site (*), and the NH2 terminus of the recombinant sAPRIL are indicated. (B) Comparison of the extracellular protein sequence of APRIL and some members of the TNF ligand family. Identical and homologous residues are represented in black and shaded boxes, respectively. TNFa, TNF-α; LTa, LTα.
Expression of APRIL mRNA in Tumors.
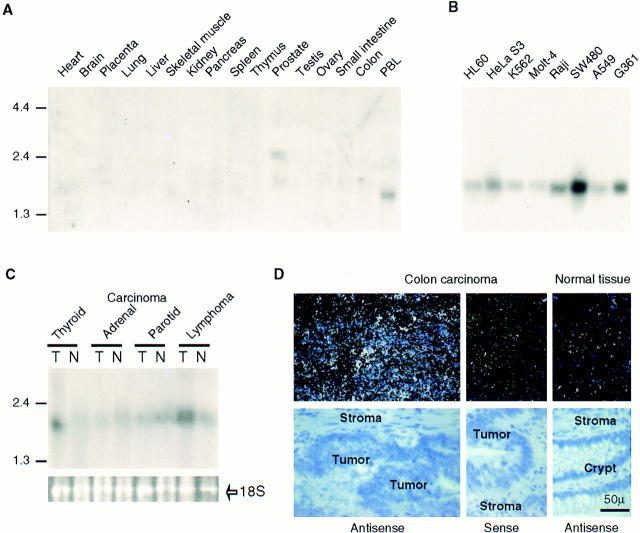
Northern blot analysis of APRIL revealed that the expression of APRIL was weak and restricted only to a few tissues (Fig. 2 A). Two transcripts of 2.1 and 2.4 kb were found in the prostate, whereas a shorter 1.8-kb transcript was found in PBLs. A longer exposure time of the Northern blot revealed that the 2.1-kb APRIL mRNA was also present in colon, spleen, and pancreas (data not shown). This restricted distribution of the APRIL mRNA was consistent with the origin of cDNA clones currently available in the EST database. Of the 23 clones identified, only two were derived from normal tissues (pregnant uterus and pancreatic islet cells). Remarkably, the remainder of the EST clones (21 clones, 91%) were present in cDNA libraries generated from tumors or tumor-derived cell lines (ovary tumor, 11; prostate tumor, 3; Gessler Wilms tumor, 1; colon carcinoma, 1; endometrial tumor, 1; parathyroid tumors, 1; pancreas tumor, 1; T cell lymphoma, 1; LNCAP adenocarcinoma–derived cell line, 1). This is in contrast to TNF-α, where only 16% of EST clones were tumor-derived. This prompted us to test transformed cell lines for the expression of APRIL mRNA (Fig. 2 B), and indeed, all tumor cell lines strongly expressed the 2.1-kb transcript of APRIL. The highest APRIL-specific signals were detected in the colorectal adenocarcinoma SW480, the Burkitt's lymphoma Raji, and the melanoma G361 cell lines.
Figure 2.
Expression of APRIL. (A) Northern blots (2 μg polyA+ RNA/lane) of various human tissues were probed with APRIL cDNA. (B) APRIL mRNA expression in various tumor cell lines: promyelocytic leukemia HL60; HeLa cell S3; chronic myelogenous leukemia K562; lymphoblastic leukemia Molt-4; Raji Burkitt's lymphoma; colorectal adenocarcinoma SW480; lung carcinoma A459; melanoma and G361. (C) APRIL mRNA expression in four different human tumors (T) and normal tissues (N). The 18S rRNA band shows equal loading. (D) APRIL mRNA expression in primary colon carcinoma. In situ hybridization reveals abundant APRIL message in human colon carcinoma. Colon tumor tissue sections and adjacent normal colon tissue were hybridized to antisense APRIL 35S-labeled cRNA, and as control, colon tumor tissue sections were also hybridized to sense APRIL 35S cRNA (negative control). Top, Dark field micrographs; bottom, the corresponding light field micrographs.
To corroborate this finding, we measured APRIL mRNA expression levels in several tumors and compared them to normal tissues. APRIL mRNA was elevated in thyroid carcinoma and in lymphoma, whereas in the corresponding normal tissues, hybridization signals were either weak or absent (Fig. 2 C). In the two other tumors analyzed by Northern blots (adrenal and parotid carcinoma), APRIL mRNA was not increased. By in situ hybridization, highly increased levels of APRIL mRNA were also detected in human colon adenocarcinomas of three different patients compared with normal colon tissue (Fig. 2 D).
APRIL Enhances Tumor Cell Proliferation.
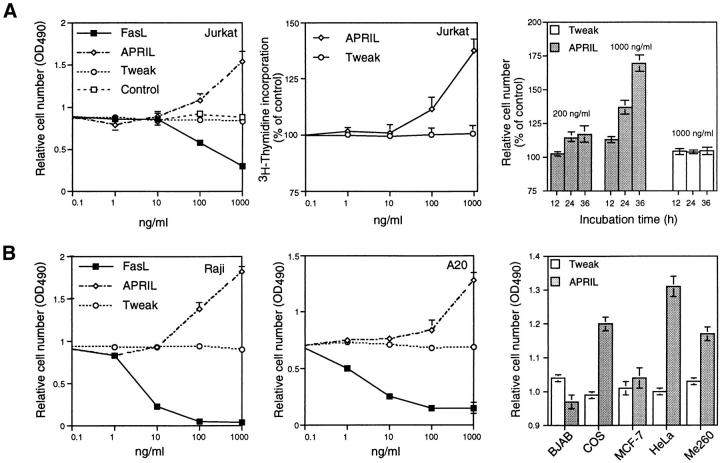
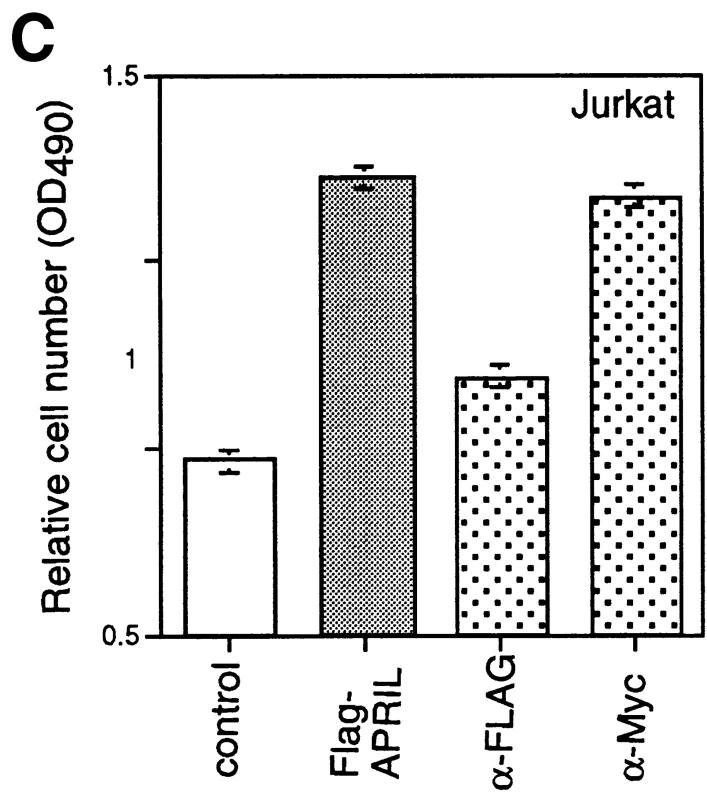
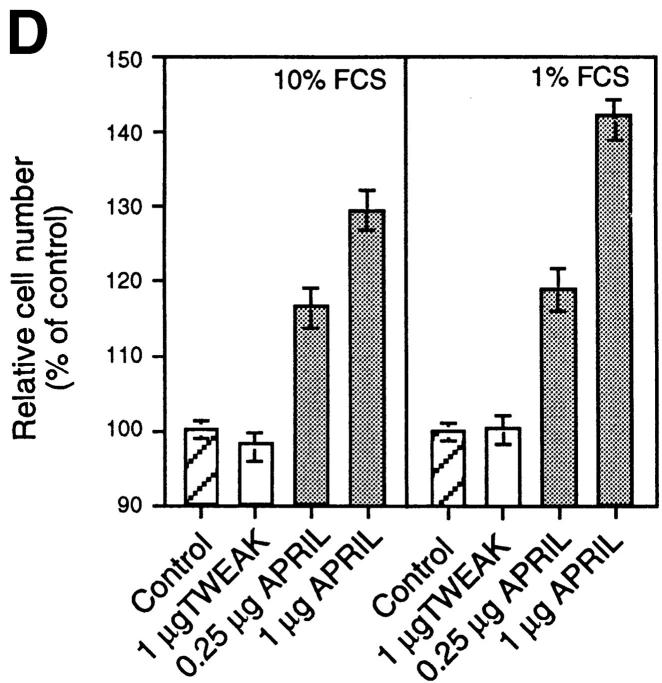
To explore the possible activities of APRIL, we expressed a FLAG-tagged form of the soluble extracellular domain of APRIL (sAPRIL) encompassing amino acids 110–250 in 293 cells. The widespread expression of APRIL in tumor cells and tissues suggested to us that APRIL may be associated with tumor growth, and we therefore incubated various tumor cell lines with purified recombinant sAPRIL. An increase in proliferation of the Jurkat T lymphoma cells in the presence of APRIL was observed in a dose-dependent manner as detected by an increase in the number (∼50%) of viable cells 24 h after ligand addition (Fig. 3 A). As expected, the addition of identically produced and purified FasL to Jurkat cells decreased the number of viable cells, whereas TWEAK had no effect. The increased cell number correlated with augmented (40%) [3H]thymidine incorporation in APRIL-treated cells (Fig. 3 A). Differences in growth rates of Jurkat cells exposed to APRIL and TWEAK were already apparent after 12 h of incubation, and after 36 h the number of Jurkat cells had almost doubled (Fig. 3 A, right). Immunodepletion of FLAG-tagged APRIL–containing medium by anti-FLAG antibodies, but not anti-myc antibodies, reduced the proliferative effect (Fig. 3 C), indicating that the stimulation of proliferation was specific and due to the presence of APRIL. Increased rates in proliferation were also seen in some B lymphomas (human Raji cells, mouse A20 cells, but not human BJAB cells) and in cell lines of epithelial origin, such as COS and HeLa, as well as melanomas (Fig. 3 B). The breast carcinoma cell MCF-7 did not respond. The effect on Jurkat cells was even more intense when the concentration of FCS was reduced from 10 to 1% (Fig. 3 D).
Figure 3.
APRIL stimulates cell growth. (A) Dose-dependent stimulation of proliferation of Jurkat cells (human leukemic T cells), determined 24 h after the addition of sAPRIL. Controls include cells treated with FasL, TWEAK, and no ligand (Control). Left, Number of viable cells; middle, [3H]thymidine incorporation; right, kinetic analysis of the effect of APRIL on Jurkat cells. The concentrations of ligands are indicated. (B) Effect of APRIL on the proliferation rate of Raji (human Burkitt lymphoma), A20 (mouse B lymphoma), BJAB (human B lymphoma), COS (SV40-transformed monkey kidney cells), MCF-7 (human breast adenocarcinoma), HeLa (human embryonic lung) cell, and ME260 (human melanoma). (C) Influence of immunodepletion of FLAG-tagged APRIL on tumor cell growth. The proliferative effect of FLAG-tagged APRIL is neutralized by Sepharose-bound anti-FLAG antibodies, but not by anti-myc antibodies. (D) Influence of FCS concentration on APRIL-induced proliferation of Jurkat cells. Data are the means ± SEM of triplicate determinations.
Tumor Cells Expressing APRIL Display an Increased Growth Rate in Mice.
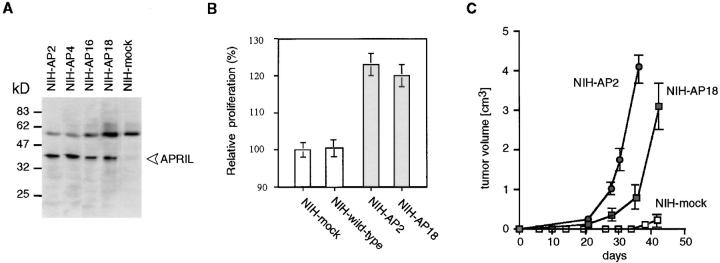
We next transfected NIH-3T3 cells with full-length human APRIL and obtained several APRIL-expressing clones (Fig. 4 A). Interestingly, APRIL transfectants proliferated faster than mock-transfected cells or wild-type cells (Fig. 4 B). Consequently, we asked the question whether the APRIL-transfected NIH-3T3 cells might also have a growth advantage in vivo. When wild-type or mock-transfected NIH-3T3 cells were injected into nude mice, small palpable tumors were observed after 5–6 wk. In contrast, the two clones of NIH-3T3 cells stably transfected with APRIL which were injected into nude mice both induced tumors after only 3–4 wk. After 6 wk, mice had to be killed due to the high tumor burden (Fig. 4 C).
Figure 4.
APRIL accelerates tumor growth. (A) Characterization of APRIL-transfected NIH-3T3 clones. FLAG-APRIL levels of the various clones were analyzed by Western blotting using an anti-FLAG antibody. Arrow, The APRIL protein; the high molecular weight protein is detected nonspecifically. (B) APRIL-expressing NIH-3T3 clones grow faster than mock-transfected clones. (C) Increased tumor growth of APRIL-expressing NIH-3T3 clones. NIH-3T3 cells (105 cells) and APRIL (NIH-AP, two different clones) transfectants (105 cells) were injected subcutaneously into nude mice, and tumor growth was monitored. Data are representative of three experiments with six mice per group.
Many of the TNF-related ligands can induce pleiotropic biological responses. Ligands trigger costimulatory signals in most cases, induce cell death, or lead to proliferation of primary cells. For instance, TNF-R2 triggering leads to proliferation of thymocytes (15), and the biological function of CD40-40L provides a clear example of costimulation (14). TNF-α was discovered as a cytokine with the capacity to induce tumor necrosis (16). Unlike these ligands, APRIL can stimulate the growth of tumor cell lines. APRIL also appears to be unique among the TNF family, as it is abundantly expressed in tumor cells. Collectively, these results suggest that the physiological function(s) of APRIL is distinct from other members of the TNF family. Preliminary results indicate that the APRIL gene is not linked to the TNF/LT locus in the MHC.
The mechanism by which APRIL increases cellular proliferation of transformed cells is currently unknown. APRIL does not appear to activate NF-κB or c-Jun NH2-terminal kinase, and the cell cycle analysis of APRIL-treated cells remains unperturbed (data not shown). Moreover, the histochemical analysis of tumors induced by APRIL-transfected NIH-3T3 cells did not reveal any apparent morphological differences compared with wild-type NIH-3T3 tumors, suggesting that APRIL is not angiogenic. Thus, it will be important to identify and characterize the receptor for APRIL (recombinant sAPRIL does not interact with Fas, LTβR, HVEM, TNF-R1, TNF-R2, GITR, TRAIL-R1, TRAIL-R2, or TRAIL-R3; data not shown).
The development of cancer is viewed as a multistep process, involving mutation and selection for cells with progressively increasing capacity for proliferation, survival, and invasion, even under conditions where the growth factor (blood) supply is limited. APRIL allows tumor cells to proliferate at a reasonable rate even in low serum conditions (see Fig. 3 D). Given that APRIL is strongly expressed in several types of tumors and that it stimulates cell proliferation in vitro and in vivo, it is possible that APRIL may play a role in tumorigenesis. Therefore, antagonistic antibodies to APRIL or the APRIL receptor may have a potential for cancer treatment.
Acknowledgments
We thank S. Hertig and G. Radlgruber for technical assistance, S. Belli for reading the manuscript, and Dr. Jeffrey Browning for helpful discussions.
This work was supported by grants from the Swiss National Science Foundation.
Footnotes
T. Kataoka, M. Schröter, K. Hofmann, and M. Irmler contributed equally to this work.
References
- 1.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 2.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 3.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 5.Wong R, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett F, Frankel W, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 6.Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu G, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 7.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 8.Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 9.Schneider P, Bodmer JL, Holler N, Mattmann C, Scuderi P, Terskikh A, Peitsch MC, Tschopp J. Characterization of Fas (Apo-1, CD95)-Fas ligand interaction. J Biol Chem. 1997;272:18827–18833. doi: 10.1074/jbc.272.30.18827. [DOI] [PubMed] [Google Scholar]
- 10.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, et al. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 12.French LE, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Muller C, Tschopp J. Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucher P, Karplus K, Moeri N, Hofmann K. A flexible search technique based on generalized profiles. Comput Chem. 1996;20:3–23. doi: 10.1016/s0097-8485(96)80003-9. [DOI] [PubMed] [Google Scholar]
- 14.Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor- human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 15.Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 16.Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]