Abstract
Interleukin (IL)-4, a crucial modulator of the immune system and an active antitumor agent, is also a potent inhibitor of angiogenesis. When incorporated at concentrations of 10 ng/ml or more into pellets implanted into the rat cornea or when delivered systemically to the mouse by intraperitoneal injection, IL-4 blocked the induction of corneal neovascularization by basic fibroblast growth factor. IL-4 as well as IL-13 inhibited the migration of cultured bovine or human microvascular cells, showing unusual dose–response curves that were sharply stimulatory at a concentration of 0.01 ng/ml but inhibitory over a wide range of higher concentrations. Recombinant cytokine from mouse and from human worked equally well in vitro on bovine and human endothelial cells and in vivo in the rat, showing no species specificity. IL-4 was secreted at inhibitory levels by activated murine T helper (TH0) cells and by a line of carcinoma cells whose tumorigenicity is known to be inhibited by IL-4. Its ability to cause media conditioned by these cells to be antiangiogenic suggested that the antiangiogenic activity of IL-4 may play a role in normal physiology and contribute significantly to its demonstrated antitumor activity.
Keywords: neovascularization, tumor angiogenesis, endothelial cell, migration, cornea assay
Interleukin (IL)-4 is a potent lymphokine able to modulate the activity of cells in all hematopoietic lineages (1). It is best known for its ability to support the TH2 arm of the T cell immune response and enable B cells to produce the IgE that plays a prominent role in the pathogenesis of allergic reactions and in resistance to parasitic infections (2, 3). The effects of IL-4, at least on cells of hematopoietic lineage, are species specific (4, 5) and result from the activation of a dimeric receptor consisting of a high affinity α subunit (IL-4Rα) that is specific for IL-4 and a second subunit (γc) that is also shared by receptors for IL-2, IL-7, IL-9, and IL-15 (6).
In contrast to its general stimulatory effects on lymphocytes, IL-4 inhibits the tumorigenicity of a variety of tumor cell lines, including those of lymphoid origin (7). Occasionally its effects may be direct in that the in vitro growth of the tumor cells themselves is also sensitive to IL-4 (8– 10). More typically, IL-4 acts through a host-dependent mechanism on IL-4–insensitive tumor cells. When such tumor cells are engineered to secrete IL-4 or are coinjected with IL-4–generating cells, they often attract an eosinophil-rich inflammatory infiltrate and, although some active immunity can develop (7), inhibition is eosinophil dependent (11, 12). However, there are other instances, usually when IL-4 is delivered systemically rather than directly to the tumor bed, where a reduction in tumor growth is seen in the absence of eosinophil infiltrates (13, 14), suggesting that IL-4 has alternate ways to curtail tumor growth.
All tumors must generate a brisk angiogenic response to support their progressive growth (15), and it is possible that IL-4 limits tumor growth in part by inhibiting angiogenesis. Two observations in the literature suggest that IL-4 might have such an inhibitory activity. First, tumor cells that secrete IL-4 can induce concomitant tumor resistance (16). For example, the growth of B16F10 melanoma cells engineered to secrete IL-4 at one site in a mouse can retard the growth of parental cells implanted at a distant site (17). In other systems, this ability to hold distant tumors in check has been shown to be due to the production by the first tumor of inhibitors of angiogenesis that accumulate in the circulation (18–20). A possible antiangiogenic role for IL-4 is also supported by the recent finding that vessel density in tumors resulting from the injection of C6 glioma was halved if the tumor cells were secreting IL-4 (21).
Although they lack the IL-4Rγc receptor subunit characteristic of lymphocytes, endothelial cells do express heterodimeric IL-4 receptors consisting of IL-4Rα and IL-13Rα. Both IL-4 and IL-13 act on endothelial cells via this receptor. Unlike the situation in cells cultured from large vessels (22–24), IL-4 does not induce vascular cell adhesion molecule 1 (VCAM-1)1 in the microvascular cells that give rise to new vessels during tumor angiogenesis (25–27), but such cells are sensitive to IL-4 as it can depress the expression of VCAM-1 in the microvessels of cardiac allografts (28) and stimulate capillary endothelial cell growth in vitro, at least over a narrow concentration range (29). Although the latter finding had suggested that IL-4 might be expected to induce angiogenesis in vivo, here we report that IL-4 is a potent inhibitor of angiogenesis, able to act both locally and systemically to block neovascularization. An analysis of media conditioned by TH0 lymphocytes and by cells whose tumorigenicity has been curtailed by overexpression of IL-4 suggests that the inhibition of angiogenesis by IL-4 could play a role in normal physiology and contribute significantly to its long-recognized antitumor activity.
Materials and Methods
Reagents.
Human recombinant basic fibroblast growth factor (bFGF), murine recombinant IL-4 (muIL-4), and goat anti– human IL-4 were from R & D Systems (Minneapolis, MN). Human recombinant IL-4 (huIL-4, specific activity 5 × 106 U/mg) was from Peprotech Inc. (Rocky Hill, NJ), as was human IL-13. Additional muIL-4 was purchased from Sigma Chemical Corp. (St. Louis, MO). Neutralizing rat mAb 11B11 (30) was a gift from Millennium Pharmaceuticals (Cambridge, MA) and was used as an ascites fluid. The muIL-4 used for systemic treatment of mice was generously supplied by Schering Plough Research Institute (Kenilworth, NJ). It had a specific activity of 2.24 × 109 U/ mg and was >99% pure as judged by silver stained SDS-PAGE reducing gels.
Conditioned Media.
Mouse mammary adenocarcinoma line K485 (31) and derivatives transfected with pSV7Neo (F1-1) or with pLT.IL-4 and pSV7Neo (D2-B1, E2A5, and E2A6; all described in reference 32) were grown in DME supplemented with 10% fetal bovine serum (FBS) and 2 mM glutamine. Serum-free conditioned media were collected as previously described (33), concentrated using a membrane with a 3-kD cut off, and then the protein was assayed with a Bio-Rad kit (Bio-Rad Laboratories, Hercules, CA).
TH0 supernatants were generated from short-term spleen cell cultures derived from BALB/c congenic αβ T cell receptor transgenic mice (D011.10) in which >85% of the CD4 T cells are specific for ovalbumin. Erythrocyte-free splenic cells (4 × 106/ml) from 8–10-wk-old mice were cultured with 18 μM ovalbumin in 24-well culture plates in Click's media (Irvine Scientific, Santa Ana, CA) supplemented with 5 × 105 2-mercaptoethanol, 3 mM glutamine, and 1% Nutridoma (a serum supplement from Boehringer Mannheim Corp., Indianapolis, IN). After 72 h of culture at 37°C in 10% CO2, supernatants were pooled, sterile filtered, and assayed for IL-4 and for angiogenic activity. All supernatants were dialyzed against PBS before use in angiogenesis assays as undialyzed Click's medium was stimulatory. Culture supernatants were assayed for IL-4 and IFN-γ using commercial ELISA kits (Endogen, Woburn, MA) and data analyzed with SoftMax Pro software (Molecular Devices, Palo Alto, CA). Test samples were diluted to fall within a standard curve containing five to seven dilution points on each assay plate.
Endothelial Cell Migration.
Human dermal microvascular endothelial cells, (HMVEC; Clonetics, San Diego, CA) were grown in endothelial basal medium supplemented with amphotericin and gentamycin sulfate, each at 50 μg/ml, 0.1 μg/ml human epidermal growth factor, 0.01 μg/ml hydrocortisone, 120 μg/ml bovine brain extract, and 5% FBS and, unless stated otherwise were used at passage <10 after starvation overnight in the same media without serum containing 0.1% BSA. Bovine adrenal capillary endothelial cells, BACE (a gift from Judah Folkman, Harvard University) were grown in DME supplemented with 10% donor calf serum, 2 mM glutamine, and endothelial cell mitogen at 50 μg/ml and used at passage 15 after starving overnight in serum-free media containing 0.1% BSA. Cells were harvested, suspended in DME with 0.1% BSA and plated at 1.75 × 104 cells/well on the lower surface of a gelatinized membrane with 5-μm pores in a modified Boyden chamber (Neuroprobe, Cabin John, MD). After attachment, chambers were inverted, test material added to the top of the well and chambers incubated 3–4 h at 37°C to allow migration. Membranes were recovered, fixed, stained, and the number of cells migrated to the top of the filter in 10 high powered fields counted. All samples were tested in quadruplicate. Conditioned media from tumor cells were used at 20 μg/ml, bFGF at 10 ng/ml, and anti–IL-4 at 10–20 μg/ml.
Endothelial Cell Proliferation.
HMVEC were plated into 96-well plates at 2.5 × 103 cells/well in Clonetics EBM containing 2% FBS and gentamycin and allowed to adhere for 4–6 h. Test substances were then added, cells incubated for 72 h, and growth measured using Cell Titer 96 (Promega Corp., Madison, WI).
Corneal Assays.
Neovascularization in the rat cornea was assayed as previously described (34). Hydron pellets containing, where indicated, bFGF at 100 ng/ml, murine IL-4 at 100 ng/ml, human IL-4 at 0.1–100 ng/ml, and/or anti-murine IL-4 at 40 μg/ml were implanted into the avascular cornea of the rat. 7 d later vessels were filled with colloidal carbon, the corneas were excised, and the growth of new vessels was assessed. Vigorous ingrowth from the limbus towards the pellet was considered a positive response.
To measure systemic effects of IL-4 in the mouse, five BALB/c mice (Jackson Laboratories, Bar Harbor, ME) were given intraperitoneal injections twice a day to give a dose of 15 μg/d/animal of murine recombinant IL-4. This dose was chosen in consultation with Dr. Bob Coffman (DNAX, Palo Alto, CA) to be saturating but not yet toxic. Five mice were treated similarly with vehicle saline only. On the second day, hydron/sucralfate pellets containing bFGF at 50 ng/pellet were implanted into their corneas as described (35), and vessels were observed using a slit lamp on days 3 and 5 after implantation.
Results
Inhibition of Neovascularization by Local and Systemic IL-4.
When tested in the classic rat cornea assay, locally released IL-4 prevented vessels from sprouting from the surrounding vascular limbus into the normally avascular cornea in response to the inducer basic fibroblast growth factor (bFGF; Fig. 1; Table 1). Both murine and human IL-4 were active in this rodent assay and inhibition was dose dependent and sensitive to a mAb against muIL-4, 11B1, which is known to neutralize other activities of IL-4 (36). As concentrations of IL-4 were reduced, inhibitory activity fell off and at the lowest concentration of 0.1 ng/ml IL-4 by itself displayed a weak ability to induce neovascularization. Simple diffusion calculations indicate that the concentration of a compound that actually reaches the vascular limbus in such cornea assays is reduced by 10–50-fold suggesting that the stimulatory concentration of IL-4 at the endothelial cell is <0.01 ng/ml.
Figure 1.
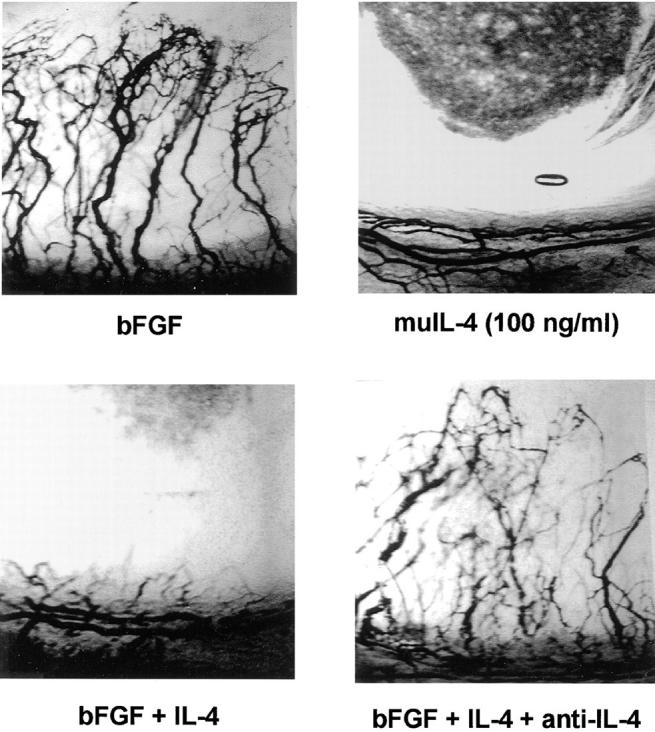
Local IL-4 blocks neovascularization in vivo in the rat cornea. Hydron pellets containing bFGF at 100 ng/ml, recombinant murine IL-4 at 100 ng/ml or the combination with or without anti–IL-4 antibody at 40 μg/ml were implanted into the avascular cornea of the rat. 7 d later vessels were filled with colloidal carbon and corneas photographed. Note the vigorous angiogenic response to bFGF and its inhibition by IL-4.
Table 1.
Inhibition of Corneal Neovascularization by Local IL-4 in the Rat
| Test sample | Concentration | Anti-murine IL-4 | Positive corneas/ total implanted | |||||
|---|---|---|---|---|---|---|---|---|
| Alone | + bFGF | |||||||
| ng/ml | ||||||||
| huIL-4 | 0.1 | − | 3*/3 | 3/3 | ||||
| 1.0 | − | 1*/3 | 3*/5 | |||||
| 10.0 | − | 0/3 | 0/3 | |||||
| muIL-4 | 100 | − | 0/2 | 0/3 | ||||
| 100 | + | nd | 2/3 | |||||
| Controls | − | − | nd | 4/4 | ||||
| − | + | 0/2 | nd | |||||
Pellets were formulated containing the indicated substances, including anti-murine IL-4 at 40 μg/ml and bFGF at 10 μg/ml, and implanted into the avascular cornea of the rat. Vigorous ingrowth of new vessels after seven days was scored as a positive response. nd = not done.
Positive responses that were weak with only a few vessels growing in from the limbus.
Systemic IL-4 also had an antiangiogenic effect. When mice received intraperitoneal injections of muIL-4, they became unable to mount a corneal angiogenic response to an implant containing an inducing concentration of bFGF (Table 2).
Table 2.
Inhibition of Corneal Neovascularization by Systemic IL-4 in the Mouse
| Systemic treatment | Positive corneas/total implanted | |||||
|---|---|---|---|---|---|---|
| Day 4 | Day 5 | Day 6 | ||||
| Saline | 3/5 | 5/5 | 5/5 | |||
| Murine IL-4 | 0/5 | 0/5 | 0/5 | |||
Mice were treated systemically with saline or with 15 μg/d/animal of muIL-4, their corneas implanted with pellets containing 1 μg/ml bFGF, and the induction of corneal neovascularization scored by slit lamp examination on the indicated days after implant. Vigorous growth of vessels into the pellet was scored as a positive response.
Effects of IL-4 on Cultured Endothelial Cells.
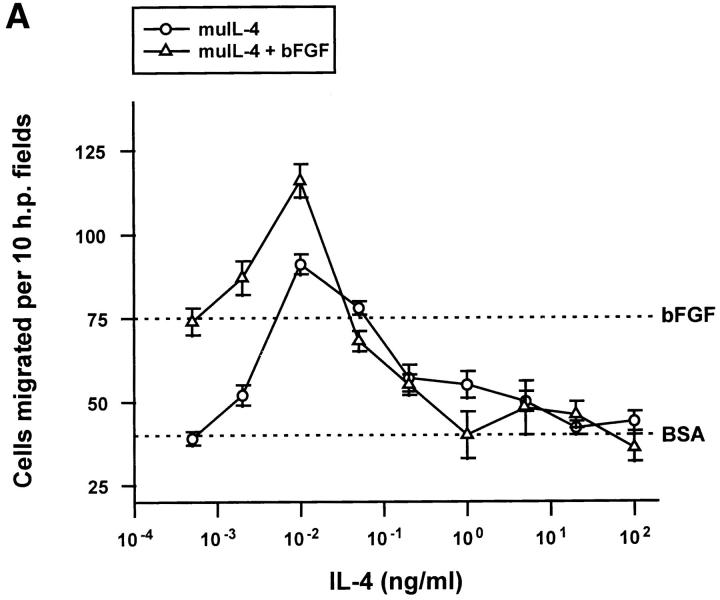
To determine if the antiangiogenic effect of IL-4 was due to a direct interaction of the cytokine with endothelial cells, its effects on cultured cells were tested. muIL-4 inhibited the migration of bovine adrenal capillary endothelial cells towards bFGF at concentrations of 1 ng/ml and greater (Fig. 2 A). huIL-4 gave an identical dose–response curve using these cells (data not shown). When their receptor message levels were measured by semiquantitative RT-PCR using primers described in references 23 and 37, human capillary endothelial cells expressed IL-4Rα and IL-13Rα subunits. These cells did not express the IL-4Rγc, the receptor subunit thought to be involved in the species-specific action of IL-4 on lymphocytes (6). The message levels of the receptors were not altered by treatment of the cells with IL-4 or with bFGF.
Figure 2.
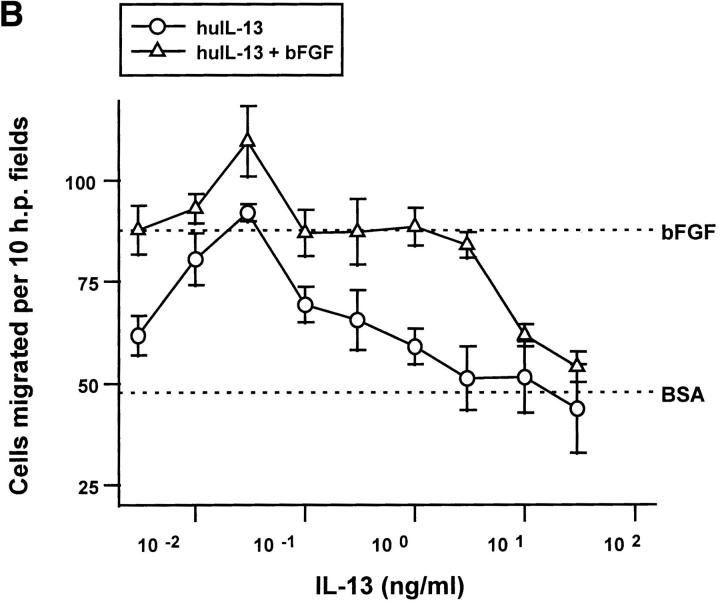
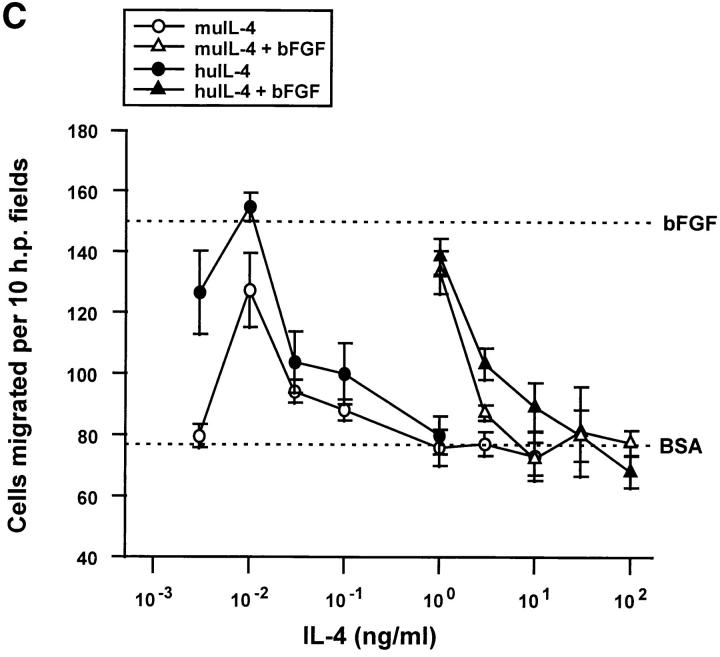
The biphasic, serum-dependent effect of recombinant IL-4 on the migration of capillary endothelial cells. (A) Serum-starved bovine adrenal capillary endothelial cells were allowed to migrate towards murine IL-4 in the absence (circles) or in the presence (triangles) of an inducing concentration of bFGF. Identical results were obtained using recombinant human IL-4. (B) IL-13 tested as was IL-4 in A. (C) Serum-starved human dermal capillary endothelial cells were allowed to migrate towards murine IL-4 (open symbols) or towards human IL-4 (filled symbols) in the absence (circles) or presence (triangles) of bFGF. Note: In all graphs, dotted lines indicate migration seen towards vehicle (0.1% bovine serum albumin, BSA) or towards 10 ng/ml bFGF (bFGF); bars indicate SE.
huIL-13, the cytokine thought to activate the same dimeric receptor on endothelial cells as does IL-4 (38), was also inhibitory (Fig. 2 B) although a higher concentration was required. Differential effects of IL-4 and IL-13 on cells like endothelial cells that lack the γc subunit of the IL-4 receptor may be explained by overproduction of IL-13Rβ, a recently identified molecule that binds IL-13 with high affinity but fails to transduce signals and may sequester IL-13 (39). Both muIL-4 and huIL-4 also blocked the migration of human microvascular cells (Fig. 2 C), although these cells required a cytokine concentrations of >10 ng/ml to achieve complete inhibition. As has been seen before with other inhibitory cytokines (40), both IL-4 and IL-13 exhibited biphasic dose-response curves. Exceedingly low doses stimulated the migration of endothelial cells (Fig. 2, A–C). huIL-4 was also effective at blocking the migration of human microvascular endothelial cells towards 100 pg/ml of the inducer VEGF and did so with an ED50 similar to that with which it inhibited migration towards bFGF (data not shown).
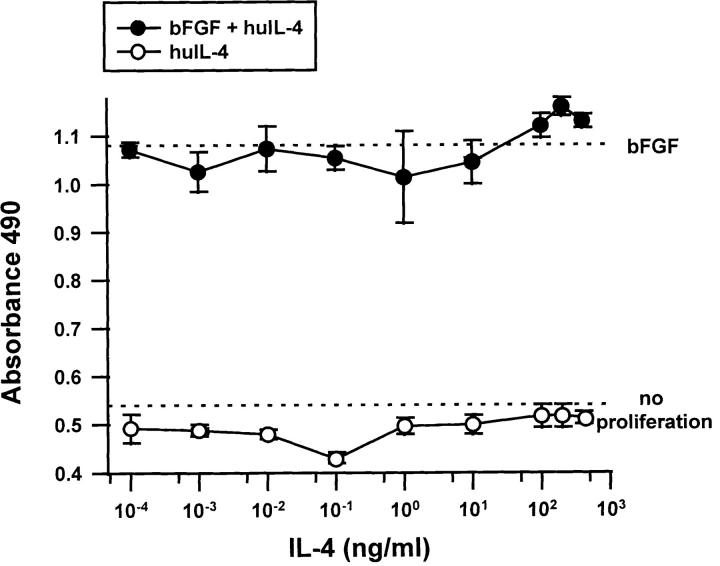
IL-4 did not have any inhibitory effect on the growth of endothelial cells. The proliferation of human dermal capillary endothelial cells (Fig. 3) and of large vessel human umbilical vein endothelial cells (data not shown) was unaffected over a wide range of doses of huIL-4.
Figure 3.
Effect of IL-4 on the proliferation of human capillary endothelial cells. Human dermal microvascular endothelial cells were allowed to grow over 72 h in the presence of increasing concentrations of IL-4 (open circles) or the combination of IL-4 and 100 ng/ml bFGF (filled circles) and proliferation quantitated. Note: Dotted lines indicate growth in the absence of any cytokine additions (no proliferation) and growth in the presence of 100 ng/ml bFGF (bFGF). Similar results were obtained if serum was used instead of bFGF or HUVEC cells instead of HMVECs. Bars indicate SE.
Antiangiogenic Activity of IL-4 in Complex Biological Fluids.
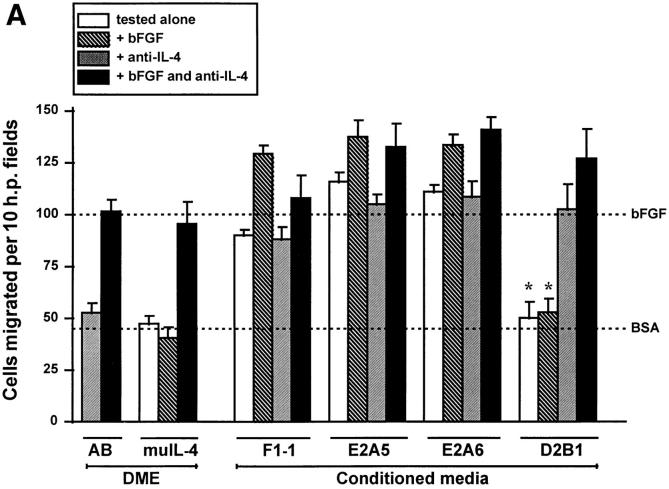
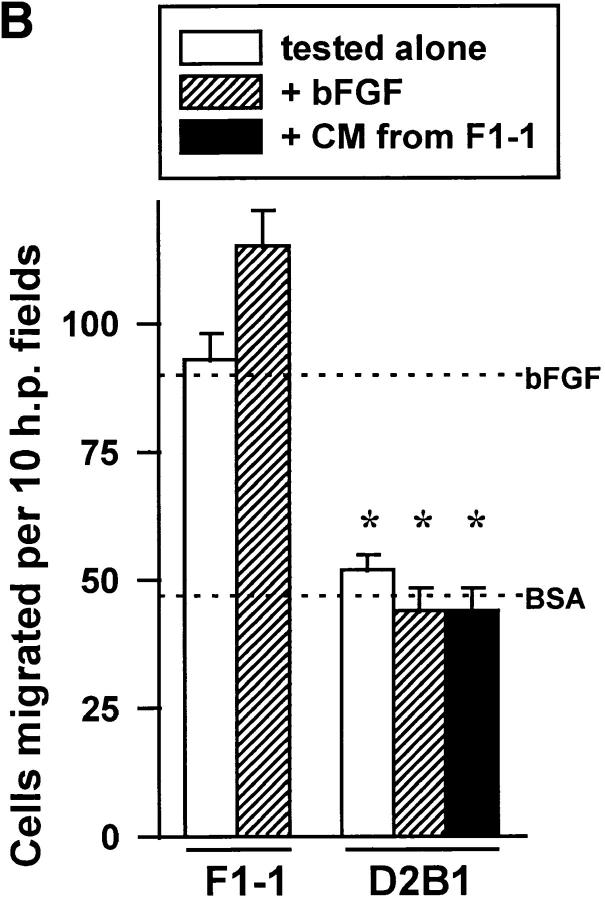
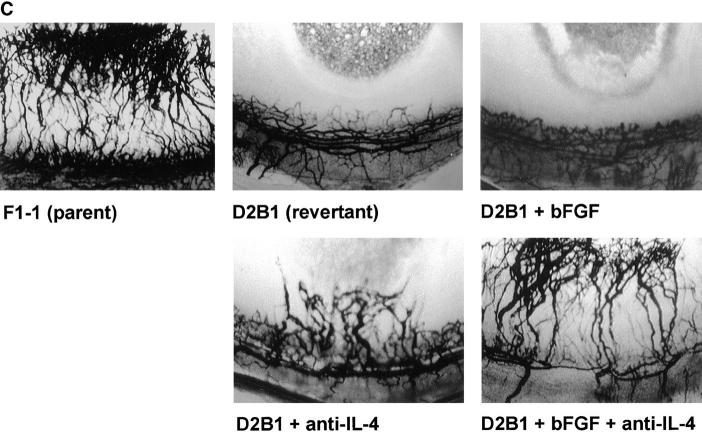
To determine if IL-4 is present in biological fluids in sufficient concentrations to be antiangiogenic, supernatants from two types of cells were tested. Serum-free conditioned media were collected from mouse mammary carcinoma tumor cell line, K485, and from its subclones that expressed IL-4 and as a result are known to produce slower growing tumors in vivo (32). Media from a vector-transfected control (F1-1, making no detectable IL-4, <0.001 ng IL-4/μg protein) and from two IL-4–transfected subclones that expressed low levels of IL-4 (E2A5 producing 0.18 ng IL-4/μg protein and E2A6 producing 0.06 ng IL-4/μg protein) were angiogenic and not sensitive to neutralizing antibody against the cytokine (Fig. 4 A). If this concentration of IL-4 were used by itself in a migration assay it would be weakly stimulatory. In contrast, medium conditioned by the IL-4 transfectant that produced high levels of IL-4 (D2B1 secreting 15 ng IL-4/μg protein), the line that was most severely retarded in in vivo tumorigenicity assays (32), was antiangiogenic despite the background of tumor angiogenic factors (Fig. 4, A–C; Table 3). The D2B1 conditioned medium blocked migration in vitro (Fig. 4 A) even towards media conditioned by the tumorigenic parent (Fig. 4 B) as well as neovascularization in vivo (Table 3; Fig. 4 C) induced by bFGF. IL-4 was the major inhibitor in this medium for its neutralization revealed underlying angiogenic activity and rendered the samples unable to inhibit angiogenesis induced by bFGF.
Figure 4.
IL-4 is responsible for the lack of in vitro angiogenic activity in revertant K485 cells. (A) Media conditioned by K485 carcinoma cells transfected with vector (F1-1) or transfected with murine IL-4 and expressing the cytokine at low levels (E2A5, E2A6) and at high levels (D2B1) were tested for ability to induce the migration of bovine capillary endothelial cells alone, when mixed with bFGF (10 ng/ml) and/or with neutralizing anti–muIL-4 antibodies (32 μl/ml). Controls labeled DME include tests of antibodies alone (AB) and of murine IL-4 with other components as indicated. Note: dotted lines as in legend to Fig. 1. (B) Supernatants of vector-transfected line F1-1 and IL-4– transfected revertant D2B1 were mixed 1:1 and tested for the ability to induce the migration of bovine capillary endothelial cells. (C) Media conditioned by parental F1-1 and revertant D2B1 cells was incorporated into pellets with the indicated additions and tested in the rat cornea for ability to induce neovascularization. An asterisk indicates significant difference from parental F1-1 line by unpaired Student's t test, P < 0.002.
Table 3.
Secretions of IL-4–producing Revertants of Mammary Carcinoma 287 Failed to Induce Neovascularization In Vivo due to High Levels of IL-4
| Test media |
Added substances | Positive corneas/ total implanted |
||||
|---|---|---|---|---|---|---|
| bFGF | anti–IL-4 | |||||
| IL-4–transfected revertant | ||||||
| D2-B1 | − | − | 0/3 | |||
| D2-B1 | + | − | 0/3 | |||
| D2-B1 | − | + | 2/3 | |||
| D2-B1 | + | + | 3/3 | |||
| Vector-transfected parental line | ||||||
| F1-1 (IL-4 deficient) | − | − | 3/3 | |||
| F1-1 + inhibitory TSP | − | − | 0/3 | |||
Media conditioned by the indicated cells was incorporated into a Hydron pellet at 200 μg/ml along with various additions, implanted into the rat cornea and neovascularization assessed 7 d later. Concentrations of additives as in legend to Table 1. TSP, human thrombospondin-1, a known inhibitor of angiogenesis used as described in 53.
In a second experiment, supernatants of stimulated murine TH0 cells were tested for angiogenic activity. These supernatants that contained 21 ng/ml of IFN-γ and 7.7 ng/ml of IL-4 were antiangiogenic due to the presence of IL-4 (Fig. 5). When IL-4 was neutralized they became able to induce the migration of capillary endothelial cells and were no longer able to inhibit migration induced by bFGF.
Figure 5.
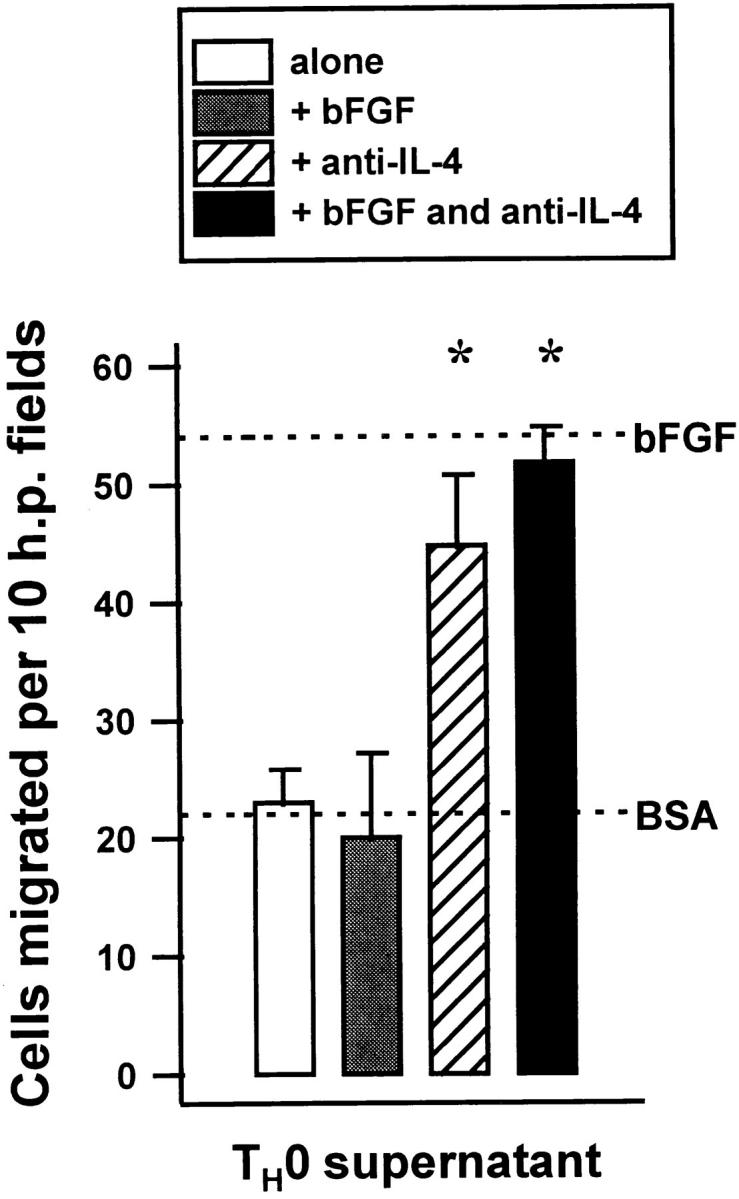
Supernatants of T0 cells are antiangiogenic due to IL-4. Supernatants obtained from splenic cells freshly derived from D011.10 T cell receptor transgenic mice and incubated with ovalbumin for 72 h were tested for the ability to induce the migration of bovine capillary endothelial cells alone and with either bFGF, anti–IL-4 or both. Dotted lines indicate migration of cells towards bFGF alone or towards media alone (BSA). An asterisk indicates significant difference from antibody-free control, P < 0.005.
Discussion
Data presented above demonstrate that IL-4 is a potent inhibitor of angiogenesis in vivo when introduced locally as well as when injected systemically. It is among the most potent inhibitors identified to date. Its ED50 measured in the migration assay was between 0.015 and 0.15 nM, better than that measured in the same assay for other potent antiangiogenic agents such as thrombospondin-1 (0.5 nM, see reference 40) and angiostatin (7 nM, see reference 41). The dose of the 13-kD IL--4 that was needed to inhibit neovascularization systemically in the mouse was a relatively modest 0.5 mg/kg/d which compares favorably with the doses needed to produce the same effect with other inhibitors of angiogenesis including the 5–10 mg/kg/d needed for 450-kD thrombospondin-1 (20), the 6–100 mg/kg/d required for 38–45-kD angiostatin (42, 43) and 20 mg/kg/d for 20-kD endostatin (44).
The inhibition of angiogenesis is a newly recognized function for IL-4 and one that helps to explain a number of its previously noted activities. Our ability to drive mice into an antiangiogenic state with IL-4 injections suggests that a systemic, IL-4–dependent antiangiogenic effect may contribute to the ability of IL-4 secreting cells to inhibit the growth of distant tumors (45, 17). The inhibition of angiogenesis may also play a role in the ability of IL-4 to ameliorate arthritis in animals (46) and reduce the cartilage degradation in patients (47) that results in part from invading endothelial cells in the synovial pannus. The local antiangiogenic activity of IL-4 could account for the decrease in tumor vessel density observed in IL-4 secreting gliomas (21) and should enhance its effectiveness as a gene therapy agent against tumor metastases (48).
IL-4 inhibits angiogenesis by acting directly on endothelial cells for it was able to block the migration of cultured cells, as are most other direct inhibitors of angiogenesis (49). At very low doses, IL-4 was stimulatory to endothelial cells in vitro and able to induce a weak neovascularization response in vivo. Such a biphasic dose–response curve is unusual although not unprecedented. TGF-β1 also has biphasic effects on endothelial cell migration, stimulating at picomolar doses but inhibiting at nanomolar concentrations (40). Although this phenomenon of stimulation giving way to inhibition has not been explained, there is an explanation for one example of the inverse situation where inhibition of migration of endothelial cells at low concentrations gives way to stimulation at high doses. This occurs with the inhibitor of angiogenesis thrombospondin-1 and can be explained by the sequential activation of two distinct receptors. An inhibitory receptor is active at low doses, but it is easily cleared from the cells so that at high doses a second more stable receptor with lower affinity is engaged and transduces a stimulatory signal (50, 51). A dual receptor hypothesis for IL-4 is supported by the observation that IL-13 replicated the activity of IL-4 in the inducing phase of the dose–response curve with reasonable fidelity yet required 10-fold more protein to reproduce the inhibitory portion. Such an observation could be explained if endothelial cells expressed a stimulatory receptor that had equal affinity for IL-4 and IL-13 in addition to a second receptor, which transduces inhibitory signals, that interacted more effectively with IL-4 than with IL-13.
In contrast to the situation in hematopoietic cells where IL-4 displays tight species specificity between mouse and human, no species limitations were observed in our experiments where human and mouse cytokines were equally effective on bovine and human endothelial cells and human IL-4 was effective in the rat cornea assay. It is possible that the species specificity that has been defined in lymphoid cells depends on the γc subunit of the receptor that is absent from endothelial cells where it is replaced by an IL-13 receptor subunit.
Despite its effects on migration, IL-4 did not appear to have any effect on the growth of endothelial cells. Although both the migration and the mitogenesis of endothelial cells are frequently blocked by agents that inhibit angiogenesis, there is a small class of inhibitors that do not seem to have major effects on mitogenesis (49). However, it may be premature to assign IL-4 to this class given the fact that another report, using microvascular endothelial cells that were brought more severely to quiescence before the start of the experiments, has described a modest mitogenic effect of IL-4 (29).
Although data presented here demonstrate most clearly the inhibition of angiogenesis induced by bFGF, IL-4 was also able to block angiogenesis induced by other factors for it was effective against tumor cell conditioned media, against the mix of inducers present in the TH0 cell supernatant as well as against media conditioned by activated macrophages (data not shown).
In identifying IL-4 as a new inhibitor of angiogenesis these results provide a new mechanism to explain its demonstrated antitumor activity at both local and distant sites, and suggest, as has been demonstrated for other inhibitors of angiogenesis (52), that it may be particularly useful in enhancing the effect of cytotoxic antitumor therapies. In addition, the ability of IL-4 to be either a positive or a negative influence on neovascularization must now be taken into account when explaining the induction and resolution of the inflammatory angiogenesis that accompanies so many pathological processes.
Abbreviations used in this paper
- bFGF
basic fibroblast growth factor
- FBS
fetal bovine serum
- HMVEC
human dermal microvascular endothelial cells
- hu
human recombinant
- mu
murine recombinant
- VCAM-1
vascular cell adhesion molecule 1
Footnotes
It is a pleasure to thank Bob Coffman of DNAX for crucial advice on in vivo dosing with IL-4 and the Schering Plough Research Institute for supplying the cytokine used for the in vivo work.
We also thank the National Institutes of Health for the grants that supported this work: CA52750 and CA64239 (N.P. Bouck), AR30692 and AR41492 (A.E. Koch) and AA08275 (C. Waltenbaugh). AEK also receives funds from the Veterans Administration Research Service, the Arthritis Foundation Illinois Chapter Wolkonsky Award for Rheumatoid Arthritis Research Grant and the Gallagher Professorship for Arthritis Research.
References
- 1.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–1870. [PubMed] [Google Scholar]
- 2.Kopf M, Le Gros G, Bachmann M, Lamers KC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 4.Ohara J, Paul WE. Receptors for B-cell stimulatory factor-1 expressed on cells of haematopoietic lineage. Nature. 1987;325:537–540. doi: 10.1038/325537a0. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Yokota T, Kastelein R, Zurawski SM, Arai N, Takebe Y. Species-specificity of T cell stimulating activities of IL 2 and BSF-1 (IL-4): comparison of normal and recombinant, mouse and human IL-2 and BSF-1 (IL-4) J Immunology. 1987;138:1813–1816. [PubMed] [Google Scholar]
- 6.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 7.Puri RK, Siegel JP. Interleukin-4 and cancer therapy. Cancer Investig. 1993;11:473–486. doi: 10.3109/07357909309018879. [DOI] [PubMed] [Google Scholar]
- 8.Toi M, Bicknell R, Harris AL. Inhibition of colon and breast carcinoma cell growth by interleukin-4. Cancer Res. 1992;52:275–279. [PubMed] [Google Scholar]
- 9.Obiri NI, Hillman GG, Haas GP, Sud S, Puri RK. Expression of high affinity interleukin-4 receptors on human renal cell carcinoma cells and inhibition of tumor cell growth in-vitro by interleukin-4. J Clin Invest. 1993;91:88–93. doi: 10.1172/JCI116205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topp MS, Papadimitriou CA, Eitelbach F, Koenigsmann M, Oelmann E, Koehler B, Oderberg D, Reufi B, Stein H, Thiel E, Berdel WE. Recombinant human interleukin 4 has antiproliferative activity on human tumor cell lines derived from epithelial and nonepithelial histologies. Cancer Res. 1995;55:2173–2176. [PubMed] [Google Scholar]
- 11.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 12.Tepper RI. The eosinophil-mediated antitumor activity of interleukin-4. J Allergy Clin Immunol. 1994;94:1225–1231. doi: 10.1016/0091-6749(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz MA, Tardelli L, Macosko HD, Sullivan LM, Narula SK, Fine JS. Interleukin 4 retards dissemination of a human B-cell lymphoma in severe combined immunodeficient mice. Cancer Res. 1995;55:3692–3696. [PubMed] [Google Scholar]
- 14.Hillman GG, Younes E, Visscher D, Ali E, Lam JS, Montecillo E, Pontes JE, Haas GP, Puri RK. Systemic treatment with interleukin-4 induces regression of pulmonary metastases in a murine renal cell carcinoma model. Cell Immunol. 1995;160:257–263. doi: 10.1016/0008-8749(95)80036-i. [DOI] [PubMed] [Google Scholar]
- 15.Folkman, J. 1995. Tumor Angiogenesis. In The Molecular Basis of Cancer. J. Mendelsohn, P.M. Howley, M.A. Israel, and L.A. Liotta, editors. W.B. Saunders Company, Philadelphia, PA. 206–232.
- 16.Prehn RT. Two competing influences that may explain concomitant tumor resistance. Cancer Res. 1993;53:3266–3269. [PubMed] [Google Scholar]
- 17.Ohira T, Ohe Y, Heike Y, Podack ER, Olsen KJ, Nishio K, Nishio M, Miyahara Y, Funayama Y, Ogasawara H, et al. In vitro and in vivo growth of B16F10 melanoma cells transfected with interleukin-4 cDNA and gene therapy with the transfectant. J Cancer Res Clin Oncol. 1994;120:631–635. doi: 10.1007/BF01245372. [DOI] [PubMed] [Google Scholar]
- 18.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 19.O'Reilly M, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 20.Volpert OV, Lawler J, Bouck NP. A human fibrosarcoma inhibits systemic angiogenesis and the growth of experimental metastases via thrombospondin-1. Proc Natl Acad Sci USA. 1998;95:6343–6348. doi: 10.1073/pnas.95.11.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saleh M, Davis ID, Wilks AF. The paracrine role of tumor-derived mIL-4 on tumor-associated endothelium. Int J Cancer. 1997;72:664–672. doi: 10.1002/(sici)1097-0215(19970807)72:4<664::aid-ijc19>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Schnyder B, Lugli S, Feng N, Etter H, Lutz RA, Ryffel B, Sugamura K, Wunderli-Allenspach H, Moser R. Interleukin-4 (IL-4) and IL-13 bind to a shared heterodimeric complex on endothelial cells mediating vascular cell adhesion molecule-1 induction in the absence of the common γ chain. Blood. 1996;87:4286–4295. [PubMed] [Google Scholar]
- 23.Palmer-Crocker RL, Hughes CCW, Pober JS. IL-4 and IL-13 activate the JAK2 tyrosine kinase and Stat6 in cultured human vascular endothelial cells through a common pathway that does not involve the γc chain. J Clin Invest. 1996;98:604–609. doi: 10.1172/JCI118829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotowicz K, Callard RE, Friederich K, Matthews DJ, Klein N. Biological activity of IL-4 and IL-13 on human endothelial cells: functional evidence that both cytokines act through the same receptor. Int Immunol. 1996;8:1915–1925. doi: 10.1093/intimm/8.12.1915. [DOI] [PubMed] [Google Scholar]
- 25.Swerlick RA, Lee KH, Li L-J, Sepp NT, Caughman SW, Lawley TJ. Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells. J Immunol. 1992;149:698–705. [PubMed] [Google Scholar]
- 26.Haraldsen G, Kvale D, Lein B, Farstad IN, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human intestinal microvascular endothelial cells. J Immunol. 1996;156:2558–2565. [PubMed] [Google Scholar]
- 27.Petzelbauer P, Bender JR, Wilson J, Pober JS. Heterogeneity of dermal microvascular endothelial cell antigen expression and cytokine responsiveness in situ and in cell culture. J Immunol. 1993;151:5062–5072. [PubMed] [Google Scholar]
- 28.Bergese SD, Huang EH, Pelletier RP, Widmer MB, Ferguson RM, Orosz CG. Regulation of endothelial VCAM-1 expression in murine cardiac grafts. Expression of allograft endothelial VCAM-1 can be manipulated with antagonist of IFN-α or IL-4 and is not required for allograft rejection. Am J Pathol. 1995;147:166–175. [PMC free article] [PubMed] [Google Scholar]
- 29.Toi M, Harris AL, Bicknell R. Interleukin-4 is a potent mitogen for capillary endothelium. Biochem Biophys Res Commun. 1991;174:1287–1293. doi: 10.1016/0006-291x(91)91561-p. [DOI] [PubMed] [Google Scholar]
- 30.Ohara J, Paul WE. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985;315:333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- 31.Leder A, Pattengale PK, Kuo A, Stewart TA, Leder P. Consequences of widespread deregulation of the c-myc gene in transgenic mice: multiple neoplasms and normal development. Cell. 1986;45:485–495. doi: 10.1016/0092-8674(86)90280-1. [DOI] [PubMed] [Google Scholar]
- 32.Tepper RI, Pattengale PK, Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989;57:503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- 33.Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 34.Polverini PJ, Bouck NP, Rastinejad F. Assay and purification of a naturally occurring inhibitor of angiogenesis. Methods Enzymol. 1991;198:440–450. doi: 10.1016/0076-6879(91)98044-7. [DOI] [PubMed] [Google Scholar]
- 35.Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Investig Ophthalmol Vis Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- 36.Finkelman FD, Katona IM, Urban JF, Snapper CM, Ohara J, Paul WE. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci USA. 1986;83:9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aman MJ, Tayebi N, Obir NI, Puri RK, Modi WS, Leonard WJ. cDNA cloning and characterization of the human interleukin 13 receptor α chain. J Biol Chem. 1996;271:29265–29270. doi: 10.1074/jbc.271.46.29265. [DOI] [PubMed] [Google Scholar]
- 38.Lugli SM, Feng N, Heim MH, Adam M, Schnyder B, Etter H, Yamage M, Eugster H-P, Lutz RA, Zurawski G, Moser R. Tumor necrosis factor α enhances the expression of the interleukin (IL)-4 receptor α-chain on endothelial cells increasing IL-4 or IL-13-induced Stat6 activation. J Biol Chem. 1997;272:5487–5494. doi: 10.1074/jbc.272.9.5487. [DOI] [PubMed] [Google Scholar]
- 39.Orchansky PL, Ayres SD, Hilton DJ, Schrader JW. An interleukin (IL)-13 receptor lacking the cytoplasmic domain fails to transduce IL-13-induced signals and inhibits responses to IL-4. J Biol Chem. 1997;272:22940–22947. doi: 10.1074/jbc.272.36.22940. [DOI] [PubMed] [Google Scholar]
- 40.Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gately S, Twardowski P, Stack MS, Patrick M, Boggio L, Cundiff DL, Schnaper HW, Madison L, Volpert O, Bouck N, et al. Human prostate carcinoma cells express enzymatic activity that converts human plasminogen to the angiogenesis inhibitor, angiostatin. Cancer Res. 1996;56:4887–4890. [PubMed] [Google Scholar]
- 42.Wu Z, O'Reilly MS, Folkman J, Shing Y. Suppression of tumor growth with recombinant murine angiostatin. Biochem Biophys Res Commun. 1997;236:651–654. doi: 10.1006/bbrc.1997.7032. [DOI] [PubMed] [Google Scholar]
- 43.Sim BKL, O'Reilly MS, Liang H, Fortier AH, He W, Madsen JW, Lapcevich R, Nacy CA. A recombinant human angiostatin protein inhibits experimental primary and metastatic cancer. Cancer Res. 1997;57:1329–1334. [PubMed] [Google Scholar]
- 44.Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 45.Golumbek PT, Lazenby AJ, Levitsky HI, Jaffee LM, Karasuyama H, Baker M, Pardoll DM. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991;254:713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- 46.Allen JB, Wong HL, Costa GL, Bienkowski MJ, Wahl SM. Suppression of monocyte function and differential regulation of IL-1 and IL-1rα by IL-4 contribute to resolution of experimental arthritis. J Immunol. 1993;151:4344–4351. [PubMed] [Google Scholar]
- 47.van-Roon JA, van-Roy JL, Gmelig-Meyling FH, Lafeber FP, Bijlsma JW. Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin-10 and interleukin-4. Arthritis Rheum. 1996;39:829–835. doi: 10.1002/art.1780390516. [DOI] [PubMed] [Google Scholar]
- 48.Hurford RK, Jr, Dranoff G, Mulligan RC, Tepper RI. Gene therapy of metastatic cancer by in vivo retroviral gene targeting. Nature Genet. 1995;10:430–435. doi: 10.1038/ng0895-430. [DOI] [PubMed] [Google Scholar]
- 49.Bouck N, Stellmach V, Hsu S. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 50.Dawson DW, Pearce SFA, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawson, D.W., and N. Bouck. 1998. Thrombospondin as an inhibitor of angiogenesis. In Antiangiogenic Agents in Cancer. B.A. Teicher, editor. Humana Press, Totowa, NJ. In press.
- 52.Teicher, B.A., Y. Emi, Y. Kakeji, and D. Northey. 1996. TNP-470/minocycline/cytotoxic therapy: a systems approach to cancer therapy. Eur. J. Cancer. 32A:2461–2466. [DOI] [PubMed]
- 53.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]