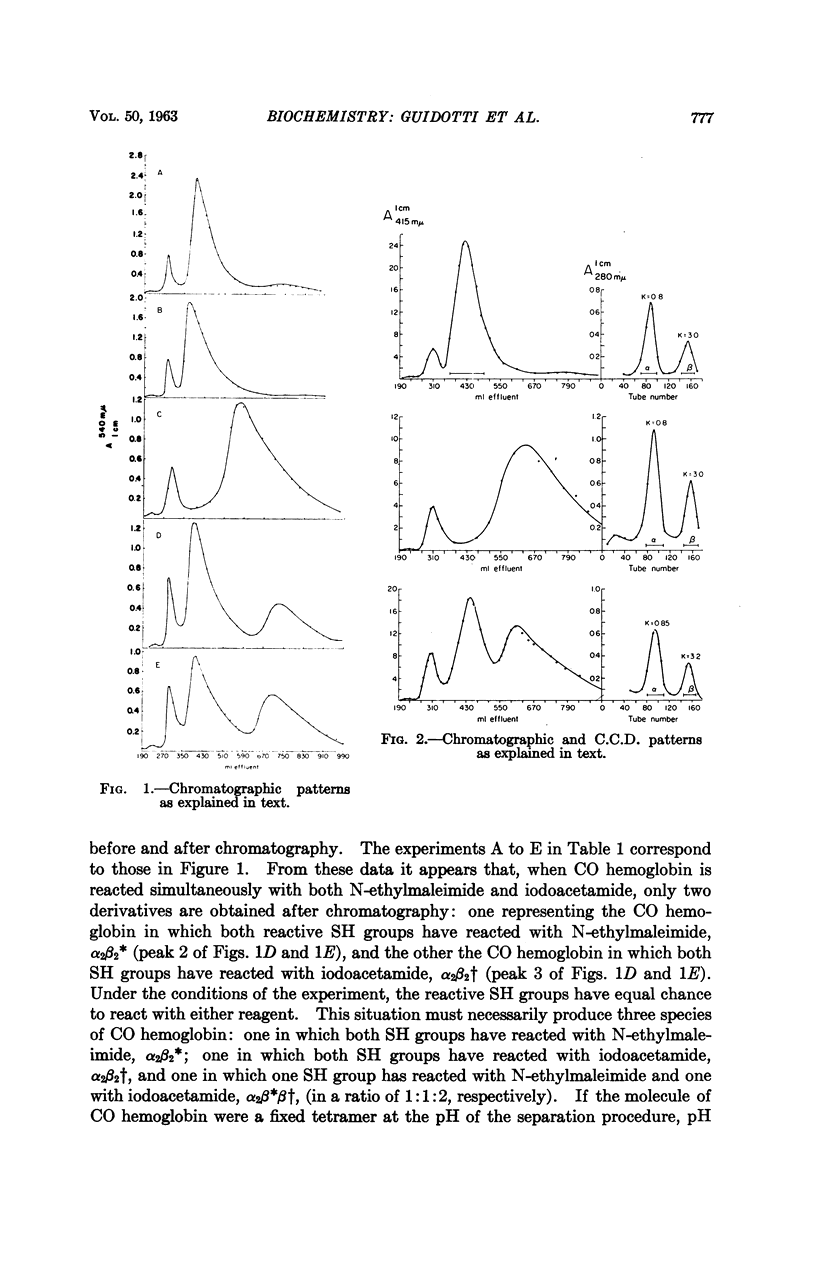
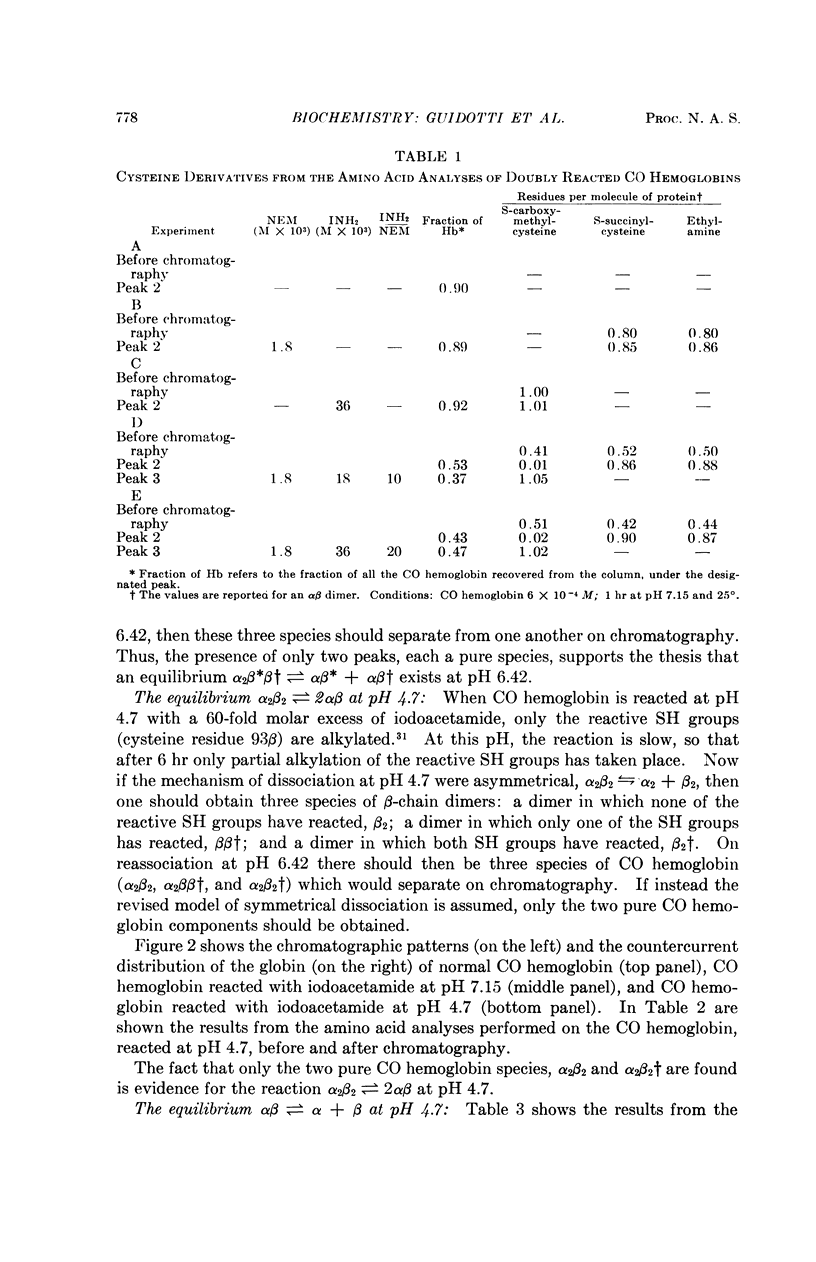
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., WYMAN J., BUCCI E., FRONTICELLI C., ROSSI-FANELLI A. The dissociation and recombination of subunits of human, horse, and canine hemoglobin. J Mol Biol. 1962 May;4:368–375. doi: 10.1016/s0022-2836(62)80017-5. [DOI] [PubMed] [Google Scholar]
- BENESCH R. E., BENESCH R., WILLIAMSON M. E. The influence of reversible oxygen binding on the interaction between hemoglobin submits. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2071–2075. doi: 10.1073/pnas.48.12.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENESCH R. E., RANNEY H. M., BENESCH R., SMITH G. M. The chemistry of the Bohr effect. II. Some properties of hemoglobin H. J Biol Chem. 1961 Nov;236:2926–2929. [PubMed] [Google Scholar]
- BENHAMOU N., DAUNE M., JACOB M., LUZZATI A., WEILL G. [Study of the action of sodium chloride on oxyhemoglobin. II. Influence of the protein concentration on the dissociation]. Biochim Biophys Acta. 1960 Jan 1;37:1–14. doi: 10.1016/0006-3002(60)90071-8. [DOI] [PubMed] [Google Scholar]
- BRAUNITZER G., GEHRING-MUELLER R., HILSCHMANN N., HILSE K., HOBOM G., RUDLOFF V., WITTMANN-LIEBOLD B. [The structure of normal adult human hemoglobins]. Hoppe Seylers Z Physiol Chem. 1961 Sep 20;325:283–286. doi: 10.1515/bchm2.1961.325.1.283. [DOI] [PubMed] [Google Scholar]
- FIELD E. O., O'BRIEN J. R. Dissociation of human haemoglobin at low pH. Biochem J. 1955 Aug;60(4):656–661. doi: 10.1042/bj0600656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIDOTTI G., HILL R. J., KONIGSBERG W. The structure of human hemoglobin. II. The separation and amino acid composition of the tryptic peptides from the alpha and beta chains. J Biol Chem. 1962 Jul;237:2184–2195. [PubMed] [Google Scholar]
- GUTTER F. J., SOBER H. A., PETERSON E. A. The effect of mercaptoethanol and urea on the molecular weight of hemoglobin. Arch Biochem Biophys. 1956 Jun;62(2):427–434. doi: 10.1016/0003-9861(56)90141-2. [DOI] [PubMed] [Google Scholar]
- Gralén N. The splitting of haemoglobin by acids. Biochem J. 1939 Dec;33(12):1907–1912. doi: 10.1042/bj0331907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL R. J., KONIGSBERG W., GUIDOTTI G., CRAIG L. C. The structure of human hemoglobin. I. The separation of the alpha and beta chains and their amino acid composition. J Biol Chem. 1962 May;237:1549–1554. [PubMed] [Google Scholar]
- HUEHNS E. R., SHOOTER E. M., DANCE N., BEAVEN G. H., SHOOTER K. V. Haemoglobin alpha A. Nature. 1961 Dec 16;192:1057–1059. doi: 10.1038/1921057a0. [DOI] [PubMed] [Google Scholar]
- HUEHNS E. R., SHOOTER E. M. Polypeptide chains of haemoglobin A2. Nature. 1961 Mar 18;189:918–919. doi: 10.1038/189918a0. [DOI] [PubMed] [Google Scholar]
- HUISMAN T. H., MARTIS E. A., DOZY A. Chromatography of hemoglobin types on carboxymethylcellulose. J Lab Clin Med. 1958 Aug;52(2):312–327. [PubMed] [Google Scholar]
- Hasserodt U., Vinograd J. DISSOCIATION OF HUMAN CARBONMONOXYHEMOGLOBIN AT HIGH pH. Proc Natl Acad Sci U S A. 1959 Jan;45(1):12–15. doi: 10.1073/pnas.45.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITANO H. A., ROBINSON E. Properties and inheritance of haemoglobin by asymmetric recombination. Nature. 1959 Nov 7;184:1468–1469. doi: 10.1038/1841468a0. [DOI] [PubMed] [Google Scholar]
- Itano H. A., Singer S. J. ON DISSOCIATION AND RECOMBINATION OF HUMAN ADULT HEMOGLOBINS A, S, AND C. Proc Natl Acad Sci U S A. 1958 Jun;44(6):522–529. doi: 10.1073/pnas.44.6.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONIGSBERG W., GOLDSTEIN J., HILL R. J. The structure of human hemoglobin. VII. The digestion of the beta chain of human hemoglobin with pepsin. J Biol Chem. 1963 Jun;238:2028–2033. [PubMed] [Google Scholar]
- KONIGSBERG W., HILL R. J. The structure of human hemoglobin. V. The digestion of the alpha chain of human hemoglobin with pepsin. J Biol Chem. 1962 Oct;237:3157–3162. [PubMed] [Google Scholar]
- LABIE D., ROSA J., DREYFUS J. C., SCHAPIRA G. Inter-specific hybridization of haemoglobin. Nature. 1962 Apr 28;194:384–385. doi: 10.1038/194384a0. [DOI] [PubMed] [Google Scholar]
- Lamm O., Polson A. The determination of diffusion constants of proteins by a refractometric method. Biochem J. 1936 Mar;30(3):528–541. doi: 10.1042/bj0300528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGAS D. A., KOLER R. D., OSGOOD E. E. Hemoglobin H; clinical, laboratory, and genetic studies of a family with a previously undescribed hemoglobin. J Lab Clin Med. 1956 Jan;47(1):51–64. [PubMed] [Google Scholar]
- RIGGS A. The binding of N-ethylmaleimide by human hemoglobin and its effect upon the oxygen equilibrium. J Biol Chem. 1961 Jul;236:1948–1954. [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Studies on the relations between molecular and functional properties of hemoglobin. I. The effect of salts on the molecular weight of human hemoglobin. J Biol Chem. 1961 Feb;236:391–396. [PubMed] [Google Scholar]
- SCHROEDER W. A., CUA J. T., MATSUDA G., FENNINGER W. D. Hemoglobin F1, an acetyl-containing hemoglobin. Biochim Biophys Acta. 1962 Oct 8;63:532–534. doi: 10.1016/0006-3002(62)90125-7. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Itano H. A. ON THE ASYMMETRICAL DISSOCIATION OF HUMAN HEMOGLOBIN. Proc Natl Acad Sci U S A. 1959 Feb;45(2):174–184. doi: 10.1073/pnas.45.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., HUTCHINSON W. D. Carbon-14 labelled hybrids of haemoglobin. Nature. 1960 Jul 16;187:216–218. doi: 10.1038/187216a0. [DOI] [PubMed] [Google Scholar]


